Abstract
Skeletal muscle tissue has an inherent capacity for regeneration following injury. However, severe trauma, such as volumetric muscle loss, overwhelms these natural muscle repair mechanisms prompting the search for a tissue engineering/regenerative medicine approach to promote functional skeletal muscle restoration. A desirable approach involves a bioscaffold that simultaneously acts as an inductive microenvironment and as a cell/drug delivery vehicle to encourage muscle ingrowth. Both biologically active, naturally derived materials (such as extracellular matrix) and carefully engineered synthetic polymers have been developed to provide such a muscle regenerative environment. Next generation naturally derived/synthetic “hybrid materials” would combine the advantageous properties of these materials to create an optimal platform for cell/drug delivery and possess inherent bioactive properties. Advances in scaffolds using muscle tissue engineering are reviewed herein.
Keywords: Extracellular matrix (ECM), Electrospinning, Hybrid materials, Hydrogel, Mesh, Volumetric muscle loss (VML)
1. Introduction
Select tissues within adult mammals (e.g., skeletal muscle, liver, among others) possess the regenerative potential to repair injured tissue. However, most postnatal mammalian tissues, such as cardiac muscle and central nervous system tissues, respond to injury by a well-defined process of inflammation and eventual downstream scar tissue formation. While skeletal muscle tissue possesses a robust innate regenerative ability, this response is incapable of regenerating severe injuries in which large volumes of muscle tissue are lost or damaged, a condition referred to as volumetric muscle loss (VML) [1,2]. Currently, limited therapeutic options for VML exist, thus tissue engineering/regenerative medicine (TE/RM) strategies for this condition have received increasing attention in recent years.
The discipline of TE/RM attempts to provide functional tissue repair for challenging medical problems such as VML. TE/RM strategies to replace/regenerate injured tissues and organs typically involve cell based approaches, bioactive molecules, biologic or synthetic scaffold materials, or combinations thereof (Fig. 1). The majority of preclinical research efforts and clinical investigations aimed at augmenting the innate response to skeletal muscle injury have been cell-centric (i.e., cell transplantation). Unfortunately, these approaches have shown limited clinical success due to factors including low cell viability and regulatory issues, among others [3–6]. Alternatively, bioscaffold materials, harvested from naturally occurring sources (e.g., extracellular matrix [ECM]) or created by artificial means using synthetic materials (e.g., PLGA), have been used as a guide or inductive template to facilitate skeletal muscle repair [2,7–12]. Hybrid devices, in which some or all of these strategies are combined, have also been attempted [13–16]. These next generation hybrid materials can be designed to deliver these bioactive molecules (e.g., small molecules, pharmaceuticals) and/or cells in a spatiotemporal manner. The use of scaffold materials to facilitate skeletal muscle reconstruction in TE/RM applications will be discussed herein.
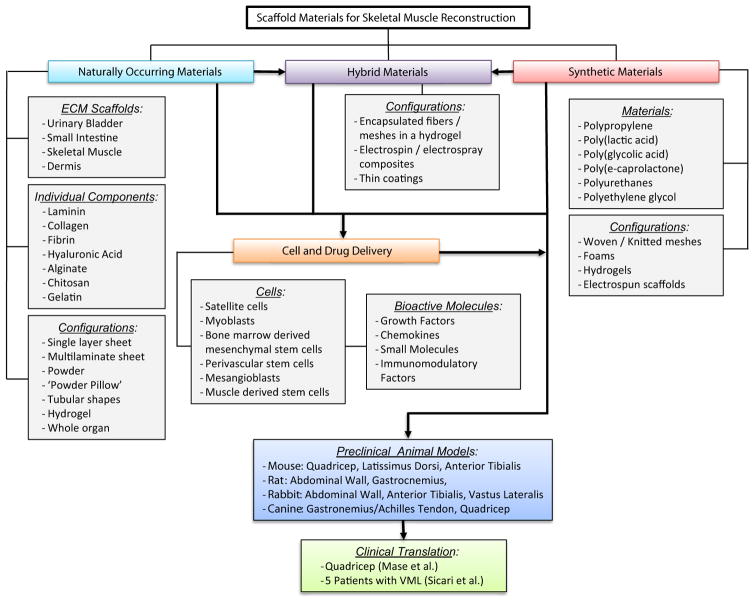
Fig. 1.
Schematic overview of scaffold materials used for skeletal muscle reconstruction in tissue engineering/regenerative medicine applications. Several overarching strategies have emerged, including the use of naturally occurring, synthetic, and/or hybrid materials. These materials have been characterized in numerous preclinical animal models and successfully translated to clinical use.
2. Scaffold materials for skeletal muscle regeneration
2.1. Naturally occurring materials
The ECM was once considered as a material that provides structural support, shape, and strength for tissues and organs. It is now widely appreciated that the ECM, in addition to its structural and mechanical properties, is an information highway for signals and molecules that augment many aspects of cell behavior. A variety of naturally occurring scaffold materials composed of ECM have been used to support skeletal muscle reconstruction/regeneration [2,7–12]. These ECM scaffold materials are derived from various species, a variety of tissues and organs, and can be configured as two-dimensional (2-D) sheets, simple tubular/hollow constructs, three-dimensional (3-D) whole organ shapes, and as hydrogels for expanded clinical applications (Fig. 2).
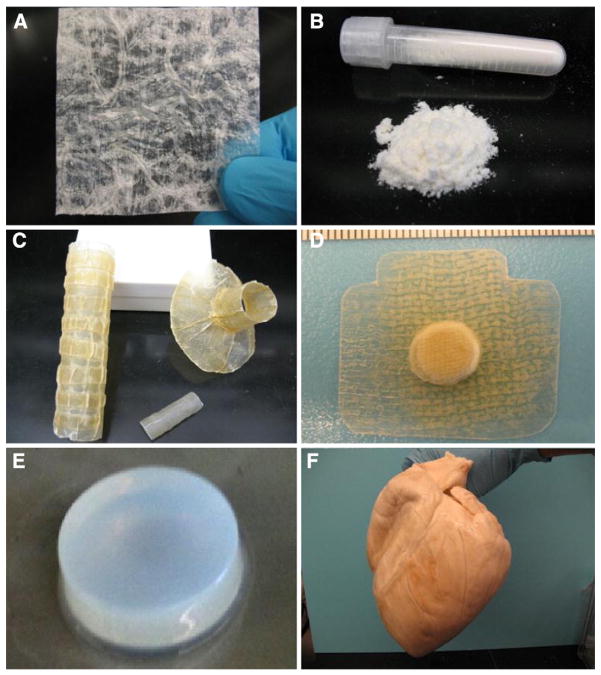
Fig. 2.
Configurations of ECM scaffold materials. (A) 2-D sheet, (B) comminuted powder, (C) tubular shapes, (D) powder pillow devices, (E) thermally responsive hydrogels, and (F) whole organ decellularization.
2.1.1. ECM scaffolds from decellularized tissues and organs
ECM is typically harvested by decellularization of source mammalian tissues and organs. The ECM of small intestine, dermis, urinary bladder, pericardium, and heart valves are all examples of FDA approved scaffolds for soft tissue repair [20]. While an array of decellularization protocols has been described, the common goal is: removal of as much of the cellular components of the source tissue as possible while preserving the structure and composition of the native ECM. The resulting acellular ECM scaffolds are composed of a specific combination of structural and functional molecules organized in a specific 3-D architecture — all of which are unique to each source material and method of decellularization. These ECM scaffolds contain collagen molecules (i.e., types I, III, IV, V, VI, VII, among others), laminin, fibronectin, and glycosaminoglycans (GAGs) including heparin, heparan sulfate, chondroitin sulfate and hyaluronic acid and an assortment of growth factors (e.g., vascular endothelial growth factor [VEGF], basic fibroblast growth factor [bFGF], transforming growth factor β [TGFβ]) [21–26]. This milieu of tissue specific structural and biochemical cues provides a microenvironment ideally suited for cell attachment, growth, and differentiation [27,28].
Individual ECM components have been isolated from native tissues to study the specific effects of these constituent molecules on cell behavior, and to manufacture scaffolds for various TE/RM applications. ECM component scaffolds have been created from purified collagens, fibronectin, fibrin, laminin, hyaluronic acid, and chondroitin sulfate, among others. Purified Type I collagen derived scaffolds are FDA approved for several clinical applications and are the most commonly purified ECM component [29–31]. Type I collagen is the most abundant ECM protein in many adult tissues and is easily extracted using acidic or enzymatic methods. Type I collagen devices have been configured into various forms including hydrogels, sponges, and films [32]. Natural materials, such as gelatin hydrogels, can be tailored by chemical crosslinking to possess mechanical properties that are similar to native muscle. Satellite cells cultured on these surfaces more effectively maintain their stem cell phenotype, have greater engraftment efficiency, and improved regeneration capacity than cells grown on softer hydrogels [33].
Other naturally derived molecules have been derived from non-mammalian sources including chitosan, silk fibroin, alginate, and agarose, which are derived from crustacean shells, silkworm cocoons, algae, and seaweed, respectively [34–36]. These materials are well tolerated in vivo and can be readily chemically modified to add specific functional groups, peptides, and/or crosslinkers. For example, alginate scaffolds can be used as both cell and drug delivery vehicles [37], and/or modified via incorporation of cell adhesion peptides such as Arg-Gly-Asp (RGD) to improve cell attachment and survival [38]. Some of these materials are also amenable to fabrication methods that allow control of structural and mechanical properties. Lyophilization of chitosan and Type I collagen solutions under a temperature gradient produces elongated, aligned micropores that can guide myotube formation [39,40]. Hydrogel forms of ECM and other naturally derived materials can be molded into shapes conducive for myogenesis. The liquid stage fills irregularly shaped spaces within a mold, and polymerize into a solid substrate during gelation. Molding a grooved microstructure into gelatin hydrogels influences the alignment and size of fused myotubes [41]. More complex patterns can be introduced in hydrogels to create myotube networks in vitro that yield improved viability and differentiation [42]. The geometry of these patterns can be adjusted to optimize myotube alignment, maturity, and contractile force generation [43].
2.1.2. Configurations of ECM scaffolds
Several configurations of ECM scaffolds have been used successfully to support constructive remodeling of numerous tissues, including skeletal muscle. For example, thin tissues such as small intestinal submucosa yield an ECM scaffold with a two-dimensional (2-D) sheet configuration (Fig. 2A). Alternative configurations such as powder (Fig. 2B), multilaminate sheets & tubular shapes (Fig. 2C), powder pillow devices (Fig. 2D), thermally responsive hydrogels (Fig. 2E), and whole organs (Fig. 2F) have been created from ECM materials and have been well characterized [20,44].
The 2-D sheet and powder configurations of ECM scaffolds are ideal for topical wound dressings and select soft tissue applications. However, these configurations have relatively low mechanical strength. Multilaminate devices can be created by stacking several single layer sheets and subsequently applying a vacuum to physically connect the layers. The resultant multilaminate is thicker than the single layer sheet and provides additional mechanical strength at the time of implantation. Simple three-dimensional (3-D) tubular/hollow devices have been created by wrapping sheet forms of ECM around a mandrel before drying. These tubular devices have been used for several cardiovascular and gastrointestinal applications [45–47]. In recent years, whole organ decellularization has emerged and methods to create a three-dimensional (3-D) biologic scaffold material that preserves the native tissue architecture and composition of the intact organ have been described [48–50]. Finally, ECM scaffold materials can be enzymatically digested into an injectable liquid dispersion, which can then be polymerized into a hydrogel under physiologic conditions in situ [51–53]. The advantages of ECM hydrogels include their ability to conform to irregular shapes following injection via minimally invasive techniques and as a robust delivery vehicle for drugs, bioactive molecules, and/or cells.
