ABSTRACT
Objective:
to evaluate the effects of trolamine in the prevention or treatment of radiation dermatitis.
Method:
systematic review and meta-analysis. Detailed individual search strategies for Cinahl, Cochrane Library Central, LILACS, PubMed, and Web of Science were developed in January 2016. A manual search was also performed to find additional references. A grey literature search was executed by using Google Scholar. Two researchers independently read the titles and abstracts from every cross-reference. The risk of bias of the included studies was analyzed by the Cochrane Collaboration Risk of Bias Tool. The quality of evidence and grading of strength of recommendations was assessed using Grades of Recommendation, Assessment, Development and Evaluation (GRADE).
Results:
seven controlled clinical trials were identified. The controls used were calendula, placebo, institutional preference / usual care, Aquaphor®, RadiaCare™, and Lipiderm™. The studies were pooled using frequency of events and risk ratio with 95% confidence intervals, in subgroups according to radiation dermatitis graduation.
Conclusion:
based on the studies included in this review, trolamine cannot be considered as a standardized product to prevent or treat radiation dermatitis in patients with breast and head and neck cancer.
Descriptors: Review, Radiodermatitis, Skin Care, Radiotherapy, Nursing
Introduction
The most common effect of radiotherapy is radiation dermatitis, which has greater impact in patients with head and neck and breast cancer 1 . About 80 to 90% of these patients treated by radiotherapy experience radiation dermatitis during treatment 2 - 3 .
The skin is an organ with high radiosensitivity and susceptible to damage by radiotherapy due to rapid cell proliferation and maturation. The epidermis loses a percentage of its basal cell exposure beginning at the first fractionated dose of radiotherapy, and the repeated exposure of the subsequent fractions leads to continuous cell destruction, which avoids tissue repair 4 .
Although the skin damage starts after the first exposure to radiation, the clinical signs are often present from the second week of radiotherapy. They are characterized by mild erythema, which can develop to dry or moist desquamation, and ulcerations in some cases 5 - 6 .
Acute skin reactions generate local discomfort, itching and varied degrees of pain that impact the quality of life of patients and affect the therapeutic efficacy and the planning of radiotherapy, considering that severe intensity lesions can cause interruption of treatment 1 , 7 .
Trolamine has been indicated to prevent and treat radiation dermatitis but, to the best of our knowledge, there is no systematic review that evaluated trolamine as a potential topical product to manage skin reactions due to radiotherapy.
Background
Skin reactions may be intensified, according to the treatment plan received, a full high dose, fractional high dose, and the extension of the irradiated area. Chemotherapy and patient related factors, such as age, skin color, smoking habits and obesity also aggravate the skin reactions 6 , 8 .
Topical products are commonly used as alternatives to manage skin reactions due to radiotherapy, although there is insufficient evidence regarding skin care products for the prevention or treatment of radiation dermatitis 6 .
Topical application of emulsions containing trolamine has bee used in clinical practice for more than three decades in Europe and in the United States for the management of radiation dermatitis. Trolamine has the capacity to heal through the recruitment of macrophages to the wound, promoting the growth of granulation tissue 9 . Trolamine emulsion is a compound with properties similar to nonsteroidal anti-inflammatory agents and has been considered as a safe and tolerable topical intervention, with low potential to develop contact dermatitis. Trolamine promotes skin hydration and reduces discomfort and pain, which contribute to the non-interruption of treatment 9 .
The evidence and clinical observations demonstrate the advantages and disadvantages between trolamine and other topical products, including steroidal creams, non-steroidal anti-inflammatory compounds, and antihistamines 1 , 10 .
The aim of this study is to systematically review the literature about the evidence of trolamine compared to other topical products in the prevention and treatment of acute radiation dermatitis in cancer patients.
Method
Protocol and registration
The reporting of this systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses PRISMA Checklist 11 . The systematic review protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO), registration number CRD42016032805 12 .
Eligibility criteria
Only original prospective studies in which the objective was to investigate the effects of the use of trolamine as the only active ingredient (without associations) to prevent and treat acute radiation dermatitis compared to other topical products in cancer patients undergoing radiotherapy were eligible. Studies published in Portuguese, English, Spanish, and French were included. There were no restrictions to the year of publication. The age of the participants, sex, previous or concurrent therapies, health status or dosage of treatment was not restricted either.
