Abstract
Purpose
This study aims to investigate the influence of different storage humidity conditions on crystallization and aerosol performance of inhalable spray dried amorphous powder formulations (Ciprofloxacin hydrochloride as the model drug).
Methods
The spray dried samples were stored at 20%, 55% and 75% relative humidity (RH). Crystallinity was monitored by Powder X-ray diffraction (PXRD), and particle morphology was measured by scanning electron microscopy (SEM) and atomic force microscopy (AFM). Aerosol performance was evaluated using a multi-stage liquid impinger (MSLI).
Results
PXRD diffractograms showed the spray dried Ciprofloxacin stored at 20% RH for three weeks were amorphous; whereas those stored at 55%RH and 75%RH started crystallizing after one hour. Fine particle fraction (FPF) of the particles was improved from 28%to 42%after storage at 55% RH for three days. Such improvement was attributed to the crystallization of amorphous powders, which led to increased particle roughness and reduced particulate contact area, as visualized by SEM and quantified by AFM. A linear relationship was observed between degree of crystallinity/crystallite size and FPF (R2 = 0.94 and R2 = 0.96, respectively). However, deterioration in aerosol performance was observed after storage at 75% RH due to formation of inter-particulate liquid/solid bridges, as confirmed by SEM.
Conclusions
This study provides a fundamental understanding in moisture-induced physical and aerosol instability of the spray dried powder formulations.
Keywords: aerosol performance, amorphous powder, crystallization, dry powder inhaler, particle surface roughness, spray drying, storage humidity
INTRODUCTION
Pulmonary drug delivery systems have become popular for treatment of respiratory diseases because direct delivery of drugs to the disease sites offers quick drug action, high drug concentration in the lungs and lower systemic drug exposure (1,2). Dry powder inhalers (DPIs) has attracted increasing attention among all inhaler devices as they are easy to carry and use, and most drugs are more stable in the solid form (3,4).
However, there are a few limitations associated with dry powder inhalers (DPIs). These include inconsistent flow rate and variable dose delivery which are mainly due to dependence of some passive DPIs on inspiration rate of patients (5) and dispersion properties of the drug powders in the inhalation formulations (6). Dispersion behavior of inhalable powders are affected by powder properties such particle size distribution, surface morphology, surface energy, hygroscopicity and electrostatic properties of the drug/carrier particles (7–10). Surface morphology affects particle cohesion/adhesion by increasing or reducing contact area. Hence, modifying surface morphological properties may be an effective way to alter cohesion/adhesion and control the aerosol performance (11). Higher roughness of the drug/carrier is shown to reduce the particle-particle contact area and cohesiveness leading to better aerosol performance (12). Crystalline properties, particularly particle surface crystallinity of both drug and carriers may also have significant influence on aerosol performance and stability of DPI formulations (13).
Though different particle engineering technologies have been used for producing the drug particles for use in DPIs, spray drying technology has attracted increasing attentions (14,15). Spray drying involves atomizing drug solutions/suspensions into small droplets and evaporating the solvents using hot and dry gas (16,17). In most cases, particularly for small-molecules, spray drying produces amorphous powders due to rapid drying (18). It has been observed that spray dried amorphous powder of different material tends to crystallize at varying rates depending on the relative humidity (RH) and molecular weight (19). Wu et al. reported that the spray dried amorphous lactose particles, a carrier for DPI formulations, tend to recrystallize when stored at the humidity >32% (20). Costantino et al. observed a drastic decrease in aerosol performance of a pharmaceutical protein rhuMabE25 (Recombinant humanized anti-IgE monoclonal antibody) when the co-spray dried excipient of mannitol powder was crystallized form the amorphous form upon storage (21). However, there is a lack of studies that systematically examine the impact of crystallization of spray dried drug particles on aerosol performance upon storage at various humidity conditions. Such significant changes in aerosol performance of DPIs upon storage may cause critical quality concerns in product stability. Therefore, there is a strong need to understand the mechanisms of such impact of crystallization on aerosol performance to ensure the quality and stability of the DPI products.
Here our study has systematically examined the impact of crystallization of spray dried drug particles on aerosol performance upon storage at different RHs. Correlations between crystallinity/crystallite size, surface roughness and aerosol performance were established for the first time. Ciprofloxacin, a broad spectrum antibacterial drug often used for the treatment of lower respiratory infections was chosen as the model compound for our current study as it showed a tendency of crystallization upon storage post spray drying in the literature (22,23) and in our preliminary studies. Additionally, Ciprofloxacin is being developed as a dry powder for inhalation product (24). Effects of change in solid-state properties and surface morphology of the spray dried Ciprofloxacin particles on in-vitro DPI performance upon exposure to various humidity conditions were evaluated. Outcomes of the present study are critical for understanding the physical instability associated with amorphous spray dried particles and are useful for developing an effective DPI formulation with consistent quality.
MATERIALS AND METHODS
Chemicals
Ciprofloxacin hydrochloride monohydrate (abbreviated as Ciprofloxacin or Cipro in the text) was purchased from βetaPharma® (Shanghai) Co., Ltd. (Wujiang City, JiangSu Province, China). High performance liquid chromatography (HPLC) grade acetonitrile was supplied by Fischer scientific (Fair Lawn, NJ, USA). Magnesium nitrate and sodium chloride were purchased from fisher scientific (Fair Lawn, NJ, USA) to prepare saturated salt solutions for generating corresponding equilibrium RHs.
Production and Storage of Powder Formulations
A spray drying feed solution (16 mg/mL) was prepared by dissolving Ciprofloxacin in MilliQ water. The drug solution was spray dried using a BUCHI B-290 mini spray dryer, − (BUCHI Labortechnik AG, Flawil, Switzerland) at a feed rate of 2 mL/min. The spray dryer was operated under the following conditions: inlet air temperature (Tin) 120±2°C, aspirator at 35 m3/h and airflow of 700 L/h. These conditions resulted in an outlet temperature (Tout) of 60 ± 2°C. The spray-dried powder was divided into 3 equal parts and stored at 20 ± 2°C in: (1) a desiccator containing silica gel to maintain 20 ± 2% RH; (2) a humidity chamber containing saturated magnesium nitrate solution to maintain 55 ± 2% RH; (3) a humidity chamber containing saturated sodium chloride solution to maintain 75 ± 2% RH.