2.1.3. Preclinical and clinical use of ECM scaffolds for skeletal muscle repair
ECM scaffolds have been studied for over two decades in attempts to characterize the host response and mechanism of bioscaffold remodeling. Preclinical animal studies have shown that ECM scaffold materials serve as an inductive and instructive template to facilitate the deposition of functional skeletal muscle tissue (i.e., constructive remodeling) when implanted into a site of muscle injury. These pre-clinical studies have been conducted using different animal model species (e.g., mouse, rat, rabbit, canine), various muscle groups (e.g., quadriceps, abdominal wall, gastrocnemius/Achilles tendon), and volumetric defect injury sizes (i.e., ~15–75% of the affected tissue). ECM scaffolds prepared from several source tissues (e.g., small intestinal submucosa [SIS], urinary bladder matrix [UBM]) show a constructive remodeling response in the large majority of these studies and models.
The use of ECM scaffolds to facilitate restoration of tissue form and function after injury has been successfully translated to the clinic. More than 4 million patients have been implanted with an ECM scaffold for a variety of conditions including extremity musculotendinous reinforcement, ventral hernia repair, and esophageal reconstruction, among others. For example Mase et al. describe a case study in which a military service member sustained a traumatic skeletal muscle injury and had exhausted all possible treatment options with limited success. An ECM scaffold was placed in the defect site following scar tissue debridement, and resulted in a significant increase in function after 16 weeks. Furthermore, Sicari et al. recently reported that an ECM scaffold used as a surgical treatment for patients with VML showed perivascular stem cell mobilization within the site of injury, de novo formation of skeletal muscle cells, increased force production of the affected muscle group, and improvements in activities of daily living. Taken together, these findings demonstrate the effectiveness of ECM scaffolds in restoring skeletal muscle structure and function following injury. The precise cellular and molecular mechanisms that drive ECM scaffold mediated constructive remodeling response are only partially understood, but scaffold degradation, recruitment of endogenous stem cells, and modulation of the innate immune system have been shown to be critical determinants.
2.1.4. Mechanisms of ECM scaffold mediated constructive remodeling
Within hours of implantation, ECM scaffolds are densely infiltrated by mononuclear cells, followed by degradation of the scaffold, modulation of the innate immune response, the recruitment, proliferation, and differentiation of multipotent stem/progenitor cells, and ultimately the formation of site appropriate tissue. Each of these events serves as a mechanism by which ECM scaffolds mediate the constructive remodeling response in skeletal muscle tissue (Fig. 3). It should be emphasized however, that these events do not occur in isolation but rather in an orchestrated temporospatial pattern in which the final product is greater than the sum of individual parts.
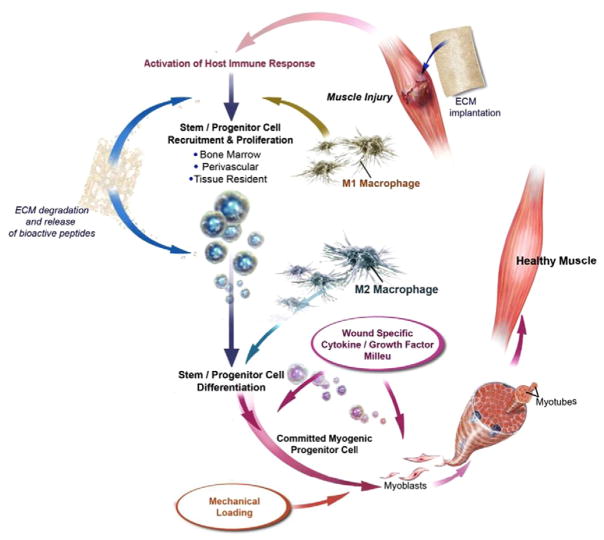
Fig. 3.
Schematic overview of skeletal muscle reconstruction using an ECM scaffold material. Implantation and host mediated degradation of an ECM scaffold elicits mononuclear cell infiltration, modulation of the innate immune response, the recruitment, proliferation, and differentiation of multi-potent stem/progenitor cells, and ultimately the formation of site appropriate skeletal muscle tissue.
Adapted and reprinted with permission from Turner et al. [54].
Rapid infiltration by host cells, especially macrophages, and subsequent degradation of ECM scaffolds via matrix metalloproteinases (MMPs) and other proteases is a requirement for constructive remodeling. Scaffold degradation releases depots of embedded bioactive molecules such as growth factors and cytokines. In addition, bioactive cryptic peptides are created by cleavage of parent molecules such as collagen, fibronectin and laminin [55,56]. The combination of these events initiates robust remodeling activity including immunomodulatory, antimicrobial, chemotactic, proliferative, and differentiation activity for a variety of cell types [57–62]. However, the type and amount of bioactive molecules that remain in the scaffolds vary significantly depending on the specific methods of decellularization and other processing steps. Chemically crosslinking ECM with carbodiimide or glutaraldehyde inhibits degradation and the aforementioned release of biologic factors, leading to impaired remodeling [11,63].
The ability of macrophages to assume a continuum of phenotypic and functional phenotypes (i.e., M1, M2a, M2b, M2c) and their ability to modulate skeletal regeneration following injury has been well reported and a number of excellent reviews are available [64,65]. Interestingly, modulation of the innate immune response, and macrophage phenotype in particular, has been shown to be a necessary and determinant factor of ECM scaffold mediated constructive remodeling. Briefly, ECM scaffold materials have been shown to promote a bias toward regulatory, M2 macrophages and that this directed macrophage polarization is strongly correlated to the downstream constructive remodeling response. These findings suggest that macrophages, namely the M2 phenotype, directly contribute to the ECM mediated tissue remodeling response, likely through differential regulation of the macrophage’s secretome (e.g., cytokines, chemokine, and growth factor secretion). However, a direct cause/effect relationship has yet to be fully identified.
The recruitment, proliferation, and differentiation of multipotent stem/progenitor cells to the site of injury/ECM scaffold implantation are critical steps in the formation of site appropriate skeletal muscle tissue. ECM scaffold degradation products have been shown to possess chemotactic activity for a number of myogenic stem/progenitor cells in vitro including: skeletal muscle cells and perivascular stem cells, among others. Furthermore, ECM scaffold degradation products have been shown to affect progenitor cell differentiation and function. Importantly, these biologic affects have been shown in vivo as ECM scaffolds induce the recruitment/accumulation of a variety of endogenous stem/progenitor cells to the site of injury/remodeling in both pre-clinical models of skeletal muscle injury and patients suffering from volumetric muscle loss.
2.2. Synthetic scaffold materials
A wide variety of synthetic materials have been used as scaffolds for tissue repair and reinforcement. Synthetic materials offer certain advantages over naturally derived materials in that they can be precisely characterized and fabricated with great control over physical and chemical properties. The range of synthetic scaffolds developed for general tissue engineering applications is very broad, and several have been studied specifically for skeletal muscle regeneration. These materials include: polypropylene, polyesters such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(ε-caprolactone) (PCL) [66,67], and their copolymers [68,69], and various polyurethanes [70,71].
2.2.1. Polypropylene
Among the earliest examples of implantable biomaterials for muscle repair is polypropylene; a non-degradable and hydrophobic plastic polymer that maintains widespread use today. Polypropylene possesses high mechanical strength and durability, is easily sterilized, and is relatively inexpensive to manufacture. One of the most desirable qualities of polypropylene for surgical applications was its consideration as “biologically inert” in vivo. This designation was applied because polypropylene is non-toxic, non-degradable in vivo (and therefore does not release potentially inflammatory degradation products), does not possess surface groups or ligands for cell receptor activation, and elicits a highly localized tissue response [72]. However, biological inertness is not an accurate description of the host response to polypropylene, or to other non-degradable polymers (such as polyethylene or ePTFE). All non-degradable polymers elicit a cascade of innate immunological events and fibrotic tissue deposition, known as the foreign body reaction [73,74]. The robustness of the foreign body reaction is dependent upon several factors such as the configuration of the material, the tissue in which it is implanted, and the degree of injury. Complications of the foreign body reaction include fibrous encapsulation of the device leading to loss of tissue compliance.
2.2.2. PLGA
The copolymer poly(lactic-co-glycolic acid) (PLGA), has been one of the most extensively used synthetic biomaterials in skeletal muscle tissue engineering. As an FDA approved polyester biomaterial, PLGA has been used in the clinic in a variety of applications over the past three decades. Due to the nontoxic nature of the degradation products (i.e. lactic acid and glycolic acid) [75,76], PLGA is a popular source for an implantable material used in devices such as biodegradable sutures in addition to tissue engineering scaffolds [77]. Extensively studied as a scaffold material for a variety of tissue engineering applications, biodegradable PLGA has been shown to promote cell adherence, proliferation, and formation of new three-dimensional tissues. Porous PLGA scaffolds have also been shown to promote host mediated vascularization and cell infiltration upon their in vivo implantation [78–80].
2.2.3. PCL
Poly(ε-caprolactone) (PCL) is an FDA approved polyester that has been used to fabricate scaffolds for several tissue engineering applications, including the skeletal muscle. PCL and PLGA are both polyesters, but possess different material properties that dictate their implementation (reviewed in [81]). Like PLGA, PCL is biodegradable in vivo, although at a much slower rate on the order of months to years depending on the specific scaffold configuration [75,76,82,83]. PCL scaffolds are therefore useful in instances where long-term mechanical or structural support is desired, or for long term drug delivery applications. PCL has several properties that are favorable from a fabrication perspective including a relatively low melting point and miscibility in several solvents and other synthetic polymers [67,81]. This versatility has been exploited to prepare an array of PCL copolymers with PGA, PLA, among others. PCL–PGA copolymer fibers are a component of partially degradable mesh materials (e.g. Ethicon Ultrapro™) to control mesh degradation and mechanical properties [84].
2.2.4. Polyurethanes
Polyurethanes represent a diverse class of polymers that are linked by urethane bonds formed between isocyanates and hydroxyl group containing compounds. Numerous other chemical moieties in these monomers can be incorporated into polyurethanes including polyesters, carbonate, urea, and ether functional groups. Such chemical modifications have been used to efficiently control scaffold material properties such as in vivo degradation rate, mechanics, and hydrophobicity (reviewed in [85]). Polyurethanes can therefore be tailored to specific applications: they may be either rapidly degradable or non-degradable in vivo, very stiff or elastomeric. These functional groups may also be used for additional scaffold modifications, such as chemical crosslinking, or conjugation of bioactive compounds and growth factors [86,87]. Like the previously described polyesters, polyurethane linkages are biodegradable via hydrolysis and generally non-toxic, depending upon the monomer units. Porous polyurethane scaffolds prepared with different copolymers had significantly altered degradation kinetics and remodeling [88]. Altering scaffold fabrication methods will affect the structure and surface chemistry of the polymer, affecting myoblast morphology and monocyte activation in vitro [89]. Polyurethanes are useful for skeletal muscle TE applications in which mechanical and structural control is necessary to influence cell phenotype or to enable physiologic muscle function.
