Abstract
Despite conservation concerns for many species of bats, factors causing mortality in bats have not been reviewed since 1970. Here we review and qualitatively describe trends in the occurrence and apparent causes of multiple mortality events (MMEs) in bats around the world.
We compiled a database of MMEs, defined as cases in which ≥ 10 dead bats were counted or estimated at a specific location within a maximum timescale of a year, and more typically within a few days or a season. We tabulated 1180 MMEs within nine categories.
Prior to the year 2000, intentional killing by humans caused the greatest proportion of MMEs in bats. In North America and Europe, people typically killed bats because they were perceived as nuisances. Intentional killing occurred in South America for vampire bat control, in Asia and Australia for fruit depredation control, and in Africa and Asia for human food. Biotic factors, accidents, and natural abiotic factors were also important historically. Chemical contaminants were confirmed causes of MMEs in North America, Europe, and on islands. Viral and bacterial diseases ranked low as causes of MMEs in bats.
Two factors led to a major shift in causes of MMEs in bats at around the year 2000: the global increase of industrial wind-power facilities and the outbreak of white-nose syndrome in North America. Collisions with wind turbines and white-nose syndrome are now the leading causes of reported MMEs in bats.
Collectively, over half of all reported MMEs were of anthropogenic origin. The documented occurrence of MMEs in bats due to abiotic factors such as intense storms, flooding, heat waves, and drought is likely to increase in the future with climate change. Coupled with the chronic threats of roosting and foraging habitat loss, increasing mortality through MMEs is unlikely to be compensated for, given the need for high survival in the dynamics of bat populations.
Keywords: bats, conservation, disease, mortality, wind turbines
INTRODUCTION
Bats number over 1300 species and occur on all continents except Antarctica (Fenton & Simmons 2014). Losses of roosting and foraging habitat and other stressors have led to widespread declines of bat populations (e.g. Mickleburgh et al. 1992, Hutson et al. 2001). Nevertheless, mortality in bats has not been reviewed since the work of Gillette and Kimbrough (1970). Many species of bat are highly gregarious and thus potentially vulnerable to ‘die-offs’, also referenced as multiple mortality events (MMEs). Additionally, bats are sources of zoonotic viral diseases (e.g. Calisher et al. 2006, Luis et al. 2013). Few viral disease-induced MMEs seem to have been documented in bats (e.g. Messenger et al. 2003, O’Shea et al. 2014), suggesting that many microparasites of bats are low in virulence or do not cause MMEs. However, documentation of die-offs due to any cause may be rare simply because bats are secretive, and thus MMEs due to disease may not be disproportionately uncommon. Here we review and qualitatively describe trends in the occurrence of MMEs, including those caused by disease, in bats around the world.
METHODS AND SCOPE
We defined an MME as a case in which ≥ 10 dead bats were counted or estimated in a given locality within a maximum timespan of one year, and more typically within a few days or a season. We included accounts in which the authors qualitatively estimated the number of deaths (as many, dozens, hundreds, etc.). With few exceptions, we did not include a report unless it involved observations ofcarcasses. Because Mickleburgh et al. (2009) reviewed the consumption of bats as human food, we did not include observations of bats at marketplaces or as imports, unless the report indicated the number of bats killed over a given period of time at a specific location. We did not include records of bats killed by researchers. We compiled published literature and internet-accessible reports that included observations of carcasses. We did not solicit unpublished material or personal communications. We searched Web of Science, Internet search engines (e.g. Google), regional mammal summaries, specialised outlets and newsletters (e.g. ‘Bats’ magazine), and other resources. Our search terms included the union of the terms “bats” or “Chiroptera” with mortality, die-offs, disease, epizootics, killing, mass mortality, multiple mortality, and so forth.
