Abstract
Objective
To study rates and predictors of HCV cure among HIV/HCV coinfected patients
To evaluate the effect of attendance to clinic visits on HCV cure
Methods
Retrospective cohort study of adult HIV/HCV coinfected patients who initiated and completed treatment for HCV with Directly Acting Antivirals (DAAs) between 1/1/2014–6/30/2015
Results
Eighty-four participants reported completing treatment. The median age was 58 years (IQR 50–66), 88% men and 50% black. A third were cirrhotic and half were HCV treatment experienced. Most commonly used regimen was Sofosbuvir/ledipasvir (40%) followed by simeprevir/sofosbuvir (30%). Cure was achieved in 83.3%, 11.9% relapsed and 2.3% experienced virological breakthrough. Two patients (2.3%) had not completed treatment based on pills counts and follow up visit documentation.
In multivariable analysis cure was associated with attendance to follow up clinic visits (OR=9.0, 95% CI=2.91–163) and use of an integrase based HIV regimen vs. other non integrase regimens such as non nucleoside analogues or protease inhibitors (OR=6.22, 95% CI 1.81–141). Age, race, genotype, presence of cirrhosis, prior HCV treatment, HCV regimen and pre treatment CD4 counts were not associated with cure.
Conclusions
Real world HCV cure rates with DAAs in HCV/HIV coinfection are lower than those seen in clinical trials. Cure is associated with attendance to follow up clinic visits and use of an integrase based HIV regimen. Future studies should evaluate best antiretroviral regimens, predictors of attendance to follow up visits, impact of different monitoring protocols on medication adherence and interventions to ensure adequate models of HIV/HCV care.
Keywords: HIV/HCV coinfection, HCV Cure, follow up, integrase inhibitors
Introduction
Globally it is estimated that up to 30% of the 33 million people living with HIV (human immunodeficiency virus) infection also have hepatitis C (HCV) coinfection. [1] The natural history of HCV infection is altered in the HIV infected host. Patients with HIV/HCV coinfection are half as likely to spontaneously clear HCV viremia, tend to have higher HCV RNA levels and have accelerated progression to hepatic fibrosis and decompensated liver disease. [2, 3] Despite the use of antiretroviral therapy, the proportion of deaths caused by HCV related end stage liver disease and hepatocellular carcinoma have increased in the HIV infected population. [4]
Historically HIV/HCV coinfected patients treated with interferon and ribavirin (IFN/RBV) had lower rates of sustained virological response (SVR) than those without HIV infection. [5] In contrast to the IFN/RBV treatment, the Directly Acting Antiviral agents (DAAs) have demonstrated similar rates of HCV cure in mono and coinfected patients in clinical trials where strict follow up is mandated. [6, 7] However, data on rates of HCV cure with the use of DAAs in HIV/HCV coinfected patients in real world settings is lacking.
Treatment of HIV/HCV coinfection poses specific challenges as it is preferred that patients are on stable ART prior to initiating H CV treatment and requires careful consideration of drug to drug interactions. In addition due to the high cost of DAAs, insurance companies may have specific criteria to approve HCV treatment in the HIV infected population. Access to care, frequent monitoring of HIV treatment and adherence to antiretroviral therapy have been associated with faster time to HIV viral suppression and increased survival in the HIV infected population.[8, 9]. In real world settings, these and other factors may be less optimal than in randomized clinical trials, hence, we aim to describe predictors of HCV cure among HIV/HCV coinfected patients in 3 large urban outpatient settings. We hypothesize that real world rates of HCV cure in the HIV infected population are lower than what has been described in clinical trials, and that attendance to clinical monitoring visits is associated with higher cure rates.
Understanding ‘real world’ data is key to inform interventions and treatment protocols and potentially increase cure rates in the HIV/HCV infected population.
Methods and Materials
Study design
This was a retrospective cohort study of adult HIV/HCV coinfected patients receiving medical care at 3 large outpatient settings in South Florida, USA:
The Miami Veterans Affairs Healthcare System (VA), serves approximately 153,000 veterans in three counties by operating 372 hospital beds, a community living center and 7 satellite clinics.
University of Miami Health system (UHealth), a private academic institution operating 3 hospitals and 30 outpatient facilities serving 4 counties.
Jackson Health System (JHS), a public academic institution with 6 hospitals, 12 specialty care centers and two long-term centers that serves as tertiary referral center for the State of Florida.