2.2.5. Configurations of synthetic scaffolds
Synthetic materials used for skeletal muscle tissue reconstruction can be processed in many configurations — meshes, foams, hydrogels, and electrospun scaffolds are among the most common. Synthetic polymer mesh configurations are most frequently used for ventral hernia repair, which is a bulge or rupture of the abdominal wall and one of the most common muscle injuries [90]. Mesh architectures have evolved over the years including woven and multifilament braided mesh, though monofilament, knitted polypropylene has become established as the gold standard for hernia repair. Traditionally, these materials were configured as “heavy-weight” designs, which were constructed with a dense knit structure, though in recent years, a large pore “light-weight” configuration has gained clinical acceptance [91]. The light-weight mesh greatly reduces the amount of material exposed to the host, resulting in a reduced foreign body reaction while still possessing adequate mechanical properties [90,92,93]. Other synthetic materials have been used in mesh configurations for hernia repair including partially degradable mesh devices consisting of PLGA, polyurethane, and PCL [84,94]. Such variations can affect the structure, mechanical behavior, and host response to a mesh device. Several studies have shown that the mechanical environment greatly influences stem cell differentiation [95]. Muscle satellite cells in particular more efficiently maintain quiescence when maintained on a substrate that matches the mechanics of healthy muscle [96]. Disrupted muscle mechanical properties in vivo results in a corresponding loss of satellite cell potential and impaired regeneration [33].
Advances in electrospinning techniques have led to the production of a variety of novel nanofibrous polymeric scaffolds (reviewed in [97]) with morphologies that can be tightly controlled by adjusting fabrication parameters. Electrospun scaffolds can be produced with biomimetic nanofibrous morphologies that consist of scaffolds with fiber sizes that are similar to fibrous ECM proteins such as Type I collagen. Such topographical cues influence cell behavior such as proliferation and cell adhesion and phenotype [98,99]. One of the most important electrospinning parameters for muscle tissue engineering is nanofiber alignment control. Scaffolds with highly aligned (anisotropic), or unidirectionally-oriented fibers have been repeatedly shown to enhance myotube formation and muscle cell alignment when compared to scaffolds with randomly aligned fibers (Fig. 4). Structural alignment and implementation has been shown for multiple types of synthetic polymers such as PLGA [66,100–103], PCL [104,105], and polyurethanes [106,71,107]. Aligned electrospun scaffolds showed improved human and rodent myoblast adherence, proliferation, and fusion into myotubes compared to randomly oriented fiber substrates. Incorporating electrically conductive materials within aligned electrospun PCL [108–110] and polyurethane [106,111] scaffolds enabled more efficient electrical stimulation to the developing cultures, further improving myotube maturity. Mechanical stimulation and substrate stiffness are parameters that have been previously shown to influence skeletal muscle development, and polymers such as polyurethane have highly controllable mechanical properties. Elastomeric polyurethane scaffolds prepared with an optimal stiffness and cyclic mechanical strain during culture resulted in the development of more mature, striated myotubes than with fiber alignment alone [111]. Porosity and cell infiltration can be controlled in electrospun scaffolds by the inclusion of “sacrificial fibers.” For example, water soluble polymers co-spun with PCL can be easily removed following scaffold fabrication. The spaces where the sacrificial fibers had resided can then act as pathways for improved cell infiltration [112]. For more controlled pore formation, post-processing techniques, such as laser ablation, can also be utilized [113].
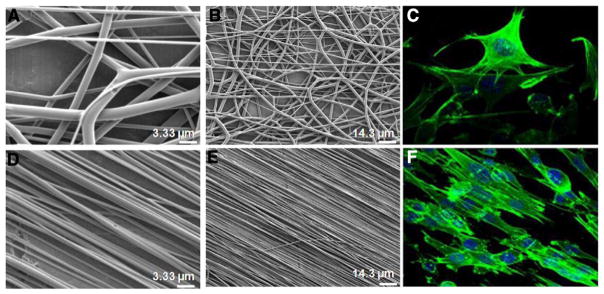
Fig. 4.
Aligned (anisotropic) and randomly-oriented electrospun scaffolds. Randomly-oriented PLGA scaffolds (A, B) were electrospun with a mandel rotation speed of 300 rpm and aligned PLGA scaffolds (D, E) were electrospun at 1500 rpm. Myoblasts cultured on the PLGA scaffolds were fixed and stained with FITC-phalloidin for the f-actin component of the cytoskeleton (green), and DAPI stained nuclei (blue). Confocal micrographs show cytoskeletal alignment after 5 h in culture in the aligned fiber scaffold (F) but not in the randomly-oriented scaffold (C).
Adapted and reprinted with permission from Aviss et al. [100].
As discussed in Section 2.2, there is generally a greater degree of control over the physical and chemical properties of synthetic materials compared to naturally occurring materials; scaffolds can be tailored with specific characteristics for mechanistic evaluation both in vitro and in vivo. Scaffold fabrication techniques are being developed to rapidly produce scaffolds with increasingly complex surfaces and 3-D structures. Lithography techniques have been effective for creating nanoscale patterns on PLGA scaffold patches, which direct myoblast alignment and differentiation in vitro, and improves myoblast engraftment and survival in vivo [114,115]. Additive microfabrication techniques such as 3-D printing act by rapidly layering small amounts of material to build structurally detailed scaffolds, and extend scaffold design control to the third dimension [116]. Similarly, pressure activated microsyringe deposition has been utilized to fabricate PLGA and PCL scaffolds with an array of 2-D and 3-D geometries that possess different mechanical properties, which can favor myoblast proliferation or differentiation [117]. For skeletal muscle TE scaffolds, it will be important to understand how the porosity, fiber diameter and density, elasticity, degradation rates, bioactivity and biocompatibility affect both local and systemic host response.
2.2.6. Preclinical and clinical use of synthetic scaffolds for skeletal muscle repair
Synthetic mesh materials have been clinically used for skeletal muscle repair for over 50 years. More than a million synthetic mesh materials are implanted worldwide each year as they are considered the gold standard for reinforcement of the abdominal wall musculature following a hernia [90]. These synthetic mesh materials have been shown to significantly reduce hernia recurrence rates by reinforcing the abdominal wall musculature, a result mediated by rapid incorporation into the host tissue and robust mechanical strength of the material [118–120]. However, the use of these materials is associated with several limitations. For example, bacteria readily adhere to non-degradable synthetic meshes and form antibiotic resistant biofilms on the material surface, contraindicating their use in potentially contaminated fields [121–123]. Likewise, multifilament mesh fiber configurations are especially susceptible to bacterial colonization [124]. The small spaces within mesh filament interstices provide an environment for bacterial colonization and are inaccessible to host phagocytes [120]. Degradable ECM derived materials are resistant to colonization and are typically indicated in situations where bacterial contamination is suspected [125].
Furthermore, synthetic materials elicit a chronic pro-inflammatory foreign body reaction, leading to dense scar plate formation within and around the mesh material resulting in chronic pain and discomfort for the patient [74,92]. The inflammatory effects of synthetic implants may also extend to nearby uninjured tissue. Subcutaneously implanted polyurethane sponges initiated an accumulation of inflammatory cells, increased cytokine production, and induced angiogenesis in the adjacent uninjured skeletal muscle [126]. There are other examples of synthetic materials for skeletal muscle repair applications that have been applied to both preclinical models and clinically. A polycarbonate–polyurethane patch was shown to reduce the incidence of musculotendinous rotator cuff failure in preclinical and clinical studies, and decreased post-operative pain during movement [127,128].
Alternative materials and configurations for skeletal muscle reconstruction have been investigated in an effort minimize the deleterious aspects of synthetic mesh materials. Several strategies have been investigated including alterations in the mesh fiber composition, the addition of bioactive coatings, and/or in combination with biologically derived materials.
2.3. Hybrid scaffold materials
Benefits of ECM and synthetic scaffold materials include facilitation of a favorable constructive remodeling response and strong mechanical reinforcement of affected tissues, respectively. However, each type of scaffold possesses inherent limitations that restrict clinical effectiveness in restoring skeletal muscle form and function after injury. For example, non-degradable synthetic materials elicit a chronic foreign body reaction, and ECM scaffold materials are typically not mechanically robust and can vary in composition. Thus, it is not surprising that hybrid materials (comprised of both synthetic and naturally derived components), have been developed (Fig. 5). Hybrid scaffolds attempt to exploit both the desirable mechanical properties of the synthetic materials and the constructive remodeling properties of ECM scaffolds. While the creation and characterization of these scaffolds have only recently begun to be explored, several general configurations have been identified [129]. Those configurations of particular interest to skeletal muscle tissue reconstruction can be described as either encapsulation of fibers/meshes in a hydrogel matrix, naturally derived coatings on synthetic mesh materials, or concurrent electrospun/electrosprayed composites.
Fig. 5.
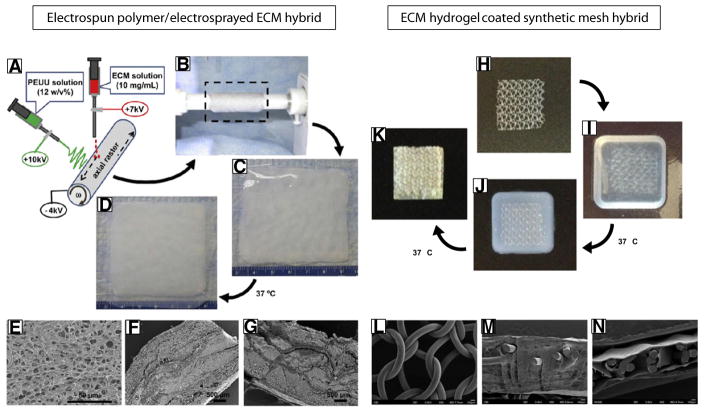
Examples of electrospun and mesh synthetic/ECM hybrid scaffold configurations. (A–B) Schematic illustrating the electrospun hybrid scaffold fabrication process. Synthetic nanofibers are electrospun concurrently with electrospraying of an ECM hydrogel around a rotating mandrel. (C–D) The hydrogel polymerized once the temperature is raised to 37 °C. (E–G) Scanning electron micrographs of the hybrid scaffold in cross section. Adapted and reprinted with permission from Hong et al. [15]. (H) Uncoated heavy-weight polypropylene mesh is submerged within a (I) liquid ECM pre-gel (pH neutralized ECM digest). (J) The polypropylene mesh within pre-gel solution is incubated at 37 °C for approximately 30 min to induce gelation around the mesh. (K) The mesh embedded within the ECM hydrogel may be further air dried at 37 °C to produce a thin, compact ECM coating. Scanning electron micrographs of (L) the surface of the uncoated polypropylene mesh, (M) a cross section of the hydrogel coated mesh, (N) a cross section of the air dried ECM hydrogel coated mesh.
A hybrid scaffold composed of a synthetic polyurethane elastomer and ECM hydrogel was generated by a concurrent polymer electrospinning/ECM hydrogel electrospraying technique [14,15]. The synthetic component of the scaffold provides elasticity, flexibility, and mechanical support and the ECM hydrogel enhances bioactivity and biocompatibility (Fig. 5A–G). Each component can by varied temporally during the process, yielding various hybrid configurations. For example, a sandwich structure was achieved with polymer fiber-rich upper and lower layers for structural support and an ECM-rich inner layer to encourage cell ingrowth [14]. Using this technique, the mechanical strength of the material can be predictably modified. Electrospun scaffolds composed of blended PCL and naturally derived chitosan at a 60:40 ratio created aligned nanofibers, which increased myoblast alignment compared to a PCL/chitosan film in vitro. The addition of chitosan microfiber bands to the electrospun nanofiber surface along the same orientation further increased myoblast differentiation and expression of skeletal muscle myosin heavy chain [130]. Aligned PCL/chitosan scaffolds in combination with Wnt3a protein supplemented media supported efficient skeletal muscle differentiation of human embryonic stem cells compared to unaligned scaffolds and collagen [131].
