Abstract
Background
Few data on HIV resistance in pregnancy are available from Mozambique, one of the countries with the highest HIV toll worldwide. Understanding the patterns of HIV drug resistance in pregnant women might help in tailoring optimal regimens for prevention of mother to child transmission of HIV (pMTCT) and antenatal care.
Objectives
To describe the frequency and characteristics of HIV drug resistance mutations (HIVDRM) in pregnant women with virological failure at delivery, despite pMTCT or antiretroviral therapy (ART).
Methods
Samples from HIV-infected pregnant women from a rural area in southern Mozambique were analysed. Only women with HIV-1 RNA >400c/mL at delivery were included in the analysis. HIVDRM were determined using MiSeq® (detection threshold 1%) at the first antenatal care (ANC) visit and at the time of delivery.
Results
Ninety and 60 samples were available at the first ANC visit and delivery, respectively. At first ANC, 97% of the women had HIV-1 RNA>400c/mL, 39% had CD4+ counts <350 c/mm3 and 30% were previously not on ART. Thirteen women (14%) had at least one HIVDRM of whom 70% were not on previous ART. Eight women (13%) had at least one HIVDRM at delivery. Out of 37 women with data available from the two time points, 8 (21%) developed at least one new HIVDRM during pMTCT or ART. Twenty seven per cent (53/191), 32% (44/138) and 100% (5/5) of the mutations that were present at enrolment, delivery and that emerged during pregnancy, respectively, were minority mutations (frequency <20%).
Conclusions
Even with ultrasensitive HIV-1 genotyping, less than 20% of women with detectable viremia at delivery had HIVDRM before initiating pMTCT or ART. This suggests that factors other than pre-existing resistance, such as lack of adherence or interruptions of the ANC chain, are also relevant to explain lack of virological suppression at the time of delivery in women receiving antiretrovirals drugs during pregnancy.
Introduction
Women are disproportionately vulnerable to HIV infection, particularly in sub-Saharan Africa (SSA), where approximately three-quarters of new infections are in women of reproductive age [1]. HIV infection in pregnancy has important public health consequences; it is responsible for the majority of new HIV pediatric infections and a significant cause of maternal mortality [2, 3]. Mother to child transmission of HIV (MTCT) can occur during pregnancy, labor and through breastfeeding [4, 5]. Among the many factors that have been suggested to increase the risk of MTCT, maternal HIV viral load at delivery has been shown to be the strongest predictor [6, 7]. Provision of antiretroviral (ARV) drugs in pregnancy is the most effective intervention for blocking viral replication and reducing MTCT and HIV/AIDS maternal related deaths [1, 8]. However, in low-income countries, increasing occurrence of HIV drug resistance mutations (HIVDRM) to first line recommended ARV drugs either for prevention of MTCT (pMTCT) or antiretroviral therapy (ART) and cross resistances to second line ARV drugs have been found [9]. Pre-existence of transmitted drug resistant viruses or selection of ARV drug resistance during pregnancy, may hinder the long-term efficacy of MTCT preventive programs, as well as severely constrain future treatment options for both mother and child.
Mozambique is one of the 10 countries with the highest HIV prevalence in the world [10]. A study showed that 90% of the subjects on ART with virological failure had developed resistance to first-line treatment options [11]. In addition, a prevalence of 12% of acquired HIVDRM was reported in HIV infected Mozambican children [12]. However, little information on HIV resistance in pregnancy has been reported in the country. As ART coverage continues to grow, better knowledge of the effectiveness of first line ARV drugs in HIV-infected pregnant women in Mozambique is key for antenatal care (ANC) and pMTCT programme optimization and planning. We describe here the frequency and characteristics of HIVDRM, at first ANC visit and at the time of delivery, in pregnant women participating in a clinical trial on malaria prevention in Southern Mozambique.
Materials and methods
Study design
This is an observational study to describe the prevalence of HIVDRM at first ANC visit and at delivery in HIV-infected pregnant women, who were enrolled, from March 2010 to April 2012, in a randomized controlled clinical trial to evaluate the safety and efficacy of MQ compared to placebo for malaria prevention (ClinicalTrials.gov, NCT0081121, and Pan African Clinical Trials, PACTR2010020001429343) [13].
Study area and population
The study was carried out in Manhiça, a semi-rural area in southern Mozambique. Since 1996, the Centro de Investigaçao em Saúde de Manhiça (CISM) is conducting a continuous demographic surveillance in the Manhiça District that covers a population of approximately 95,000 inhabitants in an area of 500km.2 [14]. Although the countrywide HIV average prevalence figure is 11% a community based study in adults undertaken in the Manhiça area in 2012 reported nearly 40% of HIV prevalence, while 30% of pregnant women attending the ANC were HIV seropositive [15, 16]. The predominantly subtype in the area is subtype C [17, 18]. At the time of this study, pMTCT relied on ARV prophylaxis, which consisted in antepartum monotherapy with zidovudine (AZT), a single dose nevirapine (sd NVP) at the onset of labor, and AZT + lamivudine (3TC) at delivery and for 7 days postpartum. National guidelines recommended ART to HIV-infected pregnant women for their own health if CD4+T cell count were <350 and/or 3–4 HIV/AIDS WHO clinical stage. The recommended first-line regimen consisted in the dual NRTI backbone (3TC and either AZT or stavudine [d4T]) plus an NNRTI (either NVP or efavirenz [EFV]). However, in 2013, Mozambique adopted “Option B+”, consisting in the initiation of lifelong ART with tenofovir (TDF) + 3TC + EFV in all HIV-positive pregnant and lactating women regardless of their immune status and clinical stage [19]. Both currently and at the time of the study, to assess intra-utero and perinatal MTCT national guidelines recommend to perform an HIV-DNA PCR test between 4–6 weeks of age in all infants born to HIV-infected mothers, with a confirmatory test on a new sample in those infants who test positive.
Study procedures
Enrolment of pregnant women
Pregnant women attending the ANC clinics for the first time were screened for eligibility to participate in the trial. Inclusion criteria were, permanent residency in the area, a gestational age ≤28 weeks, a positive HIV test, absence of history of allergy to intervention drugs, absence of history of severe renal, hepatic, psychiatric, or neurological disease, and no MQ or halofantrine treatment in the preceding four weeks. Following national guidelines, HIV status was assessed after voluntary HIV counseling. Hemoglobin (Hb) and the syphilis rapid plasma reagin test (RPR) were also assessed at this visit as part of routine ANC. Socio-demographic and clinical characteristics were recorded on standardized questionnaires and five mL of venous blood was collected for CD4+T cell count and HIV viral load determination. Administration of ARV drugs for pMTCT or for ART was registered in the study concomitant medication forms.
Following physical examination, enrolled women with gestational age ≥13 weeks received the first administration of IPTp (either placebo or MQ) under supervision. The second and third administrations of IPTp-MQ/placebo were given in the next ANC visits at least one month apart. All women also received study CTXp tablets for daily prophylaxis.
