Abstract
Although historic perfluorinated compounds are currently under scrutiny and growing regulatory control in the world, little is known about human exposure to other polyfluorinated compounds presently in use. Fluorotelomer alcohols (FTOHs) and polyfluoroalkyl phosphate esters (PAPs) are known to degrade to terminal perfluorinated acids and toxic reactive intermediates through metabolic pathways. Therefore, it is important to characterize their human exposure by the identification of unique biomarkers. With the use of liquid chromatography–mass spectrometry–time-of-flight analysis (LC–MS–TOF), we developed a workflow for the identification of metabolites for the 8:2 FTOH and 8:2 diPAP. Analysis of serum and urine of dosed rats indicated the 8:2 FTOH–sulfate and the 8:2 diPAP as potential biomarkers. These compounds, as well as 25 other fluorinated compounds and metabolites, were analyzed in human serum and urine samples from the general population (n = 100) and office workers (n = 30). The 8:2 FTOH–sulfate was measured for the first time in human samples in 5 to 10% of the serum samples, ranging from 50 to 80 pg/mL. The 8:2 diPAP was measured in 58% of the samples, ranging from 100 to 800 pg/mL. This study indicates the FTOH–sulfate conjugate as a biomarker of exposure to FTOHs and PAPs in humans.
Graphical abstract
Introduction
Perfluoroalkyl and polyfluoroalkyl substances (PFASs)1 have been used in a wide range of industrial and consumer applications for more than 50 years. These compounds have distinctive chemical properties that make them useful as surfactants, lubricants, and stain repellents and in a wide range of other uses.2 The same properties that make them ideal for industrial use also make them toxic, bioaccumulative, and persistent in the environment.3–5 The extensive use of these compounds has translated into their ubiquitous presence and, therefore, increased human exposure and potential threats to human health and the receiving environment. In the past decade, studies have highlighted the knowledge of the risk of perfluorinated materials such as perfluorooctanoic sulfonate (PFOS) and perfluorooctanoic acid (PFOA). Observations in humans have associated PFOS and PFOA with cancer, reduced fertility, developmental toxicity, suppression of the immune system, liver toxicity, immunotoxicity, hormonal alterations, and changes in fatty acid metabolism.6–10 These findings have led to regulatory efforts to limit the production of these historical perfluorinated compounds and to voluntary reductions in the production of these materials by their principal manufacturers in the United States and the European Union, leading ultimately to the phase-out of PFOS and PFOA as well as all eight-carbon-based chemistries (in 2002 for PFOS and by 2015 for PFOA).11 Nevertheless, industry still needs chemicals with the properties of PFASs. The United States and the European Union have moved to a six-carbon-based chemistry; thus, eight-carbon-based compounds are still produced in other parts of the world. Among these, fluorotelomer alcohols (FTOHs) and polyfluoroalkyl phosphate esters (mono-, di-, and triPAPs) are of particular concern. Fluorotelomer alcohols are raw material intermediates used to manufacture fluorotelomer-based products.2 They are detected as an unreacted residual material used in the production of water- and grease-proof coatings of upholstery, carpet, outdoor equipment, and clothing surface treatment.12,13 FTOHs are volatile compounds,14 and the hypothesis that part of these unbound FTOHs are released from products has been confirmed by their measurement in outdoor and indoor air in private homes, offices, furniture, and outdoor equipment stores, where it was found to be the highest in concentration.14–19 These findings indicate a potential exposure route for humans to FTOHs. PAPs are also used in coating materials, and they are of particular concern because they have also been approved for application to food-contact materials by the U.S. FDA (Food and Drug Administration)20 and the European Union. PAPs are therefore susceptible of entering the human body through ingestion regardless of the type of population concerned. Recent studies have confirmed their presence in food packaging,21–23 and their potential migration into food products has been reported.21,24,25 PAPs have also been measured in indoor air26 as well as drinking water,27 indicating other potential routes of human exposure. Numerous in vivo metabolic studies in rodents have demonstrated that FTOHs and PAPs are metabolic precursors of perfluorinated carboxylic acids (PFCAs).28–32 The transformation of FTOHs and PAPs into PFCAs during metabolism indicates that FTOHs and PAPs can contribute to PFCAs burden in humans by indirect exposure and lead to similar health implications.12,28 However, thus far, there are only limited studies that investigate FTOHs and PAPs as sources of PFCAs in humans. PAPs have been measured in human serum and whole blood in the United States and China at concentrations ranging from 0.002 to 0.067 ng/mL.33–35 Fraser et al. show a correlation between airborne FTOHs and PFOA in human serum.16 Measurement of PFCAs in serum is not conclusive on FTOHs and PAPs exposure. PFCAs are still currently in use, and therefore, their presence in the human body can come from both direct (exposure to PFCAs) and indirect (metabolism of other PFSAs) exposures.12 To investigate indirect exposure routes to PFCAs, discovery of biomarkers of exposure is necessary. Biomarkers of exposure can be defined as a xenobiotic measured in the system itself, a metabolite of that same xenobiotic, or an endogenous marker or mechanism in the system itself.36 Biomarkers of exposure can be used to assess the amount of a chemical that is present within the body and which, in combination with other data, may provide information on the importance of the exposure pathways,37 particularly if the biomarker is specific (i.e., a unique metabolite). Measurement of the parent compound or intermediate metabolites in human matrices (urine, serum, saliva, hair, plasma, etc.) can provide evidence concerning exposure in the body to given compounds. Biomarkers of exposure can be chemicals, metabolites, or endogenous changes in the organism related to the exposure. These biomarkers can give us the ability to determine if there has been an exposure to a certain chemical and provide precious information on the exposure routes.
Unique biomarkers of 8:2 FTOH and 8:2 diPAP exposure have been poorly described in human serum or urine. In this study, we dosed two groups of rats with either 8:2 FTOH or 8:2 diPAP. Serum and urine were isolated at specific time points and evaluated using nontargeted analysis approach with a liquid chromatograph–mass spectrometer–TOF (LC–MS–TOF) system to identify potential biomarkers for exposure. Targeted analysis was then carried on two groups of human populations (general population and office workers) to measure and quantify 25 historic and novel per- and polyfluorinated compounds, as well as putative metabolites and biomarkers of exposure.
Materials and Methods
Standards
A list of all standards and reagents used in this study is provided in the Supporting Information. Structures, full names, acronyms, and proposed metabolic pathway of the target analytes are shown in Figure S.1 and Table S.1. A list of all previously reported 8:2 FTOH and 8:2 diPAP metabolites in animal studies was established, and when available, standards of metabolites were analyzed by LC–MS–TOF. A database of all putative metabolites in serum and urine based on the name, formula, and exact mass of compounds was constructed (Table S.1).
Animal Treatment
Sprague–Dawley male rats (10–12 weeks old, 200–250 g) from Charles River Laboratory (Raleigh, NC) were housed individually in polycarbonate, metabolic cages. Animals (n = 3 per dose group and n = 1 for control; total n = 13) were given a single dose by oral gavage of 8:2 FTOH or 8:2 diPAP at 5 or 50 mg/kg of body weight, at a volume of 1 mL/kg. Blood from the tail vein, urine, and feces samples from metabolic cages were collected at 8, 24, 48, 72, 96, and 120 h after dosing. Samples were stored at −70 °C until analysis. Because our study was not aimed at understanding the metabolism of the 8:2 diPAP and 8:2 FTOH in dosed animals, we designed our animal study to use as few animals as possible. Only one animal was used as the control; all serum, urine, and feces of the control showed no contamination with the dosed compounds. Therefore, we believe that for our purpose this was sufficient. More details are available in the Supporting Information.
