Abstract
Introduction:
Varenicline (Chantix) is a first-line treatment for smoking cessation but does not produce cessation in many individuals. It may be possible to improve abstinence by co-administering varenicline with other medications. Zonisamide (Zonegran) has a similar pharmacologic profile to topiramate, which has been shown to reduce smoking, but is better tolerated. This study evaluated whether combined zonisamide and varenicline reduced tobacco withdrawal and increased abstinence among smokers trying to quit, relative to varenicline and placebo.
Methods:
This was a double-blind, randomized, placebo-controlled pilot trial of zonisamide + varenicline versus placebo + varenicline for smoking cessation. Smokers received brief counseling and study medications, and completed weekly assessments for 10 consecutive weeks. The primary outcome was continuous abstinence rates (biochemically verified) during the final 4 weeks of treatment.
Results:
Results are presented as intent-to-treat and completer analyses. Seventy-four individuals were enrolled; 45 completed the study. Overall, 14.9% (intent-to-treat) and 25.0% (completer) of participants maintained sustained abstinence during the final 4 weeks of treatment. There were no differences between groups for biochemically-verified smoking, but zonisamide + varenicline reduced self-reported smoking, nicotine withdrawal, and craving compared to placebo + varenicline.
Conclusions:
Zonisamide decreased nicotine withdrawal and craving, though not of sufficient magnitude to modify smoking behavior. The sample size was small and low rates of abstinence across groups suggest the study population was difficult to treat. Additional evaluation of zonisamide or other medications that increase GABA or decrease glutamate in larger or more diverse populations may yield positive clinical benefit for nicotine/tobacco cessation.
Implications:
This study provides support for layering novel medications with varenicline for smoking cessation, for investigating medications that target the GABA and glutamate system, and for assessing the contribution that reductions in nicotine withdrawal have on ultimate cessation outcomes.
Introduction
In 2012, 18.7% of the global population aged 15 and over smoked cigarettes and an estimated 6 million people worldwide died from a smoking-related illness. 1 , 2 In the United States, approximately 40 million adults (18.1%) smoke cigarettes. Smoking is blamed for more than 400 000 in the US deaths annually, and an additional 16 million adults and children are believed to suffer from smoking-related diseases. The US Surgeon General attributes 15 types of cancers and at least 24 chronic diseases to cigarette smoking, 3 and smoking has been estimated to cost $133 billion in direct healthcare and $156 billion in lost productivity. 3
Varenicline (Chantix) is a first-line treatment for smoking cessation and produces the highest rates of sustained smoking abstinence among individuals trying to quit, 4 yet, fewer than half of people who take varenicline are able achieve long-term sustained abstinence. 5–8 One potential method for enhancing the efficacy of varenicline for smoking cessation is to identify medications with effect profiles that may complement the clinical effects of varenicline. Support for this approach is apparent in nicotine/tobacco cessation research where combined pharmacotherapies often outperform single agents for smoking cessation. 4 , 9 Varenicline is well suited for this approach as it is a leading treatment for smoking cessation and has a very low rate of drug–drug interactions.
The strategy of combining varenicline with other medications to support increased smoking cessation is rapidly gaining attention, although randomized trials to date have been limited to FDA-approved medications for smoking cessation. One of three randomized controlled trials indicated that a combination of varenicline and nicotine replacement therapy improved smoking outcomes compared with varenicline + placebo. 10–12 Studies that combined varenicline and bupropion (Zyban) have also indicated significant benefits of drug combination for smoking cessation, with 39.8%–50.0% of participants randomized to varenicline + bupropion achieving abstinence compared to 25.9%–43.2% of participants receiving varenicline alone. 13 , 14
Zonisamide (Zonegran) has been FDA-approved for the treatment of epilepsy since 2000, is available generically, and may have utility for smoking cessation. Zonisamide enhances dopaminergic tone (tyrosine hydroxylase activator, monoamine oxidase B inhibitor 15–17 ), normalizes glutamate homeostasis (potentially normalizing neuroplasticity), decreases stress and anxiety, restores cysteine-glutamate exchange transporter (xCT) and glial glutamate transporter (GTT-1; both of which are affected in nicotine use 18 , 19 ), and increases GABA release. 20 Zonisamide is also associated with substantial weight loss, 21 which is not generally addressed by current smoking cessation pharmacotherapies. 22 Zonisamide is in the same class of medications as topiramate (Topamax), which has been shown to improve smoking cessation outcomes and minimize weight gain in smokers, 23 and to enhance smoking cessation in alcohol-dependent smokers. 24 , 25 Zonisamide is generally better tolerated than topiramate, 26 and does not produce the negative cognitive effects that are evident following topiramate administration, 27 suggesting that it may be preferable to topiramate for use in smoking cessation. The present pilot study was conducted to evaluate the effect of combining zonisamide with varenicline on nicotine withdrawal, craving, and smoking abstinence among smokers attempting to quit.
Methods
Participants
Smokers who wanted to quit were recruited via newspaper advertisements and flyers posted in Baltimore, MD between October 2012 and December 2013. Inclusion criteria were being 18–65 years old, smoking at least 10 cigarettes per day for at least 1 year, reporting a desire to quit smoking (≥7 on Contemplation Ladder 28 ), providing a urine sample that tested positive for the nicotine metabolite cotinine, and agreeing to adhere to the visit schedule. Participants were excluded if they used a nicotine product other than cigarettes, had a history of serious psychiatric disorder, scored at least 20 on the Beck Depression Inventory, 29 scored at least 2 on the first question or at least 8 total on the Suicidal Behaviors Questionnaire Revised, 30 used bupropion, nortriptyline, or clonidine in the past 30 days, or had medical contraindications to varenicline or zonisamide.
Recruitment methods included a brief phone screen followed by a clinic screening visit. Eligibility was determined based on blood and urine tests for medical eligibility, cotinine, and pregnancy, and responses on several self-report assessments (see Study Measures). Of 175 individuals screened, 74 participants were randomized and received at least one dose of study medication ( Supplementary Figure 1 ). All participants provided voluntary informed consent to participate, and the study was approved by the Johns Hopkins University Institutional Review Board.
Study Design
The study evaluation period was 10 weeks and participants were asked to attend visits once weekly. During visits participants received study medication, completed self-report measures of smoking, nicotine withdrawal, and craving, and provided a urine sample (see below). At three times during the study (Weeks 3, 7, and 10), vital signs and a blood sample was collected for safety monitoring. Participants who were abstinent at the end of treatment were contacted via phone 4 weeks after study discharge and invited to complete a follow-up assessment and provide a urine sample to confirm self-reported abstinence. Participants were compensated up to $400 for completion of all study visits and were eligible to receive an additional $200 as a completion bonus for study attendance and participation.
A target-quit date (TQD) was set for the 1st day of the 3rd week. All participants received the booklet “Clearing the Air: Quit Smoking Today” at entry into the study and completed three brief counseling sessions (Weeks 1–3). The counseling sessions reviewed the “Clearing the Air” booklet, provided tips for abstinence, prepared participants for their TQD, addressed concerns regarding nicotine withdrawal and weight gain, and provided strategies for maintaining abstinence. Cards with the 1-800-QUIT-NOW number were also provided to participants for additional support.
