Abstract
Objectives:
Thyroid hormone (TH) promotes marked effects on the cardiovascular system, including the development of cardiac hypertrophy. Some studies have demonstrated that the renin–angiotensin system (RAS) is a key mediator of the cardiac growth in response to elevated TH levels. Although some of the main RAS components are changed in cardiac tissue on hyperthyroid state, the potential modulation of the counter regulatory components of the RAS, such as angiotensin-converting enzyme type 2 (ACE2), angiotensin 1–7 (Ang 1–7) levels and Mas receptor induced by hyperthyroidism is unknown. The aim of this study was to investigate the effect of hyperthyroidism on cardiac Ang 1–7, ACE2 and Mas receptor levels.
Methods:
Hyperthyroidism was induced in Wistar rats by daily intraperitoneal injections of T4 for 14 days.
Results:
Although plasma Ang 1–7 levels were unchanged by hyperthyroidism, cardiac Ang 1–7 levels were increased in TH-induced cardiac hypertrophy. ACE2 enzymatic activity was significantly increased in hearts from hyperthyroid animals, which may be contributing to the higher Ang 1–7 levels observed in the T4 group. Furthermore, elevated cardiac levels of Ang 1–7 levels were accompanied by increased Mas receptor protein levels.
Conclusion:
The counter-regulatory components of the RAS are activated in hyperthyroidism and may be contributing to modulate the cardiac hypertrophy in response to TH.
Keywords: angiotensin 1–7, angiotensin-converting enzyme type 2, Mas receptor, thyroid hormone
Introduction
It is well known that cardiac hypertrophy is an independent risk factor for arrhythmia, myocardial infarction and increased mortality, which may lead to heart failure [Levy et al. 1990; Vakili et al. 2001]. Cardiac growth in response to elevated thyroid hormone (TH) levels has been described as a physiological hypertrophy [Anjos-Ramos et al. 2006], which occurs due to several TH effects on cellular processes, which are cell type specific and involves multiple regulatory mechanisms [Barreto-Chaves et al. 2010]. The major physiological cardiac effects promoted by TH are mediated through actions on specific genes by binding to nuclear TH receptors, which change regulatory and structural proteins, such as α- and β-myosin heavy chains, sarcoplasmic reticulum proteins, calcium-activated ATPase (SERCA2) and phospholamban [Dillman, 2010; Nicollini et al. 2013; Ojamaa, 2010]. At the cellular level, cardiomyocyte growth in response to TH involves increased rates of protein synthesis, changes on gene expression and activation of several signaling pathways, including the PI3K/Akt/S6kinase/mTOR pathway [Kenessey and Ojamaa, 2006; Ojamaa, 2010] and AMPK [Takano et al. 2013].
Another important system involved in several physiological and pathological effects on the cardiovascular system is the renin–angiotensin system (RAS). Angiotensin II (Ang II) emerged as one of the main peptide effector of this system, which exerts its effects mostly via binding to type 1 Ang II receptor (AT1R), resulting in vasoconstriction, proliferation, hypertrophy, inflammation and extracellular matrix remodeling, or via binding to type 2 Ang II receptor (AT2R), which is known to counteract the majority of the AT1R-mediated effects [Keidar et al. 2007]. However, over the past decade, the classical concept of the RAS was transformed by the discovery of several others peptides, receptors and enzymes which are able to counterbalance the effects of the main axis of this complex system [Balakumar and Jagadeesh, 2014]. In contrast to the deleterious actions of Ang II on the cardiovascular system, ACE2/Ang1-7/Mas directly antagonizes many actions of Ang II, providing an additional layer of counter-regulation in this system [Santos et al. 2013; Santos, 2014]. Angiotensin-converting enzyme type 2 (ACE2) was demonstrated to be able to cleave Ang I or Ang II [Crackower et al. 2002], increasing levels of Ang 1–7, which exerts its biological effects through Mas, a G-protein coupled-receptor. Activation of Mas by Ang 1–7 has been shown to induce several actions including vasodilation, diuresis, natriuresis and antihypertrophic effect [Giani et al. 2007].
Several previous reports have demonstrated that cardiac RAS is activated in hyperthyroidism. In this sense, it has been demonstrated that hyperthyroidism increases cardiac renin activity and expression and also cardiac Ang II levels [Barreto-Chaves et al. 2010; Kobori et al. 1997]. However, cardiac ACE activity is reduced in the heart of hyperthyroid rats [Carneiro-Ramos et al. 2006]. On the other hand, the cardiac AT2 receptor is upregulated in response to hyperthyroidism [Carneiro-Ramos et al. 2010; Tavares et al. 2013], suggesting that diverse components of the main axis of the RAS are altered in hyperthyroidism. Consistent with a role for the RAS in TH-induced cardiac hypertrophy, several studies have shown that the use of an AT1 receptor blocker or an ACE inhibitor was able to attenuate or prevent cardiac growth in response to elevated TH levels [Diniz et al. 2009; Hu et al. 2003; Pantos et al. 2005], suggesting that the RAS plays a key role in cardiac hypertrophy mediated by TH.
Considering that the main axis of the RAS contributes to the cardiac hypertrophy in response to hyperthyroidism, and that the counter-regulatory components of the RAS have been shown to prevent the deleterious consequences of the over activity of the classic components of the RAS, the present study aimed to investigate the cardiac levels of ACE2, Ang 1–7 and Mas receptor in TH-induced cardiac hypertrophy.
