Abstract
Background
Prior human research indicates robust, positive relations between impulsive choice (i.e., preference for smaller, immediate over larger, delayed rewards) and alcohol-use disorders. However, varied findings in the nonhuman literature reveal a relatively ambiguous relation between impulsive choice and alcohol consumption in rodents. Moreover, few rodent studies have investigated potential relations between impulsive choice and common covariates of alcohol consumption (e.g., avidity for sweet substances or anxiety-like behavior).
Methods
Ninety-two male Long-Evans rats completed an impulsive-choice task. From this larger sample, extreme high- and low-impulsive groups (n = 30 each) were retained for further testing. In separate tests, subsequent open-field behavior and consumption of oral alcohol (12% wt/vol) and isocaloric sucrose were examined. Impulsive choice was then retested to examine whether behavior remained stable over the course of the experiment.
Results
No significant relations emerged between impulsive choice and either alcohol or sucrose consumption. However, impulsive choice predicted greater anxiety-like behavior (avoidance of the center field, defecation) in the open-field test. In turn, greater anxiety predicted lower alcohol and sucrose consumption. Finally, choice remained generally stable across the experiment, although impulsive rats tended toward less impulsive choice in the retest.
Conclusions
Although impulsive choice and alcohol consumption appear to share some variance with anxiety-like behavior, the present data offer no support for a relation between impulsive choice and alcohol consumption in Long-Evans rats. Together with mixed rodent data from prior reports, these findings attenuate cross-species comparisons to human relations between impulsive choice and alcohol-use disorders.
Keywords: impulsive choice, delay discounting, alcohol, sucrose, anxiety
Impulsivity is a multi-dimensional construct comprising an array of prematurely expressed, poorly planned, or otherwise maladaptive behavioral forms (Bickel et al., 2012). Impulsive choice describes one such form and, across species, has been operationalized as preference for smaller, relatively immediate rewards over larger, more delayed rewards. In humans, impulsive choice in laboratory tasks is strongly associated with substance-use disorders (for meta-analysis, see MacKillop et al., 2011).This association is not solely due to the effects of drug toxicity on impulsive choice, as impulsive choice in human longitudinal studies has been shown to precede and predict adoption of substance use (e.g., Audrain-McGovern et al., 2009; Kim-Spoon et al., 2013). Likewise, impulsive choice in rats reliably predicts greater self-administration of psychostimulant drugs, such as cocaine (e.g., Perry et al., 2005; for review, see Stein and Madden, 2013).
When combining data across species, impulsive choice appears to play a substantive role (primary or mediational) in vulnerability to drugs of abuse. However, despite a clear association between impulsive choice and human alcohol-use disorders, the literature on impulsive choice and rodent alcohol consumption is relatively mixed. Poulos et al. (1995) first reported that impulsive choice in a screening task predicted greater alcohol consumption in outbred rats. Likewise, rat and mouse lines bred for differential alcohol consumption (or preference) have shown directional differences in impulsive choice consistent with that reported by Poulos et al. (Oberlin and Grahame, 2009; Wilhelm and Mitchell, 2008).
Further review of the literature, however, indicates mixed relations between impulsive choice and rodent alcohol consumption. For example, inbred C57BL/6J mice consume more alcohol (e.g., Belknap et al., 1993; Risinger et al., 1998) but are less impulsive (Helms et al., 2006) than DBA/2J mice, indicating that impulsive choice and alcohol consumption do not perfectly co-vary. Likewise, some rat and mouse lines bred for high alcohol consumption or preference have shown lower levels of impulsive choice than comparison lines (Wilhelm et al., 20071; Wilhelm and Mitchell, 2012). Additional work indicates greater impulsive choice in a selectively bred rat line expressing high alcohol consumption and seeking (i.e., appetitive responding), but not in a selectively bred line expressing high consumption and only moderate seeking (Beckwith and Czachowski, 2014)—a finding that suggests that the relation between impulsive choice and selective breeding is specific to the high-alcohol-seeking phenotype.
Studies of outbred rats raise additional uncertainty regarding the relation between impulsive choice and rodent alcohol consumption. In one study, impulsive choice failed to predict acquisition of instrumental alcohol self-administration and economic demand for self-administered alcohol (i.e., the degree to which rats will defend consumption against increasing response requirements; Diergaarde et al., 2012). In other studies, experimental reduction of impulsive choice (via training or pharmacological variables) has either not affected (Oberlin et al., 2010; Stein et al., 2015) or increased (Stein et al., 2013) alcohol consumption—counter to the occasionally positive relation reported between these variables (e.g., Poulos et al. 1995).
In contrast to the mixed nonhuman literature on impulsive choice and alcohol consumption, consistent positive correlations have been reported between alcohol consumption and avidity for sweet substances (e.g., Bachmanov et al., 1996; Belknap et al., 1993; Kampov-Polevoy et al., 2004). Such covariance indicates common sources of control in the consumption of both alcohol and sweetened solutions (e.g., taste or hedonic value). However, few have examined avidity for sweet substances in studies on alcohol consumption and impulsive choice.
In the present study, we examined the degree to which impulsive choice in a sample of outbred rats would predict subsequent consumption of alcohol (12% wt/vol) and isocaloric sucrose (21% wt/vol). We screened a sample of outbred Long-Evans rats on an impulsive-choice task, selecting extreme high- and low-impulsive groups (HiI and LoI, respectively; n = 30 each) for subsequent testing. We made no specific hypotheses regarding between-group differences in alcohol consumption (given the mixed data reviewed above), but sought to determine whether this additional investigation would help clarify the literature. However, given the relatively consistent literature on the relation between alcohol and avidity for sweet substances, we hypothesized that the difference in sucrose consumption between LoI and HiI rats would mirror the observed directional difference in alcohol consumption (positive, null, or negative). We also examined anxiety-like behavior in an open-field test, as prior evidence indicates associations between anxiety and alcohol consumption (e.g., Henniger et al., 2002) and because little is known about how anxiety relates to impulsive choice in nonhumans.
