Abstract
The physical act of eating or feeding involves the coordinated action of several organs like eyes and jaws, and associated neural networks. Moreover, the activity of the neural networks controlling jaw movements (branchiomotor circuits) is regulated by the visual, olfactory, gustatory and hypothalamic systems, which are largely well characterized at the physiological level. By contrast, the behavioral output of the branchiomotor circuits, and the functional consequences of disruption of these circuits by abnormal neural development are poorly understood.
To begin to address these questions, we sought to evaluate the feeding ability of zebrafish larvae, a direct output of the branchiomotor circuits, and developed a qualitative assay for measuring food intake in zebrafish larvae at 7 days post fertilization. We validated the assay by examining the effects of ablating the branchiomotor neurons. Metronidazole-mediated ablation of nitroreductase-expressing branchiomotor neurons resulted in a predictable reduction in food intake without significantly affecting swimming ability, indicating that the assay is robust. Laser-mediated ablation of trigeminal motor neurons resulted in a significant decrease in food intake, indicating that the assay is sensitive. Importantly, in larvae of a genetic mutant with severe loss of branchiomotor neurons, food intake was abolished. These studies establish a foundation for dissecting the neural circuits driving a motor behavior essential for survival.
Keywords: Facial Branchiomotor Neuron, Food Intake, Jaw, Zebrafish, Neural Circuit, Behavior
INTRODUCTION
All animals depend on the intake of food for essential nutrients and survival. Feeding largely involves the coordination of jaw muscles and associated neural networks, which are driven by branchiomotor neurons (Chandrasekhar, 2004; Guthrie, 2007). The activity of these neural networks in teleosts is likely regulated by multiple sensory inputs from brain regions such as the visual, olfactory, and hypothalamic systems (Demski and Knigge, 1971; Grimm, 1960; Sato et al., 2007). For example, physiological, anatomical or genetic ablation of the visual system in zebrafish larvae decreased prey capture (Gahtan et al., 2005). Similarly, anatomical lesions of the olfactory tract in carp reduced odor-stimulated feeding behaviors (Hamdani et al., 2001). Interactions between hormones, neurotransmitters, and neuropeptides also regulate food intake and appetite (Filosa et al., 2016; Piccinetti et al., 2010; Volkoff et al., 2005). Thus, characteristics of feeding such as prey capture, appetite regulation, and satiety involve the integration of multiple sensory and motor pathways involved in the detection and capture of food.
The motor circuits driving food intake are located in the vertebrate hindbrain. The branchiomotor neurons, a subset of cranial motor neurons (Guthrie, 2007), include the trigeminal (nV) and facial (nVII) branchiomotor neurons that innervate jaw and facial muscles (Chandrasekhar, 2004). Branchiomotor neuron organization and development have been studied extensively in zebrafish, chick and mice (Chandrasekhar et al., 1997; Higashijima et al., 2000; Lumsden and Krumlauf, 1996; McArthur and Fetcho, 2017; Studer et al., 1996). However, the formation of the neural networks driving jaw muscle movement, and the functional outputs of these circuits (jaw movement and food intake) have received less attention (McArthur and Fetcho, 2017). The zebrafish larva represents an excellent model to investigate the assembly and function of the motor circuits driving jaw movements. By 5 days post-fertilization, the embryonic yolk sac has been depleted and zebrafish larvae begin exogenous feeding for nutrition. Transgenic lines expressing fluorescent reporters, cytotoxic enzymes, and calcium sensors in branchiomotor neurons and jaw muscles have been generated (Higashijima et al., 2000; Higashijima et al., 1997; Mapp et al., 2010; McArthur and Fetcho, 2017), facilitating an investigation of the organization and functional outputs of the branchiomotor circuits.
In this study, we evaluated the role of branchiomotor neurons for the feeding ability of zebrafish larvae, mediated presumably by the jaw muscles. Using chemical and laser ablation, and a genetic mutant, we eliminated various populations of branchiomotor neurons, and measured food intake in the manipulated larvae. We devised a qualitative food intake assay using fluorescent microspheres that can provide a semi-quantitative measure of food intake in a population of feeding larvae. Using this assay with neuron-ablated larvae, we demonstrate that branchiomotor neurons are indispensible for food intake. Importantly, our data indicate that the food intake assay is robust and sensitive, and can provide a reliable and reproducible functional readout of the branchiomotor circuits.
MATERIALS AND METHODS
Animals
All experiments were carried out with protocols approved by the Animal Care and Use Committee at the University of Missouri. The following transgenic lines were used: Tg(isl1:gfp) (Higashijima et al., 2000) and Tg(zcrest1:nsfB-mCherry) (this study). The Tg(zcrest1:nsfB-mCherry) line was created by injecting AB embryos with a plasmid construct derived from the zCREST1-hsp70:GFP plasmid (a kind gift of H. Okamoto, RIKEN, Japan) in which the GFP cassette was replaced with an nsfB-mCherry cassette. Tg(zcrest1:nsfB-mCherry) will be referred to as Tg(zcrest1:NTR-mCherry) throughout.
Embryos were obtained through natural matings in breeding cages, staged at dome stage and incubated at 28.5°C in E3 embryo medium (Westerfield, 1995). The embryos were re-staged at 18 hours post-fertilization, and pigmentation was prevented by phenylthiourea treatment (0.003%). After embryos hatched at 2 days post-fertilization (dpf), they were transferred on 3 dpf to an incubator with 14 hour light, 10 hour dark cycles until the larvae were ready for use in experiments between 5-7 dpf. The detour (dtrts269) mutant allele (Karlstrom et al., 1996) was maintained in the Tg(isl1:gfp) background (Higashijima et al., 2000).
Fluorescent Microsphere Larval Food
Our procedure is adapted from one described previously (Field et al., 2009). Larval fish food (Zeigler, <100 microns; 100 mg) was mixed thoroughly with Yellow-Green fluorescent polystyrene microspheres (2 μm, Polysciences; 150 μl of a supplied 2.5% solution) and 50 μl water to form a paste. Working in dim light, the slurry was layered thinly on a microscope slide, air-dried, scraped off, crushed into a fine powder, and stored at 4°C until use.
Feeding Assay
Swimming larvae (7 dpf) were placed in wells in a 6-well tissue culture plate in 5 ml of E3 solution containing 2 mg of fluorescent microsphere food mixture per well. The number of larvae per well ranged from 15-20, ensuring that the larval density was similar between experiments and treatment conditions. As previously described (Field et al., 2009), some of the food formed a film on the surface of the E3 solution. However, food was also dispersed (suspended) in the solution, although this was not explicitly monitored. Larvae were incubated for the indicated times up to 3 hours. We determined empirically that longer incubation times confounded the analysis due to peristalsis-mediated gut clearance (Field et al., 2009). At the end of the incubation period, the larvae were anesthetized with 0.02% Tricaine, transferred to narrow molded troughs in an agar plate, mounted dorsally and embedded in 3% methylcellulose containing Tricaine, and scored based on the amount of fluorescent food in the gut.