Powder X-ray Diffraction (PXRD)
X-ray diffraction patterns of the powder samples were collected by a Rigaku Smartlab™ diffractometer (Rigaku Americas, Texas, USA) with a Cu-Kα radiation source operated at 40 kV and 44 mA and a highly sensitive D/tex ultra-detector. All the measurements were conducted at ambient conditions in Bragg − Brentano geometry with 2θ ranging from 5 to 40° 2θ at a step size of 0.02° and a scan rate of 4°/min (25).
Particle Morphology
A field emission scanning electron microscope (NOVA nanoSEM, FEI Company, Hillsboro, Oregon, USA) was employed to examine morphology of the formulations. Samples were added on a rectangular carbon tape attached to metal stubs and excess powders were removed using compressed air. The samples were then placed in a sputter coater operated at 40 mA for 1 min (208 HR, Cressington Sputter Coater, England, UK) to obtain platinum coating at 40 mA for 1 min. The images were captured at 5 kV.
Particle Size
Particle size distributions of the powder formulations were measured using an in-built software with SEM micrographs. The diameters at 10% (d10), 50% (d50) and 90% (d90) undersize were calculated in approximately 100 particles for each formulation.
Differential Scanning Calorimetry
The DSC measurement was carried out using a DSC Q2000 (TA instruments, Delaware, USA). Samples were transferred into perforated aluminum sample pans and the mass was measured. Each sample was heated from 20 to 370°C at 10°C/min with purging of nitrogen gas at a flow rate of 50 mL/min.
Dynamic Vapor Sorption
Moisture sorption behavior for the spray dried ciprofloxacin formulation was determined using dynamic vapor sorption (DVS-Intrinsic, Surface Measurement Systems Ltd., London, UK). Approximately 5–10 mg of the spray dried powder was weighed into a pan and the pan was placed inside the measuring chamber. The powder sample was equilibrated at 0% RH to provide a baseline. The sorption mass change was measured at RH ranging from 0 to 90% (10% RH steps) at 25°C and desorption mass change was measured at RH ranging from 90 to 0%.
Crystallite Size Determination
Crystallite size was determined for the spray dried Ciprofloxacin particles stored at 55% RH at specific time points. The diffraction data were collected as described in the PXRD method section.
The diffraction data were analyzed in TOPAS (26). The data were inspected to find any evidence of phase changes, and the first dataset of each phase change was fitted with a series of individual peaks, allowing the peak positions and intensities to refine freely. The peak widths were constrained to show symmetric peak broadening with both Lorentzian and Gaussian components according to the Scherrer equation. Following this refinement, the peak positions and relative intensities were fixed, and a scale factor was added to allow all intensities to move up and down together. The peaks for each phase were added to the appropriate datasets, and the background, scale factor, and peak width parameters allowed to refine freely. The contribution of the instrument to the width of the diffraction peaks was estimated through the fundamental parameters approach (27). The crystallite size (volume-weighted mean diffraction column length) was extracted from the peak width parameters by the double-Voigt approach (28). In this approach, the individual Lorentzian and Gaussian widths are combined to give a width of the equivalent Voigt profile, and a crystallite size calculated from this value.
Determination of Crystallinity
Quantification of the crystallinity in the presence of its amorphous form or other components in a powder mixture has been reported (29–31). Under the assumption that experimentally measured crystalline and amorphous X-ray intensities or areas are proportional to the crystalline and amorphous fractions of the samples (32,33), the % crystallinity of Ciprofloxacin can be calculated by the following equation:
| (1) |
It is noteworthy that Areacrystalline and Areaamorphous represent the area contributed from crystalline and amorphous phases of the mixture in the diffractograms respectively. Although peak or halo intensity of the diffraction pattern has been used to quantify the crystallinity, we found that using relative peak area in this case has minimum variation.
Because the single peak ranging from 25.7 to 27.2 two theta is shown as the most intense and obvious peak in the diffraction pattern, this particular peak were used to build the quantitative model in this study. The integration of each diffractogram at the two theta between 25.7 to 27.2 were applied by using OriginPro 2016 (OriginLab Corporation, Northampton, MA, USA, Version 9.3E) to respectively obtain the area underneath the Bragg peaks (Areacrystalline) and the overall area (Areacrystalline + Areaamorphous).
Calibration standards were prepared by gently mixing different ratios of amorphous and crystalline Ciprofloxacin powders in a motor and pestle. Crystalline Ciprofloxacin powder was used as received from the supplier. Amorphous Ciprofloxacin powder was obtained by spray drying and stored in a dry desiccator. Crystalline forms were checked by XRD and DSC. Physical mixture was prepared in a nitrogen glove box (RH < 20%) to prevent amorphous Ciprofloxacin from crystallizing. X-ray diffraction patterns of these calibration samples were collected respectively and were pre-treated by the aforementioned method. The % crystalline of each calibration standard was then calculated based on Eq. 1. The linear correlation between the calculated % crystalline and the actual % crystalline was plotted as a calibration curve along with the 95% upper and lower prediction interval (Fig. 1). In the calibration curve, good linearity and narrow prediction bands were observed indicating this model is appropriate to quantitatively estimate the crystallinity of particles at different RH conditions.
Fig. 1.
Theoretical and predicted % Ciprofloxacin crystallinity as calculated from proportional amorphous and crystalline area contributions.