Materials and Methods
Subjects
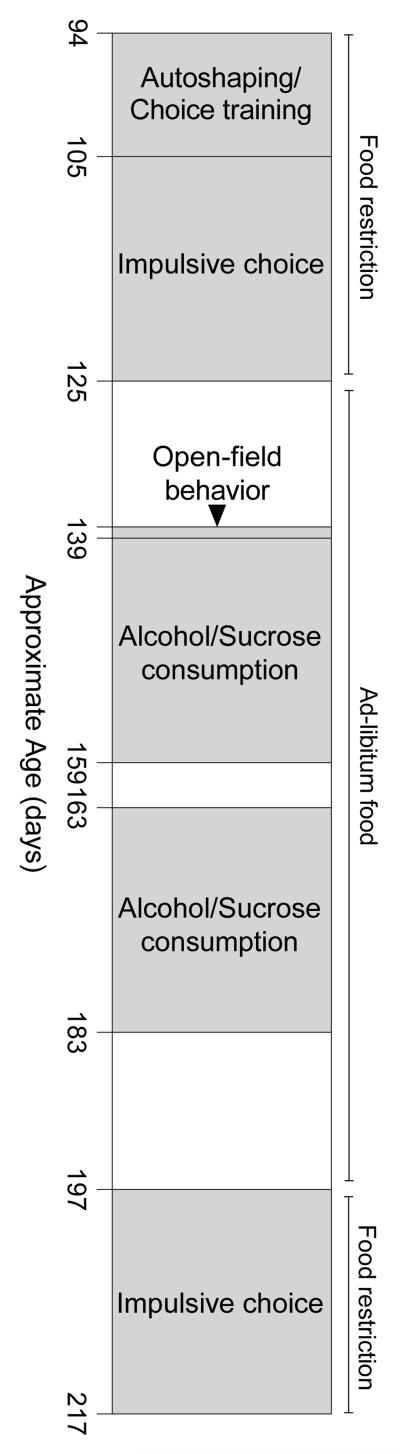
Subjects were 92 experimentally naïve, male Long-Evans rats (Harlan Sprague-Dawley, Indianapolis, IN), completing the experiment in two consecutive cohorts (n = 46 each; Cohorts 1 and 2). Rats were approximately 90 days old at intake and were housed individually in polycarbonate cages in a humidity- and temperature-controlled room on a 12-hr light/dark cycle (lights on at 7:00 a.m.). Water was available continuously in the home cage. The use of ad-libitum feeding or food restriction varied by experimental condition (see Figure 1). All animals were maintained under the standards of the Institutional Animal Care and Use Committee of Utah State University.
Fig. 1.
Order and approximate duration (in days) of experimental conditions. See textfor details. White space indicates periods in which experimental sessions were not completed.
Apparatus and Materials
Thirty identical operant conditioning chambers were used (Med Associates, St. Albans, VT). Each chamber was equipped with a white-noise speaker and housed within a sound-attenuating cubicle. In the center of the rear wall and on opposing sides of the front wall (6.5 cm above the grid floor) were retractable response levers. A cue light was positioned above all levers. A pellet feeder equipped with a photocell beam to verify reward delivery dispensed grain-based pellets (45 mg; Bio-Serv, Frenchtown, NJ) into a food receptacle between the levers.
One open-field arena was used to test anxiety-like behavior. The arena (41 × 41 × 41 cm) consisted of four black acrylic walls and a white acrylic floor. The room was equipped with a white-noise speaker and illuminated by ambient light of approximately 60 lux intensity at the level of the arena floor. Sessions were recorded using a digital video camera (Logitech, Inc., Newark, CA); behavior was analyzed using a combination of video tracking software (Smart, ver. 3.0, Coulbourn Instruments, Whitehall, PA) and manual scoring.
Fifteen identical polycarbonate cages were used to examine alcohol and sucrose consumption. Cages were equipped with two glass drinking tubes (Dyets, Inc., Bethlehem, PA) located above glassware bowls to contain potential leakage. The experimental room was equipped with a white-noise speaker and was illuminated by a 40W red light.
Procedures
Figure 1 depicts the order and approximate duration of all conditions. All behavioral procedures below, with the exception of group assignment, have been described in more detail elsewhere (Stein et al., 2015).
Autoshaping and choice training
Using an autoshaping procedure, rats were trained to respond on rear and side levers. Lever pressing was trained until rats earned ≥ 90% of available rewards for two consecutive sessions.
Next, rats completed choice-training sessions, the purpose of which was to minimize variance in sensitivity to differences in reward magnitude. At each trial, a single response on the left or right lever produced immediate delivery of either 1 or 3 food pellets, depending on lever (counterbalanced across rats). Sessions consisted of 60 trials, divided into three, 20-trial blocks.
Impulsive choice
Impulsive choice was assessed using a version of the within-session, increasing-delay task (Evenden and Ryan, 1996). Trial and session structures were identical to those described for choice-training sessions, with the following exceptions. In the first trial block, the delay to both rewards was 0 s. In the second and third trial blocks, the large-reward delay increased, respectively, to 15 and 30 s. In order to ensure continued sensitivity to differences in reward magnitude, two 0-s probe sessions (identical to choice-training sessions) were pseudorandomly interspersed among the delay sessions described above. Rats completed 20 sessions in the impulsive-choice test, with no 0-s probe sessions programmed over the terminal six sessions.
Upon completion of the impulsive-choice test, percent large-reward choice across delays (last six sessions) was used to calculate the area under each rat’s impulsive-choice curve (AUC; Myerson et al., 2001). Rats with AUC values in the approximate upper and lower tertiles of the distribution (LoI and HiI, respectively; n = 30 each) were retained for further testing.
Open-field behavior
In the open-field test, rats were placed in the arena and allowed to move freely for 10 minutes. Testing took place between 7:00 and 9:00 am.
Alcohol and sucrose consumption
Alcohol or sucrose testing began on the day following the open-field test. To determine the order of alcohol and sucrose tests, rats were matched into pairs based on AUC; from each pair, rats were randomly assigned to one of two orders: alcohol first, sucrose second (Alc-Suc) or sucrose first, alcohol second (Suc-Alc). Each test consisted of 20 daily, 30-min sessions of access to the active test solution and water in separate drinking tubes. Following each session, pre-post differences in weights (0.01 g resolution) of drinking tubes were recorded. Leakage, if present, was subtracted from consumption measures.