Study Medications
All participants received open-label varenicline, with a 10-day dose induction on the following schedule: placebo (Days 1–4), 0.5mg daily (Days 5–7), and 0.5mg twice daily (Days 8–10). The full maintenance dose of 1mg twice daily started on Day 11 (3 days prior to the TQD). Upon confirmation of study eligibility, the research pharmacist stratified participants to receive zonisamide (over-encapsulated) or weight-matched placebo capsules according to the following criteria: race (Caucasian/non-Caucasian), gender, and number of cigarettes per day (<21 vs. ≥21). Zonisamide dose induction occurred over 14 days on the following schedule: 100mg daily (Days 1–7), 200mg daily (Days 8–14), and 300mg daily beginning Day 15, coincident with the TQD and continued as maintenance dose. This dose was selected to be consistent with those administered for seizure control (100–600mg daily), while minimizing the potential for negative effects that could occur with a shorter induction period than what is used typically for seizure control. 31 At each visit, participants received a 1-week supply + 3 extra days of both varenicline and study capsules (containing either zonisamide or placebo) to provide coverage in the event an appointment needed to be re-scheduled. Medication was packaged in blister packs; all unused doses were collected at each subsequent visit and the reasons for any missed doses were documented.
Study Measures
At the Screening Visit, participants completed assessments of psychosocial functioning (see below), past 30-day smoking behavior using the Time-line Follow Back method, 32 and baseline ratings on the revised Minnesota Nicotine Withdrawal Scale (MNWS; past 7 days) 33 and the Questionnaire of Smoking Urges-Brief (QSU-B). 34 A locally developed demographic and smoking history questionnaire was administered to characterize the sample and assess prior smoking and quit attempts.
During each weekly visit, participants completed the Time-line Follow Back, MNWS, the QSU-B, and reported adverse events. Additional patient-reported outcome measures hypothesized to be sensitive to changes in smoking or study medication were collected at study Screening and periodically throughout the 10-week intervention; for brevity, the results from these measures will not be reported here. Smoking on the Time-line Follow Back was operationalized such that even 1 puff was recorded as 1 cigarette. Participants also completed the Medication Adherence Questionnaire 35 at weeks 1, 2, 3, 7, and 10, and were asked weekly to identify whether they believed they were receiving zonisamide or placebo to assess the fidelity of the medication blind. At the end of the study, participants completed an exit interview during which they were asked whether they had used any extra-study resources for smoking cessation (eg, quit-line, other pharmacotherapies or nicotine replacement), their opinion of the study medications, whether the study medication aided their quit attempt, and whether they would recommend one or both of the study medications for smoking cessation to other people. Participants who left the study early completed the exit interview on their final visit.
Urine samples were collected and tested weekly for cotinine using a semi-quantitative EMIT assay (>200ng/mL considered smoking) and for pregnancy every 4 weeks. Qualitative (positive/negative) urine testing for varenicline (1ng/mL) and zonisamide (1ng/mL) were conducted for Week 7 specimens to provide a point-prevalence assessment of medication adherence. This time-point was selected to coincide with the primary outcome variable (cessation during weeks 7–10 of the study). For participants who dropped out of the study prior to Week 7, or who missed that visit, the specimen temporally closest to their scheduled Week 7 visit was analyzed for medication adherence.
Data Analyses
The aim of this pilot study was to assess whether there was a positive signal that supported conducting a large-scale, randomized, controlled trial evaluation of zonisamide + varenicline for smoking cessation. The pilot study proposed to enroll 60 participants to assess the research hypotheses; due to successful recruitment efforts, the study was able to enroll 74 participants.
The primary study hypothesis was that zonisamide + varenicline would produce greater abstinence (defined as self-report + negative urine tests) during Weeks 7–10 compared with placebo + varenicline. Repeated measures analysis of variance was used for continuous longitudinal measures (self-reported cigarettes smoked), and repeated measures logistic regression (using Generalized Estimating Equations) was used for binary longitudinal measures (cotinine-positive urine specimens). Group comparisons of binary variables were performed using Fisher’s exact test; group comparisons of continuous variables that were not normally distributed (eg, percent abstinent and longest duration of abstinence) were analyzed using the Wilcoxon test.
Additional testing included between-group comparison of baseline demographics, cigarettes smoked per week, MNWS, QSU-B, point-prevalence medication adherence, body weight, and medication satisfaction using analysis of variance for continuous measures and Fisher’s exact tests for dichotomous measures. Secondary measures of cessation were compared across groups, and all smoking outcome data refer to the time-period following the TQD (weeks 3–10). Changes in the rate of qualitative and semi-quantitative cotinine results over time were compared across groups using Generalized Estimating Equations. Qualitative urine results were analyzed two ways: missing samples treated as missing data (missing–missing) and missing samples imputed as positive (missing–positive). Semi-quantitative results were analyzed as missing-missing only.
Smoking outcomes are presented here as intent-to-treat (ITT), which includes all participants who ingested at least one dose of study medication, and completer analyses, which includes only those participants who were continuing to attend visits at the end of the 10-week intervention. All analyses were conducted using the R statistical program version 3.1.0 (R Foundation for Statistical Computing; Vienna, Austria) and P ≤ .05 was considered statistically significant.
Results
Participants
A total of 74 participants were randomized and provided study medication (see Supplementary Figure 1 for CONSORT diagram). Participant demographics are presented in Table 1 . Thirty-four and 40 participants were randomized to receive zonisamide + varenicline (ZV) and placebo + varenicline (PV), respectively, and there were no statistically significant between-group differences for any demographic or smoking variables assessed. Of the 74 randomized participants, 61% ( n = 45) were considered study completers. Completion rate did not vary significantly as a function of medication group, with 53% ( n = 18) of the ZV and 68% ( n = 27) of the PV participants completing the study. As seen in Table 1 , participants endorsed an equal likelihood of receiving zonisamide or placebo throughout the weekly study visits, indicating the medication had been successfully blinded. Total number of visits completed ( Table 1 ) and a logrank test of survival were not significantly different across groups.
Table 1.
