Introduction
The function of acetylcholine in the striatum has perplexed basal ganglia physiologists and clinicians for decades. The effects of cholinergic and anticholinergic agents in patients with movement disorders, as well as in animal models of basal ganglia-dependent behaviors, suggest a critical role for acetylcholine in the normal function of the basal ganglia. The highest density of acetylcholine in the basal ganglia occurs in the input nucleus, the striatum, suggesting it might be the site of action. Striatal acetylcholine predominantly derives from local cholinergic interneurons, which form a dense network of cholinergic fibers throughout the striatum.1 These cholinergic interneurons represent a small percentage of all striatal neurons, but fire tonically and contribute to some of the brain's highest levels of acetylcholine.1-4 Another source of striatal acetylcholine is extrinsic cholinergic input to the striatum, deriving from brainstem structures. Acetylcholine regulates the function of the striatal microcircuit and striatal output, via a combination of nicotinic and muscarinic acetylcholine receptors. Acetylcholine also regulates striatal dopamine release, which may modulate symptoms in disorders such as Parkinson's Disease, dystonia, and Tourette Syndrome. Fluctuations in levels of both transmitters are believed to contribute to pathological circuit activity and drive symptoms, and are a prominent target of current therapeutic agents. In both normal and disease states, dopamine and acetylcholine concomitantly influence striatal projection neurons to affect a broad number of basal ganglia-mediated behaviors, including control of movement, motivation, and decision-making.
The goal of this Perspective is to discuss how recent research has modified our view of the function of striatal cholinergic interneurons. First, we will provide some background on the striatal circuit components and theories regarding acetylcholine's site of action, discussing recent studies that have clarified or altered these ideas. Next, we will discuss the hypothesized role of acetylcholine in several prominent movement disorders. Finally, we will outline some outstanding questions within the field, and how recent technological advances might allow further exploration of the mechanisms by which acetylcholine regulates striatal microcircuitry and basal ganglia-dependent behaviors. We hope this Perspective will inform clinicians and scientists interested in basal ganglia function, and provoke new inquiry regarding the role of striatal acetylcholine in both health and disease.
Striatal Cholinergic Interneurons Within Basal Ganglia Circuitry
The basal ganglia are a group of interconnected subcortical nuclei which participate in the control of movement, cognition, and mood (see Figure 1). The basal ganglia circuitry consists of (1) two input nuclei, the striatum (in primates consisting of the caudate and putamen) and subthalamic nucleus, (2) two output nuclei, the internal segment of the globus pallidus (GPi) and the substantia nigra pars reticulata (SNr), (3) one intrinsic nucleus, the globus pallidus pars externa (GPe), and (4) neuromodulatory inputs, the most clinically important of which derive from dopaminergic neurons arising from the substantial nigra pars compacta (SNc) and the ventral tegmental area (VTA). SNc dopamine neurons primarily innervate the dorsal striatum, whereas VTA neurons primarily innervate the ventral striatum.5
Figure 1.
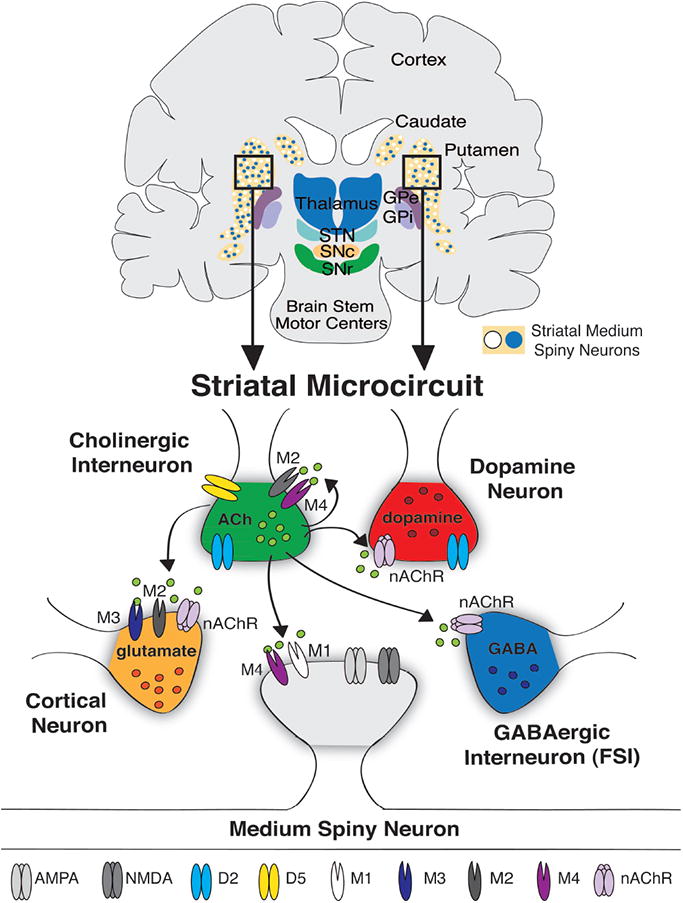
Cholinergic Interneurons in the Striatal Microcircuit. Top: Coronal schematic of the human basal ganglia. Striatal medium spiny neurons are highlighted in the caudate and putamen as blue and white circles. Bottom: Illustration of the distribution of striatal cholinergic receptors and sites of cholinergic regulation (arrows). Abbreviations: D2 (dopamine type 2-like receptor), D5 (dopamine type 5-like receptor), nAChR (nicotinic acetylcholine receptor), M1,2,3, or 4 (muscarinic acetylcholine receptor), ACh (acetylcholine), FSI (fast spiking interneuron) NMDA (N-methyl-D-aspartate receptor), AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor), GABA (γ-Aminobutyric acid).
This review will focus on the role of striatal cholinergic interneurons in regulating striatal function and basal ganglia-related behavior. However, it is critical to mention that striatal cholinergic interneurons are not the only source of acetylcholine within the striatum, and there may be distinct functions associated with cholinergic signals derived from intrastriatal versus extrastriatal sources. In fact, in many historical experiments, systemic administration of pharmacological agents, or even local administration of agents within the striatum, would tend to confuse the role of local cholinergic interneurons and long-range cholinergic neurons projecting to the striatum from other nuclei. These external sources of acetylcholine include nuclei such as the brainstem pedunculopontine tegmental area or laterodorsal tegmental area,6-9 and their distinct functions have been explored through genetic manipulations designed to alter the function of forebrain versus brainstem sources of acetylcholine.10 Extrastriatal sources of acetylcholine, however, are beyond the scope of this review.
To examine how cholinergic interneurons regulate the function of the striatum and the basal ganglia as a whole, it is important to understand where they are situated within striatal microcircuitry. The striatum is the primary input nucleus of the basal ganglia, integrating excitatory inputs from the thalamus and cortex with dopaminergic inputs from the SNc. GABAergic striatal projection neurons, also called medium spiny neurons (MSNs), comprise approximately 90% of all neurons in the striatum.11,12 MSNs can be separated by virtue of their projection targets into two distinct pathways—the direct and indirect pathways, which also have distinct cellular markers.13,14 Direct pathway-forming MSNs express the dopamine D1 receptor15 and project directly to the output nuclei, GPi and SNr. Indirect pathway-forming MSNs, conversely, express the dopamine D2 receptor15 and project via several synapses (indirectly) to the output nuclei. According to the standard model of basal ganglia function, developed by Albin, Penney, Young, and Delong,13,14,16,17 and refined by several investigators,18 the direct pathway is hypothesized to promote movement and reinforcement, while the indirect pathway is believed to inhibit movement and respond to aversive stimuli. While direct proof in vivo is currently lacking, dopaminergic inputs to the striatum are believed to positively modulate direct pathway activity and negatively modulate indirect pathway activity.19-21 This model, although oversimplified, provides a useful framework for understanding the role of cholinergic modulation of striatal output and behavior.
The remaining <10% of striatal neurons are interneurons: GABAergic interneurons22 (including parvalbumin, calretinin, and neuropeptide Y-expressing subtypes) and acetylcholine-releasing interneurons. Striatal cholinergic interneurons are intrinsically active, firing at rates of 8-12 Hz,23 setting them apart from MSNs, which fire between 0-2 Hz under most conditions. Shaped by a combination of voltage-gated sodium channels, hyperpolarization- and cyclic adenosine monophosphate (cAMP)-dependent cation (HCN) channels, and small-conductance calcium-activated (SK-type) potassium channels, cholinergic interneurons fire tonically in the absence of synaptic inputs.24-26 Importantly, the firing pattern of these neurons can change in response to synaptic input or regulation of intrinsic conductances.