Subjects and Human Sample Collection
General Population
Serum and urine samples for the general population group were obtained from 100 anonymous North Carolina residents in 2011 who had no documented occupational exposure to PFASs; samples were collected in collaboration with the National Institute of Environmental Health Sciences Clinical Research Unit (Research Triangle Park, NC). The study participants (70% women, 30% men) were predominantly Caucasian (63%) and African American (33%), with a mean age of 43.6 (±14) years. The demographics of the study population are shown in Table S.2.
Office Workers
Serum and urine samples for the office workers group were obtained in 2009 from 31 individuals living and working in the Boston, MA area as previously described.16,38 The study participants were predominantly women (84% women, 26% men), with a mean age of 45 (±13) years, who had worked at least 18 h per week in offices. Study participants were predominantly Caucasians (98%). The demographics of the study population are shown in Table S.2. Details of exposure and measurements of FTOHs in office air were previously described by Fraser et al.16,39 Serum samples were collected after blood clotting and separation by centrifugation. Serum and urine were stored in polypropylene vials at −80 °C before being shipped on dry ice for analysis.
Sample Preparation
Dosed Animals' Serum, Urine, and Feces
A pair of different methods were used for the extraction of serum, urine, and feces. Samples used for the quantitation of targeted analytes were extracted using a modified version of the liquid extraction developed by D'eon and Mabury (2011)28 based on protein precipitation with cold acetonitrile. Serum and urine samples used for untargeted analysis required a concentration step to increase the probabilities of detecting unknown metabolites. Samples were extracted with a solid phase-extraction method using an OASIS HLB (Waters) cartridge. All details on extraction are available in the Supporting Information. Limits of quantitation and recoveries for rat serum, urine, and feces are available on Table S.3.
Human Serum and Urine
Formic acid (200 μL) and acetonitrile (2.5 mL) were added to 500 μL of human serum or 15 mL of urine for protein precipitation. Supernatants were purified by solid-phase extraction using an OASIS WAX cartridge (Waters). Final samples were evaporated to 150 μL under a gentle stream of nitrogen. For quantitation, standards were spiked in blank calf serum or human urine previously tested as PFAS-free. Details on extraction procedures and quantitation methods are available in the Supporting Information. Limits of quantitation and recoveries for human serum and urine are available on Table S.4.
Analysis by LC–MS–TOF
Sample analysis was performed using an Agilent 1100 series high-performance liquid chromatograph (HPLC) interfaced with a 6200 series Accurate-Mass LC–TOF system in electrospray negative ion mode (ESI) (Agilent Technologies, Palo Alto, CA). Drifts in the mass accuracy of the TOF were corrected by continuous infusion of two reference compounds (purine [m/z = 119.03632] and hexakis (phosphazine 1H,1H,3H-tetrafluoropropoxy) measured as a formate adduct [m/z = 966.005]) via dual-ESI sprayer. The HPLC method consisted of gradient run and an initial mobile phase of 50:50 solvent A (0.4 mM ammonium formate in 95:5 DI water/methanol) and solvent B (0.4 mM ammonium formate in 5:95 DI water/methanol). For the quantitation and detection of targeted compounds, a 20 min gradient method was used (Method 1); for nontargeted analysis and the processing of data with Mass profiler software, a 36 min gradient was used (Method 2). (Details are available in Tables S1 and S2).
Data Analysis
MassHunter Workstation Data acquisition software (Agilent Technologies) was used to operate the instrumentation. Data were processed using MassHunter Qualitative Analysis software (Agilent Technologies). Compounds unique to treatment animals were extracted from the raw data using the Molecular Feature Extraction (MFE) algorithm in MassHunter Qualitative analysis software. The samples were processed using MassProfiler software (Agilent Technologies), and compound identification was performed by matching exact mass determinations, with the exact mass listed in our custom 8:2 FTOH and 8:2 diPAP Personal Metabolite Database (Table S.1) and Molecular Formula Generation software (Agilent Technologies). Statistical analysis was performed with Graph Pad Prism software (version 4.0, GraphPad Software Inc., San Diego, CA). For statistical analysis, the results were assumed to follow a normal distribution based on the results of a Shapiro–Wilk normality test (Table S.5); a Mann–Whitney test was performed for the analysis of concentration in human serum with respect to race, age, and gender. Values below the limit of quantification (LOQ) were replaced with LOQ/√2. Statistical significance was considered to be p < 0.05.
Quality Assurance and Control
For all sample analysis, procedural and analytical blanks were prepared to determine background contamination for targeted analytes. Analysis of 10% of the samples (randomly chosen) was replicated for each quantitative determination to evaluate repeatability and consistency. Mean standard error of replicates was below 15%, indicating reliability and repeatability. Quality control samples containing matching matrix and a mix of targeted analytes at high and low concentrations were analyzed with each batch of samples to evaluate the precision and accuracy of the analysis. The QC values were 100% ± 20% of nominal values, indicating a low batch-analysis variability. All blanks and control samples were below the limit of detection (LOD) for all targeted and nontargeted analysis. All of the samples from the control animals were below the LOQ (available in Table S.4) for targeted analytes in all experiments. Calibration curves were prepared in a matched matrix that was previously tested as blank for targeted compounds. Spiked QC samples were included in all targeted analysis to evaluate analytical variability between analyzes batches. Additional information on QC and blanks is available in the Supporting Information.
Results and Discussion
FTOH (8:2) and diPAP (8:2) have been reported in the literature as being precursors of PFOA28,30 in animal models. 8:2 FTOH is directly transformed into an aldehyde (fluorotelomer aldehyde, FTAL) and acid form (fluortelomer acid, FTCA), which subsequently leads to the formation of PFOA30,31 (Figures 1 and S.1). DiPAP (8:2) is suspected to be transformed into its monoester form (8:2 monoPAP), which is converted to 8:2 FTOH and then follows the same path to PFOA.28 FTOH (8:2) and diPAP (8:2) are, therefore, being considered as potential contributors to PFOA levels in humans. We analyzed the serum, urine, and feces of rats dosed with 5 and 50 mg/kg 8:2 FTOH or 8:2 diPAP and searched for the parent compound, PFOA, and other intermediates and metabolites. We quantified parent compounds (8:2 FTOH and 8:2 diPAP) as well as expected metabolites with available standards (Table S.1) in rat serum and urine. We were not able to quantify 5:3 fluorotelomer carboxylic acid (FTCA) and 7:3 FTCA in dosed rats' serum, urine, and feces because standards were not commercially available at the time of the analysis.
Figure 1.