We reviewed medical charts of individuals with HIV/HCV coinfection who initiated and completed treatment for HCV with DAAs in any of these 3 institutions between January 1 2014 and June 30 2015.
Inclusion criteria
All adults with HIV/HCV coinfection who initiated and completed HCV therapy with DAAs. HCV treatment was provided to patients older than 18 years of age with no evidence of active drug or alcohol abuse and on Antiretroviral therapy for HIV.
Outcome
Our primary outcome was HCV cure.
Cure was defined as an undetectable HCV RNA at 12 weeks after therapy completion (sustained virological response 12 weeks post treatment, SVR12). (10) A relapse was defined as recurrence of HCV RNA in a patient who had an end-of-treatment response (EOTR or undetectable HCV RNA at the end of treatment). A virologic breakthrough is appearance of HCV RNA while patient is still on HCV therapy. [10] We defined completion of treatment as self-reported completion of DAA therapy for the duration recommended by the provider. The selection and duration of HCV therapy strictly followed American Association for the Study of Liver Diseases guidelines, and was based on HCV genotype, degree of hepatic fibrosis, risk of drug interactions, etc.
Exposures
Our main exposure was attendance to follow up clinic visits.
Attendance to follow up clinic visit was defined as attendance to clinic visits at week 4, week 6–8 and week 12 of HCV treatment independent of attendance to laboratory visit. (17)
The 3 study settings have different monitoring protocols for HIV/HCV coinfected patients, however all sites required clinic visits on weeks 4, 6–8 and 12. We describe below the monitoring protocols at each site.
The VA Health System outpatient clinics: The treatment is initiated and monitored by the hepatology clinic. The treatment protocol requires clinic and laboratory visits every 2 weeks for the first 2 months and then monthly thereafter. A key characteristic is that the VA pharmacy dispenses DAAs during follow up visits only if the medication for that period has been completed.
Jackson Health System (JHS) outpatient clinics: The HIV provider initiates treatment for HCV. The treatment protocol recommends follow up clinic and laboratory visits every 4 weeks, however, during the initial visit, the patients receive prescriptions with refills to complete HCV treatment. The JHS pharmacy assists in obtaining approval for medications and informs the ordering physician when medications are approved. Medications are provided by community pharmacies based on initial prescription orders and independent of follow up visits or labs results.
UHealth: The HIV provider or the hepatologist initiates treatment. The treatment protocol recommends follow up clinic and laboratory visits every 4 weeks. Similarly to JHS, during the initial visit, the patients receive prescriptions with refills to complete HCV treatment. Pharmacy assists in obtaining approval for medications but medications are provided by community pharmacies based on initial prescription orders and independent of follow up visits or laboratory results.
Other Independent variables
Other exposure variables included the following:
Sociodemographic characteristics (age, gender, race, location of treatment)
Clinical assessments [baseline HIV viral load and Median Cd4 count, ARV regimen during HCV treatment, HCV genotype, HCV viral load, presence of cirrhosis, prior history of HCV treatment and agents used for treatment, presence of Hepatitis B coinfection, HCV treatment regimen, concurrent use of Ribavirin with DAAs for HCV treatment)
Attendance to on treatment laboratory visits was defined as lab tests done on week 4, 6–8, 12 and at the end of treatment.
Post treatment lab visits and clinic visits
Study procedures
We reviewed all medical charts/provider notes and collected exposure, outcome and Co-variate data. We also collected adverse events documented in the medical chart on each follow up visit. We reviewed information of all follow up visits as documented in the medical chart.
Statistical analysis
We used REDCap ® electronic data capture tools (Vanderbilt University, Version 6.5.2, 2015) hosted at University of Miami (10) to collect and manage study data. We used descriptive statistics to report the baseline characteristics of the population. We conducted uni-variable analysis with calculation of odds ratio, confidence interval and chi-square testing (p value of 0.05 was considered to indicate statistical significance). In order to identify predictors, we used logistic regression analysis. Our Multivariable analysis included the variables that were significant in the univariate analysis and those that have been described to be associated with cure in the literature (absence of cirrhosis, HCV genotype, AST Platelet Ratio Index or APRI score, duration of treatment, race and prior history of treatment). SPSS statistical software (version 22, Armonk, NY) was used for analysis.