Materials and methods
Animals and experimental procedures
All procedures and protocols were performed in accordance with the Ethical Principles in Animal Research set forth by the Brazilian College of Animal Experimentation and were approved by the Biomedical Sciences Institute/USP Ethics Committee for Animal Research. Male Wistar rats weighing 200–250 g were obtained from the University of São Paulo, Institute of Biomedical Sciences, in São Paulo, Brazil and were housed in a temperature- and light-controlled environment. All groups were studied concurrently and at the same age. Rats were randomized into two groups: control and hyperthyroid (T4). Hyperthyroidism was induced in the T4 group by daily intraperitoneal injections of 0.100 mg/kg of body weight (BW) of l-thyroxine (T4, Sigma Aldrich) for 14 days. The control group received daily intraperitoneal injections of saline. All rats were sacrificed 24 hours after the last dose of T4 [Hu et al. 2005].
Determination of serum TH levels
Serum levels of free T3 and T4 were evaluated in all groups (control and T4) using a commercial radioimmunoassay kit (CIS Bio International, Gif-sur-Yvette, France). T3 or T4 results are presented in ng/ml.
Analysis of cardiac hypertrophy
Cardiac hypertrophy was evaluated by the analysis of heart weight to tibia length ratio in milligrams/millimeter (mg/mm). α-Myosin heavy chain (α-MHC) mRNA expression was evaluated by real-time polymerase chain reaction (RT-PCR). In addition, transverse sections of the myocardium were prepared for histological analysis. Briefly, the left ventricle was fixed in 10% formaldehyde, embedded in paraffin and then sectioned at 5 µm thick. The myocyte cross-sectional diameter was measured in transverse section stained with hematoxylin and eosin (H&E) using Imaging Software NIS – Elements AR. For each rat, around 50 myocytes were measured and the average was calculated. Collagen content was evaluated in transverse section stained with Picrosirius Red from a minimum of 40 images from different regions (nonoverlapping), using the same software (Imaging Software NIS – Elements AR).
Analysis of Ang 1–7 levels
Hearts were homogenized with 0.045 N HCl in ethanol (10 ml/g of tissue) containing 0.9 μM p-hydroxymercury benzoate, 131.5 μM 1,10-phenanthroline, 0.9 μM phenylmethylsulfonyl fluoride (PMSF), 1.75 μM pepstatin A, 0.032 % ethylene diamine tetra-acetic acid (EDTA) and 0.0043% protease-free bovine serum albumin (BSA) and evaporated. After evaporation, the samples were dissolved in 0.003 % trifluoroacetic acid (TFA). Blood samples for angiotensin peptide measurements were collected in polypropylene tubes containing 1 mM p-hydroxymercury benzoate, 30 mM 1.1 O-phenanthroline, 1 mM PMSF, 1 mM pepstatin A and 7.5% EDTA (50 μl/ml of blood). After centrifugation, plasma samples were frozen and stored at −80°C. Peptides were extracted onto a Bond-Elut phenylsilica cartridge (Varian, USA). The columns were pre-activated by sequential washes with 10 ml of 99.9 % acetonitrile/0.1% heptafluorobutyric acid (HFBA) and 10 ml of 0.1% HFBA. Sequential washes with 10 ml of 99.9% acetonitrile/0.1% HFBA, 10 ml of 0.1% HFBA, 3 ml of 0.1% HFBA containing 0.1% BSA, 10 ml of 10% HFBA and 3 ml of 0.1 % HFBA were used to activate the columns. After sample application, the columns were washed with 20 ml of 0.1% HFBA and 3 ml of 20% HFBA. The adsorbed peptides were eluted with 3 ml of 99.9% acetonitrile/0.1% HFBA into polypropylene tubes rinsed with 0.1% BSA. After evaporation, Ang 1–7 levels were measured by radioimmunoassay (RIA), as previously described [Botelho et al. 1994]. Protein concentration in the crude homogenates was determined by the Bradford method [Bradford, 1976].
Analysis of gene expression
Total RNA was isolated from rat heart using Trizol reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. cDNA was synthesized with a qPCR-SuperMix-UDG Kit (Invitrogen) from 1 µg of total RNA in a total volume of 20 µl with an oligo (dT) primer (Invitrogen). Quantitative RT-PCR was performed in a thermocycler (Cobertt Research, Sydney, Australia) using SYBR Green PCR (Invitrogen). cDNA was used as a template for PCR with specific primers for ACE2, Mas receptor and α-MHC. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was chosen as an internal standard for PCR reactions because its expression is not affected by either TH or myocardial hypertrophy [Carneiro-Ramos et al. 2010]. The following sets of primers were used: ACE2: 5′-TTGTTGGAACGCTGCCATTT-3′ and 5′-CCAACGATCTCCCGCTTCAT-3′; GAPDH: 5′-TGGTGGACCTCATGGCCTAC-3′ and 5′-CAGCAACTGAGGGCCTCTCT-3′; Mas: 5′-CCCACCCATTCCCATAGTGC-3′ and 5′-CCGAGAGGAGAGATGCTCATG-3′ and α-MHC: 5′-CGAATTTCGGAG GGTTCTGC-3′ and 5′-ACAGAGTGCTTCGTGCCTGAT-3′. Samples were run in duplicate. The analysis of the interest gene was normalized by GAPDH levels. The data were expressed as fold of induction in relation to the control.