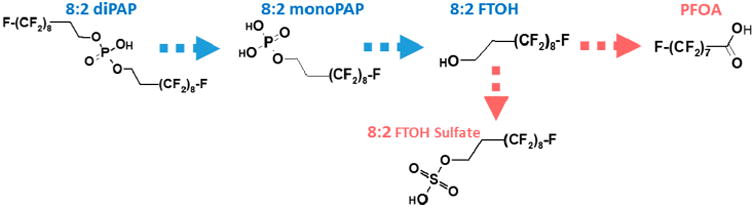
Expected biotransformation pathway of 8:2 diPAP and 8:2 FTOH, showing the combined suggestion by Martin et al. (2005), Fasano et al. (2006 and 2009), and D'eon and Mabury (2011).
Elucidating 8:2 FTOH and 8:2 DiPAP Transformation to PFOA in Dosed Animals
Metabolism of the 8:2 FTOH
In rats dosed with 8:2 FTOH, parent compound was observed only in the first time point (8 h) of serum of the 50 mg/kg dose group (Figure 2). The highest concentration of metabolites measured in serum was PFOA (1995 ng/mL) > 8:2 FTCA (1165 ng/mL) > 8:2 FTOH–sulfate (139 ng/mL) > perfluorononanoic acid (PFNA, 25.66 ng/mL) in the 50 mg/kg group (Figure 2) and PFOA (187 ng/mL) > 8:2 FTCA (84.2 ng/mL) > 8:2 FTOH–sulfate (6.7 ng/mL) > PFNA (2.6 ng/mL) in the 5 mg/kg group (Figure S.4). FTCA was present at a high concentration in serum at the first time point of examination but decreased by 3 orders of magnitude over the following 5 days. Moreover, FTCA in urine was often not detected or slightly above the LOQs. Rapid clearance of FTOH and FTCA in serum had also been reported in another similar dosing experiment,30 indicating a rapid transformation of the alcohol. Somewhat different trends were seen in urine compared to serum. In the 50 mg/kg group, PFOA (303.6 ng/mL) was the main transformation product, followed by FTOH–sulfate (0.86 ng/mL) and PFNA (0.84 ng/mL). FTCA was not measured in urine (Figure 2). In the 5 mg/kg group, only PFOA, 8:2 FTCA, and PFNA were measured at quantifiable concentrations (>LOQs; Table S.3) in urine (Figure S.4). In both serum and urine, PFOA, PFNA, and FTOH–sulfate levels were relatively steady throughout 120 h, with variations lower than 20% to 30% from the mean. FTOH and PFOA were measured in feces 24 h after dosing at levels approaching 0.1 mg/g and 0.1 μg/g, respectively. FTCA (8:2) was found in feces only at 24 h in the high-dose group; no other metabolite was detected in feces (Figure S.4). Fasano et al. also reported high levels of FTOH in feces after 8:2 FTOH dosing, with as high as 70% of the total mass delivered to the rats.30,31
Figure 2.
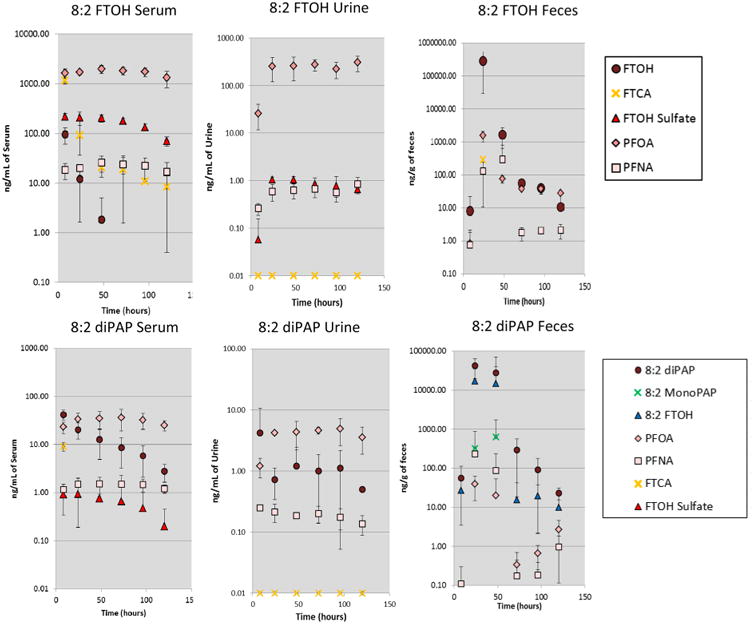
Upper panel: arithmetic mean ± SD for the concentration of the 8:2 FTOH and its expected biotransformation products in the serum, urine, and feces of the rats dosed with 50 mg/kg of 8:2 FTOH. Lower panel: arithmetic mean ± SD for the concentration of the 8:2 diPAP and its expected biotransformation products in the serum, urine, and feces of the rats dosed with 50 mg/kg of 8:2 diPAP.
Metabolism of the 8:2 diPAP
In the 8:2 diPAP-dosed animals; parent compound was detected in all serum and urine samples. In serum, the diPAP concentration peaked at 8 h after dosing at 41.8 and 17.6 ng/mL for the 50 and 5 mg/kg dosed animals, respectively (Figure 2 and Figure S.4). Values followed a downward trend through the following 5 days, but the diPAP was still measurable at 120 h after exposure in both serum and urine, indicating a slower clearance rate than the one observed for the fluorotelomer alcohol. As expected, metabolites common to the FTOH degradation pathway were observed, with concentration of metabolites in the following order: PFOA (36.1 ng/mL) > 8:2 FTCA (9.2 ng/mL) > PFNA (1.5 ng/mL) > 8:2 FTOH–sulfate (0.94 ng/mL) in the 50 mg/kg group and PFOA (4.3 ng/mL) > 8:2 FTCA (0.97 ng/mL) > PFNA (0.17 ng/mL) > 8:2 FTOH–sulfate (0.11 ng/mL) in the 5 mg/kg group. DiPAP (8:2) measured in serum at 50 mg/kg was 10-fold lower than the levels measured by D'eon and Mabury28 for the same dosing in the same strain, with similar conditions and with similar extraction recoveries, but our PFOA measurements were 2 to 10-fold higher, indicating a more-rapid metabolism in our animals. This study was the first to assess diPAP metabolism at a dose lower than 50 mg/kg in rats. Interestingly, levels of diPAP and PFOA in serum were similar for the two dosing groups, indicating a similar elimination pathway regardless of the quantity of diPAP absorbed. As we observed for the FTOH-dosed animals, PFOA, PFNA, and FTOH–sulfate (when detected) concentrations were relatively steady throughout the evaluation period, although their concentrations were much lower. This study measured diPAP in urine for the first time, with all concentrations being <10 ng/mL. PFOA was the only compound present in all urine samples. Levels for the 50 mg/kg group were in the same ranges as the one reported by D'eon and Mabury.28 All other metabolites were difficult to quantify and were present at concentration lower than 1 ng/mL, which was also in agreement with previous studies. A pair of expected intermediate metabolites, 8:2 mono-PAP and 8:2 FTOH, were not measured in serum or urine, as previously reported.28 However, these metabolites were observed in feces 24 h after dosing at high concentrations of 0.32 and 17 μg/g for the 50 mg/kg dose group and 0.7 and 1.7 μg/g in the 5 mg/kg group (Figures 2 and S.4.). This observation supports the hypothesis of Deon and Mabury:28 that dephosphorylation happens in the gut, which allows the release of the 8:2 FTOH that is further transformed into PFOA The same pathway is observed in the low-dose group, indicating that even a low dose of diPAP can lead to PFOA in serum.