Impulsive-choice retest
Following alcohol and sucrose tests, the impulsive-choice retest was conducted as described for the initial test.
Data analysis
All analyses were conducted in SPSS (ver. 21, SPSS Inc., Chicago, IL), using a significant alpha level of .05.
Dependent measures included (a) days to meet the autoshaping and choice-training criteria during training phases; (b) AUC in the impulsive-choice tests; and (c) entries into the center field (defined via software as an inner square comprising 25% of the total area), distance traveled (m), defecation count (number of fecal boluses), and latency to defecate (min) in the open-field test. Instances in which rats did not defecate during the session were coded as a 10-min latency (the length of the session). For all measures described above, data were examined using t tests or, where data were non-normally distributed and not amenable to transformation, nonparametric Mann-Whitney U or Wilcoxon ranked-sign tests.
In the alcohol and sucrose tests, the 20 test sessions were subdivided into four, 5-session blocks. Dependent measures included mean alcohol or sucrose consumption (g/kg), as well as water consumption (mL/kg), at each session block. Alcohol consumption was non-normally distributed and was natural log-transformed prior to analyses. Sucrose and water consumption did not require transformation. Alcohol and sucrose consumption were analyzed using repeated-measures MANOVA, including group (LoI/HiI) and order (Alc-Suc/Suc-Alc) as between-subjects factors, session block (1-5) as a within-subjects factor, and all two- and three-way interactions. Water consumption during alcohol and sucrose testing was examined using a separate, but identical, MANOVA. Follow-up univariate ANOVA was used where significant effects were observed in MANOVA. Greenhouse-Geisser adjusted degrees of freedom were used where data violated assumptions of sphericity.
Finally, group analyses described above were supplemented by calculating correlations between behavioral measures. Days to criterion during autoshaping was included in this analysis was included based on previously reported negative relations between cue-oriented behavior and impulsive choice (e.g., Flagel et al., 2010). Spearman rho coefficients were used because residuals in many regression analyses were non-normally distributed and heteroscedastic. Kaiser-Mayer-Olsen and Bartlett’s tests were used to examine inter-correlation between consumption across session blocks (both alcohol and sucrose) and Fisher’s z transformation was used to compare the magnitude of observed correlations by order (Alc-Suc vs. Suc-Alc; test vs. retest).
Results
In the test and retest of impulsive choice, no differences emerged between Cohorts 1 and 2 in either LoI or HiI rats (in all cases, U > 83, NS); thus, data in all subsequent analyses were collapsed across cohorts.
Lever and Choice Training
Median number of sessions to complete autoshaping was 5 (IQR: 4.00-6.00) in LoI rats and 6 (IQR: 4.75-8.00) in HiI rats. Median number of sessions to complete choice training was 5 in both LoI (IQR: 4.00-6.25) and HiI (IQR: 4.00-7.00) rats. Neither of these measures differed by group.
Impulsive Choice
Choice was stable across the terminal six sessions, as no main effect of session (F(5, 885) = 0.41, NS) or Session × Delay interaction (F(10, 885) = 0.73, NS) was observed. In the first 0-s probe session, significantly higher AUC was observed in LoI rats (median AUC: .98, IQR: .74-1.00) compared to HiI rats (median AUC: .56, IQR: .24-.84; U = 167.5 p < .0001). No difference in AUC between groups was observed in the second 0-s probe session (U = 369, NS), with median AUC values of .98 in LoI rats (IQR: .74-1.00) and .86 in HiI rats (IQR: .63-1.00).
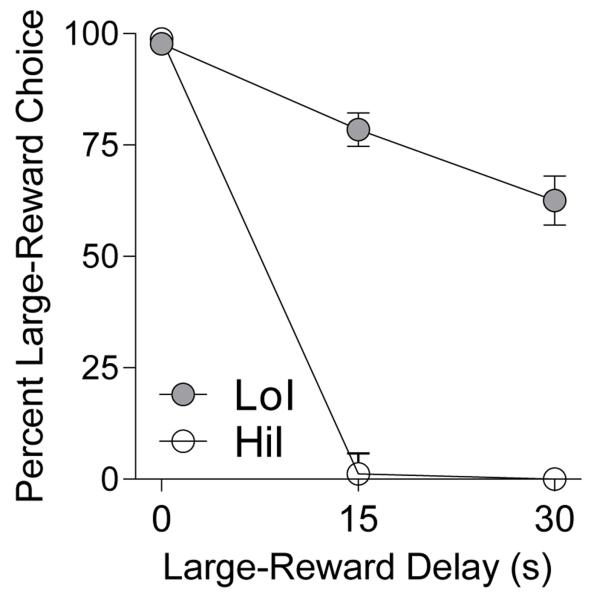
Figure 2 depicts mean (± SEM) percent large-reward choice across delays in the initial impulsive-choice test (terminal six sessions). The tertile-based selection criterion yielded a highly significant difference in AUC between groups (median LoI AUC: .85, IQR: .63-.99; median HiI AUC: .26, IQR: .25-.26; U = 0, p < .001).
Fig. 2.
Mean (±SEM) percent large-reward choice across delays for LoI and HiI rats (n = 30 each) in the initial impulsive-choice test.
