Abstract
BACKGROUND
Proton magnetic resonance spectroscopy can be used to assess brain integrity and maturation with age.
OBJECTIVE
To compare regional cerebral magnetic resonance spectroscopy metabolite ratios in extremely low birth weight and healthy term control infants measured at term-equivalent age and to evaluate association between magnetic resonance spectroscopy metabolites and cognitive and language development at 18-22 months’ corrected age.
METHODS
Single-voxel point-resolved spectroscopy sequence was performed in a prospective cohort of 43 infants. Magnetic resonance spectroscopy metabolite ratios of N-acetylaspartate to choline-containing compounds and N-acetylaspartate to myo-inositiol in the hippocampus, cortex, and subventricular zone were associated with Bayley mental, cognitive, and language scores at 18-22 months’ corrected age.
RESULTS
The mean (±S.D.) gestation of the 31 extremely low birth weight population was 25 (±1.1) weeks and mean (±S.D.) birth weight was 749 (±133.9) g. Compared with healthy term control infants, extremely low birth weight infants exhibited consistently lower N-acetylaspartate-to-choline-containing compounds ratios in our three regions of interest, with differences reaching statistical significance for the subventricular zone and cortex regions. In multiple linear regression analyses, N-acetylaspartate-to-choline-containing compounds ratio in the subventricular zone, N-acetylaspartate-to-choline-containing compounds ratio in the cortex, and N-acetylaspartate-to-myo-inositiol ratio in the subventricular zone were significantly associated with Bayley mental scores at 18-22 months’ corrected age.
CONCLUSIONS
Magnetic resonance spectroscopy metabolite abnormalities at term-equivalent age appear to be significantly associated with cognitive and language development in extremely low birth weight infants.
Keywords: proton magnetic resonance spectroscopy (MRS), extremely preterm infants, extremely low birth weight (ELBW) infants, neurodevelopmental outcome
Introduction
Up to 29% to 40% of extremely preterm survivors develop cognitive and language impairments.1–4 Yet we currently lack accurate prognostic markers for early risk stratification. It is currently not possible to accurately diagnose cognitive and language impairments until early childhood.5 Early identification of high-risk infants at term-equivalent age can reap immediate benefits through aggressive administration of early intervention services.6 Cranial ultrasound exhibits poor sensitivity for detecting diffuse white matter abnormalities7,8 and predicting neurodevelopmental impairments (NDI).9 Although conventional magnetic resonance imaging (MRI) is better at detecting diffuse white matter abnormalities, it remains subjective and inaccurate at predicting cognitive impairments.10 Quantitative MRI techniques, such as diffusion tensor imaging and proton magnetic resonance spectroscopy (MRS), offer an objective measure of cerebral microstructure and in vivo biochemistry, respectively.11–15
In MRS, different metabolites have characteristic resonant frequencies that allow their identification for quantification.16 Tissue metabolic changes likely precede macrostructural and functional changes that can be detected soon after injury or abnormal development with a high degree of specificity. Therefore tissue metabolites may serve as important early diagnostic and prognostic biomarkers. In a recent meta-analysis of MRI studies in term infants with hypoxic-ischemic encephalopathy (HIE), MRS metabolite ratios exhibited the highest accuracy in predicting neurodevelopmental outcomes.17 However, not much is known about the ability of MRS biomarkers to predict NDI in extremely preterm infants. Our goal was to compare regional cerebral MRS metabolite ratios in extremely low birth weight (ELBW; birth weight ≤1000 g) and healthy term control infants measured at term-equivalent age (term) and to evaluate the association of MRS metabolites at term-equivalent age with cognitive and language development at 18-22 months’ corrected age.
Methods
Patient population
All ELBW infants from the Children’s Memorial Hermann Hospital Neonatal Intensive Care Unit who survived to ≥34 weeks’ postmenstrual age were eligible for the study. All healthy term newborns ≥37 weeks’ gestation and with weight appropriate for gestation from the well-baby nursery were also eligible. Enrolled infants underwent MRI at 38 weeks’ postmenstrual age or before discharge, if discharge occurred earlier. ELBW infants were enrolled between January 2008 and July 2009 and term infants between July 2008 and January 2010. ELBW infants with known congenital central nervous system anomalies, chromosomal anomaly, or high mechanical ventilatory support (mean airway pressure >15 and supplemental oxygen >50%) at the time of enrollment were excluded. The exclusion criteria for the term group were gestation ≥42 weeks, any congenital or chromosomal anomaly, multiple gestation pregnancy, maternal medical or pregnancy conditions, predelivery hospital admission, intrauterine exposures (drugs of abuse, cigarettes, alcohol, and medications with known or suspected neurological effects [steroids]), high forceps or vacuum delivery, perinatal distress or complications, neonatal intensive care unit admission, abnormal neurological examination, or suspected or known infection at birth. The hospital and University Institutional Review Board approved the study, and written informed consent was obtained from each infant’s parent or guardian before enrollment.
