Abstract
Introduction:
Women have lower rates of quitting than men with both bupropion and nicotine replacement. It is unknown whether varenicline demonstrates differential efficacy for men and women. The purpose of this study was to conduct the first comprehensive meta-analysis of clinical trial data examining sex differences in the efficacy of varenicline for smoking cessation.
Methods:
Searching MEDLINE, EMBASE, and PsychINFO, 17 of 43 clinical trials of varenicline for smoking cessation published through December 31, 2014 were low-bias randomized double-blind placebo-controlled trials. Data ( n = 6710 smokers, 34% female, n = 16 studies, 96% of available data) was analyzed with Metafor program in R. Outcome endpoints were 7-day point-prevalence (PP) and continuous-abstinence (CA) at week 12 (end of treatment), week 24 (6-month follow-up), and week 52 (12-month follow-up).
Results:
Using placebo, women were less likely than men to quit (PP-12, CA-24; P < .05 for sex). Using varenicline, similar rates of abstinence for men and women were demonstrated for all six outcomes (eg, PP-12 abstinence rates were 53% in both women and men). Varenicline versus placebo outcomes demonstrated that varenicline was more effective for women for short and intermediate outcomes (PP-12, CA-12, CA-24; P < .05 sex × medication interaction). For end-of-treatment PP, varenicline was 46% more effective for women. For continuous abstinence, varenicline was 34% (CA-12) and 31% (CA-24) more effective for women.
Conclusions:
Unlike other smoking cessation medications, varenicline demonstrated greater efficacy among women smokers for short and immediate-term outcomes and equal efficacy for 1-year outcomes. Varenicline may be particularly useful for reducing the sex disparity typically seen in rates of smoking cessation.
Implications:
Varenicline is currently the most effective FDA-approved smoking cessation medication and this is the first demonstration that women compared with men have a preferred therapeutic response for a smoking cessation medication when considering short-term outcomes. Importantly, this is also the first demonstration that women have similar rates of quitting to men when considering longer-term, 1-year outcomes.
Introduction
Tobacco use is the leading preventable cause of mortality and morbidity in the United States resulting in 556 000 deaths per year 1 and an annual economic burden of $96 billion in medical expenses and $97 billion in lost productivity. 2 Currently, 21.6% of men and 16.5% of women in the United States are smokers. 2 While both men and women who smoke experience significant smoking related diseases and greater mortality than nonsmokers, 3 women exhibit greater vulnerability than men to certain serious health consequences of smoking (eg, lung cancer, heart disease). 4 , 5 Additionally, women experience sex-specific consequences including altered menstrual function, infertility, ectopic pregnancy, earlier menopause, and cancer of the cervix. 6
Smoking cessation can prevent and reduce many of the harmful consequences of smoking, and successful smoking cessation exerts greater cardiovascular 7 and respiratory 8 benefits for women compared to men. However, despite being more likely to report a quit attempt than men, 9 women are less successful in quitting smoking. This disparity in quitting has been found in numerous clinical trial investigations. 10 , 11 In pooled analysis of clinical trial data, women taking placebo were up to 50% less likely to quit than men. 11 Some population based investigations support similar findings, 12 , 13 although findings have been more mixed (Jarvis et al. 14 ). Importantly, studies which isolate single quit attempts have almost unanimously found that women have more difficulty sustaining abstinence, even when accounting for other forms of tobacco use. 12 Data from the International Tobacco Control (ITC) Four Country Survey ( n = 7825) 12 determined that women had 41% lower odds than men to achieve 30-day abstinence from smoking, when quitting “cold-turkey” (ie, without the use of smoking cessation medications). Findings from the National Epidemiologic Survey on Alcohol and Related Conditions ( n = 33 309) identified that women were 44% more likely than men to have relapsed back to smoking over a 3-year period. 13
Compounding the sex disparity in quitting are data finding differential efficacy of smoking cessation medications across women and men. Studies have demonstrated that women may be less responsive to nicotine replacement therapy as a cessation aid, 15 , 16 and findings for sex differences in bupropion efficacy have been mixed. 10 , 17 , 18 Smith et al. 12 examined sex differences in smoking cessation medication effectiveness that used population-based data from smokers attempting to quit in real-world contexts. Results demonstrated that the use of any medication attenuated the sex difference in the likelihood of successfully quitting smoking, supporting the promotion of smoking cessation medication use among women.
Varenicline (Chantix), a nicotinic acetylcholine receptor partial agonist, was approved as a first-line medication for nicotine dependence in the United States by the Food and Drug Administration (FDA) in 2006. In preclinical studies, varenicline reduces nicotine self-administration, lowers progressive ratio break points, and substitutes for nicotine. 19 In humans, varenicline reduces tobacco craving, withdrawal symptoms, and the reinforcing effects of smoking relative to placebo and other FDA-approved treatments for smoking including bupropion and transdermal nicotine patch. 10–22 Clinically, varenicline demonstrates some of the highest smoking cessation rates compared to placebo and other FDA-approved treatments for nicotine dependence (eg, bupropion, transdermal nicotine patch). 20 , 21 , 23 , 24 Two Phase-III investigations demonstrated that varenicline increased the rate of end-of-treatment continuous-abstinence (CA) by an odds of 3.85. 20 , 21 A Cochrane review determined that the pooled relative risk ratio for continuous or sustained abstinence at 6 months or longer for varenicline with standard dosing (2mg/d) versus placebo was 2.27 (95% CI = 2.02–2.55; n = 6166). 24
To date, 11 available studies of varenicline investigate sex differences in outcomes 20 , 25–34 with six of these studies providing limited data by sex. 20 , 25–29 While these studies find no sex differences in varenicline outcomes, it is unclear if these studies had sufficient statistical power to examine sex differences in medication efficacy. Supported by the Institute of Medicine, National Institutes of Health, and the Federal Drug Administration, there is a growing awareness, as well as federal regulation prompting the consideration of sex and gender in treatment development, efficacy, and reporting. 35–37 The primary objective of this study was to conduct the first meta-analytic review determining if sex differences exist in the efficacy of varenicline for smoking cessation.
Methods
Data Sources and Searches
A review protocol for this investigation is available from the authors upon request. PRISMA guidelines were followed ( www.prisma-statement.org/statement.htm ). Clinical trials of varenicline for smoking cessation published through December 31, 2014 were identified through MEDLINE, EMBASE, and PsycINFO searches in May 2013 and January 2015. The search terms “clinical trial,” “varenicline,” and “smoking cessation” were searched for in the following fields: abstracts, titles, substance words, subject headings, keywords, concept words, and unique identifiers. Duplicate records among the three databases were removed and the remaining records were screened by two authors (AHW, MK) to determine whether they were published in English and whether they were clinical trials of varenicline for smoking cessation. Discrepancies in the screening decisions were discussed among the authors until a consensus was reached.
