Abstract
The phenotypic consequences of targeted expression of mammalian biliverdin IXα reductase (BVR), an enzyme that metabolically inactivates the linear tetrapyrrole precursors of the phytochrome chromophore, are addressed in this investigation. Through comparative phenotypic analyses of multiple plastid-targeted and cytosolic BVR transgenic Arabidopsis plant lines, we show that the subcellular localization of BVR affects distinct subsets of light-mediated and light-independent processes in plant growth and development. Regardless of its cellular localization, BVR suppresses the phytochrome-modulated responses of hypocotyl growth inhibition, sucrose-stimulated anthocyanin accumulation, and inhibition of floral initiation. By contrast, reduced protochlorophyll levels in dark-grown seedlings and fluence-rate-dependent reduction of chlorophyll occur only in transgenic plants in which BVR is targeted to plastids. Together with companion analyses of the phytochrome chromophore-deficient hy1 mutant, our results suggest a regulatory role for linear tetrapyrroles within the plastid compartment distinct from their assembly with apophytochromes in the cytosol.
Light influences growth and development throughout the life cycle of a plant, including the processes of seed germination, inhibition of hypocotyl elongation, chloroplast development and greening, cotyledon and leaf expansion, internode elongation, induction of flowering, and senescence (Fankhauser and Chory, 1997). To monitor and respond to suboptimal light conditions in their environment, plants possess three distinct classes of photomorphogenetic photoreceptors, those that maximally absorb: (a) UV-B light, (b) blue/UV-A light, and (c) red/far-red light (Kendrick and Kronenberg, 1994). The most extensively characterized of these photoreceptors are the phytochromes that primarily mediate responses to red/far-red light (Furuya, 1993; Quail et al., 1995). Higher plants possess multiple phytochrome species that are encoded by a small nuclear gene family (Quail, 1994; Pratt, 1995). In Arabidopsis, five phytochrome genes, designated phyA to phyE, have been identified (Sharrock and Quail, 1989; Clack et al., 1994). Genetic analyses have established that the different phytochromes mediate overlapping, distinct aspects of photomorphogenesis in plants (Reed et al., 1994; Smith, 1995; Whitelam and Devlin, 1997).
In contrast to the genetic diversity among apophytochromes, the same linear tetrapyrrole prosthetic group, phytochromobilin (PΦB), is utilized by all higher plant phytochromes (Terry et al., 1993). Accordingly, the phenotypes of known PΦB-deficient mutants of Arabidopsis, tomato, pea, and wild tobacco lack multiple phytochrome photoregulatory activities (Koornneef et al., 1980, 1985; Chory et al., 1989; Kraepiel et al., 1994; Van Tuinen et al., 1996; Weller et al., 1996, 1997). PΦB-deficient plants exhibit defects in light-mediated growth and development both as seedlings and adults. Among other phenotypes, such mutants display reduced seed germination, possess decreased levels of chlorophyll, and fail to de-etiolate under both continuous red (Rc) and continuous far-red (FRc) light—phenotypes consistent with deficiencies in both phyA and phyB activities (Koornneef and Kendrick, 1994; Smith, 1995).
Through expression of the mammalian enzyme biliverdin IXα reductase (BVR) in transgenic Arabidopsis plants, we have demonstrated that multiple aspects of phytochrome-mediated growth and development were affected (Lagarias et al., 1997). Since PΦB biosynthesis occurs entirely within the plastid compartment of plant cells (Terry et al., 1993), we targeted BVR to plastids for greater effectiveness by fusion with a stromal transit peptide sequence (Lagarias et al., 1997). These studies revealed that plastid-targeted, constitutive expression of BVR in Arabidopsis phenocopied the phytochrome chromophore-deficient hy1 and hy2 mutants (Lagarias et al., 1997).
We previously observed that plastid-targeted expression of BVR led to a significantly reduced tolerance to high light fluences, which was characterized by a severe reduction in chlorophyll accumulation (Lagarias et al., 1997). This suggested either that BVR reduced holophytochrome to such low levels that a regulatory role for phytochrome in light tolerance was uncovered, or that accumulation of BVR and/or its rubinoid products within the plastid compartment were responsible for the high-light-intolerant phenotype. To distinguish between these alternatives, we constructed transgenic lines in which BVR was expressed in the cytoplasm. Through comparative phenotypic analyses of homozygous plastid-targeted (pBVR) and cytosolic (cBVR) transgenic plant lines, these investigations show that the subcellular localization of BVR determines the subset of phytochrome-mediated responses that are disrupted.
MATERIALS AND METHODS
Plant Material
Five transgenic, plastid-targeted BVR (35S::pBVR) lines in Arabidopsis (Nossen ecotype [No-O]) used in these studies (i.e. pBVR1 to pBVR5) were described previously (Lagarias et al., 1997). cBVR (35S::cBVR) lines in No-O were isolated using an Agrobacterium tumefaciens-mediated in planta transformation protocol (Bechtold et al., 1993). For these experiments, a rat kidney BVR cDNA was placed under control of the CaMV 35S promoter in the binary transformation vector pBIB-KAN (Becker, 1990) using a two-step process. First, plasmid p35S::cBVR was constructed by inserting the 1.3-kb NcoI-SacI BVR fragment from BVR plasmid pRKB55/NcoI (Lagarias et al., 1997) into vector pRTL2 (Carrington et al., 1990) that had been restricted with SacI and NcoI. Transformation plasmid pBIB/35S::cBVR construction was accomplished by triple ligation of HindIII-SacI-digested vector pBIB-KAN (Becker, 1990), a 1.1-kb 35S-promoter-BVR fragment isolated from p35S::cBVR by restriction with HindIII and BamHI, and an 0.8-kb BVR fragment isolated by restriction of pRKB55/NcoI with BamHI and SacI. Two homozygous, single insertion lines, cBVR1 and cBVR2, were used in the experiments described here. The chromophore-deficient mutant hy1 (21.84N) was a gift from J. Chory (Salk Institute, La Jolla, CA).
Plant Growth Conditions
Arabidopsis seeds were surface-sterilized for 15 min with 35% (v/v) commercial bleach and 0.025% (v/v) SDS, and rinsed four times with ultrapure water (Milli-Q, Millipore, Bedford, MA). Seeds were planted in 100- × 25-mm Petri dishes on media containing Murashige and Skoog salts (Gibco-BRL, Cleveland), 0.3% (w/v) Phytagel (Sigma, St. Louis), and no Suc or 1% (w/v) Suc, and adjusted to pH 6.7 with NaOH. Imbibing seeds were cold-stratified at 4°C in darkness for 4 to 5 d prior to being transferred to an appropriate light regime. All plants were grown in temperature- and humidity-controlled growth chambers. For flowering experiments, seeds sterilized as described above were germinated in pots containing Sunshine Mix no. 1 (Fisons, Bellevue, WA) and grown in a growth chamber at 20°C under short days (8-h light/16-h dark cycle) or short-day/night-break conditions (7-h light/16-h dark interrupted by 1-h light cycle).