Although they are sparse, comprising only 1-2% of all striatal neurons,27 cholinergic interneurons are large, aspiny neurons with extensive axonal arborizations densely covering the entire striatum.4,28-32 Cholinergic axonal varicosities, similar to dopaminergic varicosities, form few structurally defined synaptic connections, suggesting that acetylcholine may signal via ‘volume transmission.’ 31,33-35 Interestingly, cholinergic interneurons play a key role in signaling unexpected rewards and high salience events; evidence came from groundbreaking extracellular recordings from the dorsal striatum of awake behaving monkeys.36-41 These recordings revealed a unique class of neurons termed “tonically active neurons,” TANs, now believed to be cholinergic interneurons.23,42 During Pavlovian learning tasks, when the animal heard a tone or click, a conditioned stimulus corresponding to delivery of reward, TANs showed a brief pause in firing followed by a burst.38-40 The pause response is a neural correlate of classical conditioning within TANs, and has been termed the “conditioned pause response.” 43,44 The cellular and synaptic mechanisms of the pause are unknown.
Several hypotheses43 exist as to the origin of the conditioned pause response, including (but certainly not limited to): (1) GABAergic inhibition deriving from striatal fast spiking interneurons (FSIs) onto cholinergic interneurons, (2) dopaminergic inhibition by dopamine terminals onto cholinergic interneurons (terminal dopamine release activates D2 receptors on cholinergic interneurons, which hyperpolarizes cells via potassium channels), and (3) intrinsic properties of cholinergic interneurons themselves. Despite incomplete understanding of pause mechanisms, its characteristics suggest it may regulate striatal plasticity. This function of cholinergic interneurons is explored in depth in another Perspective by Deffains and Bergman. In addition to the pause response, cholinergic interneurons also show synchronized discharge, which will be explored in a later section.
Dopamine is likely to play a key role in the generation of the pause response. Studies showing simultaneous extracellular recordings from putative dopamine neurons in the SNc and TANs of awake behaving monkeys show that the pause in TAN neurons is coincident with an increase in firing of SNc neurons. This finding suggests that both TANs and SNc neurons signal reward-relevant information and may share synaptic inputs or connect to one another.44 Further strengthening the relationship between dopamine and the pause response, depletion of striatal dopamine with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment resulted in loss of the conditioned pause response.38 Interestingly, dopamine depletion did not alter baseline firing, suggesting a specific effect on the pause response. The pause could be rescued by administration of dopamine agonists, an even stronger finding in support of dopamine's mechanistic contribution to the pause.38 This observation suggests that dopamine is permissive for the conditioned pause response, but phasic dopamine, or the brief increase in striatal dopamine levels typically triggered by high frequency bursting of dopamine neurons, may not be required. More recent studies using slice electrophysiology, optogenetics, and pharmacology in rodents have shown that synaptically released dopamine acting on D2 receptors inhibits cholinergic interneuron firing in the dorsal striatum.45 There is additional evidence that long-range GABAergic projection neurons originating in the VTA and innervating the nucleus accumbens (NAc) in the ventral striatum may contribute to the pause in cholinergic firing during associative learning tasks.46 This mechanism may be limited to the ventral striatum, the target of VTA projection neurons, which likely carry reward-relevant information; SNc dopamine neurons generally innervate dorsal striatal areas. The dynamic firing patterns as well as the broad axonal arbors of cholinergic interneurons permit subtle changes in the firing of single cholinergic interneurons to regulate the activity of many surrounding neurons. In addition, synchronized activity of nearby cholinergic interneurons may permit even greater control.47
Striatal Muscarinic Acetylcholine Receptors
Local release of acetylcholine modulates the activity of the striatal microcircuit via multiple cholinergic receptor subtypes, including metabotropic muscarinic receptors (mAChR) and/or ionotropic nicotinic receptors (nAChR). A schematic diagram of the key striatal cell types, major synaptic inputs, and their muscarinic and nicotinic receptors can be found in Figure 1. A mixture of these receptors is expressed presynaptically on striatal afferents32,48 and postsynaptically on all striatal cell types.34,49-52 Muscarinic receptors are divided into two classes—M1 and M2 classes. M1 class receptors are composed of M1, M3, and M5 receptors and couple to Gq/11 Gα proteins to activate protein kinase C and phospholipase C. M2 class receptors, comprised of M2 and M4 type receptors, couple to Gi/o G proteins to inhibit adenylyl cyclase, subsequently closing calcium channels and opening inward-rectifying potassium channels. Although MSNs express both M1 and M4 receptors, M4 mAChRs are more prominent on direct pathway MSNs.53,54
Muscarinic receptors modulate both intrinsic excitability and synaptic inputs of MSNs. Activation of postsynaptic dendritic and somatic M1 receptors is hypothesized to increase MSN excitability.50,55-57 Dendritic M1 receptor activation decreases the probability of inhibitory potassium channels opening,57-59 whereas somatic M1 receptor activation enhances sodium currents; both would be predicted to increase neuronal excitability.60 Conversely, excitatory cortical afferents onto MSNs are heavily populated with M2 and M3 type receptors.61 Activation of these inhibitory presynaptic mAChRs decreases glutamate release from afferents and diminishes excitatory drive onto MSNs.62-67 A recent study using optical techniques confirmed this idea at the level of vesicle release.64
Muscarinic receptors have not been as well characterized on striatal GABAergic interneurons, but ex vivo physiology suggests they may regulate the function of parvalbumin positive fast spiking interneurons (FSIs). Striatal FSIs make strong inhibitory synapses onto MSNs,68,69 whose strength is reduced by acetylcholine via M1 and M4 receptors.34 While the function of striatal GABAergic interneurons in vivo is unclear, they are activated during basal-ganglia dependent decision tasks,70 so cholinergic modulation may be critical during reward-related decision making.
Finally, cholinergic interneurons themselves are regulated by acetylcholine via autoreceptors. Activation of the M4 autoreceptor leads to membrane hyperpolarization and inhibition of calcium channels. Dorsal striatal cholinergic interneurons also express M2 receptors not found in the ventral striatum.71 These finding suggest that M4 and/or M2 autoreceptors serve to homeostatically regulate acetylcholine release.71,72 Indeed, it is interesting to consider how the conditioned pause response observed in cholinergic interneurons might briefly relieve cholinergic interneurons of this autoregulation, allowing them to fire in a more concerted fashion after the pause.
Striatal Nicotinic Acetylcholine Receptors
Nicotinic receptors also play a major role in influencing striatal output. Ionotropic nAChRs are widely expressed on presynaptic terminals throughout the CNS, modulating the release of neurotransmitter; for an excellent review of this topic, see (Exley and Cragg, 2008).73 In the striatum, nAChRs are found on dopaminergic73 and cortical afferents.32 Nicotinic signaling regulates both the excitability and release properties of striatal FSIs, as well.34 Structurally, neuronal nicotinic receptors are pentameric oligomers, containing a combination of α- and β-subunits (α2-α10 and β2-β4).74-76 These pentameric receptors can homomeric or heteromeric, with each subunit conferring unique physiological properties. Differential subunit expression may allow cell-type specific regulation within the microcircuit.
The most well-known function of striatal nicotinic receptors is in cholinergic control of local dopamine release.31,48,77 Pharmacological evidence suggests that presynaptic nAChRs on dopaminergic terminals facilitate the release of dopamine.30,47,73 In fact, optogenetic activation of striatal cholinergic interneurons can locally control the release of dopamine within the striatum without a strict requirement for action potentials in midbrain dopamine neuron somata.78,79 Interestingly, expression patterns of nicotinic receptors differ across the striatum; research has suggested that α4α5β2 and α6β2β3 nAChR's regulate dopaminergic axons in the dorsal striatum, while α6α4β2β3 nAChRs dominate in the ventral striatum.80-82 Furthermore, α6 subunit expression is limited to catecholaminergic neurons.83,84 Nicotinic receptors, however, are notoriously prone to desensitization, a process by which, after activation, receptors become unresponsive for a period of time.31,85 Nicotinic receptor properties thus create a time window for striatal cholinergic interneurons to modulate dopaminergic signals, terminated by desensitization. Given tonically firing interneurons and the resultant tone on nicotinic receptors, chronic receptor desensitization may dampen dopaminergic release under baseline conditions. Brief pauses in cholinergic interneuron activity, as seen during the conditioned pause response, could relieve desensitized nAChRs and boost subsequent incoming dopaminergic signals.73 Amplification by synchronous firing of cholinergic interneurons and midbrain dopamine neurons may also occur.44 Thus, nAChRs are in an important position to filter the presynaptic signals arriving in the striatum and heading to downstream nuclei.
Cholinergic Signaling and Neurological Disease
Many of the suspected functions of striatal cholinergic interneurons derive from their (suspected) dysfunction in disease states.86,87 Below, we will discuss current literature on the potential role of striatal cholinergic interneurons in a subset of the diseases in which they are implicated.
Parkinson's Disease
Parkinson's Disease (PD) is characterized by the progressive loss of midbrain dopamine neurons. Many of the cardinal motor symptoms (rigidity, bradykinesia, postural instability, resting tremor) are believed to relate to dopamine depletion. Though abnormalities in other brainstem neuromodulatory systems or cortical neurodegeneration may contribute to cognitive and affective disturbances in PD, these symptoms may also derive from disturbances in dopamine signaling. Before the advent of dopamine replacement therapy with levodopa in the mid-1960's, anticholinergic (antimuscarinic) drugs were the primary medication class available for Parkinson patients. Clinical evidence for this use came in the late 1800's when Charcot first discovered the beneficial effects of Atropa belladonna (atropine) in reducing excessive salivation in parkinsonian patients.88 Another study supported the role of acetylcholine in motor control, noting that parkinsonian symptoms worsened when patients were treated with the cholinesterase inhibitor physostigmine.89 Though used infrequently since the advent of levodopa and potent dopamine agonists, understanding how antimuscarinic agents improve motor function in PD may also illuminate the function of cholinergic interneurons.
