Abstract
We wished to determine whether the capacity of the sugar uptake mechanisms of guard cells of the Argenteum mutant of pea (Pisum sativum L.) sufficed to support a concurrent stomatal opening movement. Sugar uptake by guard cell protoplasts was determined by silicone-oil-filtering centrifugation. The protoplasts took up [14C]glucose, [14C]fructose, and [14C]sucrose (Suc), apparently in symport with protons. Mannose, galactose, and fructose competed with Glc for transport by a presumed hexose carrier. The uptake of Glc saturated with a Km of 0.12 mm and a Vmax of 19 fmol cell−1 h−1. At external concentrations <1 mm, the uptake of Suc was slower than that of Glc. It exhibited a saturating component with a Km varying between 0.25 and 0.8 mm and a Vmax between 1 and 10 fmol cell−1 h−1, and at external concentrations >1 mm, a non-saturating component. At apoplastic sugar concentrations below 4 mm, sugar import was estimated to be mainly in the form of hexoses and too slow to support a simultaneous stomatal opening movement. If, however, during times of high photosynthesis and transpiration, the apoplastic Suc concentration rose and entered the range of non-saturating import, absorbed Suc could replace potassium malate as the osmoticum for the maintenance of stomatal opening.
During stomatal opening, the solute content of guard cells increases dramatically. For example, in the Argenteum mutant of pea (Pisum sativum L.), the subject of this study, the content of osmotica increases by 1,600 fosmol per guard cell, with an initial rate of 900 fosmol per guard cell per hour (Reckmann et al., 1990). There are several possible mechanisms for this increase. The predominant osmotically active species are K+, malate, Cl−, Suc, and possibly other sugars. More than a century ago, Kohl (1886) suggested that carbohydrates formed by the assimilatory activity in the “chlorophyll grains” of guard cells caused osmotic water uptake and turgescence. Lloyd (1908) doubted “that the substances which effect the opening are produced (in the guard cells) in great enough quantities by photosynthesis alone”; he proposed that starch “is quickly changed under appropriate stimuli into powerfully osmotic substances.” Recently, Suc accumulation in guard cells of fava bean was determined by Tallman and Zeiger (1988), Poffenroth et al. (1992), and Talbott and Zeiger (1993), and in guard cells of Commelina benghalensis by Reddy and Rama Das (1986). The controversy over the importance of salts of K+ versus that of sugars as solutes of osmotic consequence in guard cells was resolved by the discovery by Talbott and Zeiger (1996) that which osmoticum dominated depended on the phase of the photoperiod, normally correlated with the time of day; Suc gained in significance about the time when stomatal aperture culminated.
Because differentiated guard cells are not connected to neighboring cells by plasmodesmata (Palevitz and Hepler, 1985), increases in solute concentration during stomatal opening can occur via uptake across the plasmalemma; organic solutes can also be produced within. Guard cells have low levels of Rubisco activity (Outlaw et al., 1979; Gotow et al., 1988; Outlaw, 1989; Reckmann et al., 1990). In the Argenteum mutant of pea, for example, photosynthetic carbon reduction can only account for 10% of the reduced carbon required for stomatal opening if potassium malate is the osmoticum (Reckmann et al., 1990). If hexoses are produced, photosynthesis accounts for only 2% of the requirement. If Suc turns out to be the major osmoticum for stomatal opening in pea, then uptake of Suc (or other sugars) and starch breakdown must account for Suc production. Guard cells of epidermal strips floating on solutions of [14C]Glc or [14C]Suc were able to incorporate radioactivity (Dittrich and Raschke, 1977), and Lu et al. (1995) determined on whole leaves of Vicia faba that a close positive correlation existed between the Suc content of the stomatal apoplast and that of the protoplasts of the guard cells embedded in it.
In this study, isolated guard cell protoplasts from the Argenteum mutant of pea were used because the osmotic requirement for stomatal opening and Rubisco activity had already been determined in this material (Reckmann et al., 1990). Our objective was to analyze the capacity of the sugar uptake mechanisms in the plasmalemma of guard cells and to determine whether it sufficed to provide reduced carbon at a rate that would support a stomatal opening movement.
MATERIALS AND METHODS
Plant Material
Plants of the Argenteum mutant of pea (Pisum sativum L.) (Marx, 1978; Jewer et al., 1982) were grown on a hydroponic substrate (expanded clay aggregates) in a nutrient solution containing 1% (v/v) Flory 9 Hydro (Planta Düngemittel, München, Germany) and supplemented with NaCl and CaSO4, resulting in concentrations of 0.1 and 1.4 mm, respectively. The growth chamber had a light-dark cycle of 18 h/6 h, a temperature of 20°C/17°C, and a RH of 40%. Quantum flux was 200 μmol m−2 s−1 from fluorescent tubes (Universal Weiss, L 65 W/25, Osram, Munich, Germany). For protoplast preparation, fully expanded leaflets were harvested from 4-week-old plants.
Preparation of Guard Cell Protoplasts
Protoplasts were prepared from epidermal fragments using a slightly modified method of Raschke and Hedrich (1989). For each experiment, two batches comprising 200 leaflets each were minced separately in a Waring blender in three successive bursts of 50, 10, and 5 s, and rinsed with water on a 200-μm-mesh nylon net. The fragments obtained were transferred to the digestion medium (0.35 m mannitol, 0.5% [w/v] Cellulase Onozuka R-10 [Yakult Honsha, Tokyo], 0.15% [w/v] Macerozyme R-10 [Yakult Honsha], 1% [w/v] BSA, 10 mm sodium ascorbate, 1 mm calcium iminodiacetate, and 0.17% [w/v] penicillin G, pH 5.5). After incubation in a shaking water bath for 90 min at 20°C and 40 excursions min−1, green aggregations of vascular and mesophyll debris were removed with forceps or a pipette.
The remaining mesophyll protoplasts were washed away by rinsing on the 200-μm net with 0.35 m mannitol, 5 mm MES-Tris, pH 5.5, 1 mm calcium iminodiacetate, and 0.17% (w/v) penicillin G. A further 16-h incubation in the digestion medium followed, without shaking. Released guard cell protoplasts were washed through a 200-μm net with rinsing medium (0.4 m mannitol, 5 mm MES-Tris, pH 5.5, and 1 mm calcium iminodiacetate) to remove epidermal fragments, then separated from the debris by filtration through three nylon nets with mesh widths of 80, 20, and 14 μm in succession, and finally sedimented by centrifugation at 150g for 10 min at 4°C. The pellet was then resuspended in the rinsing medium. Cell density was examined with a microscope and adjusted to 1.1 × 106 cells mL−1. The obtained guard cell suspension was free of protoplasts of mesophyll or epidermal cells. Contamination by mesophyll cell fragments and aggregates of mesophyll chloroplasts (mesophyll units) was one mesophyll unit per 67 to 100 guard cell protoplasts.