Light Sources
For Wc conditions, seeds on agar media were placed in growth chambers under cool-white lights (F48FT12/CW/VHO, Sylvania, Danvers, MA) or a combination of regular and wide-spectrum Grolux lights (F20T12/GRO and F20T12/GRO/WS, Sylvania). “Monochromatic” irradiation chambers used for hypocotyl measurements included the following sources: Rc (660 ± 10 nm) and FRc (735 ± 10 nm) from FR- and R-emitting diode light sources (Tennessen et al., 1994), and continuous blue light from fluorescent tubes as previously described (Lagarias et al., 1997). Light fluence rates and spectral quality were determined using a spectroradiometer (model 1800, Li-Cor, Lincoln, NE). All irradiation chambers were maintained in constant temperature- (i.e. 20°C) and humidity-controlled growth chambers. For fluence rate curves, fluence rates were altered using neutral-density filters (Lux no. III, Rosco, Hollywood, CA).
Biochemical Fractionation
Soluble protein extracts for BVR enzyme assays were obtained as previously described (Lagarias et al., 1997). Plant material was frozen in liquid nitrogen, crushed to a powder, and soluble proteins were extracted in extraction buffer (50 mm Tris-HCl, pH 8, 100 mm NaCl, 1 mm EDTA, 1 mm EGTA, 142 mm 2-mercaptoethanol, 1 mm PMSF, and 1% [v/v] DMSO). Cleared soluble protein supernatants were obtained by ultracentrifugation at 100,000g for 15 min. For crude organellar and cytosolic fractionation experiments, 15 to 20 g fresh weight of rosette leaves from 3- to 4-week-old Arabidopsis plants were homogenized in 100 mL of ice-cold grind-resuspension buffer (330 mm sorbitol, 1 mm NaP2O7, 50 mm HEPES, pH 6.8, 2 mm Na2EDTA, 1 mm MgCl2, 1 mm MnCl2, and 5 mm isoascorbate) for 20 s using a polytron homogenizer and a method modified from that previously described (Price et al., 1994). The resulting suspension was filtered through two layers of Miracloth (Calbiochem, San Diego) to obtain the crude cell lysate. This lysate was centrifuged at 4°C in a swinging bucket rotor and brought to 4,000g. When the centrifuge reached 4,000g, it was stopped immediately. The resulting crude organellar pellet was resuspended in wash buffer (330 mm sorbitol, 2 mm Na2EDTA, 1 mm MgCl2, and 30 mm Tricine, pH 8.4) and centrifuged at 1,000g for 5 min to obtain the washed organellar fraction. The supernatant from the initial step was used as the soluble “cytoplasmic” protein fraction. Both fractions were analyzed for BVR enzyme activity and for BVR protein content via immunoblot analysis as described below.
BVR Enzyme and Immunochemical Analyses
BVR-specific enzyme activity of soluble and crude organellar fractions was measured spectrophotometrically as previously described (Lagarias et al., 1997). Specific activities were calculated using equations described previously (Kutty and Maines, 1984), and protein concentrations were determined with the bicinchoninic acid method (Smith et al., 1985) using BSA as a standard. For immunoblot analyses, protein fractions were mixed with an equal volume of preheated 2× SDS sample buffer (125 mm Tris-HCl, pH 6.8, 5% [v/v] SDS, 5% [v/v] 2-mercaptoethanol, 1 mm PMSF, and 1% [v/v] DMSO) and heated for 2 min at 100°C. Protein concentrations in SDS-solubilized crude organellar fractions were determined after methanol-chloroform extraction, as described previously (Lagarias et al., 1997). SDS-PAGE and immunoblot analyses were performed as described previously (Lagarias et al., 1997), except that a 1:5,000 dilution of rat kidney BVR-specific antiserum (kindly provided by Dr. Mahin D. Maines, University of Rochester) was used for the primary incubation.
Hypocotyl Length Measurements
Hypocotyl lengths of seedlings grown on Petri plates under appropriate light conditions were determined by scanning the plant images and then quantifying them using MacBAS 2.0 software (Fuji Medical Systems, Stamford, CT).
Chlorophyll, Protochlorophyll, and Anthocyanin Analyses
Chlorophyll extractions were performed using excised cotyledons from 7-d-old seedlings in N,N-dimethylformamide (Moran, 1982). Concentrations were calculated with equations and extinction coefficients as described previously (Inskeep and Bloom, 1985). Protochlorophyll was extracted from 7-d-old seedlings harvested under green safelights and immersed in N,N-dimethylformamide (Moran, 1982). Protochlorophyll content in extracts was determined spectrofluorometrically. Emissions curves (560–750 nm) with excitation at 438 nm were measured. Integration of emissions curves yielded relative fluorescence values for each sample. Anthocyanins were extracted from whole-plant seedlings using 1% (v/v) HCl in methanol as described previously (Feinbaum and Ausubel, 1988). Pigments were extracted overnight with shaking at 20°C using the method of Rabino and Mancinelli (Rabino and Mancinelli, 1986). Chloroform-water partitioning was performed as described previously (Kerckhoffs et al., 1997). Anthocyanin content was estimated by measuring the A535 minus the A650 of the aqueous phase.
RESULTS
Targeted Expression of BVR in Transgenic Arabidopsis Plants
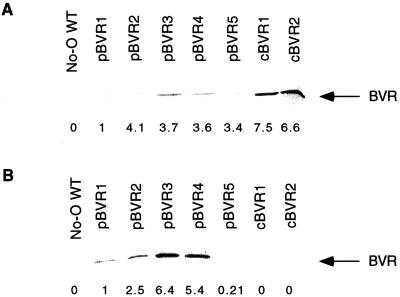
Homozygous “cytosol-targeted” BVR transgenic lines, two of which—cBVR1 and cBVR2—were the subject of the present analyses, were isolated using a protocol similar to that utilized to obtain the five homozygous transgenic lines expressing plastid-targeted BVR, pBVR1 to pBVR5 (Lagarias et al., 1997). Relative BVR activities for total soluble protein extracts from the seven transgenic lines shown in Table I revealed that both cBVR lines possessed BVR activity exceeding that of the highest expressing pBVR line. To ascertain that BVR was properly targeted, cell lysates from the cBVR and pBVR lines were fractionated into soluble “cytoplasmic” protein and crude “plastid” organelle fractions. As shown in Figure 1, immunoblot analyses and BVR enzyme assays revealed that BVR protein was found only in the soluble protein fraction in both cBVR lines. By comparison, a significant amount of BVR was found in the organelle fraction of the pBVR plants, with only one exception (i.e. pBVR5, in which very little BVR was found in the organelle fraction).
Table I.
Relative BVR soluble extract specific activities for light-grown No-O WT and BVR transgenic plants
| Plant Line | Specific Activitya |
|---|---|
| No-O WT | 0.0 |
| pBVR1 | 1.00 |
| pBVR2 | 4.03 |
| pBVR3 | 7.10 |
| pBVR4 | 3.81 |
| pBVR5 | 5.56 |
| cBVR1 | 9.46 |
| cBVR2 | 9.54 |
Seedlings were grown for 9 d at 25°C under continuous Gro-lux/Gro-lux Wide-Spectrum illumination of 129.5 μmol m−2 s−1. Whole seedlings were harvested, frozen with liquid nitrogen, and ground to a fine powder. Soluble proteins were extracted and used for BVR assays as described in “Materials and Methods.”
Values are relative to that of pBVR1 (1.19 × 10−3 IU mg−1).
Figure 1.
