Abstract
Curcumin is a popular, plant-derived compound that has been extensively investigated for diverse range of biological activities. Anti-cancer activity against various types of cancers and high safety profile associated with curcumin makes it very attractive. In this study, we report the synthesis and evaluation of pyrazole and click chemistry curcumin analogues for Head and Neck cancer. MTT assay against head and neck cancer cell lines CAL27 and UM-SCC-74A revealed the micromolar potency of the synthesized compounds. To determine the possible molecular mechanisms, effect of these analogues in the expression of pSTAT3, pFAK, pERK1/2 and pAKT was studied. Interestingly, compounds 2 and 5 significantly inhibited the pSTAT3 (Tyr 705) phosphorylation. As far as other compounds, they showed potent cytotoxicity against CAL27 however these compounds didn’t show any activity on pSTAT3 phosphorylation at IC50 concentration level. Molecular docking studies revealed the possible binding mode of pyrazole compound 2 in the SH2 domain of STAT3.
Keywords: Curcumin, Pyrazole analogue, Click-chemistry curcumin analogue, HNSCC
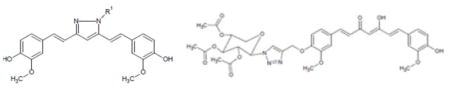
Graphical Abstract
Curcumin, a famous small molecule of natural product is being extensively explored for many biological activities. This study focuses on the synthesis and evaluation of pyrazole and click chemistry curcumin analogues for Head and Neck cancer activity. To determine the molecular mechanisms, a study was conducted to see the effect of the analogues in the expression of pSTAT3, pFAK, pERK1/2 and pAKT.
Curcumin, a natural polyphenol which is used as a food colorant, and herbal medicine in Asia. Curcumin has received a lot of attention recently due to its high safety profile and efficacy in treating various diseases (1–3). Potential anti-cancer activities associated with curcumin makes it a promising therapeutic agent. Apart from anti-cancer activity, it has shown anti-oxidant, anti-inflammatory, chemopreventive, chemotherapeutic, anti-microbial, and anti-fungal activities.
Curcumin (Figure 1) is being vigorously studied for its effect against breast cancer, prostate cancer, liver cancer, ovarian cancer, and cervical cancer (4). It demonstrated cytotoxic effects against cancer cell lines and cytoprotective effects on non-cancer cells. One of the important aspects of curcumin is its high safety profile of up to 12 g per day oral administration (5).
Figure 1.
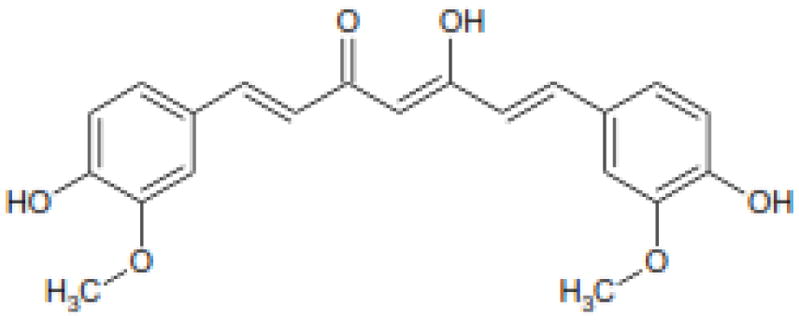
Structure of Curcumin (1)
Nevertheless, there are a number of challenges, low aqueous solubility, poor absorption, and rapid metabolism that limits its therapeutic efficacy (6). Curcumin can exitas a tautomeric mixture of keto and enol forms in solutions, and the enol form was found to be responsible for the rapid degradation of the curcumin. Chakraborti, S. et al., (7) found that the stability of curcumin was improved when the central diketone moiety of the curcumin was replaced by isooxazole and pyrazole groups. Structural modifications of curcumin were proven to be a meaningful approach to discover analogues with enhanced properties (8–12). Conjugates of curcumin with β- and γ-cyclodextrin, liposomal and loaded nanoparticles and nanoemulsion are some of the lipid-based colloidal system that have been employed to enhance water solubility and improving its bioavailability (13). Recent work supports the synergistic effect of curcumin against various cancers when administered with therapeutic cancer agents. Docetaxel, paclitaxel, bicalutamide, phenethyliosthiacyante, epigallocatechin gallate, and other agents in combination with curcumin enhanced cell growth inhibition and apoptosis (14).