The amount of food ingested by larvae was estimated and scored by an individual who was blinded to the treatment conditions. Larvae were examined using a stereomicroscope equipped with epifluorescence optics at 40-90X, and assigned a score ranging from 0-3 (Fig. 1A-E).
Figure 1.
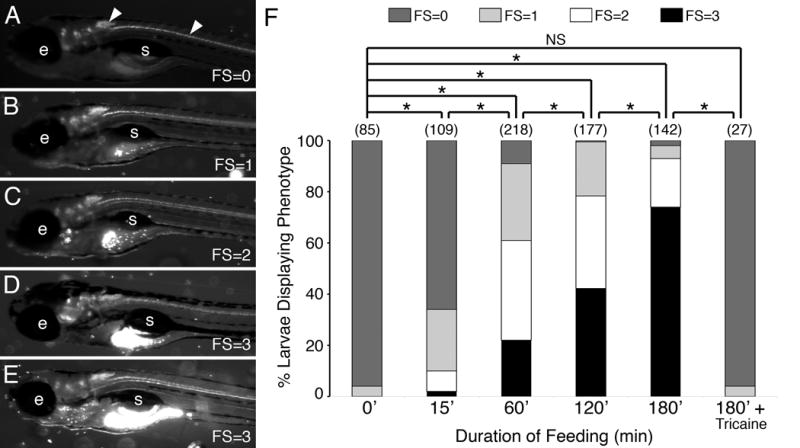
A qualitative food intake assay for zebrafish larvae. (A-E) Side views of 7 dpf larvae examined under GFP epifluorescence. The larvae were incubated with Y-G fluorescent microspheres coated with fish food (see Methods) for up to 3 hours before visualizing the fluorescent contents in their gut. The amount of fluorescence (ignoring autofluorescence) ranged from little or none (assigned a feeding score (FS) of 0), less than 25% of the gut (FS=1), about 50% of the gut (FS=2), to a full gut (FS=3). Most experiments were performed with Tg(isl1:gfp) larvae that express GFP in cranial and spinal motor neurons (arrowheads) and enabled proper sample orientation prior to scoring. e, eye; s, swim bladder. (F) Pooled data from 2-8 experiments (number of larvae in parenthesis) showing the distribution of feeding scores for various durations of feeding. For tricaine treatment, larvae were exposed to the chemical in the medium for 5 minutes before addition of labeled food. The observers assigning feeding scores were blinded to the treatment conditions of the larvae being scored. As the duration of feeding increased to 3 hours, the fraction of larvae exhibiting feeding scores of 2 and 3 increased. As expected, Tricaine-treated larvae, which were paralyzed and unable to swim, ate very poorly. Asterisk, Chi-square test with Bonferroni correction indicating significance at p < 0.003; NS=not significant.
Chemical Ablation of Neurons
Five day-old Tg(zcrest1:NTR-mCherry; isl1:gfp) larvae in E3 medium were treated with 0.5% DMSO (control) or 10 mM Metronidazole (Mtz) in 0.5% DMSO for 24 hours (until 6 dpf), washed extensively to remove Mtz, and allowed to recover overnight in E3. Recovered larvae (7 dpf) were evaluated for neuronal ablation by examining the distribution of mCherry- and GFP-expressing branchiomotor neurons (and fluorescence intensity), and estimating the degree of motor neuron loss. While we attempted to quantify motor neuron numbers in larvae using islet immunostaining as described previously for 2 dpf embryos (Bingham et al., 2002), it did not work effectively, likely due to technical issues associated with staining older animals. Therefore, motor neuron loss was estimated in these studies. Typically, about 50% of Mtz-treated Tg(zcrest1:NTR-mCherry; isl1:gfp) larvae exhibited a severe loss of branchiomotor neurons (>80% reduction; defined as a Strong effect, Fig. 2). The remaining larvae exhibited a pronounced loss (~50% loss; defined as a Weak effect, Fig. 2). In Tg(isl1:gfp) larvae not carrying the NTR transgene, Mtz treatment did not result in branchiomotor neuron loss (Fig. 2), indicating that the drug treatment was specifically ablating NTR-expressing branchiomotor neurons. Neuron-ablated larvae appeared normal morphologically and appeared to swim normally, and were used in feeding assays.
Figure 2.
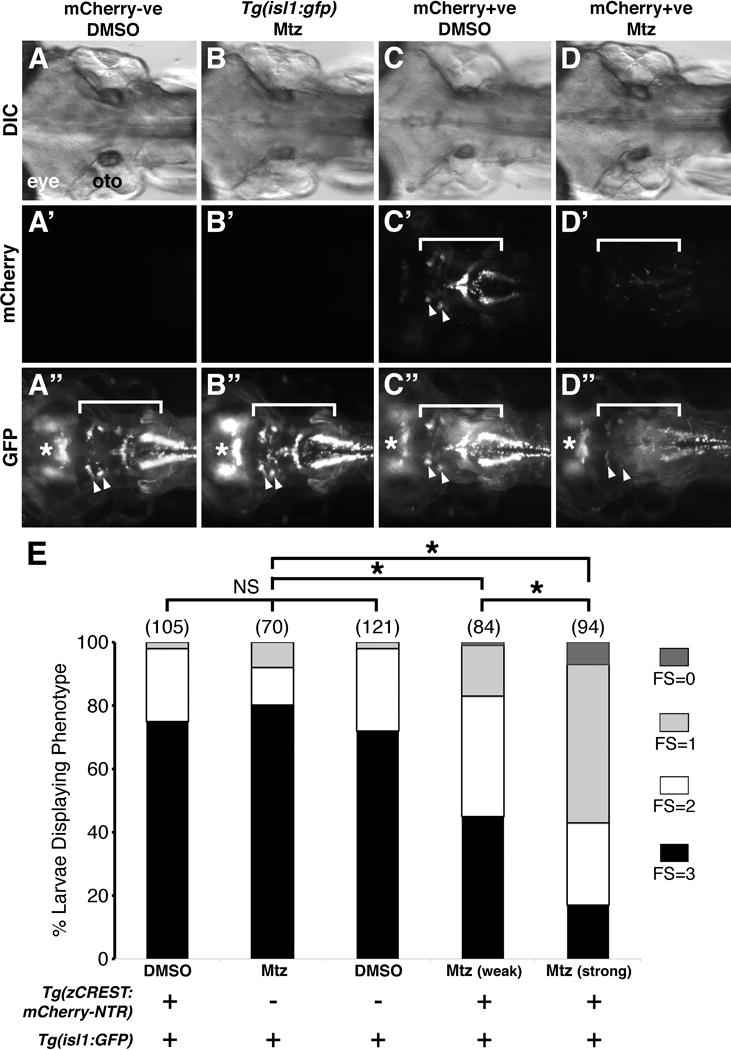
Ablation of branchiomotor neurons greatly reduces food intake. (A-D″) Dorsal views with anterior to the left of the hindbrain of 7 dpf larvae from the indicated genotypes. (A-A″) In Tg(isl1:gfp) embryos obtained from a cross of Tg(zcrest:NTR-mCherry) and Tg(isl1:gfp) fish, overnight treatment with vehicle (DMSO) has no effect on midbrain (asterisk) and hindbrain motor neurons (white bracket, arrowheads). (B-B″) Similarly, all branchiomotor and midbrain neurons are unaffected in Tg(isl1:gfp) larvae treated with 10 μM metronidazole (Mtz). (C-C″) In Tg(zcrest:NTR-mCherry); Tg(isl1:gfp) double transgenic larvae, DMSO treatment did not affect the branchiomotor neurons (white brackets) assayed using mCherry (C′) or GFP expression (C″). (D-D″) In Tg(zcrest:NTR-mCherry); Tg(isl1:gfp) double transgenic larvae treated for 24 hours with Mtz, branchiomotor neurons were severely reduced in number (mCherry (D′) or GFP (D″) expression), while trochlear and oculomotor neurons (asterisk) were not affected since they do not express NTR-mCherry. (E) Distribution of feeding scores for larvae of indicated reporter phenotypes with DMSO or metronidazole (Mtz) treatment. Data pooled from 3-6 experiments (number of larvae in parenthesis). Larvae with ablated branchiomotor neurons ate less than their control non-ablated siblings, with the larvae with the greatest neuron loss (Mtz, strong) exhibiting the largest deficit in food intake. Asterisk, Chi-square test with Bonferroni correction indicating significance at p < 0.0001; NS=not significant.