Immunoblot of soluble (A) and crude plastid organelle (B) protein fractions from 3- to 4-week-old WT and BVR transgenic plants. An equal amount of total protein (20 μg) was loaded per lane. The numbers below each lane indicate the relative BVR activity measured by BVR enzyme activity assay (see “Materials and Methods”) for soluble and sonicated crude organelle fractions. Values are relative to that measured for pBVR1, which were determined as 7.55 × 10−4 and 7.27 × 10−4 IU mg−1 for soluble and sonicated crude organelle fractions, respectively. No BVR enzyme activity was detected for protein samples from WT plants.
Due to plastid lysis and/or leakage during organelle isolation, the presence of BVR protein in soluble and organelle fractions of the pBVR lines was expected. Assuming that the amount of plastid lysis was similar for each of the five pBVR lines, however, these analyses suggested that the relative efficiency of plastid uptake and/or retention of BVR differed for the five pBVR lines. In this regard, the relative distribution of BVR between the soluble and organelle fraction was quite dissimilar for the five pBVR lines, with the amount of BVR in the plastid increasing according to pBVR5 ≪ pBVR1 < pBVR2 ≪ pBVR4 ≈ pBVR3. Moreover, the molecular size of BVR was 33 kD in all of the pBVR lines, indicating that the plastid transit peptide had been proteolytically processed to give only mature-sized enzyme. Taken together, these analyses indicate that BVR was targeted to plastids in all five pBVR lines, and to the cytoplasm in both cBVR lines.
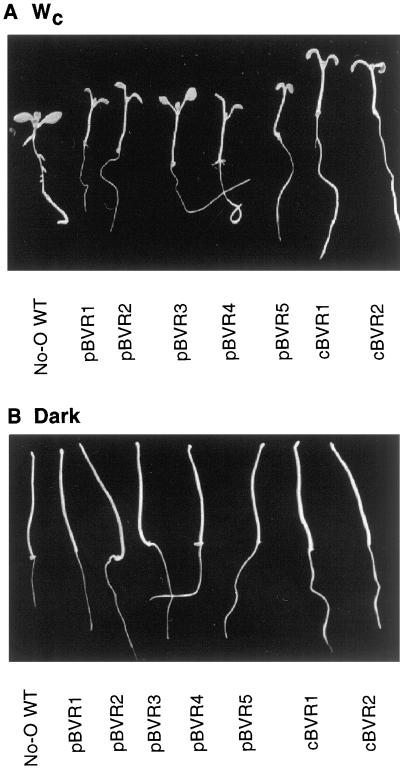
Cytosolic and Plastid-Targeted BVR Expression Similarly Affect Light-Dependent Hypocotyl Growth Inhibition
Phytochrome chromophore-deficient mutant plants exhibit altered light responsiveness throughout their life cycle due to the reduced activities of multiple phytochromes, while dark-grown phytochrome chromophore-deficient seedlings are nearly indistinguishable from wild type (WT) (for review, see Terry, 1997). Consistent with the phenotype of known phytochrome chromophore-biosynthetic mutants, both pBVR and cBVR seedlings possessed elongated hypocotyls under Wc, while the heights of dark-grown transgenic seedlings were not noticeably different from those of WT seedlings (Fig. 2). Upon closer examination, we noted that hypocotyl lengths of dark-grown pBVR seedlings were consistently shorter than No-O WT—an effect that was exaggerated in seedlings grown in the absence of Suc (Table II). This phenomenon was not observed for the cBVR plants. While not as dramatic, dark-grown hy1 seedlings were also slightly shorter than the Landsberg erecta WT (Ler WT; Table II). In view of the small magnitude of this effect, however, the possibility that this reflects a reduced rate of seed germination for the phytochrome chromophore-deficient seedlings cannot be dismissed a priori.
Figure 2.
Dark- and light-grown WT and transgenic BVR seedlings. No-O WT, pBVR (pBVR1–pBVR5), and cBVR seedlings (cBVR1 and cBVR2) were grown for 4 d under Wc of 100 μmol m−2 s−1 at 20°C (A) or in darkness (B).
Table II.
Mean hypocotyl lengths of dark-grown WT and chromophore-deficient plants on Suc-enriched or Suc-free media
| Plant Line | Hypocotyl Length
|
|
|---|---|---|
| +Suc | −Suc | |
| mm | ||
| No-O WT | 8.38 (±0.77) | 11.54 (±1.02) |
| pBVR1 | 7.45 (±1.46) | 9.40 (±1.03) |
| pBVR2 | 8.49 (±1.00) | 9.63 (±1.23) |
| pBVR3 | 8.21 (±1.62) | 9.51 (±1.27) |
| pBVR4 | 7.07 (±1.67) | 10.02 (±1.21) |
| pBVR5 | 7.98 (±1.29) | 11.17 (±0.77) |
| cBVR1 | 8.35 (±1.68) | 11.76 (±1.22) |
| Ler WT | 7.83 (±1.15) | 9.48 (±1.11) |
| hy1 | 7.56 (±1.09) | 8.41 (±1.18) |
Seedlings were grown for 4 d at 20°C in continuous darkness. Values are means (±sd).
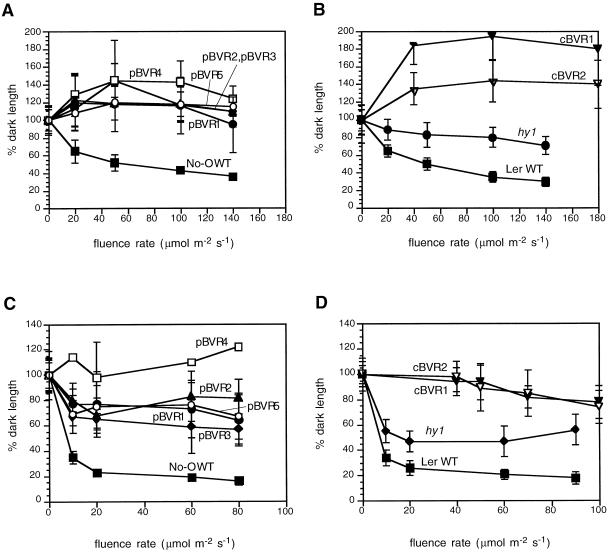
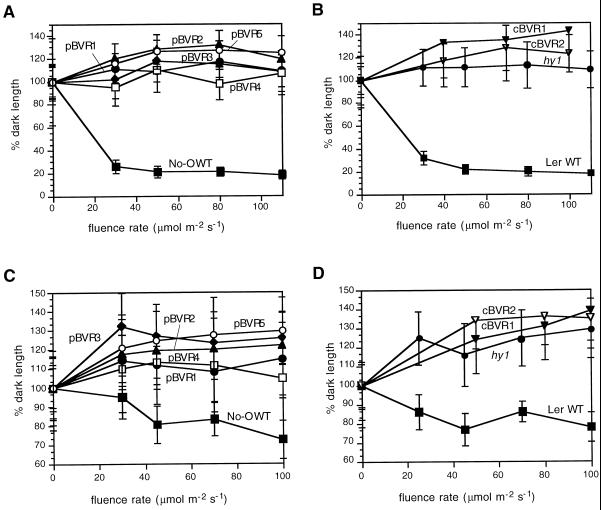
That pBVR and cBVR transgenic plants possess deficiencies in multiple phytochromes was addressed by determination of the fluence rate dependency of hypocotyl growth under different light conditions. In this regard, phyA mutants possess elongated hypocotyls in FRc but not in Rc or Wc, while phyB mutants exhibit elongated hypocotyls in Rc and Wc but not in FRc (Whitelam and Harberd, 1994). Similar to the chromophore-deficient hy1 mutant, all of the pBVR and cBVR lines displayed reduced responsiveness to Wc, Rc, and FRc (Figs. 3 and 4)—fully consistent with the loss of both phyA and phyB activities. Moreover, in both the presence and absence of Suc (Fig. 3; data not shown), all BVR transgenic plants exhibited longer hypocotyls than WT seedlings under all light regimes (including continuous blue light—data not shown). The fluence rate dependencies of hypocotyl length for all five pBVR and both cBVR transgenic lines grown under Wc, Rc, or FRc were also nearly superimposable. Additional experiments revealed no significant differences in low fluence responses (data not shown). By comparison, the hy1 mutant showed a similar, albeit less-dramatic growth inhibition under Wc (Fig. 3).