Image acquisition and processing
Before the initiation of MRI scans, all infants were fed and swaddled. Med-Vac infant vacuum splint (CFI Medical Solutions, Fenton, MI), Insta-Putty Silicone Earplugs (E.A.R. Inc, Boulder, CO), and Natus MiniMuffs (Natus Medical Inc, San Carlos, CA) were used for restraint and noise reduction. No sedation was given to any subject. All scans were supervised by an experienced neonatologist, a neonatal transport nurse, and a neonatal research nurse.
Brain proton MRS was performed on a 3-T MRI scanner (Achieva; Philips Medical Systems, Best, the Netherlands) equipped with a 32-channel receiver system. An 8-channel phased-array head coil (Philips Medical Systems) was used for data acquisition. MRS data were acquired using a single-voxel point-resolved spectroscopy sequence from a voxel that was predominantly located in subventricular zone (SVZ), hippocampus (Hip), and frontal cortex (Cortex) areas (all in the right hemisphere; Fig 1). The MRS voxel size was 18 × 10 × 10 mm3. These three regions were selected because they represent vulnerable regions in the preterm brain and for assessing the presence of neural progenitor cells in these regions18 as part of a secondary project. The acquisition parameters were repetition time = 2000 ms, echo time = 35 ms, spectral width = 2000 Hz, 1024 data points, and 128 averages. Total acquisition time for each region was about 5 minutes. Axial, coronal, and sagittal images were acquired to position the MRS voxel location and regional saturation technique bands. To guarantee consistent quality MRS data and data acquisition at the precise locations with the same acquisition parameters, the same neonatologist, MRI physicist, and technologist were involved in all data collection.
FIGURE 1.
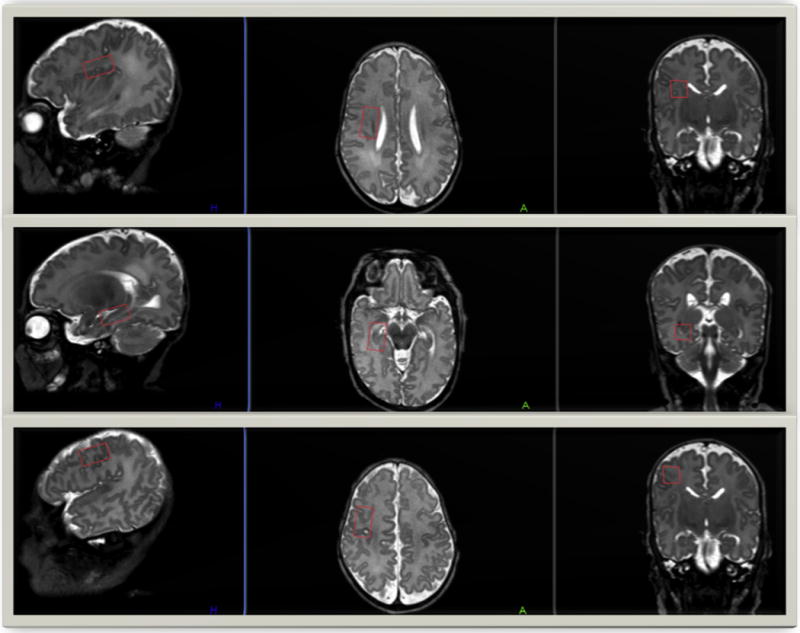
Single-voxel placement locations in the three cerebral regions of interest. T2-weighted MRI images in the sagittal, axial, and coronal planes display the single-voxel placements in our three regions of interest—the right subventricular zone (top), the right hippocampus (center), and the right frontal cortex (bottom). MRI, magnetic resonance imaging. (Color version of the figure is available in the online edition.)