Studies in PD animal models and human postmortem tissue have demonstrated increased striatal cholinergic tone, implying a hypercholinergic state in PD,90-94 although other studies have countered this assertion.95-97 Interestingly, while cholinergic interneuron firing rate appears to be unchanged,39,47 interneurons may be more likely to fire synchronously, leading to different functional properties of the striatal microcircuit.47 Together, these results suggest that either release of acetylcholine is enhanced or its breakdown and reuptake is reduced, so that for a given number of action potentials there is increased striatal acetylcholine. The increase in acetylcholine levels was long hypothesized to occur due to loss of inhibitory dopamine tone on D2 receptors of cholinergic interneurons.92,98 However, one study provided in vitro evidence that increased cholinergic tone in parkinsonian animals may occur due to RGS4-related reductions in the efficacy of M4 inhibitory autoreceptors on cholinergic interneurons.99 In PD, normal cholinergic tone could be restored by enhancing M4 autoreceptor function or by counteracting the increased RGS4 expression in cholinergic interneurons.86 This therapeutic strategy, which might avoid some of the negative side effects (such as sedation and cognitive impairment) associated with nonselective antimuscarinic agents, has not yet been pursued.
Levodopa-Induced Dyskinesia
While dopamine replacement with levodopa or dopamine agonists is an effective treatment for many of the motor symptoms of PD, long-term therapy is limited by the emergence of debilitating abnormal involuntary movements, referred to as levodopa-induced dyskinesia (LID). LID is progressive: the longer the exposure to levodopa, the more likely dyskinesias will develop.100 Recent studies suggest increased cholinergic signaling may be a key contributor to LID.101,102 To test the hypothesis that cholinergic interneurons contribute to LID, in one study cholinergic interneurons were ablated in a cell-type specific fashion via Cre-dependent viral expression of the diptheria toxin A subunit (DT-A) in hemiparkinsonian mice expressing Cre under the choline acetyltransferase (ChAT) promoter. This study found a marked decrease in abnormal involuntary movements (AIMs) in levodopa-treated mice with ablation of interneurons as compared to controls.102 Interestingly, this treatment was able to reduce LID without decreasing therapeutic efficacy of levodopa, a major limitation of many antidyskinetic therapies. Other studies have also implicated cholinergic interneurons in LID: In two PD mouse models, one group showed increased extracellular signal-regulated kinase (ERK), phosphorylation in cholinergic interneurons after repeated levodopa exposure. Furthermore, they found that inhibition of MEK1/2, an ERK phosphorylator, attenuated the motor symptoms of LID without reducing akinesia.101 Another study by the same group found that histamine H2 receptor responses were enhanced in cholinergic interneurons in mouse LID models.103 Finally, several studies have found that chronic treatment with nicotine or nicotinic drugs can ameliorate LID in multiple animal models.104,105 Together, such studies suggest that cholinergic interneurons and their signaling pathways are potential targets for future therapeutic development.
Dystonia
Dystonia is characterized by involuntary, sustained muscle contractions, with repetitive twisting movements and abnormal postures. Anticholinergics have been a first-line pharmacological therapy for dystonia, though their mechanism of action is unknown and their side effects are often dose-limiting. Animal models of dystonia have highlighted dysfunctional cholinergic signaling as a potential pathophysiological feature.86 In the most common type of primary generalized dystonia, DYT1, there is a mutation in the dyt1 gene, leading to dysfunction of the protein torsinA.106 In a mouse model of DYT1, researchers found altered D2 coupling in cholinergic interneurons, causing excitation rather than inhibition, and an attenuated in vitro pause response.107,108 Several groups have also observed reversible alterations in striatal synaptic plasticity in multiple animal models of dystonia, which appear to be related to cholinergic dysfunction, as they are reversed by treatment with anticholinergics.109-113 These findings suggest not only that cholinergic interneurons may be integral to the physiological abnormalities observed in dystonia across multiple etiologies, but that cholinergic signaling may be a therapeutic target at multiple sites within the striatum. Understanding the specific cholinergic receptor subtypes involved may allow for more targeted therapies.
Tourette Syndrome
Gilles de la Tourette Syndrome (TS) is a neurological disorder characterized by disabling tics, which are repetitive, involuntary and nonrhythmic in character. Tics affect approximately 5% of school-aged children, often arising at 3-9 years of age.114 Current research suggests that habit-related basal ganglia circuitry, specifically the dorsolateral striatum (DLS), may contribute to TS.115,116 Interestingly, postmortem human studies have shown that cholinergic interneurons are reduced by approximately 50% in patients with TS.117 To test whether loss of cholinergic interneurons is causative of TS, one group selectively ablated cholinergic interneurons in the DLS, which resulted in aberrant tic-like movements in mice after amphetamine administration and stress. This study demonstrated a potential causative role of cholinergic interneurons in TS.118
Potential Mechanisms of Striatal Cholinergic Signaling in Basal Ganglia Function
While cholinergic interneurons are likely to play a major role in striatal function in normal and disease states, the mechanism by which they control striatal output is unclear. We will focus on two (of many) hypotheses regarding regulation of striatal function by cholinergic interneurons: 1) interplay of cholinergic and dopaminergic modulation of striatal output, and 2) cholinergic interneuron synaptic integration, the pause response, and synchronous discharge. Cholinergic modulation of striatal synaptic plasticity, a third mechanism, is likely to be extremely important both in the normal function of the basal ganglia in flexible behavior, but also in disease contexts. Striatal long-term potentiation119 and depression119-124 are present at excitatory synapses onto striatal MSNs, which can be modulated by acetylcholine. As cholinergic regulation of synaptic plasticity is the topic for another, related review by Deffains and Bergman, it will not be discussed further here.
Cholinergic and Dopaminergic Interplay Within the Striatum
Striatal dopamine terminals and cholinergic interneurons have a complex bidirectional synaptic connectivity. Historically believed to antagonize each other, recent evidence supports the idea that dopamine and acetylcholine are more synergistic. Dopamine neurons regulate the function of cholinergic interneurons and vice versa, and likely do so over different timescales due to the kinetics of various cholinergic receptors. This reciprocal relationship may also depend on cotransmitters, such as glutamate and GABA (discussed in detail below; see Figure 2). While the functional corelease of two or more neurotransmitters remains controversial, particularly in higher mammals, accumulating physiological evidence of functional corelease has been fueled by the use of cell-type specific manipulations and optogenetics in rodents. Recent work suggests dissociable roles for each of several transmitters released by neuromodulatory neurons.125,126 The functional role of corelease in the striatal microcircuit is still being explored.
Figure 2.
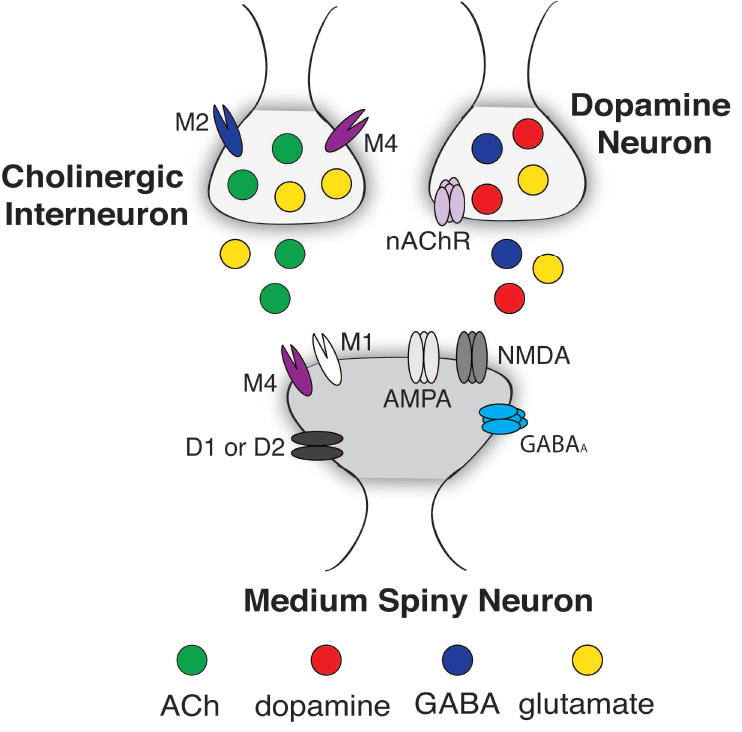
Neurotransmitter corelease from striatal neurons. Top Left: Cholinergic interneurons corelease ACh and glutamate. Top Right: Midbrain dopaminergic neurons corelease dopamine, glutamate and/or GABA. Only a subset of all receptors are illustrated. See text for details. Abbreviations: ACh (acetylcholine), GABA (γ-Aminobutyric acid), D1 or D2 (dopamine type 1 or 2-like receptor), nAChR (nicotinic acetylcholine receptor), M1 or 4 (muscarinic acetylcholine receptor), GABAA (γ-Aminobutyric acid type A receptor), NMDA (N-methyl-D-aspartate receptor), AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor).