Figure 3.
Hypocotyl lengths of WT, transgenic BVR, and chromophore-deficient hy1 seedlings under Wc. Transgenic pBVR lines (pBVR1–pBVR5), cBVR lines (cBVR1 and cBVR2), hy1, No-O, and Ler WT seedlings were grown at 20°C on Phytagel medium for 4 d under Wc of various fluence rates. Data points represent means (±sd) of 10 to 50 hypocotyls measured. A and B, 1% (w/v) Suc; C and D, 0% (w/v) Suc.
Figure 4.
Hypocotyl lengths of WT, transgenic BVR, and chromophore-deficient hy1 seedlings under Rc and FRc illumination. Transgenic pBVR lines (pBVR1–pBVR5), cBVR lines (cBVR1 and cBVR2), hy1, No-O, and Ler WT seedlings were grown at 20°C on Phytagel medium containing 1% (w/v) Suc for 4 d under Rc and FRc illumination of various fluence rates. Data points represent means (±sd) of 10 to 50 hypocotyls measured. A and B, FRc; C and D, Rc.
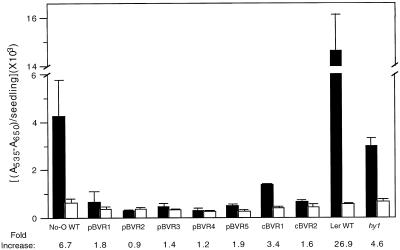
Suc-Stimulated Anthocyanin Accumulation Is Inhibited by BVR Expression
Phytochrome plays a major role in the regulation of anthocyanin synthesis in many plant species (Lange et al., 1970; Mancinelli et al., 1991; Kerckhoffs and Kendrick, 1997). For this reason, we were interested in determining the effect of BVR expression on anthocyanin accumulation in Arabidopsis. Since anthocyanin accumulation is known to peak 4 to 5 d post germination (Kubasek et al., 1992; data not shown), our experiments were performed with 5-d-old light-grown seedlings. Initial experiments were performed using media containing Suc, which we typically use to enhance germination of BVR plants. Figure 5 shows that anthocyanin accumulation in Suc-grown plants was strongly inhibited by the expression of BVR (compare black histograms). This effect was more exaggerated for the pBVR lines (i.e. 90% inhibition). However, it was also observed for cBVR plants. A similar reduction of anthocyanin was detected in light-grown hy1 seedlings compared with the Ler WT (Fig. 5).
Figure 5.
Anthocyanin content of WT, transgenic BVR, and chromophore-deficient hy1 seedlings. Transgenic pBVR lines (pBVR1–pBVR5), cBVR lines (cBVR1 and cBVR2), hy1, No-O, and Ler WT seedlings were grown at 20°C on Phytagel medium containing no Suc (white bars) or 1% (w/v) Suc (black bars) for 5 d under Wc illumination of 100 μmol m−2 s−1. Bars represent the means (±sd) of three independent measurements.
Since other investigations indicate that anthocyanin synthesis in Arabidopsis is primarily controlled by UV and blue light receptors (Kubasek et al., 1992; Jenkins, 1997), we performed experiments using Suc-free media to better mimic experimental conditions. Figure 5 shows that No-O WT plants grown on Suc-free agar media accumulated significantly less anthocyanin compared with No-O plants grown in Suc-containing medium. This result was consistent with the Suc-mediated stimulation of anthocyanin synthesis seen in other ecotypes of Arabidopsis (Tsukaya et al., 1991; Mita et al., 1997). Compared with WT plants, which exhibited a 6.7-fold Suc-mediated increase in anthocyanin accumulation, BVR-expressing lines exhibited a greatly reduced response to Suc (Fig. 5). In addition, the Suc-mediated stimulation of anthocyanin accumulation was significantly smaller for the phytochrome chromophore-deficient hy1 mutant compared with Ler WT (Fig. 5). These results show that holophytochrome and/or bilins are required for Suc-dependent stimulation of anthocyanin accumulation in light-grown Arabidopsis seedlings.
pBVR and cBVR Transgenic Plants Are Early Flowering
It is well established that phytochrome plays a regulatory role in the photoperiodic control of flowering (Jackson and Thomas, 1997). Arabidopsis is a facultative long-day plant, with short-day photoperiods delaying flowering (Coupland, 1997). Transfer of Arabidopsis plants from short- to long-day photoperiods, or interruption of the short-day photoperiod long night with a pulse of light (i.e. night break), has been shown to increase the rate of floral initiation (Halliday et al., 1994; Hempel and Feldman, 1994). Moreover, phyB mutants and the phytochrome chromophore-deficient mutants hy1 and hy2 flower early, while still retaining photoperiod sensitivity (Halliday et al., 1994; Reed et al., 1994). From these and other studies, it appears that phytochromes act to suppress floral initiation (Weigel, 1995).
To examine the effect of cytosol- and plastid-targeted expression of BVR on flowering, we compared the flowering behavior of pBVR3, cBVR1, and No-O WT lines grown under short-day and short-day with a night break photoperiod regimes. As noted previously, the highest expressing plastid-targeted BVR line, pBVR3, was chronologically delayed in its flowering response (Table III)—a result that we attributed to a general reduction in photosynthetic capacity due to a chlorophyll deficiency (Lagarias et al., 1997; also see below). In accordance with this hypothesis, cBVR1, which had nearly WT levels of chlorophyll, was not chronologically delayed in its flowering response under short-day conditions. However, as depicted by the number of rosette leaves at bolting, both pBVR3 and cBVR1 flowered earlier (i.e. with fewer leaves) than No-O WT plants under both short-day and short-day with a night break photoperiods (Table III). Like hy1, pBVR3 and cBVR1 lines both retained photoperiod responsivity, as shown by the promotive effect of the night break on floral initiation (i.e. as judged by the reduction in the number of rosette leaves). These data suggest that plastid-targeted and cytosolic expression of BVR affect flowering behavior in a qualitatively similar manner.
Table III.
Flowering responses of transgenic BVR, chromophore-deficient hy1 mutant, and WT No-O and Ler plants under short-day (SD) and short-day/night break (SD/NB) photoperiods
| Plant Line | SD
|
SD/NB
|
||
|---|---|---|---|---|
| DTFa | NRLb | DTF | NRL | |
| No-O WT | 114 (±8) | 51 (±7) | 102 (±8) | 46 (±7) |
| pBVR3 | 127 (±5) | 33 (±5) | 118 (±6) | 28 (±4) |
| cBVR1 | 113 (±17) | 44 (±6) | 122 (±8) | 38 (±5) |
| Ler WT | 62 (±5) | 53 (±5) | 59 (±2) | 22 (±2) |
| hy1 | 66 (±3) | 34 (±12) | 54 (±4) | 9 (±1) |
DTF, Days until first flower bud appeared.