The well-developed linear combination model-fitting procedure (LCModel; Steven Provencher Inc, Oakville, Ontario, Canada, version 6.2-1)19 was used for quantitative analysis of the MRS data. This software automatically identifies and quantifies relative values of several well-characterized metabolites, including N-acetylaspartate (NAA) at 2.02 ppm, phosphocreatine and/or creatine (Cr) at 3.02 ppm, choline-containing compounds (Cho) at 3.22 ppm, and myo-inositiol (mI) at 3.56 ppm. Figure 2 is a representative MRS spectrum output for a study subject depicting the metabolic profile in the SVZ and analyzed using LCModel. We identified three cerebral metabolite ratios, NAA to Cr, Cho to Cr, and mI to Cr, in our three areas of interest, SVZ, Hip, and Cortex. Based on prior research17,20 and to limit multiple comparisons, we only chose NAA-to-Cho and NAA-to-mI ratios as our main biomarkers of interest. Any estimated ratio with >20% S.D. (Cramer-Rao lower bounds) was considered to be unreliable and deleted from the data analysis.19 All analyses were blinded to clinical variables, cranial ultrasound, and anatomic MRI findings. All conventional MRI scans were read by a neuroradiologist, which included using standardized assessment or definitions of white and gray matter maturation, signal abnormalities (e.g., hemorrhage, leu- komalacia), and white matter diffuse excessive high signal intensity abnormalities.
FIGURE 2.
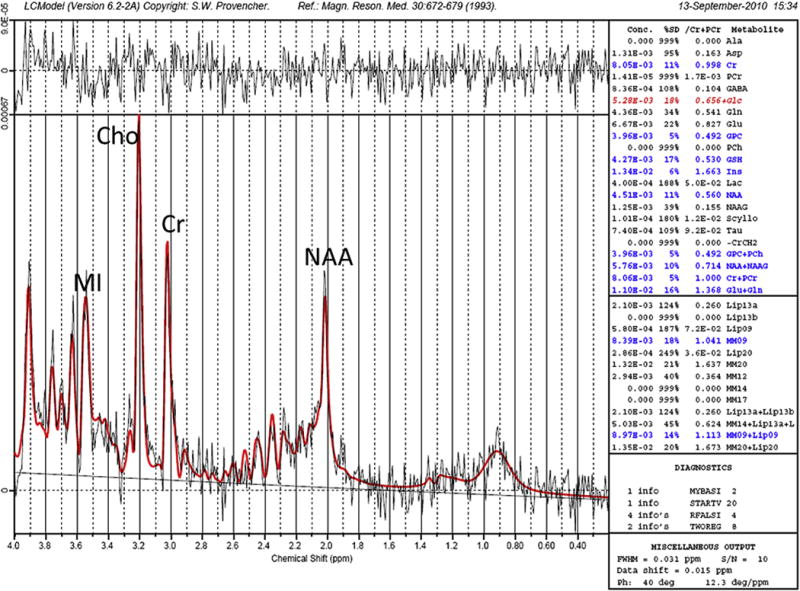
Example of an MRS spectrum from the subventricular zone in an ELBW infant. LCModel software was used to quantify N-acetylaspartate (NAA), choline- containing compounds (Cho), and myo-inositol (MI) and standardized to creatine (Cr). ELBW, extremely low birth weight. (Color version of the figure is available in the online edition.)
Neurodevelopmental follow-up and assessment
Study infants were monitored at 18-22 months’ corrected age in the High Risk Follow-up Clinic at the University of Texas Health Science Center at Houston. At the time of the follow-up visit, all patients underwent a complete neuromotor examination to assess gross motor function and ascertain presence of cerebral palsy by standardized National Institute of Child Health and Human Development Neonatal Research Network examiners. Additionally, a masked and certified examiner administered the cognitive and language subtests of the Bayley Scales of Infant and Toddler Development–III.21
Our primary outcome of interest—an individual infant’s average of the Bayley-III cognitive and language scores—was labeled the mental score. This composite score approximates the content of the Bayley II Mental Developmental Index score22 and was chosen because it permits a single primary outcome and facilitates comparison to studies using the second edition of the Bayley Scales test. Individual cognitive and language scores on the Bayley-III were evaluated independently as secondary outcomes.
Statistical analysis
Stata/IC 12 (StataCorp, College Station, TX) was used for all data analyses. Maternal, perinatal, and neonatal detailed history and data were prospectively collected for all enrolled ELBW infants during their neonatal intensive care unit stay. At the time of follow-up, additional data regarding demographics and socioeconomic and health statuses were also collected. All data were entered into a secure database with error checks by qualified neonatal research nurses. All unique identifiers were removed to protect privacy and to blind investigators to the clinical evaluation.