Sugar Uptake
The accumulation of radioactivity was used as a measure for the uptake of 14C-labeled sugars. Such a procedure will underestimate sugar absorption because of the respiratory loss of 14CO2 and a possible efflux of labeled metabolites into the medium. Absorption of radioactively labeled sugars was measured by silicone-oil-filtering centrifugation (Klingenberg and Pfaff, 1967). Aliquots (585 μL) of protoplast suspension in rinsing medium were used; the uptake processes were started by adding 65 μL of 0.23 m mannitol, 3 mm MES-Tris, pH 5.5, [3H]H2O (3 MBq mL−1), [U-14C]sugar (1 MBq mL−1), and, depending on the individual experiment, the competing sugar. The protoplasts were incubated at 20°C (the daytime temperature during plant cultivation); durations of the incubations are given in “Results.” Uptake was terminated by silicone oil centrifugation. Two-hundred-microliter aliquots of cell suspension were layered on 20 μL of 10% (v/v) HClO4 and 70 μL of silicone oil (AR 200, Wacker-Chemie, München, Germany) in 400-μL reaction tubes, followed by centrifugation in a Minifuge B (Beckman Instruments, Fullerton, CA) for 20 s at maximum speed. The tips of the tubes were separated, and the sediments they contained were resuspended in 300 μL of water. Radioactivities of the sediments and the supernatants were determined by liquid scintillation counting.
The amount of adhering medium and the space of the intact protoplasts were determined in control experiments with [3H]H2O and with [14C]sorbitol in place of the sugar (Heldt and Sauer, 1971). On average, the sorbitol-impermeable space occupied 65% of the total [3H]H2O space. From the sorbitol impermeable space, the average volume of a guard cell protoplast was estimated to be 1.1 pL. Rates of sugar uptake were related to the number of cells determined with a microscope using a hemocytometer after Thoma W. Schreck (Hofstein Ts, Germany).
Protoplast Intactness and Invertase Activity
The intactness of the protoplasts was determined by measuring the activity of the cytoplasmic marker enzyme UDP-Glc-pyrophosphorylase in the presence of 0.4 m mannitol with or without 0.1% (v/v) Triton X-100 (Borchert et al., 1993) in aliquots of the protoplast suspensions incubated for the same lengths of time as the treatments with radioactive sugars (the latter required destructive centrifugation into perchloric acid; Klingenberg and Pfaff, 1967).
The invertase activity of non-lysed protoplasts was assayed according to the method of Heineke et al. (1992). To determine the extent to which the activity of free invertase in a protoplast suspension was the result of enzyme release from damaged cells, we added known numbers of sonicated protoplasts to aliquots of a non-sonicated suspension at increasing cell number ratios, measured the invertase activities of these mixtures, and extrapolated the results to the invertase activity of a suspension free of broken cells.
Source of Chemicals
All radiochemicals were from Amersham-Buchler (Braunschweig, Germany).
RESULTS
Time Courses of Sugar Uptake
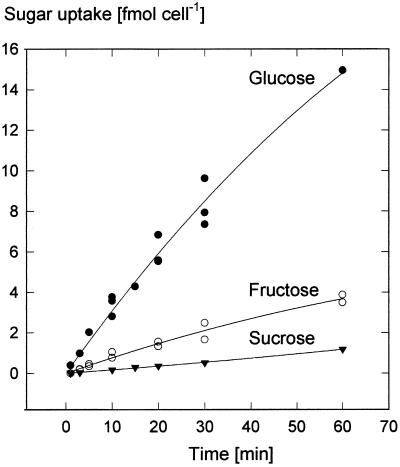
Guard cell protoplasts exposed to solutions of 0.5 mm 14C-labeled sugars took up radioactivity that indicated larger absorption rates for Glc than for Fru and Suc (Fig. 1). During the initial phase the average uptake rates of labeled Glc or Fru were 20 and 5 fmol cell−1 h−1, respectively. The import of Suc started with delays lasting between 1 and 10 min. During the following linear phase, radioactivity from Suc was incorporated at rates between 1 and 2 fmol cell−1 h−1. During the 5-year course of this study, uptake rates of Glc remained in the same order as those shown in Figure 1; however, in the experiments of Rohrig and Raschke (1991), the uptake rates of Suc were approximately 10 times larger than depicted in Figure 1 (see the following paragraph and Fig. 3B).
Figure 1.
Time courses of the uptake by guard cell protoplasts of [14C]Glc (n = 3, one datum only for uptake at 60 min), [14C]Fru (n = 2), and [14C]Suc (n = 3, the individual data are hidden behind the symbols). Uptake conditions were 0.5 mm sugar, pH 5.5, 20°C. Curves are second-order regressions. (In another series of experiments, Suc accumulation was higher than shown here; see Fig. 4.)
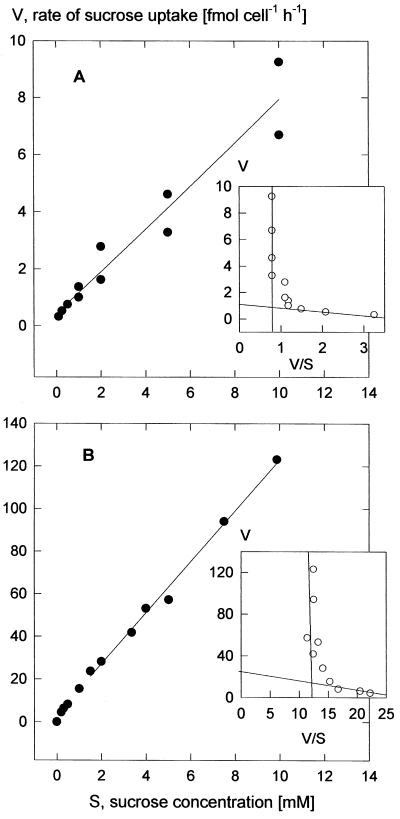
Figure 3.
