Abstract
Objectives:
In the present study, we investigated whether combination therapy of low-dose benidipine with the potent free radical scavenger edaravone has a cardioprotective effect against isoproterenol (ISO)-induced myocardial infarction (MI) in Wistar rats.
Methods:
Rats were pretreated with concurrent doses of benidipine and edaravone (1 μg/kg/day + 1 mg/kg/day and 3 μg/kg/day + 3 mg/kg/day) by intravenous (i.v.) and intraperitoneal (i.p.) routes respectively for 28 days, followed by MI induction using ISO (85 mg/kg) by subcutaneous route for two days at 24 h intervals. After the treatment period, blood was withdrawn and the heart was preserved for biochemical estimations.
Results:
The activities of the cardiac biomarkers (lactate dehydrogenase and creatine kinase-MB), and the level of malondialdehyde (MDA) significantly increased, while antioxidant markers (reduced glutathione, catalase, superoxidase dismutase, glutathione peroxidase, glutathione reductase) were significantly decreased in the ISO intoxicated group compared with the control group. Moreover, the level of C-reactive protein (CRP) and Caspase-3 activity significantly increased in ISO-intoxicated group. An ultrastructure study was also carried out. Pretreatment with a combination of benidipine and edaravone significantly attenuated the activities of the cardiac biomarkers and the level of MDA, and significantly increased the antioxidant markers compared with the ISO-intoxicated group. Furthermore, pretreatment with the combination of benidipine and edaravone significantly decreased the level of CRP and Caspase-3 activity as compared to the ISO-treated group. The ultrastructure study of myocardium revealed that pretreated groups preserved the mitochondrial shape, the membrane and its internal structures.
Conclusion:
Taken together these results suggest that the combination of benidipine and edaravone showed significant protective effect in ISO-induced MI.
Keywords: benidipine, isoproterenol, myocardial infarction, oxidative stress, ultrastructure damage
Introduction
Reactive oxygen species (ROS) are a group of highly reactive molecules such as hydroxyl anion and superoxide. ROS can significantly alter several biological processes, as well as causing myocardium dysfunction and damage [Ceconi et al. 2003; Grieve et al. 2004]. Moreover, the deleterious effects of ROS depend on the balance between the endogenous tissue antioxidant enzymes and the concentration of hydroxyl ion, superoxide ion and hydrogen peroxides. In fact, antioxidant capacity has been revealed to be reduced in myocardium dysfunction, which is related with an increase in oxidative stress [Fearon and Faux, 2009]. Oxidative stress is one of the major pathological conditions leading to the generation of ROS. ROS are unifying components in most forms of cardiovascular diseases (CVDs). CVDs are the principal cause of death in developing countries [Yusuf et al. 2001]. Among CVDs, myocardial infarction (MI) is the most prevalent and widespread. MI is caused by reduction in the myocardial blood flow due to the coronary artery occlusion. The imbalance between coronary blood supply and myocardial demand eventually leads to an acute condition of myocardial necrosis.
Isoproterenol (ISO) (4-[1-hydroxy-2-(propan-2-ylamino) ethyl] benzene-1, 2-diol) is a synthetic catecholamine and β-adrenoceptor agonist. It induces MI by multiple mechanisms, namely functional hypoxia and ischemia, alteration in metabolism, intracellular Ca+2 overloads and oxidative stress [Dhalla et al. 2010; Wexler, 1978]. In addition, pathogenesis of ISO-induced MI is also implicated in the occurrence of oxidative damage, lipid peroxidation, apoptosis and inflammatory cytokines [Shizukuda et al. 1998; Goyal et al. 2010] This model has established itself as a well-known one for evaluating the cardioprotective agents in animals under experimentation, mimicking human MI [Rona et al. 1959, Goyal et al. 2010].
Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) is a potent and novel free radical scavenger, with strong antioxidant properties, and is used in patients with acute brain infarction. In several studies it has been reported that it prevents cell damage induced by oxidative stress through inhibition of lipoxygenase metabolism of arachidonic acid, by trapping hydroxyl radicals and scavenging the ROS [Green and Ashwood, 2005; Saini et al. 2006]. Several studies have also reported anti-apoptotic and anti-inflammatory effects of edaravone and protection in experimental models of stroke cardiovascular diseases [Murota et al. 1990; Watanabe et al. 1988].
Benidipine (3-[(R*)-1-benzyl-3-piperidyl] methyl-1, 4-dihydro-2, 6-dimethyl-4-(nitro phenyl)-3, 5-pyridine dicarboxylate) is a highly potent, long-lasting, antihypertensive and antianginal agent. Benidipine has been reported to increase the coronary blood flow and suppress Ca+2 overloads [Higo et al. 1992; Kitakaze et al. 1999]. It has also been reported to reduce myocardial necrosis associated with reperfusion after coronary artery ligation [Matsubara et al. 2008; Liu et al. 2004].
We have seen in our laboratory the cardioprotective effects of the benidipine [Hassan et al. 2015b] and edaravone [Hassan et al. 2015a] ISO-induced experimental model of cardiotoxicity when administered individually. The present study was designed to assess the efficacy of a combination therapy of low doses of edaravone and benidipine in ISO-induced MI in rats. The cardioprotective effects were evaluated by the assay of malondialdehyde (MDA), antioxidant markers, and anti-apoptotic effects in cardiac tissue. We also evaluated cardiac biomarkers such as lactate dehydrogenase (LDH), creatine kinase-MB (CK-MB) and C-reactive protein (CRP) in serum. Ultrastructure study of cardiac tissues has also been carried out by transmission electron microscopy.