Simple linear regression was used to associate the two metabolite ratios (NAA to Cho and NAA to mI) in the three areas of interest with Bayley scores at 18-22 months’ corrected age. All linear regression analyses were statistically adjusted by postmenstrual age at MRI, because postmenstrual age at MRI is an important confounder that was significantly correlated with our metabolites and all three Bayley scores. Two-sample t tests and Mann-Whitney U tests were used as appropriate to compare metabolite ratios and Bayley scores between the ELBW and term cohorts. P < 0.05 was considered to be statistically significant.
Results
Thirty-eight ELBW and 16 term infants were enrolled in the study. High-quality MRS data (metabolite ratio S.D. <20%) were obtained in 31 of the 38 ELBW infants. Followup information at 18-22 months’ corrected age (mean chronological age was 22.6 months) was available in 30 of 31 (96%) ELBW infants. Of these 30, one infant died after discharge and three were too uncooperative to receive a complete Bayley examination. For 5 ELBW infants, because of time constraints, data acquisition was only feasible in the SVZ region. High-quality MRS data were available in 12 of 16 term infants. Follow-up information at 18-22 months’ corrected age was available for 11 of these 12 term infants.
Demographic and clinical characteristics of the infants are presented in Table 1. The mean (±S.D.) gestation of our ELBW population was 25 (±1.1) weeks and mean (±S.D.) birth weight was 749 (±133.9) g. There were a considerably higher proportion of boys in the ELBW cohort. The mean postmenstrual age at MRI scan was 39 weeks in both groups. Of the 31 ELBW infants, 11 had normal anatomic MRI scans, 18 exhibited mild injury, and 2 exhibited moderate-to-severe injury.
TABLE 1.
Baseline Characteristics of Study Infants
| Characteristics | Term (n = 12) | ELBW (n = 31) |
|---|---|---|
| Gestation at birth, weeks (mean ± S.D.) | 38.5 ± 0.9 | 25.2 ± 1.1 |
| Birth weight, g (mean ± S.D.) | 3135.3 ± 415.3 | 748.9 ± 133.9 |
| Men, n (%) | 4 (33.3) | 20 (64.5) |
| Maternal age, yr (mean ± S.D.) | 24.3 ± 5.7 | 25.9 ± 4.7 |
| Antenatal steroids, full course, n (%) | N/A | 17 (54.8) |
| White matter injury on HUS, n(%) | N/A | 6 (19.4) |
| Bronchopulmonary dysplasia (NIH), n (%) | N/A | 25 (80.6) |
| Late onset sepsis, n (%) | N/A | 7 (22.6) |
| Retinopathy of prematurity, n (%) | N/A | 23 (74.2) |
| Necrotizing enterocolitis, n (%) | N/A | 3 (9.7) |
| Major surgery (general anesthesia), n (%) | N/A | 3 (9.7) |
| Anatomic MRI at term, moderate-to-severe injury, n (%) | 0 (0) | 2 (6.5) |
| Postmenstrual age at MRI, weeks (mean ± S.D.) | 38.8 ± 0.9 | 38.7 ± 2.1 |
Abbreviations:
ELBW = Extremely low birth weight
MRI = Magnetic resonance imaging
N/A = Not available
NIH = National Institutes of Health
We compared the metabolite ratios between the preterm and the term populations in the three areas of interest (Table 2). As compared with healthy term control infants, ELBW infants exhibited consistently lower NAA-to-Cho ratios in our three regions of interest, with differences reaching statistical significance for the SVZ and Cortex regions. Regional NAA-to-mI values were comparable between the two study groups.
TABLE 2.
Comparison of Metabolite Ratios in Healthy Term vs ELBW Infants at Term-Equivalent Age
| Metabolite Ratios | Term (n = 12) Mean (S.D.) |
ELBW (n = 31) Mean (S.D.) |
P Value |
|---|---|---|---|
| NAA/Cho in SVZ | 1.9 (0.2) | 1.7 (0.3) | 0.03 |
| NAA/Cho in Hip | 1.7 (0.2) | 1.6 (0.3) | 0.07 |
| NAA/Cho in Cortex | 2.0 (0.3) | 1.6 (0.3) | 0.01 |
| NAA/mI in SVZ | 0.6 (0.09) | 0.6 (0.2) | 0.57 |
| NAA/mI in Hip | 0.6 (0.07) | 0.6 (0.2) | 0.67 |
| NAA/mI in Cortex | 0.5 (0.08) | 0.5 (0.1) | 0.43 |
Neurodevelopmental outcomes at 18-22 months’ corrected age between term and ELBW infants are listed in Table 3. Cerebral palsy was not considered as an outcome measure because of its low incidence and resulting limited study power. Although ELBW infants scored consistently lower than term control infants on the Bayley mental, cognitive, and language scales, these differences did not reach statistical significance. However, term control infants exhibited lower scores than reported in several recent studies.23,24
TABLE 3.