Concentration dependencies of the rate of Suc uptake at pH 5.5 (30-min incubation time). A, Rates characteristic for the majority of the experiments; B, data from one set of experiments showing particularly high rates. The curves are second-order regressions. The Eadie-Hofstee: plots of the means (insets) bring out the saturating and linear concentration dependencies. Graphs parallel to the ordinates represent the linear components. Linear regression of the data for Suc concentrations <0.5 mm (A) gave apparent Km values between 0.18 and 0.28 mm, with a mean of 0.25 mm (three experiments), and in B for concentrations <1 mm apparent Km values between 0.4 and 1.2 mm, with a mean of 0.8 mm (three experiments).
Concentration Dependencies
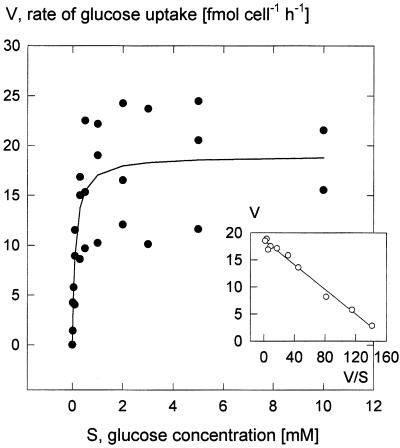
The uptake system for Glc saturated with increasing Glc concentration with an apparent Km of 0.12 mm and a Vmax of 19 fmol cell−1 h−1 (Fig. 2). One experiment with an incubation time of 2 min instead of the usual 15 min led to the same characteristics. In contrast to the experiments with Glc, the uptake of [14C]Suc did not show Michaelis-Menten behavior, but displayed two components; one saturated at low concentrations and the other rose linearly (Fig. 3A). This biphasic behavior was evident in an Eadie-Hofstee plot (Fig. 3, inset). Biphasic kinetics of Suc accumulation was also observed in our initial investigation (Rohrig and Raschke, 1991; Fig. 3B), when uptake rates were about 10 times higher than those shown in Figures 2 and 4A. Because the experiments of Rohrig and Raschke (1991) were performed in the light, we compared Suc accumulation by illuminated guard cell protoplasts with that of cells kept in darkness. Light caused enhancements in four out of five replications of the experiment (Fig. 4); these increases were, however, not sufficient to explain the observed variations. Nevertheless, it is worth noting that, as shown in Figure 4, Suc uptake rates in the dark were about twice as high as those derived from Figure 3A for a concentration of 3 mm. Evidently, there is great variability in the guard cells' capacity to absorb Suc, and this was an unexpected result of our investigation.
Figure 2.
Concentration dependence of the rate of Glc uptake at pH 5.5 (15-min incubation time). Inset, Eadie-Hofstee plot of the means. Linear regression resulted in an apparent Km of 0.12 mm (ranging from 0.10–0.15 mm among three replications) and a mean Vmax of 19 fmol cell−1 h−1 (12–24 fmol cell−1 h−1).
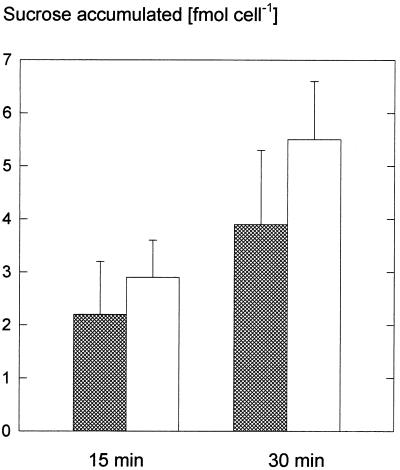
Figure 4.
Uptake of Suc in darkness and in the light. Guard cell protoplasts were incubated in 3 mm Suc at pH 5.5 and 20°C for 15 and 30 min in the dark (shaded bars) or in the light (white bars; 600 μmol m−2 s−1).
Substrate Specificity
For the determination of the substrate specificity of the Glc uptake system we conducted experiments according to the method of Heldt and Rapley (1970). Import of Glc was inhibited by Fru to 30% of the full rate. Man and Gal were also competitors, whereas Suc did not inhibit Glc uptake (Table I). The accumulation of 0.5 mm [14C]Fru was greatly reduced if 5 mm Glc was offered simultaneously; uptake was only one-seventh that of the control with unlabeled Fru (data not shown). These results indicate that guard cells possess a hexose carrier with a high affinity for Glc, and that Suc was not accepted as a substrate. The uptake of [14C]Suc was strongly reduced in the presence of unlabeled Glc; inhibition increased with increasing concentration of Glc (Fig. 5). Even at 3 mm Suc, a concentration at which the linear uptake component dominates, the addition of Glc (1 mm) caused a 90% inhibition of Suc uptake (data not shown). This result, as well as the observed lag phase in the time course for 14C accumulation after exposure to labeled Suc, indicate that the cleavage of Suc by invertase and the subsequent import of hexoses could be at least partially responsible for the accumulation of 14C from labeled Suc. However, the high rates of 14C accumulation during exposure to labeled Suc exceeded the maximum velocity of the presumed Glc transporter (Fig. 3B in conjunction with Fig. 1) and cannot be explained by the import of hexoses.
Table I.
Inhibition of [14C]Glc uptake by competing unlabeled sugars
| Competing Sugar | Inhibition
|
|
|---|---|---|
| Mean | Range | |
| % | ||
| Glc | 100 | n.a. |
| Man | 57 | 56–57 |
| Gal | 53 | 52–56 |
| Fru | 29 | 21–40 |
| Suc | 2 | −11–+15 |
Concentration of [14C]Glc was 0.5 mm, that of the competing sugars 5 mm. Inhibition is expressed in percent of an inhibition caused by 5 mm unlabeled Glc. The control solution causing 0% inhibition was 405 mm with respect to mannitol. All other essays were in 400 mm mannitol, which served as the osmoticum. Incubation time was 10 min at pH 5.5. Range: lowest and highest degree of inhibition (n = 3); n.a., not applicable.
Figure 5.
Rate of [14C]Suc uptake at pH 5.5 in the presence of increasing concentrations of unlabeled Glc (15-min incubation time) and a Suc concentration of 0.5 mm, pH 5.5. Rate of uptake in the absence of Glc was set at 100%. Inset, Semilogarithmic plot of the data.