Methods
Chemicals
Benidipine and edaravone were acquired from Tocris Bioscience (Bristol, UK). Enzyme-linked immunosorbent assay (ELISA) kits, Caspase-3 and CRP were procured from Immunology Consultant Laboratory Inc. (Portland, OR, USA) and Biovision Research Products (Milpitas, CA, USA), respectively. Cardiac biomarkers were purchased from Crest Biosystems Ltd (Delhi, India). Isoproterenol, L-glutathione oxidase and glutathione reductase were procured from Sigma-Aldrich Chemicals Pvt. Ltd (Bangalore, India).
Animals
This study was approved by the Institutional Animal Ethics Committee (IAEC), Jamia Hamdard, New Delhi, India. Wistar strain rats of both sexes, weighing 200–220 g were procured from the Central Animal House Facility of Jamia Hamdard. Rats were kept in polypropylene cages and had free access to a commercial pellet diet (Amrut rat feed, manufactured by Nav Maharashtra Chakan Oil Mills Ltd, New Delhi, India), and water ad libitum. The environmental condition of the animal house was maintained as 12 h light and 12 h dark day/night cycle, with a temperature of 25 ± 2°C.
Drug preparation
Benidipine and edaravone were dissolved in normal saline, containing 0.1% tween 80 and 0.05% CMC, respectively. Drug solutions were prepared freshly on the day of dosing. ISO was freshly prepared by being dissolved in normal saline.
Treatment schedules
Rats were allocated randomly into six groups, containing six rats each. Group I was a normal control group and received only normal saline for 28 days. Group II and III served as per se groups and were administered a combination of edaravone and benidipine, with a doses of 1 mg/kg i.p. + 1 μg/kg i.v. and 3 mg/kg i.p. + 3 μg/kg i.v., respectively, for 28 days. Group IV served as a toxic group, and were administered ISO 85 mg/kg subcutaneously (s.c.) in two doses at a 24 h interval. The doses of ISO were selected on the basis of previous studies [Hassan et al. 2015b; Goyal et al. 2010], which had established the dose at 85 mg/kg s.c. at 24 h. Group V and VI were pretreated with benidipine and edaravone (1 mg/kg i.p. + 1 μg/kg i.v. and 3 mg/kg i.p. +3 μg/kg i.v., respectively) followed by two doses of ISO 85 mg/kg, s.c. at a 24 h interval. Blood samples were collected after the experimental schedule and the animals were sacrificed by cervical decapitation under light ether anesthesia. Hearts were rapidly excised and washed with ice cold normal saline. The hearts were then stored in liquid nitrogen for biochemical estimations. Heart tissue samples were fixed in glutaraldehyde (3%) for ultrastructure study. Blood samples were centrifuged at 3000g for 30 min, and separated serum stored at −20°C for estimation of cardiac markers.
Estimation of cardiac marker enzymes
Activities of CK-MB [Lum and Gambino, 1974] and LDH [Young, 1990] in the serum were estimated using commercially available kits (Crest Biosystems Ltd, Delhi, India) as per instructions.
Estimation of myocardium MDA level
The level of MDA in the myocardium was estimated by the method of Okhawa and colleagues [Okhawa et al. 1970], using thiobarbituric acid. The 3, 3-tetramethoxypropane was used as an external standard, absorbance was recorded at 540 nm by using spectrophotometer. Level of MDA was expressed as nanomoles per milligram protein.
Estimation of antioxidant enzymes in myocardial tissues
Reduced glutathione (GSH) was measured according to Sedlak and Lindsay [Sedlak and Lindsay, 1968]. The absorbance was recorded within 5 min after the addition of Ellman’s reagent (DTNB) at 410 nm, against a reagent blank. The activities of glutathione S-transferase (GST), glutathione peroxidase (GPx) and glutathione reductase (GR) in myocardium were assayed spectrophotometrically according to the methods of Habig and colleagues [Habig et al. 1974], Wheeler and colleagues [Wheeler et al. 1990] and Carlberg and Mannervik [Carlberg and Mannervik, 1975], respectively. Superoxide dismutase (SOD) and catalase (CAT) activities in myocardial cells were estimated spectrophotometrically, as described by Marklund and Marklund [Marklund and Marklund, 1974] and Claiborne [Claiborne, 1985], respectively.
Estimation of Caspase-3 protease
The level of Caspase-3 protease in cardiac tissue was estimated by the ELISA method, using commercially available Caspase-3 colorimetric assay kit from Biovision (USA) as per the instruction leaflet [Gurtu and Kain, 1997]. A microtiter plate was observed at 405 nm in a micro reader instrument (MicroScan MS5608A Electronic Corporation of India Ltd).
Estimation of C-reactive protein
The level of CRP in serum was estimated using the commercially available kit from Immunology Consultants Laboratory Inc. (USA) as per the instruction leaflet, by the ELISA method [Eckersall, 2000].
Estimation of protein
The protein concentration in the myocardium was estimated by the method of Lowry and colleagues, using bovine serum albumin as a standard [Lowry et al. 1951].
Transmission electron microscopy studies
For the ultrastructure study, 4–5 pieces of 1.0–1.5 mm dimension were prepared from the left ventricle. These samples were rinsed several times with a 0.1 M phosphate buffer (pH 7.2). Immediately, the samples were fixed with 2.5% glutaraldehyde and 2% paraformaldehyde solution in a 0.1 M sodium phosphate buffer (pH 7.2) and stored at 4°C for 12 h. Ultrathin sections from these samples were stained with 2% uranyl acetate and 0.2% lead acetate, and observed under an electron microscope (FMI-Margagani Operated 268, the Netherlands) [Pogodina et al. 2006].
Statistical analysis
Statistical analysis was carried out by using a Prism software package (Version 4, GraphPad, USA). An analysis of variance with Dunnett’s test was also used to observe the significant difference (p < 0.05).