Data Extraction and Quality Assessment
The full-text articles were examined individually by two of the authors (AHW, MK) using a piloted coding form. The following variables were extracted from each article and entered on the coding form: publication identification (eg, first author, journal, date of publication), funding source/s, location of the study (country/countries), sample size, gender composition, racial/ethnic composition, type of sample (ie, community sample of smokers versus a subgroup of smokers such as adolescents or adults with a medical or psychiatric disorder), inclusion/exclusion criteria, design (eg, randomization, inclusion of placebo-control condition), treatment conditions (eg, dose/s of varenicline, other treatments provided, length of treatment, rates of attrition by medication condition), and smoking outcomes. Smoking outcomes that were extracted from each article included the number of male and female participants who were abstinent by condition (varenicline, placebo) for each outcome time point (week 12, week 24, week 52) and type of abstinence (7-day point-prevalence [PP], continuous). Quality assessment for each study was conducted by AHW using The Cochrane Collaboration’s tool for assessing risk of bias 38 which allows for the categorization of risk of bias as “low,” “unclear,” or “high” in each of five categories (ie, Selection Bias, Performance Bias, Detection Bias, Attrition Bias, and Reporting Bias). For a clinical trial to be included, it was judged as “low” bias for each of the five categories. Clinical trials judged to be “low bias” were randomized, double-blind, placebo-controlled studies without evidence of differential attrition.
As the published reports contained limited outcome data by sex, corresponding authors and Pfizer, Inc were contacted after the data extraction described above was completed to request data regarding sex-specific smoking outcomes. For all Pfizer, Inc studies included in this review, data were collected by investigators at academic institutions. The data requested included the number of male and female participants who (1) received varenicline, (2) received varenicline and were abstinent at each of the endpoints listed below, (3) received placebo, and (4) received placebo and were abstinent at each of the endpoints listed below. The six outcome endpoints requested were 7-day PP and CA at week 12 (end of treatment), week 24 (6-month follow-up), and week 52 (12-month follow-up). Seven-day PP was defined as no smoking in the prior 7 days of each time point (PP-12; PP-24; PP-52), and continuous abstinence was defined as no smoking for the last 4 weeks of study drug for each of the three outcomes: weeks 9 through 12 (CA-12), weeks 9 through 24 (CA-24), or weeks 9 through 52 (CA-52).
Data Synthesis and Analysis
We conducted meta-analyses using the Metafor program in R 39 for all six outcomes. We first specified a model with only fixed effects, using odds ratio as the summary measure. If there was evidence of significant heterogeneity in effect size ( P value for Q -statistic < .05), we then calculated a mixed-effects model. First, we stratified analyses by treatment group (varenicline and placebo), and examined sex differences in abstinence rates. Second, we stratified by sex and compared abstinence rates in the varenicline treatment condition versus placebo. Third, we tested a sex by treatment interaction. Finally, we conducted sensitivity analyses for treatment group sample size. For this set of analyses, we only included studies in which each analysis subgroup (eg, women receiving varenicline who achieved abstinence) contained data from at least five participants. The number 5 was selected to remove studies with the smallest subgroup sizes, while retaining adequate numbers of studies for analyses.
Role of Data and Funding Source
While all data summarized in this meta-analysis was collected at academic centers, Pfizer, Inc provided data for 15 studies and data for one study was provided by the study author ( Table 1 ). Funding for the study was supported by the National Institutes of Health. Those providing data or funding had no role in the design and conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Table 1.
Study Characteristics for Studies Included in the Meta-Analysis ( k = 16; Total n = 6710)
| First author | Data source | Sample a | % Female a | % Caucasian a | Varenicline duration (wk) | Study duration (wk) | Varenicline dose b | Outcomes | Study location(s) | Sample | Study notes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gonzales et al. 20 | P | 696 | 48 | 78 | 12 | 52 | 1mg bid | PP-12,24,52; CA-12,24,52 | United States | Adults | — |
| Jorenby et al. 21 | P | 685 | 43 | 85 | 12 | 52 | 1mg bid | PP-12,24,52; CA-12,24,52 | United States | Adults | — |
| Nides et al. 47 | P | 254 | 49 | 86 | 6 | 52 | 0.3mg qd, 1.0mg qd, or 1.0mg bid | PP-12; CA-12 | United States | Adults | — |
| Oncken et al. 40 | P | 388 | 50 | 79 | 12 | 52 | 0.5mg bid or 1mg bid | PP-12, CA-12 | United States | Adults | — |
| Nakamura et al. 43 | P | 310 | 21 | — | 12 | 52 | 1mg bid | PP-12,24,52; CA-12,24,52 | Japan | Adults | — |
| Tsai et al. 42 | P | 250 | 11 | — | 12 | 24 | 1mg bid | PP-12,24; CA-12,24 | Korea, Taiwan | Adults | — |
| Williams et al. 48 | P | 377 | 50 | 89 | 52 | 53 | 1mg bid | PP-12 | United States | Adults | 1 |
| Niaura et al. 41 | P | 320 | 48 | 90 | 12 | 52 | 0.25–1.0mg bid | PP-12,24,52; CA-12,24,52 | United States | Adults | 2 |
| Wang et al. 45 | P | 333 | 3 | 0 | 12 | 24 | 1mg bid | PP-12,24; CA-12,24 | China, Singapore, Thailand | Adults | — |
| Fagerström et al. 46 | P | 431 | 11 | — | 12 | 26 | 1mg bid | PP-12; CA-12,24 | Norway, Sweden | Adults | 3 |
| Rigotti et al. 44 | P | 703 | 21 | 81 | 12 | 52 | 1mg bid | PP-12,24,52; CA-12,24,52 | 15 countries c | Adults with CVD | — |
| Bolliger et al. 32 | P | 593 | 40 | 30 | 12 | 26 | 1mg bid | PP-12,24; CA-12,24 | 11 countries d | Adults | — |
| Steinberg et al. 50 | A | 79 | 41 | 72 | 12 | 24 | 1mg bid | PP-12,24 | United States | Hospitalized adults | — |
| Tashkin et al. 29 | P | 504 | 38 | 83 | 12 | 52 | 1mg bid | PP-12,24,52; CA-12,24,52 | France, Italy, Spain, United States | Adults with COPD | — |
| Rennard et al. 33 | P | 659 | 40 | 68 | 12 | 24 | 1mg bid | PP-12,24; CA-12,24 | 14 countries e | Adults | 4 |
| Williams et al. 49 | P | 128 | 23 | 59 | 12 | 24 | 1mg bid | PP-12,24; CA-12 | United States | Adults with SZ/SZA | — |
AUD = alcohol use disorders, CVD = cardiovascular disease, COPD = chronic obstructive pulmonary disease, SZ = schizophrenia, SZA = schizoaffective disorder. Data source: A = author; P = Pfizer. Outcomes: PP-12 = point prevalence abstinence weeks 9–12, PP-24 = point prevalence abstinence weeks 9–24, PP-52 = point prevalence abstinence weeks 9–52, CA-12 = continuous abstinence weeks 9–12, CA-24 = continuous abstinence weeks 9–24, CA-52 = continuous abstinence weeks 9–52.
a Sample size, % female, and % Caucasian are presented for the sample included in the analyses. Participants in studies who were given a treatment other than varenicline or placebo (eg, bupropion) were not included in these estimates. As a result, numbers may differ from data published in the relevant citation. All studies analyzed intent-to-treat samples except 44,47,49 which analyzed starters.
b Dose reflects steady-state dose after titration.
c Argentina, Australia, Brazil, Canada, Czech Republic, Denmark, France, Germany, Greece, Mexico, the Netherlands, Republic of Korea, Taiwan, United Kingdom, and United States.
d Brazil, Colombia, Costa Rica, Egypt, Jordan, Lebanon, Mexico, Saudi Arabia, South Africa, United Arab Emirates, and Venezuela.
e Argentina, Brazil, Canada, China, Czech Republic, France, Germany, Hungary, Italy, Mexico, Republic of Korea, Taiwan, United Kingdom, and United States.