Surface Roughness Quantification
The surface topography and roughness of spray dried Ciprofloxacin particles stored at 20% and 55% RH were evaluated using an atomic force microscopy (AFM; Veeco DI3100, Veeco Ltd. (Current as Bruker AFM), Santa Barbara, CA). AFM scanning was carried out using the tapping mode with a super sharp tip cantilever (TESP-SS-10, NanoAndMore Ltd., Watsonville, CA, USA) oscillating at 287 ± 2 kHz, with 5% offset frequency of first bending resonance. The images were captured at a scan rate of 1 Hz for a scan size of 1–2 and analyzed using the Gwyddion software (Czech Metrology Institute, Brno, Czechia) extensively used for scanning probe microscopy data analysis (34). Six images for each formulation were analyzed for the spray dried ciprofloxacin particles stored at 20%RHand 55%RH. Root mean square (RMS) was calculated based on the AFM images as the indicator of surface roughness. Higher RMS represents rougher surfaces.
Drug Quantification
The concentration of Ciprofloxacin in water was determined by high performance liquid chromatography (HPLC) using 76% v/v of 30 mM solution of sodium sulfate (adjusted to pH 2.5 with H3PO4) and 24% v/v acetonitrile as mobile phase resulting in isocratic elution of the sample for 7 min with a flow rate of 1.0 mL/min. Briefly, the HPLC system consisted of G1311C (1260 Quat Pump VL) pump, G1330B (1290 Thermostate) thermostate, G1329B (1260 ALS) autosampler, G1316A (1260 TCC) thermostated column compartment, G1314F (1260 VWD) variable wavelength detector (Agilant, Waldbronn, Germany), and an Agilant Eclipse Plus, 5 µm C18 150 × 4.60 mm column (Agilant, Waldbronn, Germany). The calibration curve for Ciprofloxacin was linear (R2 > 0.999) over the concentration range of approximately 0.25 to 0.01 mg/mL.
In-Vitro Aerosol Performance
Aerosolization performance of the powder formulations was measured using a Multi-Stage Liquid Impinger (MSLI) (Copley Scientific Limited, Nottingham, UK) with a USP induction port (USP throat). Prior to each run, Stage 1 to Stage 4 were filled with 20 mL of water and a 0.2 µm glass microfiber filter was placed at the bottom of the impinger base. The formulation (10 ± 1 mg) was weighed into a Size 3 hydroxypropyl methylcellulose capsule (Qualicaps, Whitsett, NC, USA). A standard dispersion procedure (USP 38) was carried out by passing 4 L of air through the inhaler at an airflow of 100 L/min for 2.4 s, with a pressure drop of <4 kPa (approximately 3.2 kPa at 100 L/min) across a RS01 DPI device (Plastiape S.p.A., Osnago, Italy). The RS01 DPI device has a similar design and characteristics to the Osmohaler. Stages 1–4 of the liquid impinger at 100 L/min had cutoff diameters of 10.4, 4.9, 2.4, and 1.2 µm, respectively (35). For the powder formulations stored at each relative humidity condition, four replicated experiments were carried out and results were averaged.
After the dispersion, the drug particles remained in the capsule and deposited in the inhaler device, USP throat, Stage 1–4 and filter paper were collected using MilliQ water. Drug contents were analyzed using a validated HPLC method described above. The emitted dose (ED) was determined as the drug released from the capsule and device over the recovered dose; whereas the fine particle fraction (FPF) was defined as drug particles with an aerodynamic size below 4.9 µm (cutoff diameter of Stage 2) relative to the total recovered drug.
Statistical Analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) with Tukey-Kramer post hoc tests using a GraphPad Prism Software (GraphPad Software, Inc., La Jolla, CA). Probability values of less than 0.05 were considered as a statistically significant difference and “NS” represents not significant (p > 0.05).
RESULTS AND DISCUSSION
Spray Dried Formulations upon Storage at Various RHs
In-Vitro Aerosol Performance
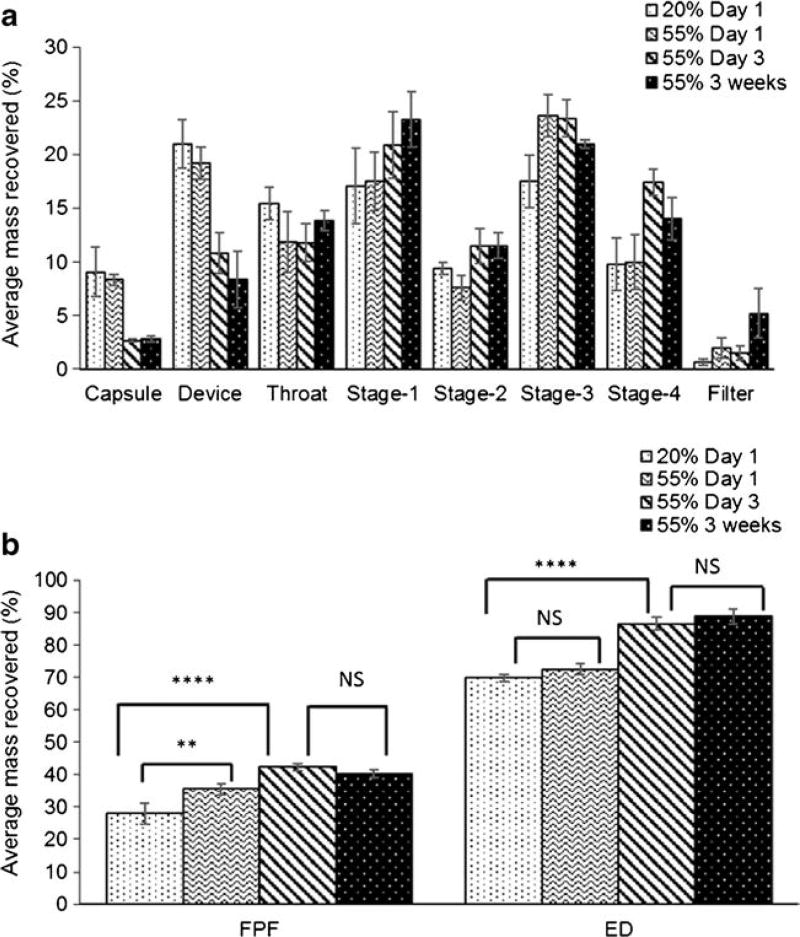
Aerosol deposition profiles of the spray dried Ciprofloxacin formulations stored under 20% RH, 55% RH and 75% RH for one day are shown in Fig. 2a. The corresponding emitted dose (ED) and fine particle fraction (FPF) values of spray dried Ciprofloxacin particles stored under three different relative humidity conditions are summarized in Fig. 2b. At 75% RH, ED of the spray dried Ciprofloxacin was found to be 87.9 ± 5.8%, which was significantly higher than that stored at 55% RH (72.5 ± 1.7%) and at 20% RH (69.9 ± 1.1%). There was no statistical difference in the ED for spray dried Ciprofloxacin formulations stored at 55% RH and 20% RH (Fig. 2, p > 0.05). The FPF increased significantly from 28.0 ± 3.2% at 20% RH to 35.6 ± 1.7% upon storage at 55% RH for one day (p < 0.05). Additionally, there was a statistically significant decrease (p < 0.01) in the FPF when the samples were stored at 75% RH (21.0 ± 5.8%) for one day as compared to those stored at 55% RH (FPF: 35.6 ± 1.7%).