We identified nine categories of MMEs, according to the cause of death: (1) intentional killing by humans; (2) biotic factors other than disease (e.g. predation, biotoxins); (3) natural abiotic factors (e.g. weather, floods, fire, volcanism); (4) exposure to environmental contaminants, including pesticides; (5) accidents (e.g. entrapment, impalement, collisions with objects other than wind turbines); (6) collisions with wind turbines; (7) infectious viral and bacterial diseases; (8) the fungal disease white-nose syndrome (WNS); and (9) unexplained causes of death. We also classified MMEs geographically, as occurring in: Africa, Asia, Australia, Europe (including the British Isles, Cyprus, Malta), North America, South America (including Trinidad, Curacao), or on islands (> 100 km from continental mainland). Primary sources and detailed information about these MMEs are tabulated as online supporting information (Appendices S1-S9, with supporting literature cited listed separately as Appendix S10). We limit our analyses to descriptive summaries because the literature we compiled has many biases.
RESULTS AND DISCUSSION
We compiled 1180 accounts of MMEs in 152 species of bats in all regions, beginning in the year 1790 (Table 1, Fig. 1). Cumulatively, collisions with wind turbines caused the highest number of MMEs (a number biased by regulatory reporting requirements in North America and Europe), followed by MMEs due to WNS (Table 1, Fig. 2). MMEs attributed to infectious viral and bacterial diseases ranked lowest (Table 1, Fig. 2).
Table 1.
Summary of numbers of mass mortality events (MMEs) reported in bats, by category and region (see Appendices S1-S10 for details and references). Order of magnitude for maximum unadjusted numbers of carcasses documented for largest MME within a category given in parentheses following number of reports for each region.
| Category | Africa | Asia | Australia | Europe | Islands | North America | South America | N Events | N Species |
|---|---|---|---|---|---|---|---|---|---|
| Intentional killing (S1) | 11 (104) | 20 (104) | 13 (104) | 21 (104) | 50 (103) | 58 (104) | 32 (105) | 205 (105) | 69 |
| Biotic (S2) | 5 (101) | 1 (101) | 19 (103) | 16 (102) | 16 (102) | 40 (103) | 10 (101) | 107 (103) | 75 |
| Abiotic (S3) | 0 | 6 (103) | 71 (103) | 0 | 13 (103) | 24 (105) | 0 | 114 (105) | 23 |
| Contaminants (S4) | 0 | 0 | 1 (101) | 27 (104) | 1 (102) | 14 (103) | 0 | 43 (104) | 16 |
| Accidental (S5) | 1 (101) | 0 | 8 (101) | 34 (102) | 1 (101) | 22 (104) | 0 | 66 (104) | 37 |
| Wind turbines (S6) | 1 (102) | 0 | 2 (101) | 59 (102) | 2 (101) | 213 (102) | 4 (101) | 281 (102) | 41 |
| Viral or bacterial disease (S7) | 1 (102) | 1 (101) | 2 (103) | 2 (103) | 6 (103) | 13 (103) | 0 | 25 (103) | 14 |
| White-nose syndrome (S8) | 0 | 0 | 0 | 0 | 0 | 266 (104) | 0 | 266 (104) | 6 |
| Unexplained (S9) | 0 | 0 | 3 (102) | 30 (103) | 2 (103) | 38 (105) | 0 | 73 (105) | 20 |
| Totals | 19 (104) | 28 (104) | 119 (104) | 189 (104) | 91 (103) | 688 (104) | 46 (105) | 1,180 | 152 |
Fig. 1.