Participant Characteristics and Study Outcomes
| ITT | Completers | |||
|---|---|---|---|---|
| Zonisamide + varenicline ( N = 34) | Placebo + varenicline ( N = 40) | Zonisamide + varenicline ( N = 18) | Placebo + varenicline ( N = 27) | |
| Participant characteristics | ||||
| Age (y) | 45.3±9.8 | 45.9±9.7 | 46.8±9.4 | 46.6±10.3 |
| Male (%) | 76.5 | 67.5 | 72.2 | 66.7 |
| Caucasian (%) | 32.4 | 32.5 | 22.2 | 33.3 |
| Hispanic (%) | 0.0 | 2.5 | 0.0 | 3.7 |
| Employed (%) | 44.1 | 32.5 | 44.4 | 29.6 |
| Cigarettes per day (past 30 days) | 17.2±6.3 | 19.5±8.3 | 17.3±3.6 | 20.5±9.2 |
| Years smoked > 10 cigarettes per day | 13.7±10.5 | 18.2±12.9 | 13.0±10.6 | 19.3±14.0 |
| Previous serious quit attempt (%) | 73.5 | 82.5 | 77.8 | 88.9 |
| Study outcomes | ||||
| Smoking related outcomes a | ||||
| Abstinent weeks 7–10 (%) | 14.7 | 15.0 | 27.8 | 22.2 |
| Longest duration continuous abstinence (wk) b | 1.79±2.9 | 2.03±3.06 | 2.83±3.43 | 2.70±3.35 |
| Total visits attended (range 0–10) | 8.3±3.5 | 9.0±3.1 | 10.0 | 10.0 |
| Believed they were receiving zonisamide (% visits) | 54.9 | 52.6 | 54.5 | 49.3 |
| Medication adherence | ||||
| Mean percent adherence (assessed via pill count) (%) | ||||
| Varenicline | 76.7 | 59.8 | 76.1 | 77.0 |
| Study capsules | 73.3 | 73.0 | 84.7 | 86.4 |
| Urine testing confirmation of adherence (%) c | ||||
| Varenicline | 90.3 | 84.6 | 81.3 | 92.6 |
| Zonisamide | 100.0 | n/a | 100.0 | n/a |
No significant between-group comparisons within the intent-to-treat (ITT) and completer analyses; P values not shown values represent mean ± SD unless otherwise noted.
a Smoking outcomes based on urinary cotinine testing.
b Assessed as missing = positive only.
c Point prevalence testing at week 7.
Smoking Outcomes
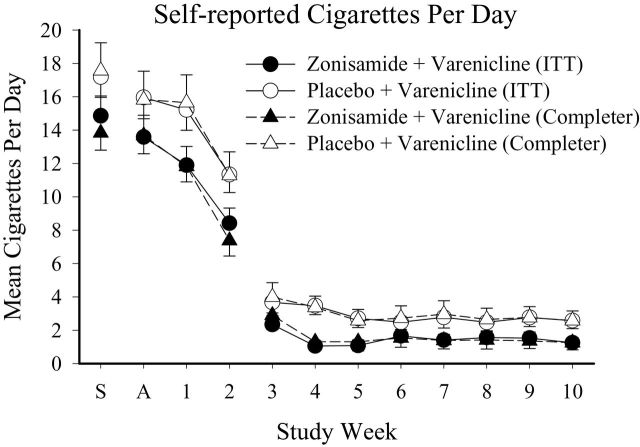
There was no difference in rates of biochemically-verified abstinence between medication conditions. Overall, 14.9% and 25.0% of participants provided continuous negative samples during weeks 7–10 of the study in the ITT and completer analyses, respectively. ZV participants were no more likely to remain abstinent during this timeframe than PV participants (ITT odds ratio [ OR ] = 0.98, 95% confidence interval [CI] = 0.21–4.30; completer OR = 1.34, 95% CI = 0.26–6.53). The abstinence rates also remained the same in both the ITT and completer analyses at the 4-week follow-up visit (14.9% and 25.0%), with no significant between-group differences, and closer inspection revealed that it was not always the same individuals abstaining at the 10 and 14-week visits. At the 4-week follow-up visit, abstinence was equally likely for the ZV compared to PV participants ( OR = 0.42, 95% CI = 0.03–4.00; completer OR = 0.42, 95% CI = 0.03–4.00). No significant between-group differences were observed for several additional secondary cessation variables ( Table 1 ) or semi-quantitative cotinine results ( Supplementary Figure 2 ) in the ITT or completer analyses. In contrast, self-reported number of cigarettes smoked per day did vary significantly across the groups ( Figure 1 ), with the ZV participants reporting smoking significantly fewer cigarettes relative to the PV participants in the ITT [ F (1,432) = 13.7, P < .001] and completer [ F (1,354) = 8.48, P < .01] analyses, though no significant effects of study visit or group × visit interactions were observed.
Figure 1.
Self-reported mean number of cigarettes smoked per day. X-axis presents study week; S = Screening, A = Admission to study. The target-quit day was Day 1 of Week 3. Results are presented as a function of intent-to-treat (ITT) (circle) and completer (triangle) analyses, and as a function of the Zonisamide + Varenicline (filled) and Placebo + Varenicline (open) groups. Error bars represent standard error of the mean ( SEM ).
Medication Adherence
The number of participants who reported forgetting to take their medication in both the ITT and completer analyses did increase slightly over time ( z = 1.99, P = .047 for ITT, z = 2.15, P = .031 for completers); though medication group was not statistically significant in these models. Weekly pill counts of medications revealed good adherence with varenicline and study capsules in both the ITT (68.3%, 73.2%) and completer (76.6%, 85.6%) groups, respectively. This was supported by point-prevalence urinalysis testing, which indicated 87.5% (ITT) and 87.0% (completer) adherence for varenicline, and 100% adherence for zonisamide; between-group differences were not significant.
Patient-Reported Outcomes
Nicotine Withdrawal
Significant main effects of study group [ F (1,713) = 9.20, P < .01] and study visit [ F (1,713) = 14.3, P < .001] were observed on the MNWS total score in the ITT analysis. Withdrawal decreased significantly throughout the study in both medication groups. Analysis of individual symptoms revealed significant main effects of group and study visit for several items in the ITT ( Table 2 ) and completer ( Table 3 ) analysis. There were no significant differences in average ( SD ) weight gain across the two groups, with the ZV and PV groups gaining 4.39 (9.0) and 3.75 (13.5) pounds, respectively.
Table 2.