Open-field Behavior
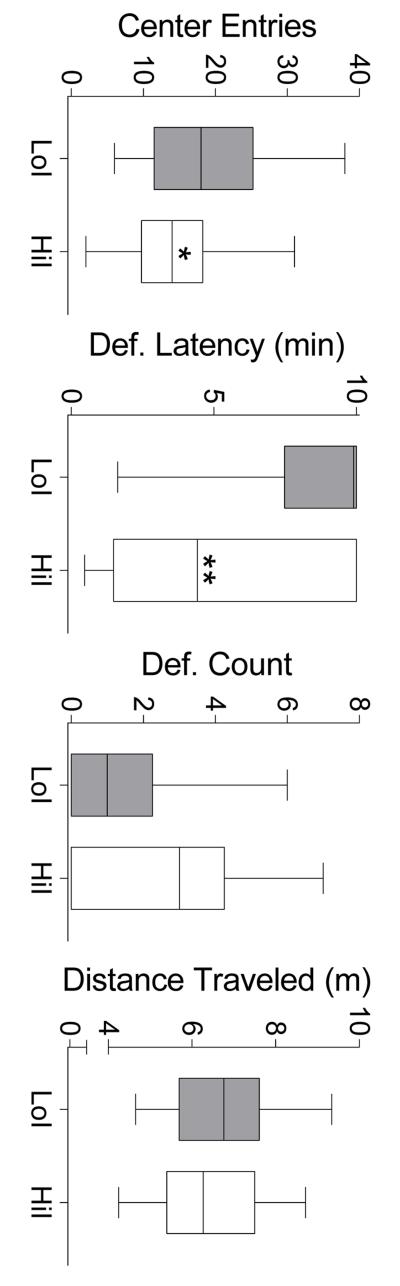
Videos for one LoI and one HiI rat were incomplete (experimenter error), so software-derived open-field measures (center entries and distance traveled) for these rats were excluded from analysis. Defecation data in these rats were unaffected, as defecation count was scored in vivo (post-session) and defecation latency could be derived from incomplete video.
Figure 3 depicts results of the open-field test. Significantly longer defecation latencies (U = 283.5, p < .01) and more center entries (t(58) = 2.57, p < .05) were observed in LoI compared to HiI rats. No group differences in defecation count (U = 343, NS) or distance traveled (t(56) = 1.06, NS) were observed.
Fig. 3.
Behavioral measures from the open-field test in LoI and HiI rats. Horizontal lines within each box indicate group medians, lower and upper box edges indicate interquartile range, and whiskers indicate minimum and maximum observed values *Significantly different than LoI rats (p < .05). **Significantly different than LoI rats (p < .01).
Alcohol and Sucrose Consumption
Data for four sessions (three alcohol and one sucrose, affecting separate rats) were lost due to equipment failure (loss of vacuum seal in drinking tube, causing unmeasurable leakage). Missing data were imputed using linear interpolation.
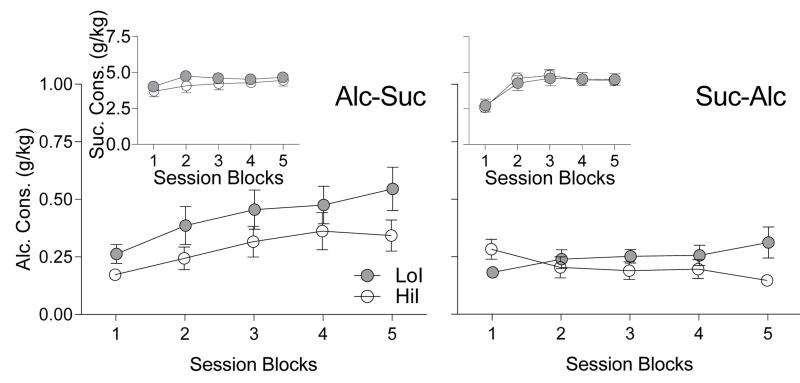
Figure 4 depicts mean alcohol and sucrose consumed (g/kg) per session block in the alcohol and sucrose tests. Results of MANOVA revealed no main effect of group on alcohol and sucrose consumption (F(2, 55) = 1.50, NS, η2 = .05) or interaction between group and any other factor (in all cases, F < 1.32, NS, η2 < .18). Likewise, no main effect of order was observed (F(2, 55) = 2.37, NS, η2 = .08). However, there was a significant main effect of session block (F(8, 49) = 14.03, p < .001, η2 = .70) and an Order × Session Block interaction (F(8, 49) = 4.82, p < .001, η2 = .44). Follow-up univariate tests revealed significant Order × Session Block interactions for both alcohol (F(3.01, 168.79) = 9.28, p < .001, η2 = .14) and sucrose (F(2.96, 165.95) = 7.56, p < .001, η2 = .12). However, no three-way Order × Session Block × Group interaction was observed for either alcohol or sucrose (in both cases, F < 1.77, NS, η2 < .04).
Fig. 4.
Mean alcohol consumed (g/kg) in LoI and HiI rats across session blocks. Insets depict mean sucrose consumed (g/kg). Error bars represent SEM. Depicted are rats from Alc-Suc and Suc-Alc orders (left and right panels, respectively; n = 15 each group, each panel). Each data point represents the mean of four sessions.
Table 1 provides mean water consumption (mL/kg) in LoI and HiI rats during alcohol and sucrose tests. A significant main effect of session block on water consumption was observed (F(8, 49) = 9.17, p < .001, η2 = .60), but no effects of order or group (in both cases, F(2, 55) < 0.41, NS, η2 < .02) or interactions between any factor (F(8, 49) < 1.10, NS, η2 < .16). Follow-up univariate tests indicated a significant effect of session block (consumption declining over blocks) when alcohol was concurrently available (F(3.15, 176.46) = 12.56, p < .001, η2 = .18), but only a marginally significant effect of block when sucrose was available (F(3.63, 203.20) = 2.25, p = .07, η2 = .04).
Table 1.
Water consumption (mL/kg; ± SEM) in LoI and HiI rats during alcohol and sucrose tests.
| Session Block | ||||||
|---|---|---|---|---|---|---|
| Solution | Group | 1 | 2 | 3 | 4 | 5 |
| Alcohol | LoI | 2.15 (0.25) | 1.91 (0.27) | 1.58 (0.17) | 0.96 (0.14) | 0.94 (0.13) |
| HiI | 2.14 (0.30) | 1.81 (0.18) | 1.73 (0.25) | 1.10 (0.15) | 1.22 (0.18) | |
| Sucrose | LoI | 0.67 (0.10) | 0.67 (0.08) | 0.54 (0.09) | 0.50 (0.07) | 0.54 (0.08) |
| HiI | 0.72 (0.08) | 0.53 (0.10) | 0.65 (0.09) | 0.47 (0.07) | 0.66 (0.09) | |
Correlational Analyses
Table 2 provides correlation coefficients between behavioral measures. Consumption measures were highly inter-correlated across session blocks (in both cases, KMO > .80; Bartlett’s χ2 > 246.65, p < .001) and comparisons of coefficients revealed that correlation magnitude differed significantly by order in only one instance (noted below); thus, sucrose and alcohol consumption were collapsed across order and session block.