Studies were excluded for the following reasons: 1. cobalt therapy; 2. studies that compared interventions to chronic radiation dermatitis only; 3. trolamine associated with others compounds; 4. trolamine compared with non-topical products; 5. study design: reviews, letters, conference abstracts, personal opinions, book chapter, retrospective study, descriptive study, case reports or case series.
Information sources and search strategy
Studies were identified using a search strategy adapted for each electronic database, with the aid of a health sciences librarian: CINAHL EBSCO, Cochrane Central Register of Controlled Trials (CENTRAL), LILACS, PubMed, and Web of Science. The hand search was performed on the reference lists from the selected articles for any additional references that might have been missed in the electronic search. In addition, a grey literature search was performed using Google Scholar.
We used the following search terms to search PubMed and adapted the strategy for the other databases: (“biafine” OR “triethanolamine” OR “trolamine” OR “trolamine emulsion” OR “emulsion containing trolamine”) AND (“radiodermatitis” OR “dermatitis” OR “radiation dermatitis” OR “radio-dermatitis” OR “skin damage” OR “skin toxicity” OR “skin reaction” OR “skin injuries” OR “radiation reaction” OR “radio-epithelitis” OR “acute skin toxicity” OR “acute skin reaction” OR “acute dermatitis” OR “acute radiodermatitis” OR “acute cutaneous toxicity” OR “acute radiation dermatitis” OR “acute radiation reactions” OR “acute radiation-induced skin reactions” OR “radiation-induced acute skin” OR “radiation induced skin injuries” OR “radiation-induced skin reaction” OR “radiation induced dermatitis” OR “radio-induced damage” OR “radiotherapy-induced skin reactions” OR “radiation skin reactions” OR “radiation-induced skin injuries”).
After obtaining all references, duplicates were excluded by using appropriate software (EndNoteBasic®, Thomson Reuters, USA). All the electronic database searches were undertaken on January 18th, 2016.
Study selection
For the phase of screening and data extraction, ©Covidence (Web-based systematic review tool designed to facilitate the process) was used.
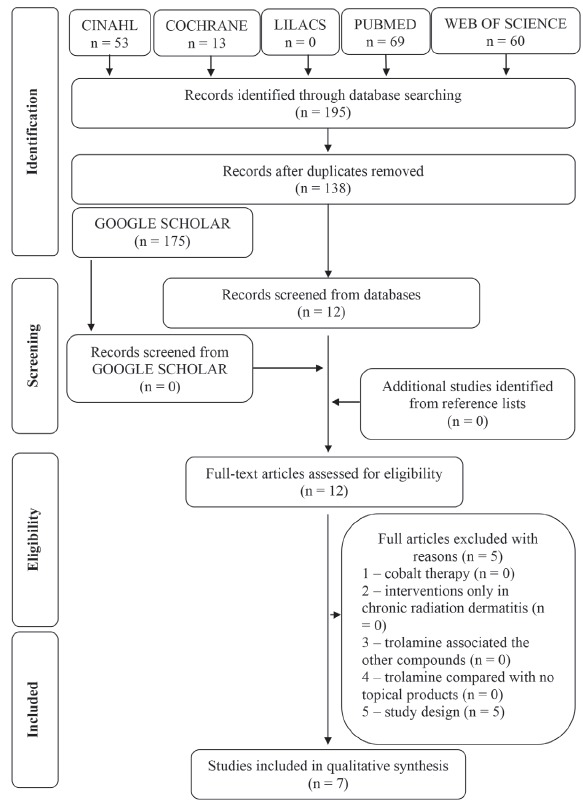
The study selection was conducted in two phases. In phase 1, two investigators (A.G.M. and E.B.F.) independently screened the titles and abstracts of potentially relevant studies and selected articles that appeared to meet the inclusion criteria based on their abstracts. In phase 2, the same reviewers independently read the full-text of all selected articles and excluded studies that did not meet the inclusion criteria. Any disagreements, either in the first or second phases, were resolved by discussion and mutual agreement between the two reviewers. In case a consensus could not be reached, a third author (P.E.D.R.) was involved to make a final decision. Studies that were excluded after full-text assessment and the reasons for their exclusion are listed in Figure 1.
Figure 1. Flow diagram of literature search and selection process. Brasília, DF, Brazil, 2016.
Data collection process and items
Two investigators (A.G.M. and E.B.F.) independently collected the data from the selected articles: study characteristics (author(s), year of publication, setting, objectives, methods), population characteristics (sample size, age, irradiated area), intervention characteristics (groups, follow-up period, primary outcomes, radiation dermatitis criteria and statistical analysis), and outcome characteristics (main results). The third author (P.E.D.R.) crosschecked all the retrieved information to make a final decision. If the required data were not complete, attempts were made to contact the authors to retrieve any pertinent missing information.