Fig. 2.
(a) Deposition profiles and (b) aerosol performance of the spray dried Ciprofloxacin formulations as reflected by ED and FPF after storage for one day at different RHs (mean ± SD, n = 4; *, p < 0.05; **, p < 0.01; ***, p < 0.001; NS, no significant difference).
The increase in FPF at 55% RH was attributed to increased deposition of drug particles on the stage 3, stage 4 and filter whereas the decrease in FPF at 75% RH was attributed to higher deposition of particles in the USP throat, stage 1 and stage 2 (Fig. 2a). The instability in aerosol performance upon storage at different humidity conditions could be of great concern in terms of clinical efficacy and safety. Thus, it is crucial to understand the physicochemical behavior of amorphous spray dried powders at an early formulation development stage as it could have significant impacts on manufacturability, stability and drug delivery performance (36). Since the physical size of inhalable particles may have a significant influence on aerosol deposition (37), it was measured for the spray dried Ciprofloxacin powders stored at different RHs.
Table I shows the physical particle size distributions of spray dried Ciprofloxacin powders stored at 20% and 55% RH. The particle size distribution of spray dried Ciprofloxacin powder stored at 75% RH could not be measured because the particles fused and formed coarse lumps. Agglomeration or increased particle size enabled easy flow of the powders from the capsule and device upon storage at 75% RH. Thus, the ED for spray dried Ciprofloxacin powders stored at 75% RH was significantly higher as compared to 20% and 55% storage humidity (Fig. 2a). No significant difference in particle size between 20% RH and 55% RH was observed and the changes in aerosol performance were not due to altered physical particle size distributions.
Table I.
Particle Size Distributions of the Spray Dried Ciprofloxacin Powders (n = 100)
| Formulation | D10 (µm) | D50 (µm) | D90 (µm) |
|---|---|---|---|
| SD Ciprofloxacin (20% RH) | 0.91 | 1.56 | 2.39 |
| SD Ciprofloxacin (55% RH) | 1.20 | 1.73 | 2.54 |
Particle Morphology
Scanning electron micrographs (SEM) showed that the spray dried Ciprofloxacin powder stored under 20% RH exhibited a near-spherical shape with relatively smooth surfaces and some concaveties (Fig. 3a). However, those powders stored at 55% RH had much rougher surfaces (Fig. 3b) compared to 20% RH samples (Fig. 3a). On the other hand, the samples stored at 75% RH were fused into irregular lumps (Fig. 3c). As suggested by Visser et al., at elevated relative humidity (≥ 65%) the particles are likely to be held together by capillary force due to presence of excess moisture thus resulting in fusion of particles (38). Similar observations were made by Zhou et al. where the spray-dried formulations of colistin were hygroscopic and the particles fused together upon storage at 90% RH (10).
Fig. 3.
SEM micrographs of the spray-dried Ciprofloxacin powders that were stored at (a) 20%RH; (b) 55%RH; and (c) 75% RH for one day.
Chew and Chan demonstrated that increasing the surface roughness of the inhalable particles could lower the area of contact, and thus reduce the powder cohesiveness (12). In our study, the altered morphology for spray dried Ciprofloxacin particles stored at 55% RH is most likely responsible for the change in aerosol performance.
Dynamic Vapor Sorption
Moisture sorption isotherm for spray dried ciprofloxacin formulation is shown in Appendix Fig. 12. With an increasing in RH from 0% to 60%, the formulation absorbed water up to 8.1% (w/w). Further increasing RH resulted in a decrease in the powder mass, likely due to crystallization (23). Approximately 5% of water was bonded to the powder at the end of desorption cycle, which indicated moisture-induced crystallization of the amorphous spray dried ciprofloxacin powder.
Solid-State Characterization
To understand the underlying cause for the changes in surface morphology of spray dried powder formulations, we examined crystallinity of Ciprofloxacin particles stored at three different humidity conditions using PXRD. Sharp peaks were observed for the spray dried Ciprofloxacin formulations stored at 55% RH and 75% RH for one day (Fig. 4). Whereas, no crystallinity was detected for the samples stored at 20% RH, indicating their amorphous nature (Fig. 4). Degree of crystallization was much higher for the spray dried Ciprofloxacin powders stored at 75% RH as opposed to the powders stored at 55% RH for one day. PXRD patterns for the samples stored at 75% RH were similar to the raw Ciprofloxacin hydrochloride monohydrate. However, PXRD pattern for the spray dried Ciprofloxacin stored at 55% RH exhibited a new metastable polymorphic form with a distinct peak at 5.8° 2 theta.
Fig. 4.