Laser Ablation of Trigeminal Neurons
Larvae (6 dpf) were anesthetized with 0.02% Tricaine, embedded in 2% LMP agarose (in E3) on a microscope slide, the agarose covering the hindbrain was excavated, and the preparation was covered in E3. The larvae were viewed with a 20X water objective (Olympus BX50WI). A Q-switched, Nd:YAG pumped, optical parametric oscillator (OPO) tunable laser (Vibrant, Opotek Inc.) was used. An optical fiber (1 mm) delivered laser light, which was collimated through a bi-convex lens resulting in a 0.5 cm2 beam spot coupled in through the camera port of the microscope. A 1 mm aperture used at the camera port blocked most of the laser light, and created a 50 μm image of itself at the focal plane. This 50 μm spot was used for all laser ablations. A camera was inserted into one of the eye ports for imaging during laser ablation. Cells were irradiated with 440 nm wavelength, 5 ns pulsed light at a repetition rate of 5 Hz for 1 minute (300 pulses total with 400-500 mJ/cm2 at the focal plane). The cells were defocused in the z-axis (~15-20 μm) after 30 seconds to ensure complete irradiation of targeted cells. Laser illumination rapidly destroyed the fluorescent signal in the irradiated cells. Irradiated larvae were placed in E3 to recover overnight (~20 hours), and imaged at 7 dpf to evaluate the efficacy of the laser ablation, and to ensure that animals with photobleached neurons were excluded from analyses.
Analysis of Larval Motility and Responsiveness
For swim analysis, larvae were placed individually in a 48-well plate in 300 μl of E3 medium per well. After a 15 minute acclimation period, larval behavior was recorded for 20 seconds at 5 frames per second using a Retiga2000 camera system. Larval motility was analyzed using DIAS (Dynamic Image Analysis Software). Touch responsiveness was assayed as described previously (Eaton et al., 1977; Granato et al., 1996), and the analysis was limited to visual scoring (i.e., no high speed recording) following touch. While response to touch appeared normal in chemically ablated larvae, subtle defects cannot be ruled out.
Since experimental larvae were embedded in methylcellulose for scoring food intake, or were incubated one per well in E3 medium for the swimming assay, we did not use the same larvae for both assays to avoid any confounding effects due to injury or manipulation.
Statistical Analysis
We employed the chi square test to identify any significant differences in the distribution of feeding scores across all experimental groups in each experiment. We then used pairwise chi square tests with Bonferroni correction to test for significant differences in the distribution of feeding scores between treatment groups within an experiment. For each initial chi square test, a p-value < 0.05 was considered significant. Bonferroni adjusted p-values were reported for each experiment to account for the relevant number of pairwise comparisons performed in the statistical analysis of each experiment. Chi square tests were performed using software available from quantpsy.org.
We performed one-way analysis of variance (ANOVA) to determine if any differences in swimming parameters between experimental groups were statistically significant. We then did a post-hoc Tukey’s HSD (honest significance difference) to compare each condition for significance.
RESULTS
Development of a Qualitative Food Intake Assay for Zebrafish Larvae
In order to investigate the role of branchiomotor neurons in the neural circuits regulating jaw movements, we have developed a robust and sensitive assay to measure the food intake of zebrafish larvae. At 7 days post fertilization (dpf), the yolk sac has been fully absorbed, the intestinal lumen is not obscured by pigmentation and is easily visualized in a lateral view, and the larvae actively forage and eat exogenously provided food such as Paramecia. We employed a previously described feeding protocol (Field et al., 2009) to prepare fluorescent food composed of larval fish food and fluorescent polystyrene microspheres (see Methods). When larvae are allowed to freely swim in E3 containing the fluorescent food, they ingest the food leading to an accumulation of non-digestible polystyrene fluorescent microspheres within the gut that can be readily visualized (Cassar et al., 2015; Farber et al., 2001; Field et al., 2009). Each larva was assigned a feeding score (0-3) based on the distribution of the fluorescent microspheres within the gut, ranging from no fluorescence observed (FS=0) to large amount of fluorescence (FS=3) (Fig. 1A-E). Since there was substantial variation within a group of identically grown larvae in the accumulation of fluorescent material within the gut, we defined feeding scores as follows: FS=3, fluorescence observed in >50% of gut extent; FS=2, ~25-50% of gut, typically at the anterior; FS=1, <25% of the gut; and FS=0, no fluorescent particles in gut. The amount of fluorescence in the gut was an estimate based upon the proportion of the gut that contained fluorescent material, and not upon the intensity of the fluorescence. The person scoring the larvae was blinded to the treatment conditions.
Using this binning approach, we plotted the distribution of feeding scores for Tg(isl1:gfp) larvae incubated with fluorescent food for different time periods ranging from 0 to 180 min. As expected, the number of well-fed (FS=3) larvae increased with longer incubation times, and the number of larvae with no food intake (FS=0) decreased (Fig. 1E). With incubation periods exceeding 180 min, there was no appreciable increase in the fraction of larvae with high feeding scores (FS=2 or 3), suggesting that larvae may be excreting fluorescent microspheres along with digested food, and consistent with a gut transit time of 4-6 hours (Field et al., 2009). To rule out that the fluorescent food accumulated in the gut was taken up passively, we measured food intake in Tricaine-treated paralyzed larvae (Fig. 1F). Treated larvae ate very little, and essentially resembled unfed larvae. These results demonstrate that the fluorescent microsphere food intake assay is robust and that it can be used to evaluate the functional output of the branchiomotor neural circuits.