Study notes:
1. Long-term safety study where participants received 52 weeks of varenicline or placebo; only data from week 12 outcomes were included in analyses.
2. Flexible dosing study.
3. Smokeless tobacco study.
4. Flexible quit date study (ie, participants could choose to quit smoking any time from 8 to 35 days after starting the study medication).
Results
Supplementary Figure 1 includes a flow diagram for study inclusion. We identified 80 abstracts through MEDLINE, 630 through EMBASE, and 83 through PsycINFO. Out of these initial 793 records, 136 records were duplicates across databases, eight were not published in English, and 606 were not clinical trials. Forty-three clinical trials of varenicline for smoking cessation were identified. Of these, 17 studies were randomized, double-blind, placebo-controlled studies without evidence of differential attrition and were thus judged to be “low bias.” Data were requested and obtained for 16 of the 17 studies. Pfizer, Inc provided outcome data by sex for 15 studies 20 , 21 , 29 , 32 , 33 , 40–49 and corresponding authors provided data for one study. 50 Data for one study was not obtained. 34 Thus, 16 of 17 eligible studies had data on sex and smoking outcomes, representing 96% of all available published clinical trial data with varenicline for smoking cessation meeting Cochrane’s low bias criteria ( n = 6710). Of the 27 clinical trial studies that were not included, 12 studies were not placebo-controlled trials, 25 , 27 , 28 , 30 , 31 , 51–57 seven studies had very small sample sizes, 58–64 five studies recruited only (or nearly only) male or female participants, 65–69 two studies 70 , 71 used the same data as Swan et al. 28
Study Characteristics
See Table 1 for the study characteristics for the 16 studies that were included in the analyses. The sample sizes of the individual studies ranged from 79 to 703 ( M = 419, SD = 202). Percentage of female participants within studies ranged from 3% to 50%, with an average of 34% ( SD = 16%).
Interactions of Sex Within Placebo and Varenicline Arms
We examined sex differences within each of the varenicline and placebo arms. Among those receiving placebo, women were less likely to achieve abstinence than men for PP-12 ( OR = 1.31; 95% CI = 1.05–1.62) and CA-24 ( OR = 1.37; 95% CI = 1.02–1.83; Table 2 , Supplementary Figure 2 ). For these two outcomes, women were 31% and 37%, respectively, less likely than men to achieve abstinence. Among those receiving varenicline, there were no significant differences between men and women for likelihood of quitting at any of the six outcome-time points. For example, at PP-12, 53.3% of men and 52.6% of women had quit smoking.
Table 2.
Meta-Analysis Outcomes for Medication Differences and Sex Interactions
| Men | Women | Interaction a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Varenicline | Placebo | Varenicline | Placebo | ||||||||||
| k b | Total n | % Abst. e | Total n | % Abst. f | OR c,d (95% CI) | Total n | % Abst. e | Total n | % Abst. f | OR c,d (95% CI) | OR c,d (95% CI) | ||
| PP | Week 12 | 16 | 2377 | 53.26 | 1986 | 26.79 § | 3.42** (2.86, 4.14) | 1389 | 52.56 | 952 | 17.44 | 4.95** (3.86, 6.36) | 1.46* (1.06, 1.97) |
| Week 24 | 11 | 1806 | 41.25 | 1565 | 22.04 | 2.51** (2.14, 2.95) | 1027 | 37.00 | 734 | 15.80 | 2.95** (2.31, 3.77) | 1.17 (0.88, 1.57) | |
| Week 52 | 6 | 986 | 31.85 | 1035 | 17.87 | 2.16** (1.75, 2.66) | 663 | 29.11 | 568 | 14.61 | 2.34** (1.75, 3.13) | 1.08 (0.76, 1.55) | |
| CA | Weeks 9–12 | 14 | 2226 | 49.28 | 1899 | 22.27 | 3.53** (3.06, 4.10) | 1249 | 47.24 | 871 | 14.70 | 4.66** (3.71, 5.81) | 1.34* (1.01, 1.72) |
| Weeks 9–24 | 11 | 1972 | 35.34 | 1738 | 17.49 § | 2.59** (2.20, 3.06) | 1035 | 31.21 | 740 | 10.27 | 3.49** (2.64, 4.57) | 1.31* (0.97, 1.84) | |
| Weeks 9–52 | 6 | 849 | 26.86 | 1035 | 10.24 | 2.53** (2.05, 3.13) | 623 | 21.67 | 568 | 7.92 | 2.66** (1.93, 3.67) | 1.05 (0.72, 1.54) | |
Abst. = abstinent; CA = continuous abstinence; CI = confidence interval; OR = odds ratio; PP = point prevalence.
a Interaction for treatment condition by sex.
b Number of studies included in meta-analysis.
c OR for varenicline vs. placebo.
d Statistical significance denotes heterogeneity in effect size between men and women.
e Comparisons of % abstinent of men vs. women within varenicline arm are all P > .05.
f Comparisons of % abstinent of men vs. women within placebo arm § < 0.05.
* P < .05; ** P < .001.
Interactions of Sex and Medication on Rates of Abstinence
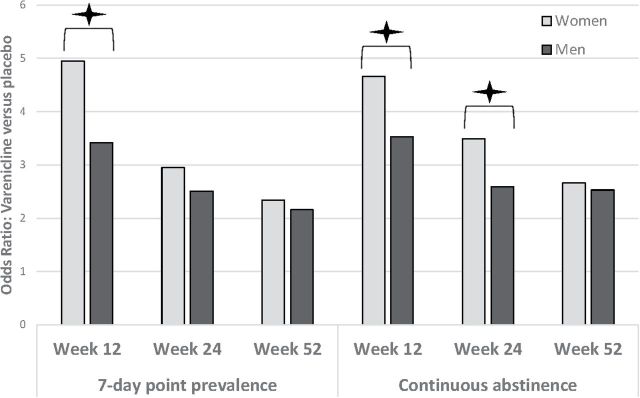
Effect size heterogeneity was found only for PP-12 ( Q = 53.93, P < .05). For this outcome, a mixed effect model was specified. For all other outcomes, fixed effects models were specified. For all six outcomes, the odds of quitting on varenicline versus placebo was significant for both men and women. In determining whether sex moderated the efficacy of varenicline, the effect size for varenicline was significantly larger for women than men for three of the six outcomes: PP-12, CA-12, and CA-24 (sex × medication interaction P < .05, Table 2 , Figure 1 ). For example, at PP-12, varenicline increased the odds of quitting in women by 4.95 times and by 3.42 times in men with the interaction demonstrating that varenicline was 46% more efficacious in women ( Figure 2 ).