Head and neck squamous cell carcinoma (HNSCC) is one of the most common types of cancer in the U.S. It includes tumors of the mouth, nose, sinuses, salivary gland, throat, and lymph nodes in the neck. The oral cavity and pharynx malignancy represent about 4% (34,780) of all cancer cases, with over 9,000 deaths twice as common in men as is women. The potential for recurrence of the malignancy is 35% within 2 years indicating its high importance in treatment (15). The effect of curcumin to inhibit the expression of matrix metalloproteases (MMP) in HNSCC cells was demonstrated. CCLL23, CAL27, UM-SCC1, and UM-SCC14A head and neck cancer cells respond differently to curcumin. There is a significant increase in cell death in CCL23 and CAL27 cell lines with 50 μmol/L of curcumin for 24 h. Treatment of 400 μM of curcumin in a time and concentration manner resulted in nearly 100 % cell death (16).
The activity of curcumin against head and neck squamous cell carcinoma in in-vitro and in vivo models is reported (17). Structural modifications of curcumin serve as a meaningful approach to discovering novel analogues to enhance the efficacy and improve its poor pharmacokinetics. A few of the structurally modified curcumin analogous have displayed efficacious anti-head and neck cancer activity (18).
Chakravarti et al reported the over expression of STAT3 in multiple head and neck cancers (19). Curcumin was found to suppress the IL-6 mediated phosphorylation of STAT3. The growth of immortalized oral mucosal epithelial cells and squamous carcinoma cells were found to be suppressed by curcumin (20).
In the previously published studies, heterocyclic modification at the keto-enol moiety of curcumin has been proven to be an important pharmacophore playing a pivotal role in various biological activities including anti-oxidant, anti-Alzheimer’s, anti-androgenic, and cytotoxicity. According to the literature, an appropriate modification at the keto-enol region could cause enhanced potency (21). In this work, design, synthesis and evaluation of few semi-synthetic curcumin analogues for cytotoxic activity against HNSCC cells are reported.
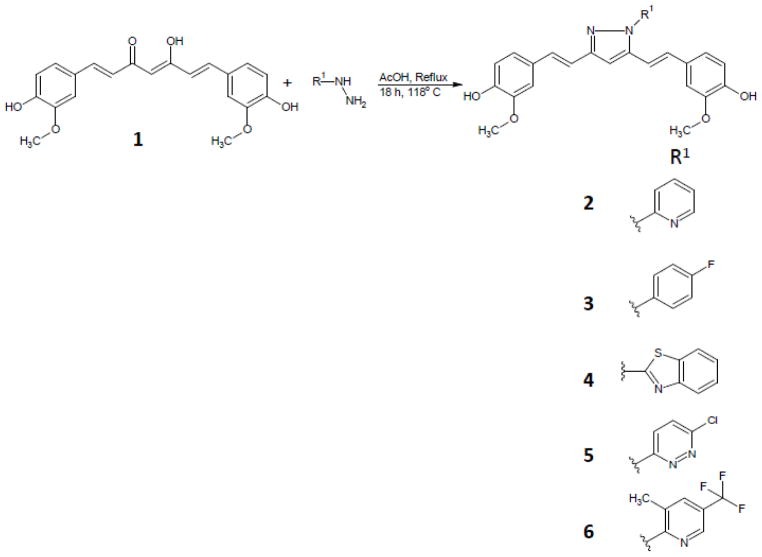
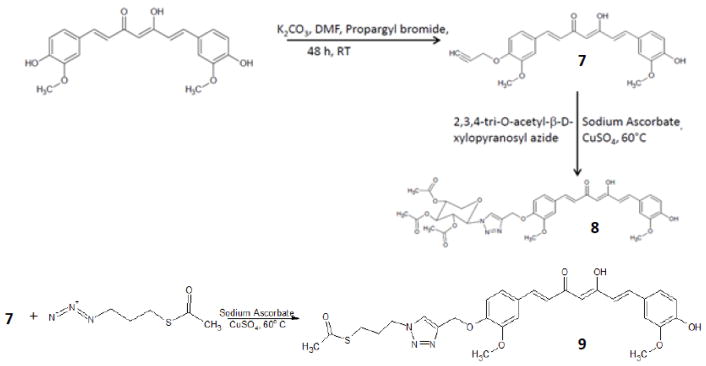
Curcumin was isolated from commercial curcumin powder by silica gel column chromatography using CH2Cl2: MeOH: AcOH. Final product was co-evaporated with toluene to remove traces of acetic acid to obtain pure curcumin 1. Pyrazole analogues, 2, 3, 4, 5, and 6 were prepared (scheme 1) by treating pure curcumin (1.2 mmol) in glacial acetic acid (9 ml) and substituted hydrazines (2.0 mmol) (22). The solution was refluxed for 8 h at 118°C and monitored by thin layer chromatography (Dichloromethane: Methanol, 95:5). The solution was evaporated under reduced pressure, co-evaporated with toluene. Crude product was further purified by silica gel chromatography, eluting with CH2Cl2-MeOH. “Click-chemistry” curcumin analogues 8 and 9 were prepared (scheme 2) by two step reaction. In the first step mono-propargyl curcumin was prepared using propargyl bromide. The mono-propargyl curcumin was treated with 2,3,4-tri-O-acetyl-β-D-xylopyranosylazide or thioacetate propyl azide, copper sulfate and sodium ascorbate with click-chemistry reaction protocol (23, 24).