Nitroreductase-mediated ablation of branchiomotor neurons reduces food intake in zebrafish larvae
To further validate the feeding assay, we sought to test whether it could detect differences in food intake between larval populations with defined neuronal lesions. Specifically, we examined the consequences of severe branchiomotor neuron loss on food intake. Using a Tg(zcrest1:NTR-mCherry) line, bacterial nitroreductase (NTR) was specifically expressed in branchiomotor neurons (and a subset of spinal motor neurons) under the control of the zcrest1 enhancer element (Uemura et al., 2005). In NTR-expressing cells, the prodrug metronidazole (Mtz) is converted into cytotoxic metabolites leading to cell death within 24-48 hours of treatment (Drabek et al., 1997). NTR-mediated cell ablation has been successfully employed in zebrafish, and demonstrated to be both effective and specific, resulting in the ablation of NTR-expressing cells while leaving neighboring cells unharmed (Curado et al., 2008; Godoy et al., 2015; Pisharath et al., 2007; Pope and Voigt, 2014).
In order to evaluate the efficacy of cell ablation independently of the NTR-mCherry fusion protein, we crossed these fish to Tg(isl1:gfp) fish to obtain double transgenic embryos expressing NTR-mCherry and GFP in the branchiomotor neurons (Fig. 2C-C″ and Fig. S1). Since mCherry expression exhibited two expression levels between embryos, we separated embryos into three categories: mCherry -ve (Fig. 2A-A″ and 2B-B″), Weak mCherry (Fig. S1), and Strong mCherry (Fig. 2C-C″ and Fig. S1). All larvae expressed GFP in the branchiomotor neurons, permitting us to evaluate the degree of Metronidazole (Mtz)-induced cell ablation. “Strong mCherry +ve” larvae exhibited much higher mCherry fluorescence than “Weak mCherry +ve” larvae (Fig. S1), and more severe neuron loss following Mtz treatment (Fig. S1), consistent with a higher level of NTR-mCherry protein in branchiomotor neurons. Intensity of GFP expression in the branchiomotor neurons was roughly comparable in all larvae (Fig. 2A″-D″). In DMSO-treated (mCherry+ve or -ve) and Metronidazole (Mtz)-treated mCherry -ve larvae, GFP-expressing branchiomotor neurons were found in normal numbers and pattern, ruling out nitroreductase-independent effects of Mtz on neuronal number and patterning. Following incubation with 10 mM Mtz for 24 hr, Tg(zCREST1:NTR-mCherry; isl1:GFP) larvae exhibited a severe loss of both mCherry- and GFP-expressing branchiomotor neurons. (Fig. 2D′-D″) compared to sibling controls (Fig. 2C′-C″). The extent of neuron loss was proportional to the brightness of NTR-mCherry expression in those larvae, and ranged from an estimated 50% loss in weak mCherry +ve larvae (Fig. S1), to over an 80% loss in Strong mCherry +ve larvae (Fig. 2C-C″ and Fig. S1). The extent of neuron loss appeared uniform across branchiomotor subtypes such as trigeminal and facial.
The ability of neuron-ablated larvae to eat was evaluated using our feeding assay. As expected, larvae with normal numbers of GFP-expressing branchiomotor neurons ate typical amounts of food as described earlier for wild-type embryos (Fig. 1). However, Mtz-treated, neuron-ablated larvae ate poorly, with the Strong mCherry +ve larvae with the greatest neuron loss exhibiting the smallest food intake (Fig. 2E). These results suggest strongly that the branchiomotor neurons are the main cell type that drives jaw movements involved in food intake, and that the feeding assay is robust.
While the reduced food intake in neuron-ablated larvae may result from the loss of branchiomotor neurons, it is possible that the food intake deficit results from poor swimming ability given that larvae paralyzed with Tricaine did not ingest any food (Fig. 1E). Since Mtz-treated larvae appeared to swim normally, we initially examined head touch-evoked escapes (Eaton et al., 1977) and found that the neuron-ablated larvae appeared to respond normally (data not shown). We next performed a quantitative analysis of swimming in 7 dpf control and neuron-ablated larvae. While neuron-ablated (Mtz-treated mCherry +ve) larvae failed to inflate swim bladders, this was a byproduct of Mtz treatment since swim bladders also failed to inflate in a large fraction of control (Mtz-treated mCherry -ve) larvae (Fig. 3D). Despite of the failure of the swim bladder to inflate, neuron-ablated larvae exhibited no significant differences in swimming ability in terms of velocity and distance traveled, although bout frequency was reduced (Fig. 3A-C). These data suggest strongly that the reduced food intake of neuron-ablated larvae results primarily from the loss of branchiomotor neurons.
Figure 3.
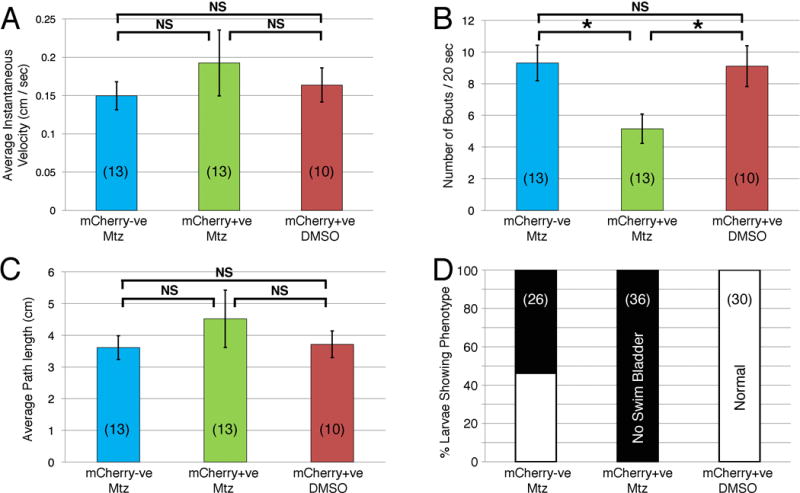
Swimming behaviors are largely unaffected in neuron-ablated larvae. Swimming parameters are compared for metronidazole (Mtz)-treated control (mCherry-ve, blue), mCherry+ve neuron-ablated (green), and mCherry+ve non-ablated (DMSO, brown) larvae (number of larvae in parenthesis). For average instantaneous velocity (A) and average path length (C) recorded over a 20-second period, the neuron-ablated larvae appeared to perform better, although there was no statistically significant difference (NS) between the three groups (unpaired t-test). Interestingly, the number of swim bouts (B) was significantly lower in the neuron-ablated larvae compared to the other groups (p<0.01). Moreover, 100% of neuron-ablated larvae failed to inflate swim bladders, which was also seen in ~55% of Mtz-treated non-ablated controls. One-way ANOVA with post-hoc Tukey’s HSD; NS=not significant; asterisk, p<0.05.