Table 2.
Spearman rho correlation coefficients between behavioral measures.
| Auto | AUC | Alc. | Suc. | Dist. | C. Ent. | Def. Count |
|
|---|---|---|---|---|---|---|---|
| AUC | −.26 | ||||||
| Alc. | −.41** | .28 | |||||
| Suc. | −.35* | .19 | .36** | ||||
| Dist. | −.14 | .19 | .11 | .26 | |||
| C.Ent. | .07 | .20 | −.01 | −.01 | .59*** | ||
| Def. Count | .43** | −.30* | −.18 | −.50*** | −.25 | −.10 | |
| Def. Lat. | −.36* | .39* | .29* | .57*** | .36* | .20 | −.89*** |
Bolded coefficients indicate statistical significance.
p < .05.
p < .01.
p < .001.
ΩCorrelation differed significantly by order: Alc-Suc rho= .07, Suc-Alc rho = −.51. Auto = days to criterion during autoshaping; AUC = area under the curve; Alc. = mean alcohol consumed (Blocks 1-5); Suc. = mean sucrose consumed (Blocks 1-5); Dist. = distance traveled (m); C. Ent. = center entries; Def. Count = defecation count; Def. Lat. = defecation latency (min).
Days to criterion during autoshaping was significantly correlated with both consumption measures and both defecation measures, indicating that delayed operant learning predicted lower alcohol and sucrose consumption and higher anxiety. However, the correlation between days to criterion and sucrose consumption varied significantly by order (z = 2.33, p < .05; see Table 2).
Consistent with group analyses, AUC was not significantly correlated with alcohol or sucrose consumption (although alcohol and sucrose consumption were significantly correlated with each other). In contrast, AUC was significantly correlated with both defecation measures, indicating that lower levels of impulsive choice were associated with lower anxiety. However, unlike group analyses, AUC did not significantly correlate with center entries.
Defecation measures were significantly correlated with both alcohol and sucrose consumption, although these relations were more consistent for defecation latency than defecation count. Such relations indicate that higher anxiety was associated with lower alcohol and sucrose consumption. Finally, distance traveled was significantly correlated with defecation latency and center entries, but not with any other behavioral measure.
Impulsive-choice Retest
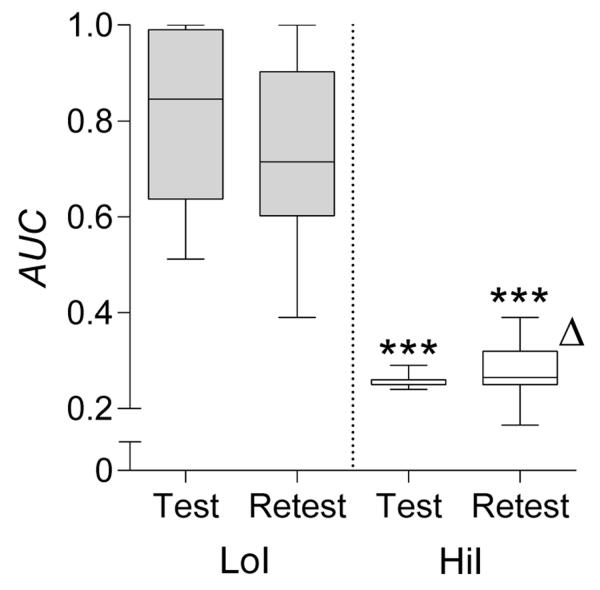
Figure 5 provides comparisons between the impulsive-choice test and retest. Group differences in AUC remained significant at the retest (U = 1, p < .001), with AUC highly correlated across tests (r = .92, p < .001). No test-retest change in AUC was observed in LoI rats (W = −145, NS). In contrast, significantly higher AUC in HiI rats was observed in the retest (W = 127, p < .05).
Fig. 5.
Test and retest AUC values for LoI and HiI rats. Horizontal lines within each box indicate group medians, lower and upper box edges indicate interquartile range, and whiskers indicate minimum and maximum observed values. ***Significantly different than LoI in same test (p < .001). ΔSignificantly different than HiI in prior test (p < .05).
Using retest AUC, we recalculated the AUC correlations presented in Table 2 (AUC × alcohol and sucrose consumption; AUC × open-field measures). In no case did correlations differ from those calculated using AUC from the initial test (in all cases, z <.79, NS). Likewise, instances in which significance was observed (AUC × defecation measures) were consistent across analyses.
Discussion
Results of the present study provide no support for a relation between impulsive choice and either alcohol or sucrose consumption in male Long-Evans rats. Alcohol and sucrose consumption were positively related to each other, but neither measure was predicted by impulsive choice. Instead, results revealed that anxiety-like behavior significantly correlated with both impulsive choice and alcohol and sucrose consumption, but in opposing directions (Fig. 3 and Table 2). That is, anxiety (defecation and center entries) was associated with greater impulsive choice and, in turn, anxiety (defecation, but not center entries) was also associated with lower alcohol and sucrose consumption. Finally, individual differences in impulsive choice remained generally stable across the course of the experiment. Although impulsive choice in HiI rats significantly decreased from test to retest, this change was modest (median AUC difference: .01) and restricted variability in initial AUC values (IQR: .25-.26) likely facilitated statistical significance.
Alcohol Consumption
The absence of a relation between impulsive choice and alcohol consumption contrasts with some prior reports of positive relations between these variables in rodents (Beckwith and Czachowski, 2014; Oberlin and Grahame, 2009; Poulos et al., 1995; Wilhelm and Mitchell, 2008). Although alcohol consumption differed visually between LoI and HiI groups in the present study (Fig. 4), this difference was not significant and tended in a direction opposite to what would be observed if greater impulsive choice predisposed organisms toward alcohol reward. Together with other reports (Diergaarde et al., 2012; Helms et al., 2006; Oberlin et al., 2010; Stein et al., 2013, 2015; Wilhelm et al., 2007; Wilhelm and Mitchell, 2012), the present data indicate that positive covariance between impulsive choice and rodent alcohol consumption is not a generalized phenomenon.