Risk of bias in individual studies
To assess the risk of bias of the included randomized controlled trials (RCT), the Cochrane Collaboration Risk of Bias Tool 13 was applied, including judgments about the sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective reporting, and other sources of bias. The risk of bias was assessed as low, high or unclear. Two investigators performed this process independently (A.G.M. and E.B.F.). Disagreements between the 2 reviewers were resolved by a third investigator (P.E.D.R.).
Summary measures
The primary outcome was the development of different grades of radiation dermatitis or the reduction of the intensity/degree of reaction. Further measures considered in this review were risk ratio (RR) or risk differences for dichotomous outcomes.
Synthesis of results
The overall data combination of the included studies was performed by a descriptive synthesis. Statistical pooling of data using meta-analysis was planned whenever trials were considered combinable and relatively homogeneous in relation to design, interventions and outcomes. Heterogeneity within studies was evaluated either by considering clinical (differences about participants, type of controls and results), methodological (design and risk of bias) and statistical (effect of studies) characteristics or by using the I2 statistical test. A value from 0 to 40% was considered of not important consistency, between 30 and 60% moderate heterogeneity, whereas 50 to 90% was considered to represent substantial heterogeneity 13 .
The Cochrane Collaboration´s Review Manager®5 (RevMan 5) was used to summarize the results by means of the Mantel-Haenszel model. The results were presented with 95% confidence intervals (95% CI).
Risk of bias across studies
The quality of evidence and grading of the strength of recommendations was assessed using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) 14 - 15 . The criteria for this assessment were study design, risk of bias, inconsistency, indirectness, imprecision and other considerations. The quality of evidence must be characterized as high, moderate, low, or very low 15 .
No funnel plot was constructed to assess the possibility of publication bias because there were few trials per subgroups of meta-analysis.
Results
Study Selection
In phase 1 of study selection, 195 citations were identified across five electronic databases. After the duplicated articles were removed, 138 citations remained. No references from grey literature were added. A thorough screening of the titles and abstracts was completed and 126 references were excluded. A manual search from the reference lists of the identified studies yielded no additional studies. Thus, 12 articles remained for a full-text screening (phase 2). This process led to the exclusion of five studies (Figure 1). In total, seven articles 16 - 22 were selected for data extraction and qualitative synthesis (Figure 2). Figure 1 (flow chart) details the process of identification, inclusion and exclusion of studies with reasons.
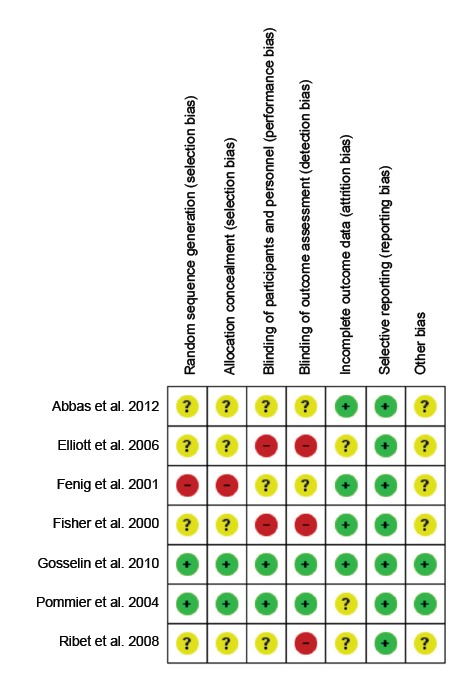
Figure 2. Risk of bias assessment for individual studies. Brasília, DF, Brazil, 2016.
Study characteristics
The studies were published in English 16 - 19 , 21 - 22 and French 20 , from 2000 to 2012.
Two studies included patients who also underwent concurrent chemotherapy 19 , 22 . Radical radiotherapy has been reported in five studies 16 - 18 , 20 - 21 . The use of tamoxifen has been described in only one study, among those including patients with breast cancer 17 .
Two studies 19 , 22 included only head and neck cancer patients, and four studies 16 - 18 , 21 included only breast cancer patients in the sample. Only one 20 of the selected studies included a heterogeneous sample of patients with different cancer types and irradiated areas: breast and head and neck cancer.