Intent-To-Treat (ITT) Withdrawal and Craving Analyses
| Means | Group | Study visit | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | A | 1 | 2 | 3/TQD | 4 | 5 | 6 | 7 | 8 | 9 | 10 | F (1,713) | P | F (1,713) | P | |
| Nicotine withdrawal a | ||||||||||||||||
| Total scale score (0–48) | ||||||||||||||||
| Placebo + Var | 5.6 | 7.0 | 6.2 | 5.7 | 6.6 | 5.9 | 4.7 | 4.0 | 4.4 | 4.0 | 4.1 | 4.8 | 9.2 | .0025 | 14.3 | .0002 |
| Zonisamide + Var | 4.6 | 5.1 | 5.3 | 4.8 | 4.7 | 3.8 | 3.8 | 3.4 | 3.8 | 2.7 | 3.1 | 2.6 | ||||
| Anger (0–4) | ||||||||||||||||
| Placebo + Var | 1.3 | 1.3 | 1.0 | 1.0 | 1.1 | 1.0 | 0.8 | 0.8 | 0.7 | 0.7 | 0.6 | 0.7 | 6.91 | .0087 | 24.3 | 1 × 10 −6 |
| Zonisamide + Var | 0.9 | 0.9 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.6 | 0.6 | 0.6 | 0.5 | 0.4 | ||||
| Anxiety (0–4) | ||||||||||||||||
| Placebo + Var | 0.9 | 0.9 | 0.9 | 0.8 | 0.8 | 0.7 | 0.6 | 0.4 | 0.5 | 0.4 | 0.3 | 0.5 | 5.34 | .02 | 26.3 | 3.8 × 10 −7 |
| Zonisamide + Var | 0.8 | 0.7 | 0.8 | 0.5 | 0.5 | 0.4 | 0.5 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | ||||
| Constipation (0–4) | ||||||||||||||||
| Placebo + Var | 0.2 | 0.4 | 0.4 | 0.4 | 0.6 | 0.4 | 0.2 | 0.1 | 0.1 | 0.2 | 0.0 | 0.2 | 5.27 | .02 | 0.97 | .33 |
| Zonisamide + Var | 0.2 | 0.2 | 0.3 | 0.7 | 0.5 | 0.5 | 0.5 | 0.3 | 0.4 | 0.5 | 0.3 | 0.4 | ||||
| Coughing (0–4) | ||||||||||||||||
| Placebo + Var | 0.6 | 0.6 | 0.4 | 0.5 | 0.7 | 0.5 | 0.5 | 0.4 | 0.2 | 0.6 | 0.4 | 0.3 | 4.22 | .04 | 15.5 | 8.9 × 10 −5 |
| Zonisamide + Var | 0.6 | 0.7 | 0.5 | 0.3 | 0.3 | 0.5 | 0.3 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | ||||
| Craving (0–4) | ||||||||||||||||
| Placebo + Var | 2.6 | 2.6 | 2.0 | 1.8 | 1.8 | 1.5 | 1.4 | 1.2 | 1.2 | 1.0 | 1.0 | 1.2 | 4.24 | .04 | 169 | 2 × 10 −16 |
| Zonisamide + Var | 2.4 | 2.4 | 2.4 | 1.8 | 1.5 | 0.8 | 1.0 | 1.2 | 0.9 | 0.8 | 0.8 | 0.9 | ||||
| Depressed (0–4) | ||||||||||||||||
| Placebo + Var | 0.4 | 0.5 | 0.7 | 0.5 | 0.4 | 0.5 | 0.5 | 0.5 | 0.4 | 0.2 | 0.4 | 0.6 | 3.94 | .05 | 1.62 | .20 |
| Zonisamide + Var | 0.4 | 0.3 | 0.4 | 0.6 | 0.4 | 0.3 | 0.3 | 0.4 | 0.4 | 0.2 | 0.3 | 0.2 | ||||
| Difficulty concentrating (0–4) | ||||||||||||||||
| Placebo + Var | 0.5 | 0.5 | 0.5 | 0.6 | 0.7 | 0.6 | 0.4 | 0.3 | 0.3 | 0.2 | 0.1 | 0.4 | 0.11 | .74 | 6.23 | .013 |
| Zonisamide + Var | 0.4 | 0.5 | 0.5 | 0.5 | 0.4 | 0.4 | 0.4 | 0.5 | 0.6 | 0.3 | 0.4 | 0.4 | ||||
| Dizziness (0–4) | ||||||||||||||||
| Placebo + Var | 0.1 | 0.1 | 0.2 | 0.3 | 0.3 | 0.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.3 | 2.36 | .13 | 0.6 | .44 |
| Zonisamide + Var | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.2 | ||||
| Impatient (0–4) | ||||||||||||||||
| Placebo + Var | 1.0 | 1.0 | 0.9 | 0.8 | 1.1 | 0.9 | 0.6 | 0.6 | 0.6 | 0.8 | 0.7 | 0.7 | 17.7 | 3 × 10 −5 | 9.23 | .0025 |
| Zonisamide + Var | 0.8 | 0.7 | 0.6 | 0.6 | 0.4 | 0.4 | 0.4 | 0.4 | 0.5 | 0.2 | 0.4 | 0.4 | ||||
| Insomnia (0–4) | ||||||||||||||||
| Placebo + Var | 0.7 | 1.4 | 0.9 | 0.6 | 0.9 | 0.9 | 0.6 | 0.6 | 0.7 | 0.7 | 0.6 | 0.8 | 5.93 | .02 | 7.57 | .0061 |
| Zonisamide + Var | 0.5 | 0.8 | 0.8 | 0.9 | 0.7 | 0.7 | 0.6 | 0.5 | 0.5 | 0.5 | 0.3 | 0.3 | ||||
| Nausea (0–4) | ||||||||||||||||
| Placebo + Var | 0.1 | 0.2 | 0.3 | 0.8 | 0.5 | 0.5 | 0.4 | 0.4 | 0.3 | 0.3 | 0.3 | 0.5 | 4.93 | .03 | 0.86 | .35 |
| Zonisamide + Var | 0.0 | 0.0 | 0.4 | 0.6 | 0.3 | 0.4 | 0.2 | 0.3 | 0.1 | 0.4 | 0.3 | 0.0 | ||||
| Restless (0–4) | ||||||||||||||||
| Placebo + Var | 0.5 | 0.9 | 0.8 | 0.7 | 0.9 | 0.7 | 0.6 | 0.5 | 0.6 | 0.6 | 0.7 | 0.7 | 11.0 | .0009 | 5.65 | .018 |
| Zonisamide + Var | 0.4 | 0.6 | 0.6 | 0.8 | 0.6 | 0.4 | 0.4 | 0.2 | 0.4 | 0.2 | 0.3 | 0.2 | ||||
| QSU-B craving b | ||||||||||||||||
| Factor 1 (0–35) | ||||||||||||||||
| Placebo + Var | 11.8 | 16.8 | 22.5 | 25.1 | 27.9 | 28.2 | 28.4 | 29.6 | 29.5 | 27.3 | 29.9 | 29.1 | 1.23 | .27 | 122.6 | 2 × 10 −16 |
| Zonisamide + Var | 14.2 | 18.9 | 23.8 | 26.8 | 28.6 | 29.1 | 29.6 | 31.2 | 30.1 | 28.7 | 29.3 | 27.1 | ||||
| Factor 2 (0–35) | ||||||||||||||||
| Placebo + Var | 20.8 | 24.4 | 26.6 | 27.4 | 29.6 | 29.4 | 29.5 | 31.4 | 31.1 | 29.0 | 30.9 | 31.1 | 0.73 | .40 | 26.8 | 2.9 × 10 −7 |
| Zonisamide + Var | 21.0 | 25.4 | 24.8 | 28.0 | 29.7 | 29.6 | 29.8 | 31.5 | 30.8 | 29.5 | 29.4 | 27.2 | ||||
A = admission to study; QSU-B = Questionnaire of Smoking Urges-Brief; S = screening; TQD = target-quit date.
a Values rated as 0 = none to 4 = severe. Higher values indicate greater withdrawal severity.
b Values rated as 1 = strongly agree to 7 = strongly disagree. Lower values indicate greater craving.
Table 3.