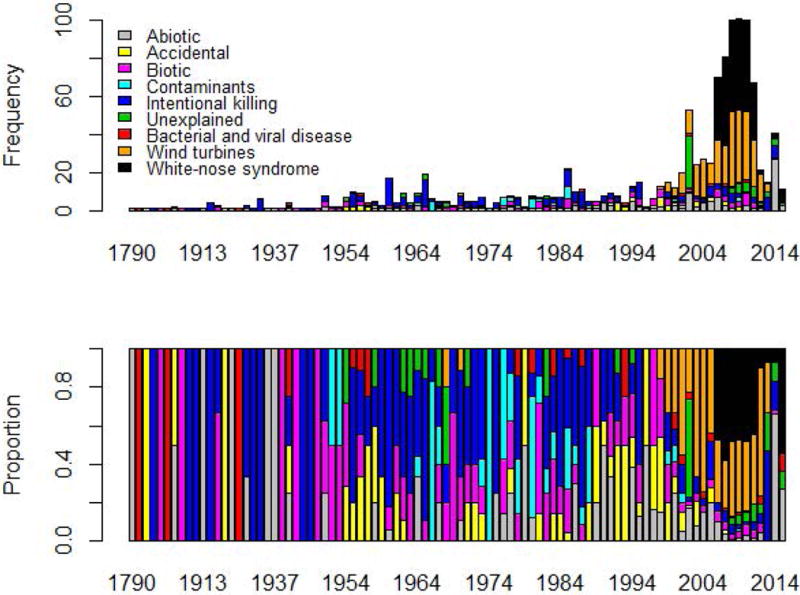
Numbers and proportions of reported multiple mortality events in bats over time (1790 – 2015). All 1180 events included in this review are shown.
Fig. 2.
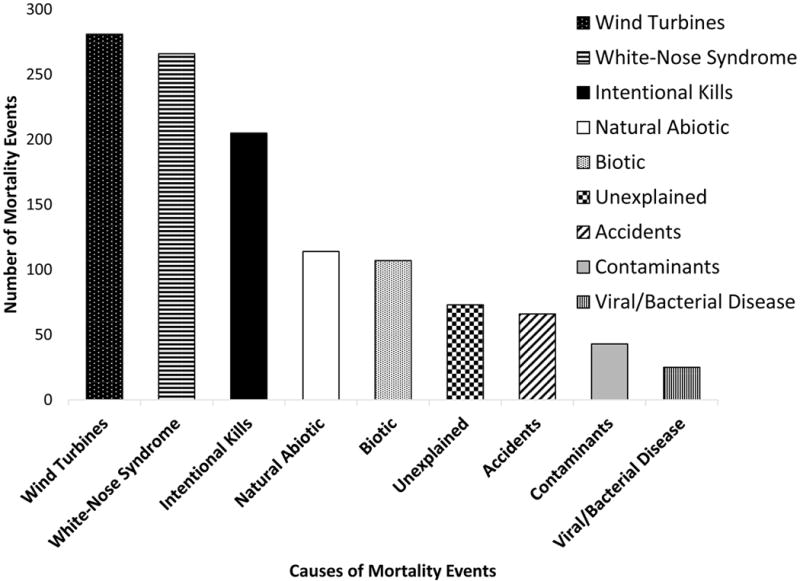
Cumulative (1790-2015) frequencies of 1180 reported multiple mortality events in bats, by causal category.
A notable temporal shift in causes of MMEs in bats took place around the year 2000 (Fig. 3). From 1790 to 1999, 58% of reported MMEs globally were due to intentional killing by humans (39%) or biotic causes (19%; Fig. 3). From 2000 onwards, 70% of all MMEs were due to collisions with wind turbines (35%) and WNS (35%; Fig. 3). These two latter categories represent new and alarming challenges to bat populations.
Fig. 3.
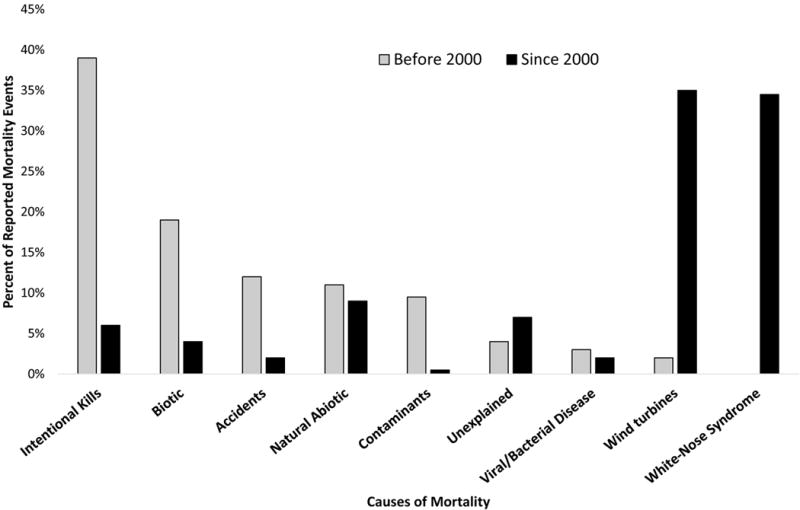
Percentages of reported multiple mortality events in bats by causal category, before the year 2000 (n = 409 events) and from 2000 and thereafter (n = 771 events).