Profiling of Metabolites in Dosed Animals Using Nontargeted Analysis
The metabolism pathways of 8:2 FTOH and 8:2 diPAP and their possible transformation into PFOA have been previously described in dosed rats28–31 (Figure 1). Thus, if we assume that a similar metabolic pathway is followed in humans, the measure of PFOA in human samples could be caused by differences sources: direct exposure to PFOA or metabolism of 8:2 FTOH and 8:2 diPAP. Measuring an intermediate metabolite of FTOH or diPAP metabolism in humans would confirm a similar metabolic pathway for FTOH and diPAPs in dosed rats and humans and indicate that indirect exposure of these compounds contributes to overall PFCAs levels in human serum. From previous publications,28,31,40 we have established a proposed metabolic pathway for the transformation of 8:2 diPAP and 8:2 FTOH to PFCAs (simplified pathway shown in Figure 1 and detailed pathway shown in Figure S.1) in humans. Based on the assumed metabolic pathway, we produced a complete list of all previously reported metabolites based on monoisotopic mass for use with TOF-based screening, “the 8:2 FTOH and 8:2 diPAP Metabolite Database” (Table S.1). This list was transformed into a database for the screening of rat serum and urine. Pooled extracts of each dosed rat's serum and urine, as well as the control, were extracted and compared following the workflow represented in Figure 3 using Mass profiler software to identify significant metabolites or compounds indicative of a biological response suitable to be considered as biomarkers of exposure. The samples (three replicates per sample) were analyzed by LC–TOF–MS according to method 2 described in the Supporting Information. The first step in the workflow used the Molecular Feature Extraction algorithm (MFE, in Mass Hunter Qualitative Analysis software) to locate all ions in the raw data for the control and the experiment (dosed animals) samples. This screening process allowed us to create a file containing all major chromatographic features present in the samples and obtain a “mass list” for all analyzed samples. A list of detected exact masses was created for each sample that contained a list of all detected ions. The second step in the workflow compared two sets of lists (i.e., two distinct groups of samples, Control and Dosed) with MassProfiler software, in which a list of differential masses was produced. This software allows us to isolate the masses that are unique to the 8:2 FTOH or 8:2 diPAP serum or urine of treated animals, (i.e., “unique to the treatment”). The calculated neutral monoisotopic mass of each feature in the list was subsequently queried against the 8:2 FTOH and 8:2 diPAP Metabolite Database (Table S.1.) for matching to compounds falling within the user-adjusted mass tolerance window (in this case, 5 ppm). The 8:2 FTOH and 8:2 diPAP Metabolite Database matched the calculated neutral mass to the monoisotopic mass value calculated from the empirical formula of compounds in the database. A mass can be attributed to more than one formula; the software helps the user choose the most likely formula on the basis of a matching scoring. Finally, only the purchase of a standard will confirm the exactitude of the identification.
Figure 3.
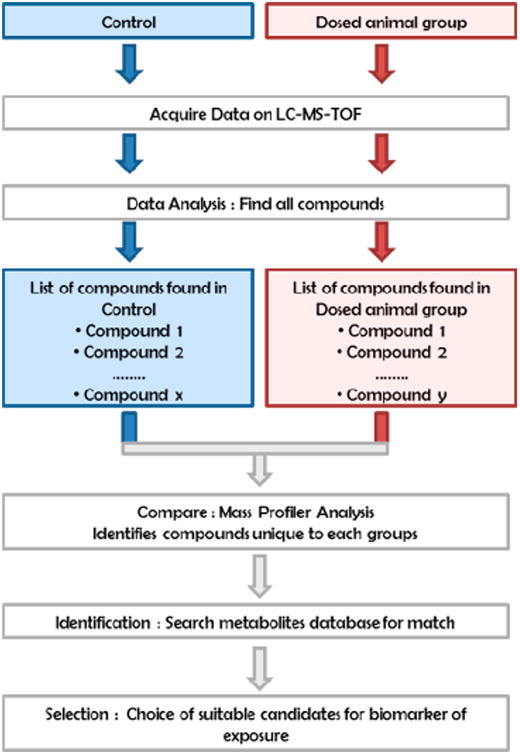
Schematic data-processing workflow for biomarker identification, generation of compound lists for a comparison between samples in MassProfiler, and identification by a search of the specific database.
For the 8:2 FTOH-dosed samples, we compared the control (three injection replicates) to all six pooled serum extracts from the two dosed groups (with three injection replicates and a total of 18 samples). In serum, 257 features were unique to the dosed animal (Figure S-3.) (i.e., were not present in the control). Dot size in Figure.S.3 is indicative of the abundance of the compound, while neutral mass, abundance, retention time, and frequency of detection in the samples are summarized in a table (data not shown). The second step of the workflow consisted of identifying as many of the 257 features as possible metabolites or parent from the exposure to the 8:2 FTOH. Therefore, we cross-searched the list of the selected features with our database (Table S.1). The identification process takes into account information based on monoisotopic mass, isotope abundances, and spacing between isotope peaks and gives results with a probability score of matching (%). With the database search in Mass Profiler, we were able to identify four features with masses of 442.005, 413.974, 543.9638, and 545.013 Da that matched in the database within 0.5 ppm of tetrahydroperfluoroalkyl carboxylate (THPFCA), PFOA, 8:2 FTOH–sulfate conjugate, and the 8:2 fluorotelomer unsaturated alcohol cysteine. These compounds were tentatively identified with their mass accuracy, but standards are needed as well as spectral analysis to confirm their identity. Because costs prohibited custom synthesis of standards for all three metabolites (THPFCA, 8:2 FTOH–sulfate, and 8:2 fluorotelomer unsaturated alcohol cysteine), we chose to pursue the identification for the 8:2 FTOH–sulfate only. FTOH–sulfate was chosen as the one to be investigated as a candidate for biomarker because it had the longest half-life (Figure S.2), was the most prevalent, and was also detected in urine. The same workflow was followed for the analysis of the 8:2 diPAP-dosed serums. Control samples (three replicates) were compared to all six pooled samples from both dose groups (with three replicates and a total of 18 samples). In serum, 869 features were unique to the dosed animals (Figure S.2.). With the database search, we were able to identify one feature with the neutral mass of 989.9694 Da matching within 0.5 ppm in the database with the 8:2 diPAP. Although the 8:2 FTOH–sulfate was not selected with the mass profiler because of its too-low abundance in the samples, we were able to detect it in all serum samples of dosed animals. The 8:2 diPAP and 8:2 FTOH–sulfate were therefore considered as the two potential biomarkers of exposure to 8:2 diPAP. Our work represents, a novel method based on accurate mass and nontarget analysis workflow for the identification of biomarkers of exposure to environmental chemicals. Similar workflow methods have been previously described with success for the identification of drug metabolites in human urine.41–43 Accurate mass and the molecular feature extraction algorithm are useful tools for the screening of complex mixtures, especially if they can be paired with database search for identification. This workflow also highlights the need of further metabolic studies to understand the mechanisms of FTOH and diPAP elimination and the health impact of their intermediate metabolites. In fact, 255 and 866 unknowns are recorded after FTOH and diPAP dosing in rats. These unknowns are not present in the control untreated rat; this means that the unknowns are likely to be linked with the exposure to the chemical. The use of MS/MS tools, combined with high-resolution mass accuracy and database search, such as a liquid chromatograph–quadrupole time-of-flight–tandem mass spectrometer (LC–quadrupole-TOF–MS/MS) would certainly allow the identification of part of these unknowns. Other studies based on metabolomics profiling of the dosed animals would be necessary to determine the identity of remaining unknowns.