Follow-up of pregnant women and their children
Women were followed-up through study visits during pregnancy, delivery and up to one month post-partum. At delivery, a sample from the mother’s peripheral blood was collected for Hb, CD4+T cell count, HIV viral load and malaria infection evaluation. Following national guidelines for pMTCT of HIV, a capillary blood sample was collected from the infant at six weeks of age for HIV PCR analysis to assess vertical transmission.
Laboratory methods
HIV serostatus was assessed using rapid test (Determine, Abbot Laboratories, USA), and positive results confirmed using Unigold rapid test (TM HIV, Trinity Biotech, Ireland), following national guidelines. HIV-1 RNA levels were determined from cryopreserved plasma samples using the COBAS AMPLICOR or AmpliPrep (Roche Diagnostics, Rotkreuz, Switzerland) devices; these assays have a lower detection limit ranging from 50 to 400 copies/mL. CD4+ T cell counts were determined by flow cytometry after staining of whole blood for CD3, CD8, CD4 and CD45 using fluorochrome-labelled antibodies and acquisition using FACSCalibur (BD Biosciences) and Trucount tubes (Becton Dickinson, San Jose, CA, USA). Remaining plasma samples from first ANC and delivery visits were stored at the CISM laboratory at -80 C°. Analysis of genotypic drug resistance of HIV in stored plasma samples was attempted in all subjects who had HIV-1 RNA ≥400 copies/mL at delivery. HIV-1 RNA was extracted from 140 mL of plasma (QIAamp Viral RNA Mini Kit,Qiagen). Pol amplicons (PR/RT) were PCR-generated, fragmented using Nextera-XT and sequenced in an Illumina MiSeqTM [20]. Next-Generation Sequencing (NGS) raw data was analyzed using an in-house pipeline (https://www.paseq.org). Briefly, we filtered low quality and potentially contaminant sequences and aligned them against a user-specified reference (NCBI Accesion: 97ZA012) using BWA. Codon-level variant calling was then performed on the resulting alignments and an amino acid variant table was generated using a 1% user specified detection threshold with a median of 5147 depth of coverage (IQR: 3295–7281). Drug resistance-associated mutations in protease and reverse transcriptase gene regions from all quality-assured sequences were interpreted with the Standford HIV database (HIVdb) program. To predict susceptibility to NRTIs, NNRTIs and PIs a resistance score was calculated as susceptible, intermediate level and high level following the Stanford HIVdb scoring system (http://hivdb.stanford.edu).
Data management, statistical methods, and definitions
Data were double-entered using the OpenClinica EnterpriseTM software for clinical data management (www.openclinica.com).
Literacy was defined as knowing how to read and/or write. Adolescent women were considered those <19 years of age. CD4+T cell count was categorized as < 350 or > = 350 cells/μl. Detectable viral load was defined as HIV-1 RNA levels ≥400copies/mL. HIV drug resistance was considered as the presence of one or more major resistance mutations as defined by the Stanford HIVdb.
Baseline characteristics of study patients were described using standard statistics. Proportions for categorical variables were assessed using the Chi-squared test or Fisher’s exact test where appropriate. Student's t-test or the Mann–Whitney test was used for comparing means and medians of quantitative variables according to variable characteristics. All statistical tests were two-tailed and statistical significance was defined as p<0.05. Data analysis was performed using Stata version 13 (Stata Corp., College Station, TX, US).
Ethics statement and participants’ safety
This is an exploratory analysis nested in a clinical trial, which protocol and informed consent forms were reviewed and approved by the Ethics Committees from the Hospital Clínic of Barcelona (Spain) and the local regulatory authorities and National Ethics Review Committee from Mozambique (209/CNBS/2014). Study participants signed a written informed consent form prior to enrolment. The study was conducted under the provisions of the Declaration of Helsinki and in accordance with Good Clinical Practices guidelines set up by the WHO and by the International Conference of Harmonization. The protocol for the HIV resistance analysis in samples of women participating in the trial was reviewed and approved by the National Ethics Review Committee from Mozambique.
Results
Study profile
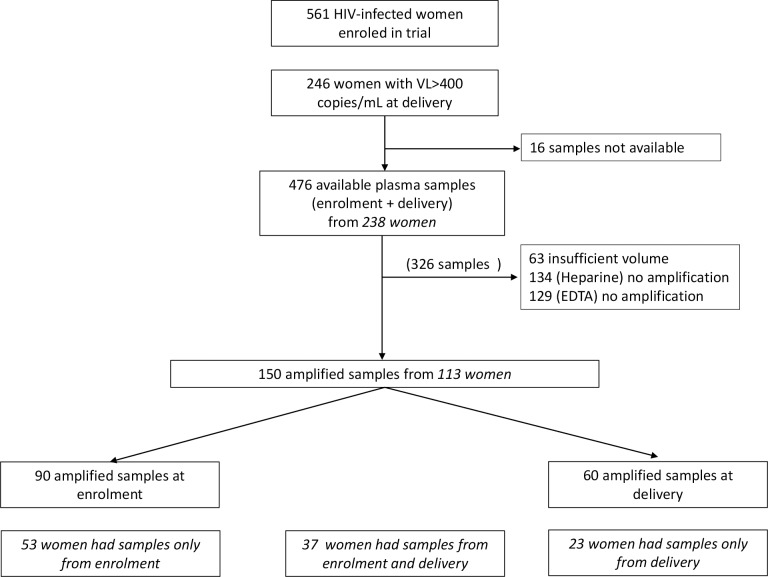
Out of 561 enrolled HIV-infected pregnant women, 246 had detectable viral load (HIV-1 RNA ≥400 copies/ml) at delivery. Out of these 246 women, there were available stored plasma samples from the first ANC and the delivery from 238 women. Out of these 476 plasma samples, 63 had insufficient plasma volume, and in 263 plasma samples RNA could not be amplified. Overall, RNA was successfully amplified in samples from 113 women, and were thus, the study population for this exploratory analysis; 53 women had samples amplified only at enrolment, 23 only at delivery and 37 at both time points (Fig 1).
Fig 1. Study profile.
Out of these 113 women, 18 (16%) were adolescents, 33 (29%) were illiterate and the median gestational age was 22 weeks, Interquartile Range, (IQR) 17–24. Twenty three (20%) of the women were primigravidae, 44 (39%) had CD4+ T-cell counts <350 cells/mm3 and almost all [109 (96%)] had detectable viral load at enrolment. Thirty five women (31%) were on previous ART with first line regime at the time of enrolment (Table 1).
