Abstract
Atrial fibrillation is a common, costly and morbid cardiovascular arrhythmia. Stroke prevention remains the mainstay of treatment for atrial fibrillation, and the recent advent of novel oral anticoagulants with direct factor IIa or factor Xa inhibition has significantly revolutionized this aspect of treatment for atrial fibrillation patients. This review focuses on the tolerability and efficacy of apixaban and tackles the generalizability of the findings with apixaban to broader patient populations than those primarily enrolled in the clinical trials, drawing from the AVERROES and ARISTOTLE trials and their subsequent secondary analyses. Taken together, findings from these trials show that apixaban is superior to warfarin in preventing stroke with a lower risk of major bleeding in the general population of patients with atrial fibrillation as well as in several key high-risk patient subgroups.
Keywords: anticoagulation, atrial fibrillation, stroke
Introduction
Atrial fibrillation is the most common cardiac arrhythmia, affecting 2.7–6.1 million US adults in 2015 [Mozaffarian et al. 2015; National Center for Health Statistics – CDC, 2014], and even more if one includes clinically undetected atrial fibrillation. Each year, approximately 750,000 hospitalizations, 130,000 deaths, and over US$6 billion dollars of healthcare spending are attributed to atrial fibrillation [Agency for Healthcare Research and Quality, 2012; National Center for Health Statistics – CDC, 2014]. Atrial fibrillation has been associated with increased all-cause mortality [hazard ratio (HR) of 9.62 in the first 4 months of diagnosis and 1.66 afterwards] [Miyasaka et al. 2007]. Women are more likely to die from atrial fibrillation than men, and Americans of European descent are more likely to die from atrial fibrillation than those of African or Asian descent [Benjamin et al. 1998; Novaro et al. 2008]. Patients with atrial fibrillation are at a 5–7 times increased risk of developing a stroke than the general population [Wolf et al. 1978; Flegel et al. 1987]. Strokes associated with atrial fibrillation are more disabling than other strokes, with the majority being either fatal or of moderate-to-high severity [Lin et al. 1996].
While oral anticoagulation has been a challenging aspect of atrial fibrillation treatment for years, the relatively recent advent of direct oral anticoagulants (DOACs) has provided improved options for patients. At the same time, the aging and highly morbid population with atrial fibrillation has posed a number of important clinical questions related to DOAC use. Atrial fibrillation disproportionately affects the elderly; while the prevalence of atrial fibrillation in the general population is approximately 1%, 4.1–8.6% of adults 65–74 and 21.6–22.7% of adults 75–84 have atrial fibrillation [Psaty et al. 1997; Mozaffarian et al. 2015]. Thus, with the aging US population, atrial fibrillation has increasingly become a disease of the elderly, with over 50% of all cases occurring in patients aged over 80 years [Feinberg et al. 1995]. Some 36% of all strokes in patients over 80 years are related to atrial fibrillation [Psaty et al. 1997]. Furthermore, atrial fibrillation is a disease of the multimorbid. Patients with atrial fibrillation are more likely to have other cardiovascular diseases such as ischemic heart disease, congestive heart failure, hypertension and valvular disease, as well as diabetes, chronic obstructive lung disease, chronic kidney disease, obesity and alcoholism [Furberg et al. 1994; Benjamin et al. 1994]. Therefore, it is critically important to examine the efficacy and tolerability profiles of any oral anticoagulant in these diverse groups of patients.
Apixaban is currently approved in the US to prevent stroke in patients with atrial fibrillation. Major trials excluded patients with serum creatinine greater than Cr = 2.5 mm/dL, or glomerular filtration rate of less than 25 ml/min/1.73 m2 (as derived from the Cockcroft–Gault equation) [Agnelli et al. 2013]. Also, apixaban has been dose reduced to 2.5 mg twice daily from the usual 5 mg twice daily in patients with at least two of the following criteria: age greater than 80 years, serum creatinine greater than 1.5, and weight less than 60 kg [Lopes et al. 2010; Eikelboom et al. 2010]. Understanding the role of apixaban in patients with different chronic kidney disease (CKD) stages is important. Likewise, a better understanding of how apixaban fares in relation to different scoring systems for stroke (such as CHA2DS2-VASc) and major bleeding (such as HAS-BLED) is equally important. A number of decision tools have been developed to identify patients who may benefit most from oral anticoagulation. In a large prospective study of over 60,000 patients with atrial fibrillation, the CHADS2, CHA2DS2-VASc and ATRIA stroke scores had C statistics of 0.68–0.70 for full-point scores, with a slight advantage in using the ATRIA score over the other two [van den Ham et al. 2015]. The HAS-BLED score has been used to predict 1-year major bleeding risk in patients on oral anticoagulants [Pisters et al. 2010], although it has poor ability to discriminate and it may potentiate underuse of anticoagulation, including among those at high risk of stroke who also tend to have high risk of bleeding. Moreover, bleeding risk-stratification tools are largely generated from patients treated with warfarin. In this paper, we will discuss the emerging evidence supporting the use of apixaban to treat patients with atrial fibrillation.
Prevention of stroke and systemic embolism
AVERROES trial
In early 2011, the apixaban versus acetyl-salicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment (AVERROES) trial was published. It was a double-blind, randomized-controlled trial comparing the use of apixaban with aspirin (two thirds received low-dose aspirin) for prevention of stroke or systemic embolism among 5599 patients who had previously failed warfarin (poor compliance, refusal or prior bleeding) and who were deemed to be unsuitable for warfarin (like a CHADS2 score of 1) [Eikelboom et al. 2010]. The trial was stopped early in 2010 because of a clear benefit of apixaban over aspirin in preventing stroke. Mean follow up was approximately 1.1 years. Overall, the primary outcome of stroke or systemic embolism occurred in 1.6% of patients per year treated with apixaban and in 3.7% of patients per year treated with aspirin [Connolly et al. 2011]. There was no significant difference between the groups in all-cause mortality, but subsequent analysis revealed that there was a significant reduction in cardiovascular hospitalizations in the apixaban group (12.3% per year) compared with the aspirin group (15.4% per year), and that cardiovascular hospitalization was predictive of mortality (HR 3.95) [Hohnloser et al. 2013]. Apixaban was less often discontinued than was aspirin, showing its favorable tolerability. Importantly, the risk of major bleeding was only modestly higher with apixaban (HR 1.13), as was the risk of minor bleeding (HR 1.24). The rates of intracranial hemorrhage were the same. Given the smaller sample size and shorter follow-up period in AVERROES, results have been interpreted with more caution, especially where they may differ from other larger trials, such as ARISTOTLE.