Figure 1.
Interactions of sex and medication efficacy on odds of point prevalence and continuous abstinence at the end of treatment (12 weeks), 6-month follow-up (24 weeks), and 12-month follow-up (52 weeks). * P < .05 indicating that varenicline was 46%, 34%, and 31%, more effective for women for PP-12, CA-12, and CA-24 respectively. Seven-day point-prevalence was defined as no smoking in the prior 7 days of each time point (12-week; 24-week, 52-week), and continuous abstinence was defined as no smoking for the last 4 weeks of study drug (ie, weeks 9 through 12; week 12), or weeks 9 through 24 (week 24), or weeks 9 through 52 (week 52).
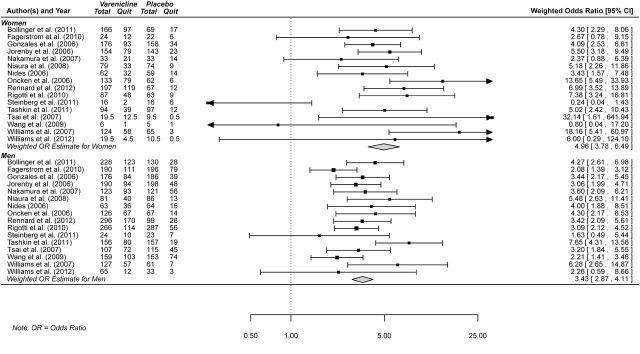
Figure 2.
Forest plot of odds ratios for 12-week (end of treatment) point-prevalence abstinence, varenicline vs. placebo (referent), by study and sex.
Sensitivity Analyses: Sample Size
We removed all studies with analysis subgroup sample sizes of n < 5. This procedure resulted in removal of the following studies for each outcome: PP-12 (42, 45, 48–50), PP-24 (42, 45, 50), CA-12 (40, 42, 45), CA-24 (42, 44, 45), CA-52 (44). When examining sex differences by treatment condition, the pattern of results were consistent with the full-sample analyses (data not shown).
Discussion
With the meta-analytic approach, we were able to pool 96% of available high-quality clinical trial data to maximize power to examine whether sex moderated the efficacy of varenicline. Results demonstrated that varenicline was more efficacious for women compared to men for short and intermediate term smoking cessation outcomes. Varenicline significantly increased the odds of quitting in women by 4.95 times compared to 3.42 times in men by the end of 12 weeks of treatment with abstinence assessed over the past 7 days, indicating that varenicline was 46% more efficacious for women. Women also had a preferred response for continuous abstinence by the end of treatment and at a 6-month follow-up, with results demonstrating that varenicline was 34% and 31% more efficacious for women, respectively. At longer-term, 1-year outcomes, women and men were quitting at equal rates.
Varenicline is currently the most effective FDA-approved smoking cessation medication, 72 and this is the first demonstration in placebo-controlled trials that women compared to men have a preferred or equal therapeutic response for a smoking cessation medication. One recent study 73 examined varenicline or varenicline plus bupropion in an adaptive design following nonresponse to nicotine patch. Although not placebo-controlled, results appear to suggest that women had a preferred response in the varenicline only group (31% women vs. 20% men). 74 Given that tobacco is the single greatest preventable cause of morbidity and mortality in the United States, these findings have significant public health relevance.
Overall, women demonstrated a pattern of lower rates of quitting in the placebo arm (eg, 31% less likely for end-of-treatment PP). This finding replicates other clinical trial findings demonstrating lower rates of smoking cessation in women receiving placebo medication. 10 While a number of factors have been identified for why women have poorer quit rates than men (stronger associations with psychiatric disorders, increased withdrawal symptoms, smoking for negative affect and stress, menstrual cycle factors, smoking for weight management), 75 it is currently unknown why women have lowered rates of quitting in placebo arms across studies of pharmacological interventions. Factors which are known to interact with medication outcomes (eg, subclinical depression, menstrual cycle status) are often not studied as part of standard clinical trial designs.
Unlike nicotine replacement and bupropion, varenicline completely attenuated sex differences in rates of smoking cessation that were found with placebo. In the current study, 53% of women and 53% of men successfully quit by the end of treatment with varenicline. In comparison, a meta-analysis with bupropion 10 demonstrated that women were 23% less likely to quit regardless of whether they received placebo or bupropion. With nicotine replacement, a meta-analytic review found that women were less likely to quit with either placebo or medication, with the interaction of sex and medication demonstrating that nicotine replacement therapy was 40% more effective for men when compared with women. 15
While sex differences in varenicline efficacy exist, it is unknown what mechanisms may underlie these differences. Smoking in women is more strongly tied to negative affect and stress 76 , 77 and varenicline may directly target negative affect and improve mood during nicotine withdrawal. 18 , 48 However, this same mechanism has been hypothesized for bupropion, 78 and as stated above, meta-analysis of bupropion demonstrate lower quit rates in women. 10 Women are also more likely to clear nicotine more quickly from their systems than men, as assessed by the nicotine metabolite ratio (3′-hydroxycotinine:cotinine). 79 This effect is known to be partially mediated by estrogen with even higher rates of nicotine clearance seen in women taking estrogen through birth control or hormone replacement. 79 Prospectively randomizing smokers based on their nicotine metabolite ratio determined that faster metabolizers had a better therapeutic response with varenicline versus nicotine replacement. 80 Given that women have greater nicotine metabolite ratios compared to men, these results support the current meta-analytic findings. Additional translational research to understand how sex-sensitive mechanisms interact with medication efficacy are important to pursue. Currently, there are no placebo-controlled studies of varenicline examining outcomes by factors that are known to differentially impact smoking behavior or smoking cessation outcomes for women (eg, stress, menstrual cycle, reproductive status, or weight concerns 81 , 82 ).