Cholinergic interneurons receive synaptic input from midbrain dopamine neurons and express both Gs-coupled dopamine D5 receptors (from the D1 family) and Gi-coupled D2 receptors.127,128 D1 agonists increase the excitability of striatal cholinergic interneurons in vitro,129-131 whereas D2 agonists inhibit the pacemaking of cholinergic interneurons.25,132 However, the net effect of synaptically released dopamine on cholinergic interneuron excitability in vivo is unclear. Furthermore, as midbrain dopamine neurons corelease dopamine, glutamate, and GABA, dopamine may not mediate all observed effects (see Figure 2).133-136
In addition, cholinergic interneurons synapse onto dopamine terminals, regulating their transmitter release. In vitro optogenetic activation of cholinergic interneurons is sufficient to drive increases in dopamine levels in the dorsal and ventral striatum.78,79 This relationship had been suspected based on pharmacological experiments, but these studies using cyclic voltammetry to measure local dopamine concentrations, provided direct evidence. Synchronous optical activation of thalamostriatal inputs, the main excitatory afferent input to cholinergic interneurons, also triggered dopamine release disynaptically.79 This mechanism may be engaged in vivo when a salient sensory stimulus (encoded by the thalamic inputs to striatal cholinergic interneurons), triggers both cholinergic interneuron activity and disynaptic dopamine release. These studies were groundbreaking in that they corroborated older pharmacological studies and provided direct evidence for a cooperative relationship between striatal dopamine and acetylcholine, in which cholinergic neurons can control dopamine release independently of activity in the midbrain. This observation may be critical in the context of learning as well as disease, as cholinergic interneurons become more synchronized during learning tasks,41 and in parkinsonism.47
The final readout of this interplay is the activity of striatal MSNs, the sole output neurons. Interestingly, modulation of MSN activity may be mediated in part by noncanonical neurotransmitter release from both cholinergic interneurons and dopamine terminals. First, cholinergic interneurons appear to release glutamate.137,138 This recent finding was fueled by the observation that striatal cholinergic interneurons express the glutamate transporter VGlut3.139,140 The advent of optogenetics permitted selective activation of groups of cholinergic interneurons, making it possible to detect modest glutamate responses in MSNs.64 Second, optogenetic stimulation of dopamine terminals in rodent striatal slice preparations triggers glutamate and/or GABA release, in addition to dopamine.133-136 The function of dopamine terminal-derived glutamate is unknown: MSN responses are relatively small and confined to the ventral striatum,134 but presumably increase activity. Dopamine terminal-derived GABA responses may also reflect cholinergic regulation. GABAergic MSN synaptic responses have been observed by several groups in response to activation of cholinergic interneurons.138,141-143 This response appears to be mediated in part by GABA released from dopamine terminals, likely triggered by the same nicotinic mechanism identified by labs exploring cholinergic control of dopamine from the same terminals.78,79,138 In addition, activation of cholinergic interneurons triggers GABA release from a type of local GABAergic interneurons neurogliaform cells, which may allow signaling over complementary timescales.141 Both components of the inhibitory response depend on activation of nicotinic receptors, and thus would be subject to the same type of desensitization effects as cholinergic interneuron-driven dopamine release, making it likely to act during specific time windows when desensitization is relieved.138,141
Synchronization of Interneuron Discharge
One intriguing property of striatal cholinergic interneurons is their tendency to fire synchronously,38,41,47,144 leading investigators to hypothesize that they form synchronous neuronal assemblies during the conditioned pause response.38,39,41 Investigators have found significant correlated firing between nearby cholinergic interneurons at a time lag of zero milliseconds, suggesting synchronized firing.44,47,144,145 This synchronization exists both in and out of task events and persists in parkinsonian animals, suggesting cholinergic interneurons provide a timing signal, which complements the reward-predictive signal of dopamine neurons in the same context.44 Candidate synaptic sources for synchrony include dopaminergic, cortical, thalamic, and intrastriatal neurons.
Cholinergic interneurons receive significant input from midbrain dopamine neurons; single dopamine neurons form large axonal arborizations within the striatum, innervating many cholinergic interneurons.146 Anatomically, these inputs are a potential source of simultaneous activation of striatal cholinergic interneurons, but as dopamine signals postsynaptically through slower G-protein coupled receptors and second messengers, it is not a likely candidate for mediating millisecond timescale synchrony. Glutamate or GABA, signaling via fast ionotropic receptors, are potential candidates underlying synchrony.
Cortical Inputs to Cholinergic Interneurons
Although the striatum as a whole receives heavy excitatory cortical input,147,148 corticostriatal projections to cholinergic interneurons are relatively sparse and have been difficult to isolate anatomically.149 Physiological evidence suggests a monosynaptic connection does exist.150-152 Thalamic terminals appear to be more numerous than cortical afferents,149,152 though a recent rabies tracing study shows many cortical neurons project to cholinergic neurons, as well.153 Further work on corticostriatal connectivity to cholinergic interneurons will be needed to determine if this is a potential source of synchrony.
Intrastriatal inputs to Cholinergic Interneurons
Cholinergic interneurons express GABAA receptors,154 and are surrounded by GABAergic neurons, including medium spiny neurons, the principal neurons of the striatum, and local GABAergic interneurons.143 The role of these neurons in controlling synchrony in cholinergic interneurons is unknown, but recent work shows there are extensive anatomical connections between medium spiny neurons and cholinergic interneurons.153,155 Interestingly, there are minimal correlations between the firing of these two cell populations in vivo in simultaneous single-unit recordings from the striatum,156 which may reflect a lack of monosynaptic connectivity, or weakness of individual connections. Local GABAergic interneurons could in principle also inhibit cholinergic interneurons, though there is not strong evidence for this connection as measured by paired recordings in vitro.69
Thalamic Inputs to Cholinergic Interneurons
The richest afferent projection to striatal cholinergic interneurons originates in the intralaminar nuclei of the thalamus, consisting of excitatory glutamatergic fibers.157 This thalamic area corresponds to the centromedian- parafascicular (CM-PF) nuclear complex in primates and the lateral and medial parafascicular (PF) nuclei in the rat. Thalamic axons, similar to dopaminergic and cholinergic axons, arborize extensively in the striatum and innervate cholinergic interneuron cell bodies as well as proximal and distal dendrites.158 There is also good evidence that thalamic inputs to cholinergic interneurons are functional and control firing, as measured in vitro.79,132 In fact, synchronized activation of thalamic inputs can drive disynaptic dopamine release via cholinergic interneurons.78 These inputs are likely to transmit relevant sensory information to the striatum: the CM-PF area of the thalamus responds to salient and unexpected events, which alert the animal to pay attention to their surrounding environment.132 Thus, given their dense projections and wide coverage of the striatum, as well as strong evidence for their functional control of striatal cholinergic interneurons, thalamic inputs are poised to contribute to interneuron synchrony.
Clinical Importance, Future Research, and Conclusions
Cholinergic interneurons powerfully regulate locomotion and procedural learning by modulating the excitability of MSNs, the plasticity of corticostriatal synapses, and the local release of dopamine through local release of acetylcholine. These neurons are pivotal for normal striatal function and their dysfunction may complicate neuropsychiatric disease. There is still much to be done to determine the precise mechanisms by which cholinergic interneurons regulate striatal output in both normal and disease states, and evolving experimental methods, such as transgenic models of disease, optogenetics, and parallel recording techniques will help elucidate these mechanisms. While there are many potential approaches to these questions, some potential experiments to address these outstanding questions are outlined below.