Results
Effects of a combination of benidipine and edaravone on the nonenzymatic and enzymatic antioxidant parameters
Table 1 demonstrates the effects on the experimental group of a combination of benidipine and edaravone on the nonenzymatic (GSH) and enzymatic (CAT, SOD, GST, GPx and GR) antioxidant parameters in myocardium. This table shows significantly (p < 0.01) decreased levels of GSH in the toxic group compared with the normal control group. Pretreatment with a combination of benidipine and edaravone (1 µg/kg + 1 mg/kg and 3 µg/kg + 3 mg/kg) significantly (p < 0.0 1) increased levels of GSH compared with the ISO-treated group. Table 1 shows that the enzymatic antioxidants such as CAT, SOD, GST, GPx and GR in myocardium are significantly (p < 0.01) decreased in the ISO-treated group compared with the normal control group. Pretreatment with a combination of benidipine and edaravone with dose of (3 µg/kg + 3 mg/kg) significantly (p < 0.01) increased these markers compared with the ISO-treated group. Also, pretreatment with a low dose combination of benidipine and edaravone (1 µg/kg + 1 mg/kg) significantly (p < 0.05) increased levels of SOD and GST as compared with the ISO-treated group.
Table 1.
Effects of a combination of benidipine and edaravone on activity of GSH and levels CAT, SOD, GST, GPx, GR in the myocardium of Wistar rats.
| Experimental group | GSH (µmol GSH/mg) | CAT (nmol of H2O2 /mg) | SOD (unit/mg) | GST (nmol CDNB /mg) | GPx (nmol NADPH /mg) | GR (nmol NADPH/mg) |
|---|---|---|---|---|---|---|
| Group I | 1.459±0.147 | 7.660±0.266 | 4.811±0.311 | 7.393±0.303 | 5.979±0.224 | 6.617±0.160 |
| Group II | 1.254±0.095 | 7.389±0.658 | 3.852±0.293 | 6.863±0.366 | 5.474±0.400 | 6.057±0.575 |
| Group III | 1.175±0.189 | 7.714±0.698 | 4.382±0.281 | 5.636±0.274 | 5.227±0.378 | 6.098±0.467 |
| Group IV | 0.257±0.052## | 1.669±0.203## | 1.440±0.198## | 2.233±0.063## | 1.720±0.186## | 1.852±0.305## |
| Group V | 1.225±0.172** | 7.218±0.753** | 2.868±0.207* | 5.375±0.377* | 4.721±0.093** | 5.254±0.377** |
| Group VI | 1.194±0.175** | 7.212±0.840** | 3.792±0.345** | 7.361±0.260** | 5.565±0.6** | 5.814±0.635** |
GSH, glutathione; CAT, catalase; SOD, superoxide dismutase; GST, glutathione S-transferase; GPx, glutathione peroxidase; GR, glutathione reductase.
p < 0.01 when group IV was compared with group I; *p < 0.05, **p < 0.01 when the pretreated groups were compared with group IV.
Effects of a combination of benidipine and edaravone on the activity of myocardial enzymes
Figure 1 reveals the activity of myocardial enzymes (LDH and CK-MB) in serum. The activity of myocardial enzymes was significantly (p < 0.01) elevated in the serum of the ISO-treated group compared with the control group. Pretreatment with a combination of benidipine and edaravone (1 µg/kg + 1 mg/kg and 3 µg/kg + 3 mg/kg) significantly (p < 0.01) reduced the activity of myocardial enzymes in serum compared to the ISO-treated group.
Figure 1.
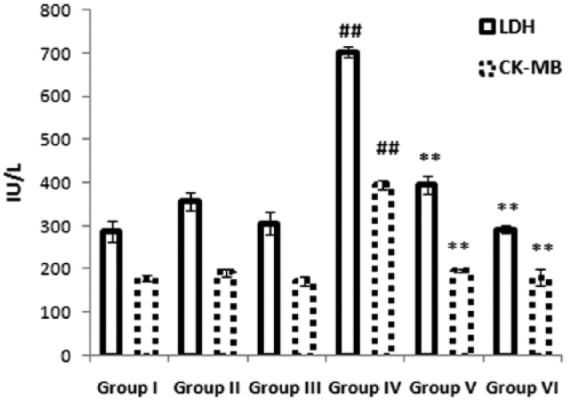
Effects of the combination of benidipine and edaravone on cardiac biomarker in serum. Serum levels of lactate dehydrogenase (LDH) and creatine kinase-MB (CK-MB) were measured in the control group (group I); combination of benidipine and edaravone without isoproterenol (ISO) in the per se groups (groups II and III); toxic group (group IV); and the groups pretreated with ISO (group V) 1 μg/kg + 1 mg/kg and (group VI) 3 μg/kg + 3 mg/kg. Data were mean ± standard error of the mean from a group of six animals. ##p < 0.01 when group IV is compared with group I; *p < 0.05, **p < 0.01, when pretreated groups were compared with group IV.
Effects of a combination of benidipine and edaravone on the inflammatory marker
Figure 2 illustrates the effects of a combination of benidipine and edaravone on levels of inflammatory marker CRP in the experimental group. The ISO-treated group showed significantly (p < 0.01) increased level of CRP in serum compared with the control group. Pretreatment with a combination of benidipine and edaravone (1 µg/kg + 1 mg/kg and 3 µg/kg + 3 mg/kg) significantly (p < 0.01) decreased the level of CRP in serum compared with the ISO-treated group.
Figure 2.