With regard to study limitations, the majority of the samples were primarily Caucasian and it is unknown how study findings would generalize to other ethnic and racial groups. Safety, attrition, or compliance were not investigated in this meta-analysis, which would be important avenues for future research. With regard to safety, evidence indicates that women taking varenicline may be more likely to report experiencing nausea than men taking varenicline. 83–85 It is important to note that while the black box warning remains in effect for varenicline, the medication has been found to be safe and well-tolerated in smokers and does not appear to exacerbate mental illness or neuropsychiatric side effects in samples ranging up to 80 000. 86–89 Results of two FDA-sponsored studies conducted by the Department of Defense ( n = 19 933) and the Veteran’s Administration ( n = 14 131) evaluating the risk of hospitalizations due to neuropsychiatric events in varenicline versus nicotine patch found no differences across the two medications. 87 With regard to compliance, Hays and colleagues 90 examined pooled data from two clinical trials 20 , 21 and reported that sex was not a predictor of medication adherence across varenicline, bupropion, and placebo conditions. Studies also varied with regards to nonmedication factors (eg, length, type, delivery of counseling) and it is possible that these factors influenced sex differences in medication outcomes.
Conclusion
Despite significant evidence that sex differences exist in smoking cessation outcomes and medication response, few studies examine and report outcomes by sex. Current federal regulations exist to support the study, analysis, and reporting of treatment efficacy by sex. 35 , 37 Results of this study support such efforts and highlight the importance of examining medication efficacy by sex. In the current clinical care guidelines for smoking cessation it is acknowledged that nicotine replacement therapy may be less effective for women and other medications, such as varenicline, should be considered. 23 Current meta-analysis of FDA-approved smoking cessation therapeutics, 10 , 15 as well as our findings, demonstrate that women are less likely to quit smoking indicating a significant sex disparity with regards to smoking cessation. Our findings indicate that varenicline, unlike prior findings with nicotine replacement and bupropion, attenuates lowered rates of quitting seen in the placebo arms thereby demonstrating greater efficacy among women smokers for short and immediate-term outcomes and equal efficacy for longer term 1-year outcomes. Varenicline may be particularly useful for reducing the sex disparity typically seen in rates of smoking cessation.
Funding
This work was supported by the National Institutes of Health grant P50DA033945 (ORWH, NIDA, FDA to SAM) and K12DA031050 (ORWH, NIDA, NIAAA to CMM).
Declaration of Interests
SAM has Investigator-Initiated grants from Pfizer to examine alcohol-varenicline interactions. Authors PHS, MK, CMM, and AHW report no conflicts of interest.
Supplementary Material
Acknowledgments
SAM conceived of the study. AHW and MK conducted the literature review. PHS conducted the statistical analysis. SAM, PHS, AHW, and CMM wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Those providing data or funding had no role in the design and conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1. Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality—beyond established causes . N Engl J Med . 2015. ; 372 ( 7 ): 631 – 640 . doi: 10.1056/NEJMsa1407211 . [DOI] [PubMed] [Google Scholar]
- 2. CDC . Current cigarette smoking among adults—United States, 2011 . MMWR Morb Mortal Wkly Rep . 2012. ; 61 ( 44 ): 889 – 894 . www.cdc.gov/mmwr/preview/mmwrhtml/mm6144a2.htm?s_cid=mm6144a2_w . Accessed July 8, 2015 . [PubMed] [Google Scholar]
- 3. USDHHS . How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General . Atlanta,GA: : U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; ; 2010. . www.cdc.gov/tobacco/data_statistics/sgr/2010/index.htm . Accessed July 8, 2015 . [PubMed] [Google Scholar]
- 4. Kiyohara C, Ohno Y . Sex differences in lung cancer susceptibility: a review. Gender Medicine . 2010. ; 7 ( 5 ): 381 – 401 . doi: 10.1016/j.genm.2010.10.002 . [DOI] [PubMed] [Google Scholar]
- 5. Huxley RR, Woodward M . Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies . Lancet . 2011. ; 378 ( 9799 ): 1297 – 1305 . doi: 10.1016/S0140-6736(11)60781–2 . [DOI] [PubMed] [Google Scholar]
- 6. USDHHS . Women and Smoking. A Report of the Surgeon General . Rockville, MD: : Department of Health and Human Services; ; 2001. . www.cdc.gov/tobacco/data_statistics/sgr/2001/index.htm . Accessed July 8, 2015 . [Google Scholar]
- 7. Mercuro G, Deidda M, Piras A, Dessalvi CC, Maffei S, Rosano G . Gender determinants of cardiovascular risk factors and diseases . J Cardiovasc Med 2010. ; 11 ( 3 ): 207 – 220 . doi: 10.2459/JCM.0b013e32833178ed . [DOI] [PubMed] [Google Scholar]
- 8. Rahmanian SD, Diaz PT, Wewers ME . Tobacco use and cessation among women: research and treatment-related issues . J Womens Health . 2011. ; 20 ( 3 ): 349 – 357 . doi: 10.1089/jwh.2010.2173 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG . Use of smoking-cessation treatments in the United States . Am J Prev Med . 2008. ; 34 ( 2 ): 102 – 111 . doi: 10.1016/j.amepre.2007.09.033 . [DOI] [PubMed] [Google Scholar]
- 10. Scharf D, Shiffman S . Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of bupropion CR . Addiction . 2004. ; 99 ( 11 ): 1462 – 1469 . doi: 10.1111/j.1360-0443.2004.00845.x . [DOI] [PubMed] [Google Scholar]
- 11. Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB . Gender differences in smoking cessation . J Consult Clin Psychol . 1999. ; 67 ( 4 ): 555 – 562 . doi: 10.1037/0022-006X.67.4.555 . [DOI] [PubMed] [Google Scholar]
- 12. Smith PH, Kasza K, Hyland A, et al. Gender differences in medication use and cigarette smoking cessation: results from the International Tobacco Control Four Country Survey . Nicotine Tob Res . 2015. ; 17 ( 40 ): 463 – 472 . doi: 10.1093/ntr/ntu212 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinberger AH, Pilver CE, Mazure CM, McKee SA . Stability of smoking status in the U.S. population: a longitudinal investigation . Addiction . 2014. ; 109 ( 9 ): 1541 – 1553 . doi: 10.1111/add.12647 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jarvis MJ, Cohen JE, Delnevo CD, Giovino GA . Dispelling myths about gender differences in smoking cessation: population data from the USA, Canada, and Britain . Tob Control . 2013. ; 22 ( 5 ): 356 – 360 . doi: 10.1136/tobaccocontrol-2011–050279 . [DOI] [PubMed] [Google Scholar]