ARISTOTLE trial
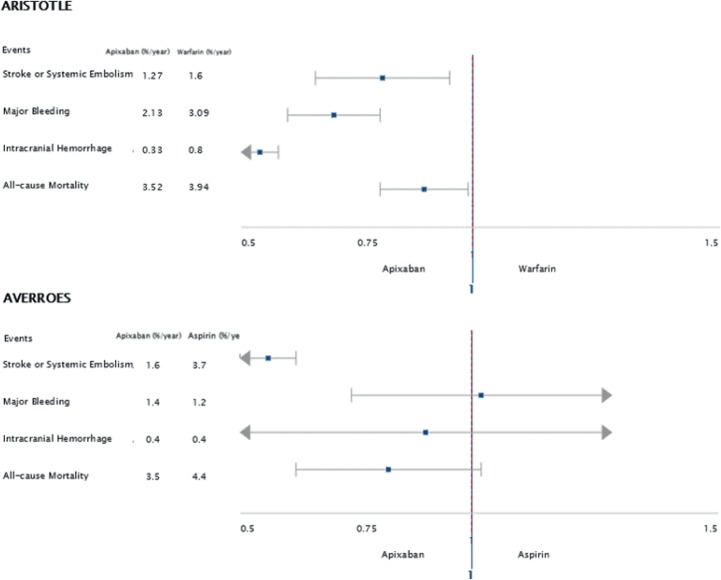
Later in 2011, the apixaban for reduction in stroke and other thrombotic events in atrial fibrillation (ARISTOTLE) trial was published. It was a double-blinded, double-dummy noninferiority trial comparing the use of apixaban versus warfarin to prevent stroke and systemic embolism among 18,201 patients with atrial fibrillation; eligible patients had two or more episodes of atrial fibrillation within the previous 12 months, as well as at least one of the following risk factors: age greater than 75, prior stroke or systemic embolism, symptomatic heart failure within the previous 3 months, or diabetes or hypertension requiring pharmacologic treatment. Median age at enrollment was 70 years with 35% of the patients being female [Lopes et al. 2010; Granger et al. 2011]. At the conclusion of the study, apixaban was not only noninferior based on predefined specifications, but actually superior to warfarin in terms of the primary outcome (stroke or systemic embolism), major bleeding, and all-cause mortality (see Table 1). Stroke or systemic embolism occurred in 1.27% per year of patients treated with apixaban and in 1.6% per year of patients treated with warfarin (HR 0.79, p < 0.001). All-cause mortality was lower in patients treated with apixaban (3.52% per year) compared with patients treated with warfarin (3.94% per year) (HR 0.89, p = 0.047) [Granger et al. 2011]. (See Figure 1 for comparison of results of the AVERROES and ARISTOTLE trials). With the other DOACs, there were also consistent modest (about 10%) reductions in mortality in each of the trials [Rao et al. 2014; Connolly et al. 2009; Patel et al. 2011; Giugliano et al. 2013] and a highly significant and consistent reduction in mortality with DOACs versus warfarin in the overview [Ruff et al. 2015].
Table 1.
Comparison of study designs and key findings of the AVERROES and ARISTOTLE trials [Connolly et al. 2011; and Granger et al. 2011].
| Trial | AVERROES | ARISTOTLE |
|---|---|---|
| Design | RCT, double blind | Noninferiority RCT, double blind, double dummy, placebo controlled. |
| Patient population | Patients ‘unsuitable’ for VKA based on being unacceptable or prior failure | Patients with AFib and at least one other stroke risk factor |
| Treatment versus comparison | Apixaban versus aspirin | Apixaban versus warfarin |
| Number of subjects | 5599 | 18,201 |
| Primary outcome: | Stroke or systemic embolism: 1.6% per year in the apixaban group and 3.7% per year in the ASA group (p < 0.001) | Stroke or systemic embolism: 1.27% per year in the apixaban group, 1.60% per year in the warfarin group (p<0.001) |
| Secondary outcomes: | Major bleeding: 1.4% per year in the apixaban group, 1.2% per year in the ASA group (p = 0.57). All-cause mortality: 3.5% per year in the apixaban group, 4.4% per year in the ASA group (p = 0.07). |
Major bleeding: 2.13% per year in the apixaban group, 3.09% per year in the warfarin group (p < 0.001). All-cause mortality: 3.52% per year in the apixaban group, 3.94% per year in the warfarin group (p = 0.047) |
AFib, atrial fibrillation; ASA, aspirin at randomization; VKA, vitamin K antagonist; RCT, randomized-controlled trial.
Figure 1.
Comparison of the effects of apixaban versus control on major clinical outcomes in the ARISTOTLE and AVERROES trials [Connolly et al. 2011; Granger et al. 2011].
It should be noted that when the ARISTOTLE trial ended, there was a 2-day bridging period, such that patients on apixaban had a 2-day overlap of continuing apixaban while being transitioned to vitamin K antagonist (VKA). Following this, there was an excess in both thrombosis and bleeding events. This was largely attributed to the liability of initiating VKA rather than an adverse effect of apixaban [Granger et al. 2015]. In the edoxaban versus warfarin in subjects with atrial fibrillation—effective anticoagulation with factor Xa next generation in atrial fibrillation (ENGAGE AF–TIMI 48 trial), which had a more careful overlap of half-dose edoxaban until the INR was at least 2.0, the transition to warfarin was not associated with higher risk [Ruff et al. 2014].
Major bleeding
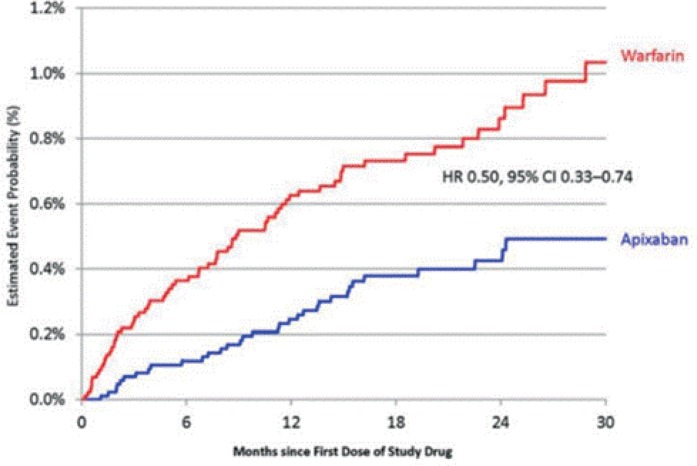
As with warfarin, major bleeding remains the most serious adverse effect of apixaban. Despite this being of special concern, given the lack of a reversal agent, patients in ARISTOTLE taking apixaban had a significantly lower risk of death within 30 days of a major bleeding event than patients taking warfarin (HR 0.5, 95% CI 0.33–0.74) (see Figure 2) [Hyleck et al. 2014]. Patients in ARISTOTLE with both intracranial and nonintracranial hemorrhage had higher mortality, higher risk of stroke and higher risk of myocardial infarction [Held et al. 2015]. Apixaban was associated with a significantly lower risk of major bleeding than warfarin in ARISTOTLE (2.13% per year in patients taking apixaban and 3.09% per year in patients taking warfarin, HR 0.69, p < 0.001) [Hohnloser et al. 2013; Granger et al. 2011].
Figure 2.