Western blot analysis
Total protein from rat hearts was obtained using 1 g of tissue to 10 ml of digestion buffer [KCl 90 mM, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 10 mM, MgCl2 3 mM, EDTA 5 mM, glycerol 1%, dithiothreitol (DTT) 1 mM, sodium dodecyl sulfate (SDS) 0.04%]. Protein concentrations were determined by the Bradford method [Bradford, 1976]. Total protein (150 μg) was resolved by electrophoresis on 5% stacking/10% polyacrylamide-SDS gels, and the resolved proteins were transferred to nitrocellulose membrane (Bio-Rad). The membrane was stained with Ponceau solution to demonstrate that the protein concentration was similar in the different samples. The membrane was then washed with TBST (Tris 50 mM, NaCl 150 mM, pH 7.5, Tween-20 2%) for 10 min at room temperature and incubated at 4ºC overnight with polyclonal antibody against Mas receptor (1:500, kindly provided by Dr Robson Santos from the Department of Physiology and Biophysics, Biological Sciences Institute, Federal University of Minas Gerais, Brazil), ACE2 (1:500, Santa Cruz Biotechnology, SC-17720) or α-actinin (1:1000, Santa Cruz Biotechnology, SC-15335) in TBST. After washing the membranes, the secondary anti-rabbit, anti-goat or anti-mouse immunoglobulin (Ig) G conjugated with peroxidase (Amersham Biosciences) at a 1:1000 dilution in TBST was added for 1 hour at room temperature. The membranes were washed again with TBST and incubated with ECL detection reagents (Amersham Biosciences), which produced a chemiluminescence signal that was detected by exposure to X-ray film. The protein bands were quantified by densitometry, and the band densities were calculated and then expressed as percentage in relation to control. α-Actinin levels were used for normalization of the proteins of interest.
ACE2 activity assay
The ACE2 activity assay was performed as previously described [Murça et al. 2012], using a microplate reader (BioTekSynergyTM2; Biotek, Winooski, VT, USA). Left ventricle samples were homogenized in Politron (Kinematica Polytron homogenizer mixer –PCU11) using ACE2 buffer (75 mM Tris-HCL, 1 mol/l NaCl, 0.5 μM ZnCl2 pH 7.5). Tissue extracts were centrifuged at 4°C for 10 minutes at 10,000g and the supernatant retained. Total protein concentrations were determined by the Bradford method [Bradford, 1976]. All assays were performed in duplicate at pH 7.4. The reaction mixture contained 70 µg of total protein from tissue extracts, 5 M Nacl, 10 µM captopril and 50 µM fluorogenic peptide substrate (Fluorogenic Peptide Substrate VI – R&D Systems) in ACE2 buffer to a final volume of 100 µl. Immediately after the addition of the substrate, kinetic assay was started and the fluorescence emitted was measured at 1-minute intervals over 1 hour at 37°C at 320 nM excitation and 405 nM emission (Biotek Instruments, Sinergy HT, USA). For a negative control, 10 µM ACE2 specific peptide inhibitor (Dx600 Linear – Phoenix Pharmaceuticals, INC) was added to the reaction and, for a positive control, 5 µM recombinant human ACE2 (rhACE2 – R&D Systems, USA) was added to the reaction. Background fluorescence readings were obtained from reactions without tissue samples and the final enzymatic activity of the samples was corrected by the obtained background value. Data were expressed as fluorescence (a.u.)/minute/µg of protein.
Statistical analysis
Data are presented as mean ± SD. For convenience some data are expressed as percentage or fold of induction in relation to the control group. Statistical analysis was performed using Student’s t-test, and values of p < 0.05 were considered statistically significant.
Results
Confirmation of hyperthyroid status
To validate the hyperthyroid state, the serum levels of free T3 and T4 were determined (Table 1). As expected, T3 and T4 serum levels were significantly increased in the hyperthyroid group (T4 group) (5.21 ± 0.34 and 67.56 ± 13.68 ng/ml in T4 group, respectively, p < 0.05) when compared with control (4.06 ± 0.65 and 34.07 ± 5.75 ng/ml in control, respectively).
Table 1.
Measurements of serum thyroid hormone levels in experimental groups (Control and T4).
Values are expressed as mean ± standard deviation.
Versus control, p <0.05.
n, number of rats for group (n = 4).
Hyperthyroidism-induced cardiac hypertrophy is accompanied by cardiac activation of ACE2/Ang 1–7/Mas axis
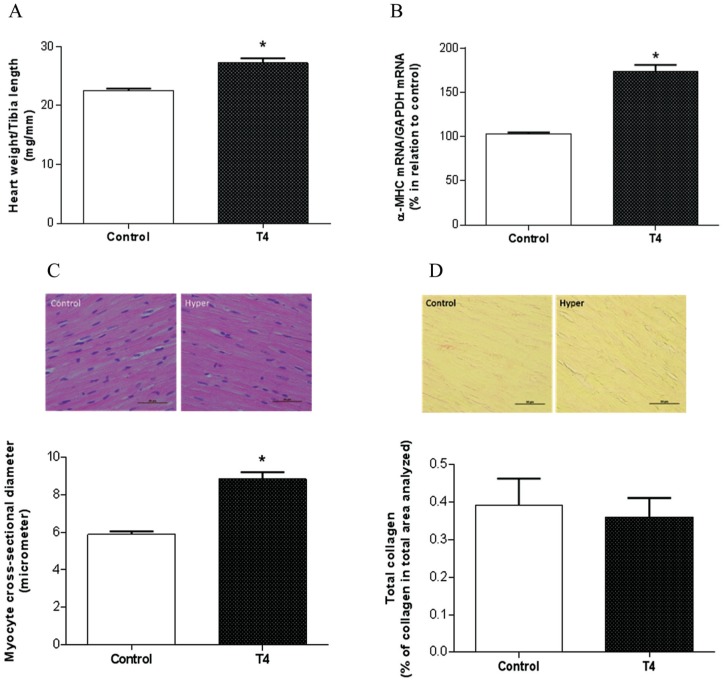
As expected, the hyperthyroid group demonstrated a significant cardiac hypertrophy evidenced by increased heart weight to tibia length ratio (27.2 ± 1.6 mg/mm in T4 group versus 22.5 ± 0.7 mg/mm in control; p < 0.05) (Figure 1A). Corroborating the cardiac hypertrophy evidenced in hyperthyroidism, RT-PCR analysis showed that α-MHC mRNA expression was increased in the T4 group compared with control (Figure 1B). In addition, the hyperthyroid group showed an increase in myocyte cross-sectional diameter (8.84 ± 0.9 μm in T4 group versus 5.88 ± 0.4 µm in control; p < 0.05) (Figure 1C), which was not accompanied by an increase in collagen deposition (0.36 ± 0.1% in T4 group versus 0.39 ± 0.1% in control) (Figure 1D).