Neurodevelopmental Outcomes at 18-22 Months’ Corrected Age in Study Infants
| Developmental Outcome | Term (n = 11) Median (IQR) |
ELBW (n = 30) Median (IQR) |
P value |
|---|---|---|---|
| Cerebral palsy, n (%) | 0 (0%) | 2 (6.7%) | N/A |
| Bayley-III mental score | 94 (90-106) | 90 (79-101)* | 0.19 |
| Bayley-III cognitive score | 90 (90-110) | 90 (80-100)† | 0.35 |
| Bayley-III language score | 97 (89-103) | 86 (74-97)‡ | 0.17 |
Abbreviations:
IQR = Interquartile range
N/A = Not available
n = 26.
n = 26.
n = 25.
In multiple linear regression analyses, NAA to Cho in the SVZ, NAA to Cho in the Cortex, and NAA to mI in the SVZ were significantly associated with Bayley mental scores at 18-22 months’ corrected age (Table 4). In secondary analyses, NAA-to-Cho ratios in the SVZ (P = 0.01) and the Cortex (P = 0.04) and NAA-to-mI ratios in the SVZ (P = 0.03) were significantly associated with Bayley language scores.
TABLE 4.
MRS Metabolite Ratios at Term-Equivalent Age and Association With Mental Scale Score at 18-22 Months’ Corrected Age in Study Infants
| MRS Metabolite | Coefficient (95% CI) | P Value |
|---|---|---|
| NAA/Cho in SVZ | 23.2 (1.7-44.7) | 0.04 |
| NAA/Cho in Hip | −11.7 (−32.9 to 9.7) | 0.27 |
| NAA/Cho in Cortex | 19.1 (0.9-37.4) | 0.04 |
| NAA/mI in SVZ | 44.2 (−1.1 to 89.5) | 0.06 |
| NAA/mI in Hip | −22.9 (−64.9 to 19.1) | 0.27 |
| NAA/mI in Cortex | 39.1 (−19.6 to 97.9) | 0.18 |
Abbreviations:
Cho = Choline-containing compounds
CI = Confidence interval
Cortex = Frontal cortex
Hip = Hippocampus
mI = Myo-Inositol
NAA = N-acetylaspartate
SVZ = Subventricular zone
Discussion
In this pilot study, we identified significantly lower NAA-to-Cho ratios in the SVZ and Cortex of ELBW infants as compared with healthy term infants. This metabolite ratio in the SVZ and Cortex also significantly correlated with standardized mental and language scores at 18-22 months’ corrected age. Only 2 of 31 study infants had severe injury on conventional MRI. The remaining 94% had mild or no injury. Van Kooij et al.25 used a similar single-voxel MRS approach in the cerebellum in very preterm infants with no or mild injury (87%) also found a significant association between NAA-to-Cho ratios and Bayley-III scores. A recent study26 found lower NAA-to-Cho and higher Cho-to-Cr ratios and worse executive function in 2- to 4-year-old healthy very low birth weight infants without any brain injury as compared with age-matched term control infants. More recently, Chau et al.,27 using multivoxel magnetic resonance spectroscopic imaging, reported on an important correlation between slower increase in NAA-to-Cho ratio in the basal ganglia and several white matter regions early in life and adverse Bayley-III scores in a large cohort of very preterm infants.
NAA is a marker of neuronal activity that is known to increase with brain maturity, whereas Cho, a marker for membrane turnover and myelination, decreases with age as the rapid neonatal brain growth slows in infancy.16 Vigneron et al.12 observed lower NAA-to-Cho ratio in the basal ganglia and thalamus of nine preterm infants as compared with eight term infants. Gadin et al.28 reported significantly lower NAA-to-Cho ratio in the periventricular parietal region of 20 very preterm infants as compared with four term infants.