Several studies have investigated applying naturally derived materials as a surface coating for synthetic mesh materials to affect the host response. Chitosan [132], cellulose [133,134], omega-3 fatty acids [134,135], hyaluronic acid [133,136], and Type I collagen [134,136,137] have all been investigated to prevent complications such as adhesion formation following mesh implantation. Polypropylene mesh with a gelatin hydrogel coating was shown to be an effective delivery method for basic fibroblast growth factor [138]. These coatings may adhere by physical interactions or via covalent chemical bonds. Myotube formation was enhanced on polyurethane microchannel scaffolds by chemically crosslinking gelatin or silk fibroin coatings on the scaffold surface [139]. A dermal ECM/polypropylene mesh hybrid material increased mechanical strength and prevented failure during enzymatic degradation of the ECM component in vitro [140].
A method was recently described by which a hydrogel form of ECM was used as a coating for a synthetic mesh (Fig. 5H–N, [141]). Briefly, a synthetic mesh was suspended in a neutralized ECM digest solution. Following ~30 min at 37 °C, the ECM digest formed a robust hydrogel around and between the synthetic mesh fibers. The ECM hydrogel embedded mesh was then air dried overnight to create a continuous ECM coating that remained adherent even after numerous washes with saline.
A wide array of alternative materials and configurations are available for use in hybrid scaffolds. As such, investigators possess the ability to control the mechanical properties, degradation profile, and bioactivity of the scaffold by using different materials and configurations; thus, offering the opportunity to tailor the scaffold to the specific needs of each application.
2.3.1. Preclinical use of hybrid scaffolds for skeletal muscle repair
While the use of hybrid scaffolds for skeletal muscle reconstruction has only recently been explored, there has been a focus upon ECM coated synthetic meshes or concurrent electrospun/electrosprayed composites for this application. Wolf et al. investigated the use of ECM hydrogels as a coating for a polypropylene mesh in a rat partial thickness abdominal wall defect model [141]. Compared to the uncoated mesh, the presence of the ECM hydrogel was associated with a reduction in the intensity of the foreign body reaction, reduced number of foreign body giant cells, and the diminished density of host deposited collagen during the initial 35 days of in vivo implantation. ECM hydrogel coatings with different nanostructures and from different tissue sources were also shown to attenuate the M1 macrophage response to polypropylene, and may be a determinant of the altered remodeling outcome [142].
A polyurethane (PEUU)/dermal ECM hybrid scaffold was evaluated in a rat full-thickness abdominal wall replacement model [14,15]. No herniation, infection, or tissue adhesion was observed 8 weeks after implantation. Compared to a pure PEUU scaffold, the hybrid scaffolds were significantly thicker at the time of explant, with greater numbers of associated smooth muscle actin-positive staining cells. It was found that a sandwich configuration maintained its thickness, showed higher collagen content 8 weeks after implantation, exhibited an increased M2 macrophage phenotype response, and developed more favorable biaxial mechanical properties compared to control scaffolds. Taken together, these investigations have shown that hybrid scaffolds offer improved mechanical properties over naturally derived scaffolds and improved bioactivity compared to synthetic scaffolds alone. Electrospun PCL scaffolds incorporating Type I collagen have been shown to enhance human stem cell viability and desired lineage specification and to improve myoblast adherence, alignment, and fusion in vitro compared to the PCL alone, which has limited biologic activity [66,143]. The improved bioactivity of a collagen/PCL hybrid translated to improved skeletal muscle infiltration in vivo [144].
3. Materials for cell and drug delivery to aid in skeletal muscle reconstruction
As discussed in Section 2.1.4, the use of acellular scaffold materials involves the recruitment of endogenous stem/progenitor cells in situ to facilitate skeletal muscle reconstruction following injury. As an alternative to this strategy, a large body of literature exists which describe ECM and/or synthetic scaffold materials as a delivery vehicle for cells, drugs, and/or small molecules.
3.1. Cell delivery
Historically, several limitations are associated with cell based approaches for skeletal muscle reconstruction, such as the poor survival/engraftment of delivered cells into host tissue, and/or their inability to migrate away from the injection site. These challenges have been attributed to a hostile microenvironment and adverse inflammatory reactions in the injured tissue. Thus, RM/TE strategies that alleviate these challenges by enhancing the survival/engraftment of transplanted cells are of great need for their potential to improve muscle regeneration.
Several strategies have been employed to increase the efficacy of cell delivery to injured skeletal muscle tissue. One approach involves the incorporation of cells within a scaffold material prior to injection. The ideal material for this application should be able to support and enhance cell viability and promote a constructive remodeling response. Both biologic and synthetic scaffolds have been investigated for this use. Many individual purified components of the ECM such as hyaluronic acid (HA), laminin, collagen, or fibrin have been used as substrates or vehicles for cell delivery. HA based hydrogels have been shown to facilitate many beneficial cellular responses including the ability to promote myogenesis in vitro. Importantly, Rossi et al. showed that a HA/cell hybrid was able to support a constructive and functional remodeling response when used in a VML injury model. Several studies have investigated the use of Type I collagen/cell hybrids in preclinical skeletal muscle injury models. Compared to control conditions, the use of collagen delivery vehicles was associated with enhanced myogenesis. However, some speculate that the use of individual purified components of the ECM in isolation does not fully recapitulate the complex ultrastructure and bioactivity of an ECM scaffold. To this end, numerous investigations have utilized ECM scaffold materials as delivery vehicles.
As tissue engineering strategies for skeletal muscle regeneration move toward the clinic, one of the major hurdles for the implantation of cellular grafts is expedient vascularization. Diffusion limits have traditionally prevented implantation of full thickness grafts with any success. In an elegant attempt to pre-vascularize a skeletal muscle construct prior to implantation, Levenberg et al. used a porous, three-dimensional, degradable scaffold comprised of PLLA and PLGA (1:1) as a platform for the co-culture of mouse myoblasts with either human embryonic endothelial cells or human umbilical vein endothelial cells (HUVECs) and demonstrated formation of endothelial networks throughout and in between differentiating skeletal muscle fibers [145]. Results after implantation showed continued differentiation, integration with the host tissue, and inosculation of the vessels in the construct with the host vasculature. This same strategy was extended to engineer a skeletal muscle flap for transplantation. Scaffolds co-cultured in vitro with myoblasts, fibroblasts, and endothelial cells were followed by transplantation adjacent to the femoral artery and veins until host vasculature connected with the scaffold endothelial network. The resulting host-vascularized flap was transplanted to a full thickness abdominal wall defect, where it remained viable [146].
3.2. Drug delivery
Pharmacologic therapies for muscle injuries include anti-inflammatory drugs, steroids, hormones, and growth factors. Although these compounds have shown efficacy in promoting muscle regeneration, they are typically limited by traditional delivery routes. Systemic administration restricts optimal dosing due to systemic toxicity and side effects, and even direct bolus intramuscular injection is ineffective due to the relatively short in vivo half lives of many of these compounds. A solution to these problems is to use scaffold biomaterials to deliver and release therapeutic drugs in a sustained manner. Controlled drug delivery is an area of intense research and is a central tenet of TE. Initial work with hybrid scaffolds included the use of PLGA microspheres delivered in alginate hydrogels to administer vascular endothelial growth factor (VEGF) in an ischemic hindlimb model in mice [147]. The use of a synthetic microsphere carrier provided a platform for prolonging delivery over what could be achieved from the alginate alone.
As with other ischemic diseases, such as in the heart post-myocardial infarction, both direct cell transplantation and angiogenic growth factor therapy have been investigated for the treatment of PAD and CLI. Though the results have been variable, cell transplantation is limited by poor survival, retention, and engraftment [148,149]. It is apparent that many of the benefits of cell therapy are likely due to paracrine effects, specifically the increase in angiogenesis [150]. Thus, angiogenic growth factor therapy is an attractive alternative without the complications of cellular interventions. The goal of growth factor delivery to ischemic skeletal muscle is to increase vascularization such that perfusion of the affected area allows for appropriate wound healing. Increased perfusion also reduces pain at rest and can prevent the need for amputation. While most of these approaches primarily focus on direct injection of cells or angiogenic growth factors, some biomaterial-based strategies have been explored. Incorporating growth factors into biomaterial scaffolds increases retention and prolongs delivery, thus enhancing the effect. Numerous synthetic materials and configurations have been designed for drug delivery. For example, implantable porous polyurethane scaffolds have been fabricated while simultaneously loading growth factors for controlled release over the course of several months in vitro [151]. Injectable forms of polyurethane scaffolds have been developed such as growth factor loaded foams that set in situ [152], or as nanoparticles [153]. Collagen–fibronectin blend, alginate, gelatin, fibrin, peptide amphiphiles, and PLGA have been investigated as growth factor delivery vehicles [147,154–160]. Electrospun PCL/gelatin hybrids were created to improve cell attachment and were chemically modified with heparin to bind proteins for drug delivery from the scaffold [161].
Alginate has been investigated for the delivery of a variety of growth factors, including VEGF [158], VEGF-encoding plasmid-DNA [156], and hepatocyte growth factor (HGF) [159]. While alginate is an abundant, naturally-derived biomaterial, with attractive properties for tissue engineering, it is not an extracellular matrix component. To better mimic the native tissue microenvironment, Kuraitis et al. incorporated stromal cell-derived factor-1 (SDF-1) in alginate microspheres which they then embedded in an injectable collagen matrix and delivered in a rabbit hindlimb ischemia model [162,163]. Similarly, alginate microparticles in a collagen–fibronectin scaffold have been used to co-deliver VEGF and endothelial cells [155]. Combinations of several growth factors and/or cell types may act in a synergistic manner for muscle repair. Alginate scaffolds loaded with VEGF, insulin-like growth factor-1 (IGF-1), and myoblasts, enhanced mouse skeletal muscle regeneration to a greater extent than any of these components alone [164]. VEGF delivery from alginate was also shown to improve muscle and nerve survival following ischemia via nerve growth factor induced mechanisms, emphasizing that multiple cell types may be targeted [165]. Scaffolds comprised of other ECM components, specifically gelatin and fibrin, have also been used to deliver basic fibroblast growth factor (bFGF) [157, 160]. Though varied in the model and length of study, the results of this work supported the use of the experimental scaffolds as delivery vehicles, demonstrating increases in cell survival, retention, and/or payload delivery with corresponding increases in neovascularization. Acellular PLLA and gelatin scaffolds implanted in a site of muscle injury are capable of recruiting endogenous satellite cells, and migration is significantly enhanced with IGF-1 or bFGF, though not SDF-1 [166]. This study emphasizes that endogenous cell recruitment is a viable target for scaffold implementation strategies.