Figure 1.
Analysis of cardiac hypertrophy evaluated by heart weight to tibia length ratio (A), α-MHC mRNA expression using RT-PCR (B), myocyte cross-sectional diameter (C) and collagen deposition (D), using transverse sections of the myocardium stained with hematoxylin and eosin (H&E) and Picrosirius Red, respectively.
*Versus control, p < 0.05 (n = 4–5); the line in the bottom of figure represents 50 μm.
α-MHC, α-myosin heavy chain; RT-PCR, real-time polymerase chain reaction.
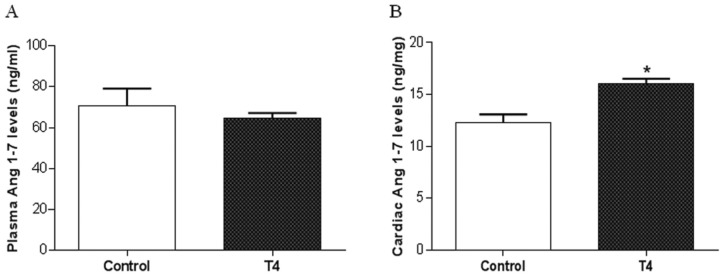
To evaluate whether TH-induced cardiac hypertrophy could be accompanied by an alteration in Ang 1–7 levels, we determined their levels in plasma and cardiac tissue by RIA. Plasma Ang 1–7 levels (in ng/ml) were not changed in the T4 group compared with the control group (64.4 ± 6.7 ng/ml versus 70.7 ± 16.7 ng/ml in control) (Figure 2A). However, cardiac Ang 1–7 levels (in ng/mg of protein) were significantly increased in the hyperthyroid group in relation to control (16 ± 1.1 versus 12.2 ± 1.8 ng/mg of protein in control; p < 0.05) (Figure 2B).
Figure 2.
Hyperthyroidism increases cardiac Ang 1–7 levels. Plasma (A) and cardiac (B) Ang 1–7 levels in control and hyperthyroid (T4) groups, evaluated by RIA.
*Versus control, p < 0.05 (n = 5).
Ang 1–7, angiotensin 1–7; RIA, radioimmunoassay.
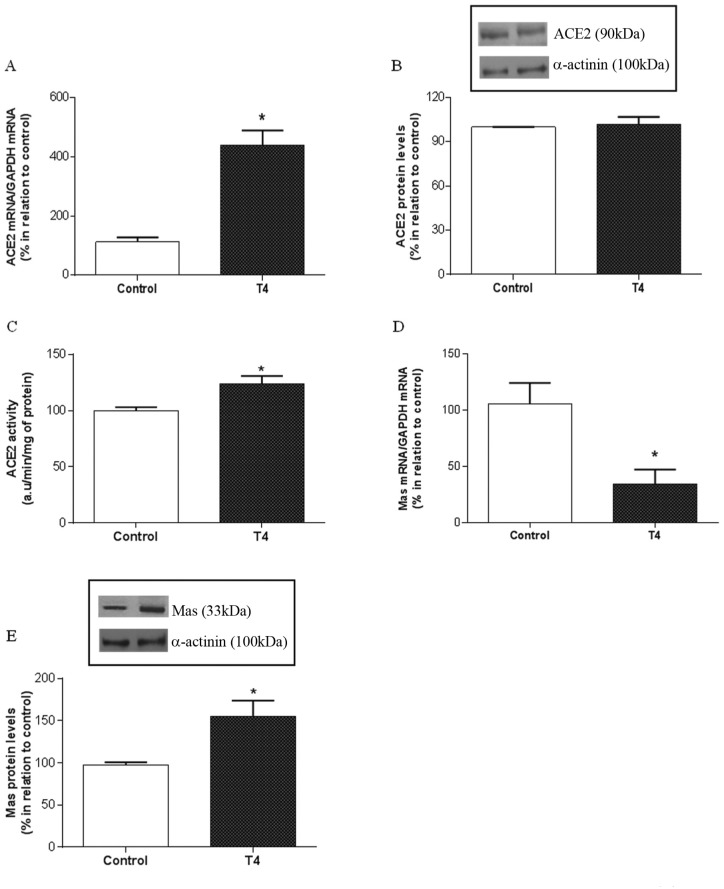
To evaluate whether the increased cardiac Ang 1–7 levels observed in the T4 group could be accompanied by changes in ACE2 expression and activity, we also analyzed the cardiac mRNA and protein levels by RT-PCR and western blotting, respectively, as well as the enzymatic activity. Cardiac ACE2 mRNA levels were significantly increased in the hyperthyroid group compared with the control (439.1 ± 99.23% versus 112 ± 35% in control; p < 0.05) (Figure 3A). Although cardiac ACE2 protein levels were unchanged in the T4 group (101.9 ± 11.2%) (Figure 3B), cardiac ACE2 activity was significantly increased in response to hyperthyroidism (123.9 ± 13.5 versus 100.1 ± 5.8 arbitrary units in the control group, p < 0.05), indicating its important contribution to the elevated levels of Ang 1–7 observed in the hyperthyroid hearts (Figure 3C).