Anthropogenic sources (human-caused categories and human-caused MMEs within other categories, e.g. accidents, contaminants) account for 54% of MMEs in all years (593 anthropogenic vs. 514 natural, unexplained cases excluded). WNS in North America was not considered anthropogenic. Comparisons with other mammals suggest that the high proportion of anthropogenic MMEs in bats is a cause for concern. In one analysis, from 1940-2012 ‘human perturbations’ were listed as a cause for 20-25% of die-offs of all animal groups combined, but only 0-25% each decade for mammals with no increase in magnitude through time (Fey et al. 2015, bats not included). In another study, anthropogenic causes accounted for 52% of 2209 MMEs involving ten or more deaths in 27 species of large and medium-sized (> 1 kg) mammals of North America, but more than half of that fraction were due to managed legal harvests (Collins & Kays 2011).
Below we present an overview of findings within the major categories of MME, highlighting regional differences where pertinent. Reports of MMEs in bats are biased regionally towards North America, Europe, Australia, and islands; comparatively few events are recorded for Africa, Asia, and South America (Table 1, Fig. 4). Similar biases are apparent in reporting of mass mortality for all animals (Fey et al. 2015). We encourage greater reporting and documentation of MMEs in bats globally, perhaps in conjunction with other ongoing bat monitoring programs.
Fig. 4.
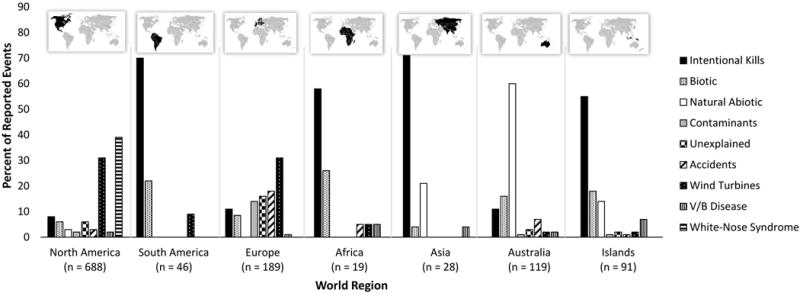
Causes of multiple mortality events in bats, expressed as percentages of all events (n), for each geographic region.
MMEs due to intentional killing
Prior to the year 2000, humans intentionally killing bats caused the greatest number of reported MMEs. These events occurred worldwide and are recorded as early as the 1800s; in total we documented 205 cases involving millions of individuals of at least 69 species (Table 1 and Appendix S1). Intentional killing was widespread, and continues today, but education and protective legislation have undoubtedly helped reduce its occurrence. Reports of people killing bats for food are common in Asia, Africa, and on islands, but extremely rare in South America and absent elsewhere (Appendix S1). Reports of people legally and illegally killing fruit bats (ostensibly for crop protection) are common in Australia, and to a lesser extent elsewhere (primarily in the Paleotropics). Killing of bats for crop protection is likely to be under-reported (e.g. Vincenot et al. 2015). In parts of the Americas within the range of common vampire bats Desmodus rotundus, state-sanctioned poisoning of bats and uncontrolled destruction of roosts aimed at reducing common vampire bat bites on livestock has affected thousands of caves and is likely to have killed millions of bats including many non-target species (see below and Appendix S1; dozens of other species share roosts with common vampire bats [Greenhall et al. 1983]). In addition to the intentional killing of bats for food, crop protection, and common vampire bat control, people have attempted to exterminate colonies by acts of vandalism, including burning, shooting, bludgeoning, and fumigation with poisons (Appendix S1). Below we provide further summaries of MMEs in this category by region.