FTOH–Sulfate Conjugate Confirmation
Following the database indications, we attempted to confirm the identification of the FTOH–sulfate in serum and urine of the dosed rats on the basis of the capacities of the LC–TOF–MS. In addition to monoisotopic mass, which was within 0.5 ppm, the first confirmation was based on isotopic matching. FTOH–sulfate possesses both carbon and sulfur atoms, with 13C at 1.078% abundance and 34S at 4.29% abundance, making it possible to use these isotopic fractions to provide evidence of identification. The mass spectrum for the putative FTOH–sulfate in serum revealed an isotope distribution pattern that had a very good mass match to the empirical formula C10H5F17O4S (Figure 4, represented by the red boxes). For confirmation, we used the MassHunter Formula Generator software for back-predicting the formula on the basis of the spectra. This software analyzes the spectrum of the selected candidate and proposes a list of molecular formulas that would match that spectrum on the basis of the monoisotopic mass, isotopic abundance, and isotopic spacing. Each proposed formula is accompanied by a score in percentage that defines how well the formula matches the criteria. The first hit corresponded to the formula of the FTOH–sulfate with a percentage of probability of 97% (Figure 4). We then compared the retention time and spectra of our candidate to our custom-made FTOH–sulfate standard, which matched perfectly. Finally, we increased the fragmentor voltage to 190 V to allow the in-source fragmentation of the compound. For both our candidate compound and the standard, we observed a fragment of 96.9596 m/z, corresponding to the HSO4, coeluting with the 542.9564 m/z (Figure 4). This evidence is in agreement with previous data on the measurement of FTOH–sulfate in rat serum.28 Altogether, these data allow us to confirm the identification of the FTOH–sulfate conjugate in the serum of dosed rats. The same identification procedures were followed to confirm the presence of FTOH–sulfate in the urine of the dosed rats (data not shown).
Figure 4.
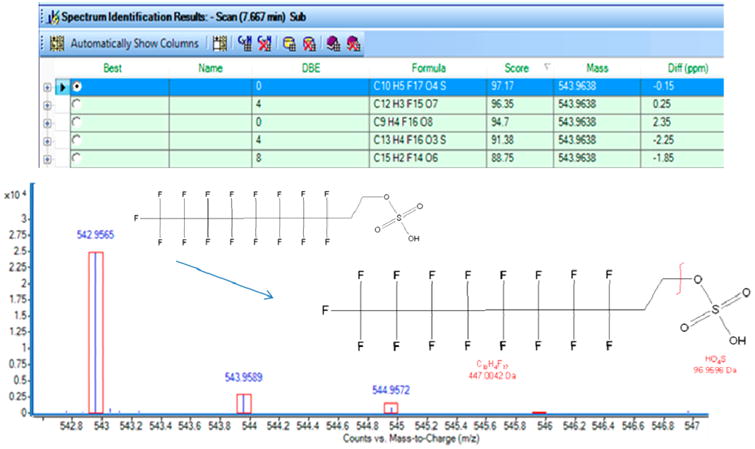
Spectra of the 8:2 FTOH–sulfate in dosed rat serum (M-H: 542.9565), as well as fragmentation of the 8:2 FTOH–sulfate. The table indicates the potential formula matching the spectra; the formula corresponding to the 8:2 FTOH–sulfate is highlighted in blue. Red boxes on spectra indicate the theoretical isotopic distribution of the 8:2 FTOH–sulfate. The graphical structure illustrates the fragmentation of the compound at 190 V fragmentor voltage.
Human Analysis
We followed up the rat experiments by investigating potential exposure biomarkers in two human populations with a range of commercially used PFASs and metabolites (23 compounds in total). The demographics of the population are described in materials and methods and in Table S.2. The list of all target compounds in human serum and urine is available in Table S4. Perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), PFOA, PFNA, perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), perfluorohexanoic sulfonate (PFHxS), perfluorobutanoic sulfonate (PFBS), and PFOS were detected in more than 90% of the serum samples. The detection frequency for pefluoroheptanoic acid (PFHpA) and perfluorotridecanoic acid (PFTrA) was higher than 70%, while 6:2 diPAP, 8:2 diPAP, perfluorododecanoic acid (PFDoA), and perfluorotetradecanoic (PFTetA) were between 30 and 60%. PFBA and 8:2 monoPAP were detected less frequently (1% and 15% detection, respectively) and at very low concentrations, often at or lower than the LOQs. MonoPAP (6.2) and FTOH (4:2, 6:2, 8:2 and 10:2), as well as all the metabolites, were not detected. The individual levels of all detected compounds in each population are represented in Figure 5. Consistent with many previously reported studies, PFOS was the most-abundant compound in serum, with a mean of 8.9 ng/mL (±7.7) followed by PFOA (3.28 ng/mL ± 2.3), PFHxS (1.8 ng/mL ± 1.7), and PFNA (0.99 ng/mL ± 0.74). PFOS, PFOA, PFNA, PFBS, PFHxS, PFHpA, PFBA, PFDA, PFUnA, and PFDoA were all in ranges similar to those reported in recent measurements from the NHANES cohort from 2009 to 2010.44 Significant gender differences (Mann–Whitney U test, p < 0.05, Table S.6) were observed for PFOA, PFHxS, and PFOS (p = 0.0073, p < 0.001, and p = 0.0018, respectively), which were higher in men than women; this was observed in other similar studies.44,45 A significant difference was observed between the general population group and the office workers for some compounds (Mann–Whitney U test, p < 0.05, Table S.6). PFHxA, PFUnA, and PFTriA were lower in office workers (p < 0.001, p = 0.019, p = 0.019, respectively), whereas PFNA, PFDA, and PFBS were higher (p = 0.0416, p < 0.0002, and p < 0.0001, respectively). Higher levels of PFNA and PFDA in the office workers might be linked to the previously reported exposure to 10:2 FTOH30,31 in their office environment. Thus, considering that presence of PFNA and PFDA in serum can be associated with both direct and indirect exposure, it is not possible here to establish a significant correlation between the exposure and the measurements. PFOA, PFNA, PFDA, PFHxS, and PFOS in the office workers measured here had an excellent correlation with serum measurements performed by a different laboratory for the same population16 (R2 = 0.88, 0.66, 0.91, 0.96, and 0.98, respectively; Figure S.6) providing further confirmation for our quality assurance and control on the basis of interlaboratory variability. Long-chain PFCAs (PFDoA, PFTrDA, and PFTet-DA) were also measured in human serum, although levels of these long-chained acids were low (<0.1 ng/mL).