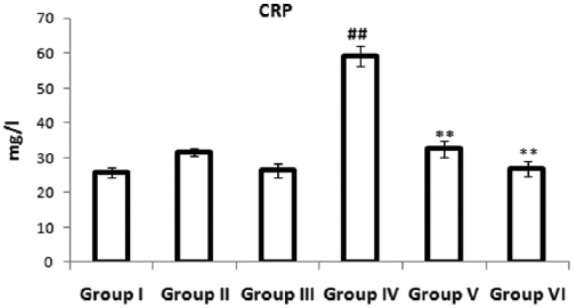
Effects of the combination of benidipine and edaravone on levels of C-reactive protein in serum in the control group (group I); combination of benidipine and edaravone without isoproterenol (ISO) in the per se group (groups II and III); toxic group (group IV); and the groups pretreated with ISO (group V) 1 μg/kg + 1 mg/kg and (group VI) 3 μg/kg+3 mg/kg. Data were mean ± standard error of the mean from a group of six animals. ##p < 0.01 when group IV was compared with group I; *p < 0.05, **p < 0.01 when the pretreated groups were compared with group IV.
Effects of a combination of benidipine and edaravone on the activity of Caspase-3
Figure 3 depicts the anti-apoptotic effect of a combination of benidipine and edaravone in the experimental group. The bar graph shows significantly (p < 0.01) elevated levels of Caspase-3 activity in the myocardium of the ISO-treated group compared with the normal control group. Pretreatment with a combination of benidipine and edaravone (1 µg/kg + 1 mg/kg and 3 µg/kg + 3 mg/kg) significantly (p < 0.01) decreased Caspase-3 activity in the myocardium as compared with the ISO-treated group.
Figure 3.
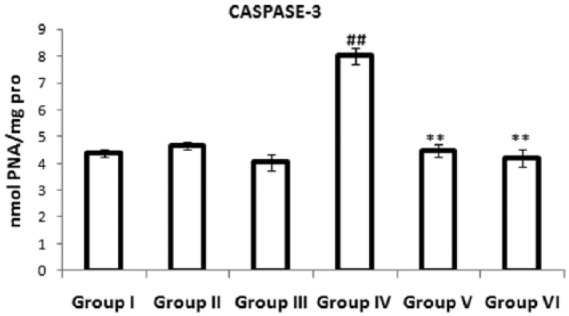
Effects of the combination of benidipine and edaravone on levels of Caspase-3 protease in cardiac tissue in the control group (group I); combination of benidipine and edaravone without isoproterenol (ISO) in the per se group (groups II and III); toxic group (group IV); and the groups pretreated with ISO (group V) 1 μg/kg + 1 mg/kg and (group VI) 3 μg/kg+3 mg/kg. Data were mean ± standard error of the mean from a group of six animals. ##p < 0.01 when group IV was compared with group I; *p < 0.05, **p < 0.01 when the pretreated groups were compared with group IV.
Effects of a combination of benidipine and edaravone on the level of MDA
Figure 4 presents the level of MDA in the myocardium of the experimental group. The ISO-treated group showed a significantly (p < 0.01) increased level of MDA in the myocardium compared with the control group. Pretreatment with a combination of benidipine and edaravone (1 µg/kg + 1 mg/kg and 3 µg/kg + 3 mg/kg) significantly (p < 0.01) reduced MDA levels in the myocardium compared with the ISO-treated group.
Figure 4.
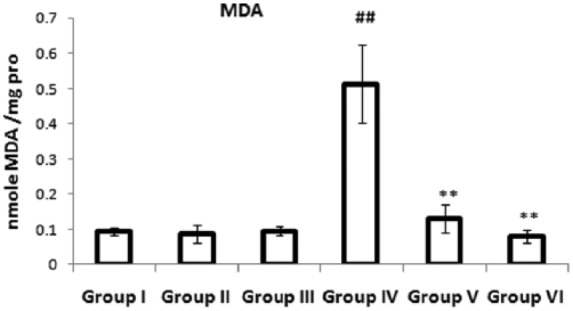
Effects of the combination of benidipine and edaravone on MDA in myocardium in the control group (group I); combination of benidipine and edaravone without isoproterenol (ISO) in the per se group (groups II and III); toxic group (group IV); and the groups pretreated with ISO (group V) 1 μg/kg + 1 mg/kg and (group VI) 3 μg/kg+3 mg/kg. Data were mean ± standard error of the mean from a group of six animals. ##p < 0.01 when group IV was compared with group I; *p < 0.05, **p < 0.01 when the pretreated groups were compared with group IV.
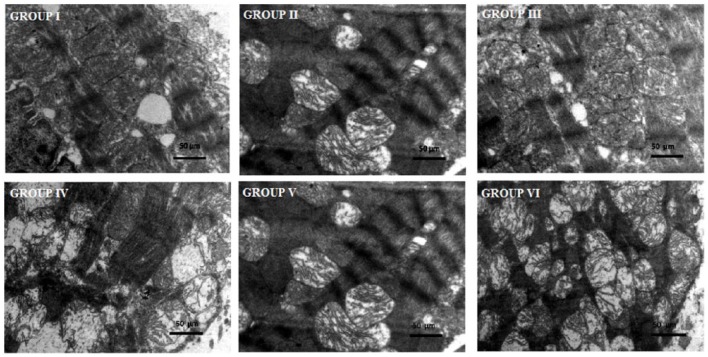
Effects of a combination of benidipine and edaravone on the ultrastructure of cardiac tissue
Figure 5 illustrates the ultrastructure of the left ventricle of the control, toxic and experimental groups. In the present study, microscopic images of the mitochondria in the ventricle of ISO-treated animals (group IV) shows an extensive destruction of cristae, and the presence of swollen and altered mitochondrial membrane. Pretreatment with a combination of benidipine and edaravone (1 µg/kg + 1mg/kg and 3 µg/kg + 3 mg/kg) in the ISO-treated group shows a preserved architecture of the cell membrane and a maintained cristae structure (groups V and VI). A combination of benidipine and edaravone per se in a dose of 1 µg/kg + 1 mg/kg and 3 µg/kg + 3 mg/kg shows no pathological alteration (groups II and III). The normal group shows a normal ultrastructure of the mitochondria of cardiac tissue (group I).