Africa
We found reports of people intentionally killing bats in Africa dated a century ago (Appendix S1). Lang and Chapin (1917a) noted “Night after night they [caverniculous insectivorous bats in central Africa] return to their accustomed roosts, which they do not abandon even when frequent raids made upon them by the natives have thinned out their numbers. Large, juicy lumps of fat, deposited in and about their abdominal cavity, stimulate the natives to kill all they can.” Lang and Chapin (1917b) provide other accounts of people killing apparently large numbers of insectivorous bats from other types of roosts. Deaths of multiple pteropodids (Family Pteropodidae) taken for food at specific locations are reported, primarily for straw-coloured fruit bats Eidolon helvum (Appendix S1), but other species have been reported at markets (Mickleburgh et al. 2009).
Australia
Starting in the 1800s, people decimated roosting camps and killed pteropodids for fruit crop protection (Appendix S1; Hall & Richards 2000). A limited number of these MMEs meet our documentation criteria (Appendix S1), however evidence suggests that millions of pteropodids were intentionally killed. Early methods included shooting, netting, trapping, explosives, fire and smoke; recent methods include electrified grids (Martin 2011). This culling was widespread and common, predicated on beliefs that local aggregations of bats consumed commercial fruit and could be controlled, and that the bats sent ‘scouts’ to search for ripening crops. Research suggests that such beliefs are largely erroneous. Grey-headed flying foxes Pteropus poliocephalus, for example, exist in large open populations in which major eruptive movements follow the availability of native food (mostly nectar and pollen from eucalypts; see reports in Eby & Lunney 2002). Exclusion nets protect fruit crops but are too expensive for some growers. From the year 2000, legal protections designated for some species led to heated conflicts over pteropodid conservation and crop protection (Eby & Lunney 2002). Some states are phasing out legal killing, or regulating take by issuing permits. For example, the government of Queensland issued permits to kill 10580 bats of four species in 2013-14 (Anonymous 2014a). Despite protections, illegal killing continues. We found little information about intentional killing of microchiropteran bats in Australia. In 1965 about 200 long-fingered bats (Miniopterus sp.) were found dead on the floor of a cave with traumatic injuries caused by vandals (Appendix S1).
Asia
Commercial fruit growers entangled and killed over 1000 pteropodids (of more than one species) in Thailand (Appendix S1). Egyptian fruit bats Rousettus aegyptiacus in Israel were fumigated with ethylene dibromide and lindane, leading to mass mortality and declines in several non-target species of cave-dwelling insectivorous bats. Mortality through fumigation was reported from 1958 to 1985 (Appendix S1) and led to the “complete extermination” of Geoffroy’s Myotis Myotis emarginatus and greater horseshoe bats Rhinolophus ferrumequinum at an Israeli nature reserve (Makin & Mendelssohn 1987). Market-hunters killed 10000 frugivorous, nectarivorous, and insectivorous bats every month in one cave in Thailand during the early 1980s. Large numbers of insectivorous as well as pteropodid bats were, and still are, killed for food markets in Laos, peninsular Malaysia, and Nagaland (India; Fig. 5; Appendix S1).
Fig. 5.


Intentional killing of bats for food. A. Dead bats on the ground outside a cave in Nagaland, India, where villagers kill several thousand bats as an annual event extending back perhaps 150 years (see Appendix S1; photograph by Pilot Dovih). B. Pteropus species for sale at a market in Manado, Northern Sulawesi, Indonesia (photograph by D.T.S. Hayman).