Figure 3.
Cardiac ACE2 and Mas receptor are upregulated in hyperthyroidism. Cardiac ACE2 mRNA (A), protein levels (B) and ACE2 activity (C) in control and hyperthyroid (T4) groups. Cardiac Mas receptor mRNA (D) and protein levels (E).
*Versus control, p < 0.05; (n = 4–5).
ACE2, angiotensin-converting enzyme type 2; a.u., arbitrary units.
Considering that cardiac Ang 1–7 levels were increased in hyperthyroidism, we investigated whether the cardiac expression of Mas receptor might be altered in response to T4. Although Mas receptor gene expression was significantly decreased in the T4 group (Figure 3D), western blotting analysis demonstrated that the T4 group had a significant increase in Mas receptor protein levels compared with the control group (154.8 ± 37%, p < 0.05) (Figure 3E).
These results demonstrate for the first time that cardiac growth in response to elevated levels of TH, which are clinically observed in hyperthyroid patients, is accompanied by activation of ACE2/Ang 1–7/Mas axis.
Discussion
The present study was designed to evaluate the potential modulation of the counter-regulatory components of the RAS, ACE2/Ang 1–7/Mas receptor in cardiac hypertrophy induced by hyperthyroidism.
Diverse reports have demonstrated that RAS is involved in the physiological regulation of blood pressure and volume homeostasis, as well as in the pathogenesis of hypertension and other cardiovascular diseases. Indeed, the modulation of some of the classical components of the RAS in TH-induced cardiac hypertrophy has been reported by several studies [Carneiro-Ramos et al. 2006, 2010; Kobori et al. 1997; Sernia, et al. 1993]. Additionally, it has been demonstrated that some of the classical components of the RAS are critical for the cardiac growth in response to hyperthyroidism, since the use of Ang II receptors blockers or ACE inhibitors was able to attenuate or to blunt cardiac hypertrophy elicited by higher TH levels [Carneiro-Ramos et al. 2010; Hu et al. 2003; Pantos et al. 2005].
The discovery of the novel components of the RAS, as Ang 1–7, ACE2 and Mas receptor, resulted in a new perception of the mechanisms through which the RAS regulates cardiac homeostasis and influences the development of several cardiovascular diseases [Farag et al. 2015; Santos, 2014]. Considering the emerging cardioprotective actions mediated by this counter-regulatory axis of the RAS, we hypothesized that the cardiac growth in response to elevated TH levels might be accompanied by a modulation of cardiac Ang 1–7, ACE2 and Mas receptor levels. In fact, the analysis of the plasma Ang 1–7 levels indicated that this peptide was not changed in the T4 group. However, cardiac Ang 1–7 levels were increased in cardiac hypertrophy in response to elevated TH levels. This result demonstrates for the first time that local Ang 1–7, present in the cardiac tissue, is increased in hyperthyroidism. Although a chronic increase on Ang II may induce several deleterious effects on the cardiovascular system, such as vasoconstriction, increased cell proliferation and inflammation [Keidar et al. 2007; Silva-Filho et al. 2011], some studies have demonstrated that Ang 1–7 promotes opposite effects to those elicited by Ang II, exerting a cardioprotective role [Santos, 2014]. In the heart, Ang 1–7 has been shown to prevent cardiac remodeling, mainly by decreasing cardiomyocyte hypertrophy and fibrosis [Benter et al. 2006; Grobe et al. 2006, 2007]. Additionally, Ang 1–7 has also been reported to decrease cardiac Ang II levels [Mendes et al. 2005] and to modulate the AT1R-mediated actions of Ang II [Jackman et al. 2002; Mahon et al. 1994]. Taking this into account, it is possible to hypothesize that the elevated cardiac Ang 1–7 levels may be contributing to minimize or to counteract the cardiac growth process in response to higher TH levels. However, future functional studies are required to corroborate this hypothesis.
The exact mechanisms underlying the increased cardiac Ang 1–7 levels induced by hyperthyroidism are uncertain. Considering that Ang 1–7 present in the myocardium appears to depend on Ang II as a substrate [Ferrario et al. 1997; Keidar et al. 2007; Zisman et al. 2003], and that cardiac Ang II levels are increased in hyperthyroidism [Carneiro-Ramos et al. 2010; Kobori et al. 1997], it is possible that the elevated cardiac Ang II levels induced by TH may be contributing, at least in part, for the higher Ang 1–7 levels observed in the T4 group. We have previously demonstrated that ACE activity is downregulated in cardiac tissue of hyperthyroid rats [Carneiro-Ramos et al. 2006]. Given that ACE may degrade Ang 1–7 into Ang 1-5 [Santos and Ferreira, 2007], it is possible that the reduced cardiac ACE activity observed in hyperthyroidism may be contributing at least in part to the higher cardiac Ang 1–7 levels found in the T4 group. However, further studies are needed to characterize the potential mechanisms involved in elevated cardiac Ang 1–7 levels in response to TH, as well as the potential biological effects mediated by this peptide in TH-induced cardiac hypertrophy.