Table 1. Baseline characteristics at enrolment of study participants (N = 113).
| Characteristics | n | % |
|---|---|---|
| Age, median (IQR) | 25 [21–30] | |
| Adolescents1 | 18 | 15.93 |
| Gestational age, median (IQR)2 | 22 [17–24] | |
| 3rd trimester | 10 | 8.85 |
| Primigravidae | 23 | 20.35 |
| Number of previous pregnancies, median (IQR) | 2 [1–3] | |
| Illiteracy3 | 33 | 29.20 |
| HIV-1 RNA (copies/mL) median (IQR) | 35660 [9821–118467] | |
| Detectable HIV-1 RNA 4 | 109 | 96.46 |
| CD4+ T-cell counts (cell/mm3), median (IQR) | 342 [196–559] | |
| CD4+ T-cell counts <350 cell/mm3 | 44 | 38.94 |
| Previously on antiretroviral therapy | 35 | 30.97 |
1 Age<19 years
2 Gestational age calculated from fundal height
3 Not being able to read and/or write
4 HIV-1 RNA>400 copies/ml
HIV drug resistance mutations at enrolment
Ninety pregnant women at enrolment were included in this part of the analysis. The median age was 25 years (IQR 21; 29), and the median gestational age 21 weeks (IQR 16; 23). Twenty one (23%) women were primigravidae, 29 (32%) were illiterate and 15 (17%) were adolescents.Nearly all women [87 (97%)] had detectable viral load with a median viral load of 47,877 copies/ml (IQR 12,807–140,129) In 35 (40%) of them, CD4+ T-cell counts were <350 cells/mm3. Only 27 women (30%) were on ART at the time of the first ANC (Table 2). Thirteen (14%) presented at least one HIVDRM, 6 (7%) had resistance to NNRTI, 8 (9%) to NRTI and 2 (2%) to PI. Among the women with at least one HIVDRM, seven (8%) had resistant mutations to at least one drug of the first-line regimen recommended at the time of the study (Table 3). Among the women with HIVDRM to NRTI, 4 (4%) had resistant mutations to ddI, 4 (4%) to TDF and 4 (4%) to 3TC. Of the women who had resistant mutations to NNRTI, 6 (7%) were to EFV, 6 (7%) to NVP and 2 (2%) to RPV (Table 4). The most frequent mutations are shown in Table 5. No significant differences were observed in baseline characteristics between women who presented at least one HIVDRM and those who did not (Table 2).
Table 2. Characteristics of women at enrolment and at delivery.
| Characteristics | Enrolment | Delivery | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (N = 90) | At least one HIVDRM1(N = 13) | No HIVDRM1(N = 77) | P-value | All (N = 60) | At least one HIVDRM1 (N = 8) | No HIVDRM1 (N = 52) | P-value | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |||||
| Characteristics at enrolment | ||||||||||||||||
| Age2,3 | 25 [21–29] | 26 [19–29] | 25 [21–29] | 0.895 | 26 [22–30] | 28 [25–31] | 26 [22–30] | 0.290 | ||||||||
| Adolescents3 | 15 | 16.67 | 3 | 23.08 | 12 | 15.58 | 0.449 | 9 | 15.00 | 0 | 0.00 | 9 | 17.31 | 0.339 | ||
| Gestational age2,4 | 21 [16–23] | 20 [12–22] | 21 [16–23] | 0.468 | 22 [18–25] | 21 [18–25] | 22 [19–25] | 0.819 | ||||||||
| 3rd trimester | 19 | 21.11 | 2 | 15.38 | 17 | 22.08 | 0.728 | 16 | 26.67 | 2 | 25.00 | 14 | 26.92 | 1.000 | ||
| Primigravidae | 21 | 23.33 | 3 | 23.08 | 18 | 23.38 | 1.000 | 12 | 20.00 | 2 | 25.00 | 10 | 19.23 | 0.655 | ||
| Number of previous pregnancies2 | 2 [1–3] | 1 [1–3] | 2 [1–2] | 0.879 | 2 [1–3] | 1 [1–5] | 2 [1–3] | 0.885 | ||||||||
| Illiteracy5 | 29 | 32.22 | 5 | 38.46 | 24 | 31.17 | 0.749 | 17 | 28.33 | 3 | 37.50 | 14 | 26.92 | 0.676 | ||
| HIV-1 RNA (copies/mL)2 | 47877 [12807–140129] | 48486 [26200–166409] | 47268 [12105–116933] | 0.578 | 32124 [6990–107693] | 208266 [16442–425395] | 30500 [6990–72500] | 0.106 | ||||||||
| Detectable HIV-1 6 | 87 | 96.66 | 13 | 100.00 | 74 | 96.10 | 1.000 | 3 | 5.00 | 1 | 12.50 | 2 | 3.85 | 0.065 | ||
| CD4+ T-cell counts (cell/mm3)2 | 402 [255.5–595.5] | 432 [194–576] | 401 [277–596] | 0.666 | 419 [269–654] | 226 [116–285] | 440 [295–664] | 0.010 | ||||||||
| CD4+ T-cell counts <350 (cell/mm3) | 35 | 38.89 | 5 | 38.46 | 30 | 38.96 | 1.000 | 23 | 38.33 | 7 | 87.50 | 16 | 30.77 | 0.008 | ||
| Previous antiretroviral therapy | 27 | 30.00 | 4 | 30.77 | 23 | 29.87 | 1.000 | 17 | 28.33 | 6 | 75.00 | 11 | 21.15 | 0.009 | ||
| Characteristics at delivery | ||||||||||||||||
| IPTp with MQ in pregnancy 7,8 | 42 | 46.67 | 5 | 38.46 | 37 | 48.05 | 0.564 | 28 | 46.67 | 4 | 50.00 | 24 | 46.15 | 1.000 | ||
| HIV-1 RNA (copies/mL)2 | 35028 [7077–81277] | 40053 [26200–60319] | 29945 [6358–81443] | 0.667 | 34943 [12891–78116] | 155732 [37537–253078] | 30500 [6990–72500] | 0.024 | ||||||||
| CD4+ T-cell counts (cell/mm3)2 | 348 [193–566] | 333 [186–559] | 349 [193–584] | 0.919 | 379 [250–584] | 129 [71–5419] | 440 [295–664] | 0.071 | ||||||||
|
CD4+ T-cell counts <350 cell/mm3 (cell/mm3) |
40 | 44.44 | 6 | 46.15 | 34 | 44.16 | 1.000 | 25 | 41.67 | 5 | 62.50 | 19 | 38.46 | 0.259 | ||
| ARV during pregnancy: -PMTCT9 | 73 | 81.11 | 8 | 61.54 | 65 | 84.42 | 0.057 | 46 | 81.67 | 4 | 50.00 | 42 | 84.00 | 0.018 | ||
| - ART10 | 9 | 10.00 | 2 | 15.38 | 7 | 9.09 | 8 | 11.67 | 4 | 50.00 | 4 | 8.00 | ||||
| - None | 8 | 8.89 | 3 | 23.08 | 5 | 6.49 | 4 | 6.67 | 0 | 0.00 | 4 | 8.00 | ||||
| MTCT 11,12 | 8 | 9.20 | 2 | 16.67 | 6 | 8.00 | 0.687 | 4 | 8.51 | 0 | 0.00 | 4 | 9.52 | 1.000 | ||
1Drug resistance mutation
2 Median (IQR)
3 Age<19 years
4 Gestational age calculated from fundal height
5 Not being able to read and/or write
6 HIV-1 RNA>400 copies/mL
7Intermittent treatment of malaria in pregnancy
8Mefloquine
9 Prevention of mother to child transmission
10Antiretroviral therapy
11 Mother to child transmission of HIV
12 Data available from 87 women at enrolment and from 47 at delivery
Table 3. Frequency of resistance mutations by drug category at enrolment, at delivery and acquired during pregnancy.