A considerable majority of infants with NDI at 2 years of age or later exhibit no structural injury on cranial ultrasound or conventional MRI at term.9,10 Regional differences in brain metabolites in ELBW infants as compared with healthy term infants may represent a delay in brain maturation or reflect abnormal changes consistent with subtle injury that is invisible or difficult to diagnose on conventional qualitative imaging at term. In a recent study, Phillips et al.26 found lower NAA-to-Cho ratio in frontal lobe white matter at 3-4 years of age in very low birth weight infants as compared with term controls. This suggests that metabolic abnormalities that likely develop in the neonatal period in preterm infants may persist into early childhood.
Unlike several other contemporary cohorts,23,24 our inner city population of healthy term controls exhibited lower Bayley scores. Most other cohorts have reported higher scores between 105 and 110 than the test mean of 100. Such low scores in our control group, in addition to our smaller sample size, may have contributed to our inability to detect significant differences in scores with the ELBW population, despite ELBW infants consistently scoring lower than the term control infants.
Brain metabolite ratios, including NAA to Cho, NAA, Cr, lactate to NAA, lactate to Cho, and lactate to Cr, have been assessed in a number of studies of term infants with HIE to predict neurodevelopmental outcomes.29,30 Basal ganglia mI-to-Cr ratios were associated with MRI changes of severe injury and poor neurodevelopmental outcome at 1 year of age in this population.20 In a recent meta-analysis,17 deep nuclear gray matter lactate-to-NAA and lactate-to-Cr ratios were found to be the most accurate predictors at term of neurodevelopmental outcomes in term infants with HIE. While studies in preterm infants are limited, one recent study found punctate white matter lesions to be associated with increased glutamate on MRS, suggesting glutamate excitotoxicity may play a key role in the pathogenesis of such white matter lesions.31
In our study, NAA-to-Cho ratios in the SVZ and Cortex also exhibited significant correlation with our primary outcome of Bayley mental scores and secondary outcome of Bayley language scores. Three reported studies have examined the relationship of MRS cerebral metabolites and neurodevelopment in preterm infants. Augustine et al.32 did not identify any correlations between metabolite ratios in 36 very low birth weight preterm infants at the level of the basal ganglia and supraventricular level and Bayley mental and psychomotor scores at 18-24 months’ corrected age. Use of categorical rather than continuous outcomes and the small number of infants (n = 8) with abnormal neurodevelopmental outcomes likely resulted in reduced study power to find meaningful correlations. Furthermore, there was a strong correlation with age at MRI scan that was not factored into the analyses. Gadin et al.28 examined motor outcomes at 6 months in 20 very preterm infants and found no correlation with NAA-to-Cho ratio. The small sample size and limited duration of follow-up may have contributed to a negative study. More recently, cerebellar NAA-to-Cho ratio in 53 preterm infants at term-equivalent age were positively associated with cognition at 2 years of age.25 Similar to our study, infants were imaged using a higher resolution 3-T scanner and tested using the third edition of the Bayley Scales developmental test, and linear regression analyses were used to predict Bayley scores on a continuous scale.
Disturbances in cerebral metabolite ratios have also been correlated with postnatal clinical factors. In a large cohort of very preterm infants with MRS scans within 2 weeks of birth, Card et al.33 demonstrated that NAA-to-Cr ratio was significantly lower and NAA-to-Cho ratios indicated a trend toward lower levels in infants with white matter injury as observed on conventional MRI. In a different cohort of preterm infants, metabolite ratios of NAA to Cho were found to be significantly lower in infants with white matter injury.34 The same group has also reported a significant negative relationship between NAA-to-Cho ratio in preterm infants and postnatal infections.35
No prior studies in preterm infants have examined metabolites in the SVZ, Cortex, and Hip, regions susceptible to brain maturational delay and/or injury. Two of these regions, the SVZ and Hip, are also rich in neural progenitor cells.18 As such, injury to these two regions could result in aberrant brain development and NDI. Therefore, long-term follow-up of our cohort is important.
We recognize the noteworthy limitations in our study. Larger voxel volume was used to improve signal-to-noise ratio. This affected tissue specificity and resulted in regions of interest including nontargeted tissues such as cerebrospinal fluid (for Hip) and gray matter (for Cortex). We were willing to accept this compromise in order to gain signal. Our method of MRS acquisition did not permit determination of absolute metabolite values. This may have allowed us to determine if altered NAA-to-Cho ratio was due to changes in NAA, Cho, or both. Owing to our small sample size, we were unable to examine the independent effects of cerebral metabolites over known perinatal factors such as intraventricular hemorrhage, maternal education, or abnormal conventional neuroimaging that are known to affect neurodevelopmental outcomes in ELBW infants. Serial longitudinal measurements may reflect brain matura- tional delay and/or injury more accurately than a single measurement at term. Also, because 2-year cognitive outcomes only modestly correlate with long-term intelligence5 it is also important to correlate MRS biomarkers with school-age neurocognitive outcomes.