Ethics statement
Institutional review board approvals from the University of Miami, Jackson Memorial Hospital and the Veterans Hospital administration was obtained prior to any study related procedures. Due to the retrospective nature of this study, a waiver of informed consent was granted.
Results
Characteristics of the study population
Eighty-four participants reported completing treatment and were evaluated 12 weeks post treatment. The median age was 58 years (IQR 50–66), 88% were men, and 50% were black. (Table 1) Approximately 90% of the patients had undetectable HIV viral load at baseline and a median CD4 count of 604 cells/mm3 (IQR 812–374). Use of ARV was as follows: over half of the patients were on an integrase based HIV regimen and 20% were on a non-nucleoside reverse transcriptase inhibitor (NNRTI) based regimen. More than two thirds of our patients were infected with genotype 1a with a median HCV viral load of 14.9 log10IU/ml (IQR 12.4–17.4). A third of the population had cirrhosis and half had prior history of HCV treatment. Among the prior HCV treatment regimens used about 40% were interferon based. Hepatitis B coinfection was found in 16% of the patients. The most commonly used regimen was sofosbuvir and ledipasvir, which was prescribed to approximately 40% of the patients. The second most common regimen was simeprevir with sofosbuvir (without Ribavirin) prescribed to about 30% of the patients followed by simeprevir with sofosbuvir (with Ribavirin) prescribed to about 15% of the patients. The regimens appeared to be well tolerated with two thirds of the patients reporting no adverse effects. Patients on ribavirin were noted to have more fatigue and anemia (n=9 and 8) vs. those not on ribavirin (n=5 and 0) respectively.
Table 1.
Baseline demographic characteristics of patients (N=84) and distribution by site
| Baseline Characteristic | Total N=84 |
VA N=48 |
JHS N=20 |
UHealth N=16 |
|---|---|---|---|---|
| Age, Years (IQR) | 58 (50–66) | 60(47–57) | 57 (60–52) | 54 (61–49) |
| Male, n (%) | 74 (88.1) | 47(97.9) | 15 (75.0) | 12 (75.0) |
| Race | ||||
| Caucasian, n (%) | 28 (33.3) | 15(31.3) | 7 (35.0) | 6 (37.5) |
| Black, n (%) | 42 (50.0) | 28(58.3) | 9 (45.0) | 5 (31.3) |
| Hispanic, n (%) | 14 (16.7) | 5(10.4) | 4 (20.0) | 5 (31.3) |
| HIV RNA <50 IU/ml, Mean (SD) | 76 (90.4) | 44(91.7) | 18 (90.0) | 14 (87.5) |
| Median CD4 count cells/mm3 (IQR) | 604 (812–374) | 570(775–412) | 615 (750–466) | 688 (888–580) |
| HIV Antiviral Regimen | ||||
| Integrase + TDF-FTC, n (%) | 25 (29.8) | 11(22.9) | 4(20.0) | 10(62.5) |
| Integrase+ ABC-3TC, n (%) | 12(14.3) | 7(14.6) | 3(15.0) | 2(12.5) |
| PI/r +, TDF-FTC n (%) | 11 (13.1) | 8(16.7) | 3(15.0) | 0 |
| Rilpivrine+TDF-FTC, n (%) | 5 (6.0) | 0 | 4(20.0) | 1(6.3) |