NRL, Number of rosette leaves at appearance of first flower bud.
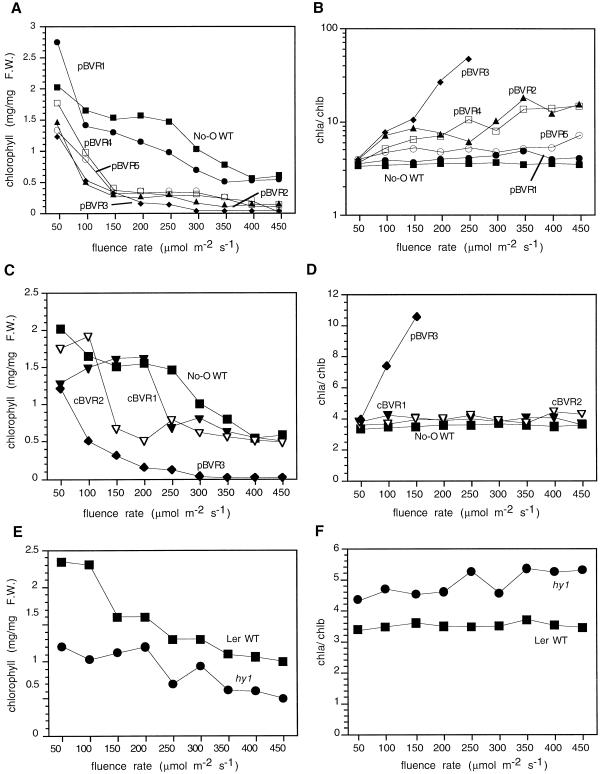
Fluence Rate Dependence of Chlorophyll Accumulation Depends on the Subcellular Targeting of BVR
In contrast to the other phenotypes examined, chlorophyll accumulation in BVR transgenic plants was strongly dependent on the subcellular localization of BVR. As noted previously, plastid-targeted BVR expression yielded plants whose chlorophyll accumulation profiles were very sensitive to increasing light intensity (Lagarias et al., 1997). With the exception of the poorest expressing pBVR1 line, pBVR plants exhibited a severe reduction in total chlorophyll levels that was coincident with an increased chlorophyll a/b ratio, especially at high fluence rates (Fig. 6, A and B). By comparison, cBVR plants also possessed lower levels of total chlorophyll than the WT at low light intensities (i.e. 100–300 μmol m−2 s−1); however, at higher fluence rates (i.e. >350 μmol m−2 s−1), chlorophyll levels of both cBVR lines were indistinguishable from the WT (Fig. 6C).
Figure 6.
Fluence-rate-dependent chlorophyll accumulation of WT, transgenic BVR, and chromophore-deficient hy1 seedlings. Transgenic pBVR lines (pBVR1–pBVR5), cBVR lines (cBVR1 and cBVR2), hy1, No-O, and Ler WT seedlings were grown at 20°C on Phytagel medium containing 1% (w/v) Suc for 7 d under Wc of various fluence rates. Data points represent means obtained from three measurements. A, C, and E, Total chlorophyll; B, D, and F, Chl a/b ratios. F.W., Fresh weight.
With regard to the fluence rate dependence of the chlorophyll a/b ratio in the pBVR lines, the threshold intensity at which this ratio increased significantly correlated well with the level of BVR expression. Indeed, low-fluence light effected a dramatic increase in this ratio for the highest expressing line, pBVR3—a threshold that was shifted to higher fluences for the moderate-expressing pBVR2 and pBVR4 lines (Fig. 6B). Consistent with this correlation, the lowest expressing pBVR1 line showed no fluence-rate-dependent increase in the chlorophyll a/b ratio, as was observed for the WT and both cBVR lines (Fig. 6, compare B and D). The behavior of the pBVR5 line, which despite its high level of BVR expression required very high fluence rates to effect a significant increase in the chlorophyll a/b ratio, appeared to be at variance with the generalization that the BVR expression level was correlated with the loss of light tolerance. As we shown in Figure 1, BVR protein accumulated poorly in plastids of pBVR5 plants, which presumably accounted for this discrepancy.
For comparative purposes, chlorophyll accumulation in hy1 and Ler WT was also analyzed (Fig. 6, E and F). With regard to chlorophyll content, hy1 plants accumulated less chlorophyll than Ler WT (Fig. 6E). As previously noted, but extended to higher fluences rates in Figure 6F, the chlorophyll a/b ratio of hy1 seedlings was higher than Ler WT, but showed no significant responses to elevated light fluences (Lagarias et al., 1997).
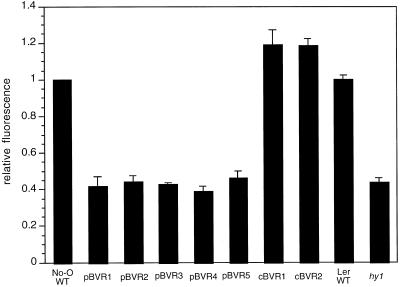
Protochlorophyll Accumulation in Dark-Grown Seedlings Depends on the Subcellular Targeting of BVR
Because of reports of reduced levels of protochlorophyll in phytochrome chromophore-deficient plants (Terry, 1997; Terry and Kendrick, 1999), we examined the effect of pBVR- and cBVR-mediated phytochrome chromophore deficiency on protochlorophyll accumulation in dark-grown seedlings. As depicted in Figure 7, plastid-targeted BVR expression led to a 60% reduction in protochlorophyll levels in pBVR seedlings compared with No-O WT. A similar level of reduction in protochlorophyll content was observed for hy1 seedlings compared with the Ler WT ecotype. By contrast, cBVR lines exhibited WT levels of protochlorophyll (Fig. 7). Thus, a basal level of BV and/or PΦB within the chloroplast appears to be necessary for the accumulation of WT levels of protochlorophyll during skotomorphogenesis of Arabidopsis seedlings.
Figure 7.
Protochlorophyll accumulation in WT, transgenic BVR, and chromophore-deficient hy1 seedling extracts. Transgenic pBVR lines (pBVR1–pBVR5), cBVR lines (cBVR1 and cBVR2), hy1, No-O, and Ler WT seedlings were grown at 20°C on Phytagel medium containing 1% (w/v) Suc for 7 d in darkness. Bars indicate the relative fluorescence values calculated by normalizing to the fluorescence value of No-O WT seedling extracts. Values represent the means (± sd) of three independent measurements.
DISCUSSION
BVR-Expressing Transgenic Plants Exhibit Phytochrome-Deficient Phenotypes
These studies demonstrate that both cytosolic and plastid-targeted expression of BVR significantly alter light-mediated growth and development of Arabidopsis plants. As with phytochrome chromophore-deficient mutants, light-grown BVR transgenic plants possess elongated hypocotyls, exhibit decreased light-dependent anthocyanin synthesis, have poorly germinating seeds, and flower with fewer leaves. In particular, phytochrome-mediated inhibition of hypocotyl elongation under Rc and FRc was effectively absent in both cytosol- and plastid-targeted BVR transgenic plant lines in a manner similar to that observed for hy1 and hy2 mutants (Koornneef et al., 1980; Chory et al., 1989). Regardless of the ultimate subcellular destination for BVR, light-modulated inhibition of hypocotyl elongation was lacking even in the lowest expressing lines. A qualitatively similar result was observed for the initiation of flowering. Although pBVR plants were chronologically delayed in the onset of flowering, both pBVR and cBVR lines flowered earlier than WT plants, as determined by the number of rosette leaves at bolting. These findings are in good agreement with the role of phytochromes in the suppression of flowering in light-grown Arabidopsis plants (Weigel, 1995; Coupland, 1997).