30-day mortality related to major bleeding events in patients taking apixaban versus warfarin [Hylek et al. 2014].
HR, hazard ratio; CI, confidence interval.
Apixaban, along with all of the DOACs reduced the risk of intracranial hemorrhage compared with warfarin. Apixaban was associated with a similar risk of gastrointestinal bleeding compared with warfarin [Granger et al. 2011; Hess et al. 2013]. Among patients taking apixaban, age, prior hemorrhage, prior stroke or transient ischemic attack (TIA), diabetes, chronic kidney disease, anemia, aspirin use and nonsteroidal anti-inflammatory drug (NSAID) use were independent predictors of major hemorrhage (see Table 2) [Hylek et al. 2014]. After adjustment, women were significantly less likely to bleed than men [Vinereanu et al. 2015]. The HAS-BLED score was predictive of major hemorrhage in patients taking apixaban, although not much more predictive of bleeding than was the CHADS2 score; the greatest relative benefit of apixaban over warfarin was seen in a reduction of intracranial hemorrhages in patients with a HAS-BLED score of at least 3 [Lopes et al. 2012]. Apixaban was associated with less major bleeding than warfarin regardless of prior warfarin use, international normalized ratio or predicted time in the therapeutic range [Garcia et al. 2013; Wallentin et al. 2013].
Table 2.
Baseline characteristics independently associated with major bleeding in ARISTOTLE [Hylek et al. 2014].
| Parameter | HR | 95% CI | p |
|---|---|---|---|
| Hematocrit < 45% | 1.38 | (1.24–1.52) | <0.001 |
| History of bleeding | 1.38 | (1.17–1.63) | 0.002 |
| Age (per 10 years) | 1.36 | (1.23–1.51) | <0.001 |
| NSAIDs at randomization | 1.33 | (1.07–1.65) | 0.0096 |
| Aspirin at randomization | 1.31 | (1.14–1.52) | 0.002 |
| Diabetes | 1.24 | (1.06–1.45) | 0.0067 |
| Prior stroke | 1.23 | (1.04–1.45) | 0.016 |
| Creatinine clearance (<81 ml/min/1.73 m2) | 1.11 | (1.06–1.17) | <0.001 |
| Female sex | 0.74 | (0.63–0.87) | 0.002 |
| Liver disease | 0.44 | (0.22–0.88) | 0.02 |
CI, confidence interval; HR, hazard ratio; NSAIDs, nonsteroidal anti-inflammatory drugs.
Anticoagulation of specific patient groups with apixaban
Numerous specific patient groups have been shown to benefit more from apixaban compared with warfarin. Of note, the majority of findings in this section are derived from subgroup analysis, which can be subject to a number of inherent weaknesses, and thus should be interpreted with appropriate caution. (Please see Table 3 for a summary of key subgroup analysis.)
Table 3.
Summary of subgroup analysis from the ARISTOTLE trial [Hohnloser et al. 2012; Halvorsen et al. 2014; Vinereanu et al. 2015; Easton et al. 2012; Alexander et al. 2014; Avezum et al. 2015].
| Subgroup | Stroke and systemic embolism (% per year) |
Major bleeding (% per year) |
All-cause mortality (% per year) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Apixaban | Warfarin | p | Apixaban | Warfarin | p | Apixaban | Warfarin | p | |
| Cr Cl ⩾ 80 | 0.99 | 1.12 | 0.71 | 1.46 | 1.84 | 0.03 | 2.33 | 2.71 | 0.63 |
| 50 < Cr Cl < 80 | 1.24 | 1.69 | 2.45 | 3.21 | 3.41 | 3.56 | |||
| Cr Cl ⩽ 50 | 2.11 | 2.67 | 3.21 | 6.44 | 7.12 | 8.30 | |||
| Age < 80 | 1.23 | 1.55 | 0.91 | 1.93 | 2.78 | 0.74 | 3.03 | 3.42 | 0.73 |
| Age > 80 | 1.53 | 1.90 | 3.55 | 5.41 | 6.86 | 7.44 | |||
| Women | 1.35 | 1.81 | 0.45 | 1.91 | 3.29 | 0.06 | 3.11 | 3.41 | 0.83 |
| Men | 1.22 | 1.49 | 2.26 | 2.98 | 3.37 | 3.75 | |||
| Prior CVA | 2.46 | 3.24 | 0.71 | 2.84 | 3.91 | 0.69 | 4.22 | 4.77 | 0.89 |
| No prior CVA | 1.01 | 1.23 | 1.98 | 2.91 | 3.37 | 3.75 | |||
| On ASA | 1.12 | 1.91 | 0.10 | 3.10 | 3.92 | 0.29 | 3.02 | 3.61 | 0.10 |
| Not on ASA | 1.11 | 1.32 | 1.82 | 2.78 | 4.95 | 4.88 | |||
| Valvular disease | 1.20 | 1.43 | 0.38 | 3.78 | 5.69 | 0.12 | 3.02 | 3.61 | 0.10 |
| No valvular disease | 1.46 | 2.08 | 4.90 | 6.36 | 4.95 | 4.88 | |||
Cr Cl, creatinine clearance (calculated by Cockcroft–Gault equation); ASA, aspirin at randomization; CVA, cerebrovascular accident.
Patients with chronic kidney disease
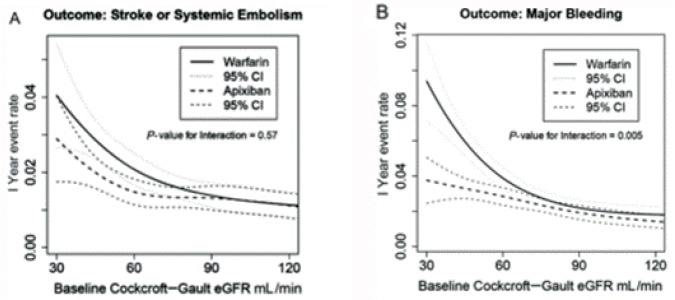
Renal impairment is a known independent predictor of stroke, bleeding and all-cause mortality in patients with atrial fibrillation [Lip et al. 2013]. Because all currently available DOACs are at least partially excreted by the kidneys, there is a theoretical risk of increased bleeding in patients with impaired renal function. For this reason, patients with serum creatinine greater than 2.5 or glomerular filtration rate of less than 25 ml/min/1.73 m2 were excluded from ARISTOTLE and AVERROES [Hohnloser et al. 2013; Granger et al. 2011]. However, compared with dabigatran that is cleared approximately 80% by the kidneys, apixaban and rivaroxaban are excreted approximately 30% renally and edoxaban about 50% renally [Raghavan et al. 2009]. In AVERROES, apixaban reduced primary events by 68% in patients with stage III chronic kidney disease and by 43% in patients with a creatinine clearance greater than 60 ml/min/1.73 m2 [Eikelboom et al. 2012]. In ARISTOTLE, apixaban was associated with fewer strokes or systemic embolisms and with significantly less major bleeding (p = 0.03) than warfarin irrespective of creatinine clearance, and more particularly, in those with a creatinine clearance < 60 ml/min/1.73 m2 (p = 0.005) (see Figure 3) [Hohnloser et al. 2012].