Our study was strengthened by the use of a high-resolution 3-T scanner, a meticulously selected term control cohort with strict exclusion criteria, use of a well-established automated MRS postprocessing program, and enrollment of a higher risk population of ELBW infants than previously reported.
Conclusions
ELBW infants exhibit regional metabolic abnormalities at term as measured by spectroscopy. These abnormalities likely reflect delays in neural maturation or abnormal changes consistent with subtle injury as suggested by the correlation with cognitive and language development at 18-22 months’ corrected age. A larger longitudinal study will be essential to determine if such metabolite abnormalities and correlation with long-term cognitive, language, and behavioral outcomes are present in this high-risk population. If our findings are validated, MRS biomarkers could play a complementary role in identifying ELBW infants with normal conventional MRI scans who remain at high-risk for poor neurodevelopmental outcomes.
Acknowledgments
The funding agencies played no role in the design, conduct, or analysis of the trial. The authors take full responsibility for the integrity of the data and analyses. We sincerely thank Vipulkumar S. Patel, RT, for assistance with magnetic resonance imaging data acquisition. Grant funding was from National Center for Research Resources (NCRR) grant UL1 RR024148 (University of Texas Health Science Center at Houston Center for Clinical and Translational Sciences) and NCRR/Eunice Shriver National Institute of Child Health and Human Development grant UL1 RR024148-04S3 (Best Pharmaceuticals for Children Act), and the Research Institute at Nationwide Children’s Hospital (NAP). The 3-T scanner was partially funded by NCRR/National Institutes of Health through a grant to Ponnada A. Narayana (grant S10 RR19186).
References
- 1.Tyson JE, Parikh NA, Langer J, et al. Intensive care for extreme prematurity—moving beyond gestational age. N Engl J Med. 2008;358:1672–1681. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potharst ES, van Wassenaer AG, Houtzager BA, et al. High incidence of multi-domain disabilities in very preterm children at five years of age. J Pediatr. 2011;159:79–85. doi: 10.1016/j.jpeds.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 3.Serenius F, Källén K, Blennow M, et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA. 2013;309:1810–1820. doi: 10.1001/jama.2013.3786. [DOI] [PubMed] [Google Scholar]
- 4.Moore T, Hennessy EM, Myles J, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. doi: 10.1136/bmj.e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116:333–341. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 6.Spittle A, Orton J, Anderson P, Doyle LW. Early developmental intervention programmes post-hospital discharge to prevent motor and cognitive impairments in preterm infants. Cochrane Database Syst Rev. 2012;12:CD005495. doi: 10.1002/14651858.CD005495.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Inder TE, Anderson NJ, Spencer C, et al. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am J Neuroradiol. 2003;24:805–809. [PMC free article] [PubMed] [Google Scholar]
- 8.Maalouf EF, Duggan PJ, Counsell SJ, et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics. 2001;107:719–727. doi: 10.1542/peds.107.4.719. [DOI] [PubMed] [Google Scholar]
- 9.Laptook AR, O’Shea TM, Shankaran S, et al. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115:673–680. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- 10.Woodard LE, Anderson PJ, Austin NC, et al. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 11.Bartha AI, Yap KR, Miller SP, et al. The normal neonatal brain: MR imaging, diffusion tensor imaging, and 3D MR spectroscopy in healthy term neonates. AJNR Am J Neuroradiol. 2007;28:1015–1021. doi: 10.3174/ajnr.A0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vigneron DB, Barkovich AJ, Noworolski SM, et al. Three-dimensional proton MR spectroscopic imaging of premature and term neonates. AJNR Am J Neuroradiol. 2001;22:1424–1433. [PMC free article] [PubMed] [Google Scholar]
- 13.Ward P, Counsell S, Allsop J, et al. Reduced fractional anisotropy on diffusion tensor magnetic resonance imaging after hypoxic- ischemic encephalopathy. Pediatrics. 2006;117:e619–e630. doi: 10.1542/peds.2005-0545. [DOI] [PubMed] [Google Scholar]