Targeted Expression of BVR Reveals a Regulatory Role for Linear Tetrapyrroles within the Plastid Compartment
Of all the phenotypes examined, a striking dependence on the subcellular localization of BVR was observed for chlorophyll and protochlorophyll accumulation. As was previously reported and corroborated here, plastid-targeted BVR transgenic plants were intolerant to elevated light fluences—displaying a fluence-rate-dependent reduction in chlorophyll levels together with an increase in the chlorophyll a/b ratio (Lagarias et al., 1997). By contrast, despite the higher level of BVR expression in both cBVR lines, cytoplasmic targeting of BVR never produced light-intolerant plants. There are many possible explanations to account for the light-intolerant phenotype of pBVR plants. This phenotype might reflect the greater reduction in phytochrome levels in these plants compared with cBVR plants, since PΦB is synthesized within the plastid (Terry et al., 1993). A small but sufficient level of photoactive phytochrome may thus be produced in the cBVR lines due to the inability of BVR to metabolically inactivate all of the PΦB prior to its “competitive” assembly with apophytochrome in the cytosol. This scenario suggests that higher levels of cBVR expression should be able to reduce the levels of phytochrome sufficiently to induce the light-intolerant phenotype. Since light-intolerant cBVR plants were not isolated in our studies (including additional cBVR lines not described here), we favor the hypothesis that BV and/or PΦB perform a key regulatory role within the plastid compartment.
Since the entire pathway of PΦB biosynthesis is plastid localized (Terry, 1997; Terry et al., 1993), the plastid bilin-regulatory hypothesis suggests that phytochrome chromophore-deficient mutants should be intolerant to elevated light fluences. This has been supported for the phytochrome chromophore-deficient mutants of pea (Weller et al., 1996, 1997), tobacco (Kraepiel et al., 1994), and tomato (Koornneef et al., 1985; Van Tuinen et al., 1996). While our investigations on the hy1 mutant of Arabidopsis did not display the striking fluence-rate-dependent reduction of chlorophyll seen for pBVR transgenic plants, this difference might be due to the more effective reduction of plastid bilin levels by BVR. In this regard, the recent cloning of HY1 has revealed the presence of a second HY1-related gene in the Arabidopsis genome that likely accounts for the leakiness of all known hy1 alleles (Davis et al., 1999; Muramoto et al., 1999).
Recent studies have established that HY1 encodes a plastid-localized heme oxygenase (Davis et al., 1999; Muramoto et al., 1999). For this reason, the pool sizes of both BV and PΦB are expected to be reduced in plastids of hy1 mutants. By analogy to the known PΦB biosynthesis mutants in pea and tomato (Koornneef et al., 1985; Van Tuinen et al., 1996; Weller et al., 1996, 1997), HY2 likely encodes the catalytic subunit of phytochromobilin synthase, the enzyme that converts BV to PΦB (Terry and Lagarias, 1991). Plastid levels of PΦB but not BV are thus expected to be reduced in hy2 plants. Based on the chlorophyll-deficient phenotype of hy2 plants (Chory et al., 1989), we propose that the lack of PΦB within the plastid is responsible for the light-intolerant, chlorophyll-deficient phenotype of pBVR transgenic plants. Based on this rationale, we speculate that a phytochrome-like molecule(s) is present in plastids. The presence of a phytochrome in the plastid compartment has received support from the recent finding of phytochromes in cyanobacteria (Hughes et al., 1997; Yeh et al., 1997), ancestors of which are thought to be the evolutionary precursor of higher plant chloroplasts.
An alternative hypothesis to explain the light-intolerant phenotype of pBVR plants is based on recent studies of the phytochrome chromophore-deficient aurea and yellow-green-2 mutants of tomato (Terry and Kendrick, 1999). In that study, evidence was presented that the reduced chlorophyll accumulation in these mutants is due to an increase in heme levels, which feedback-inhibits the synthesis of 5-aminolevulinic acid, the first committed precursor of all known tetrapyrroles in plants. The chlorophyll-deficient phenotype of aurea and yellow-green-2 mutants, which is also more pronounced under elevated light fluences, thus arises from the insufficient biosynthesis of chlorophyll to compensate for the light-dependent turnover of the photosynthetic light-harvesting apparatus. This hypothesis is understandable for plants that lack heme oxygenase (i.e. yellow-green-2) or phytochromobilin synthase (i.e. aurea), in which the buildup of heme would be expected.
How pBVR expression might effect an increased heme level is less intuitively obvious. It is conceivable that the reduced level of bilin in the plastids of pBVR plants could affect the stability of phytochromobilin synthase due to reduced substrate stabilization of the enzyme. Substrate level stabilization has been documented for numerous enzymes of important metabolic pathways (Kuhn-Velten and Lohr, 1996). The indirect effect of reduced bilin level on heme pool size could thus arise via destabilization of a phytochromobilin synthase-heme oxygenase complex, which may be required to efficiently drive heme oxygenase, an enzyme known to be strongly product inhibited. In this way, pBVR expression could lead to increased plastid heme levels along with a concomitant inhibition of chlorophyll biosynthesis. A rigorous test of this hypothesis requires antibodies to heme oxygenase and phytochromobilin synthase, which are not presently available.
BVR Expression Disrupts Suc-Mediated Signaling in Arabidopsis
Sugars have been shown to play a role in distinct aspects of light-mediated de-etiolation in plants. A number of experiments have shown that the presence of Suc is correlated with a significant increase in anthocyanin accumulation (Tsukaya et al., 1991; Mita et al., 1997) and inhibition of hypocotyl elongation, most likely as a result of sugar perception by sugar sensing moieties (Jang and Sheen, 1997). Chromophore-deficient pBVR and hy1 seedlings exhibited a reduced inhibition of hypocotyl growth by Suc compared with their respective WT seedlings (Table II and data not shown). Additionally, the Suc-dependent stimulation of anthocyanin synthesis in light-grown plants was largely disrupted by BVR expression, an effect also observed for hy1. This effect was even more pronounced in the hy1phyAphyB triple-mutant background (data not shown). These results support the previously postulated existence of a phytochrome-mediated component of sugar signaling, including potential interactions with sugar regulators such as hexokinase or Suc transporters (Dijkwel et al., 1996). Additionally, these data provide further evidence for an intrinsic connection between Suc and light-signaling pathways, as uncovered in the study of Suc-uncoupled (sun) mutants (Dijkwel et al., 1997).
Are Phytochromes Active in the Dark?
The central dogma of the phytochrome field is that Pfr is the active form and that Pr, the form of phytochrome synthesized in dark-grown tissues, is biologically inactive. However, several reports have indicated an active regulatory role for Pr in such processes as gravitropism, germination, flowering, and hypocotyl elongation (Liscum and Hangarter, 1993; Reed et al., 1993, 1994; Shinomura et al., 1994; Saefkow et al., 1995). Unfortunately, many of these experiments are confounded by experimental protocols that include red-light pre-treatment (Liscum and Hangarter, 1993; Reed et al., 1993) or growth under FRc (Reed et al., 1994; Shinomura et al., 1994) in which some Pfr is produced (Smith and Whitelam, 1990; Robson and Smith, 1996). Still other investigations suggest that Pr can influence a number of physiological responses under continuous darkness, conditions that avoid the formation of Pfr during germination and growth (Saefkow et al., 1995). However, with these experiments the potential for carryover of preexisting Pfr in the embryo or seed is unresolved.