Normal Function of Cholinergic Interneurons In Vivo
To understand the role of cholinergic neurons in vivo under normal conditions, it will be important to both observe and manipulate these neurons during basal ganglia-dependent behaviors. Historically, there have been two major obstacles impeded these goals. First, as cholinergic interneurons are relatively sparse, it is challenging to record numerous neurons simultaneously using traditional recording devices, which use either one or a small bundle of metal electrodes. Second, there existed no method to manipulate these neurons specifically in vivo, as pharmacological agents and stimulating electrodes would nonspecifically activate many surrounding cells. However, the advent of new recording techniques, such as larger and less tissue-disruptive electrode arrays,159 as well as deep calcium imaging in awake-behaving animals, may help overcome the first of these issues. Researchers have already taken advantage of Choline Acetyltransferase (ChAT) – Cre recombinase driver lines, which are available in mice and rats, to drive cell-type specific expression of optogenetic tools142,160,161 and calcium indicators in cholinergic neurons.162-166 Genetically-encoded calcium indicators can thus be expressed in cholinergic interneurons, combined with techniques such as fiber photometry, in which a surgically implanted optical fiber can detect bulk fluorescence signals in deep structures such as the basal ganglia.153 While technically challenging, such techniques have already been used for other cell types in the striatum in animals performing behavioral tasks.167,168 To monitor individual cholinergic interneurons independently within the overall ensemble during behavioral tasks, these indicators can also be combined with implanted GRIN lenses and head-mounted mini-microscopes.169 These observational techniques will allow investigators to determine if small ensembles of cholinergic interneurons are engaged during specific aspects of behavior. Of particular interest would be looking at the conditioned pause response across many neurons, to further explore the synchrony seen in pairs of neurons recorded in monkeys.47
To establish causal relationships between cholinergic interneuron firing and specific behaviors, it is crucial to manipulate the activity of cholinergic interneurons on a physiologic timescale. Historically, this was not possible with electrical or pharmacological stimulation due to lack of specificity in the former and nonphysiological timescale in the latter. However, the combination of cell-type specific tools and optogenetics allows for selective activation or inhibition of neuronal ensembles at millisecond timescales, and patterns of stimulation can mimic what is seen in vivo during behavior. While optogenetic manipulations can often synchronize neurons in a nonphysiological way, in the case of cholinergic interneurons it may be a way to mimic their natural synchrony. The effects of stimulation or inhibition of interneurons can now be studied as it alters locomotor behavior, decision-making, and reward-related behavior. A pioneering study used optical inhibition of cholinergic interneurons during reinforcement behavior,142 finding that optical inhibition of striatal cholinergic interneurons blocked cocaine conditioned place preference. These same tools for recording and manipulating the activity of cholinergic interneurons can be used in the context of animal models of disease to probe their role in disease phenotypes. Although these experiments present technical challenges, the pioneering work of several laboratory groups is making such technology more feasible and accessible to the scientific community.
Long-term modulation of cholinergic interneuron activity can be achieved in parallel with these recording techniques. These chronic manipulations may trigger adaptive changes in basal ganglia circuits, but nevertheless provide an interesting way to address the role of interneurons in chronic disease context. Cell type-specific chronic manipulation can be achieved using Cre-dependent neuromodulatory or ablation techniques. Specific neuronal populations can now be eliminated using caspase or diphtheria toxin signaling,118,170,171 or their synaptic output can be selectively manipulated using tetanus toxin or designer G-protein coupled receptors, also called DREADDs (designer receptors exclusively activated by designer drugs).172,173 Given the hypothesized function of cholinergic interneurons in movement disorders, chronic cell type-specific manipulations in animal models may ameliorate or worsen disease symptoms. Such methods have been validated in two recent studies finding that cell-type specific ablation of cholinergic interneurons (1) reduced levodopa-induced dyskinesia102 and (2) reduced the threshold for tics.118 Similar experiments in other disease models would further support the centrality of cholinergic interneurons in these disorders. Building on historic pharmacological studies, these newer manipulations have the ability to dissociate the role of striatal cholinergic interneurons versus other sources of acetylcholine in the striatum and brain at large.
Finally, the complex relationship of striatal dopaminergic and cholinergic neurons might be parsed in vivo using these novel techniques for both neuronal recording and manipulation. For example, though pharmacological studies and in vitro physiology have identified a number of potential mechanisms by which dopamine release may regulate the activity of cholinergic interneurons (and consequently, striatal output neurons), these findings have not been verified under physiological conditions in vivo. We know that firing of midbrain dopamine neurons and the cholinergic pause response are approximately synchronous,44 but we do not know whether this relationship is causal. Investigators might probe this relationship by simultaneous optical stimulation of dopamine terminals and recording of cholinergic interneurons (and/or output neurons) in vivo, during relevant decision-making or reward-related tasks. Such experiments could demonstrate causal relationships between activity in these two populations.
Function of Thalamostriatal Inputs to Cholinergic Interneurons
While the anatomic connection between the thalamus and the striatum, and the dense innervation of striatal cholinergic interneurons is well-established, the function of these inputs is unclear. Using new recording and optical stimulation techniques to investigate the role of thalamostriatal inputs during both normal basal ganglia-dependent behaviors and in disease models may shed light on the role of thalamostriatal inputs onto MSNs versus cholinergic interneurons. For example, the specific sources of cholinergic interneuron synchrony, the synaptic inputs of tonically active neurons could be silenced in a cell-type specific fashion during in vivo recordings. Thalamostriatal inputs might be inactivated using optogenetic or chemogenetic techniques, observing both the resulting changes in striatal activity and behavioral effects in movement, decision-making, and reward responses.
Pharmacological Dissection of Striatal Cholinergic Signaling
Though optogenetics and new recording techniques will shed light on the function of cholinergic interneurons, these techniques do not currently translate to patient care. Cholinergic and anticholinergic drugs have major shortcomings therapeutically, largely due to off-target effects. Developing a deeper understanding of how individual nicotinic and muscarinic receptor subtypes participate in regulation of striatal microcircuitry will be an important step toward harnessing the positive effects of these powerful drugs while minimizing harmful off-target effects. Many of the functions investigated so far do have some receptor subtype specificity, such as the specific nicotinic receptor subunits expressed on dopamine neurons and not on other neighboring cells75,84 and more specific functions may motivate development of additional therapeutic agents. In addition, cell-type specific deletion of particular receptor subtypes or downstream signaling molecules may permit even finer dissection of the receptors involved in striatal cholinergic signaling in vivo.174,175
Building on the pioneering work of clinicians and primate neurophysiologists, we conclude that this is a pivotal time in the investigation of striatal cholinergic interneurons. We can now utilize novel neurophysiology techniques and cell-type specific manipulations to discover the role of cholinergic interneurons in both health and disease.
Acknowledgments
AEG is supported by the NSF Graduate Research Fellowship. ABN is supported by a K08 grant from the NINDS, the UCSF Physician Scientist Scholar Program, and an endowment in honor of Richard and Shirley Cahill.
Footnotes
No conflict of interest
References
- 1.Mesulam MM, Mash D, Hersh L, Bothwell M, Geula C. Cholinergic innervation of the human striatum, globus pallidus, subthalamic nucleus, substantia nigra, and red nucleus. The Journal of comparative neurology. 1992;323:252–68. doi: 10.1002/cne.903230209. [DOI] [PubMed] [Google Scholar]
- 2.Macintosh FC. The distribution of acetylcholine in the peripheral and the central nervous system. The Journal of physiology. 1941;99:436–42. doi: 10.1113/jphysiol.1941.sp003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebb CO. Biochemical evidence for the neural function of acetylcholine. Physiological reviews. 1957;37:196–220. doi: 10.1152/physrev.1957.37.2.196. [DOI] [PubMed] [Google Scholar]
- 4.Contant C, Umbriaco D, Garcia S, Watkins KC, Descarries L. Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience. 1996;71:937–47. doi: 10.1016/0306-4522(95)00507-2. [DOI] [PubMed] [Google Scholar]
- 5.Gerfen CR, Herkenham M, Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1987;7:3915–34. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dautan D, Huerta-Ocampo I, Witten IB, et al. A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:4509–18. doi: 10.1523/JNEUROSCI.5071-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends in neurosciences. 2004;27:585–8. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Saper CB, Loewy AD. Projections of the pedunculopontine tegmental nucleus in the rat: evidence for additional extrapyramidal circuitry. Brain research. 1982;252:367–72. doi: 10.1016/0006-8993(82)90404-8. [DOI] [PubMed] [Google Scholar]
- 9.Smith Y, Parent A. Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus) Neuroscience. 1986;18:347–71. doi: 10.1016/0306-4522(86)90159-4. [DOI] [PubMed] [Google Scholar]