Several studies have suggested a beneficial role for ACE2 in the cardiovascular system. These observations are consistent with the findings demonstrating elevated ACE2 expression at the initial stage of several pathologies, which is reduced with the progression of disease, pointing to the critical function of this enzyme for the regulation of the cardiac homeostasis [Keidar et al. 2007]. Moreover, recombinant human ACE2 (rhACE2) administration suppressed myocardial fibrosis and cardiac dysfunction [Zhong et al. 2010] decreasing right ventricular hypertrophy and improving right ventricular systolic and diastolic function [Johnson et al. 2011] in a pressure overload model. Also, rhACE2 antagonized Ang II-induced pressor response in Wistar-Kyoto rats and spontaneously hypertensive rats (SHR) [Lo et al. 2013]. However, ACE2 knockout mice displayed increased cardiac inflammation, exacerbated cardiac dysfunction and myocardial injury in response to Ang II [Song et al. 2013]. Additionally, loss of ACE2 is associated with increased plasma Ang II levels and decreased plasma Ang 1–7 levels, which indicates a cardioprotective role of ACE2 [Song et al. 2013]. ACE2 has been shown to control the Ang II levels, since this enzyme degrades this peptide into Ang 1–7. Indeed, Ang I also can be hydrolyzed by ACE2, forming Ang 1-9 [Donoghue et al. 2000], which can be converted by ACE into Ang 1–7. Herein we found that TH-induced cardiac hypertrophy was accompanied by higher cardiac ACE2 gene expression and unchanged ACE2 protein expression. Interestingly, independently of unaltered ACE2 protein levels, hyperthyroidism promoted an increase in cardiac ACE2 activity. Considering that Ang 1–7 is one of the major enzymatic products of ACE2 [Santos et al. 1988], the increase in ACE2 activity may be contributing to the elevated cardiac Ang 1–7 levels evidenced in hyperthyroidism. It is also important to note that others enzymes, such prolyl endopeptidase and prolyl carboxypeptidase, are also able to cleave Ang II into Ang 1–7 [Santos and Ferreira, 2007]. Taking this into account, it is plausible that the higher cardiac Ang 1–7 levels found in TH-induced cardiac hypertrophy may also be promoted by others enzymes besides ACE2, as discussed previously.
The present study also demonstrated that cardiac growth in response to elevated TH levels was accompanied by an increase in cardiac Mas receptor protein levels. Thus, it is possible that the higher levels of cardiac Mas receptor induced by hyperthyroidism could be contributing to mediate some of the beneficial effects of the elevated cardiac Ang 1–7 levels observed in the hyperthyroid group. However, further studies are required to determine the potential role of Mas receptor in cardiac hypertrophy induced by TH.
Some studies have revealed a functional interaction of Mas receptor with AT1R and AT2R in the cardiac tissue of mouse [Castro et al. 2005; Villela et al. 2015; Von Bohlen und Halbach et al. 2000]. This interaction may be attributable to hetero oligomerization between Mas and the AT1R, which leads to inhibition of Ang II effects mediated by AT1R [Kostenis et al. 2005]. However, whether the increased cardiac Mas receptor levels observed in the T4 group may be interacting with the Ang II receptors, and contributing to counteract some of the Ang II actions in the cardiac tissue, is unknown and must be investigated.
The predominant physiological role of the cardiac RAS appears to be the maintenance of an appropriate cellular milieu balancing stimuli inducing and inhibiting cell growth and proliferation, as well as mediating adaptive responses to myocardial stress [Paul et al. 2006]. A growing body of evidence suggests that the ACE2/Ang 1–7/Mas axis acts as the main counter-regulatory mechanism for the central axis ACE/Ang II/AT1R [Santos, 2014]. However, whether the modulation of cardiac Ang 1–7/ACE2/Mas axis evidenced in hyperthyroidism plays a counter regulatory action during the TH-induced cardiac hypertrophy is unclear and further studies are needed to elucidate this hypothesis.
Conclusion
Our results indicate for the first time that hyperthyroidism activates the counter-regulatory axis of the RAS by increasing cardiac ACE2 activity and elevating Ang 1–7 levels and Mas receptor protein levels in the heart, which may be contributing to attenuate some actions exerted by TH in the heart.
Acknowledgments
G.P. Diniz and N. Senger contributed equally.
All the authors are grateful to Marina Fevereiro for the technical assistance provided.
Footnotes
Funding: This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Foundation for the Support of Research in the State of São Paulo; Grants 07-53993-6 and 13/16348-6) and CNPq (National Council of Technological and Scientific Development).
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Gabriela P. Diniz, Department of Anatomy, Laboratory of Cell Biology and Functional Anatomy, University of São Paulo, Sao Paulo, Brazil
Nathalia Senger, Department of Anatomy, Laboratory of Cell Biology and Functional Anatomy, University of São Paulo, Sao Paulo, Brazil.
Marcela S. Carneiro-Ramos, Human and Natural Sciences Center, Federal University of ABC, São Paulo, Brazil
Robson A. S. Santos, Department of Physiology and Biophysics, Biological Sciences Institute, Federal University of Minas Gerais, Belo Horizonte, Brazil Cardiology Institute of Rio Grande do Sul, University Foundation of Cardiology.
Maria Luiza M. Barreto-Chaves, Laboratory of Cellular Biology and Functional Anatomy, Department of Anatomy, Biomedical Sciences Institute, University of São Paulo, Av. Prof. Lineu Prestes 2415, Cidade Universitária, São Paulo, SP 05508-900, Brazil.