Figure 3.
(A) Stroke and systemic embolism (% per year) by baseline glomerular filtration rate (GFR). (B) Major bleeding events (% per year) by baseline GFR [Hohnloser et al. 2012].
eGFR, estimated glomerular filtration rate.
The elderly
Anticoagulation in the elderly is particularly problematic because patients over 80 years are at significantly higher risk of both strokes and bleeding, even when compared with patients aged 65–79 years [Hess et al. 2012]. In a recent systematic review, only about 50% of patients with atrial fibrillation over 80 years were adequately anticoagulated, based on their risk of stroke [Garwood and Corbett, 2008]. Of elderly patients started on oral anticoagulants, approximately 26% discontinue their medication within the first year. Of the elderly patients who discontinue anticoagulation, 81% cite safety concerns, such as falls, as the main reason for discontinuation. Of concern, the rates of termination have been observed to be highest among patients with a CHADS2 score of at least 3 [Hylek et al. 2007].
Risk of falling has been very commonly cited as a reason for not anticoagulating the elderly. However, falling does not appear to be a strong risk factor for major bleeding in patients on systemic anticoagulation regardless of age or baseline stroke risk [Man-Son-Hing et al. 1999]. One study suggested that a patient would need to fall over 300 times for the risk of systemic anticoagulation to outweigh the benefit [Sellers and Newby, 2011].
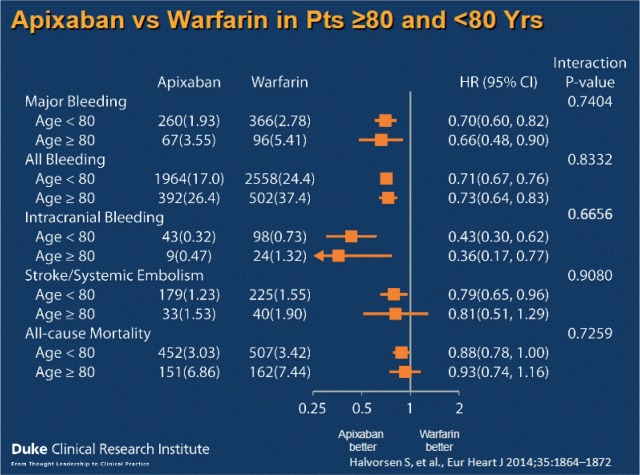
Both ARISTOTLE and AVERROES have shown that apixaban is safe to use in the elderly: compared with aspirin use in 1898 patients aged over 75 years, apixaban was associated with a significant reduction in the risk of stroke (1% versus 7% per year, respectively, or an 87% risk reduction), with no difference in bleeding risk (4.7% versus 4.9% per year, respectively) [Ng et al. 2015]. In ARISTOTLE, apixaban resulted in fewer stroke and systemic embolism events, and fewer major bleeding events than warfarin regardless of age (p for interaction = 0.11); patients over 80-years old had less major bleeding and intracranial bleeding and a trend towards less risk of stroke and systemic embolism, as well as all-cause mortality when taking apixaban compared with warfarin (see Figure 4) [Halvorsen et al. 2014].
Figure 4.
Study outcomes from the ARISTOTLE trial by age [Halvorsen et al. 2014].
HR, hazard ratio; CI, confidence interval; Pts, patients; Yrs, years.
Patients with prior stroke or transischemic attack
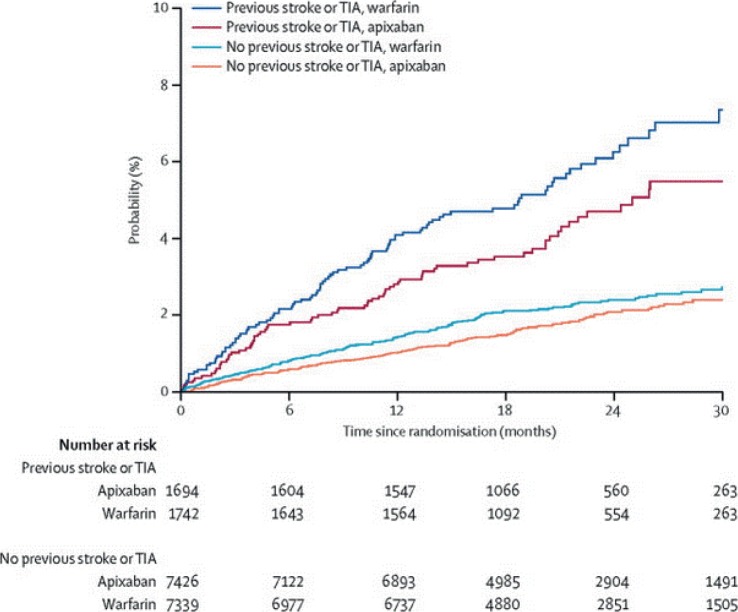
The single most significant predictor of stroke risk in patients with atrial fibrillation is prior stroke or TIA [Hess et al. 2013]. Despite this known fact, a large systematic review showed that patients with prior stroke and atrial fibrillation were anticoagulated less than 60% of the time [Ogilvie et al. 2010]. In AVERROES, patients with previous stroke or TIA were three times more likely to experience the primary outcome of stroke and systemic embolism. Patients with prior stroke or TIA benefited even more from apixaban than those without prior stroke or TIA (HR 0.29 versus 0.51, respectively) [Diener et al. 2012]. Patients in ARISTOTLE with prior stroke had an even greater absolute benefit of taking apixaban with regard to stroke and systemic embolism (absolute risk reduction 0.77/100 person-years) compared with those without prior stroke (0.22/100 person-years; p for interaction = 0.71) (see Figure 5) [Easton et al. 2012].
Figure 5.
Stroke and systemic embolism in patients with and without previous stroke and treated with warfarin versus apixaban. With prior CVA: 2.46% per year on apixaban versus 3.24% per year on warfarin [hazard ratio (HR) for apixaban 0.76]; without prior CVA: 1.01% per year on apixaban versus 1.32 per year on warfarin (HR for apixaban 0.82); p for interaction = 0.71 [Easton et al. 2012].
TIA, transient ischemic attack.
Women
Women in ARISTOTLE had a similar overall stroke risk as men and the benefits of apixaban over warfarin were consistent regardless of sex (p for interaction = 0.45) [Vinereanu et al. 2015]. This is thought to be more accurate than the finding in AVERROES that women suffered nearly twice as many strokes as men. The latter observation may have resulted from the fact that women, on average, were older than men with a significantly larger proportion of women than men who were older than 75 years. Despite this difference, patients treated with apixaban had about 60% risk reduction in the primary outcome of stroke and systemic embolism, regardless of sex [Lip et al. 2014].