| Resistance category | Enrolment (N = 90) | Delivery (N = 60) | Acquired during pregnancy (N = 37) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| At least one DRM1 | 13 | 14.4 | 8 | 13.3 | 5 | 13.5 |
| At least one NNRTI DRM2 | 6 | 6.7 | 5 | 8.3 | 2 | 5.4 |
| At least one NRTI DRM3 | 8 | 8.9 | 6 | 10.0 | 2 | 5.4 |
| At least one PI DRM4 | 2 | 2.2 | 0 | 0.0 | 1 | 2.7 |
| At least one drug in first-line regime | 7 | 7.8 | 6 | 10.0 | 1 | 2.7 |
1 Drug resistance mutation
2 Non-nucleoside reverse-transcriptase inhibitors
3 Nucleoside reverse-transcriptase inhibitors
4 Protease inhibitors
Table 4. Predicted drug susceptibility in women at enrolment and at delivery.
| Drug type | Enrolment (N = 90) | Delivery (N = 60) | |||||
|---|---|---|---|---|---|---|---|
| Drug | S1 | I2 | R3 | S | I | R | |
| NRTI4 | ABC | 82 (91.1%) | 8 (8.9%) | 0 (0%) | 54 (90.0%) | 5 (8.3%) | 1 (1.7%) |
| AZT | 87 (96.7%) | 3 (3.3%) | 0 (0%) | 58 (96.7%) | 2 (3.3%) | 0 (0%) | |
| DDI | 86 (95.6%) | 0 (0%) | 4 (4.4%) | 56 (93.3%) | 1 (1.7%) | 3 (5.0%) | |
| D4T | 83 (92.2%) | 7 (7.8%) | 0 (0%) | 56 (93.3%) | 3 (5.0%) | 1 (1.7%) | |
| FTC | 82 (91.1%) | 4 (4.4%) | 4 (4.4%) | 55 (91.7%) | 2 (3.3%) | 3 (5.0%) | |
| TDF | 86 (95.6%) | 0 (0%) | 4 (4.4%) | 58 (96.7%) | 0 (0%) | 2 (3.3%) | |
| 3TC | 82 (91.1%) | 4 (4.4%) | 4 (4.4%) | 55 (91.7%) | 2 (3.3%) | 3 (5.0%) | |
| NNRTI5 | EFV | 84 (93.3%) | 0 (0%) | 6 (6.7%) | 54 (90.0%) | 2 (3.3%) | 4 (6.7%) |
| ETR | 87 (96.7%) | 3 (3.3%) | 0 (0%) | 57 (95.0%) | 1 (1.7%) | 2 (3.3%) | |
| NVP | 82 (91.1%) | 2 (2.2%) | 6 (6.7%) | 52 (86.7%) | 3 (5.0%) | 5 (8.3%) | |
| RPV | 79 (87.8%) | 9 (10.0%) | 2 (2.2%) | 51 (85.0%) | 7 (11.7%) | 2 (3.3%) | |
| PI6 | ATV/r | 89 (98.9%) | 1 (1.1%) | 0 (0%) | 60 (100%) | 0 (0%) | 0 (0%) |
| DRV/r | 89 (98.9%) | 1 (1.1%) | 0 (0%) | 60 (100%) | 0 (0%) | 0 (0%) | |
| FPV/r | 86 (95.6%) | 3 (3.3%) | 1 (1.1%) | 60 (100%) | 0 (0%) | 0 (0%) | |
| IDV/r | 86 (95.6%) | 4 (4.4%) | 0 (0%) | 60 (100%) | 0 (0%) | 0 (0%) | |
| LPV/r | 86 (95.6%) | 4 (4.4%) | 0 (0%) | 60 (100%) | 0 (0%) | 0 (0%) | |
| NFV | 69 (76.7%) | 20 (22.2%) | 1 (1.1%) | 48 (80.0%) | 12 (20.0%) | 0 (0%) | |
| SQV/r | 86 (95.6%) | 4 (4.4%) | 0 (0%) | 60 (100%) | 0 (0%) | 0 (0%) | |
| TPV/r | 88 (97.8%) | 2 (2.2%) | 0 (0%) | 60 (100%) | 0 (0%) | 0 (0%) | |
1 Intermediate resistance
2 Resistant
3 Susceptible
4 Nucleoside reverse-transcriptase inhibitors
5Non-nucleoside reverse-transcriptase inhibitors
6 Protease inhibitors
Table 5. HIV resistant mutations in HIV-infected pregnant women at enrolment, at delivery and acquired during pregnancy.