- 10.Patel JC, Rossignol E, Rice ME, Machold RP. Opposing regulation of dopaminergic activity and exploratory motor behavior by forebrain and brainstem cholinergic circuits. Nature communications. 2012;3:1172. doi: 10.1038/ncomms2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graveland GA, DiFiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain research. 1985;327:307–11. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- 12.Chang HT, Wilson CJ, Kitai ST. A Golgi study of rat neostriatal neurons: light microscopic analysis. The Journal of comparative neurology. 1982;208:107–26. doi: 10.1002/cne.902080202. [DOI] [PubMed] [Google Scholar]
- 13.Penney JB, Jr, Young AB. Speculations on the functional anatomy of basal ganglia disorders. Annual review of neuroscience. 1983;6:73–94. doi: 10.1146/annurev.ne.06.030183.000445. [DOI] [PubMed] [Google Scholar]
- 14.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in neurosciences. 1989;12:366–75. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 15.Gerfen CR, Engber TM, Mahan LC, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–32. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 16.DeLong MR. The neurophysiologic basis of abnormal movements in basal ganglia disorders. Neurobehavioral toxicology and teratology. 1983;5:611–6. [PubMed] [Google Scholar]
- 17.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends in neurosciences. 1990;13:281–5. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 18.Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Archives of neurology. 2003;60:1365–8. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- 19.Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:10178–82. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez-Lopez S, Tkatch T, Perez-Garci E, et al. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:8987–95. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Planert H, Berger TK, Silberberg G. Membrane Properties of Striatal Direct and Indirect Pathway Neurons in Mouse and Rat Slices and Their Modulation by Dopamine. PloS one. 2013:8. doi: 10.1371/journal.pone.0057054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tepper JM, Tecuapetla F, Koos T, Ibanez-Sandoval O. Heterogeneity and diversity of striatal GABAergic interneurons. Frontiers in neuroanatomy. 2010;4:150. doi: 10.3389/fnana.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10:508–19. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett BD, Wilson CJ. Spontaneous activity of neostriatal cholinergic interneurons in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:5586–96. doi: 10.1523/JNEUROSCI.19-13-05586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurice N, Mercer J, Chan CS, et al. D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10289–301. doi: 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson CJ. The mechanism of intrinsic amplification of hyperpolarizations and spontaneous bursting in striatal cholinergic interneurons. Neuron. 2005;45:575–85. doi: 10.1016/j.neuron.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 27.Woolf NJ, Butcher LL. Cholinergic neurons in the caudate-putamen complex proper are intrinsically organized: a combined Evans blue and acetylcholinesterase analysis. Brain research bulletin. 1981;7:487–507. doi: 10.1016/0361-9230(81)90004-6. [DOI] [PubMed] [Google Scholar]
- 28.Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy Neuroscience. 1984;12:711–8. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- 29.Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends in neurosciences. 2000;23:120–6. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- 30.Graybiel AM, Baughman RW, Eckenstein F. Cholinergic neuropil of the striatum observes striosomal boundaries. Nature. 1986;323:625–7. doi: 10.1038/323625a0. [DOI] [PubMed] [Google Scholar]
- 31.Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nature neuroscience. 2001;4:1224–9. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- 32.Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. Journal of neurobiology. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- 33.Descarries L, Gisiger V, Steriade M. Diffuse transmission by acetylcholine in the CNS. Progress in neurobiology. 1997;53:603–25. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- 34.Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:529–35. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aznavour N, Mechawar N, Watkins KC, Descarries L. Fine structural features of the acetylcholine innervation in the developing neostriatum of rat. The Journal of comparative neurology. 2003;460:280–91. doi: 10.1002/cne.10660. [DOI] [PubMed] [Google Scholar]
- 36.Apicella P, Scarnati E, Schultz W. Tonically discharging neurons of monkey striatum respond to preparatory and rewarding stimuli. Experimental brain research. 1991;84:672–5. doi: 10.1007/BF00230981. [DOI] [PubMed] [Google Scholar]
- 37.Kimura M, Rajkowski J, Evarts E. Tonically discharging putamen neurons exhibit set-dependent responses. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:4998–5001. doi: 10.1073/pnas.81.15.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994;265:412–5. doi: 10.1126/science.8023166. [DOI] [PubMed] [Google Scholar]
- 39.Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate's striatum. Journal of neurophysiology. 1995;73:1234–52. doi: 10.1152/jn.1995.73.3.1234. [DOI] [PubMed] [Google Scholar]
- 40.Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:3969–84. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–31. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 42.Inokawa H, Yamada H, Matsumoto N, Muranishi M, Kimura M. Juxtacellular labeling of tonically active neurons and phasically active neurons in the rat striatum. Neuroscience. 2010;168:395–404. doi: 10.1016/j.neuroscience.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg JA, Reynolds JN. Spontaneous firing and evoked pauses in the tonically active cholinergic interneurons of the striatum. Neuroscience. 2011;198:27–43. doi: 10.1016/j.neuroscience.2011.08.067. [DOI] [PubMed] [Google Scholar]
- 44.Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–43. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Straub C, Tritsch NX, Hagan NA, Gu C, Sabatini BL. Multiphasic modulation of cholinergic interneurons by nigrostriatal afferents. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:8557–69. doi: 10.1523/JNEUROSCI.0589-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown MT, Tan KR, O'Connor EC, Nikonenko I, Muller D, Luscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–6. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- 47.Raz A, Feingold A, Zelanskaya V, Vaadia E, Bergman H. Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. Journal of neurophysiology. 1996;76:2083–8. doi: 10.1152/jn.1996.76.3.2083. [DOI] [PubMed] [Google Scholar]
- 48.Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nature neuroscience. 2004;7:583–4. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki T, Miura M, Nishimura K, Aosaki T. Dopamine-dependent synaptic plasticity in the striatal cholinergic interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:6492–501. doi: 10.1523/JNEUROSCI.21-17-06492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howe AR, Surmeier DJ. Muscarinic Receptors Modulate N-Type, P-Type, and L-Type Ca2+ Currents in Rat Striatal Neurons through Parallel Pathways. Journal of Neuroscience. 1995;15:458–69. doi: 10.1523/JNEUROSCI.15-01-00458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. Journal of Neuroscience. 1996;16:2592–604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alcantara AA, Mrzljak L, Jakab RL, Levey AI, Hersch SM, Goldman-Rakic PS. Muscarinic m1 and m2 receptor proteins in local circuit and projection neurons of the primate striatum: anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways. The Journal of comparative neurology. 2001;434:445–60. doi: 10.1002/cne.1186. [DOI] [PubMed] [Google Scholar]
- 53.Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:3591–600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan Z, Flores-Hernandez J, Surmeier DJ. Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience. 2001;103:1017–24. doi: 10.1016/s0306-4522(01)00039-2. [DOI] [PubMed] [Google Scholar]
- 55.Galarraga E, Hernandez-Lopez S, Reyes A, et al. Cholinergic modulation of neostriatal output: A functional antagonism between different types of muscarinic receptors. Journal of Neuroscience. 1999;19:3629–38. doi: 10.1523/JNEUROSCI.19-09-03629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez-Rosello T, Figueroa A, Salgado H, et al. Cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. Journal of neurophysiology. 2005;93:2507–19. doi: 10.1152/jn.00853.2004. [DOI] [PubMed] [Google Scholar]
- 57.Figueroa A, Galarraga E, Bargas J. Muscarinic receptors involved in the subthreshold cholinergic actions of neostriatal spiny neurons. Synapse. 2002;46:215–23. doi: 10.1002/syn.10114. [DOI] [PubMed] [Google Scholar]
- 58.Akins PT, Surmeier DJ, Kitai ST. Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons. Nature. 1990;344:240–2. doi: 10.1038/344240a0. [DOI] [PubMed] [Google Scholar]
- 59.Shen W, Tian X, Day M, et al. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nature neuroscience. 2007;10:1458–66. doi: 10.1038/nn1972. [DOI] [PubMed] [Google Scholar]
- 60.Carrillo-Reid L, Tecuapetla F, Vautrelle N, et al. Muscarinic enhancement of persistent sodium current synchronizes striatal medium spiny neurons. Journal of neurophysiology. 2009;102:682–90. doi: 10.1152/jn.00134.2009. [DOI] [PubMed] [Google Scholar]
- 61.Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of M1-M4 Muscarinic Receptor Proteins in the Rat Striatum - Light and Electron-Microscopic Immunocytochemistry Using Subtype-Specific Antibodies. Journal of Neuroscience. 1994;14:3351–63. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barral J, Galarraga E, Bargas J. Muscarinic presynaptic inhibition of neostriatal glutamatergic afferents is mediated by Q-type Ca2+ channels. Brain research bulletin. 1999;49:285–9. doi: 10.1016/s0361-9230(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 63.Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Blockade of M2-like muscarinic receptors enhances long-term potentiation at corticostriatal synapses. Eur J Neurosci. 1998;10:3020–3. doi: 10.1111/j.1460-9568.1998.00348.x. [DOI] [PubMed] [Google Scholar]