All studies evaluated trolamine as an intervention to prevent radiation dermatitis and only one evaluated trolamine as treatment 19 . The topical controls were usual care/institution routine 16 , 19 , 22 , calendula 18 , water thermal gel 20 , placebo, Aquaphor®, RadiaCare™ 21 , Lipiderm and no intervention 17 .
Table 1 summarizes the descriptive characteristics of the studies.
Table 1. Summary of descriptive characteristics of included articles (n=7). Brasília, DF, Brazil, 2016.
| Study characteristics | Population characteristics | Intervention characteristics | Outcome characteristics | ||||||
| Year, Country | Objective | Total n Irradiated Area | Age Mean (years) | Intervention (n) | Control (n) | Follow-Up (months) | Primary outcomes | RD* Criteria | Main Results |
| 2012( 22 ) Egypt | To compare trolamine with usual care for patients with head and neck cancer undergoing radiotherapy with concurrent chemotherapy | 30 Head and neck | 54.5 | Trolamine emulsion (15) | Usual care (15) | 16 | Development of mild reaction (grades 1 and 2), and higher-grade RD* | RTOG† Acute Radiation Toxicity Criteria | Grade 1-2 TA‡: 80% (12/15) CA§: 46.6% (7/15) P< 0.01 Grade 3 TA‡: 20% (3) CA§: 53.4% (8) P<0.01 Grade 4: none |
| 2006( 19 ) Canada | To compare trolamine emulsion, as a prophylactic agent and as an interventional agent, with declared institutional preference in reducing the incidence of higher-grade RD* | 494 Head and neck | 59.0 | Trolamine emulsion Prevention (163) Treatment (172) | Institutional preference (159) | 19 | Reduction of grade 2 or higher RD* | NCI/CTC|| version 2.0 ONS¶ - toxicity scoring system | Grade 0** TA‡: 3% (5/163) CA§: 1% (2/159) Grade 1 TA‡: 18% (30/163) CA§: 20% (31/159) Grade 2 TA‡: 54% (88/163) CA§: 57% (90/159) Grade 3 TA‡: 21% (35/163) CA§: 20% (31/159) Grade 4 TA‡: 3% (5/163) CA§: 3% (5/159) P = 0.82 |
| 2001( 17 ) Israel | To evaluate the effectiveness of Biafine and Lipiderm in preventing RD* | 75 Breast | 69 | Biafine (25) | Lipiderm (24) Control (25) | - | Incidence of RD* | RTOG† | Grade 3-4 reaction†† TA‡: 25% (6/25) Lipiderm: 23% (5/24) Control: 25% (6/25) P = 0.98 |
| 2000( 16 ) United States of America | To compare Biafine to best supportive care in preventing RD* | 140 Breast | 61 | Trolamine (66) | Best supportive care (74) | 4 | Prevention or reduction of RD* - Time to develop grade 2 or high skin toxicity | RTOG† | Grade 0 TA‡: 9% (6/66) CA§: 7% (5/74) Grade 1 TA‡: 50% (33/66) CA§: 58% (43/74) Grade 2 TA‡: 41% (27/66) CA§: 32% (24/74) Grade 3 TA‡: 0% (0/66) CA§: 3% (2/74) |
| 2010( 21 ) United States of America | To evaluate three commonly used skin care products for women receiving whole-breast radiotherapy against a placebo | 208 Breast | Placebo 55.8 Aquaphor® 54.8 Biafine® RE 56 RadiaCare™ 55.6 | Trolamine (Biafine® ) (53) | Placebo (49) Aquaphor® (53) RadiaCare™ (53) | 48 | Prevention or reduction of RD* | RTOG† | Grade 2 to 4‡‡ TA‡: 90% (47.7/53) Placebo: 80% (39.2/49) Aquaphor®: 80% (42.4/53) RadiaCare™ 72% (38.16/53) |
| 2004( 18 ) France | To assess the effectiveness of calendula for the prevention of acute RD* of grade 2 or higher during postoperative radiotherapy for breast cancer, compared with trolamine | 254 Breast | Calendula 56.5 Trolamine 55.1 | Trolamine (128) | Calendula (126) | 20 | Occurrence of acute RD* of grade 2 or higher | RTOG† | Grade 2 to 3 TA‡: 63% (95% CI§§, 59 to 68) CA§: 41% (95% CI§§, 37 to 46) P < 0.001 Grade 4: none |
| 2008( 20 ) France | To evaluate the efficacy and tolerance Avène Thermal Spring Water anti burning gel versus trolamine cream in the prevention of RD* | 69 Head and neck Breast | 57.9 | Trolamine cream (34) | Avène Thermal Spring Water anti burning gel (35) | - | Time to onset of the first signs of RD* | National Cancer Institute | Grade 0 TA‡: 24.1% (7/29) CA§: 23.3% (7/30) Grade 1 TA‡: 34.5% (10/29) CA§: 46.7% (14/30) Grade 2 TA‡: 34.5% (10/29) CA§: 26.7% (8/30) P = 0.347 |
*RD: Radiation Dermatitis; †RTOG: Radiation Therapy Oncology Group; ‡TA: Trolamine Arm; §CA: Control Arm; ||NCI/CTC: National Cancer Institute/Common Toxicity Criteria; ¶ONS: Oncology Nursing Society; **Prevention group; ††Nurse’s impression; ‡‡Data calculated by review authors; §§CI: Confidence Interval.