During the main part of our investigation, the uptake of 14C was significantly lower if cells were incubated with [14C]Suc than if they were incubated with[14C]Glc (Fig. 1). We measured invertase activities in guard cell protoplast suspensions (intactness between 76% and 88% of the examined cells), and found them to be between 9.6 and 20.4 fmol cell−1 h−1, with a mean of 16.2 fmol cell−1 h−1 (n = 3). When all cells were broken, the invertase activity was 65 fmol cell−1 h−1. (Hite et al. [1993] found for guard cells of fava bean a value of 12 mmol kg−1 h−1, which, according to a personal communication with W.H. Outlaw Jr., translates to 36 fmol cell −1 h−1.) Measurements of invertase activity in suspensions containing varying ratios of intact and ruptured (sonicated) guard cell protoplasts allowed us to extrapolate invertase activity to that of a suspension completely free of broken cells. The result was an activity of 5.3 ± 6.7 fmol cell−1 h−1, which indicated that most, if not all, of the invertase activity resulted from broken protoplasts.
Guard cell protoplasts with low Suc uptake capacity exposed to a 5 mm [14C]Suc solution for 30 min accumulated 14C at about 4 fmol cell−1 h−1 (Fig. 3A), and those of high import capacity, at about 60 fmol cell−1 h−1 (Fig. 3B). Assuming that invertase was working at its maximum velocity, concentrations of Glc and Fru would reach 1.3 μm after 5 min, the average duration of the lag phase, and 8 μm after 30 min. In a solution of 20 μm [14C]Glc, a cell was able to take up Glc at about 3 fmol cell−1 h−1 (Fig. 1). It seems unlikely that the absorption of Suc was only apparent and the observed rates of 14C uptake by protoplasts suspended in 5 mm [14C]Suc entirely due to the absorption of hexoses following the hydrolysis of Suc.
H+ Symport
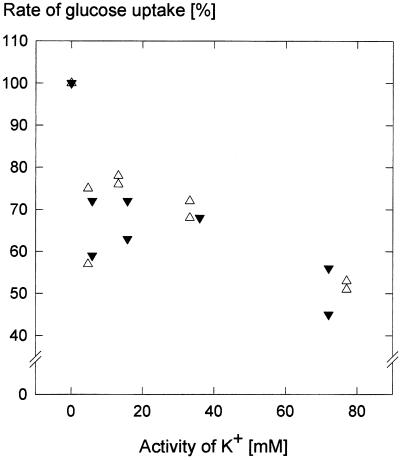
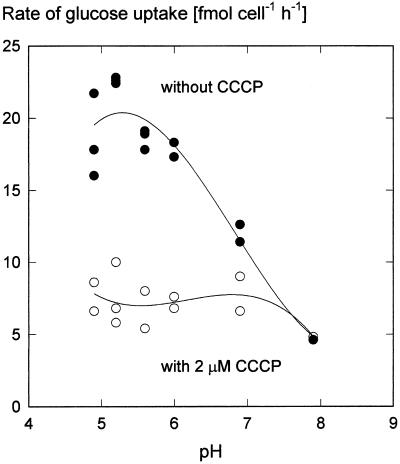
Generally, sugar uptake into plant cells occurs in symport with protons (Bush, 1993). If a mechanism of this type also operates in guard cells, then sugar uptake in guard cell protoplasts should increase with decreasing pH. Figure 7 shows that this was the case. Maximum Glc uptake occurred at pH 5.2; Suc uptake was affected by pH in a similar manner (data not shown). However, the activity of virtually all enzymes depends on pH; support for the notion of H+ cotransport comes from the leveling of the pH dependence in the presence of the uncoupler carbonyl cyanide m-chlorophenyl-hydrazone (CCCP) (Fig. 6). Short-circuiting the proton gradient inhibited sugar uptake (Table II). These results are consistent with the idea that sugar transport into guard cell protoplasts was driven by the chemical potential difference of H+. We also attempted to reduce the electrical component of the proton motive force through variations of the diffusion potential of K+. Raising the activity of K+ in the medium to about 75 mm reduced the rate of Glc uptake to approximately one-half, independent of whether the counterion to K+ was the absorbable Cl− (Raschke and Schnabl, 1978; Van Kirk and Raschke, 1978) or the poorly absorbable SO42− (Fig. 7). A replication of the experiment with Suc and KCl yielded a similar result, except that the depression of the uptake rate at a K+ activity of 5 mm did not occur. Presumably, therefore, the inhibitory effect of K+ was caused by a breakdown of the membrane potential and not by competition for protons between anions and Glc or Suc.
Figure 7.
Relative rates of Glc uptake as depending on the activity of K+ in the medium, counter ions were either Cl− (▵) or SO42− (▾); establishment of the K+ diffusion potentials was ensured by the presence of 2.5 μm valinomycin. Osmolality was adjusted to 0.42 osmol by adding mannitol, and was determined with a vapor pressure osmometer (Wescor, Logan, Utah). The incubation media had a 0.5 mm Glc concentration; the pH was kept at 5.5 with 33 mm MES-Tris buffer. Incubation time was 10 min. During this time, changes in protoplast volume due to the absorption of K+ did not occur (according to determinations of the sorbitol-impermeable space; see “Materials and Methods”). Glc uptake rates in the absence of K+ were set to 100%.
Figure 6.
Dependence of Glc uptake rate on the pH of the medium in the presence or absence of the uncoupler CCCP (2 μm). The Glc concentration in the incubation medium was 0.5 mm and incubation times were 5 min. CCCP in ethanol was added 1 min before the addition of [14C]Glc. In both treatments the ethanol concentration was 0.8%. The pH values of the MES-Tris buffers (33 mm) were determined with a pH electrode. Curves are third-order regressions.
Table II.
Inhibition of the uptake of [14C]Glc or [14C]Suc by CCCP
| Concentration of CCCP | Glc Uptake | Suc Uptake |
|---|---|---|
| μm | % of uninhibited uptake | |
| 0 | 100 | 100 |
| 2 | 34 | 58 |
| 5 | 23 | 41 |
| 10 | 15 | n.d.a |
Ethanolic CCCP was added 1 min before the addition of [14C]Glc or simultaneous with [14C]Suc, respectively. Incubation time was 5 min (Glc) or 15 min (Suc) at pH 5.5. The concentration of Glc and Suc was 0.5 mm; that of ethanol 0.8% (v/v), also in the controls (n = 3).
n.d., Not determined.