| Type of mutation | Enrolment (N = 90) |
Delivery (N = 60) |
Acquired during pregnancy (N = 37) |
|||
|---|---|---|---|---|---|---|
| All mutations |
Minority Mutations (freq <20%) |
All mutations |
Minority mutations (freq <20%) |
All mutations |
Minority mutations (freq <20%) |
|
| N (%) | n (%) | N (%) | n (%) | N (%) | n (%) | |
| PR mutations1 | ||||||
| A71T | 7 (7.78) | 3 (42.86) | 2 (3.33) | 0 (0) | 0 (0) | 0 (0) |
| A71V | 3 (3.33) | 2 (66.67) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| D60E | 20 (22.22) | 3 (15.00) | 12 (20.00) | 1 (8.33) | 0 (0) | 0 (0) |
| G16E | 11 (12.22) | 4 (36.36) | 11 (18.33) | 4 (36.36) | 0 (0) | 0 (0) |
| I62V | 12 (13.33) | 2 (16.67) | 6 (10.00) | 1 (16.67) | 0 (0) | 0 (0) |
| I64L | 4 (4.44) | 0 (0) | 2 (3.33) | 0 (0) | 0 (0) | 0 (0) |
| I64V | 2 (2.22) | 2 (100.00) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| K20M | 3 (3.33) | 2 (66.67) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| K20R | 32 (35.56) | 3 (9.38) | 24 (40.00) | 1 (4.17) | 1 (2.70) | 0 (0) |
| L10I | 5 (5.56) | 2 (40.00) | 3 (5.00) | 1 (33.33) | 0 (0) | 0 (0) |
| L10V | 2 (2.22) | 0 (0) | 3 (5.00) | 1 (33.33) | 0 (0) | 0 (0) |
| L33V | 1 (1.11) | 0 (0) | 1 (1.67) | 1 (100.00) | 0 (0) | 0 (0) |
| M46I | 3 (3.33) | 2 (66.67) | 1 (1.67) | 1 (100.00) | 0 (0) | 0 (0) |
| V11I | 2 (2.22) | 0 (0) | 1 (1.67) | 0 (0) | 0 (0) | 0 (0) |
| V77I | 20 (22.22) | 2 (10.00) | 13 (21.67) | 1 (7.69) | 0 (0) | 0 (0) |
| V82I | 15 (75.00) | 4 (26.67) | 11 (18.33) | 5 (45.45) | 1 (2.70) | 0 (0) |
| Others: D30N, F53L,G48V,G73C,G73S,I50V,I54V,I64M,K20I,L10F,L76V,M46L,N88S | 9 (10.00) | 9 (100.00) | 4 (6.67) | 4 (100.00) | 0 (0) | 0 (0) |
| RT mutations2 | ||||||
| E138A | 8 (8.89) | 0 (0) | 4 (6.67) | 0 (0) | 0 (0) | 0 (0) |
| V90I | 3 (3.33) | 1 (3.33) | 3 (5.00) | 2 (6.67) | 0 (0) | 0 (0) |
| V179D | 1 (1.11) | 0 (0) | 3 (5.00) | 2 (6.67) | 0 (0) | 0 (0) |
| V106M | 1 (1.11) | 1 (100.00) | 2 (3.33) | 1 (50.00) | 1 (2.70) | 1 (2.70) |
| M41L | 1 (1.11) | 1 (100.00) | 1 (1.67) | 1 (100.00) | 0 (0) | 0 (0) |
| M184V | 3 (3.33) | 0 (0) | 3 (5.00) | 0 (0) | 0 (0) | 0 (0) |
| M184I | 1 (1.11) | 1 (100.00) | 1 (1.67) | 1 (100.00) | 0 (0) | 0 (0) |
| K65R | 4 (4.44) | 4 (100.00) | 2 (3.33) | 2 (100.00) | 1 (2.70) | 1 (2.70) |
| K103S | 0 (0.00) | 0 (0) | 2 (3.33) | 2 (100.00) | 1 (2.70) | 1 (2.70) |
| K103N | 4 (4.44) | 1 (25.00) | 2 (3.33) | 1 (50.00) | 0 (0) | 0 (0) |
| K101E | 3 (3.33) | 0 (0) | 2 (3.33) | 0 (0) | 0 (0) | 0 (0) |
| H221Y | 0 (0.00) | 0 (0) | 2 (3.33) | 1 (50.00) | 0 (0) | 0(0) |
| G190A | 3 (3.33) | 0 (0) | 1 (1.67) | 0 (0) | 0 (0) | 0 (0) |
| E138G | 4 (3.33) | 2 (66.67) | 1 (1.67) | 0 (0) | 0 (0) | 0 (0) |
| Others: A62V, A98G,D67N,E138K,F77L,K101P,K219Q,K70R,L74V,M184I,M230I,P225H,V106A,V108I,V75I,Y181C,Y188H,Y188L | 4 (4.44) | 2 (50.00) | 15 (25.00) | 10 (6.67) | 0 (0) | 0 (0) |
Proportion of minority mutations were calculated as the number of mutations with a frequency under 20% among all existing mutations.
1 Protease
2 Reverse transcriptase
HIV drug resistance mutations at delivery
There were 60 women included in the analysis at the time of delivery. The median viral load was of 34943 copies/ml (IQR: 12891–78116), and it was significantly higher in the women who presented HIVDRM (p = 0.024) (Table 2). Twenty five women (42%) had CD4+ T cell counts < 350 cells/mm3. Forty six (82%) women had received ARV drugs for pMTCT and 8 (12%) were on ART for their own health during this pregnancy. Four women (7%) had received neither pMTCT nor ART during this pregnancy. None of the women presenting HIVDRM at delivery transmitted HIV to their infants at first month of age (Table 2). Eight women (13%) presented at least one HIVDRM, 5 (8%) to NNRTIs and 6 (10%) to NRTIs. None of the women had mutations conferring resistance to PIs (Table 3). Among the women presenting HIVDRM to NNRTI, 5 (8%) women had resistant mutations to NVP, 4 (7%) to EFV, 2 (3%) to RPV and 2 (3%) to ETR. Of the women with HIVDRM to NRTI, 3 (5%) had resistant mutations to ddI, 3 (5%) to FTC, 3 (5%) to 3TC, 2 (3%) to TDF, 1 (2%) to ABC and 1 (2%) to d4T (Table 4). The most frequent HIVDR mutations are shown in Table 5.
HIV drug resistance mutations at enrolment and at delivery
Thirty seven women had plasma samples from both enrolment and delivery available for this analysis. Of these, 5 (14%) developed new drug resistances during this pregnancy, though only one of them (3%) developed HIVDRM to drugs included in the first-line regimen (Table 3). Among those who developed HIVDRM, 4 (80%) had received pMTCT during pregnancy. In addition, among women who developed HIVDRM, none transmitted HIV to their infant at first month of age, while among those women who did not developed HIVDRM, 4 (8%) transmitted HIV to their infants (p = 0.344) (S1 Table).
Discussion
This is the first study undertaken in Mozambique, -one of the countries with the highest prevalence and incidence of HIV infection in the world-, addressing HIV drug resistance in pregnant women and using NGS. A previous study reported NVP resistance in puerperal women who had received sd-NVP during labor, and all other reports on HIV drug resistance were conducted in either pediatric or adult (non-pregnant) populations [11, 21–23]. This study showed that about 14% of women with detectable viral load at delivery had at least one HIVDRM at enrolment, 10% even before initiating ARV drugs. On the other hand, 3% of the women without HIVDRM at enrolment developed at least one HIVDRM to first line ARV drugs while on pMTCT or ART during pregnancy. Thus, virological failure may occur despite a low proportion of women with pre-existance or acquired HIVDRM, indicating that other factors,—lack of adherence to treatment or prophylaxis, or interruptions of the ANC ‘cascade’, may play a role in explaining the lack of viral suppression at the time of delivery in women receiving ARV drugs during pregnancy.