Direct injection of sphingosine-1-phosphate (S1P) into the injured area promoted significantly greater mean muscle fiber cross-sectional area when compared to controls in their mouse model [167]. Similarly, mice treated with the small molecule 2-acetyl-4(5)-tetrahydroxybutlimidazole (THI), previously shown to increase S1P expression, had a 4-fold increase in the number of cells expressing myf5, a protein that plays a key role in regulating myogenesis and a 3.6-fold increase in the number of muscle cell fibers [168]. Another interesting approach that has been explored is using small molecules to dedifferentiate multinucleated myoblasts into mononuclear proliferatory cells and satellite cells which can then contribute to the regeneration at a different site [169,170]. The use of small molecule therapy for skeletal muscle regeneration has been recently reviewed [171].
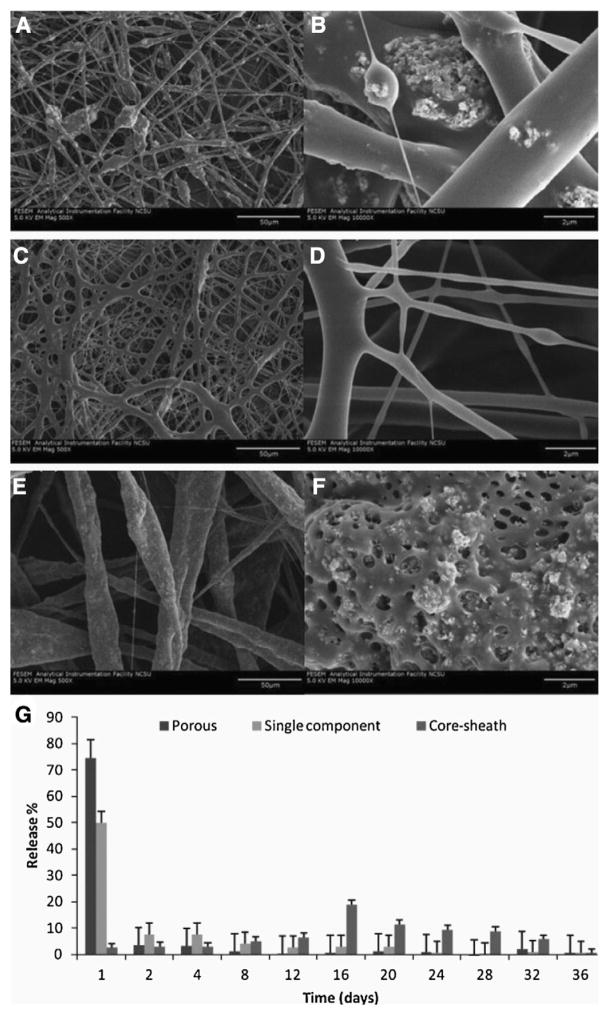
As drug delivery scaffolds designed for skeletal muscle TE improve, an understanding of pharmacokinetics will become increasingly important. It is likely that growth factors, cytokines, or small molecules will need to be delivered simultaneously, in sequence, and/or for different lengths of time to achieve the desired effects. Greater control over drug release from electrospun scaffolds may be achieved by varying fiber morphologies within the scaffold, altering drug release profiles. Like fiber alignment, fiber morphology can be manipulated by varying the electrospinning/electrospraying parameters (Fig. 6). Changing fiber morphology (e.g. porous, single-component, or core-sheath) has been demonstrated to be an effective way to control the release of nanoparticle payloads incorporated in the scaffolds, though these scaffolds have not yet been specifically implemented for skeletal muscle reconstruction or regeneration [172].
Fig. 6.
Controlled release with electrospun fiber morphology. Functional PLA scaffolds with porous (A, B), single-component (C, D), and core–sheath (E, F) fiber morphology are shown here at 500× (A, C, E) and 10,000× (B, D, F) doped with tricalcium phosphate nanoparticles. Pharmacokinetic analysis (G) indicates variation in release profiles for the three different scaffolds, with single-component and porous fibers exhibiting a burst release profile and the core–sheath fibers providing a slower, more constant release.
Adapted and reprinted with permission from Mohiti-Asli et al. [172].
4. Conclusions and future directions
The field of RM/TE is dedicated to developing strategies that facilitate the restoration of form and function to damaged tissues and organs, including skeletal muscle. As discussed herein, a variety of naturally occurring, synthetic, and hybrid materials have been developed in an attempt to achieve this goal. Each of these respective approaches possesses a large body of either proof of concept, preclinical, and/or clinical data that support their use for skeletal muscle reconstruction; however each has various technical and translational challenges that limit their widespread clinical use. Thus more work is needed to develop next generation scaffold materials that can build on this success.
Both synthetic and naturally derived materials have been rapidly evolving, though it is clear that there is room for improvement. Synthetic scaffolds are being developed with enhanced bioactivity in an attempt to mimic properties of native tissues and engage the host response rather than striving for biologic inertness. Cell adhesion peptides, signaling compounds, and enzymatic cleavage sites are being incorporated into synthetics to this end. Likewise, control over ECM mechanics and degradation however, is lacking. Although an ECM scaffold may recapitulate the native microenvironment of healthy tissue, injured tissues may require additional mechanical support.
Hybrid materials provide a necessary bridge between synthetic and biologic scaffolds with controlled material properties and favorable biologic properties. Hybrids are an attractive option for both cell and drug delivery applications. Next generation hybrid materials used for cell delivery will need to provide a microenvironmental niche that maintains cell viability and phenotype during delivery and enhances cell survival and differentiation. To achieve this goal future research will need to be aimed at further understanding of the complexities of cell/delivery material combination, the damaged tissue microenvironment, and importantly the interaction between the two.
Acknowledgments
The authors apologize to those colleagues whose work they could not cite due to space limitations. The authors would like to thank Scott Johnson of the Badylak Lab for providing photographs of ECM scaffold configurations. The authors gratefully acknowledge their funding support (MTW was partially supported by the NIH-NHLBI training grant (T32-HL76124-6) entitled “Cardiovascular Bioengineering Training Program” through the University of Pittsburgh’s Department of Bioengineering and SBS is supported by a grant from the National Institute of General Medical Sciences, Division of Training, Workforce Development, and Diversity under the Institutional Research and Academic Career Development Award, grant #K12-GM000678.)
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Scaffolds, Cells, Biologics: At the Crossroads of Musculoskeletal Repair”.
References
- 1.Grogan BF, Hsu JR. Volumetric muscle loss. J Am Acad Orthop Surg. 2011;19(Suppl 1):S35–S37. doi: 10.5435/00124635-201102001-00007. [DOI] [PubMed] [Google Scholar]
- 2.Sicari BM, Agrawal V, Siu BF, Medberry CJ, Dearth CL, Turner NJ, Badylak SF. A murine model of volumetric muscle loss and a regenerative medicine approach for tissue replacement. Tissue Eng A. 2012;18:1941–1948. doi: 10.1089/ten.tea.2012.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerette B, Asselin I, Skuk D, Entman M, Tremblay JP. Control of inflammatory damage by anti-LFA-1: increase success of myoblast transplantation. Cell Transplant. 1997;6:101–107. doi: 10.1177/096368979700600203. [DOI] [PubMed] [Google Scholar]
- 4.Fan Y, Maley M, Beilharz M, Grounds M. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 1996;19:853–860. doi: 10.1002/(SICI)1097-4598(199607)19:7<853::AID-MUS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Camargo FD, Chambers SM, Drew E, McNagny KM, Goodell MA. Hematopoietic stem cells do not engraft with absolute efficiencies. Blood. 2006;107:501–507. doi: 10.1182/blood-2005-02-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glimm H, Oh IH, Eaves CJ. Human hematopoietic stem cells stimulated to proliferate in vitro lose engraftment potential during their S/G(2)/M transit and do not reenter G(0) Blood. 2000;96:4185–4193. [PubMed] [Google Scholar]
- 7.Agrawal V, Brown BN, Beattie AJ, Gilbert TW, Badylak SF. Evidence of innervation following extracellular matrix scaffold-mediated remodelling of muscular tissues. J Tissue Eng Regen Med. 2009;3:590–600. doi: 10.1002/term.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly KA, Wolf M, Johnson SA, Badylak SF. A rabbit model of peripheral compartment syndrome with associated rhabdomyolysis and a regenerative medicine approach for treatment. Tissue Eng Part C Methods. 2011;17:631–640. doi: 10.1089/ten.TEC.2010.0699. [DOI] [PubMed] [Google Scholar]
- 9.Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347:759–774. doi: 10.1007/s00441-011-1185-7. [DOI] [PubMed] [Google Scholar]
- 10.Turner NJ, Yates AJ, Jr, Weber DJ, Qureshi IR, Stolz DB, Gilbert TW, Badylak SF. Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction. Tissue Eng A. 2010;16:3309–3317. doi: 10.1089/ten.TEA.2010.0169. [DOI] [PubMed] [Google Scholar]
- 11.Valentin JE, Turner NJ, Gilbert TW, Badylak SF. Functional skeletal muscle formation with a biologic scaffold. Biomaterials. 2010;31:7475–7484. doi: 10.1016/j.biomaterials.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mase VJ, Jr, Hsu JR, Wolf SE, Wenke JC, Baer DG, Owens J, Badylak SF, Walters TJ. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics. 2010;33:511. doi: 10.3928/01477447-20100526-24. [DOI] [PubMed] [Google Scholar]
- 13.Takanari K, Hong Y, Hashizume R, Huber A, Amoroso NJ, D’Amore A, Badylak SF, Wagner WR. Abdominal wall reconstruction by a regionally distinct biocomposite of extracellular matrix digest and a biodegradable elastomer. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1834. [DOI] [PubMed] [Google Scholar]
- 14.Hong Y, Takanari K, Amoroso NJ, Hashizume R, Brennan-Pierce EP, Freund JM, Badylak SF, Wagner WR. An elastomeric patch electrospun from a blended solution of dermal extracellular matrix and biodegradable polyurethane for rat abdominal wall repair. Tissue Eng Part C Methods. 2012;18:122–132. doi: 10.1089/ten.tec.2011.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong Y, Huber A, Takanari K, Amoroso NJ, Hashizume R, Badylak SF, Wagner WR. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber–extracellular matrix hydrogel biohybrid scaffold. Biomaterials. 2011;32:3387–3394. doi: 10.1016/j.biomaterials.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stankus JJ, Freytes DO, Badylak SF, Wagner WR. Hybrid nanofibrous scaffolds from electrospinning of a synthetic biodegradable elastomer and urinary bladder matrix. J Biomater Sci Polym Ed. 2008;19:635–652. doi: 10.1163/156856208784089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sicari BM, Dearth CL, Badylak SF. Tissue engineering and regenerative medicine approaches to enhance the functional response to skeletal muscle injury. Anat Rec. 2014;297:51–64. doi: 10.1002/ar.22794. [DOI] [PubMed] [Google Scholar]
- 18.Koning M, Harmsen MC, van Luyn MJ, Werker PM. Current opportunities and challenges in skeletal muscle tissue engineering. J Tissue Eng Regen Med. 2009;3:407–415. doi: 10.1002/term.190. [DOI] [PubMed] [Google Scholar]
- 19.Klumpp D, Horch RE, Kneser U, Beier JP. Engineering skeletal muscle tissue—new perspectives in vitro and in vivo. J Cell Mol Med. 2010;14:2622–2629. doi: 10.1111/j.1582-4934.2010.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonvillain RW, Danchuk S, Sullivan DE, Betancourt AM, Semon JA, Eagle ME, Mayeux JP, Gregory AN, Wang G, Townley IK, Borg ZD, Weiss DJ, Bunnell BA. A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng A. 2012;18:2437–2452. doi: 10.1089/ten.tea.2011.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, Christman KL. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One. 2010;5:e13039. doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown B, Lindberg K, Reing J, Stolz DB, Badylak SF. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12:519–526. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 24.Hodde JP, Record RD, Liang HA, Badylak SF. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001;8:11–24. doi: 10.3109/10623320109063154. [DOI] [PubMed] [Google Scholar]