- 15. Perkins KA, Scott J . Sex differences in long-term smoking cessation rates due to nicotine patch . Nicotine Tob Res . 2008. ; 10 ( 7 ): 1245 – 1251 . doi: 10.1080/14622200802097506 . [DOI] [PubMed] [Google Scholar]
- 16. Weinberger AH, Smith PH, Kaufman M, McKee SA . Consideration of sex in clinical trials of transdermal nicotine patch: a systematic review . Exp Clin Psychopharmacol . 2014. ; 22 ( 5 ): 373 – 383 . doi: 10.1037/a0037692 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aubin HJ, Lebargy F, Berlin I, Bidaut-Mazel C, Chemali-Hudry J, Lagrue G . Efficacy of bupropion and predictors of successful outcome in a sample of French smokers: a randomized placebo-controlled trial . Addiction . 2004. ; 99 ( 9 ): 1206 – 1218 . doi: 10.1111/j.1360-0443.2004.00814.x . [DOI] [PubMed] [Google Scholar]
- 18. Gonzales D, Jorenby DE, Brandon TH, Arteaga C, Lee TC . Immediate versus delayed quitting and rates of relapse among smokers treated successfully with varenicline, bupropion SR or placebo . Addiction . 2010. ; 105 ( 11 ): 2002 – 2013 . doi: 10.1111/j.1360-0443.2010.03058.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE . Rationale, pharmacology and clinical efficacy of partial agonists of α4β2 nACh receptors for smoking cessation . Trends Pharmacol Sci . 2007. ; 28 ( 7 ): 316 – 325 . doi: 10.1016/j.tips.2007.05.003 . [DOI] [PubMed] [Google Scholar]
- 20. Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial . JAMA . 2006. ; 296 ( 1 ): 47 – 55 . doi: 10.1001/jama.296.1.47 . [DOI] [PubMed] [Google Scholar]
- 21. Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline an α4β2 nicotinic acetylcholine receptor partial agonist vs placebo or sustained-release bupropion for smoking cessation. A randomized controlled trial . JAMA . 2006. ; 296 ( 1 ): 56 – 63 . doi: 10.1001/jama.296.1.56 . [DOI] [PubMed] [Google Scholar]
- 22. Foulds J, Russ C, Yu C-R, et al. Effect of varenicline on individual nicotine withdrawal symptoms: a combined analysis of eight randomized, placebo-controlled trials . Nicotine Tob Res . 2013. ; 15 ( 11 ): 1849 – 1857 . doi: 10.1093/ntr/ntt066 . [DOI] [PubMed] [Google Scholar]
- 23. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update . Rockville, MD: : U.S. Department of Health and Human Services; ; 2008. . www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf . Accessed July 8, 2015 . [Google Scholar]
- 24. Cahill K, Stead LF, Lancaster T . Nicotine receptor partial agonists for smoking cessation . Cochrane Database Syst Rev . 2012. ; 2 : CD006103 . doi: 10.1002/14651858.CD006103.pub3 . [DOI] [PubMed] [Google Scholar]
- 25. Heydari G, Talischi F, Tafti SF, Masjedi MR . Quitting smoking with varenicline: parallel, randomised efficacy trial in Iran . Int J Tuberc Lung Dis . 2012. ; 16 ( 2 ): 268 – 272 . doi: 10.5588/ijtld.11.0183 . [DOI] [PubMed] [Google Scholar]
- 26. Nides M, Glover ED, Reus VI, et al. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis . Am J Health Behav . 2008. ; 32 ( 6 ): 664 – 675 . doi: 10.5993/AJHB.32.6.10 . [DOI] [PubMed] [Google Scholar]
- 27. Ramon JM, Bruguera E . Real world study to evaluate the effectiveness of varenicline and cognitive-behavioural interventions for smoking cessation . Int J Environ Res Public Health . 2009. ; 6 ( 4 ): 1530 – 1538 . doi: 10.3390/ijerph6041530 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Swan GE, McClure JB, Jack LM, et al. Behavioral counseling and varenicline treatment for smoking cessation . Am J Prev Med . 2010. ; 38 ( 5 ): 482 – 490 . doi: 10.1016/j.amepre.2010.01.024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tashkin DP, Rennard S, Hays JT, Ma W, Lawrence D, Lee TC . Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial . Chest . 2011. ; 139 ( 3 ): 591 – 599 . doi: 10.1378/chest.10-0865 . [DOI] [PubMed] [Google Scholar]
- 30. Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR . Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates . Arch Intern Med . 2011. ; 171 ( 8 ): 770 – 777 . doi: 10.1001/archinternmed.2011.138 . [DOI] [PubMed] [Google Scholar]
- 31. Park ER, Japuntich S, Temel J, et al. A smoking cessation intervention for thoracic surgery and oncology clinics: a pilot trial . J Thorac Oncol . 2011. ; 6 ( 6 ): 1059 – 1065 . doi: 10.1001/archinternmed.2011.138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bolliger CT, Issa JS, Posadas-Valay R, et al. Effects of varenicline in adult smokers: a multinational, 24-week, randomized, double-blind, placebo-controlled study . Clin Ther . 2011. ; 33 ( 4 ): 465 – 477 . doi: 10.1016/j.clinthera.2011.04.013 . [DOI] [PubMed] [Google Scholar]
- 33. Rennard S, Hughes J, Cinciripini PM, et al. A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates . Nicotine Tob Res . 2012. ; 14 ( 3 ): 343 – 350 . doi: 10.1093/ntr/ntr220 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wong J, Abrishami A, Yang Y, et al. A perioperative smoking cessation intervention with varenicline: a double-blind, randomized, placebo-controlled trial . Anesthesiology . 2012. ; 117 ( 4 ): 755 – 764 . doi: 10.1097/01.sa.0000428893.19142.6b . [DOI] [PubMed] [Google Scholar]
- 35. Clayton JA, Collins FS . NIH to balance sex in cell and animal studies . Nature . 2014. ; 509 ( 7500 ): 282 – 283 . doi: 10.1038/509282a . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. FDA . FDASIA section 907: Inclusion of demographic subgroups in clinical trials . 2014. . www.fda.gov/RegulatoryInformation/Legislation/Federal FoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/ucm389100.htm . Accessed July 8, 2015 .
- 37. Institute of Medicine (US) Board on Population Health and Public Health Practice . Sex-Specific Reporting of Scientific Research: A Workshop Summary . Washington, DC: : National Academies Press (US) ; 2012. . www.iom.edu/Reports/2012/Sex-Specific-Reporting-of-Scientific-Research.aspx . Accessed July 8, 2015 . [PubMed] [Google Scholar]
- 38. Higgins JPT, Green S , eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 . 2011http://community.cochrane.org/handbook . Accessed July 8, 2015 .