- 14.Hüppi PS, Fusch C, Boesch C, et al. Regional metabolic assessment of human brain during development by proton magnetic resonance spectroscopy in vivo and by high-performance liquid chromatography/gas chromatography in autopsy tissue. Pediatr Res. 1995;37:145–150. doi: 10.1203/00006450-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Roelants-van Rijn AM, van der Grond J, et al. Cerebral structure and metabolism and long-term outcome in small-for-gestational-age preterm neonates. Pediatr Res. 2004;56:285–290. doi: 10.1203/01.PDR.0000132751.09067.3F. [DOI] [PubMed] [Google Scholar]
- 16.Xu D, Bonifacio SL, Charlton NN, et al. MR spectroscopy of normative premature newborns. J Magn Reson Imaging. 2011;33:306–311. doi: 10.1002/jmri.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thayyil S, Chandrasekaran M, Taylor A, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics. 2010;125:e382–e395. doi: 10.1542/peds.2009-1046. [DOI] [PubMed] [Google Scholar]
- 18.Manganas LN, Zhang X, Li Y, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 20.Robertson NJ, Lewis RH, Cowan FM, et al. Early increases in brain myo-inositol measured by proton magnetic resonance spectroscopy in term infants with neonatal encephalopathy. Pediatr Res. 2001;50:692–700. doi: 10.1203/00006450-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Bayley N. Bayley Scales of Infant and Toddler Development. 3rd. San Antonio: Psychological Corporation; 2006. [Google Scholar]
- 22.Moore T, Johnson S, Haider S, et al. Relationship between test scores using the second and third editions of the Bayley Scales in extremely preterm children. J Pediatr. 2012;160:553–558. doi: 10.1016/j.jpeds.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 23.Anderson PJ, De Luca CR, Hutchinson E, et al. Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med. 2010;164:352–356. doi: 10.1001/archpediatrics.2010.20. [DOI] [PubMed] [Google Scholar]
- 24.Vohr BR, Stephens BE, Higgins RD, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr. 2012;161:222–228.e3. doi: 10.1016/j.jpeds.2012.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Kooij BJ, Benders MJ, Anbeek P, et al. Cerebellar volume and proton magnetic resonance spectroscopy at term, and neuro-development at 2 years of age in preterm infants. Dev Med Child Neurol. 2012;54:260–266. doi: 10.1111/j.1469-8749.2011.04168.x. [DOI] [PubMed] [Google Scholar]
- 26.Phillips JP, Ruhl D, Montague E, et al. Anterior cingulate and frontal lobe white matter spectroscopy in early childhood of former very LBW premature infants. Pediatr Res. 2011;69:224–229. doi: 10.1203/PDR.0b013e3182091d52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chau V, Synnes A, Grunau RE, et al. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology. 2013;81:2082–2089. doi: 10.1212/01.wnl.0000437298.43688.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadin E, Lobo M, Paul DA, et al. Volumetric MRI and MRS and early motor development of infants born preterm. Pediatr Phys Ther. 2012;24:38–44. doi: 10.1097/PEP.0b013e31823e069d. [DOI] [PubMed] [Google Scholar]
- 29.Barkovich AJ, Baranski K, Vigneron D, et al. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol. 1999;20:1399–1405. [PMC free article] [PubMed] [Google Scholar]
- 30.Cheong JL, Cady EB, Penrice J, et al. Proton MR spectroscopy in neonates with perinatal cerebral hypoxic-ischemic injury: metabolite peak-area ratios, relaxation times, and absolute concentrations. AJNR Am J Neuroradiol. 2006;27:1546–1554. [PMC free article] [PubMed] [Google Scholar]
- 31.Wisnowski JL, Bluml S, Paquette L, et al. Altered glutamatergic metabolism associated with punctate white matter lesions in pre-term infants. PLoS One. 2013;8:e56880. doi: 10.1371/journal.pone.0056880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Augustine EM, Spielman DM, Barnes PD, et al. Can magnetic resonance spectroscopy predict neurodevelopmental outcome in very low birth weight preterm infants? J Perinatol. 2008;28:611–618. doi: 10.1038/jp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Card D, Nossin-Manor R, Moore AM, et al. Brain metabolite concentrations are associated with illness severity scores and white matter abnormalities in very preterm infants. Pediatr Res. 2013;74:75–81. doi: 10.1038/pr.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chau V, Poskitt KJ, McFadden DE, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009;66:155–164. doi: 10.1002/ana.21713. [DOI] [PubMed] [Google Scholar]
- 35.Chau V, Brant R, Poskitt KJ, et al. Postnatal infection is associated with widespread abnormalities of brain development in premature newborns. Pediatr Res. 2012;71:274–279. doi: 10.1038/pr.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]