Figure 5.
Transmission electron microscopic study of ventricle tissue of rat heart. Group I: normal control group showing normal architecture of the heart mitochondria. Groups II and III: per se groups treated with benidipine + edaravone (1 μg/kg + 1 mg/kg and 3 μg/kg + 3 mg/kg) dose showing normal mitochondria along with good appearance of myofilaments of the heart mitochondria. Group IV: toxic group treated with isoproterenol (ISO; 85 mg/kg, subcutaneous) heart mitochondria showing ruptured mitochondrial cell membrane, bulging of mitochondria and disorder of cristae with vacuolation. Group V: group treated with benidipine + edaravone (1 μg/kg + 1 mg/kg) + ISO showing disarrangement of cristae with some swelling and vacuolation. Group VI: pretreated group, treated with benidipine + edaravone (3 μg/kg + 3 mg/kg) + ISO showing heart mitochondria with maintained architecture and slight fibrosis of mitochondria space. Scale bar = 50 μm.
Discussion
ISO is a β-adrenoceptor agonist and synthetic catecholamine. It has been reported that catecholamine produces membrane alterations and intracellular Ca2+ overload, leading to myocardial damage [Green and Ashwood, 2005]. Adrenochrome and hydroxyl radicals are an oxidative product of catecholamine, leading to contractile failure and causing myocardial cell damage [Singal et al. 1981]. Benidipine is a calcium channel blocker and edaravone has a novel free radical scavenger moiety. Therefore, a combination of these may be a potent protective agent in the experimental model of cardiotoxicity. In the present study, protective effects of a low dose combination of benidipine and edaravone were evaluated by estimation of myocardial biomarkers and inflammatory markers in serum, enzymatic and nonenzymatic antioxidant markers, anti-apoptotic effects, and an ultrastructure study by electron transmission microscope in cardiac tissue.
The present study revealed that the ISO-treated group showed a significant elevation of the cardiac biomarkers LDH and CK-MB in the serum, compared with the normal control group. The results of our study are in accordance with previous studies [Wang et al. 2009; Upaganlawar et al. 2009; Kurian et al. 2005]. The cardiotoxic effect of ISO, due to the deficiency of oxygen supply and glucose, results in cellular damage, loss of integrity and permeability of the cell membrane [Thomes et al. 2010]. It leads to a leakage of cardiac biomarkers in the cardiac interstitium, and finally into the blood [Thomes et al. 2010; Senthil et al. 2007]. Pretreatment with the combination of benidipine and edaravone significantly (p < 0.01) restored the normal levels of cardiac biomarkers in serum. The combination of benidipine and edaravone might arguably have preserved the structural and functional integrity and permeability of the cardiac membrane due to the Ca2+ channel blocker and antioxidant effects, thus restricting the leakage of these cardiac biomarkers.
The antioxidant defense system comprises enzymatic and nonenzymatic antioxidants, namely GSH and GST (enzymatic) and GPx, GR, SOD and CAT (nonenzymatic) [Palanisamy et al. 2009]. Oxidative stress and pathological conditions generate ROS-like hydroxyl ions and superoxide free radicals, which alter the antioxidant defense system [Gauthier and Leblais, 1998; Dhalla et al. 2000]. In the present study, the ISO-treated group showed a significant decrease in the concentration of enzymatic antioxidant and the activities of nonenzymatic antioxidants in cardiac tissue of the rats, as compared to normal control rats. The activities of GPx, GR and GST may have been decreased due to the consumption of GSH. The level of GSH is decreased due to formation of ROS in myocardium, which scavenges the superoxide radical, hydroxyl radical and singlet oxygen, and itself changes to oxidized glutathione (GSSH) [Meister, 1988]. Furthermore, in the present study, we observed reduction in the activities of SOD and CAT in ISO-treated groups, which may be due to the increased generation of highly cytotoxic free radicals by auto-oxidation of catecholamines. This leads to reduction in the activities of SOD and CAT. In our study, pretreatment with combination of benidipine and edaravone significantly suppressed the activities of antioxidant markers compared with the ISO-treated group. The above results indicate that this combination suppressed the oxidative stress induced by ISO.
Lipid peroxides can be detected by the thiobarbituric acid reactive substance (TBARS) assay which is estimated as MDA. The level of MDA significantly increased in the ISO-treated group compared with the normal control group. MDA is formed due to the oxidation of the plasma membrane polyunsaturated fatty acids in the myocardium, which are oxidized by oxidative products of ISO. Elevation in the level of MDA in ISO-treated rats due to the oxidation of lipids in the myocardium leads to the irreversible deterioration of the myocardial membrane [Zhou et al. 2008]. The level of MDA, which is considered as one of the best markers of oxidative stress, also significantly decreased in pretreatment group.