Scheme 1.
Synthesis of Curcumin pyrazole analogues.
Scheme 2.
Synthesis of Curcumin click chemistry analogues
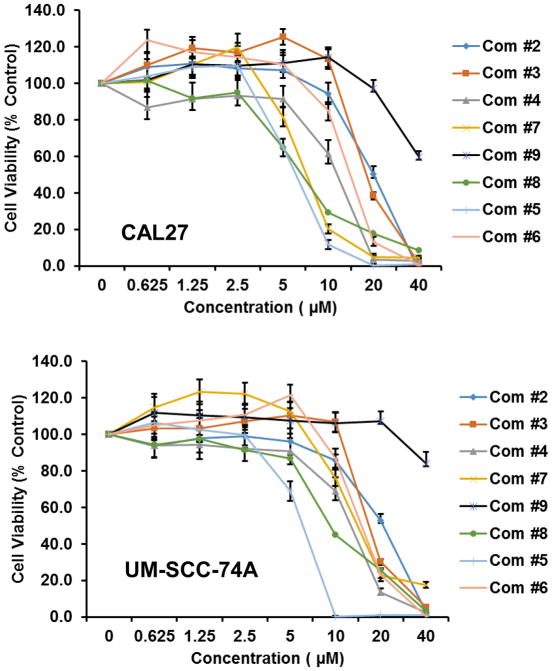
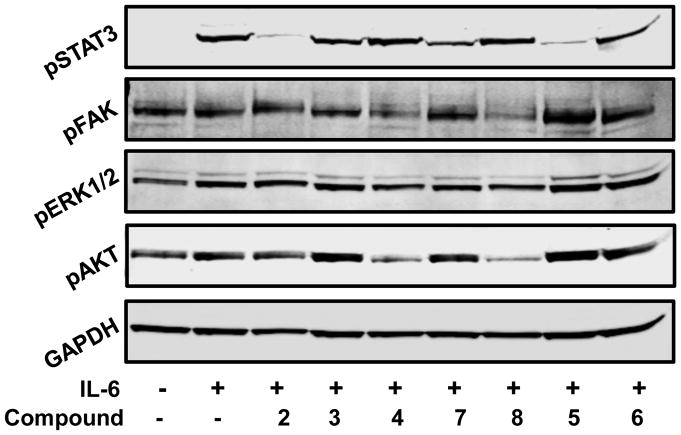
The in-vitro cytotoxicity of the synthesized curcumin analogues (2–9) were evaluated by MTT assay method against head and neck cancer cell lines CAL27 and UM-SCC-74A (18). Results showed that all the compounds except compound 9 were significantly active (Figure 2). To determine the possible molecular mechanisms, a detailed study was done. STAT3 activation in intracellular signaling has been cited as one of the most critical steps in HNSCC progression. Approximately 82 % of HNSCC exhibit up-regulation of STAT3 expression. However, limited data exists on the role of semi-synthetic curcumin analogue on STAT3 pathway in head and neck cancer. To find out the mechanism of the analogues, the effects on pSTAT3, pFAK, pERK1/2 and pAKT signaling was investigated at IC50 dose level as shown in figure 3. Interestingly, Compounds 2 and 5 showed good activity against CAL27 cell line (Figure 2), while inhibiting pSTAT3 (Tyr 705) phosphorylation at IC50 dose level (Figure 3). Moreover 7 showed weak inhibitory activity on pSTAT3 phosphorylation. Compound 8 appears to be acting on pFAK and pAKT pathways. Although compounds 3, 4, 6 and 7, exhibited potent cytotoxicity against CAL27 and UM-SCC-74A (Figure 2), but these compounds didn’t show any activity on pSTAT3, pFAK, PERK1/2 and pAKT signaling pathways. Further studies are needed to support the mechanism of these compounds.