References
- Anjos-Ramos L., Carneiro-Ramos M.S., Diniz G.P., Martins-Silva J., Barreto-Chaves M. (2006) Early cardiac hypertrophy induced by thyroxine is accompanied by an increase in VEGF-A expression but not by an increase in capillary density. Virchows Arch 448: 472–479. [DOI] [PubMed] [Google Scholar]
- Balakumar P., Jagadeesh G. (2014) A century old renin-angiotensin system still grows with endless possibilities: AT1 receptor signaling cascades in cardiovascular physiopathology. Cell Signal 26: 2147–2160. [DOI] [PubMed] [Google Scholar]
- Barreto-Chaves M., Carrillo-Sepulveda M., Carneiro-Ramos M., Gomes D., Diniz G. (2010) The crosstalk between thyroid hormones and the renin–angiotensin system. Vascul Pharmacol 52: 166–170. [DOI] [PubMed] [Google Scholar]
- Benter I., Yousif M., Anim J., Cojocel C., Diz D. (2006) Angiotensin-(1–7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Am J Physiol Heart Circ Physiol 290: H684–H691. [DOI] [PubMed] [Google Scholar]
- Botelho L., Block C., Khosla M., Santos R. (1994) Plasma angiotensin-(1–7) immunoactivity is increased by salt load, water deprivation, and hemorrahage. Peptides 15: 723–729. [DOI] [PubMed] [Google Scholar]
- Bradford M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Carneiro-Ramos M., Diniz G., Nadu A., Almeida J., Vieira R., Santos R., et al. (2010) Blockage of angiotensin II type 2 receptor prevents thyroxine-mediated cardiac hypertrophy by blocking Akt activation. Basic Res Cardiol 105: 325–35. [DOI] [PubMed] [Google Scholar]
- Carneiro-Ramos M., Silva V., Santos R., Barreto-Chaves M. (2006) Tissue-specific modulation of angiotensin-converting enzyme (ACE) in hyperthyroidism. Peptides 27: 2942–2949. [DOI] [PubMed] [Google Scholar]
- Castro C., Santos R., Ferreira A., Bader M., Alenina N., Almeida A. (2005) Evidence for a functional interaction of the angiotensin-(1–7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension 46: 937–942. [DOI] [PubMed] [Google Scholar]
- Crackower M., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S., et al. (2002) Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417: 822–828. [DOI] [PubMed] [Google Scholar]
- Dillman W. (2010) Cardiac hypertrophy and thyroid hormone signaling. Heart Fail Rev 15: 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz G., Carneiro-Ramos M., Barreto-Chaves M. (2009) Angiotensin type 1 receptor mediates thyroid hormone-induced cardiomiocyte hypertrophy through the Akt/GSK-3beta/mTOR signaling pathway. Basic Res Cardiol 104: 653–667. [DOI] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., et al. (2000) A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87: E1–9. [DOI] [PubMed] [Google Scholar]
- Farag E., Maheshwari K., Morgan J., Sark Esa W., Doyle D. (2015) An update of the role of Renin Angiotensin in cardiovascular homeostasis. Anesth Analg 120: 275–92. [DOI] [PubMed] [Google Scholar]
- Ferrario C., Chappell M., Tallant E., Brosnihan K., Diz D. (1997) Counterregulatory actions of angiotensin-(1–7). Hypertension 30: 535–541. [DOI] [PubMed] [Google Scholar]
- Giani J., Gironacci M., Munoz M., Pena C., Turyn D., Dominici F. (2007) Angiotensin-(1–7) stimulates the phosphorylation of JAK2, IRS-1 and Akt in rat heart in vivo: role of the AT1 and Mas receptors. Am J Physiol Heart Circ Physiol 293: H1154–1163. [DOI] [PubMed] [Google Scholar]
- Grobe J., Mecca A., Lingis M., Shenoy V., Bolton T., Machado J., et al. (2007) Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1–7). Am J Physiol Heart Circ Physiol 292: H736–742. [DOI] [PubMed] [Google Scholar]
- Grobe J., Mecca A., Mao H., Katovich M. (2006) Chronic angiotensin-(1–7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol 290: 2417–2423. [DOI] [PubMed] [Google Scholar]
- Hu L., Benvenuti L., Liberti E., Carneiro-Ramos M., Barreto-Chaves M. (2003) Thyroxine-induced cardiac hypertrophy: influence of adrenergic nervous system versus renin-angiotensin system on myocyte remodeling. Am J Physiol Regul Integr Comp Physiol 285: 1473–1480. [DOI] [PubMed] [Google Scholar]
- Hu L., Liberti E., Barreto-Chaves M. (2005) Myocardial ultrastructure in cardiac hypertrophy induced by thyroid hormone – an acute study in rats. Virchows Arch 446: 265–269. [DOI] [PubMed] [Google Scholar]
- Jackman H., Massad M., Sekosan M., Tan F., Brovkovych V., Marcic B., et al. (2002) Angiotensin 1–9 and 1–7 release in human heart: role of cathepsin A. Hypertension 39: 976–981. [DOI] [PubMed] [Google Scholar]
- Johnson J., West J., Maynard K., Hemnes A. (2011) ACE2 improves right ventricular function in a preassure overload model. PloS One 6: 20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keidar S., Kaplan M., Gamliel-Lazarovich A. (2007) ACE2 of the heart: from angiotensin I to angiotensin (1–7). Cardiovasc Res 73: 463–469. [DOI] [PubMed] [Google Scholar]
- Kenessey A., Ojamaa K. (2006) Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J Biol Chem 281: 20666–20672. [DOI] [PubMed] [Google Scholar]