X-ray powder diffraction (PXRD) patterns of the spray dried Ciprofloxacin formulations stored at 20% RH, 55% RH, and 75% RH for one day and raw Ciprofloxacin.
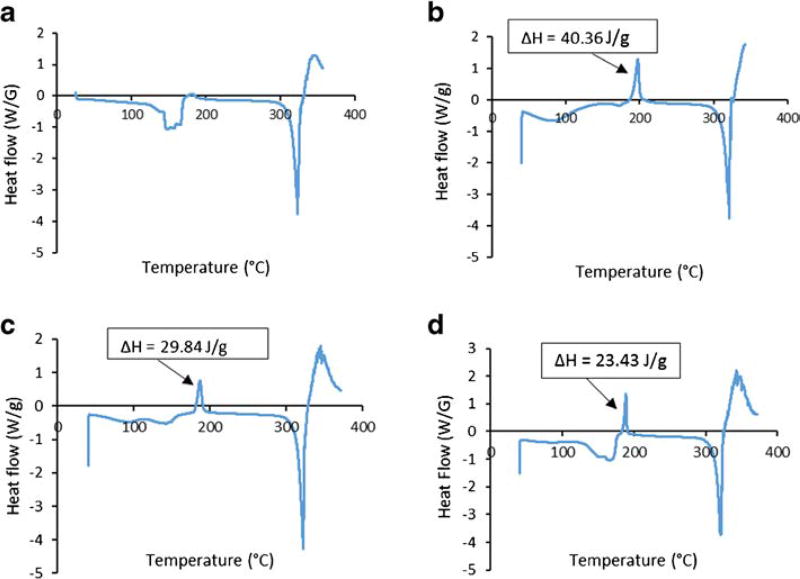
DSC thermograms of the raw and spray dried Ciprofloxacin samples stored at different relative humidity conditions after one day are shown in Fig. 5. For the raw Ciprofloxacin powder, first endothermic peak around 158°C could be attributed to dehydration (Ciprofloxacin hydrocloride monohydrate was used here) (39). Second endothermic peak between 305°C to 320°C corresponded to the characteristic melting peak of Ciprofloxacin hydrochloride (39). For the spray dried Ciprofloxacin samples stored at 20% RH, an exothermic peak (heat of crystallization, ΔH = 40.36 J/g) was observed around 198°C indicative of a phase transition of amorphous Ciprofloxacin samples; whereas, the endothermic peak around 321°C represents melting of the sample. Similarly, exothermic peaks were observed around 198°C for the spray dried Ciprofloxacin samples stored at 55% RH and 75% RH with heat of crystallization corresponding to 29.84 J/g and 23.43 J/g, respectively. The heat of crystallization decreased from 40.36 J/g for amorphous spray dried Ciprofloxacin at 20% RH to 23.43 J/g for the recrystallized samples stored at 75% RH, indicating a decrease in amorphous content/polymorphic transition upon storage at the higher humidity.
Fig. 5.
DSC thermograms of (a) raw Ciprofloxacin; (b) spray-dried Ciprofloxacin at 20% RH; (C) spray-dried Ciprofloxacin at 55% RH; and (D) spray-dried Ciprofloxacin at 75% RH for one day. ΔH is the heat of crystallization.
Mechanisms of Moisture-induced Crystallization on Aerosol Performance
Physical instability is a common drawback associated with the spray-dried amorphous particles (19). Depending upon moisture, temperature and time of storage, these metastable amorphous spray dried particles may tend to convert to its more stable crystalline form (19). Presence of water may promote the crystallization of spray dried samples as water molecules increase the mobility of the drug molecule (40). Crystallization of amorphous powders upon storage could cause severe stability issues. There have been studies showing water-induced crystallization of different spray dried powders upon exposure to higher relative humidity (19) but impact of crystallization on in-vitro aerosol performance has not been systematically studied. Our work has demonstrated the substantial impacts of moisture-induced crystallization on aerosol performance of the spray dried drug powders such as Ciprofloxacin (Fig. 6).
Fig. 6.
Schematic diagram showing different effects of moisture-induced crystallization on particle morphology and aerosol performance when spray dried amorphous Ciprofloxacin particles were stored at various RHs for one day.
Our hypothesis is that crystallization of amorphous spray dried Ciprofloxacin on exposure to 55%RH is responsible for the increased surface roughness and decreased particle-particle interactions. The cohesive forces between particles with corrugated surfaces are lower thereby reducing the contact areas between particles and improving the aerosolization performance (Fig. 6). However, storage of the spray dried Ciprofloxacin powders at 75% RH leads to fusion of particles along with crystallization due to presence of excess moisture resulting in decreased aerosol performance. Since the spray dried Ciprofloxacin stored at 55%RH did not crystallize fully as compared to spray dried Ciprofloxacin powders stored at 75% RH (Figs. 4 and 5), the crystallization of spray dried Ciprofloxacin formulations at 55%RH was further examined for up to 3 weeks to study longer-term stability.
Correlations between Crystallinity, Crystallite Size and Aerosol Performance upon Storage at 55% RH
Crystallinity and Crystallite Size
The crystallinity of spray dried Ciprofloxacin formulations stored at 55% RH at different time points was estimated by calculating the proportional amorphous and crystalline area contributions in a specific 2θ range (25.7 to 27.2°) of X-ray diffraction patterns. The amorphous nature of the spray dried Ciprofloxacin formulations stored at 20% RH was well maintained for up to 3 weeks; In contrast, a progressive increase in the crystallinity was observed for the spray dried Ciprofloxacin samples stored at 55% RH (Fig. 7). To be specific, crystallization (49%) can be readily induced even after 1 h exposure at 55% RH storage condition. The crystallization was further increased (78%) during the three-day storage at the same condition. Afterwards, only marginal increase in crystallinity (86%) was observed at 3 weeks. Likewise, an increase in crystallite size was observed with an increase in degree of crystallinity. The crystallite size for the spray dried Ciprofloxacin formulations stored at 55% RH increased over the time from 0.7 nm at 0 h to 24.7 nm at the end of 3 weeks. There was a significant increase in the crystallite size from 15.5 nm at Day 1 to 26.6 nm at Day 3; however, the crystallite size did not show a significant increase from Day 3 up to3weeks (24.7 nm). Impact of increasing degree of crystallinity and crystallite size on aerosol performance was further investigated.