CRP is a member of the pentraxin family of protein, and a prototypical acute phase reactant that elevates plasma concentration during tissue damage. The tissue damage in myocardial necrosis causes an inflammatory reaction, leading to increased levels of CRP [Balbay et al. 2001; Hedlund, 1961]. The concentration of CRP reflects the extent of myocardial necrosis [de Beer et al. 1982]. Several studies report that the level of CRP significantly increased in acute MI and angina pectoris [Suleiman et al. 2006; Cusack et al. 2002]. In the present study, the ISO-treated group showed significantly elevated serum CRP level as compared with the normal control group. Oxidative metabolites of ISO develop the oxidative stress that generates the ROS. These ROS alter the myocardium and are responsible for eliciting an inflammatory cascade through the generation of cytokines [Lefer and Granger, 2000; Dhalla et al. 2010]. In our study, a combination of benidipine and edaravone showed a normal serum CRP level in MI-induced rats. Our results suggest that a combination of benidipine and edaravone inhibits the inflammatory mediator, as evidenced by the decreased the serum CRP level.
Caspase-3 (cysteinyl aspartate-specific proteinases) is an apoptotic enzyme that activates a biochemical cell-suicide pathway, leading to programmed cell death [Dhalla et al. 2010]. Several studies have demonstrated the involvement of Caspase-3 in the development and progression of CVDs including MI [Ferrari et al. 1990; Sahu et al. 2014]. In the present study, the activity of Caspase-3 was significantly increased in ISO-treated group compared with the normal control group. Increased activity in Caspase-3 indicates a deterioration of the myocardium, a finding which is also supported by our ultrastructure study. Our result is also in accord with various previous studies [Lin et al. 2014; Gu et al. 2000]. Pretreatment with a combination of benidipine and edaravone significantly restored the activity of Caspase-3 towards the normal. Our result suggests that the combination of benidipine and edaravone has potential cardioprotective action against ISO-induced MI. This is possibly due to multiple effects of these combined drugs, one of which controls the intracellular Ca2+ overload while the other attenuates the oxidative stress induced by oxidative products of ISO.
Transmission electron microscopy was also carried out for an ultrastructural evaluation of myocardial mitochondria. The myocardium of ISO-treated rats showed numerous necrotic foci within the myocardium, the destruction of cristae, and the presence of swollen and broken mitochondria, largely in the left ventricle subendocardium. These ultrastructural changes of the ventricle were in accordance with several other studies [Hassan et al. 2015a; Goldspink et al. 2004]. In the present study, the combination of benidipine and edaravone has significantly preserved the ultrastructure of myocardial mitochondria.
The calcium influx induced by ISO damages the myocardium and is one of the most important primary factors in various myocardial dysfunctions [Sharov et al. 1988]. It has also been reported that ISO-induced cardiomyopathy was mediated by an increased level of intracellular Ca2+, through L-type calcium channels [Singh et al. 2001]. Treatment with a combination of benidipine and edaravone has significantly preserved the ultrastructural architecture of the ventricle tissue of rats. This may be due to L-type calcium channel blocker property of benidipine and the free radical scavenger property of edaravone.
In conclusion, our results and observations suggest that a low dose combination of benidipine and edaravone shows a potentially protective effect in ISO-induced MI. This may be due to the combination attenuating oxidative stress, causing apoptotic signaling and preventing alteration in myocardial biomarkers, as well as maintaining the ultrastructure of cardiac tissue.
Footnotes
Funding: The present study was supported by University Grant Commission, New Delhi who provided a Junior Research Fellowship (JRF) to the first author.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Md. Quamrul Hassan, Department of Pharmacology, Faculty of Pharmacy, Jamia Hamdard, New Delhi, India.
Md. Sayeed Akhtar, Department of Pharmacology, Faculty of Pharmacy, Jamia Hamdard, New Delhi, India.
Mohd. Akhtar, Department of Pharmacology, Faculty of Pharmacy, Jamia Hamdard, New Delhi, India
Javed Ali, Department of Pharmacology, Faculty of Pharmacy, Jamia Hamdard, New Delhi, India.
Syed Ehtaishamul Haque, Department of Pharmacology, Faculty of Pharmacy, Jamia Hamdard, New Delhi, India.
Abul Kalam Najmi, Department of Pharmacology, Faculty of Pharmacy, Jamia Hamdard, New Delhi 110062, India.
References
- Balbay Y., Tikiz H., Baptiste R., Ayaz S., Sasmaz H., Korkmaz S. (2001) Circulating interleukin-1 beta, interleukin-6, tumor necrosis factor-alpha, and soluble ICAM-1 in patients with chronic stable angina and myocardial infarction. Angiology 52: 109–114. [DOI] [PubMed] [Google Scholar]
- Carlberg I., Mannerviek B. (1975) Glutathione reductase levels in rat brain. J Biol Chem 250: 5475–5580. [PubMed] [Google Scholar]
- Ceconi C., Boraso A., Cargnoni A., Ferrari R. (2003) Oxidative stress in cardiovascular disease: myth or fact? Arch Biochem Biophys 420: 217–221. [DOI] [PubMed] [Google Scholar]