Risk of bias within studies
The risk of bias was analyzed individually in all studies included. One randomized clinical trial was graded as having a low risk of bias in the six domains assessed 21 (Figure 2). Four studies 16 , 19 - 20 , 22 exhibited an unclear risk of selection bias due to the poor description of the randomization strategy. One of the studies 17 had a high risk of bias due to randomization description of the inclusion of participants in the intervention groups consecutively. The domain “selective reporting” showed predominantly low risk of bias in the evaluation of the studies (100%).
Four studies were classified as high risk of bias because they contained one or more compromised domains 16 - 17 , 19 - 20 . Two studies were classified as uncertain risk of bias 18 , 22 . One of them received positive bias ratings, with low risk of bias in 91% of the evaluated domains 18 . Only one study presented low risk of bias in all domains evaluated 21 , allowing us to ascribe the results of the study as of increased reliability.
Results of individual studies
The studies used trolamine to prevent or treat radiation dermatitis and reported different results for all 7 articles. Characteristics and results of the included studies are listed in Table 1.
Synthesis of results
Regarding the rating scales, five studies used exclusively the RTOG scale (71.4%) 16 - 18 , 21 - 22 , one of them used only NCI-CTC (14,1%) 20 , and one study used both NCI-CTC and ONS scales to assess the skin reactions of their patients 19 .
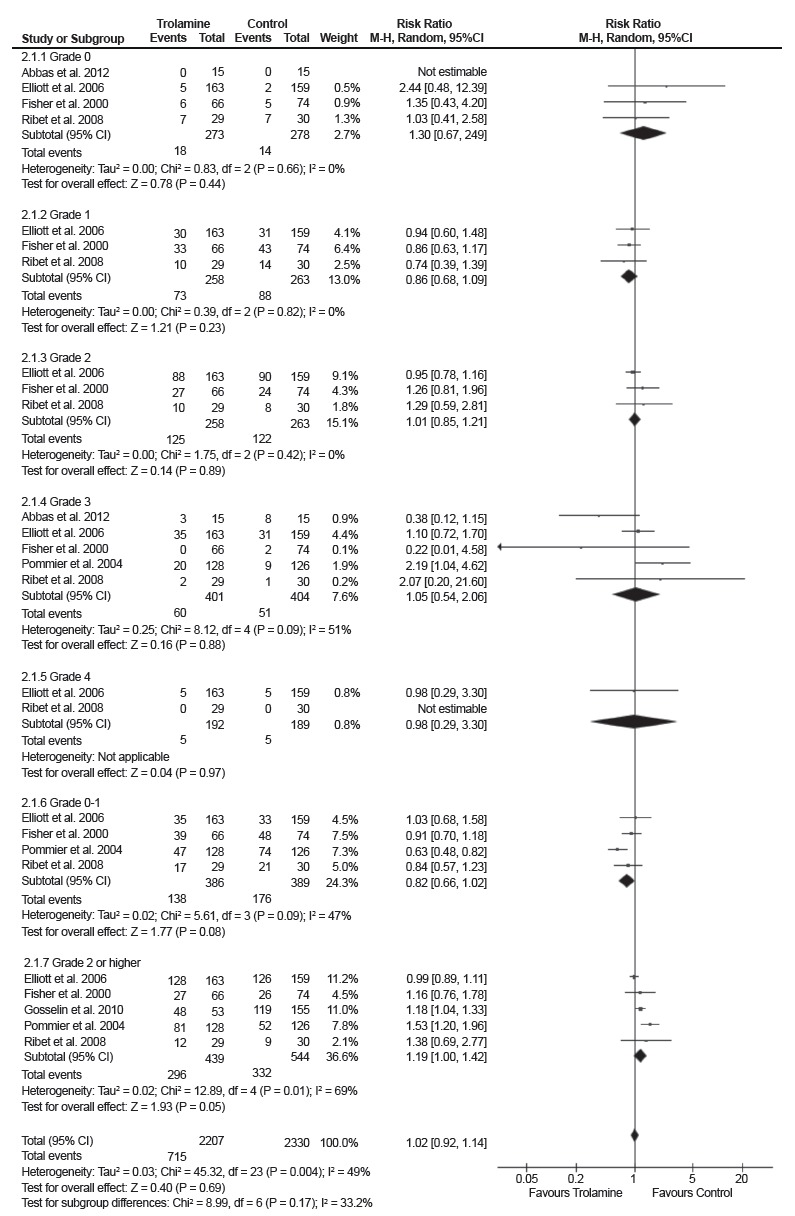
The studies were grouped into subgroups according to the graduation of radiation dermatitis 16 , 18 - 22 . Overall, the results of this random-effect meta-analysis demonstrate that there is no difference between the use of trolamine and evaluated controls to prevent radiation dermatitis (RR 1.02, 95% CI: 0.92 - 1.14. I2 = 49%) (Figure 3).