The prevalence of pre-treatment HIVDRM in this study was 10%, which is higher than that reported (5%) in a previous study carried out in adults in the capital, Maputo, and higher than the 7% found in HIV-infected pregnant women in the south-Africa neighboring region of Kwazulu-Natal [24]. Reports from other countries in the region show prevalences ranging from 5% in Malawi to 12% in Tanzania [24–26]. The prevalence found here exceeds the WHO recommended threshold of 5% transmitted HIV drug resistances at a population-level [9, 27]. Though prior to 2007, most African countries reported transmitted resistance prevalence estimates of <5%, attributed mainly to low ARV drugs coverage, a trend towards increasing prevalence has been observed correlating with time since the initiation of ARV drugs rollout [28, 29]. However, in this study the prevalence of pre-treatment HIVDRM at enrolment may have been over-estimated since only women with detectable viral load at delivery were selected. It is important, to highlight that the use of an ultrasensitive genotyping in this study might have increased the detection of HIVDRM compared to other studies using less sensitive techniques. It is also possible that exposure to ARV drugs for pMTCT in previous pregnancies in study participants could have favored a higher prevalence of resistance compared to the general population. Though differences were not significant, the proportion of multigravidae was higher among women who did not present HIVDRM compared to those who did at enrolment, suggesting that exposure to ARVs in previous pregnancies might not have been frequent among this group. Up to the time of this study, pMTCT in low-income countries consisted of simple regimens, which, combined with lack of virological monitoring or resistance testing, could have fostered the emergence of HIVDRM in pregnancy [30]. Despite the long duration of administration of sdNVP as part of pMTCT in Mozambique, HIV susceptibility to NVP was preserved both at enrolment and at delivery in almost all study women. Although still recommended in the current WHO guidelines, NVP is being phased out from first-line recommended regimens. Similarly, older NRTIs such as the thymidine analogue drugs (AZT and D4T) are being replaced by TDF as part of the NRTI backbone in first-line regimens in resource-limited settings. Our data shows that HIV resistance to regimens used to prevent MTCT is still very limited in the Manhiça district of Mozambique. Moreover, we did not observe a major degree of selection of HIV resistance during pMTCT. Thereby, our findings support current WHO recommendations for the region.
At enrolment, no significant differences were found between women presenting HIVDRM and those who did not. However, among women who had HIVDRM at enrolment there was an increased proportion of adolescents and primigravidae, whom are less likely to have been exposed previously to ARVs, than in those with no HIVDRM. This is in line with our hypothesis that previous exposure to ARV drugs among study participants was low. At the time of delivery, among women that presented HIVDRM, CD4 cell counts were lower and mean viral load was higher compared to women with no HIVDRM. More women transmitted HIV to their infants among those without HIVDRM despite having lower viral loads at the time of delivery. This difference was not statistically significant and may be just due to chance given the small numbers. Other factors favoring MTCT, such as type of delivery, breastfeeding options or uptake of infant prophylaxis for pMTCT were not evaluated. In addition, among the women with HIVDRM at delivery, a higher proportion had been on ART during pregnancy compared to those with no HIVDRM. Poor adherence to ART in those that were already on treatment before pregnancy could explain the presence of drug resistance in this group. In women who received pMTCT, duration of exposure to ARV drugs might have been insufficient to promote the emergence of resistances within the current pregnancy, despite a poor adherence.
In this study, all HIVDRM that emerged during pregnancy were minority mutants. The clinical implications of these minority mutations on developing virological failure in these participants cannot be identified, as all participants had virological failure at delivery as per study inclusion criteria. However, there is evidence suggesting an association between the minority mutations and the risk of virological failure to first-line NNRTI therapy. Similarly, in women in low and middle-income countries (LMIC) previously exposed to NNRTI as regimens to prevent mother-to-child transmission (pMTCT), detection of NNRTI minority mutations also proved to be clinically relevant, also leading to increased risk of virological failure [31–33]. Contrarily, no clinical implications have been reported in the presence of minority resistances when PI-based regimens are used [34]. Further studies should be performed for assessing the impact of current recommended drug-regimens under the Option B+ approach.
The Mozambican national ethics review committee and the Data and Safe Monitoring Board (DSMB) of the trial requested the investigation of the increased viral load and MTCT of HIV in the women who received MQ compared to those who received placebo. This explains the low number of samples in which viral RNA could be amplified, since samples were not stored in the conditions required for this purpose. On the other hand, the limited sample size did not allow making inferences as to whether a possible association between the administration of MQ and the development of HIVDRM during pregnancy, could have explained the observed increased risk of MTCT in the women who received MQ. There is still insufficient information on the potential interactions between antimalarial and ARV drugs [35, 36]. Drug interactions could favor the selection of HIV drug resistance by reducing the plasma concentration of ARV drugs to suboptimal levels. Artemether/lumefantrine for example, has been shown to reduce significantly plasma levels of NVP [37]. In addition, the existing information is mainly limited to artemisinin derivatives [37, 38]. A recent study reported potential pharmacokinetic interactions between MQ-Artesunate (MQ-AS) and lopinavir (LPV/r) in adults, with a reduction in systemic exposure of both drug combinations, suggesting a higher risk of treatment failure for both malaria and HIV infection when the two drugs are co-administered [39]. Another limitation of our study was that data on adherence and on average duration of ARV drugs administration before delivery, which are known to determine viral suppression, was not available. Finally, as the scope of this study was focused on addressing HIVDRM in pregnant women, NGS was not performed in HIV-infected infants. This limits the ability of this study to inform on potential vertical transmission of HIVDRM and should be considered when performing future studies aiming to guide maternal and pediatric care and HIV/AIDS control strategies.
Conclusions
The latest WHO recommendation of providing all pregnant women with lifelong ART regardless of CD4 count/disease stage (option B+), seems to offer important programmatic and operational advantages and thus, could accelerate progress towards eliminating new pediatric infections. Now that global elimination of HIV pediatric infection is within our reach making NGS accessible for HIV drug resistance surveillance also in LMICs should be a priority considering the increasing HIV-1 resistance and the limited availability of treatment options in these settings. HIV drug resistance surveillance to evaluate the risks for selection of drug resistance under the B+ approach should be performed alongside implementation at the country level.
Supporting information
(DOCX)
(DOCX)
(PDF)
Acknowledgments
We are grateful to all the study participants and to all the staff from the Manhiça District Hospital and the Centro de Investigação em Saúde de Manhiça who contributed to the study.
Data Availability
Data cannot be made publicly available due to ethical restrictions and restrictions in the consent forms signed by participants. Data will be available to all interested researchers upon request, from the Malaria in Pregnancy Preventive Alternative Drugs (MiPPAD) executive committee (for more information please refer to http://www.isglobal.org/). Requests should be submitted to the co-author Dr. Clara Menéndez (clara.menendez@isglobal.org) as she is the coordinator of the Malaria in Pregnancy Preventive Alternative Drugs (MiPPAD) study, and other committee members are unable to field data requests.