Patients on aspirin
Approximately 35% of all patients taking oral anticoagulants are also on aspirin, and of those patients, 39% do not have atherosclerotic disease [Steinberg et al. 2013]. In ARISTOTLE, there was nearly a 50% higher rate of bleeding in patients taking aspirin (compared with patients not taking aspirin) with the oral anticoagulant [Granger et al. 2011]. These data suggest that avoiding unnecessary aspirin with apixaban may be an important strategy to reducing bleeding risk. However, regardless of concomitant aspirin use, apixaban was associated with less major bleeding than warfarin (3.1% per year on apixaban with aspirin and 3.92% per year on warfarin with aspirin), as well as improvement in the primary outcome of stroke and systemic embolism (p for interaction = 0.10) [Alexander et al. 2014].
Patients with persistent or permanent atrial fibrillation
Patients in ARISTOTLE with persistent or permanent atrial fibrillation had a higher stroke risk than patients with paroxysmal atrial fibrillation [Vanassche et al. 2015]. The relative benefit of apixaban over warfarin was true regardless of atrial fibrillation type, but it was more pronounced in patients with persistent and permanent atrial fibrillation (p for all interactions > 0.13) [Al-Khatib et al. 2013].
Patients with valvular abnormalities
Apixaban is currently approved by the Food and Drug Administration for use in the US in patients with atrial fibrillation who do not have valvular heart disease, or what has been termed ‘nonvalvular’ atrial fibrillation. In ARISTOTLE, 4808 (26.4%) of the enrolled patients had significant (moderate or severe) valvular disease [Granger et al. 2011]. When comparing patients with and without valvular disease on apixaban in the ARISTOTLE trial, there was no significant difference regarding the benefits of apixaban versus warfarin on stroke or systemic embolism (p = 0.38), all-cause mortality (p = 0.10) or on major bleeding (p = 0.23) [Avezum et al. 2015]. Thus, apixaban (and the other DOACs) have consistent benefits and are appropriate to use for patients with most valvular abnormalities, with the exceptions of moderate or severe mitral stenosis (excluded from the trials) or mechanical prosthetic heart valves [who had more strokes, bleeding, and valve thrombosis with dabigatran than with warfarin in a randomized, phase II study to evaluate the safety and pharmacodynamics of oral dabigatran etexilate in patients after heart valve replacement (RE-ALIGN) study] [Eikelboom et al. 2013].
Patients who are systematically underdosed with apixaban
As mentioned previously, in ARISTOTLE, patients were systematically dose reduced to apixaban 2.5 mg twice daily (versus 5 mg twice daily) if they met two of the following three criteria: age ⩾ 80 years, serum creatinine ⩾1.5 or weight ⩽60 kg. This accounted for approximately 5% of the total patient population. Such patients were also less likely to experience the primary outcome of stroke and systemic embolism (1.7% versus 3.3% per year, respectively; p = 0.22) and to have major bleeding events (3.3% versus 6.7% per year, respectively; p = 0.21) if they were on apixaban compared with warfarin. Patients taking apixaban had fewer major bleeding events than patients taking warfarin, regardless of the number of dose-reduction criteria present [Granger et al. 2011].
Patients with varying risk of stroke
Risk stratification in the AVERROES trial showed that patients with a CHADS2 score of 1 had a 0.9% annual stroke risk, compared with 2.1% in patients with a CHADS2 score of at least 2 [Coppens et al. 2013]. Yet, there was significant benefit from apixaban over aspirin regardless of the CHADS2 score [Flaker et al. 2012]. Also, there was similar benefit from apixaban over aspirin in patients with a low CHADS2 score compared with those with previous intolerance of a vitamin K antagonist [Coppens et al. 2014]. Multivariable analysis later revealed that age over 75 years, previous stroke, chronic kidney disease and nonparoxysmal atrial fibrillation were the strongest predictors of stroke [Lip et al. 2013].
As was the case in the AVERROES trial, in ARISOTLE, the net benefit of apixaban over warfarin was present regardless of the CHADS2 or the CHA2DS2-VASc score (p = 0.12 for stroke and systemic embolism, and p = 0.94 for major bleeding) [Lopes et al. 2012]. Because of this difficulty in risk stratification among patients taking apixaban using existing scoring systems, other means of stratification have been evaluated within the ARISTOTLE trial. For example, in ARISTOTLE patients, elevated high-sensitivity troponin levels was found to be independently associated with a higher risk of stroke, cardiovascular death and major bleeding [Hijazi et al. 2014a]. The benefit of apixaban over warfarin was consistent regardless of the high-sensitivity troponin level [Hijazi et al. 2014b]. Similarly, n-terminal prohormone of brain natriuretic peptide (NT pro-BNP) was shown to be strongly associated with increased stroke and mortality risk in ARISTOTLE; the benefit of taking apixaban over warfarin was observed regardless of the NT pro-BNP level [Hijazi et al. 2013]. The net benefit of apixaban over warfarin was also observed regardless of whether the patients had previously taken warfarin, whether patients were taking amiodarone or whether they had chronic obstructive pulmonary disease [Garcia et al. 2013; Flaker et al. 2014; Durheim et al. 2015].
Future directions
One of the largest concerns related to apixaban (as with the other DOACs) has been the lack of a reversal agent (although the case fatality rate of a major bleeding event on apixaban is lower than on warfarin, as has been previously pointed out) [Hylek et al. 2014]. Currently, andexanet alfa is being investigated for potential use as a reversal agent. It is a recombinant factor-Xa molecule with higher affinity for the natural factor-Xa inhibtor than endogenous factor Xa. Animal studies have shown that andexanet alfa is capable of reversing Xa inhibition in a dose-dependent fashion without being prothrombotic [Ghadimi et al. 2015]. More recently, andexanet alfa was administered to healthy older volunteers taking apixaban or rivaroxaban; it caused a 94% reduction in anti-Xa activity in patients taking apixaban [Siegal et al. 2015]. Further investigation is underway to test the efficacy of this agent in patients with Xa-related bleeding events. In addition, idarucizumab has been shown to rapidly reverse the antithrombin effects of dabigatran in studies of healthy volunteers and in patients with clinically significant bleeding [Eikelboom et al. 2015].
Patients with renal dysfunction and atrial fibrillation have more stroke and major bleeding episodes [Lip et al. 2013]. While the differential benefit of apixaban over warfarin in treating atrial fibrillation has been previously demonstrated in patients with mild and moderate chronic kidney disease, trials have excluded patients with a creatinine clearance of less than 25 l/min. Thus, the tolerability and efficacy of apixaban in patients with advanced chronic kidney disease and end-stage renal disease (ESRD) are currently unknown [Patel and Piccini, 2015]. A recent study of the pharmacokinetics and pharmacodynamics of apixaban in eight patients with ESRD on hemodialysis showed no significant difference in drug concentrations or clearance, when compared with eight healthy subjects [Wang et al. 2015]. Given the lack of data on the safety of administering apixaban to patients with ESRD without dose adjustment, a randomized clinical trial comparing the use of apixaban with warfarin in patients with atrial fibrillation and ESRD is needed.