Laser-mediated ablation of trigeminal motor neurons reduces food intake
Since the Mtz-mediated neuron ablation experiments indicated that the feeding assay was semi-quantitative and robust, we wanted to test whether the assay was also sensitive, with the ability to detect relatively small changes in food intake. Therefore, we sought to ablate a subset of branchiomotor neurons and determine effects on food intake. Since there is no transgenic strain that drives nitroreductase expression in only a subset of branchiomotor neurons, for example, the trigeminal, we used laser irradiation to selectively kill small neuronal populations. We chose to ablate the trigeminal motor neurons by this method because 1) these neurons innervate a subset of jaw muscles and their ablation would be expected to compromise jaw movements and food intake, and 2) these neurons occupy a relatively small tissue volume compared to the facial branchiomotor neurons, generating confidence that they could be laser-irradiated effectively and killed.
Six day-old Tg(isl1:gfp) larvae were embedded in agarose, and irradiated with a Q-switched, OPO laser (see Materials and Methods). To ensure that the embedding and irradiation protocols did not affect feeding ability, we included several controls (Fig. 4A, B, D; Fig. S3), along with the experimental larvae (Fig. 4C). Embedded larvae (Fig. 4B) were placed in agarose and treated exactly as the laser-ablated animals except that they were not exposed to any laser light. Sham ablated larvae (Figs. 4D and S3C) were embedded and irradiated with laser in the tissue just rostral to the GFP-expressing trigeminal motor neurons for the same duration as the experimental larvae (Fig. S3C′). After overnight recovery, only healthy larvae (all treatment conditions) were analyzed further. None of the embedded or sham ablated control larvae exhibited trigeminal motor neuron loss (Fig. 1B′, D′). By contrast, laser ablation effectively reduced trigeminal motor neuron number by 50% or higher (Fig. 4C′). Since the larvae recuperated overnight, the reduction in GFP-expressing cells likely reflects motor neuron loss rather than a failure to recover fluorescence in photo-bleached but live neurons. Importantly, other GFP-expressing cells like the facial, vagal, oculomotor and trochlear motor neurons were unaffected in laser-ablated and control animals. Food intake as measured in the feeding assay was sharply and significantly lower in the trigeminal neuron-ablated larvae compared to the various control larval populations (Fig. 4E). This result demonstrates that the feeding assay described here, while qualitative, is also partially quantitative, and has sufficient sensitivity to measure the functional impact of relatively small deficits in branchiomotor neuron number.
Figure 4.
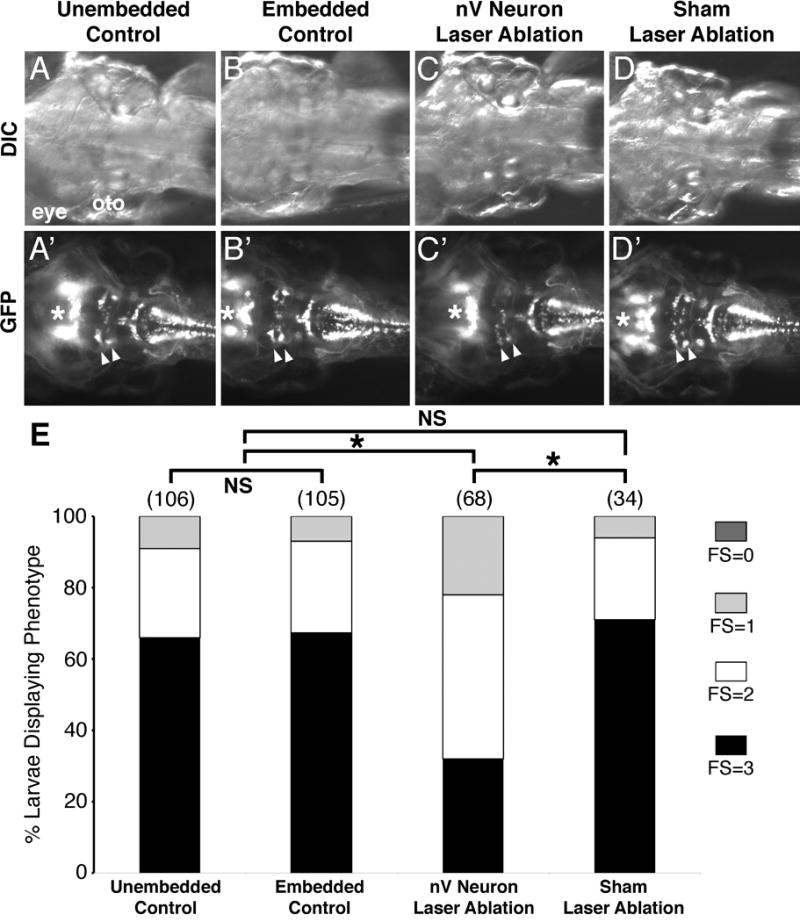
The qualitative feeding assay is sensitive. (A-D′) Dorsal views with anterior to the left of the hindbrain of 7 dpf Tg(isl1:gfp) larvae from the indicated treatments. In control larvae that were either not manipulated in any way (A, A′) or embedded in agarose to mimic laser ablation conditions (B, B′), all branchiomotor neurons, including the trigeminal motor neurons (nV, arrowheads), were unaffected. (C, C′) In this embedded larva, the nV motor neurons were ablated by laser irradiation at 6 dpf and examined 1 day later. Very few nV neurons (arrowheads) remain, reflecting the loss of most of these neurons due to ablation. (D, D′) The sham-ablated larva was embedded and laser-irradiated bilaterally in a region immediately rostral to the nV neurons, for the same duration as nV-ablated larvae. The nV neurons were unaffected in these larvae. The cranial motor neurons in the midbrain (asterisk) are not affected under these conditions. (E) Distribution of feeding scores for larvae undergoing different treatments. Data pooled from 3-6 experiments (number of larvae in parenthesis). Larvae with ablated trigeminal motor (nV) neurons ate significantly less than their control non-ablated siblings. Asterisk, Chi-square test with Bonferroni correction indicating significance at p < 0.004; NS=not significant.
Genetic ablation of branchiomotor neurons abolishes food intake
The above studies clearly indicated that the food intake assay is robust and sensitive, and could reproducibly measure the effect of deficits in subsets of branchiomotor neurons. Therefore, we wondered if eliminating branchiomotor neurons entirely would abolish food intake. To test this, we measured food intake in detour (gli1−/−) mutants, in which cranial motor neurons are not induced but spinal motor neurons develop normally (Fig. 5C,D) (Chandrasekhar et al., 1999; Vanderlaan et al., 2005). Food intake was measured in 7 dpf detour (ts269) mutant larvae with relatively straight trunks and normal swimming activity. As predicted, no fluorescence was detected in the gut (Fig. 5H, I) indicating that the larvae were unable to ingest any food without branchiomotor innervation. In some larvae, fluorescent particles were located in the oral cavity, likely due to inadvertent trapping of microspheres entering thru the gaping mouth (Fig. 5F).