Figure 3. Forest plot of trolamine vs. controls according to the degree of radiation dermatitis.
Risk of bias across studies
The quality of the evidence from the outcomes evaluated by the GRADE system was assessed as very low (Figure 4), suggesting very low confidence in the estimated effect based on the outcomes assessed. It means that the true effect is likely to be substantially different from the estimate of effect. The important limitations in the studies and inconsistency were the main factors responsible for the low quality of the evidence from the studies evaluated.
Figure 4. GRADE assessment. Brasília, DF, Brazil, 2016.

*Two studies had no blinded sample and indicate that the absence of blinding can entail bias. The random sequence generation of three studies is unclear; †I2=69%; ‡I2=47%.
Discussion
In this review, seven studies evaluating trolamine to prevent or treat radiation dermatitis were included. In four studies 17 - 19 , 21 , no benefits were shown for the use of trolamine to prevent radiation dermatitis and, in two studies 16 , 20 there was no difference to prevent radiation dermatitis between trolamine and evaluated controls. Only one study 22 showed satisfactory use of trolamine in the prevention of radiation dermatitis, but its results showed benefit only to prevent grade 3 radiation dermatitis.
Trolamine has been considered because of its good tolerability and its ability to moisturize skin and reduce local discomfort. However, it has not been proven that trolamine is a topical skin radioprotective agent 9 . Some controls presented greater or similar efficacy when compared to trolamine 16 - 21 . According to the meta-analysis, there is no difference between trolamine and controls to prevent radiation dermatitis 16 , 18 - 22 .
The skin moisture and the skin reactions from the radiotherapy could be influenced by the number of intervention applications along the day. Some studies instructed the patients to apply the intervention three times a day 16 , 19 , 22 or twice daily 17 , 21 or five times a day 20 . Only one study 18 allowed patients to apply the intervention twice a day or more according to the frequence of radiation dermatitis and pain. None of this studies described a relation between the frequence of intervention and control applications and the skin moisture. One of the studies 17 asked patients to start the product application ten days before the onset of radiotherapy, but no contribution was added to prevent radiation dermatitis.
The product quantity in each application was not measured by the studies, except in one of the studies 18 in which the mean total number of tubes was 1.62 times more used in the trolamine group than in the calendula group.
Patients considered trolamine use more satisfactory than controls when compared to calendula 18 and AquaphorR and RadiaCareR( 21 .
Some studies have shown that chemotherapy and tamoxifen increased the intensity of skin reactions in patients undergoing radiotherapy 23 - 26 . Two studies used chemoradiotherapy 19 , 22 and, in one study, tamoxifen was used concomitantly with radiotherapy in breast cancer patients 17 , but these studies did not report significant differences in the skin reactions between the groups using trolamine or controls.
Only one study evaluated the efficacy of trolamine to treat radiation dermatitis, and considered no efficacy of trolamine in head and neck cancer patients 19 . It is important that other studies evaluate trolamine to treat grade 1 and grade 2 radiation dermatitis, because these grades require products with moisturizing and anti-inflammatory action. One of the studies 22 considered that trolamine prevents grade 3 of radiation dermatitis in head and neck cancer patients, but this conclusion is only based on those patients who did not develop grade 3 of radiation dermatitis. Moreover, the non-development of maximum grades of radiation dermatitis depends on extrinsic (total dose, fractionation, radiation energy, volume of treated regions, treatment duration, boost aplication, and treatment site) and intrinsic factors (age, comorbid conditions, skin phototype, and genetic predisposition) 27 .