- Claiborne A. (1985) Catalase activity. In: Greenwald R. (ed.) Handbook of Methods for Oxygen Free Radical Research. Boca Raton, FL: CRC Press, pp. 283–284. [Google Scholar]
- Cusack M., Marber M., Lambiase P., Bucknall C., Redwood S. (2002) Systemic inflammation in unstable angina is the result of myocardial necrosis. J Am Coll Cardiol 39: 1917–1923. [DOI] [PubMed] [Google Scholar]
- de Beer F., Hind C., Fox K., Allan R., Maseri A., Pepys M. (1982) Measurement of serum C-reactive protein concentration in myocardial ischaemia and infarction. Br Heart J 47: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalla N., Adameova A., Kaur M. (2010) Role of catecholamine oxidation in sudden cardiac death. Fundam Clin Pharmacol 24: 539–546. [DOI] [PubMed] [Google Scholar]
- Dhalla N., Elmoselhi A., Hata T., Makino N. (2000) Status of myocardial antioxidants in reperfusion injury. Cardiovasc Res 47: 446–456. [DOI] [PubMed] [Google Scholar]
- Eckersall P. (2000) Recent advances and future prospects for the use of acute phase proteins and markers of disease in animals. Rev Med Vet 151: 577–584. [Google Scholar]
- Fearon I., Faux S. (2009) Oxidative stress and cardiovascular disease: novel tools give (free) radical insight. J Mol Cell Cardiol 47: 372–381. [DOI] [PubMed] [Google Scholar]
- Ferrari R., Alfieri O., Curello S., Ceconi C., Cargnoni A., Marzollo P. (1990) Occurrence of oxidative stress during reperfusion of the human heart. Circulation 81: 201–211. [DOI] [PubMed] [Google Scholar]
- Gauthier C., Leblais V. (1998) The negative inotropic effect of b3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest 102: 1377–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D., Burniston J., Ellison G., Clark W., Tan L. (2004) Catecholamine-induced apoptosis and necrosis in cardiac and skeletal myocytes of the rat in vivo: the same or separate death pathways? Exp Physiol 89: 407–416. [DOI] [PubMed] [Google Scholar]
- Goyal S., Arora S., Sharma A., Joshi S., Ray R., Bhatia J., et al. (2010) Preventive effect of crocin of crocus sativus on hemodynamic, biochemical, histopathological and ultrastructural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine 17: 227–232. [DOI] [PubMed] [Google Scholar]
- Green A., Ashwood T. (2005) Free radical trapping as a therapeutic approach to neuroprotection in stroke: experimental and clinical studies with NXY-059 and free radical scavengers. Curr Drugs Targets CNS Neurol Disord 4: 109–118. [DOI] [PubMed] [Google Scholar]
- Grieve D., Byrne J., Cave A., Shah A. (2004) Role of oxidative stress in cardiac remodeling after myocardial infarction. Heart Lung Circ 13: 132–138. [DOI] [PubMed] [Google Scholar]
- Gu C., Ma Y., Benjamin J., Littman D., Chao M., Huang X. (2000) Apoptotic signaling through the beta-adrenergic receptor. A new GS effector pathway. J Biol Chem 275: 20726–20733. [DOI] [PubMed] [Google Scholar]
- Gurtu V., Kain S., Zhang G. (1997) Fluorometric and colorimetric detection of caspase activity associated with apoptosis. Anal Biochem 251: 98–102. [DOI] [PubMed] [Google Scholar]
- Habig W., Pabst M., Jakoby W. (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249: 7130–7139. [PubMed] [Google Scholar]
- Hassan M., Akhtar M., Akhtar M., Ali J., Haque S., Najmi A. (2015a) Edaravone protects rats against oxidative stress and apoptosis in experimentally induced myocardial infarction: Biochemical and ultrastructural evidence. Redox Rep 20 April. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M., Akhtar M., Akhtar M., Ansari S., Ali J., Haque S., et al. (2015b) Benidipine prevents oxidative stress, inflammatory changes and apoptosis related myofibril damage in isoproterenol-induced myocardial infarction in rats. Toxicol Mech Methods 25: 26–33. [DOI] [PubMed] [Google Scholar]
- Hedlund P. (1961) Clinical and experimental studies on C-reactive protein (acute phase protein). Acta Med Scand Suppl 361: 1–71. [PubMed] [Google Scholar]
- Higo K., Karasawa A., Kubo K. (1992) Protective effects of benidipine hydrochloride (KW-3049), a calcium antagonist, against experimental arterial calcinosis and endothelial dysfunction in rats. J Pharmacobiodyn 15: 113–120. [DOI] [PubMed] [Google Scholar]
- Kitakaze M., Node K., Minamino T., Asanuma H., Kuzuya T., Hori M. (1999) A Ca channel blocker, benidipine, increases coronary blood flow and attenuates the severity of myocardial ischemia via NO dependent mechanisms in dogs. J Am Coll Cardiol 33: 242–249. [DOI] [PubMed] [Google Scholar]
- Kurian G., Philip S., Varghese T. (2005) Effect of aqueous extract of the Desmodium gangeticum DC root in the severity of myocardial infarction. J Ethnopharmacol 97: 457–461. [DOI] [PubMed] [Google Scholar]
- Lefer D., Granger D. (2000) Oxidative stress and cardiac disease. Am J Med. 4: 315–323. [DOI] [PubMed] [Google Scholar]
- Lin Y., Zhang X., Wang L., Zhao Y., Li H., Xiao W., et al. (2014) Polyamine depletion attenuates isoproterenol-induced hypertrophy and endoplasmic reticulum stress in cardiomyocytes. Cell Physiol Biochem 34: 1455–1465. [DOI] [PubMed] [Google Scholar]
- Liu H., Gao F., Tao L., Yan W., Gao E., Christopher T., et al. (2004) Antiapoptotic mechanisms of benidipine in the ischemic/reperfused heart. Br J Pharmacol 142: 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O., Rosebrough N., Farr A., Randall R. (1951) Protein measurements with the Folin Phenol Reagent. J Biol Chem 193: 265–275. [PubMed] [Google Scholar]