Figure 5.
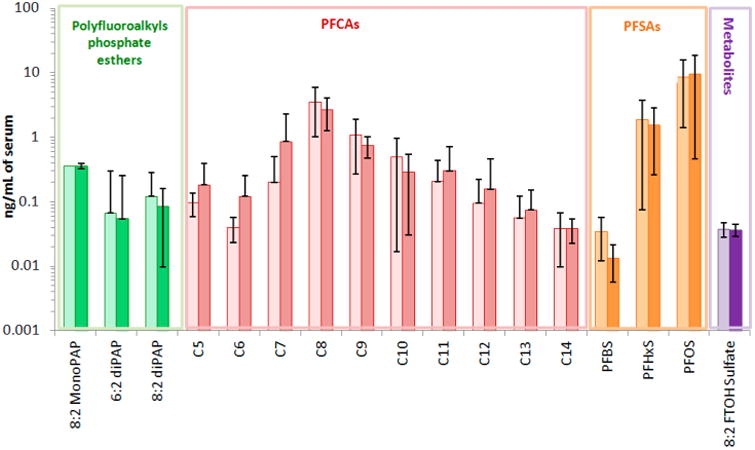
Arithmetic mean concentrations and standard deviation for all target analytes analyzed in human serum samples. Lighter colors represent means from the general population group; darker colors represent means from the office workers group.
All selected compounds were analyzed in matched urine samples from the same individuals. Even though a small percentage of urine samples showed traces of PFOA, levels were too low for quantitation. Recent study reported perfluorinated compounds in urine, with PFOA ranging from 214 to 2890 ng/L and PFBA being the major chemical (average of 48 000 ng/L).46 The levels reported in the literature are extremely high, and similar measurements have not yet been repeated. This leads us to believe that the measurement of PFBA in urine reported in this study might be biased, either by the choice of the population or by the analytical approach, and would need further confirmation. Our LOQs ranged from 5 to 50 ng/L; this suggests that our samples did not have comparable concentrations as was reported in that study. In our study, only 15 mL of urine was available for analysis; in future work, higher volumes of urine, such as 50 mL, would allow lower detection limits and may permit the quantitation of perfluorinated compounds in this matrix.
Of all of the investigated metabolites with available standards reported in Table S.1, only 8:2 FTOH–sulfate was detected. The conjugate was measurable (≥LOQ) in 5% of the samples of the general population group and 10% of the office workers. Presence of FTOH–sulfate in serum was confirmed using an authentic standard as well as consistent spectra analysis. A chromatogram example of 8:2 FTOH–sulfate in serum compared to the standards is available in the Figure S.7. FTOH–sulfate (8:2) concentrations ranged from the LOQ (0.05 ng/mL) to 0.08 ng/mL. The low detection levels and the proximity of the measured values to the LOQ required additional quality control to ensure that the values were not a false positive. All FTOH–sulfate spectra of samples in which the compound was detected were scrutinized to ensure repeatability of the measurement, accurate mass, and isotope distribution matching. Procedural blanks were introduced every four samples to ensure detection of FTOH Sulfate was not an analytical artifact. Extracted ion chromatograms of procedural blanks are available in Figure S.8. Although their accurate masses are similar, no confusion between 8:2 FTOH–sulfate and 8:2 monoPAP was possible. This difference was ensured by the accuracy of our LC–MS–TOF (5 ppm), well below the mass difference of the two compounds (9.5 ppm), as well as the distinctive isotopic pattern of the FTOH–sulfate given by the sulfur isotopes.
The low detection frequency makes statistical comparison of the two populations difficult. However, FTOH–sulfate was detected twice as much in the office workers. The small number of serum samples with detectable sulfate conjugate in the office workers was too small to determine correlation with the FTOH measurement in office air available from previous study.16 In ski waxers highly exposed to FTOH, metabolites of fluorotelomer alcohols such as 6:2, 8:2, and 10:2 FTUCA and 5:3 and 7:3 FTCA have been detected in serum,47 with an average of 0.03 to 2 ng/mL. We were not able to measure these compounds in our study populations, who had presumably much lower exposures. The 8:2 diPAP was more frequently detected (58%) than the 6:2 diPAP (47%), with mean values of 0.10 and 0.06 ng/mL, respectively, and ranging from the LOD to 1.43 ng/mL. The diPAPs were detected less frequently in our samples with respect to previous measurements of the U.S. population33 but with similar frequency and in the same range as recent measurements.34 Variations of diPAPs levels with previous studies could be related to modifications in diPAPs production and use as well as differences in analytical approaches. The 8:2 monoPAP was present in 11% of the samples and has never been reported before in human serum. No difference was observed between office workers and the general population or between males and females (Table S.6).
The use of a high-resolution instrument based on exact mass measurements, such as the LC–TOF–MS coupled with software for data analysis, is extremely useful for the identification of metabolites in biological samples. This workflow can easily be applied for biomarker discovery of other chemicals, not just fluorinated materials. Mass accuracy and isotopic matching as well as formula generator software are precious instruments for the identification and confirmation of an unknown in a sample that might avoid the unnecessary, early purchase of an expensive standard. Measurement of the 8:2 FTOH–sulfate and 8:2 diPAPs in human serum is an indication of the exposure to FTOH and PAPs in humans. It is not certain whether diPAPs and FTOHs have similar metabolic pathways in dosed animals and humans; thus, this study provides an additional argument for this hypothesis. Additionally, it is likely that exposure to FTOH and diPAPs will contribute to the total burden of PFCAs in humans; therefore, the phase-out and toxicological measurements should be focused on both PFCAs and their precursors.
diPAPs are now reportedly detected in food packaging; their migration from packaging into food has been demonstrated, and very recently, their presence has been detected in food products, drinking water, and house dust. FTOHs have been detected repetitively in indoor environment and are largely used as coating agents for outdoor wear. There is, therefore, a real concern over human exposure to these compounds. Our study suggests FTOH–sulfate as a potential biomarker of exposure to FTOHs. This biomarker may be more interesting to pursue for highly exposed populations such as ski waxers or, potentially, manufacturers or sales personnel for outdoor recreational equipment, as its concentration would be easier to detect in serum and might be present in urine.
Supplementary Material
Additional descriptions of experimental methods, including chemicals, animal treatment, and quality assurance and control. Tables showing database of known metabolites and transformation products of the 8:2 FTOH and 8:2 diPAP, descriptive demographics of the studied population, summary of instrumental parameters for LC–MS–TOF, LOQ and recoveries of analytes, results of p-values for the Shapiro–Wilk normality test, and p-values from the Mann–Whitney U test. Figures showing the expected biotransformation pathway of 8:2,8:2 diPAP and 8:2 FTOH, metabolites of 8:2 FTOH in serum, mass profiler of 8:2 diPAP-dosed animal serum, feces analysis, plots for the Spearman correlation of analysis of selected compounds in the office workers from the present study and the analysis from previous work, FTOH–sulfate in human serum, and an extracted ion chromatogram for FTOH–sulfate in blanks and standard curve points. (PDF)
Acknowledgments
This research was partially supported by an appointment to the Research Participation Program at the National Exposure Research Laboratory administered by the Oak Ridge Institute for Science and Education. This research was also partially supported by funds from the Division of Intramural Research, National Institute of Environmental Health Sciences. We thank Agilent Technologies for their support of this effort through a TOFMS (U.S. EPA, CRADA 437-A-12) and in particular Joe Weitzel for his support of this work. We thank Imma Ferrer and Michael Thurman for training and advice on TOF–MS. We also thank Matthew Stiegel for providing support on statistical analysis. M.D.M. and T.F.W. were supported in part by R01 ES015829. This article has been reviewed in accordance with the policy of the National Exposure Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the view and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Abbreviations Used
- ESI
electrospray ionization
- LC
liquid chromatography
- LOQ
limit of quantification
- MS
mass spectrometer
- NIEHS
National Institute for Environmental Health Sciences
- QC
quality control
- SPE
solid-phase extraction
- TOF
time-of-flight
- US EPA
United States Environmental Protection Agency
Footnotes
Supporting Information: The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.6b01170.