- 39. Wolfgang V . Conducting meta-analyses in R with the metafor package . J Stat Softw . 2010. ; 36 ( 3 ): 1 – 48 . www.jstatsoft.org/v36/i03/ . Accessed July 8, 2015 . [Google Scholar]
- 40. Oncken C, Gonzales D, Nides M, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation . Arch Intern Med . 2006. ; 166 ( 15 ): 1571 – 1577 . doi: 10.1001/archinte.166.15.1571 . [DOI] [PubMed] [Google Scholar]
- 41. Niaura R, Hays JT, Jorenby DE, et al. The efficacy and safety of varenicline for smoking cessation using a flexible dosing strategy in adult smokers: a randomized controlled trial . Curr Med Res Opin . 2008. ; 24 ( 7 ): 1931 – 1941 . doi: 10.1185/03007990802177523 . [DOI] [PubMed] [Google Scholar]
- 42. Tsai ST, Cho HJ, Cheng HS, et al. A randomized, placebo-controlled trial of varenicline, a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers . Clin Ther . 2007. ; 29 ( 6 ): 1027 – 1039 . doi: 10.1016/j.clinthera.2007.06.011 . [DOI] [PubMed] [Google Scholar]
- 43. Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR . Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers . Clin Ther . 2007. ; 29 ( 6 ): 1040 – 1056 . doi: 10.1016/j.clinthera.2007.06.012 . [DOI] [PubMed] [Google Scholar]
- 44. Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S . Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial . Circulation . 2010. ; 121 ( 2 ): 221 – 229 . doi: 10.1161/CIRCULATIONAHA.109.869008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang C, Xiao D, Chan KP, Pothirat C, Garza D, Davies S . Varenicline for smoking cessation: a placebo-controlled, randomized study . Respirology . 2009. ; 14 ( 3 ): 384 – 392 . doi: 10.1111/j.1440-1843.2008.01476.x . [DOI] [PubMed] [Google Scholar]
- 46. Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M . Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial . BMJ . 2010. ; 341 : c6549 . doi: 10.1136/bmj.c6549 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up . Arch Intern Med . 2006. ; 166 ( 15 ): 1561 – 1568 . doi: 10.1001/jama.296.1.47 . [DOI] [PubMed] [Google Scholar]
- 48. Williams KE, Reeves KR, Billing CB, Jr, Pennington AM, Gong J . A double-blind study evaluating the long-term safety of varenicline for smoking cessation . Curr Med Res Opin . 2007. ; 23 ( 4 ): 793 – 801 . doi: 10.1185/030079907X182185 . [DOI] [PubMed] [Google Scholar]
- 49. Williams JM, Anthenelli RM, Morris CD, et al. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder . J Clin Psychiatry . 2012. ; 73 ( 5 ): 654 – 660 . doi: 10.4088/JCP.11m07522 . [DOI] [PubMed] [Google Scholar]
- 50. Steinberg MB, Randall J, Greenhaus S, Schmelzer AC, Richardson DL, Carson JL . Tobacco dependence treatment for hospitalized smokers: a randomized, controlled, pilot trial using varenicline . Addict Behav . 2011. ; 36 ( 12 ): 1127 – 1132 . doi: 10.1016/j.addbeh.2011.07.002 . [DOI] [PubMed] [Google Scholar]
- 51. Aubin HJ, Bobak A, Britton JR, et al. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial . Thorax . 2008. ; 63 ( 8 ): 717 – 724 . doi: 10.1136/thx.2007.090647 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boudrez H, Gratziou C, Messig M, Metcalfe M . Effectiveness of varenicline as an aid to smoking cessation: results of an inter-European observational study . Curr Med Res Opin . 2011. ; 27 ( 4 ): 769 – 775 . doi: 10.1185/03007995.2011.557718 . [DOI] [PubMed] [Google Scholar]
- 53. Andreas S, Chenot JF, Diebold R, Peachey S, Mann K . Effectiveness of varenicline as an aid to smoking cessation in primary care: an observational study . Eur Addict Res . 2013. ; 19 ( 1 ): 47 – 54 . doi: 10.1159/000341638 . [DOI] [PubMed] [Google Scholar]
- 54. Nollen NL, Cox LS, Nazir N, et al. A pilot clinical trial of varenicline for smoking cessation in black smokers . Nicotine Tob Res . 2011. ; 13 ( 9 ): 868 – 873 . doi: 10.1093/ntr/ntr063 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schnoll RA, Cappella J, Lerman C, et al. A novel recruitment message to increase enrollment into a smoking cessation treatment program: preliminary results from a randomized trial . Health Commun . 2011. ; 26 ( 8 ): 735 – 742 . doi: 10.1080/10410236.2011.566829 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hays JT, Croghan IT, Schroeder DR, Ebbert JO, Hurt RD . Varenicline for tobacco dependence treatment in recovering alcohol-dependent smokers: an open-label pilot study . J Subst Abuse Treat . 2011. ; 40 ( 1 ): 102 – 107 . doi: 10.1016/j.jsat.2010.08.009 . [DOI] [PubMed] [Google Scholar]
- 57. Hawk LW, Jr, Ashare RL, Lohnes SF, et al. The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial . Clin Pharmacol Ther . 2012. ; 91 ( 2 ): 172 – 180 . doi: 10.1038/clpt.2011.317 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS . A preliminary investigation of varenicline for heavy drinking smokers . Psychopharmacology (Berl) . 2011. ; 215 ( 4 ): 655 – 663 . doi: 10.1007/s00213-010-2160-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gray KM, Carpenter MJ, Lewis AL, Klintworth EM, Upadhyaya HP . Varenicline versus bupropion XL for smoking cessation in older adolescents: a randomized, double-blind pilot trial . Nicotine Tob Res . 2012. ; 14 ( 2 ): 234 – 239 . doi: 10.1093/ntr/ntr130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weiner E, Buchholz A, Coffay A, et al. Varenicline for smoking cessation in people with schizophrenia: a double blind randomized pilot study . Schizophr Res . 2011. ; 129 ( 1 ): 94 – 95 . doi: 10.1016/j.schres.2011.02.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Dios MA, Anderson BJ, Stanton C, Audet DA, Stein M . Project Impact: a pharmacotherapy pilot trial investigating the abstinence and treatment adherence of Latino light smokers . J Subst Abuse Treat . 2012. ; 43 ( 3 ): 322 – 330 . doi: 10.1016/j.jsat.2012.01.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wu BS, Weinberger AH, Mancuso E, et al. A preliminary feasibility study of varenicline for smoking cessation in bipolar disorder . J Dual Diagn . 2012. ; 8 ( 2 ): 131 – 132 . doi: 10.1080/15504263.2012.671067 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Castle D, Baker AL, Richmond R, Filia SL, Harris D, Pirola-Merlo AJ . Varenicline plus healthy lifestyle intervention for smoking cessation in psychotic disorders . Ann Clin Psychiatry . 2012. ; 24 ( 4 ): 285 – 291 . www.aacp.com/Pages.asp?AID=10852&issue=November%202012&page= C&UID =. Accessed July 8, 2015 . [PubMed] [Google Scholar]