Figure 5.
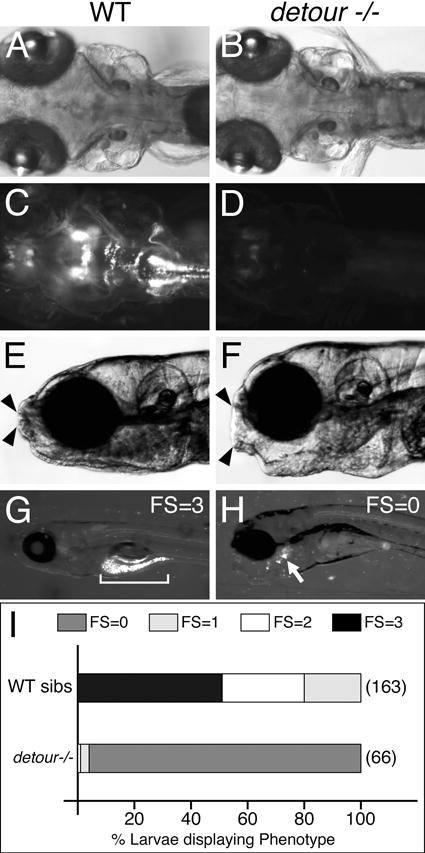
Detour mutants are unable to ingest food. (A-D) Dorsal views with anterior to the left of the hindbrain of 7 dpf Tg(isl1:gfp) larvae. (C) In a wildtype embryo, the cranial motor neurons are distributed in the expected number and pattern. (D) In a detour mutant (detour −/−), cranial motor neurons are completely absent. (E-H) Side views of 7 dpf larvae embedded in agarose to view jaw movements (E, F), or mounted to reveal the distribution of ingested fluorescent microspheres (G, H). While the opening of the mouth (arrowheads) in a wildtype larva is small compared to head size (E), the mouth opening is abnormally large in a detour mutant larva (F). Nevertheless, mutant larvae eat very poorly (H) with a few fluorescent particles stuck in the oral cavity (arrow), compared to a gut filled with fluorescent food (bracket) in a wildtype larva (G). (I) Distribution of feeding scores for 7 dpf wildtype and detour mutant larvae. Data pooled from 3-6 experiments (number of larvae in parenthesis). Mutant larvae ate very little or no food at all compared to wildtype siblings (sibs). Chi-square test indicating significance at p < 0.00001.
DISCUSSION
The zebrafish is an excellent model system to dissect the neural circuits underlying complex behaviors such as feeding (food intake), and how such circuits are modulated during development and disease. Further, due to the central role of the hypothalamus in altering food intake by regulating appetite and satiety in mammals, the relatively simple organization of the hypothalamic axis in juvenile (larval) and adult zebrafish provides a good model to investigate underlying structural and physiological mechanisms (Cassar et al., 2015; Clift et al., 2014; Farber et al., 2001; Filosa et al., 2016; Jordi et al., 2015; Minchin et al., 2013; Piccinetti et al., 2010; Shimada et al., 2012; Volkoff et al., 2005; Yokobori et al., 2012). However, the motor circuits that drive food intake have received little attention. In fact, there was no functional data supporting a role for the hindbrain branchiomotor neurons in controlling food intake. Here we used chemical, laser and genetic approaches to eliminate different subsets of (or all) zebrafish branchiomotor neurons, leading to pronounced reductions in food intake. The feeding deficits resulting from branchiomotor neuron ablation are likely due to effects on the movement of jaw muscles, the primary target of the trigeminal and facial branchiomotor neurons. However, food intake also involves coordination with gas exchange and swallowing, which are respectively controlled by opercular/gill muscles innervated by vagal (and some facial) branchiomotor neurons (Chandrasekhar et al., 1997; Higashijima et al., 2000; McArthur and Fetcho, 2017), and by oropharyngeal muscles (Kohei Hatta, University of Hyogo, personal communication). Our results suggest strongly that branchiomotor neurons are indispensible for controlling the motor programs involved in feeding, and establish a foundation for dissecting the neural circuits controlling food intake.
This study employs a food intake protocol to evaluate the ability of a large number of animals in an experimental sample to acquire food. While our protocol is qualitative, it is also robust and sensitive. Several labs have fed labeled Paramecia, a natural and live food source, to 5-7 dpf larvae (Filosa et al., 2016; Jordi et al., 2015; Shimada et al., 2012), and two of these groups were able to quantify the amount of Paramecia consumed by individual larvae (Jordi et al., 2015; Shimada et al., 2012). While both methods are quantitative, sensitive and robust, one is also high-throughput (Jordi et al., 2015). However, these protocols also require live Paramecia cultures and labeling, and specialized equipment, which may reduce broad applicability. Another food intake assay employed fluorescent lipids and liposomes to study digestive physiology (Farber et al., 2001; Otis and Farber, 2016). While this method provides exquisite detail on the molecular mechanisms and intestinal cell types regulating lipid metabolism, it is not very effective at measuring food intake on an individual larva basis in a population, and it is relatively slow and requires specialized equipment (Otis and Farber, 2016). Our food intake protocol is adapted from the method of Field et al. (Field et al., 2009) that employed fluorescent microspheres coated with powdered flake food to examine gut transit in zebrafish larvae. Our method is quick, simple, and does not need specialized equipment. However, it is qualitative, so the measurements must be performed in blinded fashion. Despite its qualitative nature, a large number of larvae in various treatment groups can be processed quickly (a trained experimenter can process 60-80 larvae over a seven hour period that includes the feeding period of 3 hours). Over the course of three years, our feeding protocol has generated consistent results independent of the lab personnel, generating confidence that these results are reproducible and reflect a true measure of the larval food intake under different experimental conditions. We are currently attempting to adapt our food intake protocol and assay to make it more quantitative and higher throughput by incorporating the plate reader method (Shimada et al., 2012).
In summary, we have used a qualitative assay to definitively establish an indispensible role for branchiomotor neurons in driving the motor circuits underlying food intake in zebrafish larvae. These studies provide the foundation for detailed studies into the roles of specific jaw muscles, branchiomotor axonal pathways and neuromuscular junctions, and of neuronal position in the implementation of these motor programs. Further, the well-established influence of visual, olfactory, gustatory, and hypothalamic pathways on the regulation of food intake can now be examined at the structural level within branchiomotor circuits.
Supplementary Material
Figure S1:
Chemical ablation of branchiomotor neurons. All panels show dorsal views of the hindbrain in 6 dpf (before treatment with Metronidazole (Mtz)) and 7 dpf (after treatment with Mtz) larvae. Tg(zcrest:NTR-mCherry) fish were sorted as “weak” and “strong” according to intensity of mCherry expression in branchiomotor neurons (brackets, Pre-treatment, mCherry). GFP expression in branchiomotor neurons due to the islet1:GFP transgene (brackets and arrowheads, Pre-treatment, GFP) was equally strong in all mCherry-expressing larvae. White asterisks indicate oculomotor and trochlear motor neurons in the midbrain, which do not express the NTR-mCherry transgene. Following overnight incubation with Mtz, NTR-mCherry-expressing larvae exhibited either a significant decrease (~50% loss, “weak effect”) or a severe loss (>80% loss, “strong effect”) of branchiomotor neurons, assayed by examining mCherry and GFP expression (brackets and arrowheads, Post-treatment). As expected, midbrain motor neurons (white asterisks) were not affected by Mtz treatment. The bright patch of mCherry expression (black asterisk) is an artifact.