With the increasing number of patients who have continuous heart monitoring via cardiac-implantable electronic devices (CIED), it has become evident that a large number of patients with no prior known atrial fibrillation experience episodes of incidental or subclinical atrial fibrillation. Recent studies of patients with a CIED have shown that up to 1/3 of all patients with atrial fibrillation detected by device have not had any previous clinical atrial fibrillation [Patel and Piccini, 2015]. The asymptomatic atrial fibrillation and stroke evaluation in pacemaker patients and the atrial fibrillation reduction atrial pacing trial (ASSERT) showed that there was a significantly increased risk of stroke and systemic embolism, as well as of developing clinical atrial fibrillation in 2500 patients with device-detected brief episodes of atrial fibrillation [Healey et al. 2012]. Accordingly, the current European Heart Society guidelines recommend considering anticoagulation of patients with device-detected atrial fibrillation [Camm et al. 2012]. However, no study has ever shown that the benefit-to-risk ratio of using an oral anticoagulant in such patients is favorable. In order to clarify the net benefit of anticoagulation in this group, the ‘apixaban for the reduction of thromboembolism in patients with device-detected sub-clinical atrial fibrillation’ (ARTESiA) trial is currently underway. This randomized-controlled trial will compare the use of apixaban with aspirin in patients with device-detected atrial fibrillation. Also, the ‘nonvitamin K antagonist oral anticoagulants in patients with atrial high-rate episodes’ (NOAH AFNET 6) trial is currently underway to test whether patients with high atrial rates may benefit from treatment with edoxaban versus aspirin versus placebo.
Direct current (DC) cardioversion is a very frequent component of the treatment of atrial fibrillation; however, the efficacy and the safety of apixaban use pre- and postcardioversion have only been inferred from data in the ARISTOTLE trial. In ARISTOTLE, there was no difference between warfarin and apixaban in stroke or systemic embolism or in major bleeding events in patients who underwent cardioversion [Camm et al. 2012]. A phase IV trial to assess the effectiveness of apixaban compared with usual care anticoagulation in subjects with nonvalvular atrial fibrillation undergoing cardioversion (EMANATE) trial is currently recruiting patients to compare the use of apixaban with warfarin in patients with atrial fibrillation who are planned to undergo DC cardioversion.
Another key question related to the use of apixaban is its use in patients with atrial fibrillation and coronary artery disease who require the use of antiplatelet therapy and systemic anticoagulation. In ARISTOTLE, patients with atrial fibrillation and coronary artery disease had fewer myocardial infarctions, strokes, bleeding episodes and death on apixaban compared with warfarin, whether or not they were on antiplatelet therapy [Bahit et al. 2013]. The apixaban versus vitamin k antagonist and aspirin versus placebo in patients with atrial fibrillation and acute coronary syndrome or percutaneous coronary intervention (AUGUSTUS) trial is currently underway to compare the use of warfarin with apixaban in the first 6 months following an acute coronary syndrome with or without percutaneous coronary intervention.
Conclusion
Apixaban is superior to warfarin in preventing stroke and systemic embolism and causes significantly less major bleeding. This has been shown to be the case in a number of high-risk patient populations such as the elderly, patients with moderate CKD, and patients with prior stroke as reviewed in this paper. Several knowledge gaps in relation to the safety and efficacy of apixaban still exist and are being addressed by ongoing randomized clinical trials.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Benjamin E. Peterson, Duke University Hospital, 2301 Erwin Road, Durham, NC 27710, USA.
Sana M. Al-Khatib, Duke University Hospital, Durham, NC, USA
Christopher B. Granger, Duke University Hospital, Durham, NC, USA
References
- Agency for Healthcare Research and Quality. (2012) Weighted national estimates. HCUP National Inpatient Sample. Available from: http://hcupnet.ahrq.gov (accessed 28 December 2015).
- Agnelli G., Buller H., Cohen A., Curto M., Gallus A., Johnson M., et al. (2013) Apixaban for extended treatment of venous thromboembolism. N Engl J Med 368: 699–708. [DOI] [PubMed] [Google Scholar]
- Al-Khatib S., Thomas L., Wallentin L., Lopes R., Gersh B., Garcia D., et al. (2013) Outcomes of apixaban versus warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J 34: 2464–2471. [DOI] [PubMed] [Google Scholar]
- Alexander J., Lopes R., Thomas L., Alings M., Atar D., Aylward P., et al. (2014) Apixaban versus warfarin with concomitant aspirin in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 35: 224–232. [DOI] [PubMed] [Google Scholar]
- Avezum A., Lopes R., Schulte P., Lanas F., Gersh B., Hanna M., et al. (2015). Apixaban in comparison with warfarin in patients with atrial fibrillation and valvular heart disease: findings from the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Circulation 132: 624–632. [DOI] [PubMed] [Google Scholar]
- Bahit M., Lopes R., Wojdyla D., Hohnloser S., Alexander J., Lewis B., et al. (2013) Apixaban in patients with atrial fibrillation and prior coronary artery disease: insights from the ARISTOTLE trial. Int J Cardiol 170: 215–220. [DOI] [PubMed] [Google Scholar]
- Benjamin E., Levy D., Vaziri S., D’Agostino R., Belanger A., Wolf P. (1994) Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 271: 840–844. [PubMed] [Google Scholar]
- Benjamin E., Wolf P., D’Agostino R., Silbershatz H., Kannel W., Levy D. (1998) Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 98: 946–952. [DOI] [PubMed] [Google Scholar]
- Camm A., Lip G., De Caterina R., Savelieva I., Atar D., Hohnloser S., et al. (2012) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 33: 2719–2747. [DOI] [PubMed] [Google Scholar]
- Connolly S., Eikelboom J., Joyner C., Diener H., Hart R., Golitsyn S., et al. (2011) Apixaban in patients with atrial fibrillation. N Engl J Med 364: 806–817. [DOI] [PubMed] [Google Scholar]
- Connolly S., Ezekowitz M., Yusuf S., Eikelboom J., Oldgren J., Parekh A., et al. (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- Coppens M., Eikelboom J., Hart R., Yusuf S., Lip G., Dorian P., et al. (2013) The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J 34: 170–176. [DOI] [PubMed] [Google Scholar]
- Coppens M., Synhorst D., Eikelboom J., Yusuf S., Shestakovska O., Connolly S. (2014) Efficacy and safety of apixaban compared with aspirin in patients who previously tried but failed treatment with vitamin K antagonists: results from the AVERROES trial. Eur Heart J 35: 1856–1863. [DOI] [PubMed] [Google Scholar]