| EFV+ TDF-FTC, n (%) | 12 (14.4) | 7(14.6) | 3(15.0) | 2(12.5) |
| Others | 19(22.4) | 15(31.2) | 3(15.0) | 1(6.3) |
| HCV genotype- | ||||
| 1a, n (%) | 59 (70.2) | 32(66.7) | 13(65.0) | 14(87.5) |
| 1b, n (%) | 20 (23.8) | 13(27.1) | 6(30.0) | 1(6.3) |
| 2b, n (%) | 3 (3.6) | 2(4.2) | 1(5.0) | 0 |
| Others (3a and 4), n (%) | 2 (2.4) | 1(2.1) | 0 | 1(6.3) |
| Median HCV RNA (IQR), log10IU/ml | 14.9 (12.4–17.4) | 6.4(6.9–5.8) | 6.4(6.6–5.7) | 6.5(7.0–6.3) |
| Cirrhosis, n (%) | 24 (28.6) | 15(31.3) | 6(30.0) | 3(18.8) |
| Prior HCV treatment experienced, n (%) | 49 (58.3) | 25(52.0) | 13(65.0) | 11(68.7) |
| Prior HCV treatment agents | ||||
| PEG IFN/RBV | 30(40.0) | 21(43.8) | 6(33.3) | 3(33.3) |
| Boceprevir/teleprevir | 2(2.7) | 1(2.1) | 1(5.6) | 0 |
| DAAs | 1(1.3) | 1(2.1) | 0 | 0 |
| Other/not known | 8(10.7) | 2(4.2) | 5(27.8) | 1(11.1) |
| Hepatitis B coinfection, n (%) | 13 (15.9) | 8(16.7) | 4(22.2) | 1(6.3) |
| APRI Score, Median (IQR) | 0.57(0.92–0.37) | 0.54(0.75–0.31) | 1.2(2.4–0.6) | 0.48(0.67–0.38) |
| HCV treatment regimen | ||||
| Sofosbuvir+ledipasvir, n (%) | 33 (39.3) | 19(39.6) | 5(25.0) | 9(56.3) |
| Simeprevir+sofosbuvir, n (%) | 25 (29.8) | 10(20.8) | 10(50.0) | 5(31.3) |
| Simeprevir+sofosbuvir+RBV, n (%) | 13(15.5) | 13(27.1) | 0 | 0 |
| Sofosbuvir/IFN/RBV, n (%) | 7 (8.3) | 2(4.2) | 4(20.0) | 1(6.3) |
| Sofosbuvir+RBV, n (%) | 4 (4.8) | 2(4.2) | 1(5.0) | 1(6.3) |
| Ombitasvir+paritaprevir+dasabuvir/r n (%) | 2(2.3) | 2(4.2) | 0 | 0 |
| Concurrent RBV use for HCV treatment, n % | 28(33.7) | 21(43.8) | 5(26.3) | 2(12.5) |
| Attendance to follow up clinic visit, n % | 62(73.8) | 45(93.7) | 6(30.0) | 11(68.7) |
VA: The Miami Veterans Affairs Healthcare System, JHS: Jackson Health System
UHealth: University of Miami Health system
TDF-FTC: Tenofovir-Emtricitabine, ABC-3TC: Abacavir-Lamivudine, PI/r: Ritonavir boosted protease inhibitors, EFV: Efavirenz, APRI: AST to Platelet Ratio Index, /r: Ritonavir, RBV: Ribavirin
Outcomes
Cure
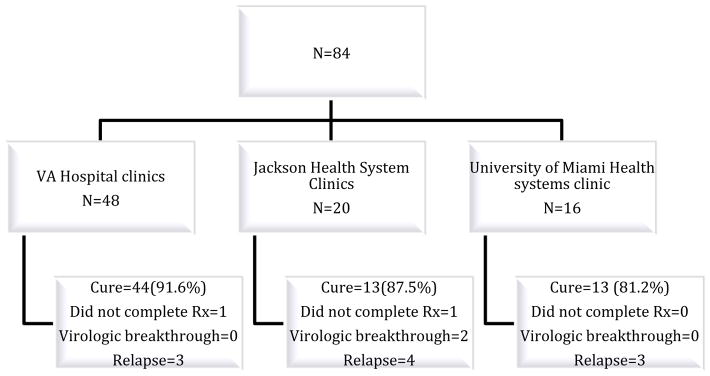
Among the 84 participants included in the study, 83.3% achieved SVR12 (n=70), 11.9% relapsed (n=10), 2.3% experienced virological breakthrough (n=2) and two patients (2.3%) were deemed to have not completed treatment based on pill counts and follow up visit documentation. (Figure 1)
Figure 1.
Outcomes per site
Virologic breakthrough : A virologic breakthrough is appearance of HCV RNA while patient is still on HCV therapy, Relapse : A relapse was defined as recurrence of HCV RNA in a patient who had an end-of-treatment response (EOTR or undetectable HCV RNA at the end of treatment)
Factors associated with cure
Univariate and multivariate analysis of demographics and clinical factors and their association with cure was conducted and illustrated in table 2 and 3.
Table 2.