- 25.Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478–491. [PubMed] [Google Scholar]
- 26.Marcal H, Ahmed T, Badylak SF, Tottey S, Foster LJ. A comprehensive protein expression profile of extracellular matrix biomaterial derived from porcine urinary bladder. Regen Med. 2012;7:159–166. doi: 10.2217/rme.12.6. [DOI] [PubMed] [Google Scholar]
- 27.Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, Bedelbaeva K, McIntosh D, Dewilde A, Braunhut SJ, Badylak SF. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng A. 2009;15:605–614. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 28.Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, Cordero K, Bedelbaeva K, Gourevitch D, Heber-Katz E, Badylak SF, Braunhut SJ. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29:690–700. doi: 10.1016/j.matbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Wangensteen KJ, Kalliainen LK. Collagen tube conduits in peripheral nerve repair: a retrospective analysis. Hand (N Y) 2010;5:273–277. doi: 10.1007/s11552-009-9245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) Int Orthop. 2007;31:729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spencer SJ, Saithna A, Carmont MR, Dhillon MS, Thompson P, Spalding T. Meniscal scaffolds: early experience and review of the literature. Knee. 2012;19:760–765. doi: 10.1016/j.knee.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Sano A, Hojo T, Maeda M, Fujioka K. Protein release from collagen matrices. Adv Drug Deliv Rev. 1998;31:247–266. doi: 10.1016/s0169-409x(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 33.Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, Vozzi G, Rando TA, Bonaldo P. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun. 2013;4:1964. doi: 10.1038/ncomms2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murakami K, Aoki H, Nakamura S, Takikawa M, Hanzawa M, Kishimoto S, Hattori H, Tanaka Y, Kiyosawa T, Sato Y, Ishihara M. Hydrogel blends of chitin/chi-tosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials. 2010;31:83–90. doi: 10.1016/j.biomaterials.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 35.Meinel L, Kaplan DL. Silk constructs for delivery of musculoskeletal therapeutics. Adv Drug Deliv Rev. 2012;64:1111–1122. doi: 10.1016/j.addr.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khaing ZZ, Schmidt CE. Advances in natural biomaterials for nerve tissue repair. Neurosci Lett. 2012;519:103–114. doi: 10.1016/j.neulet.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 37.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Shansky J, Borselli C, Mooney D, Vandenburgh H. Design and fabrication of a biodegradable, covalently crosslinked shape-memory alginate scaffold for cell and growth factor delivery. Tissue Eng A. 2012;18:2000–2007. doi: 10.1089/ten.tea.2011.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jana S, Cooper A, Zhang M. Chitosan scaffolds with unidirectional microtubular pores for large skeletal myotube generation. Adv Healthc Mater. 2013;2:557–561. doi: 10.1002/adhm.201200177. [DOI] [PubMed] [Google Scholar]
- 40.Kroehne V, Heschel I, Schugner F, Lasrich D, Bartsch JW, Jockusch H. Use of a novel collagen matrix with oriented pore structure for muscle cell differentiation in cell culture and in grafts. J Cell Mol Med. 2008;12:1640–1648. doi: 10.1111/j.1582-4934.2008.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hosseini V, Ahadian S, Ostrovidov S, Camci-Unal G, Chen S, Kaji H, Ramalingam M, Khademhosseini A. Engineered contractile skeletal muscle tissue on a microgrooved methacrylated gelatin substrate. Tissue Eng A. 2012;18:2453–2465. doi: 10.1089/ten.tea.2012.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bian W, Bursac N. Engineered skeletal muscle tissue networks with controllable architecture. Biomaterials. 2009;30:1401–1412. doi: 10.1016/j.biomaterials.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bian W, Juhas M, Pfeiler TW, Bursac N. Local tissue geometry determines contractile force generation of engineered muscle networks. Tissue Eng A. 2012;18:957–967. doi: 10.1089/ten.tea.2011.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379:943–952. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 45.Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, Freytes DO, Thapa A, Gilbert TW, Nieponice A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87–97. doi: 10.1016/j.jss.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Badylak SF, Hoppo T, Nieponice A, Gilbert TW, Davison JM, Jobe BA. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng A. 2011;17:1643–1650. doi: 10.1089/ten.tea.2010.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21:2215–2231. doi: 10.1016/s0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 48.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 49.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 50.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29:1630–1637. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 52.Wolf MT, Daly KA, Brennan-Pierce EP, Johnson SA, Carruthers CA, D’Amore A, Nagarkar SP, Velankar SS, Badylak SF. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. 2012;33:7028–7038. doi: 10.1016/j.biomaterials.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turner NJ, Badylak SF. Biologic scaffolds for musculotendinous tissue repair. Eur Cell Mater. 2013;25:130–143. doi: 10.22203/ecm.v025a09. [DOI] [PubMed] [Google Scholar]
- 55.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156:1489–1498. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156:1489–1498. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 59.Moore AJ, Beazley WD, Bibby MC, Devine DA. Antimicrobial activity of cecropins. J Antimicrob Chemother. 1996;37:1077–1089. doi: 10.1093/jac/37.6.1077. [DOI] [PubMed] [Google Scholar]
- 60.Moore AJ, Devine DA, Bibby MC. Preliminary experimental anticancer activity of cecropins. Pept Res. 1994;7:265–269. [PubMed] [Google Scholar]
- 61.Li F, Li W, Johnson S, Ingram D, Yoder M, Badylak S. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium. 2004;11:199–206. doi: 10.1080/10623320490512390. [DOI] [PubMed] [Google Scholar]
- 62.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng A. 2008;14:1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 64.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 65.Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173–R1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJ. The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008;29:2899–2906. doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 67.Hoque ME, San WY, Wei F, Li S, Huang MH, Vert M, Hutmacher DW. Processing of polycaprolactone and polycaprolactone-based copolymers into 3D scaffolds, and their cellular responses. Tissue Eng A. 2009;15:3013–3024. doi: 10.1089/ten.TEA.2008.0355. [DOI] [PubMed] [Google Scholar]
- 68.Saxena AK, Marler J, Benvenuto M, Willital GH, Vacanti JP. Skeletal muscle tissue engineering using isolated myoblasts on synthetic biodegradable polymers: preliminary studies. Tissue Eng. 1999;5:525–532. doi: 10.1089/ten.1999.5.525. [DOI] [PubMed] [Google Scholar]
- 69.Saxena AK, Willital GH, Vacanti JP. Vascularized three-dimensional skeletal muscle tissue-engineering. Biomed Mater Eng. 2001;11:275–281. [PubMed] [Google Scholar]
- 70.Mulder MM, Hitchcock RW, Tresco PA. Skeletal myogenesis on elastomeric substrates: implications for tissue engineering. J Biomater Sci Polym Ed. 1998;9:731–748. doi: 10.1163/156856298x00118. [DOI] [PubMed] [Google Scholar]
- 71.Riboldi SA, Sampaolesi M, Neuenschwander P, Cossu G, Mantero S. Electrospun degradable polyesterurethane membranes: potential scaffolds for skeletal muscle tissue engineering. Biomaterials. 2005;26:4606–4615. doi: 10.1016/j.biomaterials.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 72.Ratner BD. Biomaterials Science: An Introduction to Materials in Medicine. 2. Elsevier Academic Press; Amsterdam. Boston: 2004. [Google Scholar]
- 73.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klinge U, Klosterhalfen B, Muller M, Schumpelick V. Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur J Surg. 1999;165:665–673. doi: 10.1080/11024159950189726. [DOI] [PubMed] [Google Scholar]
- 75.Li S. Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. J Biomed Mater Res. 1999;48:342–353. doi: 10.1002/(sici)1097-4636(1999)48:3<342::aid-jbm20>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 76.Grizzi I, Garreau H, Li S, Vert M. Hydrolytic degradation of devices based on poly(DL-lactic acid) size-dependence. Biomaterials. 1995;16:305–311. doi: 10.1016/0142-9612(95)93258-f. [DOI] [PubMed] [Google Scholar]
- 77.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 78.Peters MC, Polverini PJ, Mooney DJ. Engineering vascular networks in porous polymer matrices. J Biomed Mater Res. 2002;60:668–678. doi: 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- 79.Harris LD, Kim BS, Mooney DJ. Open pore biodegradable matrices formed with gas foaming. J Biomed Mater Res. 1998;42:396–402. doi: 10.1002/(sici)1097-4636(19981205)42:3<396::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 80.Smith MK, Peters MC, Richardson TP, Garbern JC, Mooney DJ. Locally enhanced angiogenesis promotes transplanted cell survival. Tissue Eng. 2004;10:63–71. doi: 10.1089/107632704322791709. [DOI] [PubMed] [Google Scholar]
- 81.Woodruff MA, Hutmacher DW. The return of a forgotten polymer—polycaprolactone in the 21st century. Prog Polym Sci. 2010;35:1217–1256. [Google Scholar]
- 82.Lam CX, Hutmacher DW, Schantz JT, Woodruff MA, Teoh SH. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J Biomed Mater Res A. 2009;90:906–919. doi: 10.1002/jbm.a.32052. [DOI] [PubMed] [Google Scholar]
- 83.Lam CX, Savalani MM, Teoh SH, Hutmacher DW. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: accelerated versus simulated physiological conditions. Biomed Mater. 2008;3:034108. doi: 10.1088/1748-6041/3/3/034108. [DOI] [PubMed] [Google Scholar]
- 84.Bellon JM, Rodriguez M, Garcia-Honduvilla N, Pascual G, Bujan J. Partially absorbable meshes for hernia repair offer advantages over nonabsorbable meshes. Am J Surg. 2007;194:68–74. doi: 10.1016/j.amjsurg.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 85.Guelcher SA. Biodegradable polyurethanes: synthesis and applications in regenerative medicine. Tissue Eng B Rev. 2008;14:3–17. doi: 10.1089/teb.2007.0133. [DOI] [PubMed] [Google Scholar]
- 86.Lee JH, Ju YM, Kim DM. Platelet adhesion onto segmented polyurethane film surfaces modified by addition and crosslinking of PEO-containing block copolymers. Biomaterials. 2000;21:683–691. doi: 10.1016/s0142-9612(99)00197-0. [DOI] [PubMed] [Google Scholar]