- Diener H., Eikelboom J., Connolly S., Joyner C., Hart R., Lip G., et al. (2012) Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. Lancet Neurol 11: 225–231. [DOI] [PubMed] [Google Scholar]
- Durheim M., Cyr D., Lopes R., Thomas L., Tsuang W., Gersh B., et al. (2015) Chronic obstructive pulmonary disease in patients with atrial fibrillation: Insights from the ARISTOTLE trial. Int J Cardiol 202: 589–594. [DOI] [PubMed] [Google Scholar]
- Easton J., Lopes R., Bahit M., Wojdyla D., Granger C., Wallentin L., et al. (2012) Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol 11: 503–511. [DOI] [PubMed] [Google Scholar]
- Eikelboom J., Connolly S., Brueckmann M., Granger C., Kappetein A., Mack M., et al. (2013) Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 369: 1206–1214. [DOI] [PubMed] [Google Scholar]
- Eikelboom J., Connolly S., Gao P., Paolasso E., De Caterina R., Husted S., et al. (2012) Stroke risk and efficacy of apixaban in atrial fibrillation patients with moderate chronic kidney disease. J Stroke Cerebrovasc Dis 21: 429–435. [DOI] [PubMed] [Google Scholar]
- Eikelboom J., O’Donnell M., Yusuf S., Diaz R., Flaker G., Hart R., et al. (2010) Rationale and design of AVERROES: apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. Am Heart J 159: 348–353. e1. [DOI] [PubMed] [Google Scholar]
- Eikelboom J., Quinlan D., van Ryn J., Weitz J. (2015). Idarucizumab: the antidote for reversal of dabigatran. Circulation 132: 2412–2422. [DOI] [PubMed] [Google Scholar]
- Feinberg W., Blackshear J., Laupacis A., Kronmal R., Hart R. (1995) Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med 155: 469–473. [PubMed] [Google Scholar]
- Flaker G., Eikelboom J., Shestakovska O., Connolly S., Kaatz S., Budaj A., et al. (2012) Bleeding during treatment with aspirin versus apixaban in patients with atrial fibrillation unsuitable for warfarin: the apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment (AVERROES) trial. Stroke 43: 3291–3297. [DOI] [PubMed] [Google Scholar]
- Flaker G., Lopes R., Hylek E., Wojdyla D., Thomas L., Al-Khatib S., et al. (2014) Amiodarone, anticoagulation, and clinical events in patients with atrial fibrillation: insights from the ARISTOTLE trial. J Am Coll Cardiol 64: 1541–1550. [DOI] [PubMed] [Google Scholar]
- Flegel K., Shipley M., Rose G. (1987) Risk of stroke in non-rheumatic atrial fibrillation. Lancet 1: 526–529. [DOI] [PubMed] [Google Scholar]
- Furberg C., Psaty B., Manolio T., Gardin J., Smith V., Rautaharju P. (1994) Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am J Cardiol 74: 236–241. [DOI] [PubMed] [Google Scholar]
- Garcia D., Wallentin L., Lopes R., Thomas L., Alexander J., Hylek E., et al. (2013) Apixaban versus warfarin in patients with atrial fibrillation according to prior warfarin use: results from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 166: 549–58. [DOI] [PubMed] [Google Scholar]
- Garwood C., Corbett T. (2008) Use of anticoagulation in elderly patients with atrial fibrillation who are at risk for falls. Ann Pharmacother 42 523–532. [DOI] [PubMed] [Google Scholar]
- Ghadimi K., Dombrowski K., Levy J., Welsby I. (2015) Andexanet alfa for the reversal of factor Xa inhibitor related anticoagulation. Expert Rev Hematol 21 December. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Giugliano R., Ruff C., Braunwald E., Murphy S., Wiviott S., Halperin J., et al. (2013) Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 369: 2093–2104. [DOI] [PubMed] [Google Scholar]
- Granger C., Alexander J., McMurray J., Lopes R., Hylek E., Hanna M., et al. (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365: 981–992. [DOI] [PubMed] [Google Scholar]
- Granger C., Lopes R., Hanna M., Ansell J., Hylek E., Alexander J., et al. (2015). Clinical events after transitioning from apixaban versus warfarin to warfarin at the end of the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Am Heart J 169: 25–30. [DOI] [PubMed] [Google Scholar]
- Halvorsen S., Atar D., Yang H., De Caterina R., Erol C., Garcia D., et al. (2014) Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J 35: 1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey J., Connolly S., Gold M., Israel C., Van Gelder I., Capucci A., et al. (2012) Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 366: 120–129. [DOI] [PubMed] [Google Scholar]
- Held C., Hylek E., Alexander J., Hanna M., Lopes R., Wojdyla D., et al. (2015) Clinical outcomes and management associated with major bleeding in patients with atrial fibrillation treated with apixaban or warfarin: insights from the ARISTOTLE trial. Eur Heart J 36: 1264–1272. [DOI] [PubMed] [Google Scholar]
- Hess C., Al-Khatib S., Granger C., Lopes R. (2013) A review of apixaban for stroke prevention in atrial fibrillation: insights from ARISTOTLE. Expert Rev Cardiovasc Ther 11: 1105–1114. [DOI] [PubMed] [Google Scholar]
- Hess C., Broderick S., Piccini J., Alexander K., Newby L., Shaw L., et al. (2012) Antithrombotic therapy for atrial fibrillation and coronary artery disease in older patients. Am Heart J 164: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijazi Z., Siegbahn A., Andersson U., Granger C., Alexander J., Atar D., et al. (2014a) High-sensitivity troponin I for risk assessment in patients with atrial fibrillation: insights from the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Circulation 129: 625–634. [DOI] [PubMed] [Google Scholar]
- Hijazi Z., Wallentin L., Siegbahn A., Andersson U., Alexander J., Atar D., et al. (2014b) High-sensitivity troponin T and risk stratification in patients with atrial fibrillation during treatment with apixaban or warfarin. J Am Coll Cardiol 63: 52–61. [DOI] [PubMed] [Google Scholar]
- Hijazi Z., Wallentin L., Siegbahn A., Andersson U., Christersson C., Ezekowitz J., et al. (2013) N-terminal pro-B-type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE trial (apixaban for the prevention of stroke in subjects with atrial fibrillation). J Am Coll Cardiol 61: 2274–2284. [DOI] [PubMed] [Google Scholar]
- Hohnloser S., Hijazi Z., Thomas L., Alexander J., Amerena J., Hanna M., et al. (2012) Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 33: 2821–2830. [DOI] [PubMed] [Google Scholar]
- Hohnloser S., Shestakovska O., Eikelboom J., Franzosi M., Tan R., Zhu J., et al. (2013) The effects of apixaban on hospitalizations in patients with different types of atrial fibrillation: insights from the AVERROES trial. Eur Heart J 34: 2752–2759. [DOI] [PubMed] [Google Scholar]
- Hylek E., Evans-Molina C., Shea C., Henault L., Regan S. (2007) Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation 115: 2689–2696. [DOI] [PubMed] [Google Scholar]