Figure 2.
Anticancer activities of Compound 2–9 against CAL27 and UM-SCC-74A cell line
Figure 3.
Effect of curcumin analogues on pSTAT3, pFAK, pERK1/2 and pAKT signaling pathways at IC50 dose level.
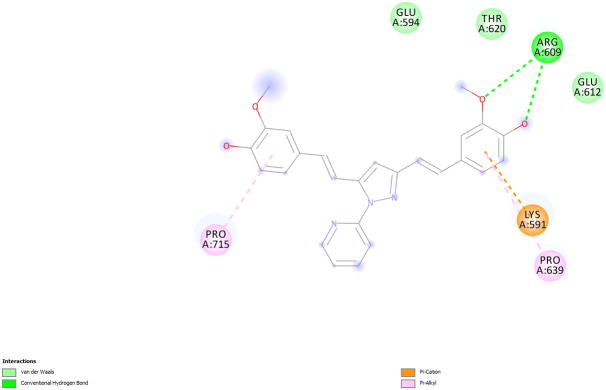
To determine the binding mode, molecular docking of one of the pSTAT3 active compounds, 2 into the SH2 domain of STAT3 was carried out using Fred (25–27) module. Omega2 (28, 29) was used to generate conformations of the ligand. The crystal structure of STAT3β (pdb code 1BG1) (30) was used to generate the receptor grid with the help of makereceptor (25–27) and a hydrogen bond constraint was applied for Arg609, one of the crucial amino acid in the phospho-tyrosine binding pocket.
The predicted binding mode of pyrazole compound 2 showed hydrogen bond formation between the methoxy substituent and Arg609 (Figure 4, 5). There is a cation-pi interaction between Lys 591 and the phenyl ring of pyrazole compound. Recent docking studies by Zhang et al (31) report the interaction of these two residues in the binding of compounds S3I-1757 and S3I-1756. The other phenyl ring has hydrophobic interaction with Pro715.
Figure 4.
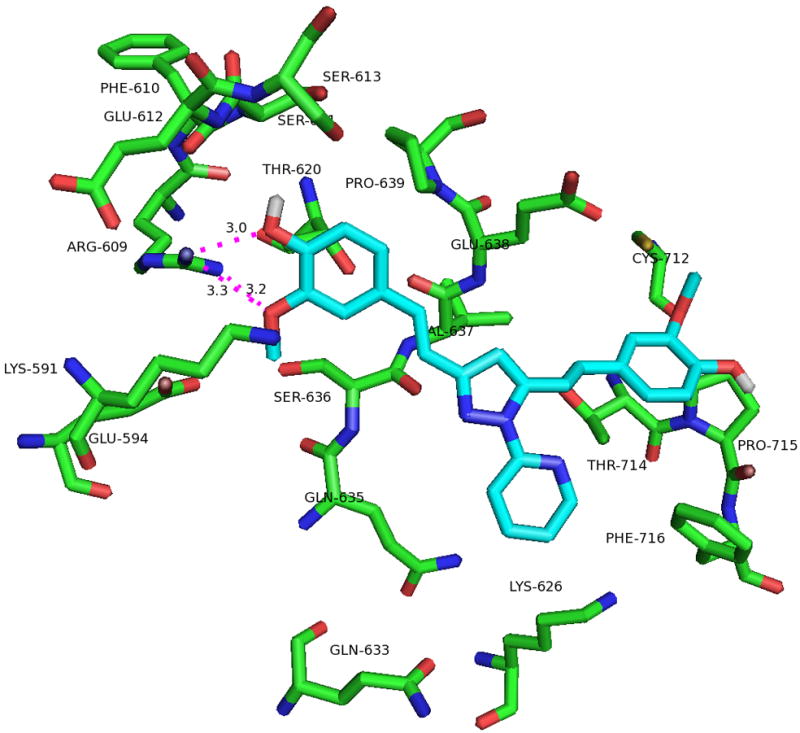
(a). Predicted binding mode of pyrazole compound 2 in the active site of STAT3-SH2. (b). 2-D interaction diagram
Figure 5.