- 87.Li D, Chen H, Glenn McClung W, Brash JL. Lysine-PEG-modified polyurethane as a fibrinolytic surface: effect of PEG chain length on protein interactions, platelet interactions and clot lysis. Acta Biomater. 2009;5:1864–1871. doi: 10.1016/j.actbio.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Yu J, Takanari K, Hong Y, Lee KW, Amoroso NJ, Wang Y, Wagner WR, Kim K. Non-invasive characterization of polyurethane-based tissue constructs in a rat abdominal repair model using high frequency ultrasound elasticity imaging. Biomaterials. 2013;34:2701–2709. doi: 10.1016/j.biomaterials.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin DT, Young TH, Fang Y. Studies on the effect of surface properties on the biocompatibility of polyurethane membranes. Biomaterials. 2001;22:1521–1529. doi: 10.1016/s0142-9612(00)00308-2. [DOI] [PubMed] [Google Scholar]
- 90.Cobb WS, Kercher KW, Heniford BT. The argument for lightweight polypropylene mesh in hernia repair. Surg Innov. 2005;12:63–69. doi: 10.1177/155335060501200109. [DOI] [PubMed] [Google Scholar]
- 91.Klosterhalfen B, Junge K, Klinge U. The lightweight and large porous mesh concept for hernia repair. Expert Rev Med Devices. 2005;2:103–117. doi: 10.1586/17434440.2.1.103. [DOI] [PubMed] [Google Scholar]
- 92.Leber GE, Garb JL, Alexander AI, Reed WP. Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg. 1998;133:378–382. doi: 10.1001/archsurg.133.4.378. [DOI] [PubMed] [Google Scholar]
- 93.Weyhe D, Schmitz I, Belyaev O, Grabs R, Muller KM, Uhl W, Zumtobel V. Experimental comparison of monofile light and heavy polypropylene meshes: less weight does not mean less biological response. World J Surg. 2006;30:1586–1591. doi: 10.1007/s00268-005-0601-0. [DOI] [PubMed] [Google Scholar]
- 94.Zieren J, Paul M, Osei-Agyemang T, Maecker F, Muller JM. Polyurethane-covered dacron mesh versus polytetrafluoroethylene DualMesh for intraperitoneal hernia repair in rats. Surg Today. 2002;32:884–886. doi: 10.1007/s005950200172. [DOI] [PubMed] [Google Scholar]
- 95.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 98.Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials. 1999;20:573–588. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 99.Badami AS, Kreke MR, Thompson MS, Riffle JS, Goldstein AS. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27:596–606. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 100.Aviss KJ, Gough JE, Downes S. Aligned electrospun polymer fibres for skeletal muscle regeneration. Eur Cell Mater. 2010;19:193–204. doi: 10.22203/ecm.v019a19. [DOI] [PubMed] [Google Scholar]
- 101.Xu CY, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 2004;25:877–886. doi: 10.1016/s0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 102.Neumann T, Hauschka SD, Sanders JE. Tissue engineering of skeletal muscle using polymer fiber arrays. Tissue Eng. 2003;9:995–1003. doi: 10.1089/107632703322495637. [DOI] [PubMed] [Google Scholar]
- 103.Cronin EM, Thurmond FA, Bassel-Duby R, Williams RS, Wright WE, Nelson KD, Garner HR. Protein-coated poly(L-lactic acid) fibers provide a substrate for differentiation of human skeletal muscle cells. J Biomed Mater Res A. 2004;69:373–381. doi: 10.1002/jbm.a.30009. [DOI] [PubMed] [Google Scholar]
- 104.Williamson MR, Adams EF, Coombes AG. Gravity spun polycaprolactone fibres for soft tissue engineering: interaction with fibroblasts and myoblasts in cell culture. Biomaterials. 2006;27:1019–1026. doi: 10.1016/j.biomaterials.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 105.Guex AG, Birrer DL, Fortunato G, Tevaearai HT, Giraud MN. Anisotropically oriented electrospun matrices with an imprinted periodic micropattern: a new scaffold for engineered muscle constructs. Biomed Mater. 2013;8:021001. doi: 10.1088/1748-6041/8/2/021001. [DOI] [PubMed] [Google Scholar]
- 106.Sirivisoot S, Harrison BS. Skeletal myotube formation enhanced by electrospun polyurethane carbon nanotube scaffolds. Int J Nanomedicine. 2011;6:2483–2497. doi: 10.2147/IJN.S24073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Riboldi SA, Sadr N, Pigini L, Neuenschwander P, Simonet M, Mognol P, Sampaolesi M, Cossu G, Mantero S. Skeletal myogenesis on highly orientated microfibrous polyesterurethane scaffolds. J Biomed Mater Res A. 2008;84:1094–1101. doi: 10.1002/jbm.a.31534. [DOI] [PubMed] [Google Scholar]
- 108.McKeon-Fischer KD, Flagg DH, Freeman JW. Coaxial electrospun poly(epsilon-caprolactone), multiwalled carbon nanotubes, and polyacrylic acid/polyvinyl alcohol scaffold for skeletal muscle tissue engineering. J Biomed Mater Res A. 2011;99:493–499. doi: 10.1002/jbm.a.33116. [DOI] [PubMed] [Google Scholar]
- 109.Ku SH, Lee SH, Park CB. Synergic effects of nanofiber alignment and electroactivity on myoblast differentiation. Biomaterials. 2012;33:6098–6104. doi: 10.1016/j.biomaterials.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 110.Chen MC, Sun YC, Chen YH. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 2013;9:5562–5572. doi: 10.1016/j.actbio.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 111.Liao IC, Liu JB, Bursac N, Leong KW. Effect of electromechanical stimulation on the maturation of myotubes on aligned electrospun fibers. Cell Mol Bioeng. 2008;1:133–145. doi: 10.1007/s12195-008-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baker BM, Gee AO, Metter RB, Nathan AS, Marklein RA, Burdick JA, Mauck RL. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008;29:2348–2358. doi: 10.1016/j.biomaterials.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McCullen SD, Gittard SD, Miller PR, Pourdeyhimi B, Narayan RJ, Loboa EG. Laser ablation imparts controlled micro-scale pores in electrospun scaffolds for tissue engineering applications. Ann Biomed Eng. 2011;39:3021–3030. doi: 10.1007/s10439-011-0378-2. [DOI] [PubMed] [Google Scholar]
- 114.Boldrin L, Elvassore N, Malerba A, Flaibani M, Cimetta E, Piccoli M, Baroni MD, Gazzola MV, Messina C, Gamba P, Vitiello L, De Coppi P. Satellite cells delivered by micro-patterned scaffolds: a new strategy for cell transplantation in muscle diseases. Tissue Eng. 2007;13:253–262. doi: 10.1089/ten.2006.0093. [DOI] [PubMed] [Google Scholar]
- 115.Yang HS, Ieronimakis N, Tsui JH, Kim HN, Suh KY, Reyes M, Kim DH. Nanopatterned muscle cell patches for enhanced myogenesis and dystrophin expression in a mouse model of muscular dystrophy. Biomaterials. 2014;35:1478–1486. doi: 10.1016/j.biomaterials.2013.10.067. [DOI] [PubMed] [Google Scholar]
- 116.Seyednejad H, Gawlitta D, Kuiper RV, de Bruin A, van Nostrum CF, Vermonden T, Dhert WJ, Hennink WE. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(epsilon-caprolactone) Biomaterials. 2012;33:4309–4318. doi: 10.1016/j.biomaterials.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 117.Cei D, Malena A, de Maria C, Loro E, Sandri F, Del Moro G, Bettio S, Vergani L, Vozzi G. In vitro development of engineered muscle using a scaffold based on the pressure-activated microsyringe (PAM) technique. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1894. [DOI] [PubMed] [Google Scholar]
- 118.George CD, Ellis H. The results of incisional hernia repair: a twelve year review. Ann R Coll Surg Engl. 1986;68:185–187. [PMC free article] [PubMed] [Google Scholar]
- 119.Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240:578–583. doi: 10.1097/01.sla.0000141193.08524.e7. (discussion 583–575.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown CN, Finch JG. Which mesh for hernia repair? Ann R Coll Surg Engl. 2010;92:272–278. doi: 10.1308/003588410X12664192076296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Taylor SG, O’Dwyer PJ. Chronic groin sepsis following tension-free inguinal hernioplasty. Br J Surg. 1999;86:562–565. doi: 10.1046/j.1365-2168.1999.01072.x. [DOI] [PubMed] [Google Scholar]
- 122.Tolino MJ, Tripoloni DE, Ratto R, Garcia MI. Infections associated with prosthetic repairs of abdominal wall hernias: pathology, management and results. Hernia. 2009;13:631–637. doi: 10.1007/s10029-009-0541-y. [DOI] [PubMed] [Google Scholar]
- 123.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 124.Klinge U, Junge K, Spellerberg B, Piroth C, Klosterhalfen B, Schumpelick V. Do multifilament alloplastic meshes increase the infection rate? Analysis of the polymeric surface, the bacteria adherence, and the in vivo consequences in a rat model. J Biomed Mater Res. 2002;63:765–771. doi: 10.1002/jbm.10449. [DOI] [PubMed] [Google Scholar]
- 125.Rosen MJ, Krpata DM, Ermlich B, Blatnik JA. A 5-year clinical experience with single-staged repairs of infected and contaminated abdominal wall defects utilizing biologic mesh. Ann Surg. 2013;257:991–996. doi: 10.1097/SLA.0b013e3182849871. [DOI] [PubMed] [Google Scholar]
- 126.Castro PR, Marques SM, Campos PP, Cardoso CC, Sampaio FP, Ferreira MA, Andrade SP. Kinetics of implant-induced inflammatory angiogenesis in abdominal muscle wall in mice. Microvasc Res. 2012;84:9–15. doi: 10.1016/j.mvr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 127.Cole BJ, Gomoll AH, Yanke A, Pylawka T, Lewis P, Macgillivray JD, Williams JM. Biocompatibility of a polymer patch for rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2007;15:632–637. doi: 10.1007/s00167-006-0187-6. [DOI] [PubMed] [Google Scholar]
- 128.Encalada-Diaz I, Cole BJ, Macgillivray JD, Ruiz-Suarez M, Kercher JS, Friel NA, Valero-Gonzalez F. Rotator cuff repair augmentation using a novel polycarbonate polyurethane patch: preliminary results at 12 months’ follow-up. J Shoulder Elbow Surg. 2011;20:788–794. doi: 10.1016/j.jse.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bosworth LA, Turner LA, Cartmell SH. State of the art composites comprising electrospun fibres coupled with hydrogels: a review. Nanomedicine. 2013;9:322–335. doi: 10.1016/j.nano.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 130.Jana S, Leung M, Chang J, Zhang M. Effect of nano- and micro-scale topological features on alignment of muscle cells and commitment of myogenic differentiation. Biofabrication. 2014;6:035012. doi: 10.1088/1758-5082/6/3/035012. [DOI] [PubMed] [Google Scholar]
- 131.Leung M, Cooper A, Jana S, Tsao CT, Petrie TA, Zhang M. Nanofiber-based in vitro system for high myogenic differentiation of human embryonic stem cells. Biomacromolecules. 2013;14:4207–4216. doi: 10.1021/bm4009843. [DOI] [PubMed] [Google Scholar]
- 132.Altinel Y, Ozturk E, Ozkaya G, Akyildiz EU, Ulcay Y, Ozguc H. The effect of a chitosan coating on the adhesive potential and tensile strength of polypropylene meshes. Hernia. 2012 doi: 10.1007/s10029-012-0950-1. [DOI] [PubMed] [Google Scholar]