Funding Statement
This work was supported by the European Developing Countries Clinical Trials Partnership (EDCTP; IP.2007.31080.002) to Clara Menéndez, the Malaria in Pregnancy Consortium and the following national agencies: Instituto de Salud Carlos III (PI08/0564), Spain to Clara Menéndez. Raquel González and María Rupérez were partially supported by grants from the Spanish Ministry of Health (ref. CM07/0015 and CM11/00278, respectively). The CISM receives core funding from the Spanish Agency for International Cooperation (AECI). Manhiça District Hospital HIV programme receives funding from the Agencia Catalana de Cooperació al Desenvolupament. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. Global update on HIV treatment 2013:results, impacts and opportunities Geneva: World Health Organization; 2013. [Google Scholar]
- 2.UNAIDS/WHO. Progress Report on the Global Plan towards elimination of new HIV infections among children by 2015 and keeping their mothers alive. In: JC2509/2/E JUNPoHAaWHOU, editor. 2013.
- 3.Zaba B, Calvert C, Marston M, Isingo R, Nakiyingi-Miiro J, Lutalo T, et al. Effect of HIV infection on pregnancy-related mortality in sub-Saharan Africa: secondary analyses of pooled community-based data from the network for Analysing Longitudinal Population-based HIV/AIDS data on Africa (ALPHA). Lancet. 2013;381(9879):1763–71. Epub 2013/05/21. doi: 10.1016/S0140-6736(13)60803-X . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John GC, Nduati RW, Mbori-Ngacha DA, Richardson BA, Panteleeff D, Mwatha A, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis. 2001;183(2):206–12. Epub 2000/12/20. doi: 10.1086/317918 . [DOI] [PubMed] [Google Scholar]
- 5.Kourtis AP, Bulterys M, Nesheim SR, Lee FK. Understanding the timing of HIV transmission from mother to infant. JAMA. 2001;285(6):709–12. Epub 2001/02/15. . [DOI] [PubMed] [Google Scholar]
- 6.Arvold ND, Ngo-Giang-Huong N, McIntosh K, Suraseranivong V, Warachit B, Piyaworawong S, et al. Maternal HIV-1 DNA load and mother-to-child transmission. AIDS Patient Care STDS. 2007;21(9):638–43. Epub 2007/10/09. doi: 10.1089/apc.2006.0169 . [DOI] [PubMed] [Google Scholar]
- 7.Mock PA, Shaffer N, Bhadrakom C, Siriwasin W, Chotpitayasunondh T, Chearskul S, et al. Maternal viral load and timing of mother-to-child HIV transmission, Bangkok, Thailand. Bangkok Collaborative Perinatal HIV Transmission Study Group. AIDS. 1999;13(3):407–14. Epub 1999/04/13. . [DOI] [PubMed] [Google Scholar]
- 8.Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. The Cochrane database of systematic reviews. 2011;(7):Cd003510 Epub 2011/07/08. doi: 10.1002/14651858.CD003510.pub3 . [DOI] [PubMed] [Google Scholar]
- 9.WHO. WHO HIV drug resistance report 2012 In: Programme HA, editor.: World Health Oganization; 2012. [Google Scholar]
- 10.WHO. Mozambique: WHO statistical profile World Health Organization; 2013. [Google Scholar]
- 11.Ruperez M, Pou C, Maculuve S, Cedeno S, Luis L, Rodriguez J, et al. Determinants of virological failure and antiretroviral drug resistance in Mozambique. J Antimicrob Chemother. 2015;70(9):2639–47. doi: 10.1093/jac/dkv143 . [DOI] [PubMed] [Google Scholar]
- 12.Vaz P, Augusto O, Bila D, Macassa E, Vubil A, Jani IV, et al. Surveillance of HIV drug resistance in children receiving antiretroviral therapy: a pilot study of the World Health Organization's generic protocol in Maputo, Mozambique. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54 Suppl 4:S369–74. Epub 2012/05/11. doi: 10.1093/cid/cis006 ; PubMed Central PMCID: PMCPMC3572867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez R, Desai M, Macete E, Ouma P, Kakolwa MA, Abdulla S, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-infected women receiving cotrimoxazole prophylaxis: a multicenter randomized placebo-controlled trial. PLoS medicine. 2014;11(9):e1001735 Epub 2014/09/24. doi: 10.1371/journal.pmed.1001735 ; PubMed Central PMCID: PMCPmc4172537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacoor C, Nhacolo A, Nhalungo D, Aponte JJ, Bassat Q, Augusto O, et al. Profile: Manhica Health Research Centre (Manhica HDSS). International journal of epidemiology. 2013;42(5):1309–18. Epub 2013/10/26. doi: 10.1093/ije/dyt148 . [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez R, Augusto OJ, Munguambe K, Pierrat C, Pedro EN, Sacoor C, et al. HIV Incidence and Spatial Clustering in a Rural Area of Southern Mozambique. PloS one. 2015;10(7):e0132053 Epub 2015/07/07. doi: 10.1371/journal.pone.0132053 ; PubMed Central PMCID: PMCPMC4493140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calverton M, EUA:INS,INE e ICF. Inquérito Nacional de Prevalência, Riscos Comportamentais e Informaçao sobre o HIV e SIDA em Moçambique 2009. In: Instituto-Nacional-de-, Instituto-Nacional-de-Saúde E, editors. 2010.
- 17.Bila DC, Young P, Merks H, Vubil AS, Mahomed M, Augusto A, et al. Evolution of primary HIV drug resistance in a subtype C dominated epidemic in Mozambique. PloS one. 2013;8(7):e68213 Epub 2013/08/13. doi: 10.1371/journal.pone.0068213 ; PubMed Central PMCID: PMCPMC3728366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahuerta M, Aparicio E, Bardaji A, Marco S, Sacarlal J, Mandomando I, et al. Rapid spread and genetic diversification of HIV type 1 subtype C in a rural area of southern Mozambique. AIDS research and human retroviruses. 2008;24(2):327–35. Epub 2008/02/15. doi: 10.1089/aid.2007.0134 . [DOI] [PubMed] [Google Scholar]
- 19.Saúde RdMMd. Guia de tratamento antiretroviral e infecções oportunistas no adulto, adolescente, gràvida e criança 2014. In: Médica DNdA, editor. Moçambique2014.