- 64. Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M . The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: a pilot study . Am J Addict . 2010. ; 19 ( 5 ): 401 – 408 . doi: 10.1111/j.1521-0391.2010.00066.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ebbert JO, Croghan IT, North F, Schroeder DR . A pilot study to assess smokeless tobacco use reduction with varenicline . Nicotine Tob Res . 2010. ; 12 ( 10 ): 1037 – 1040 . doi: 10.1093/ntr/ntq134 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ebbert JO, Croghan IT, Severson HH, Schroeder DR, Hays JT . A pilot study of the efficacy of varenicline for the treatment of smokeless tobacco users in Midwestern United States . Nicotine Tob Res . 2011. ; 13 ( 9 ): 820 – 826 . doi: 10.1093/ntr/ntr078 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tsukahara H, Noda K, Saku K . A randomized controlled open comparative trial of varenicline vs nicotine patch in adult smokers: efficacy, safety and withdrawal symptoms (the VN-SEESAW study) . Circ J . 2010. ; 74 ( 4 ): 771 – 778 . doi: 10.1253/circj.CJ-09-0803 . [DOI] [PubMed] [Google Scholar]
- 68. Kikkawa H, Maruyama N, Fujimoto Y, Hasunuma T . Single- and multiple-dose pharmacokinetics of the selective nicotinic receptor partial agonist, varenicline, in healthy Japanese adult smokers . J Clin Pharmacol . 2011. ; 51 ( 4 ): 527 – 537 . doi: 10.1177/0091270010372388 . [DOI] [PubMed] [Google Scholar]
- 69. Cui Q, Robinson L, Elston D, et al. Safety and tolerability of varenicline tartrate (Champix([REGISTERED])/Chantix([REGISTERED])) for smoking cessation in HIV-infected subjects: a pilot open-label study . AIDS Patient Care STDS . 2012. ; 26 ( 1 ): 12 – 19 . doi: 10.1089/apc.2011.0199 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McClure JB, Swan GE, Jack L, et al. Mood, side-effects and smoking outcomes among persons with and without probable lifetime depression taking varenicline . J Gen Intern Med . 2009. ; 24 ( 5 ): 563 – 569 . doi: 10.1007/s11606-009-0926-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McClure JB, Swan GE, Catz SL, et al. Smoking outcome by psychiatric history after behavioral and varenicline treatment . J Subst Abuse Treat . 2010. ; 38 ( 4 ): 394 – 402 . doi: 10.1016/j.jsat.2010.03.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cahill K, Stevens S, Perera R, Lancaster T . Pharmacological interventions for smoking cessation: an overview and network meta-analysis . Cochrane Database Syst Rev . 2013. ; 5 : CD009329 . doi: 10.1002/14651858.CD009329.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rose JE, Behm FM . Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm . Am J Psychiatry . 2014. ; 171 ( 11 ): 1199 – 1205 . doi: 10.1176/appi.ajp.2014.13050595 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McKee SA, Weinberger AH . Innovations in translational sex and gender-sensitive tobacco research . Nicotine Tob Res . 2015. ; 17 ( 4 ): 379 – 381 . doi: 10.1093/ntr/ntu335 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gorelick DA . Sex difference in response to varenicline for smoking cessation . Am J Psychiatry . 2015. ; 172 ( 4 ): 394 – 395 . doi: 10.1176/appi.ajp.2015.14111429 . [DOI] [PubMed] [Google Scholar]
- 76. Nakajima M, al’Absi M . Predictors of risk for smoking relapse in men and women: a prospective examination . Psychol Addict Behav . 2012. ; 26 ( 3 ): 633 – 637 . doi: 10.1037/a0027280 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. McKee SA, Maciejewski PK, Falba T, Mazure CM . Sex differences in the effects of stressful life events on changes in smoking status . Addiction . 2003. ; 98 ( 6 ): 847 – 855 . doi: 10.1046/j.1360-0443.2003.00408.x . [DOI] [PubMed] [Google Scholar]
- 78. Lerman C, Roth D, Kaufmann V, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment . Drug Alcohol Depend . 2002. ; 67 ( 2 ): 219 – 223 . doi: 10.1016/S0376-8716(02)00067-4 [DOI] [PubMed] [Google Scholar]
- 79. Chenoweth MJ, Novalen M, Hawk LW, Jr, et al. Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers . Cancer Epidemiol Biomarkers Prev . 2014. ; 23 ( 9 ): 1773 – 1782 . doi: 10.1158/1055–9965.EPI-14-0427 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lerman C, Schnoll RA, Hawk LW, Jr, et al. ; PGRN-PNAT Research Group . Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial . Lancet Respir Med . 2015. ; 3 ( 2 ): 131 – 138 . doi: 10.1016/S2213-2600(14)70294-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Perkins KA . Smoking cessation in women: special considerations . CNS Drugs . 2001. ; 15 ( 5 ): 391 – 411 . doi: 10.2165/00023210-200115050-00005 . [DOI] [PubMed] [Google Scholar]
- 82. Weinberger AH, Smith PH, Allen SS, et al. . Systematic and meta-analytic review of research examining the impact of menstrual cycle phase and ovarian hormones on smoking and cessation . Nicotine Tob Res . 2015. ; 17 ( 4 ): 407 – 421 . doi: 10.1093/ntr/ntu249 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Halperin AC, McAfee TA, Jack LM, et al. Impact of symptoms experienced by varenicline users on tobacco treatment in a real world setting . J Subst Abuse Treat . 2009. ; 36 ( 4 ): 428 – 434 . doi: 10.1016/j.jsat.2008.09.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ravva P, Gastonguay MR, French JL, Tensfeldt TG, Faessel HM . Quantitative assessment of exposure-response relationships for the efficacy and tolerability of varenicline for smoking cessation . Clin Pharmacol Ther . 2010. ; 87 ( 3 ): 336 – 344 . doi: 10.1038/clpt.2009.282 . [DOI] [PubMed] [Google Scholar]
- 85. Faessel H, Ravva P, Williams K . Pharmacokinetics, safety, and tolerability of varenicline in healthy adolescent smokers: a multicenter, randomized, double-blind, placebo-controlled, parallel-group study . Clin Ther . 2009. ; 31 ( 1 ): 177 – 189 . doi: 10.1016/j.clinthera.2009.01.003 . [DOI] [PubMed] [Google Scholar]
- 86. Tonstad S, Davies S, Flammer M, Russ C, Hughes J . Psychiatric adverse events in randomized, double-blind, placebo-controlled clinical trials of varenicline: a pooled analysis . Drug Saf . 2010. ; 33 ( 4 ): 289 – 301 . doi: 10.2165/11319180-000000000-00000 . [DOI] [PubMed] [Google Scholar]
- 87. FDA . FDA Drug Safety Communication: Safety review update of Chantix (varenicline) and risk of neuropsychiatric adverse events . 2011. . www.fda.gov/Drugs/DrugSafety/ucm276737.htm . Accessed July 8, 2015 .
- 88. Gunnell D, Irvine D, Wise L, Davies C, Martin RM . Varenicline and suicidal behaviour: a cohort study based on data from the General Practice Research Database . BMJ . 2009. ; 339 : b3805 . doi: 10.1136/bmj.b3805 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stapleton JA, Watson L, Spirling LI, et al. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness . Addiction . 2007. ; 103 : 146 – 154 . doi: 10.1111/j.1360-0443.2007.02083.x . [DOI] [PubMed] [Google Scholar]
- 90. Hays JT, Leischow SJ, Lawrence D, Lee TC . Adherence to treatment for tobacco dependence: association with smoking abstinence and predictors of adherence . Nicotine Tob Res . 2010. ; 12 ( 6 ): 574 – 581 . doi: 10.1093/ntr/ntq047 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.