By contrast, the relation between human impulsive choice and alcohol-use disorders is relatively robust. In a recent meta-analysis, MacKillop et al. (2011) reviewed 17 studies comparing impulsive choice in alcohol abusing populations and controls. These authors reported greater impulsive choice in alcohol abusers in 65% of the studies examined, with the remaining studies reporting no relation. Of particular interest, group differences in impulsive choice were significantly more robust in populations meeting clinical criteria for alcohol dependence than in subclinical populations. Given this finding, examination of outbred rats in this and prior studies (Diergaarde et al., 2012; Poulos et al., 1995; Stein et al., 2013) may be of limited utility in understanding the phenomenology of human impulsive choice and alcohol dependence. However, we note that examinations of alcohol-preferring inbred or selectively bred rodents (putative models of alcohol dependence) have yielded mixed results similar to those observed in outbred rats (Beckwith and Czachowski, 2014; Helms et al., 2006; Oberlin et al., 2009; Wilhelm et al., 2007; Wilhelm and Mitchell, 2008, 2012). Thus, in consideration of the concerns outlined above, caution is warranted when interpreting rodent data on impulsive choice and alcohol consumption.
Sucrose Consumption
In the present study, we observed no relation between impulsive choice and sucrose consumption (for a similar finding, see Koffarnus and Woods, 2013). However, sucrose and alcohol consumption were positively related to each other, reproducing prior data in outbred and selectively bred rats (e.g., Bachmanov et al., 1996). Importantly, such covariance is not unique to the nonhuman literature, as greater avidity for sweet substances has also been associated with alcohol dependence in humans (see Kampov-Polevoy et al., 1999).
Across species, consumption of alcohol and sweet substances is thought to be mediated by similar biological mechanisms, including taste, feeding-related neuropeptides, and dopamine and opioid systems (for reviews, see Fortuna, 2010; Leggio et al., 2011). Thus, because consumption of alcohol and sucrose share similar sources of control, the failure of impulsive choice to predict alcohol consumption in the present study is consistent with its failure to predict sucrose consumption. Nonetheless, impulsive choice and avidity for sweet substances have previously been shown to co-vary with selective breeding. Specifically, Perry et al. (2007) reported lower levels of impulsive choice in rats bred for high saccharin consumption (HiS) compared to rats bred for low saccharin consumption (LoS). Interestingly, more-impulsive HiS rats have been shown elsewhere to consume more alcohol than LoS rats (Dess et al., 1998); however, scarcity of additional data prevents further interpretation.
Anxiety
Impulsive choice in the present study predicted greater anxiety-like behavior (i.e., more defecation, shorter defecation latencies, and more center entries). This finding represents a relatively novel contribution to the nonhuman literature, although the underlying mechanism remains unclear. One possibility is that pre-existing anxiety interfered with learning of response-reward contingencies (evidenced by significant correlations between defecation measures and days to criterion during autoshaping; Table 2). Such interference would likely be more pronounced when imposing response-reward delays during impulsive-choice testing, producing preference for immediate over delayed food in anxious rats. Whatever the mechanism, a relation between anxiety and impulsive choice would not be unique to rodents, as self-report measures of anxiety (e.g., Rounds et al., 2007) and elevated cortisol levels (Takahashi, 2004) have been associated with impulsive choice in humans. However, because experimental studies have indicated mixed effects of anxiolytic drugs in humans (Acheson et al., 2006; Reynolds et al., 2004) and nonhumans (e.g., Cardinal et al., 2000; Evenden and Ryan, 1996; Huskinson and Anderson, 2012), further investigation is necessary to determine whether anxiety plays a causal role in impulsive choice.
Whereas impulsive choice predicted greater anxiety-like behavior in the present study, anxiety-like behavior predicted lower alcohol and sucrose consumption. This negative relation between anxiety and consumption reproduces some prior findings (e.g., Henniger et al., 2002; Möller et al., 2006) and is consistent with reports in which anxiolytic and anxiogenic drugs have been shown to increase and decrease, respectively, alcohol consumption in rodents (e.g., Hedlund and Wahlström, 1997; June et al., 1994). However, our findings conflict with additional data in which anxiety is instead associated with higher consumption of alcohol in both rodents and humans (e.g., Spanagel et al., 2002). Given these conflicting reports (for review, see Sharko et al., 2013), the role of anxiety in alcohol consumption requires further study. Nonetheless, anxiety in the present study may have obstructed the otherwise positive relation between impulsive choice and alcohol consumption reported previously (e.g., Poulos et al., 1995). Future research may be designed to explore this putative influence by administering anxiolytic medication prior to alcohol sessions or examining whether the direction in which impulsive choice relates to anxiety in the sample under investigation (e.g., outbred strains or selectively bred lines) determines the relation between impulsive choice and alcohol consumption.
Limitations
Three limitations of the present study deserve note. First, the level of alcohol consumption we observed was relatively modest. If alcohol’s pharmacological effects (as opposed to taste) drive the relation between consumption and impulsive choice, the present methods (e.g., use of outbred rats) were perhaps not ideal to test this relation. However, considering only rats exposed to alcohol first, mean rate of consumption across the final three session blocks was 0.46-0.55 in LoI rats and 0.32-0.45 g/kg/30 in HiI rats, indicating that consumption in the majority of rats far outpaced alcohol’s elimination rate (0.15 g/kg/30 min in outbred strains; Wallgren and Barry, 1970). Consumption levels in individual LoI rats at or above group means were consistent with those producing pharmacologically relevant blood alcohol concentration in prior studies (approximately 35 mg/dl and above; e.g., Rassnick et al., 1993; Roberts et al., 1999). Conversely, consumption in LoI rats below group means (and the majority of HiI rats) likely did not produce pharmacologically relevant blood alcohol levels. With these considerations in mind, the relation between impulsive choice and alcohol consumption can still be tested meaningfully with the relatively modest consumption observed here, although our conclusions more closely regard “acquisition,” rather than degree, of pharmacologically motivated consumption.