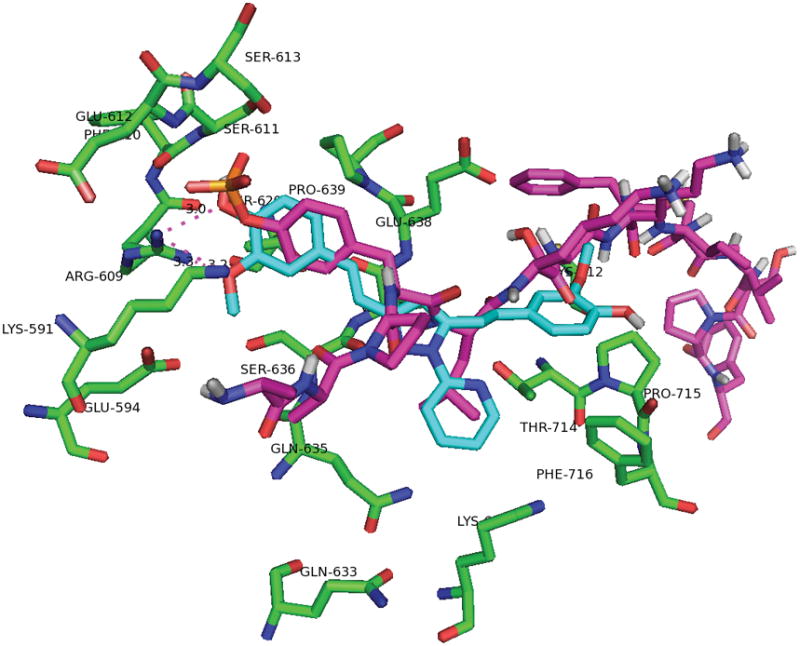
Overlay of phosphor-tyrosine peptide (magenta) and pyrazole compound 2 (cyan).
In conclusion, we synthesized curcumin analogues (compounds 2–9) and tested against head and neck cancer cell lines CAL27 and UM-SCC-74A. Among them, compounds 5 and 8 showed potent cytotoxic activity against HNSCC cell lines. Interestingly, Two analogues, 2, 5 have significant effect on pSTAT3 phosphorylation. Interruption of pFAK and pAKT phosphorylation signaling is shown with the compound 8. Compound 5, is the first reported click-chemistry curcumin analogue showing good cytotoxic activity. Thus, we investigated the cytotoxic activity of curcumin analogues and studied the possible molecular mechanisms. Moreover, possible binding mode of compound 2 was predicted by docking into the SH2 domain of STAT3. Overall, this study resulted in important findings and it paves a path to further explore the design and development of more potent compounds for head and neck cancer.
Supplementary Material
Acknowledgments
This publication was made possible, in part, by RCMI Pilot Project (Dr. Selvam Chelliah) and molecular biology research infrastructure support from grant number 2G12MD007605 from the NIMHD/NIH. Financial support of this research by Texas Southern University is also gratefully acknowledged. We thank the Openeye Inc for providing the academic license to use the docking tools.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
Additional Supporting Information may be found online in the supporting information tab for this article
References
- 1.Mock CD, Jordan BC, Selvam C. RSC Adv. 2015;5(92):75575. doi: 10.1039/C5RA14925H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Harikumar KB. Int J Biochem Cell Biol. 2009;41(1):40. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan BC, Mock CD, Thilagavathi R, Selvam C. Life Sci. 2016;152:135. doi: 10.1016/j.lfs.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shehzad A, Lee J, Lee YS. Biofactors. 2013;39(1):56. doi: 10.1002/biof.1068. [DOI] [PubMed] [Google Scholar]
- 5.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Cell Mol Life Sci. 2008;65(11):1631. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Mol Pharm. 2007;4(6):807. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 7.Chakraborti S, Chakraborti G, Dhar G, Basu G, Bhattacharyya B, Dwivedi V, Chakrabarti P, Das A, Surolia A, Poddar A. Biochemistry. 2013;52(42):7449. doi: 10.1021/bi400734e. [DOI] [PubMed] [Google Scholar]
- 8.Wang R, Zhang X, Chen C, Chen G, Zhong Q, Zhang Q, Zheng Q, Zheng S, Wang G, Chen Q-H. Eur J Med Chem. 2016;110:164. doi: 10.1016/j.ejmech.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biochem Pharmacol. 2008;76(11):1590. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q-H. Anti-Cancer Agents Med Chem. 2015;15(2):138. doi: 10.2174/1871520615666150116102442. [DOI] [PubMed] [Google Scholar]