The present study shows that BVR-expressing plants display measurable phenotypic responses when germinated and grown in continuous darkness. Most notable is the reduction of protochlorophyll in dark-grown pBVR and hy1 seedlings, a phenomenon that has also been noted for other chromophore-deficient mutants (Terry, 1997; Terry and Kendrick, 1999). This phenotype could result from a general BVR-induced defect in protochlorophyllide biosynthesis, the lack of preexisting Pfr in the embryo, or the lack of Pr regulation of PChl accumulation in these seedlings. As these seedlings are capable of accumulating WT levels of chlorophyll under low-light conditions, and because cytosolic BVR expression has no observable negative effect on PChl accumulation, the third hypothesis is the most likely. It is also conceivable that, aside from their role as phytochrome chromophore precursors, bilins perform a regulatory role during etioplast development in dark-grown plants.
Future Directions: Selective Expression of BVR in Transgenic Plants
The present study shows that both subcellular localization and the level of BVR expression in transgenic plants effect distinct combinations of phenotypic responses in the growth and development of Arabidopsis. Regulated expression of BVR is expected to facilitate selective control of phytochrome levels, which should enable us to distinguish between cell-autonomous and cell-to-cell signaling systems that are mediated by phytochrome, as well as to localize and thereby regulate sites of photoperception within the plant. Thus, cell- and tissue-specific expression of BVR should impart more information in regard to distinct sites of photoperception of phytochrome and its role in specific aspects of plant growth and development.
ACKNOWLEDGMENTS
We thank Lucy Pham for performing some of the hypocotyl length analyses, Arnold Bloom for the use of the R and FR LED sources, and Mike McDowell for critical reading of the manuscript.
Footnotes
This research was supported in part by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (grant no. AMD–9801768 to J.C.L.).
LITERATURE CITED
- Bechtold N, Ellis J, Pelletier G. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. CR Acad Sci Ser III. 1993;316:1194–1199. [Google Scholar]
- Becker D. Binary vectors which allow the exchange of plant selectable markers and reporter genes. Nucleic Acids Res. 1990;18:203. doi: 10.1093/nar/18.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington JC, Freed DD, Oh C-C. Expression of potyviral polyproteins in transgenic plants reveals three proteolytic activities required for complete processing. EMBO J. 1990;9:1347–1353. doi: 10.1002/j.1460-2075.1990.tb08249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J, Peto C, Ashbaugh M, Saganich R, Pratt L, Ausubel F. Different roles for phytochrome in etiolated and green plants deduced from characterization of Arabidopsis thaliana mutants. Plant Cell. 1989;1:867–880. doi: 10.1105/tpc.1.9.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PhyD and PhyE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Coupland G. Regulation of flowering by photoperiod in Arabidopsis. Plant Cell Environ. 1997;20:785–789. [Google Scholar]
- Davis SJ, Kurepa J, Vierstra RD. The Arabidopsis thaliana HY1 locus, required for phytochrome-chromophore biosynthesis, encodes a protein related to heme oxygenases. Proc Natl Acad Sci USA. 1999;96:6541–6546. doi: 10.1073/pnas.96.11.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel PP, Huijser C, Weisbeek PJ, Chua N-H, Smeekens SCM. Sucrose control of phytochrome A signaling in Arabidopsis. Plant Cell. 1997;9:582–595. doi: 10.1105/tpc.9.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel PP, Kock P, Bezemer R, Weisbeek PJ, Smeekens SCM. Sucrose represses the developmentally controlled transient activation of the plastocyanin gene in Arabidopsis thaliana seedlings. Plant Physiol. 1996;110:455–463. doi: 10.1104/pp.110.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Chory J. Light control of plant development. Annu Rev Cell Dev Biol. 1997;13:203–229. doi: 10.1146/annurev.cellbio.13.1.203. [DOI] [PubMed] [Google Scholar]
- Feinbaum RL, Ausubel FM. Transcriptional regulation of the Arabidopsis thaliana chalcone synthase gene. Mol Cell Biol. 1988;8:1985–1992. doi: 10.1128/mcb.8.5.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M. Phytochromes: their molecular species, gene families, and functions. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:617–645. [Google Scholar]
- Halliday KJ, Koornneef M, Whitelam GC. Phytochrome B and at least one other phytochrome mediate the accelerated flowering response of Arabidopsis thaliana L. to low red/far-red ratio. Plant Physiol. 1994;104:1311–1315. doi: 10.1104/pp.104.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel FD, Feldman LJ. Bi-directional inflorescence development In Arabidopsis-thaliana: acropetal initiation of flowers and basipetal initiation of paraclades. Planta. 1994;192:276–286. [Google Scholar]
- Hughes J, Lamparter T, Mittmann F, Hartmann E, Gartner W, Wilde A, Borner T. A prokaryotic phytochrome (Scientific Correspondence) Nature. 1997;386:663. doi: 10.1038/386663a0. [DOI] [PubMed] [Google Scholar]
- Inskeep WP, Bloom PR. Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Thomas B. Photoreceptors and signals in the photoperiodic control of development. Plant Cell Environ. 1997;20:790–795. [Google Scholar]
- Jang JC, Sheen J. Sugar sensing in higher plants. Trends Plant Sci. 1997;2:208–214. [Google Scholar]
- Jenkins GI. UV and blue light signal transduction in Arabidopsis. Plant Cell Environ. 1997;20:773–778. doi: 10.1046/j.1365-3040.1997.d01-105.x. [DOI] [PubMed] [Google Scholar]
- Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Dordrecht, The Netherlands: Martinus Nijhoff; 1994. [Google Scholar]
- Kerckhoffs LH, Kendrick RE. Photocontrol of anthocyanin biosynthesis in tomato. J Plant Res. 1997;110:141–149. doi: 10.1007/BF02506853. [DOI] [PubMed] [Google Scholar]
- Kerckhoffs LHJ, Schreuder MEL, VanTuinen A, Koornneef M, Kendrick RE. Phytochrome control of anthocyanin biosynthesis in tomato seedlings: analysis using photomorphogenic mutants. Photochem Photobiol. 1997;65:374–381. [Google Scholar]
- Koornneef M, Cone JW, Dekens RG, O'Herne-Robers EG, Spruit CJP, Kendrick RE. Photomorphogenetic responses of long hypocotyl mutants of tomato. J Plant Physiol. 1985;120:153–165. [Google Scholar]
- Koornneef M, Kendrick RE. Photomorphogenic mutants of higher plants. In: Kendrick R, Kronenberg G, editors. Photomorphogenesis in Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 601–628. [Google Scholar]
- Koornneef M, Rolff E, Spruit CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana L. Heynh Z Pflanzenphysiol. 1980;100:147–160. [Google Scholar]
- Kraepiel Y, Jullien M, Cordonnier-Pratt MM, Pratt L. Identification of two loci involved in phytochrome expression in Nicotiana plumbaginifolia and lethality of the corresponding double mutant. Mol Gen Genet. 1994;242:559–565. doi: 10.1007/BF00285279. [DOI] [PubMed] [Google Scholar]