- Hylek E., Held C., Alexander J., Lopes R., De Caterina R., Wojdyla D., et al. (2014) Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: The ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation): predictors, characteristics, and clinical outcomes. J Am Coll Cardiol 63: 2141–2147. [DOI] [PubMed] [Google Scholar]
- Lin H., Wolf P., Kelly-Hayes M., Beiser A., Kase C., Benjamin E., et al. (1996) Stroke severity in atrial fibrillation. The Framingham Study. Stroke 27: 1760–1764. [DOI] [PubMed] [Google Scholar]
- Lip G., Connolly S., Yusuf S., Shestakovska O., Flaker G., Hart R., et al. (2013). AVERROES Investigators. Modification of outcomes with aspirin or apixaban in relation to CHADS(2) and CHA(2)DS(2)-VASc scores in patients with atrial fibrillation: a secondary analysis of the AVERROES study. Circ Arrhythm Electrophysiol 6: 31–38. [DOI] [PubMed] [Google Scholar]
- Lip G., Eikelboom J., Yusuf S., Shestakovska O., Hart R., Connolly S. (2014) Modification of outcomes with aspirin or apixaban in relation to female and male sex in patients with atrial fibrillation: a secondary analysis of the AVERROES study. Stroke 45: 2127–2130. [DOI] [PubMed] [Google Scholar]
- Lopes R., Al-Khatib S., Wallentin L., Yang H., Ansell J., Bahit M., et al. (2012) Efficacy and safety of apixaban compared with warfarin according to patient risk of stroke and of bleeding in atrial fibrillation: a secondary analysis of a randomised controlled trial. Lancet 380: 1749–1758. [DOI] [PubMed] [Google Scholar]
- Lopes R., Alexander J., Al-Khatib S., Ansell J., Diaz R., Easton J., et al. (2010) Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J 159: 331–339. [DOI] [PubMed] [Google Scholar]
- Man-Son-Hing M., Nichol G., Lau A., Laupacis A. (1999) Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 159: 677–685. [DOI] [PubMed] [Google Scholar]
- Miyasaka Y., Barnes M., Bailey K., Cha S., Gersh B., Seward J., et al. (2007) Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol 49: 986–992. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D., Benjamin E., Go A., Arnett D., Blaha M., Cushman M., et al. (2015) American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation 131: e29–322. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D., Benjamin E., Go A., Arnett D., Blaha M., Cushman M., et al. (2015) Heart disease and stroke statistics – 2016 update: a report from the American Heart Association. Circulation 133: e38–e360. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics – CDC. (2014) About multiple cause of death 1999–2011. CDC WONDER Online Database. Available from: http://wonder.cdc.gov (Accessed 28 December 2015).
- Ng K., Shestakovska O., Connolly S., Eikelboom J., Avezum A., Diaz R., et al. (2015) Efficacy and safety of apixaban compared with aspirin in the elderly: a subgroup analysis from the AVERROES trial. Age Ageing 45: 77–83. [DOI] [PubMed] [Google Scholar]
- Novaro G., Asher C., Bhatt D., Moliterno D., Harrington R., Lincoff A., et al. (2008) Meta-analysis comparing reported frequency of atrial fibrillation after acute coronary syndromes in Asians versus whites. Am J Cardiol 101: 506–509. [DOI] [PubMed] [Google Scholar]
- Ogilvie I., Newton N., Welner S., Cowell W., Lip G. (2010) Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med 123: 638–645. e4. [DOI] [PubMed] [Google Scholar]
- Patel M., Piccini J. (2015) The need to evaluate net clinical effect of stroke prevention therapy in patients with end stage renal disease and atrial fibrillation. Circulation 17 December 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Patel M., Mahaffey K., Garg J., Pan G., Singer D., Hacke W., et al. (2011). Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365: 883–891. [DOI] [PubMed] [Google Scholar]
- Pisters R., Lane D., Nieuwlaat R., de Vos C., Crijns H., Lip G. (2010) A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 138: 1093–1100. [DOI] [PubMed] [Google Scholar]
- Psaty B., Manolio T., Kuller L., Kronmal R., Cushman M., Fried L., et al. (1997) Incidence of and risk factors for atrial fibrillation in older adults. Circulation 96: 2455–2461. [DOI] [PubMed] [Google Scholar]
- Raghavan N., Frost C., Yu Z., He K., Zhang H., Humphreys W., et al. (2009). Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos 37: 74–81. [DOI] [PubMed] [Google Scholar]
- Rao M., Pokorney S., Granger C. (2014) Atrial fibrillation: a review of recent studies with a focus on those from the duke clinical research institute. Scientifica (Cairo) 2014: 901586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff C., Giugliano R., Braunwald E., Mercuri M., Curt V., Betcher J., et al. (2014) Transition of patients from blinded study drug to open-label anticoagulation: the ENGAGE AF-TIMI 48 trial. J Am Coll Cardiol 64: 576–584. [DOI] [PubMed] [Google Scholar]
- Ruff C., Giugliano R., Braunwald E., Morrow D., Murphy S., Kuder J., et al. (2015) Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet 385: 2288–2295. [DOI] [PubMed] [Google Scholar]
- Sellers M., Newby L. (2011) Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J 161: 241–246. [DOI] [PubMed] [Google Scholar]
- Siegal D., Curnutte J., Connolly S., Lu G., Conley P., Wiens B., et al. (2015) Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med 373: 2413–2424. [DOI] [PubMed] [Google Scholar]
- Steinberg B., Kim S., Piccini J., Fonarow G., Lopes R., Thomas L., et al. (2013) Use and associated risks of concomitant aspirin therapy with oral anticoagulation in patients with atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Registry. Circulation 128: 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ham H., Klungel O., Singer D., Leufkens H., van Staa T. (2015) Comparative Performance of ATRIA, CHADS2, and CHA2DS2-VASc Risk Scores Predicting Stroke in Patients With Atrial Fibrillation: Results From a National Primary Care Database. J Am Coll Cardiol 66: 1851–1859. [DOI] [PubMed] [Google Scholar]
- Vanassche T., Lauw M., Eikelboom J., Healey J., Hart R., Alings M., et al. (2015) Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur Heart J 36: 281–287. [DOI] [PubMed] [Google Scholar]
- Vinereanu D., Stevens S., Alexander J., Al-Khatib S., Avezum A., Bahit M., et al. (2015) Clinical outcomes in patients with atrial fibrillation according to sex during anticoagulation with apixaban or warfarin: a secondary analysis of a randomized controlled trial. Eur Heart J 36: 3268–3275. [DOI] [PubMed] [Google Scholar]
- Wallentin L., Lopes R., Hanna M., Thomas L., Hellkamp A., Nepal S., et al. (2013) Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation 127: 2166–2176. [DOI] [PubMed] [Google Scholar]
- Wang X., Tirucherai G., Marbury T., Wang J., Chang M., Zhang D., et al. (2015) Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol 56: 628–636. [DOI] [PubMed] [Google Scholar]
- Wolf P., Dawber T., Thomas H., Jr., Kannel W. (1978) Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology 28: 973–977. [DOI] [PubMed] [Google Scholar]