- Kobori H., Ichihara A., Suzuki H., Takenaka T., Miyashita Y., Hayashi M., et al. (1997) The role of renin-angiotensin system in cardiac hypertrophy induced in rats by hyperthyroidism. Am J Physiol 273: H593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostenis E., Milligan G., Christopoulos A., Sanchez-Ferrer C., Heringer-Walther S., Sexton P., et al. (2005) G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation 111: 1806–1813. [DOI] [PubMed] [Google Scholar]
- Levy D., Garrison R.J., Savage D., Kannel W., Castelli W. (1990) Prognostic implications of echocardiographicall y determinated left ventricular mass in the Framingham Heart Study. N Engl J Med 322: 1561–1566. [DOI] [PubMed] [Google Scholar]
- Lo J., Patel V., Wang Z., Levasseur J., Kaufman S., Penninger J., Oudit G. (2013) Angiotensin-converting enzyme 2 antagonizes angiotensin II-induced pressor response and NADPH oxidase activation in Wistar-Kyoto rats and spontaneously hypertensive rats. Exp Physiol 98:109–122. [DOI] [PubMed] [Google Scholar]
- Mahon J., Carr R., Nicol A., Henderson I. (1994) Angiotensin(1–7) is an antagonist at the type 1 angiotensin II receptor. J Hypertens 12: 1377–1381. [PubMed] [Google Scholar]
- Mendes A., Ferreira A., Pinheiro S., Santos R. (2005) Chronic infusion of angiotensin-(1–7) reduces heart angiotensin II levels in rats. Regul Pept 125: 29–34. [DOI] [PubMed] [Google Scholar]
- Murça T., Morais P., Capuruço C., Santos S., Melo H., Santos R., et al. (2012) Oral administration of an angiotensin-converting enzyme 2 activator ameliorates diabetes-induced cardiac dysfunction. Regul Pept 177: 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicollini G., Pitto L., Kusmic C., Balzan S., Sabatino L., Iervasi G., et al. (2013) New insights into mechanisms of cardioprotection mediated by thyroid hormones. J Thyroid Res 2013: 264387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojamaa K. (2010) Signaling mechanism in thyroid hormone-induced cardiac hypertrophy. Vascul Pharmacol 52: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantos C., Paizis I., Mourouzis I., Moraitis P., Tzeis S., Karamanoli E., et al. (2005) Blockade of angiotensin II type 1 receptor diminishes cardiac hypertrophy, but does not abolish thyroxin-induced preconditioning. Horm Metab Res 37: 500–504. [DOI] [PubMed] [Google Scholar]
- Paul M., Poyan Mehr A., Kreutz R. (2006) Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803. [DOI] [PubMed] [Google Scholar]
- Santos R. (2014) Angiotensin-(1–7). Hypertension 63: 1138–1147. [DOI] [PubMed] [Google Scholar]
- Santos R., Brosnihan K., Chappell M., Pesquero J., Chemicky C., Greene L., et al. (1988) Converting enzyme activity and angiotensin metabolism in the dog brainstem. Hypertension 11: 153–157. [DOI] [PubMed] [Google Scholar]
- Santos R., Ferreira A. (2007) Angiotensin-(1–7) and the renin-angiotensin system. Curr Opin Nephrol Hypertens 16: 122–128. [DOI] [PubMed] [Google Scholar]
- Santos R., Ferreira A., Verano-Braga T., Bader M. (2013) Angiotensin-converting enzyme 2, angiotensin-(1–7) and Mas: new players of the renin-angiotensin system. J Endocrinol 216: R1–R17. [DOI] [PubMed] [Google Scholar]
- Sernia C., Marchant C., Brown L., Hoey A. (1993) Cardiac angiotensin receptors in experimental hyperthyroidism in dogs. Cardiovasc Res 27: 423–428. [DOI] [PubMed] [Google Scholar]
- Silva-Filho J., Souza M., Henriques M., Morrot A., Savino W., Nunes M., et al. (2011) AT1 receptor-mediated angiotensin II activation and chemotaxis of T lymphocytes. Mol Immunol 48: 1835–1843. [DOI] [PubMed] [Google Scholar]
- Song B., Zhang Z., Zhong J., Yu X., Oudit G., Jin H., et al. (2013) Loss of angiotensin-converting enzyme 2 exacerbates myocardial injury via activation of the CTGF-fractalking signaling pathway. Circ J 77: 2997–3006. [DOI] [PubMed] [Google Scholar]
- Takano A., Diniz G., Barreto-Chaves M. (2013) AMPK signaling pathway is rapidly by T3 and regulates the cardiomycyte growth. Mol Cell Endocrinol 376: 43–50. [DOI] [PubMed] [Google Scholar]
- Tavares F., Silva I., Gomes D., Barreto-Chaves M. (2013) Angiotensin II type 2 receptor (AT2R) is associated with increased tolerance of the hyperthyroid heart to ischemia-reperfusion. Cardiovasc Drugs Ther 27: 393–402. [DOI] [PubMed] [Google Scholar]
- Vakili B., Okin P., Devereux R. (2001) Prognostic implication of left ventricular hypertrophy. Am Heart J 141: 334–341. [DOI] [PubMed] [Google Scholar]
- Villela D., Leonhardt J., Patel N., Joseph J., Kirsch S., Hallberg A., et al. (2015) Angiotensin type 2 receptor (ATR2) and receptor Mas: a complex liasion. Clin Sci 128: 227–234. [DOI] [PubMed] [Google Scholar]
- Von Bohlen und Halbach O., Walther T., Bader M., Albrecht D. (2000) Interaction between Mas and the angiotensin AT1 receptor in the amygdala. J Neurophysiol 83: 2012–2021. [DOI] [PubMed] [Google Scholar]
- Zisman L., Meixell G., Bristow M., Canver C. (2003) Angiotensin-(1–7) formation in the intact human heart: in vivo dependence on angiotensin II as substrate. Circulation 108:1679–1681. [DOI] [PubMed] [Google Scholar]
- Zhong J., Basu R., Guo D., Chow F., Byrns S., Schuster M., et al. (2010) Angiotensin-converting enzyme 2 suppresses pathological hyperthrophy, myocardial fibrosis, and cardiac dysfunction. Circulation 122:717–728. [DOI] [PubMed] [Google Scholar]