Fig. 7.
PXRD patterns of the spray dried Ciprofloxacin formulations at (a) 20%RH and (b) 55% RH over the period of 3 weeks. The percentage values in (b) are determined crystallinity based on PXRD diffractograms.
In-Vitro Aerosol Performance
Aerosol deposition profiles of the spray dried Ciprofloxacin formulations stored at 55% RH at specific time points (Day 1, Day 3 and 3 weeks) and at 20% RH for one day are shown in Fig. 8a. The corresponding ED and FPF values are summarized in Fig. 8b. ED of the spray dried Ciprofloxacin particles increased significantly from Day 1 (72.5 ± 1.7%) to Day 3 (86.5 ± 2.0%) upon storage at 55%RH (p < 0.0001). FPF of the spray dried Ciprofloxacin formulation stored at 55% RH increased significantly from 35.5 ± 1.7% at Day 1 to 42.3 ± 0.9% at Day 3 (p < 0.01). The increase in FPF of spray dried Ciprofloxacin particles at 55% RH could be attributed to much higher deposition in the stage 4 at Day 3 in comparison to Day 1 (Fig. 8a). Thus, the powders showed improved flowability along with improved aerosol performance. However, the FPF did not increase any further after Day 3 and was plateaued until 3 weeks (40.2 ± 1.2%).
Fig. 8.
(a) Aerosol deposition profiles (b) aerosol performance of the spray dried formulations stored at 55% RH at the specific days (mean ± SD, n = 4; *, p < 0.05; **, p < 0.01; ****, p < 0.0001; NS, no significant difference).
Correlations between Crystallinity, Crystallite Size and Aerosol Performance
Correlations between degree of crystallinity, crystallite size and FPF were established (Fig. 9). A linear relationship was observed between degree of crystallinity and crystallite size (R2 = 0.92) for spray dried Ciprofloxacin powders stored at 55% RH for up to 3 weeks. The increase in degree of crystallinity and crystallite size with time was further correlated to aerosol performance of the spray dried Ciprofloxacin powders stored at 55% RH. The degree of crystallinity increased from Day 1 (~54%) to Day 3 (~78%), and a proportional increase was observed in the FPF from 35.5±1.7% at Day 1 to 42.3 ± 0.9% at Day 3 (Figs. 7b and 8b). Linear relationships were established between the degree of crystallinity and FPF (R2 = 0.94) and between the crystallite size and FPF (R2 = 0.96) for the spray dried Ciprofloxacin stored at 55% RH (Fig. 9).
Fig. 9.
Correlations between crystallinity, crystallite size and FPF for the spray dried Ciprofloxacin formulations stored at 55% RH (Mean ± SD, n = 4).
Further, a progressive change in surface morphology of the spray dried Ciprofloxacin particles from smooth to rough was observed upon storage at 55% RH for 3 weeks (Fig. 10). The increase in surface roughness was attributed to the progressive increase in crystallinity and crystallite size of the spray dried Ciprofloxacin particles upon exposure to humidity. The increase in surface roughness for the spray dried Ciprofloxacin particles stored at 55% RH compared to 20% RH was quantified using an AFM (Fig. 11). From the AFM images it is visually evident that the spray dried Ciprofloxacin particles stored at 55% RH (crystallized) are rougher in comparison to the spray dried Ciprofloxacin particles stored at 20% RH (amorphous) (Fig. 11). Quantitatively, RMS value for the spray dried Ciprofloxacin particle at 55% RH (24.7 ± 4.3 nm) was significant higher than that of the amorphous Ciprofloxacin particles stored at 20% RH (9.7 ± 3.2 nm). Increased crystallinity and crystallite size led to proportional increase in surface roughness and reduction in particle-particle interaction, thereby improved aerosol performance. This is in agreement with the previous findings that rougher surfaces resulted in less particle-particle contact area and therefore weaker interpaticulate interactions (12,41).
Fig. 10.
SEM micrographs showed changes in surface roughness of: (a) amorphous spray dried Ciprofloxacin particles stored at 20% RH, and the crystallized spray dried Ciprofloxacin particles stored at 55% RH for: (b) 1 day; (c) 3 days; and (d) 3 weeks.
Fig. 11.
Representative AFM 3D–surface topography of the spray dried Ciprofloxacin particles stored at: (a) 20% RH (amorphous); and (b) 55% RH (crystallized).
The AFM data support our hypothesis that crystallinity has an influence on surface roughness and aerosol performance. Surface roughness increased with degree of crystallinity and thereby impacted aerosol performance for the spray dried Ciprofloxacin particles stored at 55% RH.,
CONCLUSION
The present study provides fundamental understanding in physical instability of spray dried drug particles and the impact on aerosol performance. Changes in aerosol performance of the spray dried Ciprofloxacin powder formulations upon storage were attributed to crystallization that altered surface morphology. Improvement in aerosol performance for the spray dried Ciprofloxacin formulation stored at 55% RH was due to moisture-induced crystallization thereby causing an increase in surface roughness and decrease in particle-particle interactions. The decrease in aerosol performance at 75% RH was the consequence of particle fusion in presence of excessive moisture. The degree of crystallinity and crystallite size were found to increase with storage time and resulted in a proportional increase in surface roughness and aerosol performance for the spray dried Ciprofloxacin formulations stored at 55% RH. We have successfully established quantitative linear relationships between degree of crystallization/crystallite size and aerosol performance for the spray dried Ciprofloxacin formulation stored at 55% RH. The results of this study emphasize the importance of storage humidity on physical stability and aerosol performance of the spray dried powder formulations. For the amorphous DPI formulations that are physically unstable when they are exposed to moisture, strict packaging or moisture protection strategies such as particle coating (42) should be considered to maintain the quality of the products.