If Glc uptake occurs in cotransport with H+, then fusicoccin, a toxin activating an electrogenic plasmalemma H+-ATPase (Marrè, 1979), should stimulate the uptake of sugars, and this was indeed the case. When fusicoccin (10 μm) was given to a protoplast suspension 10 min prior to administering [14C]Glc (0.5 mm), the rate of Glc uptake was enhanced by 65% of its initial rate, from 4.1 to 6.8 fmol cell−1 h−1 within 10 min. In this experiment, the protoplasts were incubated with 100 mm KCl, 200 mm mannitol, and 50 mm MES-Tris at a pH of 7.0.
DISCUSSION
Sugar Symport with H+
Although the import of carbohydrate is of primary importance for the functioning of guard cells, they do not differ from other plant cells with respect to the machinery for the acquisition of sugars. The pH dependence of uptake rates for Glc (Fig. 6) and Suc by guard cells (Rohrig and Raschke, 1991), the abolishment of this pH dependence by the administration of the uncoupler CCCP (Fig. 6), and the reduction of uptake rates by depolarizations of the plasma membrane (Fig. 7) indicate that sugars are absorbed in symport with protons, as in other types of plant cells (Bush, 1993). Increases in the proton-motive force across the plasmalemma of guard cell protoplasts following an application of fusicoccin enhanced sugar uptake. Similarly, Reddy and Rama Das (1986) reported that Suc uptake into guard cell protoplasts of Commelina benghalensis was sensitive to pH, inhibited by an uncoupler, and stimulated by fusicoccin. Recently, it was discovered that the sugar transporter STP1 of Arabidopsis occurs predominantly in guard cells (R. Stadler and N. Sauer, personal communication). The transporter STP1 is a H+-hexose cotransporter (Sauer et al., 1990) with an apparent affinity sequence for sugars similar to the competition sequence shown in Table I.
Kinetics of Sugar Uptake
The Km values for Glc uptake and the saturating component of Suc uptake were 0.12 and 0.25 to 0.8 mm, respectively, and were thus of the same magnitude as those in sugarcane suspension cells (Komor et al., 1981), barley mesophyll cells (Martinoia et al., 1987), and transformed cells of Nicotiana tabacum (Verstappen et al., 1991). The cloned potato H+-Suc transporter StSUT1 had, at pH 5.0, a Km for Suc between 0.5 and 1.1 mm (Boorer et al., 1996). Suc uptake followed biphasic kinetics (Fig. 3), as was reported for some other plant tissues (Lin et al., 1984; Buckhout, 1989; Estruch et al., 1989; Lemoine and Delrot, 1989; Thom and Maretzki, 1992). The maximum rates of sugar uptake into guard cell protoplasts were 19 fmol cell−1 h−1 for Glc (Fig. 1) and 1 fmol cell−1 h−1 for the saturating component of Suc uptake. This was true for the main part of our investigation, but great variation existed in the cells' ability to absorb Suc, and there were experiments (Rohrig and Raschke, 1991) during which Suc was taken up with a velocity 1 order of magnitude faster than during most of the other experiments. Rates of Suc uptake by complete guard cells in fava bean tissue were determined previously (Outlaw, 1995), and fell between the low and high ranges of our results with pea. A ratio of roughly 10 between the highest and the lowest maximum velocities of Suc uptake appears large, but is comparable in magnitude with a ratio of 6:1 in the annual variance in the proton pump activity of guard cell protoplasts (Lohse and Hedrich, 1992), and is smaller than the ratio of 28:1 derived from the anion-current densities of guard cells over a span of 3 years (at fairly constant K+-current densities; Schulz-Lessdorf et al., 1996). We consider the wide variation in the activities of some specific transport proteins in guard cells a noteworthy result indicating possible activation or inactivation or synthesis and breakdown according to varying demands.
Uncertainty remained whether Suc was absorbed as such, or only after its hydrolysis and import of the hexoses thus formed. The observed lag in Suc uptake and the inhibition of Suc uptake by Glc that was observed simultaneously (Fig. 5) would support such a view, but the absence of inhibition of Glc uptake by Suc (Table I) does not agree with this hypothesis. Most importantly, the invertase activities we determined (see “Results”) would not have been sufficient to explain the measured rates of apparent Suc uptake in the range of linear concentration dependence, and certainly not the rates of cells that displayed high Suc absorption capacity (Fig. 3B). Presumably, most of the Suc was taken up directly through a Suc transport mechanism exhibiting saturating kinetics, as well as a component with linear concentration dependence and with properties similar to those of the Suc-binding protein isolated from soybean embryos (Overvoorde et al., 1996).
The data presented here on Suc uptake by pea guard cell protoplasts differ from those of Reddy and Rama Das (1986) on the absorption of Suc by guard cell protoplasts of C. benghalensis; protoplasts of C. benghalensis took up Suc from a 0.5 mm medium at rates similar to 1.3 pmol per cell per hour, about 1,000 times faster than guard cell protoplasts of P. sativum. It is possible that guard cells of C. benghalensis, in contrast to those of pea, use Suc as the major osmoticum for stomatal opening.
Uptake by Protoplasts Compared with Uptake by Epidermal Tissue of Pea
One could argue that the uptake mechanisms of isolated guard cells were injured during protoplast preparation and that this was responsible for the low uptake rates. The investigation of Aked and Hall (1993) into sugar absorption by the lower epidermis of leaves of the Argenteum mutant of pea provided us with information with which to test this possibility. Aked and Hall found that uptake of [14C]Glc had a saturating and a linear component. The saturating component was characterized by a Vmax of about 7 μmol m−2 h−1. With a stomatal density of the lower epidermis between 120 and 160 mm−2 (our determinations) or 180 mm−2 (Jewer et al., 1982) and a Vmax for Glc uptake by guard cell protoplasts of 19 fmol cell−1 h−1, we arrived at maximum rates between 5 and 7 μmol m−2 h−1, which is in good agreement with the determinations made on epidermis containing complete guard cells. In this estimation, we should have subtracted the rates of Glc uptake by the common epidermal cells from those given by Aked and Hall for the whole epidermis. However, the Glc uptake rates of common epidermal cells are not known, so it is possible that they are low. In conclusion, conformity exists between data derived from experiments with guard cell protoplasts and those obtained with complete epidermis. This agreement indicates that the Glc uptake mechanism does not suffer damage during protoplast preparation. With respect to Suc uptake, Aked and Hall (1993) reported a Vmax of the saturable component that was roughly one-tenth of that for Glc uptake, again, in agreement with our results.