Notes: The authors declare no competing financial interest.
References
- 1.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manage. 2011;7(4):513–41. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kissa E. Fluorinated Surfactants. CRC Press; Boca Raton, FL: 2002. [Google Scholar]
- 3.Giesy JP, Kannan K. Global distribution of perfluorooctanesulfonate in wildlife. Environ Sci Technol. 2001;35(7):1339–42. doi: 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- 4.Organisation for Economic Co-operation and Development Directorate, Environment Directorate. Hazard Assesment of PFOS and its Salts. Joint Meeting of the Chemicals Comitee and the working Party on Chemicals, Pesticides and Biotechnology (ENV/JM/RD(2002)17/Final); Organisation for Economic Co-operation and Development; Crystal City, VA: 2002. https://www.oecd.org/env/ehs/risk-assessment/2382880.pdf. [Google Scholar]
- 5.Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, Strynar MJ. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci. 2005;90(2):510–518. doi: 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- 6.Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007;115(11):1677–82. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granum B, Haug LS, Namork E, Stolevik SB, Thomsen C, Aaberge IS, van Loveren H, Lovik M, Nygaard UC. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol. 2013;10(4):373–379. doi: 10.3109/1547691X.2012.755580. [DOI] [PubMed] [Google Scholar]
- 8.Klaunig JE, Hocevar BA, Kamendulis LM. Mode of Action analysis of perfluorooctanoic acid (PFOA) tumorigenicity and Human Relevance. Reprod Toxicol. 2012;33(4):410–418. doi: 10.1016/j.reprotox.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect. 2013;121(3):318–23. doi: 10.1289/ehp.1205829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watkins DJ, Josson J, Elston B, Bartell SM, Shin HM, Vieira VM, Savitz DA, Fletcher T, Wellenius GA. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ Health Perspect. 2013;121(5):625–30. doi: 10.1289/ehp.1205838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.United States Environmental Protection Agency. Fact Sheet: 2010/2015 PFOA Stewardship Program. [accessed 01/10/2013]; https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program.
- 12.D'Eon JC, Mabury SA. Is indirect exposure a significant contributor to the burden of perfluorinated acids observed in humans? Environ Sci Technol. 2011;45(19):7974–7984. doi: 10.1021/es200171y. [DOI] [PubMed] [Google Scholar]
- 13.Dinglasan-Panlilio MJA, Mabury SA. Significant Residual Fluorinated Alcohols Present in Various Fluorinated Materials. Environ Sci Technol. 2006;40(5):1447–1453. doi: 10.1021/es051619+. [DOI] [PubMed] [Google Scholar]
- 14.Young CJ, Mabury SA. Atmospheric perfluorinated acid precursors: chemistry, occurrence, and impacts. Rev Environ Contam Toxicol. 2010;208:1–109. doi: 10.1007/978-1-4419-6880-7_1. [DOI] [PubMed] [Google Scholar]
- 15.Barber JL, Berger U, Chaemfa C, Huber S, Jahnke A, Temme C, Jones KC. Analysis of per- and polyfluorinated alkyl substances in air samples from Northwest Europe. J Environ Monit. 2007;9(6):530–41. doi: 10.1039/b701417a. [DOI] [PubMed] [Google Scholar]
- 16.Fraser AJ, Webster TF, Watkins DJ, Nelson JW, Stapleton HM, Calafat AM, Kato K, Shoeib M, Vieira VM, McClean MD. Polyfluorinated compounds in serum linked to indoor air in office environments. Environ Sci Technol. 2012;46(2):1209–15. doi: 10.1021/es2038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlummer M, Gruber L, Fiedler D, Kizlauskas M, Muller J. Detection of fluorotelomer alcohols in indoor environments and their relevance for human exposure. Environ Int. 2013;57–58:42–9. doi: 10.1016/j.envint.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Shoeib M, Harner T, Lee SC, Lane D, Zhu J. Sorbent-impregnated polyurethane foam disk for passive air sampling of volatile fluorinated chemicals. Anal Chem. 2008;80(3):675–82. doi: 10.1021/ac701830s. [DOI] [PubMed] [Google Scholar]
- 19.Shoeib M, Harner T, Webster GM, Lee SC. Indoor Sources of Poly- and Perfluorinated Compounds (PFCS) in Vancouver, Canada: Implications for Human Exposure. Environ Sci Technol. 2011;45(19):7999–8005. doi: 10.1021/es103562v. [DOI] [PubMed] [Google Scholar]
- 20.United States Food and Drug Administration. List of Indirect Additives Used in Food Contact Substances. [accessed 01/10/2013]; http://www.fda.gov/Food/IngredientsPackagingLabeling/PackagingFCS/IndirectAdditives/default.html.