Acknowledgments
ACKNOWLEDGMENTS AND DISCLOSURES
Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R01AI132681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Qi (Tony) Zhou is a recipient of the Ralph W. and Grace M. Showalter Research Trust Award. Kind donations of RS01 DPI device from Plastiape S.p.A. and HPMC capsules from Qualicaps®, Inc are acknowledged.
ABBREVIATIONS
- AFM
Atomic force microscopy
- Cipro
Ciprofloxacin hydrochloride monohydrate
- DPI
Dry powder inhaler
- DSC
Differential scanning calorimetry
- DVS
Dynamic vapor sorption
- ED
Emitted dose
- FPF
Fine particle fraction
- MSLI
Multi-stage liquid impinger
- PXRD
Powder X-ray diffractometer
- RH
Relative humidity
- RMS
Root mean square
- SEM
Scanning electron microscopy
APPENDIX
Fig. 12.
Moisture sorption isotherms for the spray dried ciprofloxacin formulation.
References
- 1.Zhou QT, Leung SS, Tang P, Parumasivam T, Loh ZH, Chan HK. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv Drug Deliv Rev. 2015;85:83–99. doi: 10.1016/j.addr.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 2.Williams RO, Taft DR, McConville JT. Advanced drug formulation design to optimize therapeutic outcomes. Boca Raton, Florida: CRC Press; 2007. [Google Scholar]
- 3.de Boer AH, Hagedoorn P, Hoppentocht M, Buttini F, Grasmeijer F, Frijlink HW. Dry powder inhalation: past, present and future. Expert Opin Drug Deliv. 2017;14(4):499–512. doi: 10.1080/17425247.2016.1224846. [DOI] [PubMed] [Google Scholar]
- 4.Smith IJ, Parry-Billings M. The inhalers of the future? A review of dry powder devices on the market today. Pulm Pharmacol Ther. 2003;16(2):79–95. doi: 10.1016/S1094-5539(02)00147-5. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Q, Tang P, Leung SSY, Chan JGY, Chan H-K. Emerging inhalation aerosol devices and strategies: where are we headed? Adv Drug Deliv Rev. 2014;75:3–17. doi: 10.1016/j.addr.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Hoppentocht M, Hagedoorn P, Frijlink HW, de Boer AH. Technological and practical challenges of dry powder inhalers and formulations. Adv Drug Deliv Rev. 2014;75:18–31. doi: 10.1016/j.addr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Hassan MS, Lau RW. Effect of particle shape on dry particle inhalation: study of flowability, aerosolization, and deposition properties. AAPS Pharm Sci Tech. 2009;10(4):1252–62. doi: 10.1208/s12249-009-9313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavan V, Dalby R. Effect of rise in simulated inspiratory flow rate and carrier particle size on powder emptying from dry powder inhalers. AAPS Pharm Sci. 2000;2(2):E10. doi: 10.1208/ps020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chew NY, Bagster DF, Chan HK. Effect of particle size, air flow and inhaler device on the aerosolisation of disodium cromoglycate powders. Int J Pharm. 2000;206(1–2):75–83. doi: 10.1016/s0378-5173(00)00516-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhou QT, Morton DA, Yu HH, Jacob J, Wang J, Li J, et al. Colistin powders with high aerosolisation efficiency for respiratory infection: preparation and in vitro evaluation. J Pharm Sci. 2013;102(10):3736–47. doi: 10.1002/jps.23685. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q, Morton DAV. Drug–lactose binding aspects in adhesive mixtures: Controlling performance in dry powder inhaler formulations by altering lactose carrier surfaces. Adv Drug Deliv Rev. 2012;64(3):275–84. doi: 10.1016/j.addr.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Chew NY, Chan H-K. Use of solid corrugated particles to enhance powder aerosol performance. Pharm Res. 2001;18(11):1570–7. doi: 10.1023/a:1013082531394. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y-W, Wong J, Qu L, Chan H-K, Zhou QT. Powder production and particle engineering for dry powder inhaler formulations. Curr Pharm Des. 2015;21(27):3902–16. doi: 10.2174/1381612821666150820111134. [DOI] [PubMed] [Google Scholar]
- 14.Bohr A, Ruge CA, Beck-Broichsitter M. Preparation of nanoscale pulmonary drug delivery formulations by spray drying. Adv Exp Med Biol. 2014;811:183–206. doi: 10.1007/978-94-017-8739-0_10. [DOI] [PubMed] [Google Scholar]
- 15.Chow AH, Tong HH, Chattopadhyay P, Shekunov BY. Particle engineering for pulmonary drug delivery. Pharm Res. 2007;24(3):411–37. doi: 10.1007/s11095-006-9174-3. [DOI] [PubMed] [Google Scholar]
- 16.Vehring R, Foss WR, Lechuga-Ballesteros D. Particle formation in spray drying. J Aerosol Sci. 2007;38(7):728–46. [Google Scholar]
- 17.Anandharamakrishnan CaI SP. Introduction to spray drying, in Spray Drying Techniques for Food Ingredient Encapsulation. John Wiley & Sons, Ltd; Chichester: 2015. [Google Scholar]
- 18.Niazi MBK, Broekhuis AA. Production of amorphous starch powders by solution spray drying. J Appl Polym Sci. 2012;126(S1):E143–53. [Google Scholar]
- 19.Chiou D, Langrish TAG. Crystallization of amorphous components in spray-dried powders. Dry Technol. 2007;25(9):1427–35. [Google Scholar]
- 20.Wu L, Miao X, Shan Z, Huang Y, Li L, Pan X, et al. Studies on the spray dried lactose as carrier for dry powder inhalation. Asian J Pharm Sci. 2014;9(6):336–41. [Google Scholar]