- 11.Samaan N, Zhong Q, Fernandez J, Chen G, Hussain AM, Zheng S, Wang G, chen Q-H. Eur J Med Chem. 2014;75:123. doi: 10.1016/j.ejmech.2014.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Chen C, Zhang X, Zhang C, Zhong Q, Chen G, Zhang Q, Zheng S, Wang G, Chen Q-H. J Med Chem. 2015;58(11):4713. doi: 10.1021/acs.jmedchem.5b00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salem M, Rohani S, Gillies ER. RSC Adv. 2014;4(21):10815. [Google Scholar]
- 14.Strimpakos AS, Sharma RA. Antioxid Redox Signal. 2008;10(3):511. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 15.Siegel RL, Miller KD, Jemal A. CA Cancer J Clin. 2016;66(1):7. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 16.LoTempio MM, Veena MS, Steele HL, Ramamurthy B, Ramalingam TS, Cohen AN, Chakrabarti R, Srivatsan ES, Wang MB. Clin Cancer Res. 2005;11(19):6994. doi: 10.1158/1078-0432.CCR-05-0301. [DOI] [PubMed] [Google Scholar]
- 17.Cohen AN, Veena MS, Srivatsan ES, Wang MB. Arch Otolaryngol Head Neck Surg. 2009;135(2):190. doi: 10.1001/archotol.135.2.190. [DOI] [PubMed] [Google Scholar]
- 18.Kumar B, Yadav A, Hideg K, Kuppusamy P, Teknos TN, Kumar P. Plos One. 2014;9(3):e93208. doi: 10.1371/journal.pone.0093208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakravarti N, Myers JN, Aggarwal BB. Int J Cancer. 2006;119(6):1268. doi: 10.1002/ijc.21967. [DOI] [PubMed] [Google Scholar]
- 20.Chakravarti N, Kadara H, Yoon DJ, Shay JW, Myers JN, Lotan D, Sonenberg N, Lotan R. Cancer Prev Res. 2010;3(3):331. doi: 10.1158/1940-6207.CAPR-09-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamed O, Mehdawi N, Taha AA, Hamed E, Al-Nuri M, Hussein A. Iranian J Pharm. 2013;12(1):47. [PMC free article] [PubMed] [Google Scholar]
- 22.Selvam C, Jachak DM, Thilagavathi R, Chakraborti AK. Bioorg Med Chem Lett. 2005;15(7):1793. doi: 10.1016/j.bmcl.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 23.Mutisya D, Selvam C, Kennedy SD, Rozners E. Bioorg Med Chem Lett. 2011;21(11):3420. doi: 10.1016/j.bmcl.2011.03.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi W, Dolai S, Rizk S, Hussain A, Tariq H, Averick S, L’Amoreaux W, Idrissi A, Banerjee P, Raja K. Org Lett. 2007;9(26):5461. doi: 10.1021/ol702370m. [DOI] [PubMed] [Google Scholar]
- 25.McGann M. J Chem Inf Model. 2011;51(3):578–596. doi: 10.1021/ci100436p. [DOI] [PubMed] [Google Scholar]
- 26.McGaughey GB, Sheridan RP, Bayly CI, Culberson JC, Kreatsolas C, Lindsley S, Maiorov V, Truchon J-F, Cornell WD. J Chem Inf Model. 2007;47(4):1504–1519. doi: 10.1021/ci700052x. [DOI] [PubMed] [Google Scholar]
- 27.Ramasamy T, Selvam C. Bioorg Med Chem Lett. 2015;25(20):4632. doi: 10.1016/j.bmcl.2015.08.040. [DOI] [PubMed] [Google Scholar]
- 28.Hawkins PCD, Skillman AG, Warren GL, Ellingson BA, Stahl MT. J Chem Inf Model. 2010;50(4):572. doi: 10.1021/ci100031x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawkins PCD, Nicholls A. J Chem Inf Model. 2012;52(11):2919. doi: 10.1021/ci300314k. [DOI] [PubMed] [Google Scholar]
- 30.Becker S, Groner B, Muller CW. Nature. 1998;394(6689):145. doi: 10.1038/28101. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Sun Y, Pireddu R, Yang H, Urlam MK, Lawrence HR, Guida WC, Lawrence NJ, Sebti SM. Cancer Res. 2013;73(6):1922. doi: 10.1158/0008-5472.CAN-12-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.