Univariate predictors of HCV cure.
| Predictor | OR | 95%CI | P |
|---|---|---|---|
| Age < 58 | 0.62 | 0.19–2.04 | 0.433 |
| Race- | |||
| Caucasian | 1.30 | 0.37–4.59 | 0.679 |
| Black | 0.70 | 0.22–2.25 | 0.558 |
| Hispanic | 1.24 | 0.24–6.28 | 0.793 |
| Veterans hospital Clinic Site | 3.98 | 1.13–13.9 | 0.024 |
| Baseline CD4 | 0.75 | 0.23–2.43 | 0.643 |
| Integrase based HIV regimen | 3.88 | 0.99–15.12 | 0.040 |
| HCV genotype 1a | 0.34 | 0.07–1.65 | 0.165 |
| Cirrhosis | 0.67 | 0.19–2.25 | 0.517 |
| APRI score >1* | 0.20 | 0.06–0.70 | 0.007 |
| HCV treatment history | 0.67 | 0.21–2.11 | 0.488 |
| Hepatitis B coinfection | 0.63 | 0.15–2.67 | 0.531 |
| Use of sofosbuvir+ledipasvir | 1.03 | 0.85–1.26 | 0.695 |
| Ribavirin use | 0.90 | 0.27–2.99 | 0.284 |
| Undetectable HCV viral load on week 4 of Treatment | 0.74 | 0.23–2.37 | 0.613 |
| Attendance to on treatment lab visits ^ | 6.60 | 1.92–22.65 | 0.001 |
| Attendance to follow up to clinic visit** | 7.89 | 2.26–27.49 | 0.000 |
| Presence of adverse effects to HCV therapy | 0.36 | 0.11–1.18 | 0.086 |
Bolded rows show univariate analysis which were statistically significant
APRI score >1 : AST to Platelet Ratio Index score >1 has a sensitivity of 76% and specificity of 72% for predicting cirrhosis.
Appropriate follow up clinic visit was defined as attendance to clinic visits at week 4, week 6–8 and week 12 of HCV treatment independent of attendance to laboratory visit
Attendance to on treatment laboratory visits at week 4, 6–8,12 and end of treatment.
Table 3.
Multivariate model of HCV Cure.
| Predictor | OR | 95% CI | P |
|---|---|---|---|
| HCV genotype 1a | 2.21 | 0.02–1.62 | 0.137 |
| APRI score | 0.004 | 0.52–1.83 | 0.948 |
| Integrase based HIV regimen | 6.22 | 1.81–141 | 0.013 |
| Attendance to follow up clinic visit | 9.00 | 2.91–163 | 0.003 |
| Cirrhosis | 0.119 | 0.13–4.05 | 0.73 |
Model for the outcome cure was adjusted for HCV genotype, APRI score, integrase HIV regimen and appropriate follow up clinic visit. Due to colinearity with appropriate follow up visit, VA clinic site and laboratory visits were excluded from the model.
Factors associated with cure in univariate analysis were: Attendance to follow up visits (OR=7.89, 95% CI=2.26–27.4), receiving care at the Miami VA site (OR=3.98 95% C.I. 1.13–13.9), Attendance to on treatment laboratory visits (OR 6.6, 95% CI=1.92–22.6). Age, race, genotype, presence of cirrhosis, prior HCV treatment, HCV regimen, HBV coinfection, week 4 SVR, CD4 counts and pre-treatment HCV RNA were not associated with cure.
In multivariable analysis, factors associated with cure were attendance to follow up clinic visits (OR=9.0, 95% CI=2.91–163), and use of an integrase based HIV regimen vs. a non integrase based regimen such as NNRTI or protease inhibitors (OR=6.22, 95% CI 1.81–141). We excluded attendance to laboratory visits and treatment at the VA from the model due to colinearity with attendance to follow up clinic visits.
Of the 12 patients who failed treatment 8 were black, 4 were cirrhotic, 10 were infected with Genotype 1a, 2 were genotype 1b, 5 were treatment experienced and 7 were treated with simeprevir + sofosbuvir. Three of the seven patients treated with simeprevir +sofosbuvir were cirrhotic. About two thirds of the patients who failed therapy did not have attendance to follow up clinic visit. However, race, presence of cirrhosis, HCV genotype and drugs used for treatment of HCV were not associated with cure. (For detailed description of the patients who failed treatment and adverse effects please see supplementary appendix).