Estimation of Sugar Uptake Rates under Presumed Physiological Conditions
Sugar levels in the apoplast of pea leaves are not known. However, information is available for the related leguminous plant, V. faba (Lohaus, 1995). These values are quasi-equilibrium values for the apoplast of the whole leaf obtained by infiltration and centrifugation and therefore after leveling concentration gradients. The concentrations were similar to 0.3 mm Glc, 0.5 mm Fru, and 3 to 4 mm Suc. Lu et al. (1995, 1997) determined Suc concentrations in the guard cell apoplast, again in fava bean, that were higher than the Suc values of Lohaus (1995) by 1 or 2 orders of magnitude; they ranged between 25 and 130 mm. If the Suc contents were related to the estimated volume of free water in the apoplast, Lu et al. (1997) arrived at concentrations that were higher by a factor of 2.5 and could exceed 300 mm. Consequently, sugar concentrations in the vicinity of guard cells can vary between values in the millimolar range or below when they are in equilibrium with those of the apoplast of the whole leaf, and high values prevailing at times of strong Suc release from the mesophyll and high transpiration currents to the epidermis.
We attempted to estimate rates of sugar uptake by guard cells for these two situations, and combined results obtained with pea with those from fava bean. Such a procedure appears justified in view of the similarity of the osmotic relationships, carboxylase activities, and chlorophyll contents of the guard cells of these two species (appendix to Reckmann et al., 1990; G. Ritte, unpublished analyses). In the presence of 0.3 mm Glc, uptake into guard cell protoplasts of pea would proceed at about 15 fmol cell−1 h−1 (Fig. 1), and that of Fru at a concentration of 0.5 mm, with an initial rate of 5 fmol cell−1 h−1 (Fig. 1). Because of the competition between Glc and Fru, the combined uptake rates of hexoses would probably not exceed the maximum rate for Glc uptake of about 20 fmol cell−1 h−1. At 3.5 mm Suc in the apoplast, this sugar could have been absorbed at 3 fmol cell−1 h−1 (Fig. 3A). However, because of the presence of Glc, the rate of Suc uptake would be decreased to one-half (Fig. 5), and we estimate that Suc would be incorporated at a rate of 2 fmol cell−1 h−1. This order of magnitude would be in agreement with the data of Aked and Hall (1993).
A guard cell of pea is therefore able to import hexose units at a rate of about 24 fmol cell−1 h−1 in the absence of photosynthesis and transpiration. Under this condition, carbohydrates are taken up mainly in the form of hexoses. However, we can envisage Suc as important in guard cells exhibiting the import mechanism of increased capacity (Fig. 3B). We consider the wide variation in the absorption of Suc by guard cells to be the result of our investigation and concur with Lu et al. (1995), who, upon referring to the disagreements between various Suc measurements in guard cells, suggested that “these differences may imply that considerable flexibility in the extent and the means by which guard cells metabolize carbohydrates awaits discovery.” Suc is expected to become prominent among the sugars taken up by the guard cells when it is released by the mesophyll, swept to the epidermis with the transpiration stream, and accumulates around the guard cells. The data of Lu et al. (1995) indicate about 25 mm Suc in the epidermal apoplast in the morning, much higher than the quasi-equilibrium data of Lohaus (1995).
During times of high rates of CO2 assimilation and transpiration, the Suc concentration in guard cell walls may reach values between 110 and 160 mm, perhaps even 300 mm, relative to the estimated water space in the cell walls (Lu et al., 1997). If we apply these values to pea, extrapolation of our data shown in Figure 3A results in Suc uptake rates of about 20 fmol cell−1 h−1 in the morning and 100 fmol cell−1 h−1 before noon (three times higher if based on the free water space). These magnitudes agree with the determinations of Lu et al. (1997), 360 to 630 fmol cell−1 h−1, and also with those of Talbott and Zeiger (1996), whose time courses of Suc accumulation indicated uptake rates between 70 and 440 fmol cell−1 h−1. We recognize that during conditions favoring photosynthesis in the mesophyll, guard cells take up mainly Suc; hexose import contributes one-tenth or less to the total absorption of sugars.
Sugar Uptake in Relation to the Osmotic Requirement for Stomatal Opening in Pea
A guard cell of the Argenteum mutant of pea accumulates solutes at a rate of 900 fosmol cell−1 h−1 during the initial linear phase of stomatal opening movement and, for attaining a full aperture, 1,600 fosmol cell−1 must be accumulated (Reckmann et al., 1990). If potassium malate is the major solute (with an activity coefficient of 0.8), then 670 fmol malate must be produced at an initial rate of 380 fmol cell−1 h−1 (Reckmann et al., 1990). If malate were made from concurrently imported sugars, then the uptake of hexose units would have to proceed at a rate of 190 fmol cell−1 h−1. At a quasi-equilibrium of the sugar concentrations in the apoplast (fava bean data of Lohaus, 1995) the combined uptake rates of hexose equivalents of 24 fmol cell−1 h−1 (at low activity of the Suc transporter) or 40 fmol cell−1 h−1 (at high activity) could meet not more than one-eighth or one-fifth of the requirement for a concurrent opening movement.
The situation changes entirely if Suc accumulates in the guard cell apoplast as a result of photosynthesis in the mesophyll (Lu et al., 1995, 1997) and is imported by the guard cells through the mechanism with linear concentration dependence. In this case, estimated uptake rates can exceed the rates required for malate production (95 fmol Suc cell−1 h−1) and provide sugars as additional or alternative stomatal solutes, as was suggested by Poffenroth et al. (1992); Talbott and Zeiger (1993, 1996), and Tallman and Zeiger (1988). The full rate of 900 fosmol cell−1 h−1, however, cannot be met by sugar import alone. Lu et al. (1995) observed that the Suc concentration within guard cells reached only approximately 40 mm. (In a personal communication, X. He and W.H. Outlaw Jr. informed us recently that the Suc concentration in guard cells can go as high as 130 mm under conditions of low transpiration). Nevertheless, during times of low sugar concentrations in the apoplast, intracellular sources of reduced carbon would need to be present and mobilized; this need highlights the importance of guard cell chloroplasts for the storage of carbohydrates. Lloyd (1908) recognized their function by stating that “the guard-cell plastid is normally and chiefly a leucoplast.” Indeed, substrate specificities and kinetic constants of the phosphate translocators in guard cell chloroplasts and in root amyloplasts of pea are similar (Borchert et al., 1993; Overlach et al., 1993). Isolated guard cell protoplasts of the Argenteum mutant of pea contained between 150 and 430 fmol hexose equivalents per protoplast in the form of starch, and additional reduced carbon was found stored in the form of fructans at levels of about 20 to 110 fmol glycosyl units per guard cell (B. Frank and K. Raschke, unpublished data). These amounts are sufficient to open the stomata of pea without a supply of carbohydrates from the mesophyll, e.g. in the early morning.