- 20.Casadella M, Noguera-Julian M, Sunpath H, Gordon M, Rodriguez C, Parera M, et al. Treatment options after virological failure of first-line tenofovir-based regimens in South Africa: an analysis by deep sequencing. Aids. 2016;30(7):1137–40. Epub 2016/01/26. doi: 10.1097/QAD.0000000000001033 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaz P, Chaix ML, Jani I, Macassa E, Bila D, Vubil A, et al. Risk of extended viral resistance in human immunodeficiency virus-1-infected Mozambican children after first-line treatment failure. The Pediatric infectious disease journal. 2009;28(12):e283–7. Epub 2009/11/13. doi: 10.1097/INF.0b013e3181ba6c92 . [DOI] [PubMed] [Google Scholar]
- 22.Bila DC, Boullosa LT, Vubil AS, Mabunda NJ, Abreu CM, Ismael N, et al. Trends in Prevalence of HIV-1 Drug Resistance in a Public Clinic in Maputo, Mozambique. PloS one. 2015;10(7):e0130580 Epub 2015/07/08. doi: 10.1371/journal.pone.0130580 ; PubMed Central PMCID: PMCPMC4494809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Micek MA, Dross S, Blanco AJ, Beck IA, Matunha L, Seidel K, et al. Transmission of nevirapine-resistant HIV type 1 via breast milk to infants after single-dose nevirapine in Beira, Mozambique. J Infect Dis. 2014;210(4):641–5. Epub 2014/03/07. doi: 10.1093/infdis/jiu130 ; PubMed Central PMCID: PMCPmc4133577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parikh UM, Kiepiela P, Ganesh S, Gomez K, Horn S, Eskay K, et al. Prevalence of HIV-1 drug resistance among women screening for HIV prevention trials in KwaZulu-Natal, South Africa (MTN-009). PloS one. 2013;8(4):e59787 Epub 2013/04/16. doi: 10.1371/journal.pone.0059787 ; PubMed Central PMCID: PMCPMC3621859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vairo F, Nicastri E, Liuzzi G, Chaula Z, Nguhuni B, Bevilacqua N, et al. HIV-1 drug resistance in recently HIV-infected pregnant mother's naive to antiretroviral therapy in Dodoma urban, Tanzania. BMC infectious diseases. 2013;13:439 Epub 2013/09/24. doi: 10.1186/1471-2334-13-439 ; PubMed Central PMCID: PMCPMC3849050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wadonda-Kabondo N, Banda R, Moyo K, M'Bang'ombe M, Chiwaula M, Porter C, et al. Prevalence of transmitted HIV drug resistance among newly diagnosed antiretroviral therapy-naive pregnant women in Lilongwe and Blantyre, Malawi. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54 Suppl 4:S324–7. Epub 2012/05/11. doi: 10.1093/cid/cir993 ; PubMed Central PMCID: PMCPMC3338309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett DE, Myatt M, Bertagnolio S, Sutherland D, Gilks CF. Recommendations for surveillance of transmitted HIV drug resistance in countries scaling up antiretroviral treatment. Antiviral therapy. 2008;13 Suppl 2:25–36. Epub 2008/06/26. . [PubMed] [Google Scholar]
- 28.Hamers RL, Wallis CL, Kityo C, Siwale M, Mandaliya K, Conradie F, et al. HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. The Lancet Infectious diseases. 2011;11(10):750–9. Epub 2011/08/02. doi: 10.1016/S1473-3099(11)70149-9 . [DOI] [PubMed] [Google Scholar]
- 29.Gupta RK, Jordan MR, Sultan BJ, Hill A, Davis DH, Gregson J, et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet. 2012;380(9849):1250–8. Epub 2012/07/26. doi: 10.1016/S0140-6736(12)61038-1 ; PubMed Central PMCID: PMCPmc3790969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paredes R, Marconi VC, Lockman S, Abrams EJ, Kuhn L. Impact of antiretroviral drugs in pregnant women and their children in Africa: HIV resistance and treatment outcomes. J Infect Dis. 2013;207 Suppl 2:S93–100. Epub 2013/05/25. doi: 10.1093/infdis/jit110 ; PubMed Central PMCID: PMCPMC3657116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boltz VF, Bao Y, Lockman S, Halvas EK, Kearney MF, McIntyre JA, et al. Low-frequency nevirapine (NVP)-resistant HIV-1 variants are not associated with failure of antiretroviral therapy in women without prior exposure to single-dose NVP. J Infect Dis. 2014;209(5):703–10. Epub 2014/01/21. doi: 10.1093/infdis/jit635 ; PubMed Central PMCID: PMCPMC3923545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362(9387):859–68. Epub 2003/09/19. doi: 10.1016/S0140-6736(03)14341-3 . [DOI] [PubMed] [Google Scholar]
- 33.Johnson JA, Li JF, Morris L, Martinson N, Gray G, McIntyre J, et al. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J Infect Dis. 2005;192(1):16–23. Epub 2005/06/09. doi: 10.1086/430741 . [DOI] [PubMed] [Google Scholar]
- 34.Casadella M, Paredes R. Deep sequencing for HIV-1 clinical management. Virus research. 2017;239:69–81. Epub 2016/11/08. doi: 10.1016/j.virusres.2016.10.019 . [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez R, Ataide R, Naniche D, Menendez C, Mayor A. HIV and malaria interactions: where do we stand? Expert review of anti-infective therapy. 2012;10(2):153–65. Epub 2012/02/22. doi: 10.1586/eri.11.167 . [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez R, Sevene E, Jagoe G, Slutsker L, Menendez C. A Public Health Paradox: The Women Most Vulnerable to Malaria Are the Least Protected. PLoS medicine. 2016;13(5):e1002014 Epub 2016/05/04. doi: 10.1371/journal.pmed.1002014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byakika-Kibwika P, Lamorde M, Mayito J, Nabukeera L, Namakula R, Mayanja-Kizza H, et al. Significant pharmacokinetic interactions between artemether/lumefantrine and efavirenz or nevirapine in HIV-infected Ugandan adults. J Antimicrob Chemother. 2012;67(9):2213–21. Epub 2012/06/13. doi: 10.1093/jac/dks207 ; PubMed Central PMCID: PMCPMC3465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiang TK, Wilby KJ, Ensom MH. Clinical pharmacokinetic drug interactions associated with artemisinin derivatives and HIV-antivirals. Clinical pharmacokinetics. 2014;53(2):141–53. Epub 2013/10/26. doi: 10.1007/s40262-013-0110-5 . [DOI] [PubMed] [Google Scholar]
- 39.Rattanapunya S, Cressey TR, Rueangweerayut R, Tawon Y, Kongjam P, Na-Bangchang K. Pharmacokinetic interactions between artesunate-mefloquine and ritonavir-boosted lopinavir in healthy Thai adults. Malaria journal. 2015;14(1):400 Epub 2015/10/11. doi: 10.1186/s12936-015-0916-8 ; PubMed Central PMCID: PMCPMC4600319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(PDF)
Data Availability Statement
Data cannot be made publicly available due to ethical restrictions and restrictions in the consent forms signed by participants. Data will be available to all interested researchers upon request, from the Malaria in Pregnancy Preventive Alternative Drugs (MiPPAD) executive committee (for more information please refer to http://www.isglobal.org/). Requests should be submitted to the co-author Dr. Clara Menéndez (clara.menendez@isglobal.org) as she is the coordinator of the Malaria in Pregnancy Preventive Alternative Drugs (MiPPAD) study, and other committee members are unable to field data requests.