- Lum G., Gambino S. (1974) A comparison of serum vs heparinised plasma for routine chemistry tests. Am J Clin Pathol 61: 108–113. [DOI] [PubMed] [Google Scholar]
- Marklund S., Marklund G. (1974) Involvement of superoxide anion radical in auto oxidation by pyrogallol and a convenient assay of superoxide dismutase. Eur J Biochem 47: 469–474. [DOI] [PubMed] [Google Scholar]
- Matsubara M., Akizuki O., Ikeda J., Saeki K., Yao K., Sasaki K. (2008) Benipidine, an anti-hypertensive drug, inhibits reactive oxygen species production in polymorphonuclear leukocytes and oxidative stress in salt-loaded stroke-prone spontaneously hypertensive rat. Eur J Pharmacol 580: 201–213. [DOI] [PubMed] [Google Scholar]
- Meister A. (1988) Glutathione metabolism and its selective modification. J Biol Chem 263: 17205–17208. [PubMed] [Google Scholar]
- Murota S., Morita I., Suda N. (1990) The control of vascular endothelial cell injury. Ann N Y Acad Sci 598: 182–187. [DOI] [PubMed] [Google Scholar]
- Okhawa H., Ohishi N., Yagi K. (1970) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 34: 30–38. [DOI] [PubMed] [Google Scholar]
- Palanisamy P., Rao Y., Farook J., Saravanan G., Bakthavathsalam G. (2009) Oxidative stress and cardiac biomarkers in patients with acute myocardial infarction. Eur J Sci Res 27: 275–285. [Google Scholar]
- Pogodina L., Shornikova M., Chentsov I. (2006) Electron microscopy description of cardiomyocytes from the left ventricle of rat heart after apoptosis induction by isoproterenol. Izv Akad Nauk Ser Biol 1: 26–37. [PubMed] [Google Scholar]
- Rona G., Chappel C., Balazs T., Gaudry R. (1959) An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. AMA Arch Pathol 67: 443–445. [PubMed] [Google Scholar]
- Sahu B., Putcha U., Kuncha M., Rachamalla S., Sistla R. (2014) Carnosic acid promotes myocardial antioxidant response and prevents isoproterenol-induced myocardial oxidative stress and apoptosis in mice. Mol Cell Biochem 394: 163–176. [DOI] [PubMed] [Google Scholar]
- Saini A., Patel R., Sharma S., Kumar A. (2006) Edaravone attenuates hydroxyl radical stress and augmented angiotensin II response in diabetic rats. Pharmacol Res 54: 6–10. [DOI] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R. (1968) Estimation of total, protein bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25: 192–205. [DOI] [PubMed] [Google Scholar]
- Senthil S., Sridevi M., Pugalendi K. (2007) Cardioprotective effect of oleanolic acid on isoproterenol-induced myocardial ischemia in rats. Toxicol Pathol 35: 418–423. [DOI] [PubMed] [Google Scholar]
- Sharov V., Irgashev S., Mavridi D., Mogilevskii G. (1988) Specific features of calcium damage in cardiomyocytes. In Ul’trastruktura serdtsa [Ultrastructure of the Heart]. Tashkent: Meditsina, pp. 57–62. [Google Scholar]
- Shizukuda Y., Buttrick P., Geenen D., Borczuk A., Kitsis R., Sonnenblick E. (1998) Beta-adrenergic stimulation causes cardiocyte apoptosis: influence of tachycardia and hypertrophy. Am J Physiol 275: 961–968. [DOI] [PubMed] [Google Scholar]
- Singal P., Yates J., Beamish R., Dhalla N. (1981) Influence of reducing agents on adrenochrome-induced changes in the heart. Arch Pathol Lab Med 105: 664–669. [PubMed] [Google Scholar]
- Singh K., Xiao L., Remondino A., Sawyer D., Colucci W. (2001) Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol 189: 257–265. [DOI] [PubMed] [Google Scholar]
- Suleiman M., Khatib R., Agmon Y., Mahamid R., Boulos M., Kapeliovich M., et al. (2006) Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction: predictive role of C-reactive protein. J Am Coll Cardiol 47: 962–968. [DOI] [PubMed] [Google Scholar]
- Thomes P., Rajendran M., Pasanban B., Rengasamy R. (2010) Cardioprotective activity of Cladosiphon okamuranus fucoidan against isoproterenol induced myocardial infarction in rats. Phytomedicine 18: 52–57. [DOI] [PubMed] [Google Scholar]
- Upaganlawar A., Gandhi C., Balaraman R. (2009) Effect of green tea and vitamin E combination in Isoproterenol-induced myocardial infarction in rats. Plant Food Hum Nutr 64: 75–80. [DOI] [PubMed] [Google Scholar]
- Wang S., Tian S., Yang F., Yang H., Yang X., Du G. (2009) Cardioprotective effect of salvianolic acid A on isoproterenol-induced myocardial infarction in rats. Eur J Pharmacol 615: 125–132. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Morita I., Nishi H., Murota S. (1988) Preventive effect of MCI-186 on 15-HPETE induced vascular endothelial cell injury in vitro. Prostaglandins Leukot Essent Fatty Acids 33: 81–87. [DOI] [PubMed] [Google Scholar]
- Wexler B. (1978) Myocardial infarction in young vs old male rats: pathophysiologic changes. Am Heart J 96: 70–80. [DOI] [PubMed] [Google Scholar]
- Wheeler R., Jhaine A., Elsayeed M., Omaye T., Korte J. (1990) Automated assays for superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase activity. Anal Biochem 184: 193–199. [DOI] [PubMed] [Google Scholar]
- Young D. (1990) Effects of Drugs on Clinical Laboratory Tests. Washington, DC: American Association of Clinical Chemistry Press, pp. 120–22. [Google Scholar]
- Yusuf S., Reddy S., Ôunpuu S., Anand S. (2001) Global burden of cardiovascular diseases: Part I – General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 104: 2746–2753. [DOI] [PubMed] [Google Scholar]
- Zhou R., Xu Q., Zheng P., Yan L., Zheng J., Dai G. (2008) Cardioprotective effect of fluvastatin on isoproterenol-induced myocardial infarction in rat. Eur J Pharmacol 586: 244–250. [DOI] [PubMed] [Google Scholar]