Completer Withdrawal and Craving Analyses
| Means | Group | Study visit | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | A | 1 | 2 | 3/TQD | 4 | 5 | 6 | 7 | 8 | 9 | 10 | F (1,534) | P | F (1,534) | P | |
| Nicotine withdrawal a | ||||||||||||||||
| Total scale score (0–48) | ||||||||||||||||
| Placebo + Var | 4.7 | 6.0 | 4.3 | 4.6 | 5.7 | 4.9 | 4.1 | 4.1 | 4.5 | 4.0 | 4.1 | 3.7 | 2.12 | .15 | 8.74 | .0033 |
| Zonisamide + Var | 4.5 | 5.7 | 4.1 | 5.3 | 5.4 | 4.1 | 3.4 | 3.2 | 4.0 | 2.6 | 3.2 | 1.6 | ||||
| Anger (0–4) | ||||||||||||||||
| Placebo + Var | 1.0 | 1.0 | 0.7 | 0.9 | 0.9 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.6 | 0.6 | 1.36 | .24 | 8.48 | .0037 |
| Zonisamide + Var | 0.7 | 1.1 | 0.6 | 0.8 | 0.9 | 0.7 | 0.6 | 0.6 | 0.6 | 0.5 | 0.6 | 0.4 | ||||
| Anxiety (0–4) | ||||||||||||||||
| Placebo + Var | 0.8 | 0.9 | 0.6 | 0.6 | 0.6 | 0.5 | 0.5 | 0.4 | 0.5 | 0.4 | 0.3 | 0.4 | 0.93 | .33 | 13.7 | .0002 |
| Zonisamide + Var | 0.6 | 0.7 | 0.4 | 0.7 | 0.6 | 0.4 | 0.4 | 0.3 | 0.4 | 0.3 | 0.3 | 0.2 | ||||
| Constipation (0–4) | ||||||||||||||||
| Placebo + Var | 0.2 | 0.3 | 0.3 | 0.3 | 0.5 | 0.4 | 0.2 | 0.1 | 0.1 | 0.2 | 0.0 | 0.1 | 1.93 | .17 | 2.74 | .10 |
| Zonisamide + Var | 0.2 | 0.1 | 0.2 | 0.6 | 0.6 | 0.5 | 0.4 | 0.2 | 0.4 | 0.3 | 0.3 | 0.3 | ||||
| Coughing (0–4) | ||||||||||||||||
| Placebo + Var | 0.4 | 0.8 | 0.3 | 0.6 | 0.7 | 0.6 | 0.5 | 0.4 | 0.2 | 0.6 | 0.4 | 0.3 | 9.39 | .0017 | 10.3 | .0014 |
| Zonisamide + Var | 0.5 | 0.4 | 0.3 | 0.3 | 0.3 | 0.6 | 0.2 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | ||||
| Craving (0–4) | ||||||||||||||||
| Placebo + Var | 2.5 | 2.4 | 1.6 | 1.6 | 1.6 | 1.3 | 1.3 | 1.2 | 1.2 | 1.0 | 1.0 | 1.0 | 1.81 | .18 | 100.6 | 2 × 10 −16 |
| Zonisamide + Var | 2.2 | 2.2 | 2.2 | 2.0 | 1.5 | 0.7 | 0.9 | 1.2 | 0.8 | 0.7 | 0.8 | 0.7 | ||||
| Depressed (0–4) | ||||||||||||||||
| Placebo + Var | 0.4 | 0.4 | 0.3 | 0.2 | 0.4 | 0.4 | 0.4 | 0.5 | 0.5 | 0.2 | 0.4 | 0.3 | 0.57 | .45 | 1.06 | .3 |
| Zonisamide + Var | 0.3 | 0.3 | 0.4 | 0.6 | 0.4 | 0.3 | 0.1 | 0.4 | 0.4 | 0.2 | 0.3 | 0.1 | ||||
| Difficulty concentrating (0–4) | ||||||||||||||||
| Placebo + Var | 0.3 | 0.5 | 0.4 | 0.5 | 0.5 | 0.5 | 0.3 | 0.3 | 0.3 | 0.2 | 0.1 | 0.2 | 3.15 | .08 | 6.23 | .01 |
| Zonisamide + Var | 0.4 | 0.5 | 0.6 | 0.7 | 0.6 | 0.4 | 0.4 | 0.5 | 0.5 | 0.3 | 0.4 | 0.3 | ||||
| Dizziness (0–4) | ||||||||||||||||
| Placebo + Var | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.11 | .74 | 4.24 | .04 |
| Zonisamide + Var | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0.3 | 0.1 | 0.0 | 0.2 | 0.1 | 0.0 | 0.1 | ||||
| Impatient (0–4) | ||||||||||||||||
| Placebo + Var | 0.9 | 0.7 | 0.7 | 0.7 | 0.9 | 0.7 | 0.4 | 0.6 | 0.6 | 0.8 | 0.7 | 0.6 | 10.7 | .0011 | 2.78 | .10 |
| Zonisamide + Var | 0.7 | 0.8 | 0.4 | 0.5 | 0.4 | 0.4 | 0.3 | 0.3 | 0.6 | 0.2 | 0.4 | 0.2 | ||||
| Insomnia (0–4) | ||||||||||||||||
| Placebo + Var | 0.7 | 1.3 | 0.7 | 0.7 | 0.9 | 0.8 | 0.6 | 0.6 | 0.8 | 0.7 | 0.6 | 0.6 | 3.57 | .06 | 11.8 | .0006 |
| Zonisamide + Var | 0.6 | 0.9 | 0.7 | 1.0 | 1.1 | 0.8 | 0.5 | 0.4 | 0.6 | 0.4 | 0.3 | 0.1 | ||||
| Nausea (0–4) | ||||||||||||||||
| Placebo + Var | 0.1 | 0.1 | 0.2 | 0.6 | 0.4 | 0.4 | 0.4 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | 3.22 | .07 | 0.42 | .52 |
| Zonisamide + Var | 0.0 | 0.0 | 0.3 | 0.6 | 0.2 | 0.4 | 0.2 | 0.4 | 0.2 | 0.2 | 0.3 | 0.0 | ||||
| Restless (0–4) | ||||||||||||||||
| Placebo + Var | 0.4 | 0.6 | 0.6 | 0.6 | 0.7 | 0.6 | 0.5 | 0.5 | 0.6 | 0.6 | 0.7 | 0.6 | 4.64 | .03 | 2.00 | .16 |
| Zonisamide + Var | 0.4 | 0.7 | 0.7 | 0.7 | 0.7 | 0.5 | 0.4 | 0.2 | 0.5 | 0.2 | 0.2 | 0.1 | ||||
| QSU-B craving b | ||||||||||||||||
| Factor 1 (0–35) | ||||||||||||||||
| Placebo + Var | 12.1 | 18.1 | 22.7 | 26.2 | 29.3 | 29.3 | 29.5 | 29.5 | 29.3 | 27.3 | 29.9 | 30.3 | 0.20 | .66 | 79.2 | 2 × 10 −16 |
| Zonisamide + Var | 15.5 | 18.6 | 23.7 | 24.8 | 28.1 | 28.0 | 28.1 | 29.9 | 29.1 | 28.6 | 29.0 | 27.8 | ||||
| Factor 2 (0–35) | ||||||||||||||||
| Placebo + Var | 20.7 | 24.4 | 26.4 | 28.3 | 30.7 | 30.4 | 30.7 | 31.3 | 31.0 | 29.0 | 30.9 | 31.7 | 5.32 | .02 | 20.6 | 7 × 10 −16 |
| Zonisamide + Var | 21.0 | 23.7 | 24.4 | 25.9 | 27.7 | 27.9 | 28.3 | 30.4 | 29.9 | 28.9 | 29.1 | 28.0 | ||||
A = admission to study; QSU-B = Questionnaire of Smoking Urges-Brief; S = screening; TQD = target-quit date.