- 133.Borrazzo EC, Belmont MF, Boffa D, Fowler DL. Effect of prosthetic material on adhesion formation after laparoscopic ventral hernia repair in a porcine model. Hernia. 2004;8:108–112. doi: 10.1007/s10029-003-0181-6. [DOI] [PubMed] [Google Scholar]
- 134.Schreinemacher MH, Emans PJ, Gijbels MJ, Greve JW, Beets GL, Bouvy ND. Degradation of mesh coatings and intraperitoneal adhesion formation in an experimental model. Br J Surg. 2009;96:305–313. doi: 10.1002/bjs.6446. [DOI] [PubMed] [Google Scholar]
- 135.Pierce RA, Perrone JM, Nimeri A, Sexton JA, Walcutt J, Frisella MM, Matthews BD. 120-day comparative analysis of adhesion grade and quantity, mesh contraction,and tissue response to a novel omega-3 fatty acid bioabsorbable barrier macroporous mesh after intraperitoneal placement. Surg Innov. 2009;16:46–54. doi: 10.1177/1553350608330479. [DOI] [PubMed] [Google Scholar]
- 136.van’t Riet M, de Vos van Steenwijk PJ, Bonthuis F, Marquet RL, Steyerberg EW, Jeekel J, Bonjer HJ. Prevention of adhesion to prosthetic mesh: comparison of different barriers using an incisional hernia model. Ann Surg. 2003;237:123–128. doi: 10.1097/00000658-200301000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van’t Riet M, Burger JW, Bonthuis F, Jeekel J, Bonjer HJ. Prevention of adhesion formation to polypropylene mesh by collagen coating: a randomized controlled study in a rat model of ventral hernia repair. Surg Endosc. 2004;18:681–685. doi: 10.1007/s00464-003-9054-4. [DOI] [PubMed] [Google Scholar]
- 138.Takaoka R, Hikasa Y, Tabata Y. Vascularization around poly(tetrafluoroethylene) mesh with coating of gelatin hydrogel incorporating basic fibroblast growth factor. J Biomater Sci Polym Ed. 2009;20:1483–1494. doi: 10.1163/092050609X12457419038465. [DOI] [PubMed] [Google Scholar]
- 139.Shen Z, Guo S, Ye D, Chen J, Kang C, Qiu S, Lu D, Li Q, Xu K, Lv J, Zhu Y. Skeletal muscle regeneration on protein-grafted and microchannel-patterned scaffold for hypopharyngeal tissue engineering. Biomed Res Int. 2013;2013:146953. doi: 10.1155/2013/146953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sahoo S, DeLozier KR, Dumm RA, Rosen MJ, Derwin KA. Fiber-reinforced dermis graft for ventral hernia repair. J Mech Behav Biomed Mater. 2014;34:320–329. doi: 10.1016/j.jmbbm.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 141.Wolf MT, Carruthers CA, Dearth CL, Crapo PM, Huber A, Burnsed OA, Londono R, Johnson SA, Daly KA, Stahl EC, Freund JM, Medberry CJ, Carey LE, Nieponice A, Amoroso NJ, Badylak SF. Polypropylene surgical mesh coated with extracellular matrix mitigates the host foreign body response. J Biomed Mater Res A. 2014;102:234–246. doi: 10.1002/jbm.a.34671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wolf MT, Dearth CL, Ranallo CA, LoPresti ST, Carey LE, Daly KA, Brown BN, Badylak SF. Macrophage polarization in response to ECM coated polypropylene mesh. Biomaterials. 2014;35:6838–6849. doi: 10.1016/j.biomaterials.2014.04.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Haslauer CM, Moghe AK, Osborne JA, Gupta BS, Loboa EG. Collagen–PCL sheath–core bicomponent electrospun scaffolds increase osteogenic differentiation and calcium accretion of human adipose-derived stem cells. J Biomater Sci Polym Ed. 2011;22:1695–1712. doi: 10.1163/092050610X521595. [DOI] [PubMed] [Google Scholar]
- 144.Zhao W, Ju YM, Christ G, Atala A, Yoo JJ, Lee SJ. Diaphragmatic muscle reconstruction with an aligned electrospun poly(epsilon-caprolactone)/collagen hybrid scaffold. Biomaterials. 2013;34:8235–8240. doi: 10.1016/j.biomaterials.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 145.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 146.Shandalov Y, Egozi D, Koffler J, Dado-Rosenfeld D, Ben-Shimol D, Freiman A, Shor E, Kabala A, Levenberg S. An engineered muscle flap for reconstruction of large soft tissue defects. Proc Natl Acad Sci U S A. 2014;111:6010–6015. doi: 10.1073/pnas.1402679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee J, Bhang SH, Park H, Kim BS, Lee KY. Active blood vessel formation in the ischemic hindlimb mouse model using a microsphere/hydrogel combination system. Pharm Res. 2010;27:767–774. doi: 10.1007/s11095-010-0067-0. [DOI] [PubMed] [Google Scholar]
- 148.Menasche P. Cell therapy for peripheral arterial disease. Curr Opin Mol Ther. 2010;12:538–545. [PubMed] [Google Scholar]
- 149.Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209:10–17. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 150.Tongers J, Roncalli JG, Losordo DW. Therapeutic angiogenesis for critical limb ischemia: microvascular therapies coming of age. Circulation. 2008;118:9–16. doi: 10.1161/CIRCULATIONAHA.108.784371. [DOI] [PubMed] [Google Scholar]
- 151.Nelson DM, Baraniak PR, Ma Z, Guan J, Mason NS, Wagner WR. Controlled release of IGF-1 and HGF from a biodegradable polyurethane scaffold. Pharm Res. 2011;28:1282–1293. doi: 10.1007/s11095-011-0391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hafeman AE, Li B, Yoshii T, Zienkiewicz K, Davidson JM, Guelcher SA. Injectable biodegradable polyurethane scaffolds with release of platelet-derived growth factor for tissue repair and regeneration. Pharm Res. 2008;25:2387–2399. doi: 10.1007/s11095-008-9618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bouchemal K, Briancon S, Perrier E, Fessi H, Bonnet I, Zydowicz N. Synthesis and characterization of polyurethane and poly(ether urethane) nanocapsules using a new technique of interfacial polycondensation combined to spontaneous emulsification. Int J Pharm. 2004;269:89–100. doi: 10.1016/j.ijpharm.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 154.Webber MJ, Tongers J, Newcomb CJ, Marquardt KT, Bauersachs J, Losordo DW, Stupp SI. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. Proc Natl Acad Sci U S A. 2011;108:13438–13443. doi: 10.1073/pnas.1016546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jay SM, Shepherd BR, Bertram JP, Pober JS, Saltzman WM. Engineering of multifunctional gels integrating highly efficient growth factor delivery with endothelial cell transplantation. FASEB J. 2008;22:2949–2956. doi: 10.1096/fj.08-108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kong HJ, Kim ES, Huang YC, Mooney DJ. Design of biodegradable hydrogel for the local and sustained delivery of angiogenic plasmid DNA. Pharm Res. 2008;25:1230–1238. doi: 10.1007/s11095-007-9526-7. [DOI] [PubMed] [Google Scholar]
- 157.Doi K, Ikeda T, Marui A, Kushibiki T, Arai Y, Hirose K, Soga Y, Iwakura A, Ueyama K, Yamahara K, Itoh H, Nishimura K, Tabata Y, Komeda M. Enhanced angiogenesis by gelatin hydrogels incorporating basic fibroblast growth factor in rabbit model of hind limb ischemia. Heart Vessel. 2007;22:104–108. doi: 10.1007/s00380-006-0934-0. [DOI] [PubMed] [Google Scholar]
- 158.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 159.Ruvinov E, Leor J, Cohen S. The effects of controlled HGF delivery from an affinity-binding alginate biomaterial on angiogenesis and blood perfusion in a hindlimb ischemia model. Biomaterials. 2010;31:4573–4582. doi: 10.1016/j.biomaterials.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 160.Layman H, Spiga MG, Brooks T, Pham S, Webster KA, Andreopoulos FM. The effect of the controlled release of basic fibroblast growth factor from ionic gelatin-based hydrogels on angiogenesis in a murine critical limb ischemic model. Biomaterials. 2007;28:2646–2654. doi: 10.1016/j.biomaterials.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee J, Yoo JJ, Atala A, Lee SJ. Controlled heparin conjugation on electrospun poly(epsilon-caprolactone)/gelatin fibers for morphology-dependent protein delivery and enhanced cellular affinity. Acta Biomater. 2012;8:2549–2558. doi: 10.1016/j.actbio.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 162.Kuraitis D, Zhang P, McEwan K, Zhang J, McKee D, Sofrenovic T, Griffith M, Cao X, Ruel M, Suuronen EJ. Controlled release of stromal cell-derived factor-1 for enhanced progenitor response in ischemia. J Control Release. 2011;152(Suppl 1):e216–e218. doi: 10.1016/j.jconrel.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 163.Kuraitis D, Zhang P, Zhang Y, Padavan DT, McEwan K, Sofrenovic T, McKee D, Zhang J, Griffith M, Cao X, Musaro A, Ruel M, Suuronen EJ. A stromal cell-derived factor-1 releasing matrix enhances the progenitor cell response and blood vessel growth in ischaemic skeletal muscle. Eur Cell Mater. 2011;22:109–123. doi: 10.22203/ecm.v022a09. [DOI] [PubMed] [Google Scholar]
- 164.Wang L, Cao L, Shansky J, Wang Z, Mooney D, Vandenburgh H. Minimally invasive approach to the repair of injured skeletal muscle with a shape-memory scaffold. Mol Ther. 2014 doi: 10.1038/mt.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shvartsman D, Storrie-White H, Lee K, Kearney C, Brudno Y, Ho N, Cezar C, McCann C, Anderson E, Koullias J, Tapia JC, Vandenburgh H, Lichtman JW, Mooney DJ. Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling. Mol Ther. 2014;22:1243–1253. doi: 10.1038/mt.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ju YM, Atala A, Yoo JJ, Lee SJ. In situ regeneration of skeletal muscle tissue through host cell recruitment. Acta Biomater. 2014 doi: 10.1016/j.actbio.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 167.Danieli-Betto D, Peron S, Germinario E, Zanin M, Sorci G, Franzoso S, Sandona D, Betto R. Sphingosine 1-phosphate signaling is involved in skeletal muscle regeneration. Am J Physiol Cell Physiol. 2010;298:C550–C558. doi: 10.1152/ajpcell.00072.2009. [DOI] [PubMed] [Google Scholar]
- 168.Ieronimakis N, Pantoja M, Hays AL, Dosey TL, Qi J, Fischer KA, Hoofnagle AN, Sadilek M, Chamberlain JS, Ruohola-Baker H, Reyes M. Increased sphingosine-1-phosphate improves muscle regeneration in acutely injured mdx mice. Skelet Muscle. 2013;3:20. doi: 10.1186/2044-5040-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim WH, Jung DW, Kim J, Im SH, Hwang SY, Williams DR. Small molecules that recapitulate the early steps of urodele amphibian limb regeneration and confer multipotency. ACS Chem Biol. 2012;7:732–743. doi: 10.1021/cb200532v. [DOI] [PubMed] [Google Scholar]
- 170.Jung DW, Williams DR. Reawakening atlas: chemical approaches to repair or replace dysfunctional musculature. ACS Chem Biol. 2012;7:1773–1790. doi: 10.1021/cb3003368. [DOI] [PubMed] [Google Scholar]
- 171.Juhas M, Bursac N. Engineering skeletal muscle repair. Curr Opin Biotechnol. 2013;24:880–886. doi: 10.1016/j.copbio.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Asli MM, Pourdeyhimi B, Loboa EG. Release profiles of tricalcium phosphate nanoparticles from poly(L-lactic acid) electrospun scaffolds with single component, core–sheath, or porous fiber morphologies: effects on hASC viability and osteogenic differentiation. Macromol Biosci. 2012;12:893–900. doi: 10.1002/mabi.201100470. [DOI] [PubMed] [Google Scholar]