- 64.Higley MJ, Soler-Llavina GJ, Sabatini BL. Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nature neuroscience. 2009;12:1121–8. doi: 10.1038/nn.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malenka RC, Kocsis JD. Presynaptic actions of carbachol and adenosine on corticostriatal synaptic transmission studied in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8:3750–6. doi: 10.1523/JNEUROSCI.08-10-03750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pakhotin P, Bracci E. Cholinergic interneurons control the excitatory input to the striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:391–400. doi: 10.1523/JNEUROSCI.3709-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sugita S, Uchimura N, Jiang ZG, North RA. Distinct muscarinic receptors inhibit release of gamma-aminobutyric acid and excitatory amino acids in mammalian brain. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:2608–11. doi: 10.1073/pnas.88.6.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nature neuroscience. 1999;2:467–72. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- 69.Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2223–34. doi: 10.1523/JNEUROSCI.4870-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gage GJ, Stoetzner CR, Wiltschko AB, Berke JD. Selective activation of striatal fast-spiking interneurons during choice execution. Neuron. 2010;67:466–79. doi: 10.1016/j.neuron.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Threlfell S, Clements MA, Khodai T, et al. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:3398–408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang WL, Basile AS, Gomeza J, Volpicelli LA, Levey AI, Wess J. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. Journal of Neuroscience. 2002;22:1709–17. doi: 10.1523/JNEUROSCI.22-05-01709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. British journal of pharmacology. 2008;153(Suppl 1):S283–97. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corringer PJ, Le Novere N, Changeux JP. Nicotinic receptors at the amino acid level. Annual review of pharmacology and toxicology. 2000;40:431–58. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- 75.Le Novere N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. Journal of neurobiology. 2002;53:447–56. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- 76.Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annual review of neuroscience. 1993;16:403–43. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- 77.Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nature neuroscience. 2004;7:581–2. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- 78.Cachope R, Mateo Y, Mathur BN, et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell reports. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal Dopamine Release Is Triggered by Synchronized Activity in Cholinergic Interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 80.Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–66. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- 81.Exley R, Maubourguet N, David V, et al. Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7577–82. doi: 10.1073/pnas.1103000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Exley R, McIntosh JM, Marks MJ, Maskos U, Cragg SJ. Striatal alpha5 nicotinic receptor subunit regulates dopamine transmission in dorsal striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:2352–6. doi: 10.1523/JNEUROSCI.4985-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.LeNovere N, Zoli M, Changeux JP. Neuronal nicotinic receptor alpha 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur J Neurosci. 1996;8:2428–39. doi: 10.1111/j.1460-9568.1996.tb01206.x. [DOI] [PubMed] [Google Scholar]
- 84.Quik M, Polonskaya Y, Kulak JM, McIntosh JM. Vulnerability of 125I-alpha-conotoxin MII binding sites to nigrostriatal damage in monkey. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:5494–500. doi: 10.1523/JNEUROSCI.21-15-05494.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. Journal of neurobiology. 2002;53:457–78. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- 86.Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends in neurosciences. 2007;30:545–53. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 87.Goldberg JA, Ding JB, Surmeier DJ. Muscarinic modulation of striatal function and circuitry. Handbook of experimental pharmacology. 2012:223–41. doi: 10.1007/978-3-642-23274-9_10. [DOI] [PubMed] [Google Scholar]
- 88.Lang AEL A. Anticholinergic therapies in the treatment of Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2002;17(Suppl 4):S7–12. doi: 10.1002/mds.5556. [DOI] [PubMed] [Google Scholar]
- 89.Duvoisin RC. Cholinergic-anticholinergic antagonism in parkinsonism. Archives of neurology. 1967;17:124–36. doi: 10.1001/archneur.1967.00470260014002. [DOI] [PubMed] [Google Scholar]
- 90.Barbeau A. The pathogenesis of Parkinson's disease: a new hypothesis. Canadian Medical Association journal. 1962;87:802–7. [PMC free article] [PubMed] [Google Scholar]
- 91.DeBoer P, Abercrombie ED, Heeringa M, Westerink BH. Differential effect of systemic administration of bromocriptine and L-dopa on the release of acetylcholine from striatum of intact and 6-OHDA-treated rats. Brain research. 1993;608:198–203. doi: 10.1016/0006-8993(93)91459-6. [DOI] [PubMed] [Google Scholar]
- 92.DeBoer P, Heeringa MJ, Abercrombie ED. Spontaneous release of acetylcholine in striatum is preferentially regulated by inhibitory dopamine D2 receptors. European journal of pharmacology. 1996;317:257–62. doi: 10.1016/s0014-2999(96)00761-3. [DOI] [PubMed] [Google Scholar]
- 93.Lehmann J, Langer SZ. The striatal cholinergic interneuron: synaptic target of dopaminergic terminals? Neuroscience. 1983;10:1105–20. doi: 10.1016/0306-4522(83)90102-1. [DOI] [PubMed] [Google Scholar]
- 94.McGeer PL, Boulding JE, Gibson WC, Foulkes RG. Drug-induced extrapyramidal reactions. Treatment with diphenhydramine hydrochloride and dihydroxyphenylalanine. Jama. 1961;177:665–70. doi: 10.1001/jama.1961.03040360001001. [DOI] [PubMed] [Google Scholar]
- 95.Herrera-Marschitz M, Goiny M, Utsumi H, et al. Effect of unilateral nucleus basalis lesion on cortical and striatal acetylcholine and dopamine release monitored in vivo with microdialysis. Neuroscience letters. 1990;110:172–9. doi: 10.1016/0304-3940(90)90807-l. [DOI] [PubMed] [Google Scholar]
- 96.Herrera-Marschitz M, Luthman J, Ferre S. Unilateral neonatal intracerebroventricular 6-hydroxydopamine administration in rats: II. Effects on extracellular monoamine, acetylcholine and adenosine levels monitored with in vivo microdialysis. Psychopharmacology. 1994;116:451–6. doi: 10.1007/BF02247477. [DOI] [PubMed] [Google Scholar]
- 97.Robertson GS, Hubert GW, Tham CS, Fibiger HC. Lesions of the mesotelencephalic dopamine system enhance the effects of selective dopamine D1 and D2 receptor agonists on striatal acetylcholine release. European journal of pharmacology. 1992;219:323–5. doi: 10.1016/0014-2999(92)90313-s. [DOI] [PubMed] [Google Scholar]
- 98.MacKenzie RG, Stachowiak MK, Zigmond MJ. Dopaminergic inhibition of striatal acetylcholine release after 6-hydroxydopamine. European journal of pharmacology. 1989;168:43–52. doi: 10.1016/0014-2999(89)90631-6. [DOI] [PubMed] [Google Scholar]
- 99.Ding J, Guzman JN, Tkatch T, et al. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nature neuroscience. 2006;9:832–42. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- 100.Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Movement disorders : official journal of the Movement Disorder Society. 2015;30:80–9. doi: 10.1002/mds.26125. [DOI] [PubMed] [Google Scholar]
- 101.Ding Y, Won L, Britt JP, Lim SA, McGehee DS, Kang UJ. Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:840–5. doi: 10.1073/pnas.1006511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Won L, Ding Y, Singh P, Kang UJ. Striatal cholinergic cell ablation attenuates L-DOPA induced dyskinesia in Parkinsonian mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:3090–4. doi: 10.1523/JNEUROSCI.2888-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lim SA, Xia R, Ding Y, et al. Enhanced histamine H2 excitation of striatal cholinergic interneurons in l-DOPA-induced dyskinesia. Neurobiology of disease. 2015;76:67–76. doi: 10.1016/j.nbd.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bordia T, Campos C, McIntosh JM, Quik M. Nicotinic Receptor-Mediated Reduction in L-DOPA-Induced Dyskinesias May Occur via Desensitization. J Pharmacol Exp Ther. 2010;333:929–38. doi: 10.1124/jpet.109.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Quik M, Cox H, Parameswaran N, O'Leary K, Langston JW, Di Monte D. Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Annals of neurology. 2007;62:588–96. doi: 10.1002/ana.21203. [DOI] [PubMed] [Google Scholar]
- 106.Ozelius LJ, Hewett JW, Page CE, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nature genetics. 1997;17:40–8. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- 107.Pisani A, Martella G, Tscherter A, et al. Altered responses to dopaminergic D2 receptor activation and N-type calcium currents in striatal cholinergic interneurons in a mouse model of DYT1 dystonia. Neurobiology of disease. 2006;24:318–25. doi: 10.1016/j.nbd.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 108.Sciamanna G, Tassone A, Mandolesi G, et al. Cholinergic Dysfunction Alters Synaptic Integration between Thalamostriatal and Corticostriatal Inputs in DYT1 Dystonia. Journal of Neuroscience. 2012;32:11991–2004. doi: 10.1523/JNEUROSCI.0041-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Avchalumov Y, Volkmann CE, Ruckborn K, et al. Persistent changes of corticostriatal plasticity in dt(sz) mutant hamsters after age-dependent remission of dystonia. Neuroscience. 2013;250:60–9. doi: 10.1016/j.neuroscience.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 110.Dang MT, Yokoi F, Cheetham CC, et al. An anticholinergic reverses motor control and corticostriatal LTD deficits in Dyt1 DeltaGAG knock-in mice. Behavioural brain research. 2012;226:465–72. doi: 10.1016/j.bbr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Loscher W, Fredow G. Effects of pharmacological manipulation of dopaminergic and cholinergic neurotransmission in genetically dystonic hamsters. European journal of pharmacology. 1992;213:31–9. doi: 10.1016/0014-2999(92)90229-w. [DOI] [PubMed] [Google Scholar]
- 112.Maltese M, Martella G, Madeo G, et al. Anticholinergic drugs rescue synaptic plasticity in DYT1 dystonia: role of M1 muscarinic receptors. Movement disorders : official journal of the Movement Disorder Society. 2014;29:1655–65. doi: 10.1002/mds.26009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martella G, Tassone A, Sciamanna G, et al. Impairment of bidirectional synaptic plasticity in the striatum of a mouse model of DYT1 dystonia: role of endogenous acetylcholine. Brain : a journal of neurology. 2009;132:2336–49. doi: 10.1093/brain/awp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Du JC, Chiu TF, Lee KM, et al. Tourette syndrome in children: an updated review. Pediatr Neonatol. 2010;51:255–64. doi: 10.1016/S1875-9572(10)60050-2. [DOI] [PubMed] [Google Scholar]