a Values rated as 0 = none to 4 = severe. Higher values indicate greater withdrawal severity.
b Values rated as 1 = strongly agree to 7 = strongly disagree. Lower values indicate greater craving.
Withdrawal as a function of group was also compared among participants classified as abstainers (eg, continuously biochemically-negative during weeks 7–10; n = 11; ZV = 5, PV = 6) and non-abstainers ( n = 63, ZV = 29, PV = 34). Analyses indicated a main effect of zonisamide on withdrawal symptoms within both the abstainer ( P = .04) and non-abstainer ( P < .001) categories, but no significant effect of visit or group × visit interaction. A nonsignificant trends towards a group × visit interaction was observed among abstainers who received zonisamide, relative to abstainers who received placebo.
Craving
The ITT analysis revealed that craving measured by the QSU-B decreased significantly throughout the study in both groups as a function of study visit, though no significant between-group or group × visit interactions were observed. These effects were evident across both Factor 1 (craving related to positive effects of smoking) and Factor 2 (craving related to avoidance of withdrawal) within the ITT ( Table 2 ) and completer ( Table 3 ) analyses. The completer analysis also revealed a significant effect of study group on Factor 2 of the QSU-B.
Adverse Effects
A total of 57 participants (77% of sample) reported an adverse event ( Supplementary Table 1 ). There were no significant between-group differences in the frequency of adverse events rated as mild [ F (1,72) = 16.3, P = .06], moderate [ F (1,72) = 0.67, P = .42], or severe [ F (1,72) = 0.93, P = .34]. Self-reported responses to questions regarding negative medication effects were collapsed across all visits and study groups and only a minority of participants endorsed feeling sad/depressed (23.6%) or hostile (8.3%). More participants reported feeling easily agitated (43.1%), though this result overlapped with the time course of nicotine withdrawal. Results did not vary significantly between the two groups.
Study Satisfaction
The ITT analysis revealed that participants rated the counseling favorably (7.6 [2.4] out of 10), and few participants reported using other smoking cessation resources during the study (14.5%) or contacting the quit line (14.5%). The majority of participants in both the ZV and PV groups reported that the medication helped them to abstain from smoking (95.4% vs. 93.9%), that they would request this combination of medications from their doctor if it were available (81.8% vs. 72.7%), and that they would recommend this combination of medications to someone who was trying to stop smoking (90.9% vs. 87.8%), respectively. None of these values differed significantly as a function of medication group.
Discussion
This was a double-blind, randomized, placebo-controlled pilot study to evaluate whether zonisamide combined with varenicline would increase rates of smoking abstinence relative to varenicline alone. This study adds to the growing approach of combining varenicline with other medications to enhance overall smoking cessation outcomes. 10 , 11 , 13 , 14 , 36 The results of this study indicate that zonisamide did not significantly enhance cotinine-verified smoking cessation among participants who were prescribed open-label varenicline, relative to placebo. However, results do suggest that zonisamide may have some efficacy in reducing the self-report of smoking, the magnitude of nicotine withdrawal, and elements of craving, suggesting that drugs that target novel systems, such as the GABA and glutamate system, may be a valuable treatment strategy for smoking cessation that warrants additional research attention.
Meta-analytic reviews vary regarding the rate of abstinence that is expected following varenicline administration, with a recent report estimating 49% of patients receiving varenicline are abstinent during the final 4 weeks of treatment. 5 The rates of biochemically-verified abstinence achieved in the ITT and completer analyses here (14.9%–25.0%) are lower than what has been previously reported, however the current methods differ from existing research in a critical way by relying on urinary cotinine (vs. carbon monoxide) as an index of abstinence. This is a departure from previous studies, which relied exclusively on point-prevalence carbon monoxide testing for assessing abstinence 5 and therefore had a detection period of only approximately 6 hours, 37 versus 5–7 days with cotinine. The results obtained here are consistent with the results of a limited number of other studies that utilized cotinine as the primary index of abstinence (0%–23.6% at end of a 12-week treatment 38 , 39 ), which serves as a positive control for the varenicline effect.
It should be noted that self-reported cigarettes per day were significantly reduced among participants who received zonisamide + varenicline, in both the ITT and completer analyses. Though these reductions were not large enough to be reflected in urinary cotinine testing, they suggest that zonisamide may have reduced some smoking behavior. Research using larger doses of zonisamide could be considered to assess whether they might produce larger reductions in smoking. These results add to previous smoking cessation trials that reported a positive signal on smoking cessation using topiramate, a medication with a mechanism of action similar to zonisamide, 23–25 and administered at doses considered equipotent to the dose of zonisamide utilized here. Ultimately, these data support additional research evaluating medications that target the GABA and glutamate systems for smoking cessation. 23–25 , 40
This study hypothesized that zonisamide may enhance varenicline-induced smoking outcomes by treating symptoms of nicotine withdrawal not otherwise addressed by varenicline. Results revealed significant between-group differences for both the ITT and completer analyses on the MNWS and in the completer analysis for Factor 2 of the QSU-B (which captures craving related to avoidance of smoking withdrawal symptoms), on several individual withdrawal symptoms (including craving, anger, anxiety, insomnia, restless, impatient, and coughing), and within participants who completely abstained during weeks 7–10 of the study. Inspection of these latter data revealed a nonsignificant trend towards a group × visit interaction in MNWS ratings among participants who received zonisamide. This effect was likely underpowered, but these results suggest that zonisamide may have a persistent effect on withdrawal that could potentially delay relapse to smoking; this effect warrants additional research attention. Differences between Factor 2 of the QSU-B and the craving item of the MNWS are likely attributable to the fact that the QSU-B subscale score is derived from several different items, whereas the MNWS asks a single craving item that is not able to differentiate between craving for positive effects or avoidance of negative effects. As a result, these two values represent qualitatively different domains. Objective indicators of adherence did not differ between the groups, suggesting the outcomes are not due to differential medication adherence. No significant effect on weight gain was observed in this study. The lack of between-group difference in weight may be due, in part, to the short duration of the study (10 weeks). Ideally, this analysis would have evaluated changes in weight during the first few weeks of abstinence, when cessation was just occurring and nicotine withdrawal ratings were highest. However, due to logistical constraints, there were 4 weeks imposed between the first and second weight measurement, which reduced the sensitivity needed to more closely evaluate changes in weight during the active quit attempt.