Conclusion
Based on the studies included in this review, trolamine cannot be considered as a standardized product to prevent or treat radiation dermatitis in patients with breast and head and neck cancer. Further well-structured blinded studies using trolamine as a treatment are required to evaluated the moisturizing and anti-inflammatory action.
References
- 1.Cui Z, Xin M, Yin H, Zhang J, Han F. Topical use of olive oil preparation to prevent radiodermatitis: results of a prospective study in nasopharyngeal carcinoma patients. Int J Clin Exp Med. 2015;8(7):11000–11006. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4565279 [PMC free article] [PubMed] [Google Scholar]
- 2.Häfner MF, Fetzner L, Hassel JC, Debus J, Potthoff K. Prophylaxis of Acute Radiation Dermatitis with an Innovative FDA-Approved Two-Step Skin Care System in a Patient with Head and Neck Cancer Undergoing a Platin-Based Radiochemotherapy: A Case Report and Review of the Literature. Dermatology. 2013;227:171–174. doi: 10.1159/000353974. http://dx.doi.org/10.1159/000353974 [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Castro M, Martín-Gil B. Efectiveness of topical therapies in patients with breast cancer that experience radiodermatitis. A systematic review. Enferm Clin. 2015;25(6):327–343. doi: 10.1016/j.enfcli.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 4.McQuestion M. Evidence-Based Skin Care Management in Radiation Therapy: Clinical Update. Semin Oncol Nurs. 2011;27(2):e1–e17. doi: 10.1016/j.soncn.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 5.González-Sanchís A, Vicedo-González A, Brualla-González L, Gordo-Partearroyo JC, Iñigo-Valdenebro R, Sánchez-Carazo J, et al. Looking for complementary alternatives to CTCAE for skin toxicity in radiotherapy: quantitative determinations. Clin Transl Oncol. 2014;16(10):892–897. doi: 10.1007/s12094-014-1163-0. [DOI] [PubMed] [Google Scholar]
- 6.O’Donovan A, Coleman M, Harris R, Herst P. Prophylaxis and management of acute radiation-induced skin toxicity: a survey of practice across Europe and the USA. Eur J Cancer Care (Engl) 2015;24(3):425–435. doi: 10.1111/ecc.12213. [DOI] [PubMed] [Google Scholar]
- 7.Bazire L, Fromantin I, Diallo A, de la Lande B, Pernin V, Dendale R, et al. Hydrosorb® versus control (water based spray) in the management of radio-induced skin toxicity: Results of multicentre controlled randomized trial. Radiother Oncol. 2015;117(2):229–233. doi: 10.1016/j.radonc.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Manas A, Santolaya M, Ciapa VM, Belinchón B, Tully F. Topical R1 and R2 Prophylactic Treatment of Acute Radiation Dermatitis in Squamous Cell Carcinoma of the Head and Neck and Breast Cancer Patients Treated With Chemoradiotherapy. Eplasty. 2015;15:e25. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4485614 [PMC free article] [PubMed] [Google Scholar]
- 9.Del Rosso JQ, Bikowski J. Trolamine-containing topical emulsion: clinical applications in dermatology. Cutis. 2008;81(3):209–214. [PubMed] [Google Scholar]
- 10.Salvo N, Barnes E, van Draanen J, Stacey E, Mitera G, Breen D, et al. Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr Oncol. 2010;17(4):94–112. doi: 10.3747/co.v17i4.493. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2913836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. http://annals.org/aim/article/744664/preferred-reporting-items-systematic-reviews-meta-analyses-prisma-statement [DOI] [PubMed] [Google Scholar]
- 12.PROPERO International Prospective Register of Systematic Reviews. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016032805
- 13.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; www.cochrane-handbook.org [Google Scholar]
- 14.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group; 2013. www.guidelinedevelopment.org/handbook [Google Scholar]