Figure S2:
Quantification of swimming in 7 dpf larvae. (A) Individual larvae were placed in 24 well plates and their swimming behavior was recorded over a 20 second period by time-lapse imaging (5 frames per sec). (B) The outline of a larva (A) in successive frames was generated manually and the swim path was generated using DIAS image analysis software. (C) The instantaneous velocity was plotted for the larval path generated in (B) for each interval (0.2 sec). (D) Extracting swimming bouts. An instantaneous velocity of 0.2 cm/sec or lower was considered noise. Any peaks of instantaneous velocity above 0.2 cm/sec were defined as bouts, when the larva swam rapidly to a new location.
Figure S3:
Effects of laser ablation procedure. (A-C′) Dorsal views with anterior to the left of the hindbrain of 7 dpf Tg(isl1:gfp) larvae from the indicated treatments. Upper panels (A-C) show brightfield (partial DIC) images and lower panels (A′-C′) show corresponding epifluorescent images from the same Tg(isl1:gfp) larvae. Manipulating the larvae for laser ablation by embedding in agarose (B,B′) had no effect on any neuronal populations (arrowheads, asterisk) compared to an unembedded larva (A,A′). Even laser irradiation of tissue (white ovals) in an embedded larva (C,C′) had no effect on the number of trigeminal motor neurons (arrowheads) and midbrain cranial motor neurons (asterisk) adjacent to the irradiated areas.
Acknowledgments
We thank members of the Chandrasekhar lab for discussion and help with animal care. This work was supported by NIH grant NS040449 (AC), the MU-PREP Scholar program (JRA) through NIH grant GM064120 (John David, PI), the MU-IMSD program (EA) through NIH grant GM056901 (Mark Hannink, PI), NIH grant NS087574 (MV) and bridging funds from the University of Missouri Research Board and the Bond Life Sciences Center (AC).
BIBLIOGRAPHY
- Cassar S, Huang X, Cole T. A high-throughput method for predicting drug effects on gut transit time using larval zebrafish. J Pharmacol Toxicol Methods. 2015;76:72–75. doi: 10.1016/j.vascn.2015.08.156. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar A. Turning heads: development of vertebrate branchiomotor neurons. Dev Dyn. 2004;229:143–161. doi: 10.1002/dvdy.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar A, Moens CB, Warren JT, Jr, Kimmel CB, Kuwada JY. Development of branchiomotor neurons in zebrafish. Development. 1997;124:2633–2644. doi: 10.1242/dev.124.13.2633. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar A, Schauerte HE, Haffter P, Kuwada JY. The zebrafish detour gene is essential for cranial but not spinal motor neuron induction. Development. 1999;126:2727–2737. doi: 10.1242/dev.126.12.2727. [DOI] [PubMed] [Google Scholar]
- Clift D, Richendrfer H, Thorn RJ, Colwill RM, Creton R. High-throughput analysis of behavior in zebrafish larvae: effects of feeding. Zebrafish. 2014;11:455–461. doi: 10.1089/zeb.2014.0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curado S, Stainier DY, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat Protoc. 2008;3:948–954. doi: 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demski LS, Knigge KM. The telencephalon and hypothalamus of the bluegill (Lepomis macrochirus): Evoked feeding, aggressive and reproductive behavior with representative frontal sections. The Journal of Comparative Neurology. 1971;143:1–16. doi: 10.1002/cne.901430102. [DOI] [PubMed] [Google Scholar]
- Drabek D, Guy J, Craig R, Grosveld F. The expression of bacterial nitroreductase in transgenic mice results in specific cell killing by the prodrug CB1954. Gene Ther. 1997;4:93–100. doi: 10.1038/sj.gt.3300366. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Farley RD, Kimmel CB, Schabtach E. Functional development in the Mauthner cell system of embryos and larvae of the zebra fish. J Neurobiol. 1977;8:151–172. doi: 10.1002/neu.480080207. [DOI] [PubMed] [Google Scholar]
- Farber SA, Pack M, Ho SY, Johnson ID, Wagner DS, Dosch R, Mullins MC, Hendrickson HS, Hendrickson EK, Halpern ME. Genetic analysis of digestive physiology using fluorescent phospholipid reporters. Science. 2001;292:1385–1388. doi: 10.1126/science.1060418. [DOI] [PubMed] [Google Scholar]
- Field HA, Kelley KA, Martell L, Goldstein AM, Serluca FC. Analysis of gastrointestinal physiology using a novel intestinal transit assay in zebrafish. Neurogastroenterol Motil. 2009;21:304–312. doi: 10.1111/j.1365-2982.2008.01234.x. [DOI] [PubMed] [Google Scholar]
- Filosa A, Barker AJ, Dal Maschio M, Baier H. Feeding State Modulates Behavioral Choice and Processing of Prey Stimuli in the Zebrafish Tectum. Cell reports. 2016;13:30361–30368. doi: 10.1016/j.neuron.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Gahtan E, Tanger P, Baier H. Visual prey capture in larval zebrafish is controlled by identified reticulospinal neurons downstream of the tectum. J Neurosci. 2005;25:9294–9303. doi: 10.1523/JNEUROSCI.2678-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy R, Noble S, Yoon K, Anisman H, Ekker M. Chemogenetic ablation of dopaminergic neurons leads to transient locomotor impairments in zebrafish larvae. J Neurochem. 2015;135:249–260. doi: 10.1111/jnc.13214. [DOI] [PubMed] [Google Scholar]
- Granato M, van EF, Schach U, Trowe T, Brand M, Furutani SM, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nusslein VC. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- Grimm RJ. Feeding behavior and electrical stimulation of the brain of Carassius auratus. Science. 1960;131:162–163. doi: 10.1126/science.131.3394.162. [DOI] [PubMed] [Google Scholar]
- Guthrie S. Patterning and axon guidance of cranial motor neurons. Nat Rev Neurosci. 2007;8:859–871. doi: 10.1038/nrn2254. [DOI] [PubMed] [Google Scholar]
- Hamdani EH, Kasumyan A, Doving KB. Is feeding behaviour in crucian carp mediated by the lateral olfactory tract? Chem Senses. 2001;26:1133–1138. doi: 10.1093/chemse/26.9.1133. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Hotta Y, Okamoto H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J Neurosci. 2000;20:206–218. doi: 10.1523/JNEUROSCI.20-01-00206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, Okamoto H, Ueno N, Hotta Y, Eguchi G. High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin. Dev Biol. 1997;192:289–299. doi: 10.1006/dbio.1997.8779. [DOI] [PubMed] [Google Scholar]