Discussion
HCV cure rates among HIV/HCV co-infected patients seeking care in real world settings are lower than those reported in clinical trials [6, 7, 11, 12]. Achieving cure (defined by SVR12 in this study) was closely associated with attendance to follow up clinic visits and to the use of an integrase based HIV regimen compared to a non integrase based regimens such as NNRTI or protease inhibitors. Our study did not show an association of black race or cirrhosis with decreased cure rates even though others have previously found this association [6]. Nevertheless, our study may lack the sufficient statistical power to detect this association.
The main contribution of our study to real world clinical practice is the relationship between attendance to follow up clinic visits and cure rates. Treatment of HCV in the HIV infected population is challenging due to accelerated liver disease, concomitant comorbidities and difficulties managing HCV-HIV drug to drug interactions. The importance of adequate adherence to clinical care has previously been described in populations infected only with HIV where greater frequency of visits and early linkage to care were associated with faster time to HIV viral suppression [9, 13, 14]. The reason for this relationship has not been fully described in HIV although these authors hypothesize medication adherence as a possible mediator. Frequency of clinic visits has been associated to higher medication adherence in populations with other chronic diseases. [15] In our study we found that attendance to visits was more common among the Veterans. This group was very similar to the other 2 groups with the exception of higher prevalence of black race and of first time HCV treatment. However, the Miami VA, being a closed system, used key strategies to incentivize attendance like scheduling more frequent visits and only refilling medications at follow up visits, once the pharmacist checked that all medication bottles were empty. This contrasts with the two fee for service sites that had less frequent scheduled visits and provided the entire regimen at the beginning of the treatment. Even though this latter protocol may have intended to improve the access to the medications, our findings suggest that providing refills during follow up visits may be more effective. We could not evaluate the mechanism through which the VA treatment protocol may influence cure however we can hypothesize that a likely mechanism is an improvement in medication adherence. Frequent clinic visits may address known barriers to adherence such as misconceptions on the efficacy of the medication, fear of side effects, low self-efficacy, mistrust, depression and other psychological factors to name a few. [16, 17]
Future studies should include measures of DAAs adherence to better elucidate the mechanisms through which different treatment protocols can achieve HCV cure. Nevertheless, our findings suggest that fee for service health systems should work with payers and other stakeholders to develop feasible and successful models of care for HCV treatment in the HIV infected population. If medical visits facilitate cure through medication adherence, models of care could include ancillary services such as in person or phone-based behavioral interventions led by counselors, which have been shown to increase medication adherence among patients with HIV [18, 19]. Such interventions could be introduced as a model of care that does not use clinic infrastructure already at maximum use and could be a more feasible strategy to provide cost-efficient high quality care. Interventions to increase adequate follow up for HCV treatment in patients coinfected with HIV have the potential to increase cure rates and reduce treatment costs by ensuring that treatment is provided as prescribed.
In this study we also found that patients receiving integrase inhibitors for the treatment of HIV had higher rates of cure when treated for HCV. This may be related to the fact that integrase inhibitors have less drug-drug interactions with HCV medications (hence lower chances of sub therapeutic drug levels for HCV) than NNRTIs or protease inhibitors. [20]. Future studies should evaluate the hypothesis that better tolerability of the integrase-based regimen may improve adherence to the HCV regimen as well.
This study has several limitations related to the retrospective nature of its design and the small sample size. 1.We could not compare medication adherence among the three groups (which may mediate the effect of follow up visits) because all clinics used different strategies to address adherence 2. We did not collect socio economic status, health literacy, depression which have also been linked to medication adherence and outcomes raising the possibility of residual confounding. 3. Sample size: although this study involves 3 large hospital sites the small sample size precludes generalization of the study findings and increases the chances of type 2 error such as ability to detect the association between cirrhosis, black race and cure. It should be noted that of the 12 patients who failed treatment 3 were cirrhotic who were recommended treatment for only 12 weeks with simeprevir + sofosbuvir which might have resulted in inadequate treatment and therefore spuriously low cure rates. 4. Finally, we could not adjust the model by provider or specialty as several providers could deliver follow up care to the same patient.
Conclusions
Real world HCV cure rates when using DAA in HIV/HCV coinfection are lower than those seen in clinical trials. Cure is associated with attendance to follow up clinic visits and the use of integrase based HIV regimens as opposed NNRTIs or protease inhibitors. Future studies should evaluate predictors of adequate attendance to follow up visits, the role of medication adherence and effect of integrase based regimen on HCV cure to inform best models of HCV/HIV care.
Supplementary Material
Take-Away points.