Europe
Stebbings (1988) summarised multiple instances of people intentionally killing bats at roosts in Europe from the 1920s through the 1980s. Many thousands of bats were killed by using fires, shooting, bludgeoning, gassing, explosives, and direct application of insecticides as poisons. Nine species in three families were subject to this intentional killing in England, Scotland, Norway, Gibraltar, Malta, France, Germany, Cyprus, and the former Yugoslavia (Appendix S1; Stebbings 1988). People directly fumigated roosts with insecticides in Europe, causing MMEs such as the loss of 2000 individuals of Pipistrellus sp. at a building in Scotland during 1971 (Stebbings 1988).
Islands
On Borneo, hundreds of individuals of the nearly extinct Bulmer’s fruit bat Aproteles bulmerae were shot in their cave roost for food on a single day, and about 4500 large flying foxes Pteropus vampyrus natunae were killed by hunters at a forest patch in 2003 (Appendix S1). Goodman (2006) reported recent food hunts for three species of insectivorous bats at caves on Madagascar, including about 2700 Commerson’s leaf-nosed bats Hipposideros commersoni killed in a 3-month season (Appendix S1). Currently a cull targeting tens of thousands of greater Mascarene flying foxes Pteropus niger is underway on Mauritius in the belief that the bats depredate fruit crops (Aldred 2015).
North America
People intentionally killed at least eleven species of North American bats by burning, shooting, bludgeoning, poisoning and other activities (Appendix S1). Reports of eradication extend over 100 years into the past. For example, “over two washtubfulls of the pesky critters” (presumed Brazilian free-tailed bats Tadarida brasiliensis) were destroyed at a Texas building in 1908; Appendix S1.
Discovery of rabies in North American bats in the 1950s increased public fear of health risks, leading to attempted eradication of entire bat colonies, especially in buildings. Killing was widespread and became commercialised within the pest control industry. Additionally, fear or misunderstanding of bats also motivated killing at colonies, often referred to as “vandalism”. Some MMEs from deliberate eradication are documented in the literature (Appendix S1). People used chemical control methods (primarily fumigation with dichlorodiphenyltrichloroethane - DDT) to poison colonies of multiple species at buildings in the USA, Canada, and Mexico (Appendix S1). Considering the large number of pest control companies operating in North America, the true number of MMEs from chemical eradication until the 1980s is probably orders of magnitude larger than reported in the literature. Killing bats with chemicals may continue in some parts of North America, but is more restricted and is considered less effective than physical exclusion.
South America
In the late 1950s, people used organochlorine pesticides to kill insectivorous bats considered nuisances in houses in Trinidad: 1592 Pallas’s mastiff bats Molossus molossus and 339 black mastiff bats Molossus rufus were found dead after up to 15 houses were sprayed (Appendix S1). Killing of bats for vampire control, however, is the most heavily reported cause of MMEs in South America. Common vampire bats have been recognised as an economic and health problem in Latin America for at least a century. These bats consume blood from livestock, reducing animal health and productivity; more significantly, common vampire bats transmit rabies to cattle, which has been estimated to cause tens to hundreds of thousands of deaths annually (Baer 1991). Recently, vampire-bat transmission of rabies to humans has also become recognised as a serious health problem in rural areas (e.g., Streicker et al. 2012). People use various methods to control common vampire bats in response to livestock and human health problems, including lighting or covering corrals, hanging spiny branches around cattle, capturing vampires in livestock corrals or roosts with nets and bat traps, clubbing and shooting bats at roosts, poisoning, setting fires in roosts, and offering bounties for vampire bat carcasses (Constantine 1970, Flores-Crespo & Arellano-Sota 1991). Some control methods at roosts have been indiscriminate, killing many bats of other species, whereas other methods specifically target common vampire bats. More targeted state-sanctioned killing of common vampire bats now makes use of gels with oral anticoagulants applied to the bodies of common vampire bats captured at ranches; the bats return to roosts, spread the substance within the colony, and ingest the toxic compounds during grooming (including allogrooming; Arellano-Sota 1988, Flores-Crespo & Arellano-Sota 1991, Johnson et al. 2014). Bite wounds on cattle are also treated with anticoagulants that are then ingested by common vampire bats during feeding (Flores-Crespo & Arellano-Sota 1991).