- Kubasek WL, Shirley BW, Mckillop A, Goodman HM, Briggs W, Ausubel FM. Regulation of flavonoid biosynthetic genes in germinating Arabidopsis seedlings. Plant Cell. 1992;4:1229–1236. doi: 10.1105/tpc.4.10.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn-Velten WN, Lohr JB. Ligand dependence of cytochrome P450c17 protection against proteolytic inactivation: structural, methodological and functional implications. FEBS Lett. 1996;388:21–25. doi: 10.1016/0014-5793(96)00466-8. [DOI] [PubMed] [Google Scholar]
- Kutty RK, Maines MD. Hepatic heme metabolism: possible role of biliverdin in the regulation of heme oxygenase activity. Biochem Biophys Res Commun. 1984;122:40–46. doi: 10.1016/0006-291x(84)90436-4. [DOI] [PubMed] [Google Scholar]
- Lagarias DM, Crepeau MW, Maines MD, Lagarias JC. Regulation of photomorphogenesis by expression of mammalian biliverdin reductase in transgenic Arabidopsis plants. Plant Cell. 1997;9:675–788. doi: 10.1105/tpc.9.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange H, Shropshire WJ, Mohr H. An analysis of phytochrome-mediated anthocyanin synthesis. Plant Physiol. 1970;47:649–655. doi: 10.1104/pp.47.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Hangarter RP. Genetic evidence that the red-absorbing form of phytochrome-B modulates gravitropism in Arabidopsis thaliana. Plant Physiol. 1993;103:15–19. doi: 10.1104/pp.103.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli AL, Rossi F, Moroni A. Cryptochrome, phytochrome, and anthocyanin production. Plant Physiol. 1991;96:1079–1085. doi: 10.1104/pp.96.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita S, Murano N, Akaike M, Nakamura K. Mutants of Arabidopsis thaliana with pleitropic effects on the expression of the gene for beta-amylase and on the accumulation of anthocyanin that are inducible by sugars. Plant J. 1997;11:841–851. doi: 10.1046/j.1365-313x.1997.11040841.x. [DOI] [PubMed] [Google Scholar]
- Moran R. Formulae for determination of chlorophyllous pigments extracted with dimethylformamide. Plant Physiol. 1982;69:1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto T, Kohchi T, Yokota A, Hwang I, Goodman HM. The Arabidopsis photomorphogenetic mutant hy1 is deficient in phytochrome chromophore biosynthesis as a result of a mutation in a plastid heme oxygenase. Plant Cell. 1999;11:335–348. doi: 10.1105/tpc.11.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LH. Phytochromes: differential properties, expression patterns and molecular evolution. Photochem Photobiol. 1995;61:10–21. [Google Scholar]
- Price CA, Hadjeb N, Newman L, Reardon EM. Isolation of chloroplasts and chloroplast DNA. In: Gelvin S, Schilperoort R, editors. Plant Molecular Biology Manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 1–15. [Google Scholar]
- Quail PH. Phytochrome genes and their expression. In RE Kendrick, GHM Kronenberg, eds, Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 71–104. [Google Scholar]
- Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D. Phytochromes: photosensory perception and signal transduction. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- Rabino I, Mancinelli AL. Light, temperature and anthocyanin production. Plant Physiol. 1986;81:922–924. doi: 10.1104/pp.81.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red-far-red light receptor phytochrome-B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson PRH, Smith H. Genetic and transgenic evidence that phytochromes A and B act to modulate the gravitropic orientation of Arabidopsis thaliana hypocotyls. Plant Physiol. 1996;110:211–216. doi: 10.1104/pp.110.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saefkow RL, Alliston TN, Shinkle JR. Absence of PHYB inhibits hypocotyl elongation in dark-grown LH cucumber seedlings: an active role for PrB. Plant Cell Environ. 1995;18:831–835. [Google Scholar]
- Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Chory J, Furuya M. The induction of seed germination in Arabidopsis thaliana is regulated principally by phytochrome B and secondarily by phytochrome A. Plant Physiol. 1994;104:363–371. doi: 10.1104/pp.104.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Physiological and ecological function within the phytochrome family. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:289–315. [Google Scholar]
- Smith H, Whitelam GC. Phytochrome, a family of photoreceptors with multiple physiological roles. Plant Cell Environ. 1990;13:695–707. [Google Scholar]
- Smith PK, Krohn RI, Hemanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olsen BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tennessen D, Singsaas EL, Sharkey TD. Light-emitting diodes as a light source for photosynthesis research. Photosynth Res. 1994;39:85–92. doi: 10.1007/BF00027146. [DOI] [PubMed] [Google Scholar]
- Terry MJ. Phytochrome chromophore-deficient mutants. Plant Cell Environ. 1997;20:740–745. [Google Scholar]
- Terry MJ, Kendrick RE. Feedback inhibition of chlorophyll synthesis in the phytochrome chromophore-deficient aurea and yellow-green-2 mutants of tomato. Plant Physiol. 1999;119:143–152. doi: 10.1104/pp.119.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MJ, Lagarias JC. Holophytochrome assembly: coupled assay for phytochromobilin synthase in organello. J Biol Chem. 1991;266:22215–22221. [PubMed] [Google Scholar]
- Terry MJ, Wahleithner JA, Lagarias JC. Biosynthesis of the plant photoreceptor phytochrome. Arch Biochem Biophys. 1993;306:1–15. doi: 10.1006/abbi.1993.1473. [DOI] [PubMed] [Google Scholar]
- Tsukaya H, Oshima T, Naito S, Chino M, Komeda Y. Sugar-dependent expression of the CHS-A gene for chalcone synthase from petunia in transgenic Arabidopsis. Plant Physiol. 1991;97:1414–1421. doi: 10.1104/pp.97.4.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tuinen A, Hanhart C, Kerckhoffs LHJ, Nagatani A, Boylan MT, Quail PH, Kendrick RE, Koornneef M. Analysis of phytochrome-deficient yellow-green-2 and aurea mutants of tomato. Plant J. 1996;9:173–182. [Google Scholar]
- Weigel D. The genetics of flower development: from floral induction to ovule morphogenesis. Annu Rev Genet. 1995;29:19–39. doi: 10.1146/annurev.ge.29.120195.000315. [DOI] [PubMed] [Google Scholar]
- Weller JL, Terry MJ, Rameau C, Reid JB, Kendrick RE. The phytochrome-deficient pcd1 mutant of pea is unable to convert heme to biliverdin IXα. Plant Cell. 1996;8:55–67. doi: 10.1105/tpc.8.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JL, Terry MJ, Reid JB, Kendrick RE. The phytochrome-deficient pcd2 mutant of pea is unable to convert biliverdin IXα to 3Z-phytochromobilin. Plant J. 1997;11:1171–1186. doi: 10.1105/tpc.8.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Devlin PF. Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Environ. 1997;20:752–758. [Google Scholar]
- Whitelam GC, Harberd NP. Action and function of phytochrome family members revealed through the study of mutant and transgenic plants. Plant Cell Environ. 1994;17:615–625. [Google Scholar]
- Yeh K-C, Wu S-H, Murphy JT, Lagarias JC. A cyanobacterial phytochrome two-component light sensory system. Science. 1997;277:1505–1508. doi: 10.1126/science.277.5331.1505. [DOI] [PubMed] [Google Scholar]