- 16.Fisher J, Scott C, Stevens R, Marconi B, Champion L, Freedman GM, et al. Randomized phase III study comparing best supportive care to biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation therapy oncology group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000;48(5):1307–1310. doi: 10.1016/s0360-3016(00)00782-3. http://dx.doi.org/10.1016/S0360-3016(00)00782-3 [DOI] [PubMed] [Google Scholar]
- 17.Fenig E, Brenner B, Katz A. Topical Biafine and Lipiderm for the prevention of radiation dermatitis: a randomized prospective trial. Oncol Rep. 2001;8(2):305–309. http://dx.doi.org/10.3892/or.8.2.305 [PubMed] [Google Scholar]
- 18.Pommier P, Gomez F, Sunyach MP, D’Hombres A, Carrie C, Montbarbon X. Phase III randomized trial of Calendula officinalis compared with trolamine for the prevention of acute dermatitis during irradiation for breast cancer. J Clin Oncol. 2004;22(8):1447–1453. doi: 10.1200/JCO.2004.07.063. http://dx.doi.org/10.1200/JCO.2004.07.063 [DOI] [PubMed] [Google Scholar]
- 19.Elliott EA, Wright JR, Swann RS, Nguyen-Tân F, Takita C, Bucci MK, et al. Phase III Trial of an emulsion containing trolamine for the prevention of radiation dermatitis in patients with advanced squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Trial 99-13. J Clin Oncol. 2006;24(13):2092–2097. doi: 10.1200/JCO.2005.04.9148. http://dx.doi.org/10.1200/JCO.2005.04.9148 [DOI] [PubMed] [Google Scholar]
- 20.Ribet V, Salas S, Levecq JM, Bastit L, Alfonsi M, De Rauglaudre G, et al. Interest of a sterilised anti-burning gel in radiation dermatitis: results of a comparative study. Ann Dermatol Vénéréol. 2008;1:5–10. doi: 10.1016/S0151-9638(08)70091-7. http://dx.doi.org/10.1016/S0151-9638(08)70091-7. [DOI] [PubMed] [Google Scholar]
- 21.Gosselin TK, Schneider SM, Plambeck MA, Rowe K. A Prospective Randomized, Placebo-Controlled Skin Care Study in Women Diagnosed With Breast Cancer Undergoing Radiation Therapy. Oncol Nurs Forum. 2010;37(5):619–626. doi: 10.1188/10.ONF.619-626. http://dx.doi.org/10.1188/10.ONF.619-626 [DOI] [PubMed] [Google Scholar]
- 22.Abbas H, Bensadoun RJ. Trolamine emulsion for the prevention of radiation dermatitis in patients with squamous cell carcinoma of the head and neck. Support Care Cancer. 2012;20(1):185–190. doi: 10.1007/s00520-011-1110-3. http://dx.doi.org/10.1007/s00520-011-1110-3 [DOI] [PubMed] [Google Scholar]
- 23.Giro C, Berger B, Bölke E, Ciernik IF, Duprez F, Locati L, et al. High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: results of a survey in EORTC institutes. Radiother Oncol. 2009;90(2):166–171. doi: 10.1016/j.radonc.2008.09.007. http://dx.doi.org/10.1016/j.radonc.2008.09.007 [DOI] [PubMed] [Google Scholar]
- 24.Merlano M, Russi E, Benasso M, Corvò R, Colantonio I, Vigna-Taglianti R, et al. Cisplatin-based chemoradiation plus cetuximab in locally advanced head and neck cancer: a phase II clinical study. Ann Oncol. 2011;22(3):712–717. doi: 10.1093/annonc/mdq412. http://dx.doi.org/10.1093/annonc/mdq412 [DOI] [PubMed] [Google Scholar]
- 25.Studer G, Brown M, Salgueiro EB, Schmückle H, Romancuk N, Winkler G, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys. 2011;81(1):110–117. doi: 10.1016/j.ijrobp.2010.05.018. http://dx.doi.org/10.1016/j.ijrobp.2010.05.018 [DOI] [PubMed] [Google Scholar]
- 26.De Langhe S, Mulliez T, Veldeman L, Remouchamps V, van Greveling A, Gilsoul M, et al. Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. BMC Cancer. 2014;14:711–711. doi: 10.1186/1471-2407-14-711. http://dx.doi.org/10.1186/1471-2407-14-711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franco P, Potenza I, Moretto F, Segantin M, Grosso M, Lombardo A, et al. Hypericum perforatum and neem oil for the management of acute skin toxicity in head and neck cancer patients undergoing radiation or chemo-radiation: a single-arm prospective observational study. Radiat Oncol. 2014;9:297–297. doi: 10.1186/s13014-014-0297-0. http://dx.doi.org/10.1186/s13014-014-0297-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