- 21.Costantino HR, Andya JD, Nguyen PA, Dasovich N, Sweeney TD, Shire SJ, et al. Effect of mannitol crystallization on the stability and aerosol performance of a spray-dried pharmaceutical protein, recombinant humanized anti-IgE monoclonal antibody. J Pharm Sci. 1998;87(11):1406–11. doi: 10.1021/js9800679. [DOI] [PubMed] [Google Scholar]
- 22.Adi H, Young PM, Chan HK, Stewart P, Agus H, Traini D. Cospray dried antibiotics for dry powder lung delivery. J Pharm Sci. 2008;97(8):3356–66. doi: 10.1002/jps.21239. [DOI] [PubMed] [Google Scholar]
- 23.Adi H, Young PM, Chan H-K, Agus H, Traini D. Co-spray-dried mannitol–ciprofloxacin dry powder inhaler formulation for cystic fibrosis and chronic obstructive pulmonary disease. Eur J Pharm Sci. 2010;40(3):239–47. doi: 10.1016/j.ejps.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Antoniu S, Azoicai D. Ciprofloxacin DPI in non-cystic fibrosis bronchiectasis: a phase II randomized study. Expert Opin Investig Drugs. 2013;22(5):671–3. doi: 10.1517/13543784.2013.783565. [DOI] [PubMed] [Google Scholar]
- 25.Nie H, Xu W, Ren J, Taylor LS, Marsac PJ, John CT, et al. Impact of metallic stearates on disproportionation of hydrochloride salts of weak bases in solid-state formulations. Mol Pharm. 2016;13(10):3541–52. doi: 10.1021/acs.molpharmaceut.6b00630. [DOI] [PubMed] [Google Scholar]
- 26.Uvarov V, Popov I. Metrological characterization of X-ray diffraction methods for determination of crystallite size in nano-scale materials. Mater Charact. 2007;58(10):883–91. [Google Scholar]
- 27.Cheary RW, Coelho AA, Cline JP. Fundamental parameters line profile fitting in laboratory diffractometers. J Res Natl Inst Stand Technol. 2004;109(1):1–25. doi: 10.6028/jres.109.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balzar D. Voigt function model in diffraction-line broadening analysis. In: Synder RL, Fiala J, Bunge HJ, editors. Defect and microstructure analysis by diffraction. Oxford: Oxford University Press; 1999. pp. 94–126. [Google Scholar]
- 29.Surana R, Suryanarayanan R. Quantitation of crystallinity in substantially amorphous pharmaceuticals and study of crystallization kinetics by X-ray powder diffractometry. Powder Diffract. 2000;15(1):2–6. [Google Scholar]
- 30.Suryanarayanan R, Herman CS. Quantitative analysis of the active ingredient in a multi-component tablet formulation by powder X-ray diffractometry. Int J Pharm. 1991;77(2):287–95. doi: 10.1023/a:1015814119985. [DOI] [PubMed] [Google Scholar]
- 31.Nie H, Liu Z, Marks BC, Taylor LS, Byrn SR, Marsac PJ. Analytical approaches to investigate salt disproportionation in tablet matrices by Raman spectroscopy and Raman mapping. J Pharm Biomed Anal. 2016;118:328–37. doi: 10.1016/j.jpba.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Rumondor ACF, Taylor LS. Application of partial least-squares (PLS) modeling in quantifying drug crystallinity in amorphous solid dispersions. Int J Pharm. 2010;398(1):155–60. doi: 10.1016/j.ijpharm.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 33.Shah B, Kakumanu VK, Bansal AK. Analytical techniques for quantification of amorphous/crystalline phases in pharmaceutical solids. J Pharm Sci. 2006;95(8):1641–65. doi: 10.1002/jps.20644. [DOI] [PubMed] [Google Scholar]
- 34.Nečas D, Klapetek P. Gwyddion: an open-source software for SPM data analysis. Open Phys. 2012;10(1):181–8. [Google Scholar]
- 35.Zhou QT, Loh ZH, Yu J, Sun SP, Gengenbach T, Denman JA, et al. How much surface coating of hydrophobic azithromycin is sufficient to prevent moisture-induced decrease in aerosolisation of hygroscopic amorphous colistin powder? AAPS J. 2016;18(5):1213–24. doi: 10.1208/s12248-016-9934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price R, Young PM. Visualization of the crystallization of lactose from the amorphous state. J Pharm Sci. 2004;93(1):155–64. doi: 10.1002/jps.10513. [DOI] [PubMed] [Google Scholar]
- 37.Glover W, Chan H-K, Eberl S, Daviskas E, Verschuer J. Effect of particle size of dry powder mannitol on the lung deposition in healthy volunteers. Int J Pharm. 2008;349(1–2):314–22. doi: 10.1016/j.ijpharm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Van der Visser J. Waals and other cohesive forces affecting powder fluidization. Powder Technol. 1989;58(1):1–10. [Google Scholar]
- 39.Silva-Júnior AA, Scarpa MV, Pestana KC, Mercuri LP, de Matos JR, de Oliveira AG. Thermal analysis of biodegradable microparticles containing ciprofloxacin hydrochloride obtained by spray drying technique. Thermochim Acta. 2008;467(1–2):91–8. [Google Scholar]
- 40.Pilcer G, Wauthoz N, Amighi K. Lactose characteristics and the generation of the aerosol. Adv Drug Deliv Rev. 2012;64(3):233–56. doi: 10.1016/j.addr.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Chew NY, Tang P, Chan HK, Raper JA. How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm Res. 2005;22(1):148–52. doi: 10.1007/s11095-004-9020-4. [DOI] [PubMed] [Google Scholar]
- 42.Zhou QT, Gengenbach T, Denman JA, Heidi HY, Li J, Chan HK. Synergistic antibiotic combination powders of colistin and rifampicin provide high aerosolization efficiency and moisture protection. AAPS J. 2014;16(1):37–47. doi: 10.1208/s12248-013-9537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]