Although the study hypothesized that zonisamide would reduce nicotine withdrawal symptoms, these data indicate that reductions in nicotine withdrawal severity and craving were not sufficient to affect cotinine-based quit rates. These outcomes are consistent with two previous studies that combined nicotine replacement products (nicotine replacement therapy) with varenicline to increase rates of smoking abstinence, which reported that varenicline + nicotine replacement therapy significantly reduced MNWS ratings relative to varenicline alone but did not impact cessation. 10 , 11 The only study that showed a positive benefit of varenicline + nicotine replacement therapy, relative to varenicline + placebo, on smoking cessation outcomes reported no effect of the combined product on craving for smoking or withdrawal ratings. 12 Together with our results, these data suggest that efforts to reduce nicotine withdrawal symptoms in individuals on varenicline may need to produce a greater effect in order to impact smoking cessation.
Despite the overall lack of between-group differences in the smoking outcomes observed, participants generally liked the resources provided by the study and were willing to recommend the study medications to others. There was also minimal endorsement of psychiatric events and varenicline and zonisamide appeared to have been well-tolerated by the participants, which increases the feasibility of evaluating medications that impact novel systems for smoking cessation.
This study has several strengths, including a double-blind, randomized treatment design and reliance upon urinary cotinine as an objective index of smoking status. Although there was a null effect of zonisamide on cotinine outcomes, there are several positive controls in the study that strengthen the validity of the results, including the fact that cotinine decreased and self-reported abstinence increased in the week following the TQD day, a finding consistently observed in individuals who are attempting to quit. Moreover, nicotine withdrawal and craving both decreased as the number of cigarettes smoked per day decreased. Limitations of this study include the fact that participants did not receive the indicated 12-week treatment of varenicline and that we did not exclude or control for ongoing illicit drug use during the study. We learned that several participants were concurrently enrolled in methadone maintenance programs, which has been previously associated with a reduced response to varenicline for smoking interventions 41 and with increasing reinforcing effects of smoking, 42 and therefore may have impacted outcomes.
In conclusion, the results of this study suggest that, consistent with the study hypothesis, zonisamide may reduce the severity of certain nicotine withdrawal symptoms when given in combination with varenicline, though the magnitude of this effect may not yet be sufficient to translate into differences in biochemically-confirmed cessation outcomes. Nevertheless, this study adds to previous studies that demonstrated a positive effect of topiramate on smoking outcomes, and provides additional evidence that the GABA and/or glutamate system may be a promising target for future studies on pharmacologic interventions for nicotine dependence and the identification of new therapeutic modalities for smoking cessation.
Funding
This study was funded by National Institute on Drug Abuse (DA034164).
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
We would like to thank Claudia Yepez-Laubach, Hye Jeong Han, Sharon Henderson, Ashley Crowner, Vishka Correya, and Leticia Nanda for their assistance with the study. This study was registered on http://clinicaltrials.gov (NCT01685996).
References
- 1. Ng M, Freeman MK, Fleming TD, et al. . Smoking prevalence and cigarette consumption in 187 countries, 1980-2012 . JAMA . 2014. ; 311 ( 2 ): 183 – 192 . [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Tobacco . Fact sheet N339 . 2015. . www.who.int/mediacentre/factsheets/fs339/en/ . Accessed October 26, 2015. [Google Scholar]
- 3. U.S. Department of Health and Human Services . The Health Consequences of Smoking- 50 Year of Progress. A Report of the Surgeon General . Atlanta, GA: U.S: . Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; . 2014. . [Google Scholar]
- 4. Cahill K, Stevens S, Perera R, Lancaster T . Pharmacological interventions for smoking cessation: an overview and network meta-analysis . Cochrane Database Syst Rev . 2013. ; 5 : CD009329 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agboola SA, Coleman T, McNeill A, Leonardi-Bee J . Abstinence and relapse amongst smokers who use varenicline in a quit attempt - a pooled analysis of randomized controlled trials . Addiction . 2015. ; 110 ( 7 ): 1182 – 1193 . doi: 10.1111/add.12941 . [DOI] [PubMed] [Google Scholar]
- 6. Gonzales D, Rennard SI, Nides M, et al. ; Varenicline Phase 3 Study Group . Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial . JAMA . 2006. ; 296 ( 1 ): 47 – 55 . [DOI] [PubMed] [Google Scholar]
- 7. Nides M, Oncken C, Gonzales D, et al. . Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up . Arch Intern Med . 2006. ; 166 ( 15 ): 1561 – 1568 . [DOI] [PubMed] [Google Scholar]
- 8. Oncken C, Gonzales D, Nides M, et al. . Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation . Arch Intern Med . 2006. ; 166 ( 15 ): 1571 – 1577 . [DOI] [PubMed] [Google Scholar]
- 9. Stapleton J, West R, Hajek P, et al. . Randomized trial of nicotine replacement therapy (NRT), bupropion and NRT plus bupropion for smoking cessation: effectiveness in clinical practice . Addiction . 2013. ; 108 ( 12 ): 2193 – 2201 . doi: 10.1111/add.12304 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hajek P, Smith KM, Dhanji AR, McRobbie H . Is a combination of varenicline and nicotine patch more effective in helping smokers quit than varenicline alone? A randomised controlled trial . BMC Med . 2013. ; 11 : 140 . doi: 10.1186/1741-7015-11-140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramon JM, Morchon S, Baena A, Masuet-Aumatell C . Combining varenicline and nicotine patches: a randomized controlled trial study in smoking cessation . BMC Med . 2014. ; 12 : 172 . doi: 10.1186/s12916-014-0172-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koegelenberg CF, Noor F, Bateman ED, et al. . Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial . JAMA . 2014. ; 312 ( 2 ): 155 – 161 . doi: 10.1001/jama.2014.7195 . [DOI] [PubMed] [Google Scholar]