CONCLUSION
We suggest that guard cells of P. sativum possess two major mechanisms for the acquisition of sugars: one is essential for Glc uptake during periods of no or low photosynthesis in the mesophyll, the other one for the import of Suc during times of high assimilatory activity in the leaf. The uptake of Glc occurs by a saturable hexose transporter that is probably specific for guard cells and similar to the hexose transporter STP1 of Arabidopsis, whose expression is restricted to the guard cells (R. Stadler and N. Sauer, personal communication, in conjunction with Sauer et al., 1990). It provides the substrate for the accumulation of starch in the guard cells in preparation for an opening movement without simultaneous production of reduced carbon, e.g. before dawn. The transporter for Suc has yet to be identified.
The rate of hexose uptake through the hexose transporter is insufficient to support a concurrent stomatal opening movement, but suffices to “trickle charge” the guard cell chloroplasts with carbohydrate. In contrast, the estimated rates of Suc accumulation at high apoplastic Suc concentrations suffice to meet the carbon requirement for malate production during a concurrent opening movement; and they could contribute to a replacement of K malate as the osmoticum in the guard cells as the photoperiod advances during the course of the day (Talbott and Zeiger, 1996). However, the rates of Suc accumulation are not large enough for this sugar to function as the sole solute for guard cell swelling.
ACKNOWLEDGMENTS
The authors thank Annette Bielefeld for skillful technical assistance, W.H. Outlaw Jr. and J.M. Ward for valuable comments on the manuscript, and M. Läsche and B. Raufeisen for help during the preparation of the article.
Footnotes
This work was supported grants to K.R. by the Deutsche Forschungsgemeinschaft.
LITERATURE CITED
- Aked J, Hall JL. The uptake of glucose, fructose and sucrose into the lower epidermis of leaf discs of pea (Pisum sativum L. cv. Argenteum) New Phytol. 1993;123:271–276. [Google Scholar]
- Boorer KJ, Loo DDF, Frommer WB, Wright EM. Transport mechanism of the cloned potato H+-sucrose cotransporter StSUT1. J Biol Chem. 1996;271:25139–25144. doi: 10.1074/jbc.271.41.25139. [DOI] [PubMed] [Google Scholar]
- Borchert S, Grosse H, Heldt HW. Specific transport of inorganic phosphate, dihydroxyacetone phosphate and 3-phosphoglycerate into amyloplasts from pea roots. FEBS Lett. 1989;253:183–186. [Google Scholar]
- Borchert S, Harborth J, Schünemann D, Hoferichter P, Heldt HW. Studies of the enzymic capacities and transport properties of pea root plastids. Plant Physiol. 1993;101:303–312. doi: 10.1104/pp.101.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckhout TJ. Sucrose transport in isolated plasma-membrane vesicles from sugar beet (Beta vulgaris L.): evidence for an electrogenic sucrose-proton symport. Planta. 1989;178:393–399. doi: 10.1007/BF00391867. [DOI] [PubMed] [Google Scholar]
- Bush DR. Proton-coupled sugar and amino acid transporters in plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:513–542. [Google Scholar]
- Dittrich P, Raschke K. Uptake and metabolism of carbohydrates by epidermal tissue. Planta. 1977;134:83–90. doi: 10.1007/BF00390099. [DOI] [PubMed] [Google Scholar]
- Estruch JJ, Peretó JG, Vercher Y, Beltrán JP. Sucrose loading in isolated veins of Pisum sativum: regulation by abscisic acid, gibberellic acid, and cell turgor. Plant Physiol. 1989;91:259–265. doi: 10.1104/pp.91.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotow K, Taylor S, Zeiger E. Photosynthetic carbon fixation in guard cell protoplasts of Vicia faba L.: evidence from radiolabel experiments. Plant Physiol. 1988;86:700–705. doi: 10.1104/pp.86.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineke D, Sonnewald U, Büssis D, Günter G, Leidreiter K, Wilke I, Raschke K, Willmitzer L, Heldt HW. Apoplastic expression of yeast-derived invertase in potato: effects on photosynthesis, leaf solute composition, water relations, and tuber composition. Plant Physiol. 1992;100:301–308. doi: 10.1104/pp.100.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt HW, Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970;10:143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Heldt HW, Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971;234:83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Hite DRC, Outlaw WH, Jr, Tarczynski MC. Elevated levels of both sucrose-phosphate synthase and sucrose synthase in Vicia guard cells indicate cell-specific carbohydrate interconversions. Plant Physiol. 1993;101:1217–1221. doi: 10.1104/pp.101.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewer PC, Incoll LD, Shaw J. Stomatal responses of Argenteum: a mutant of Pisum sativum L. with readily detachable leaf epidermis. Planta. 1982;155:146–153. doi: 10.1007/BF00392545. [DOI] [PubMed] [Google Scholar]
- Klingenberg M, Pfaff E. Means of terminating reactions. Methods Enzymol. 1967;10:680–684. [Google Scholar]
- Kohl FG. Die Transpiration der Pflanzen und ihre Einwirkung auf die Ausbildung pflanzlicher Gewebe. Braunschweig, Germany: Harald Bruhn; 1886. [Google Scholar]
- Komor E, Thom M, Maretzki A. The mechanism of sugar uptake by sugarcane suspension cells. Planta. 1981;153:181–192. doi: 10.1007/BF00384100. [DOI] [PubMed] [Google Scholar]
- Lemoine R, Delrot S. Proton-motive-force-driven sucrose uptake in sugar beet plasma membrane vesicles. FEBS Lett. 1989;249:129–133. [Google Scholar]
- Lin W, Schmitt MR, Hitz WD, Giaquinta RT. Sugar transport into protoplasts isolated from developing soybean cotyledons. I. Protoplast isolation and general characteristics of sugar transport. Plant Physiol. 1984;75:936–940. doi: 10.1104/pp.75.4.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd FE. The Physiology of Stomata, Publication No. 82. Washington, DC: Carnegie Institution of Washington; 1908. [Google Scholar]