- 115.Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M. Neurobiological substrates of Tourette's disorder. J Child Adolesc Psychopharmacol. 2010;20:237–47. doi: 10.1089/cap.2009.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–76. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 117.Kataoka Y, Kalanithi PS, Grantz H, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. The Journal of comparative neurology. 2010;518:277–91. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu M, Kobets A, Du JC, et al. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:893–8. doi: 10.1073/pnas.1419533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:4224–33. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nature neuroscience. 2002;5:446–51. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 121.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–7. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- 122.Lovinger DM, Tyler EC, Merritt A. Short- and long-term synaptic depression in rat neostriatum. Journal of neurophysiology. 1993;70:1937–49. doi: 10.1152/jn.1993.70.5.1937. [DOI] [PubMed] [Google Scholar]
- 123.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–51. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang ZF, Kai L, Day M, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–52. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 125.Liu ZX, Zhou JF, Li Y, et al. Dorsal Raphe Neurons Signal Reward through 5-HT and Glutamate. Neuron. 2014;81:1360–74. doi: 10.1016/j.neuron.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McDevitt RA, Tiran-Cappello A, Shen H, et al. Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell reports. 2014;8:1857–69. doi: 10.1016/j.celrep.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yan Z, Song WJ, Surmeier J. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. Journal of neurophysiology. 1997;77:1003–15. doi: 10.1152/jn.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]
- 128.Yan Z, Surmeier DJ. D5 dopamine receptors enhance Zn2+-sensitive GABA(A) currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron. 1997;19:1115–26. doi: 10.1016/s0896-6273(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 129.Aosaki T, Kiuchi K, Kawaguchi Y. Dopamine D1-like receptor activation excites rat striatal large aspiny neurons in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:5180–90. doi: 10.1523/JNEUROSCI.18-14-05180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Centonze D, Grande C, Usiello A, et al. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:6245–54. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pisani A, Bonsi P, Centonze D, Calabresi P, Bernardi G. Activation of D2-like dopamine receptors reduces synaptic inputs to striatal cholinergic interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:RC69. doi: 10.1523/JNEUROSCI.20-07-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hnasko TS, Chuhma N, Zhang H, et al. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–56. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8229–33. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tecuapetla F, Patel JC, Xenias H, et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7105–10. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–6. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Higley MJ, Gittis AH, Oldenburg IA, et al. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PloS one. 2011;6:e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nelson AB, Hammack N, Yang CF, Shah NM, Seal RP, Kreitzer AC. Striatal cholinergic interneurons Drive GABA release from dopamine terminals. Neuron. 2014;82:63–70. doi: 10.1016/j.neuron.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seal RP, Edwards RH. The diverse roles of vesicular glutamate transporter 3. Handbook of experimental pharmacology. 2006:137–50. doi: 10.1007/3-540-29784-7_7. [DOI] [PubMed] [Google Scholar]
- 140.Gras C, Herzog E, Bellenchi GC, et al. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:5442–51. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.English DF, Ibanez-Sandoval O, Stark E, et al. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nature neuroscience. 2012;15:123–30. doi: 10.1038/nn.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Witten IB, Lin SC, Brodsky M, et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–81. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sullivan MA, Chen H, Morikawa H. Recurrent inhibitory network among striatal cholinergic interneurons. Journal of Neuroscience. 2008;28:8682–90. doi: 10.1523/JNEUROSCI.2411-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goldberg JA, Rokni U, Boraud T, Vaadia E, Bergman H. Spike synchronization in the cortex/basal-ganglia networks of Parkinsonian primates reflects global dynamics of the local field potentials. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6003–10. doi: 10.1523/JNEUROSCI.4848-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kimura M, Matsumoto N, Okahashi K, et al. Goal-directed, serial and synchronous activation of neurons in the primate striatum. Neuroreport. 2003;14:799–802. doi: 10.1097/00001756-200305060-00004. [DOI] [PubMed] [Google Scholar]
- 146.Dimova R, Vuillet J, Nieoullon A, Kerkerian-Le Goff L. Ultrastructural features of the choline acetyltransferase-containing neurons and relationships with nigral dopaminergic and cortical afferent pathways in the rat striatum. Neuroscience. 1993;53:1059–71. doi: 10.1016/0306-4522(93)90489-3. [DOI] [PubMed] [Google Scholar]
- 147.Kemp JM, Powell TP. The termination of fibres from the cerebral cortex and thalamus upon dendritic spines in the caudate nucleus: a study with the Golgi method. Philos Trans R Soc Lond B Biol Sci. 1971;262:429–39. doi: 10.1098/rstb.1971.0105. [DOI] [PubMed] [Google Scholar]
- 148.Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J Comp Neurol. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- 149.Lapper SR, Bolam JP. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience. 1992;51:533–45. doi: 10.1016/0306-4522(92)90293-b. [DOI] [PubMed] [Google Scholar]
- 150.Reynolds JN, Wickens JR. The corticostriatal input to giant aspiny interneurons in the rat: a candidate pathway for synchronising the response to reward-related cues. Brain research. 2004;1011:115–28. doi: 10.1016/j.brainres.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 151.Sharott A, Doig NM, Mallet N, Magill PJ. Relationships between the Firing of Identified Striatal Interneurons and Spontaneous and Driven Cortical Activities In Vivo. Journal of Neuroscience. 2012;32:13221–36. doi: 10.1523/JNEUROSCI.2440-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Doig NM, Magill PJ, Apicella P, Bolam JP, Sharott A. Cortical and thalamic excitation mediate the multiphasic responses of striatal cholinergic interneurons to motivationally salient stimuli. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:3101–17. doi: 10.1523/JNEUROSCI.4627-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guo Q, Wang D, He X, et al. Whole-brain mapping of inputs to projection neurons and cholinergic interneurons in the dorsal striatum. PloS one. 2015;10:e0123381. doi: 10.1371/journal.pone.0123381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bennett BD, Wilson CJ. Synaptic regulation of action potential timing in neostriatal cholinergic interneurons. J Neurosci. 1998;18:8539–49. doi: 10.1523/JNEUROSCI.18-20-08539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gonzales KK, Pare JF, Wichmann T, Smith Y. GABAergic inputs from direct and indirect striatal projection neurons onto cholinergic interneurons in the primate putamen. J Comp Neurol. 2013;521:2502–22. doi: 10.1002/cne.23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Adler A, Katabi S, Finkes I, Prut Y, Bergman H. Different correlation patterns of cholinergic and GABAergic interneurons with striatal projection neurons. Frontiers in systems neuroscience. 2013;7:47. doi: 10.3389/fnsys.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–7. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 158.Deschenes M, Bourassa J, Doan VD, Parent A. A single-cell study of the axonal projections arising from the posterior intralaminar thalamic nuclei in the rat. Eur J Neurosci. 1996;8:329–43. doi: 10.1111/j.1460-9568.1996.tb01217.x. [DOI] [PubMed] [Google Scholar]
- 159.Tooker A, Liu D, Anderson EB, et al. Towards a large-scale recording system: demonstration of polymer-based penetrating array for chronic neural recording. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:6830–3. doi: 10.1109/EMBC.2014.6945197. [DOI] [PubMed] [Google Scholar]
- 160.Witten IB, Steinberg EE, Lee SY, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–33. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rossi J, Balthasar N, Olson D, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Broussard GJ, Liang R, Tian L. Monitoring activity in neural circuits with genetically encoded indicators. Frontiers in molecular neuroscience. 2014;7:97. doi: 10.3389/fnmol.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen TW, Wardill TJ, Sun Y, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jennings JH, Stuber GD. Tools for Resolving Functional Activity and Connectivity within Intact Neural Circuits. Curr Biol. 2014;24:R41–R50. doi: 10.1016/j.cub.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 2006;50:823–39. doi: 10.1016/j.neuron.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 166.Akerboom J, Chen TW, Wardill TJ, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:13819–40. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cui G, Jun SB, Jin X, et al. Deep brain optical measurements of cell type-specific neural activity in behaving mice. Nature protocols. 2014;9:1213–28. doi: 10.1038/nprot.2014.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cui G, Jun SB, Jin X, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–42. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ziv Y, Burns LD, Cocker ED, et al. Long-term dynamics of CA1 hippocampal place codes. Nature neuroscience. 2013;16:264–6. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang CF, Chiang MC, Gray DC, et al. Sexually Dimorphic Neurons in the Ventromedial Hypothalamus Govern Mating in Both Sexes and Aggression in Males. Cell. 2013;153:896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Traka M, Arasi K, Avila RL, et al. A genetic mouse model of adult-onset, pervasive central nervous system demyelination with robust remyelination. Brain : a journal of neurology. 2010;133:3017–29. doi: 10.1093/brain/awq247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 173.Krashes MJ, Koda S, Ye C, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. The Journal of clinical investigation. 2011;121:1424–8. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kamsler A, McHugh TJ, Gerber D, Huang SY, Tonegawa S. Presynaptic m1 muscarinic receptors are necessary for mGluR long-term depression in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1618–23. doi: 10.1073/pnas.0912540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sciamanna G, Napolitano F, Pelosi B, et al. Rhes regulates dopamine D2 receptor transmission in striatal cholinergic interneurons. Neurobiology of disease. 2015;78:146–61. doi: 10.1016/j.nbd.2015.03.021. [DOI] [PubMed] [Google Scholar]