The same cannot be said, however, for rats exposed to alcohol second, as these rats consumed considerably less alcohol than those in the opposite order (i.e., 0.25-0.31 and 0.19-0.15 g/kg/30 in LoI and HiI rats, respectively, across the final three session blocks). Although we observed no significant main effect of order on consumption, the significant Order × Session Block interaction (perhaps due to negative contrast; Crespi, 1942) indicates that crossover designs may be inappropriate for future research on this topic.
Second, because we examined each phenotype using only a single task, our conclusions are specific to the individual tasks chosen. Whether similar results would be general across varying impulsive-choice, alcohol, or anxiety paradigms remains unresolved. Among many possibilities, future research could be designed to determine whether relations between impulsive choice and alcohol consumption depend on the task used to measure impulsive choice (e.g., the increasing-delay task used here vs. the adjusting-delay task; Mazur, 1987). Such investigations may indicate that inconsistent relations owe to the recruitment of varying behavioral processes across tasks (Peterson et al., 2015; Tanno et al., 2014; but also see Craig et al., 2014).
Third, and finally, we examined only the extreme upper and lower tertiles of the impulsive-choice distribution on subsequent behavioral measures. Examination of the behavior of extreme groups is a long-standing practice in psychological research (Kelley, 1939) that increases statistical power compared to full-range methods (e.g., Feldt 1961). Moreover, this method is common in the literature on impulsive choice and drug self-administration (e.g., Perry et al., 2005). However, elimination of the middle portion of the impulsive-choice distribution may prohibit identification of potentially nonlinear relations. In future research, we advocate for examination of the full range of the impulsive-choice distribution.
Conclusions
Despite relatively robust relations between impulsive choice and alcohol- and other substance-use disorders in humans (for review, see MacKillop et al., 2011) and psychostimulant self-administration in rodents (for review, see Stein and Madden, 2013), the present and prior studies have revealed few consistent relations between impulsive choice and alcohol consumption in rodents. The relation between impulsive choice and anxiety-like behavior in the present study represents a potentially important finding, but one that should be examined in multiple paradigms in future research (e.g., the elevated-plus maze or direct measurement of stress hormones).
Acknowledgements
Portions of these data were presented at the 2013 and 2014 domestic meetings of the Association for Behavior Analysis International, the 2013 international meeting of the Association for Behavior Analysis International, and the 2013 Behavior Change, Health, and Health Disparities Conference. A report on this research will be submitted by the first author in partial fulfillment of the degree of Doctor of Philosophy. The first author thanks Mrs. Marie Borg for her generous donations to maintain the Walter R. Borg Scholarship Fund. All authors thank Patrick Johnson for assistance in the design of this experiment, as well as Dallin Everitt, Kaitlin Olsen, and McKelle Tobey for assistance in data collection. The first author is now at Virginia Tech Carilion Research Institute.
This research was supported by the National Institutes of Health grant 1R01DA029605 (GJM) and the Walter R. Borg Scholarship from the Department of Psychology, Utah State University (JSS).
Footnotes
In Wilhelm et al. (2007), this effect was specific to short delays (2-4 s) and was not observed in global measures of impulsive choice.
References
- Acheson A, Reynolds B, Richards JB, de Wit H. Diazepam impairs behavioral inhibition but not delay discounting or risk taking in healthy adults. Exp Clin Psychopharmacol. 2006;14:190–198. doi: 10.1037/1064-1297.14.2.190. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend. 2009;103:99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behav Genet. 1996;26:563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith S, Czachowski CL. Increased delay discounting tracks with a high ethanol-seeking phenotype and subsequent ethanol seeking but not consumption. Alcohol Clin Exp Res. 2014;38:2607–2614. doi: 10.1111/acer.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM, McClure SM. Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology. 2012;221:361–387. doi: 10.1007/s00213-012-2689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacol. 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Craig AR, Maxfield AD, Stein JS, Renda CR, Madden GJ. Do the adjusting-delay and increasing-delay tasks measure the same construct: delay discounting? Behav Pharmacol. 2014;25:306–315. doi: 10.1097/FBP.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi LP. Quantitative variation of incentive and performance in the white rat. Am J Psychol. 1942:467–517. [Google Scholar]
- Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16:275–278. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, van Mourik Y, Pattij T, Schoffelmeer AN, de Vries TJ. Poor impulse control predicts inelastic demand for nicotine but not alcohol in rats. Addict Biol. 2012;17:576–587. doi: 10.1111/j.1369-1600.2011.00376.x. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacol. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Feldt LS. The use of extreme groups to test for the presence of a relationship. Psychometrika. 1961;26:307–316. [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacol. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs. 2010;42:147–151. doi: 10.1080/02791072.2010.10400687. [DOI] [PubMed] [Google Scholar]
- Hedlund L, Wahlström G. The effect of diazepam on voluntary ethanol intake in a rat model of alcoholism. Alcohol Alcoholism. 1998;33:207–219. doi: 10.1093/oxfordjournals.alcalc.a008384. [DOI] [PubMed] [Google Scholar]
- Helms CM, Reeves JM, Mitchell SH. Impact of strain and d-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology. 2006;188:144–151. doi: 10.1007/s00213-006-0478-0. [DOI] [PubMed] [Google Scholar]