- 21.Begley TH, White K, Honigfort P, Twaroski ML, Neches R, Walker RA. Perfluorochemicals: potential sources of and migration from food packaging. Food Addit Contam. 2005;22(10):1023–31. doi: 10.1080/02652030500183474. [DOI] [PubMed] [Google Scholar]
- 22.Trier X, Granby K, Christensen JH. Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ Sci Pollut Res. 2011;18(7):1108–20. doi: 10.1007/s11356-010-0439-3. [DOI] [PubMed] [Google Scholar]
- 23.Trier X, Nielsen N, Christensen J. Structural isomers of polyfluorinated di- and tri-alkylated phosphate ester surfactants present in industrial blends and in microwave popcorn bags. Environ Sci Pollut Res. 2011;18(8):1422–1432. doi: 10.1007/s11356-011-0488-2. [DOI] [PubMed] [Google Scholar]
- 24.Gebbink WA, Ullah S, Sandblom O, Berger U. Polyfluoroalkyl phosphate esters and perfluoroalkyl carboxylic acids in target food samples and packaging-method development and screening. Environ Sci Pollut Res. 2013;20(11):7949–58. doi: 10.1007/s11356-013-1596-y. [DOI] [PubMed] [Google Scholar]
- 25.Herzke D, Olsson E, Posner S. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway - a pilot study. Chemosphere. 2012;88(8):980–7. doi: 10.1016/j.chemosphere.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 26.De Silva AO, Allard CN, Spencer C, Webster GM, Shoeib M. Phosphorus-containing fluorinated organics: polyfluoroalkyl phosphoric acid diesters (diPAPs), perfluorophosphonates (PFPAs), and perfluorophosphinates (PFPIAs) in residential indoor dust. Environ Sci Technol. 2012;46(22):12575–82. doi: 10.1021/es303172p. [DOI] [PubMed] [Google Scholar]
- 27.Ding H, Peng H, Yang M, Hu J. Simultaneous determination of mono- and disubstituted polyfluoroalkyl phosphates in drinking water by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A. 2012;1227:245–52. doi: 10.1016/j.chroma.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 28.D'Eon JC, Mabury SA. Exploring indirect sources of human exposure to perfluoroalkyl carboxylates (PFCAs): Evaluating uptake, elimination, and biotransformation of polyfluoroalkyl phosphate esters (PAPs) in the rat. Environ Health Perspect. 2011;119(3):344–350. doi: 10.1289/ehp.1002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Eon JC, Mabury SA. Production of perfluorinated carboxylic acids (PFCAs) from the biotransformation of polyfluoroalkyl phosphate surfactants (PAPS): exploring routes of human contamination. Environ Sci Technol. 2007;41(13):4799–805. doi: 10.1021/es070126x. [DOI] [PubMed] [Google Scholar]
- 30.Fasano WJ, Carpenter SC, Gannon SA, Snow TA, Stadler JC, Kennedy GL, Buck RC, Korzeniowski SH, Hinderliter PM, Kemper RA. Absorption, distribution, metabolism, and elimination of 8–2 fluorotelomer alcohol in the rat. Toxicol Sci. 2008;102(2):455. doi: 10.1093/toxsci/kfj160. [DOI] [PubMed] [Google Scholar]
- 31.Fasano WJ, Sweeney LM, Mawn MP, Nabb DL, Szostek B, Buck RC, Gargas ML. Kinetics of 8–2 fluorotelomer alcohol and its metabolites, and liver glutathione status following daily oral dosing for 45 days in male and female rats. Chem-Biol Interact. 2009;180(2):281–95. doi: 10.1016/j.cbi.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Himmelstein MW, Serex TL, Buck RC, Weinberg JT, Mawn MP, Russell MH. 8:2 fluorotelomer alcohol: a one-day nose-only inhalation toxicokinetic study in the Sprague-Dawley rat with application to risk assessment. Toxicology. 2012;291(1–3):122–32. doi: 10.1016/j.tox.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 33.D'Eon JC, Crozier PW, Furdui VI, Reiner EJ, Libelo EL, Mabury SA. Observation of a commercial fluorinated material, the polyfluoroalkyl phosphoric acid diesters, in human sera, wastewater treatment plant sludge, and paper fibers. Environ Sci Technol. 2009;43(12):4589–94. doi: 10.1021/es900100d. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Mabury SA. A pilot survey of legacy and current commercial fluorinated chemicals in human sera from United States donors in 2009. Environ Sci Technol. 2011;45(19):8067–74. doi: 10.1021/es200167q. [DOI] [PubMed] [Google Scholar]
- 35.Loi EI, Yeung LW, Mabury SA, Lam PK. Detections of commercial fluorosurfactants in Hong Kong marine environment and human blood: a pilot study. Environ Sci Technol. 2013;47(9):4677–85. doi: 10.1021/es303805k. [DOI] [PubMed] [Google Scholar]
- 36.Committee on Biological Markers of the National Research Council. Biological markers in environmental health research. Biological markers in environmental health research. Environ Health Perspect. 1987;74:3–9. doi: 10.1289/ehp.74-1474499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.United States Environmental Protection Agency. Defining Biomarkers. [accessed 10/06/2016]; http://www.epa.gov/pesticides/science/biomarker.html.
- 38.Carignan CC, McClean MD, Cooper EM, Watkins DJ, Fraser AJ, Heiger-Bernays W, Stapleton HM, Webster TF. Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environ Int. 2013;55:56–61. doi: 10.1016/j.envint.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraser AJ, Webster TF, Watkins DJ, Strynar MJ, Kato K, Calafat AM, Vieira VM, McClean MD. Polyfluorinated compounds in dust from homes, offices, and vehicles as predictors of concentrations in office workers' serum. Environ Int. 2013;60:128–36. doi: 10.1016/j.envint.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin JW, Chan K, Mabury SA, O'Brien PJ. Bioactivation of fluorotelomer alcohols in isolated rat hepatocytes. Chem-Biol Interact. 2009;177(3):196–203. doi: 10.1016/j.cbi.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Sana TR, Roark JC, Li X, Waddell K, Fischer SM. Molecular formula and METLIN Personal Metabolite Database matching applied to the identification of compounds generated by LC/TOF-MS. J Biomol Technol. 2008;19(4):258–66. [PMC free article] [PubMed] [Google Scholar]
- 42.Thurman EM, Ferrer I. Liquid chromatography/quadrupole-time-of-flight mass spectrometry with metabolic profiling of human urine as a tool for environmental analysis of dextromethorphan. J Chromatogr A. 2012;1259:158–66. doi: 10.1016/j.chroma.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 43.McMahen RL, Strynar MJ, Dagnino S, Herr DW, Moser VC, Garantziotis S, Andersen EM, Freeborn DL, McMillan L, Lindstrom AB. Identification of fipronil metabolites by time-of-flight mass spectrometry for application in a human exposure study. Environ Int. 2015;78:16–23. doi: 10.1016/j.envint.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.CDC. Fourth National Report on Human Exposure to Environmental Chemicals. [accessed 10/11/2014]; https://www.cdc.gov/exposurereport/pdf/fourthreport.pdf.
- 45.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in Exposure to Polyfluoroalkyl Chemicals in the U.S. Population: 1999–2008†. Environ Sci Technol. 2011;45(19):8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- 46.Perez F, Llorca M, Farre M, Barcelo D. Automated analysis of perfluorinated compounds in human hair and urine samples by turbulent flow chromatography coupled to tandem mass spectrometry. Anal Bioanal Chem. 2012;402(7):2369–78. doi: 10.1007/s00216-011-5660-5. [DOI] [PubMed] [Google Scholar]
- 47.Nilsson H, Karrman A, Rotander A, van Bavel B, Lindstrom G, Westberg H. Biotransformation of fluorotelomer compound to perfluorocarboxylates in humans. Environ Int. 2013;51:8–12. doi: 10.1016/j.envint.2012.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional descriptions of experimental methods, including chemicals, animal treatment, and quality assurance and control. Tables showing database of known metabolites and transformation products of the 8:2 FTOH and 8:2 diPAP, descriptive demographics of the studied population, summary of instrumental parameters for LC–MS–TOF, LOQ and recoveries of analytes, results of p-values for the Shapiro–Wilk normality test, and p-values from the Mann–Whitney U test. Figures showing the expected biotransformation pathway of 8:2,8:2 diPAP and 8:2 FTOH, metabolites of 8:2 FTOH in serum, mass profiler of 8:2 diPAP-dosed animal serum, feces analysis, plots for the Spearman correlation of analysis of selected compounds in the office workers from the present study and the analysis from previous work, FTOH–sulfate in human serum, and an extracted ion chromatogram for FTOH–sulfate in blanks and standard curve points. (PDF)