- Jordi J, Guggiana-Nilo D, Soucy E, Song EY, Lei Wee C, Engert F. A high-throughput assay for quantifying appetite and digestive dynamics. Am J Physiol Regul Integr Comp Physiol. 2015;309:R345–357. doi: 10.1152/ajpregu.00225.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstrom RO, Trowe T, Klostermann S, Baier H, Brand M, Crawford AD, Grunewald B, Haffter P, Hoffmann H, Meyer SU, Muller BK, Richter S, van-Eeden FJ, Nusslein-Volhard C, Bonhoeffer F. Zebrafish mutations affecting retinotectal axon pathfinding. Development. 1996;123:427–438. doi: 10.1242/dev.123.1.427. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–1115. doi: 10.1126/science.274.5290.1109. [DOI] [PubMed] [Google Scholar]
- Mapp OM, Wanner SJ, Rohrschneider MR, Prince VE. Prickle1b mediates interpretation of migratory cues during zebrafish facial branchiomotor neuron migration. Dev Dyn. 2010;239:1596–1608. doi: 10.1002/dvdy.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur KL, Fetcho J. Key features of structural and functional organization of zebrafish facial motor neurons are resilient to disruption of neuronal migration. Curr Biol. 2017 doi: 10.1016/j.cub.2017.05.033. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchin JE, Williams VC, Hinits Y, Low S, Tandon P, Fan CM, Rawls JF, Hughes SM. Oesophageal and sternohyal muscle fibres are novel Pax3-dependent migratory somite derivatives essential for ingestion. Development. 2013;140:2972–2984. doi: 10.1242/dev.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JP, Farber SA. High-fat Feeding Paradigm for Larval Zebrafish: Feeding, Live Imaging, and Quantification of Food Intake. J Vis Exp. 2016;(116) doi: 10.3791/54735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinetti CC, Migliarini B, Olivotto I, Coletti G, Amici A, Carnevali O. Appetite regulation: the central role of melatonin in Danio rerio. Hormones and behavior. 2010;58:780–785. doi: 10.1016/j.yhbeh.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HM, Voigt MM. Peripheral glia have a pivotal role in the initial response to axon degeneration of peripheral sensory neurons in zebrafish. PLoS ONE. 2014;9:e103283. doi: 10.1371/journal.pone.0103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Hamaoka T, Aizawa H, Hosoya T, Okamoto H. Genetic single-cell mosaic analysis implicates ephrinB2 reverse signaling in projections from the posterior tectum to the hindbrain in zebrafish. J Neurosci. 2007;27:5271–5279. doi: 10.1523/JNEUROSCI.0883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Hirano M, Nishimura Y, Tanaka T. A high-throughput fluorescence-based assay system for appetite-regulating gene and drug screening. PLoS One. 2012;7:e52549. doi: 10.1371/journal.pone.0052549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer M, Lumsden A, Ariza-McNaughton L, Bradley A, Krumlauf R. Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1. Nature. 1996;384:630–634. doi: 10.1038/384630a0. [DOI] [PubMed] [Google Scholar]
- Uemura O, Okada Y, Ando H, Guedj M, Higashijima S, Shimazaki T, Chino N, Okano H, Okamoto H. Comparative functional genomics revealed conservation and diversification of three enhancers of the isl1 gene for motor and sensory neuron-specific expression. Dev Biol. 2005;278:587–606. doi: 10.1016/j.ydbio.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Vanderlaan G, Tyurina OV, Karlstrom RO, Chandrasekhar A. Gli function is essential for motor neuron induction in zebrafish. Dev Biol. 2005;282:550–570. doi: 10.1016/j.ydbio.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkoff H, Canosa LF, Unniappan S, Cerda-Reverter JM, Bernier NJ, Kelly SP, Peter RE. Neuropeptides and the control of food intake in fish. Gen Comp Endocrinol. 2005;142:3–19. doi: 10.1016/j.ygcen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book University of Oregon. Eugene, OR: 1995. [Google Scholar]
- Yokobori E, Azuma M, Nishiguchi R, Kang KS, Kamijo M, Uchiyama M, Matsuda K. Neuropeptide Y stimulates food intake in the Zebrafish, Danio rerio. J Neuroendocrinol. 2012;24:766–773. doi: 10.1111/j.1365-2826.2012.02281.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1:
Chemical ablation of branchiomotor neurons. All panels show dorsal views of the hindbrain in 6 dpf (before treatment with Metronidazole (Mtz)) and 7 dpf (after treatment with Mtz) larvae. Tg(zcrest:NTR-mCherry) fish were sorted as “weak” and “strong” according to intensity of mCherry expression in branchiomotor neurons (brackets, Pre-treatment, mCherry). GFP expression in branchiomotor neurons due to the islet1:GFP transgene (brackets and arrowheads, Pre-treatment, GFP) was equally strong in all mCherry-expressing larvae. White asterisks indicate oculomotor and trochlear motor neurons in the midbrain, which do not express the NTR-mCherry transgene. Following overnight incubation with Mtz, NTR-mCherry-expressing larvae exhibited either a significant decrease (~50% loss, “weak effect”) or a severe loss (>80% loss, “strong effect”) of branchiomotor neurons, assayed by examining mCherry and GFP expression (brackets and arrowheads, Post-treatment). As expected, midbrain motor neurons (white asterisks) were not affected by Mtz treatment. The bright patch of mCherry expression (black asterisk) is an artifact.
Figure S2:
Quantification of swimming in 7 dpf larvae. (A) Individual larvae were placed in 24 well plates and their swimming behavior was recorded over a 20 second period by time-lapse imaging (5 frames per sec). (B) The outline of a larva (A) in successive frames was generated manually and the swim path was generated using DIAS image analysis software. (C) The instantaneous velocity was plotted for the larval path generated in (B) for each interval (0.2 sec). (D) Extracting swimming bouts. An instantaneous velocity of 0.2 cm/sec or lower was considered noise. Any peaks of instantaneous velocity above 0.2 cm/sec were defined as bouts, when the larva swam rapidly to a new location.
Figure S3:
Effects of laser ablation procedure. (A-C′) Dorsal views with anterior to the left of the hindbrain of 7 dpf Tg(isl1:gfp) larvae from the indicated treatments. Upper panels (A-C) show brightfield (partial DIC) images and lower panels (A′-C′) show corresponding epifluorescent images from the same Tg(isl1:gfp) larvae. Manipulating the larvae for laser ablation by embedding in agarose (B,B′) had no effect on any neuronal populations (arrowheads, asterisk) compared to an unembedded larva (A,A′). Even laser irradiation of tissue (white ovals) in an embedded larva (C,C′) had no effect on the number of trigeminal motor neurons (arrowheads) and midbrain cranial motor neurons (asterisk) adjacent to the irradiated areas.