- 13. Ebbert JO, Hatsukami DK, Croghan IT, et al. . Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial . JAMA . 2014. ; 311 ( 2 ): 155 – 163 .doi: 10.1001/jama.2013.283185 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rose JE, Behm FM . Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm . Am J Psychiatry . 2014. ; 171 ( 11 ): 1199 – 1205 . doi: 10.1176/appi.ajp.2014.13050595 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okada M, Kaneko S, Hirano T, et al. . Effects of zonisamide on dopaminergic system . Epilepsy Res . 1995. ; 22 ( 3 ): 193 – 205 . [DOI] [PubMed] [Google Scholar]
- 16. Murata M, Horiuchi E, Kanazawa I . Zonisamide has beneficial effects on Parkinson’s disease patients . Neurosci Res . 2001. ; 41 ( 4 ): 397 – 399 . [DOI] [PubMed] [Google Scholar]
- 17. Sonsalla PK, Wong LY, Winnik B, Buckley B . The antiepileptic drug zonisamide inhibits MAO-B and attenuates MPTP toxicity in mice: clinical relevance . Exp Neurol . 2010. ; 221 ( 2 ): 329 – 334 . doi: 10.1016/j.expneurol.2009.11.018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knackstedt LA, LaRowe S, Mardikian P, et al. . The role of cystine-glutamate exchange in nicotine dependence in rats and humans . Biol Psychiatry . 2009. ; 65 ( 10 ): 841 – 845 . doi: 10.1016/j.biopsych.2008.10.040 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knackstedt LA, Melendez RI, Kalivas PW . Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking . Biol Psychiatry . 2010. ; 67 ( 1 ): 81 – 84 . doi: 10.1016/j.biopsych.2009.07.018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamamura S, Saito H, Suzuki N, et al. . Effects of zonisamide on neurotransmitter release associated with inositol triphosphate receptors . Neurosci Lett . 2009. ; 454 ( 1 ): 91 – 96 . doi: 10.1016/j.neulet.2009.02.065 . [DOI] [PubMed] [Google Scholar]
- 21. Domecq JP, Prutsky G, Leppin A, et al. . Drugs commonly associated with weight change: a systematic review and meta-analysis . J Clin Endocrinol Metab . 2015. ; 100 ( 2 ): 363 – 370 . doi: 10.1210/jc.2014-3421 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farley AC, Hajek P, Lycett D, Aveyard P . Interventions for preventing weight gain after smoking cessation . Cochrane Database Syst Rev . 2012. ; 1 : CD006219 . doi: 10.1002/14651858.CD006219.pub3 . [DOI] [PubMed] [Google Scholar]
- 23. Oncken C, Arias AJ, Feinn R, et al. . Topiramate for smoking cessation: a randomized, placebo-controlled pilot study . Nicotine Tob Res . 2014. ; 16 ( 3 ): 288 – 296 . doi: 10.1093/ntr/ntt141 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baltieri DA, Daró FR, Ribeiro PL, Andrade AG . Effects of topiramate or naltrexone on tobacco use among male alcohol-dependent outpatients . Drug Alcohol Depend . 2009. ; 105 ( 1–2 ): 33 – 41 . doi: 10.1016/j.drugalcdep.2009.05.025 . [DOI] [PubMed] [Google Scholar]
- 25. Johnson BA, Ait-Daoud N, Akhtar FZ, Javors MA . Use of oral topiramate to promote smoking abstinence among alcohol-dependent smokers: a randomized controlled trial . Arch Intern Med . 2005. ; 165 ( 14 ): 1600 – 1605 . [DOI] [PubMed] [Google Scholar]
- 26. Verrotti A, Loiacono G, Di Sabatino F, Zaccara G . The adverse event profile of zonisamide: a meta-analysis . Acta Neurol Scand . 2013. ; 128 : 297 – 304 . doi: 10.1111/ane.12147 . [DOI] [PubMed] [Google Scholar]
- 27. Mula M . Topiramate and cognitive impairment: evidence and clinical implications . Ther Adv Drug Saf . 2012. ; 3 ( 6 ): 279 – 289 . doi: 10.1177/2042098612455357 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Biener L, Abrams DB . The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation . Health Psychol . 1991. ; 10 ( 5 ): 360 – 365 . [DOI] [PubMed] [Google Scholar]
- 29. Beck AT, Brown G, Steer RA. Beck Depression Inventory II Manual . San Antonio, TX: The Psychological Corporation; 1996.
- 30. Osman A, Bagge CL, Gutierrez PM, Konick LC, Kopper BA, Barrios FX . The Suicidal Behaviors Questionnaire-Revised (SBQ-R): validation with clinical and nonclinical samples . Assessment . 2001. ; 8 ( 4 ): 443 – 454 . [DOI] [PubMed] [Google Scholar]
- 31. Leppik IE . Practical prescribing and long-term efficacy and safety of zonisamide . Epilepsy Res . 2006. ; 68 ( suppl 2 ): S17 – S24 . [DOI] [PubMed] [Google Scholar]
- 32. Sobell LC, Sobell MB . Timeline follow-back: a technique for assessing self-reported alcohol consumption . In: Litten RZ, Allen JP , eds. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods . Totowa, NJ: : Humana Press; ; 1992. : 41 . [Google Scholar]
- 33. Hughes JR, Hatsukami D . Signs and symptoms of tobacco withdrawal . Arch Gen Psychiatry . 1986. ; 43 ( 3 ): 289 – 294 . [DOI] [PubMed] [Google Scholar]
- 34. Cox LS, Tiffany ST, Christen AG . Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings . Nicotine Tob Res . 2001. ; 3 ( 1 ): 7 – 16 . [DOI] [PubMed] [Google Scholar]
- 35. Toll BA, McKee SA, Martin DJ, Jatlow P, O’Malley SS . Factor structure and validity of the Medication Adherence Questionnaire (MAQ) with cigarette smokers trying to quit . Nicotine Tob Res . 2007. ; 9 ( 5 ): 597 – 605 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ebbert JO, Croghan IT, Sood A, Schroeder DR, Hays JT, Hurt RD . Varenicline and bupropion sustained-release combination therapy for smoking cessation . Nicotine Tob Res . 2009. ; 11 ( 3 ): 234 – 239 . [DOI] [PubMed] [Google Scholar]
- 37. SRNT Subcommittee on Biochemical Verification . Biochemical verification of tobacco use and cessation . Nicotine Tob Res . 2002. ; 4 ( 2 ): 149 – 159 . [DOI] [PubMed] [Google Scholar]
- 38. Gray KM, Carpenter MJ, Lewis AL, Klintworth EM, Upadhyaya HP . Varenicline versus bupropion XL for smoking cessation in older adolescents: a randomized, double-blind pilot trial . Nicotine Tob Res . 2012. ; 14 ( 2 ): 234 – 239 . doi: 10.1093/ntr/ntr130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nollen NL, Cox LS, Nazir N, et al. . A pilot clinical trial of varenicline for smoking cessation in black smokers . Nicotine Tob Res . 2011. ; 13 ( 9 ): 868 – 873 . doi: 10.1093/ntr/ntr063 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McClure EA, Baker NL, Gipson CD, et al. . An open-label pilot trial of N-acetylcysteine and varenicline in adult cigarette smokers . Am J Drug Alcohol Abuse . 2015. ; 41 ( 1 ): 52 – 56 . doi: 10.3109/00952990.2014.933839 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stein MD, Caviness CM, Kurth ME, Audet D, Olson J, Anderson BJ . Varenicline for smoking cessation among methadone-maintained smokers: a randomized clinical trial . Drug Alcohol Depend . 2013. ; 133 ( 2 ): 486 – 493 . doi: 10.1016/j.drugalcdep.2013.07.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chait LD, Griffiths RR . Effects of methadone on human cigarette smoking and subjective ratings . J Pharmacol Exp Ther . 1984. ; 229 ( 3 ): 636 – 640 . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.