- Lohaus G. Vom Source zum Sink: Phloemtransport verschiedener Kohlenstoff- und Stickstoffverbindungen. Doctoral dissertation. Göttingen, Germany: Georg-August-Universität Göttingen; 1995. [Google Scholar]
- Lohse G, Hedrich R. Characterization of the plasma-membrane H+-ATPase from Vicia faba guard cells: modulation by extracellular factors and seasonal changes. Planta. 1992;188:206–214. doi: 10.1007/BF00216815. [DOI] [PubMed] [Google Scholar]
- Lu P, Outlaw WH, Jr, Smith BG, Freed GA. A new mechanism for the regulation of stomatal aperture size in intact leaves: accumulation of mesophyll-derived sucrose in the guard-cell wall of Vicia faba. Plant Physiol. 1997;114:109–118. doi: 10.1104/pp.114.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Zhang SQ, Outlaw WH, Jr, Riddle KA. Sucrose: a solute that accumulates in the guard-cell apoplast and guard-cell symplast of open stomata. FEBS Lett. 1995;362:180–184. doi: 10.1016/0014-5793(95)00239-6. [DOI] [PubMed] [Google Scholar]
- Marrè E. Fusicoccin: a tool in plant physiology. Annu Rev Plant Physiol. 1979;30:273–288. [Google Scholar]
- Martinoia E, Kaiser G, Schramm MJ, Heber U. Sugar transport across the plasmalemma and the tonoplast of barley mesophyll protoplasts: evidence for different transport systems. J Plant Physiol. 1987;131:467–478. [Google Scholar]
- Marx GA. Argenteum: a mutant under nuclear and extranuclear control. Pisum Newsletter. 1978;10:34–37. [Google Scholar]
- Outlaw WH., Jr Critical examination of the quantitative evidence for and against photosynthetic CO2 fixation by guard cells. Physiol Plant. 1989;77:275–281. [Google Scholar]
- Outlaw WH., Jr . Stomata and sucrose: a full circle. In: Madore MA, Lucas WJ, editors. Carbon Partitioning and Source-Sink Interactions in Plants. Rockville, MD: The American Society of Plant Physiologists; 1995. pp. 56–67. [Google Scholar]
- Outlaw WH, Jr, Manchester J, DiCamelli CA, Randall DD, Rapp B, Veith GM. Photosynthetic carbon reduction pathway is absent in chloroplasts of Vicia faba guard cells. Proc Natl Acad Sci USA. 1979;76:6371–6375. doi: 10.1073/pnas.76.12.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overlach S, Diekmann W, Raschke K. Phosphate translocator of isolated guard cell chloroplasts from Pisum sativum L. transports glucose-6-phosphate. Plant Physiol. 1993;101:1204–1207. doi: 10.1104/pp.101.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overvoorde PJ, Frommer WB, Grimes HD. A soybean sucrose binding protein independently mediates nonsaturable sucrose uptake in yeast. Plant Cell. 1996;8:271–289. doi: 10.1105/tpc.8.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palevitz BA, Hepler PK. Changes in dye coupling of stomatal cells of Allium and Commelina demonstrated by microinjection of lucifer yellow. Planta. 1985;164:473–479. doi: 10.1007/BF00395962. [DOI] [PubMed] [Google Scholar]
- Poffenroth M, Green DB, Tallman G. Sugar concentrations in guard cells of Vicia faba illuminated with red or blue light: analysis by high performance liquid chromatography. Plant Physiol. 1992;98:1460–1471. doi: 10.1104/pp.98.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke K, Hedrich R. Patch clamp measurements on isolated guard cell protoplasts and vacuoles. Methods Enzymol. 1989;174:312–330. [Google Scholar]
- Raschke K, Schnabl H. Availability of chloride affects the balance between potassium chloride and potassium malate in guard cells of Vicia faba L. Plant Physiol. 1978;62:84–87. doi: 10.1104/pp.62.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckmann U, Scheibe R, Raschke K. Rubisco activity in guard cells compared with the solute requirement for stomatal opening. Plant Physiol. 1990;92:246–253. doi: 10.1104/pp.92.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AR, Rama Das VS. Stomatal movement and sucrose uptake by guard cell protoplasts of Commelina benghalensis L. Plant Cell Physiol. 1986;27:1565–1570. [Google Scholar]
- Rohrig K, Raschke K. [14C]Sucrose uptake by guard cell protoplasts of Pisum sativum, Argenteum mutant (abstract no. 317) Plant Physiol. 1991;96:S-47. [Google Scholar]
- Sauer N, Friedländer K, Gräml-Wicke U. Primary structure, genomic organization and heterologous expression of a glucose transporter from Arabidopsis thaliana. EMBO J. 1990;9:3045–3050. doi: 10.1002/j.1460-2075.1990.tb07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Lessdorf B, Lohse G, Hedrich R. GCAC1 recognizes the pH gradient across the plasma membrane: a pH-sensitive and ATP-dependent anion channel links guard cell membrane potential to acid and energy metabolism. Plant J. 1996;10:993–1004. [Google Scholar]
- Talbott LD, Zeiger E. Sugar and organic acid accumulation in guard cells of Vicia faba in response to red and blue light. Plant Physiol. 1993;102:1163–1169. doi: 10.1104/pp.102.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E. Central roles for potassium and sucrose in guard-cell osmoregulation. Plant Physiol. 1996;111:1051–1057. doi: 10.1104/pp.111.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallman G, Zeiger E. Light quality and osmoregulation in Vicia guard cells: evidence for involvement of three metabolic pathways. Plant Physiol. 1988;88:887–895. doi: 10.1104/pp.88.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, Maretzki A. Evidence for direct uptake of sucrose by sugarcane stalk tissue. J Plant Physiol. 1992;139:555–559. [Google Scholar]
- Van Kirk CA, Raschke K. Presence of chloride reduces malate production in epidermis during stomatal opening. Plant Physiol. 1978;61:361–364. doi: 10.1104/pp.61.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstappen R, Ranostaj S, Rausch T. The hexose transporters at the plasma membrane and the tonoplast of transformed plant cells: kinetic characterization of two distinct carriers. Biochim Biophys Acta. 1991;1073:366–373. doi: 10.1016/0304-4165(91)90144-6. [DOI] [PubMed] [Google Scholar]