- Henniger MS, Spanagel R, Wigger A, Landgraf R, Hölter SM. Alcohol self-administration in two rat lines selectively bred for extremes in anxiety-related behavior. Neuropsychopharmacol. 2002;26:729–736. doi: 10.1016/S0893-133X(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Huskinson SL, Anderson KG. Effects of acute and chronic administration of diazepam on delay discounting in Lewis and Fischer 344 rats. Behav Pharmacol. 2012;23:315–330. doi: 10.1097/FBP.0b013e3283564da4. [DOI] [PubMed] [Google Scholar]
- June HL, Murphy JM, Mellor-Burke JJ, Lumeng L, Li TK. The benzodiazepine inverse agonist Ro19-4603 exerts prolonged and selective suppression of ethanol intake in alcohol-preferring (P) rats. Psychopharmacol. 1994;115:325–331. doi: 10.1007/BF02245073. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Janowsky DS. Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol Alcoholism. 1999;34:386–395. doi: 10.1093/alcalc/34.3.386. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Eick C, Boland G, Khalitov E, Crews FT. Sweet liking, novelty seeking, and gender predict alcoholic status. Alcohol Clin Exp Res. 2004;28:1291–1298. doi: 10.1097/01.alc.0000137808.69482.75. [DOI] [PubMed] [Google Scholar]
- Kelley TL. The selection of upper and lower groups for the validation of test items. J Educ Psychol. 1939;30:17. [Google Scholar]
- Kim-Spoon J, McCullough ME, Bickel WK, Farley JP, Longo GS. Longitudinal associations among religiousness, delay discounting, and substance use initiation in early adolescence. J Res Adol. 2014;43:745–756. doi: 10.1111/jora.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Woods JH. Individual difference in discount rate are associated with demand for self-administered cocaine, but not sucrose. Addict Biol. 2013;18:8–18. doi: 10.1111/j.1369-1600.2011.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Addolorato G, Cippitelli A, Jerlhag E, Kampov-Polevoy AB, Swift RM. Role of feeding-related pathways in alcohol dependence: a focus on sweet preference, NPY, and ghrelin. Alcohol Clin Exp Res. 2011;35:194–202. doi: 10.1111/j.1530-0277.2010.01334.x. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analyses of Behavior: V. The Effect of Delay and of Intervening Events on Reinforcement Value. Lawrence Erlbaum; Hillsdale, New Jersey: 1987. pp. 55–73. [Google Scholar]
- Möller C, Wiklund L, Thorsell A, Hyytiá P, Heilig M. Decreased measures of experimental anxiety in rats bred for high alcohol preference. Alcohol Clin Exp Res. 1997;21:656–660. [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res. 2009;33:1294–1303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Bristow RE, Heighton ME, Grahame NJ. Pharmacologic dissociation between impulsivity and alcohol drinking in high alcohol preferring mice. Alcohol Clin Exp Res. 2010;64:1363–1375. doi: 10.1111/j.1530-0277.2010.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JR, Hill CC, Kirkpatrick K. Measurement of impulsive choice in rats: Same- and alternate-form test–retest reliability and temporal tracking. J Exp Anal Behav. 2015 doi: 10.1002/jeab.124. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Be. 2007;86:822–837. doi: 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–814. [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. SDZ-205,152, a novel dopamine receptor agonist, reduces oral ethanol self-administration in rats. Alcohol. 1993;10:127–132. doi: 10.1016/0741-8329(93)90091-2. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Dassinger M, de Wit H. Therapeutic doses of diazepam do not alter impulsive behavior in humans. Pharmacol Biochem Behav. 2004;79:17–24. doi: 10.1016/j.pbb.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Brown MM, Doan AM, Oakes RA. Mouse strain differences in oral operant ethanol reinforcement under continuous access conditions. Alcohol Clin Exp Res. 1998;22:677–684. doi: 10.1111/j.1530-0277.1998.tb04311.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Koob GF. Operant self administration of sweetened versus unsweetened ethanol: effects on blood alcohol levels. Alcohol Clin Exp Res. 1999;23:1151–1157. [PubMed] [Google Scholar]
- Rounds JS, Beck JG, Grant DM. Is the delay discounting paradigm useful in understanding social anxiety? Behav Res Ther. 2007;45:729–735. doi: 10.1016/j.brat.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Sharko AC, Fadel JR, Wilson MA. Mechanisms and mediators of the relationship between anxiety disorders and alcohol use disorders: focus on amygdalar NPY. J Addict Res Ther. 2013;S 4:2–11. [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, Shoaib M, Holsboer F, Landgraf R. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology. 1995;122:369–373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- Stein JS, Johnson PS, Renda CR, Smits RR, Liston KJ, Shahan TA, Madden GJ. Early and prolonged exposure to reward delay: effects of impulsive choice on alcohol self-administration in male rats. Exp Clin Psychopharmacol. 2013;21:172–180. doi: 10.1037/a0031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Madden GJ. Delay discounting and drug abuse: empirical, conceptual, and methodological considerations, in The Wiley-Blackwell Handbook of Addiction Psychopharmacology. In: MacKillop J, de Wit H, editors. Wiley-Blackwell; Oxford, UK: 2013. pp. 165–208. [Google Scholar]
- Stein JS, Renda CR, Hinnenkamp JE, Madden GJ. Impulsive choice, alcohol consumption, and pre exposure to delayed rewards: II. Potential mechanisms. J Exp Anal Behav. 2015 doi: 10.1002/jeab.116. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T. Cortisol levels and time-discounting of monetary gain in humans. Neuroreport. 2004;15:2145–2147. doi: 10.1097/00001756-200409150-00029. [DOI] [PubMed] [Google Scholar]
- Tanno T, Maguire DR, Henson C, France CP. Effects of amphetamine and methylphenidate on delay discounting in rats: interactions with order of delay presentation. Psychopharmacol. 2014;231:85–95. doi: 10.1007/s00213-013-3209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallgren H, Barry H. Actions of alcohol: I. Biochemical, physiological and psychological aspects. Elsevier Publishing; New York: 1970. [Google Scholar]
- Wilhelm CJ, Reeves JM, Phillips TJ, Mitchell SH. Mouse lines selected for alcohol consumption differ on certain measures of impulsivity. Alcohol Clin Exp Res. 2007;31:1839–1845. doi: 10.1111/j.1530-0277.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes Brain Behav. 2008;7:705–713. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Acute ethanol does not always affect delay discounting in rats selected to prefer or avoid ethanol. Alcohol alcohol. 2012;47:518–524. doi: 10.1093/alcalc/ags059. [DOI] [PMC free article] [PubMed] [Google Scholar]