Clinical trials have shown excellent cure rates for HCV among the HIV/HCV population. We examined data for real world cure rates of HCV and predictors of cure in this population.
Real world HCV cure when using DAAs (Directly Acting Antivirals) in HIV/HCV coinfection are lower than those seen in clinical trials.
Cure is associated with attendance to follow up clinic visits and the use of integrase based antiretroviral regimen compared to a non integrase based HIV regimen such as non nucleoside analogues and protease inhibitors.
Future studies should evaluate best antiretroviral regimens, predictors of attendance to follow up visits, impact of different monitoring protocols on medication adherence and interventions to ensure adequate models of HIV/HCV care.
Acknowledgments
The authors gratefully acknowledge use of the services of the Miami Center for AIDS Research (CFAR) at the University of Miami (P30AI073961).
Contributor Information
Seetha Lakshmi, Jackson Memorial Hospital, Miami, USA and University of Miami, Miami, USA.
Maria Alcaide, University of Miami, Miami, USA.
Ana M Palacio, Jackson Memorial Hospital, Miami, USA.
Mohammed Shaikhomer, Jackson Memorial Hospital, Miami, USA and University of Miami, Miami, USA.
Abigail L Alexander, University of Miami Miller School of Medicine, Miami, USA.
Genevieve Wiehl, University of Miami Miller School of Medicine, Miami, USA.
Aman Pandey, University of Miami Miller School of Medicine, Miami, USA.
Kunal Patel, University of Miami Miller School of Medicine, Miami, USA.
Dushyantha Jayaweera, University of Miami, Miami, USA.
Maria Del Pilar Hernandez, University of Miami, Miami, USA and Veterans Affairs Hospital, Miami, USA.
Bibliography
- 1.Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8(12):1002–12. doi: 10.1016/j.cgh.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham CS, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33(4):562–9. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 3.Soto B, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26(1):1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal E, et al. Liver-related deaths in HIV-infected patients between 1995 and 2005 in the French GERMIVIC Joint Study Group Network (Mortavic 2005 study in collaboration with the Mortalite 2005 survey, ANRS EN19) HIV Med. 2009;10(5):282–9. doi: 10.1111/j.1468-1293.2008.00686.x. [DOI] [PubMed] [Google Scholar]
- 5.Soriano V, et al. New paradigms in the management of HIV and hepatitis C virus coinfection. Curr Opin Infect Dis. 2005;18(6):550–60. doi: 10.1097/01.qco.0000191509.56104.ec. [DOI] [PubMed] [Google Scholar]
- 6.Naggie S, et al. Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015;373(8):705–13. doi: 10.1056/NEJMoa1501315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sulkowski MS, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA. 2014;312(4):353–61. doi: 10.1001/jama.2014.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall HI, et al. HIV care visits and time to viral suppression, 19 U.S. jurisdictions, and implications for treatment, prevention and the national HIV/AIDS strategy. PLoS One. 2013;8(12):e84318. doi: 10.1371/journal.pone.0084318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giordano TP, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–9. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 10.Ghany MG, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feld JJ, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594–603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 12.Afdhal N, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–93. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 13.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131(2):81–7. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 14.Mugavero MJ, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–56. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey JE, et al. Risk factors associated with antihypertensive medication nonadherence in a statewide Medicaid population. Am J Med Sci. 2014;348(5):410–5. doi: 10.1097/MAJ.0b013e31825ce50f. [DOI] [PubMed] [Google Scholar]
- 16.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–7. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 17.Langebeek N, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. Bmc Medicine. 2014:12. doi: 10.1186/s12916-014-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palacio AM, et al. Can phone-based motivational interviewing improve medication adherence to antiplatelet medications after a coronary stent among racial minorities? A randomized trial. J Gen Intern Med. 2015;30(4):469–75. doi: 10.1007/s11606-014-3139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holstad MM, et al. Motivational groups support adherence to antiretroviral therapy and use of risk reduction behaviors in HIV positive Nigerian women: a pilot study. Afr J Reprod Health. 2012;16(3):14–27. [PMC free article] [PubMed] [Google Scholar]
- 20.Kiser JJ, Burton JR, Jr, Everson GT. Drug-drug interactions during antiviral therapy for chronic hepatitis C. Nat Rev Gastroenterol Hepatol. 2013;10(10):596–606. doi: 10.1038/nrgastro.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.