People caused MMEs at common vampire bat roosts using such methods as flamethrowers, dynamite, fumigation with gases and poisons, electrocution, introduction of disease agents, shooting, netting, trapping, and application of anticoagulants (Greenhall & Schmidt 1988, Brown 1994; Appendix S1). Concerned biologists and conservationists have issued resolutions against destroying multi-species roosts for common vampire bat control (Anonymous 1968, 1998) and some governments have banned the destruction of bat roosts on state-owned reserves (e.g. Kikuti et al 2011). Nonetheless, agencies and private individuals continue to destroy multi-species roosts and misapply anticoagulants to non-target species (e.g. Mayen 2003, Aguiar et al. 2010, Streicker et al. 2012, D. Streicker, personal observation). Recent quantitative analyses suggest that culling vampire bats may be ineffective for controlling bovine rabies (e.g. Streicker et al. 2012, Blackwood et al. 2013, Johnson et al. 2014).
Governments still try to control common vampire bats as part of national rabies control plans (D. Streicker, personal observation) and improving methods of control is an area of active research (Corrêa-Scheffer et al. 2014), but reports chronicling the current extent and effects of these practices on non-target species are limited. Earlier literature suggests a severe impact that may be ongoing. In Venezuela, people indiscriminately dynamited and discharged poisonous organophosphate gas into caves, killing an estimated 900000 bats of many species each year from 1964 to 1967 (see Constantine 1970 for references). In 1963-1968, authorities in the state of Rio Grande do Sul, Brazil, indiscriminately killed bats in 8240 caves (Constantine 1970). In an unusual approach in Colombia, a cave was fumigated with atomised Newcastle’s disease virus (a Paramyxovirus of poultry), and an estimated 5000 common vampire bats were later found sick or dead, apparently from the virus (Constantine 1970).
MMEs due to biotic factors other than disease
MMEs caused by biotic factors other than disease principally involve predation; however, impalement on burrs of plants, paralysis from tick neurotoxins, and poisoning by a toxic algal bloom were also reported (Appendix S2). We tabulated 107 MMEs beginning in the 1890s, involving over 75 species of bat globally (Table 1 and Appendix S2). Bats have numerous predators, nearly all opportunistic, and the literature includes many anecdotal accounts of individual cases (Gillette & Kimbrough 1970). However, MMEs involving ten or more bats taken as prey at specific locations have been reported for well over a century and have occurred globally. The predators known to cause MMEs in bats include invertebrates, snakes, birds, and mammalian mesocarnivores (Appendix S2). Birds are most widely cited as predators of bats globally, and may be a selective force for nocturnality (Mikula et al. in press). Two species of raptor specialise on bats, one in the Paleotropics (Macheiramphus alcinus) and one in the Neotropics (Falco rufigularis). These specialists hunt at dusk, particularly during emergence of bats, but rely on other prey as well. Predation by birds can be substantial globally, but estimates are unavailable. The author of the most extensive regional study estimated that avian predation in the British Isles accounted for over 200000 bat deaths per year (Speakman 1991). In some areas, predation by house cats also can be extensive: in one study it was estimated that cats kill 170000 bats annually in Britain (Appendix S2).
MMEs due to natural abiotic factors
Unseasonably cold weather, snow storms, flooding of roosts during heavy rainfall, overheating during unusual hot spells, burning or suffocation during landscape-level fires, and volcanic eruptions all cause MMEs in bats (Appendix S3). MMEs due to such abiotic factors have been known since the 1790s. Reports are unevenly spread globally: most observations are made in Australia, North America, and on islands. We documented 114 MMEs due to natural abiotic factors involving 23 species (Table 1), but found no published reports of such events in Africa, Europe, or South America.
Asia
MMEs in pteropodid bats were reported at several locations in India as a result of unusually hot periods during 2010 and 2015 (Appendix S3).
Australia
Deaths of many thousands of pteropodid bats due to extremely hot spells were reported in eastern Australia as recently as 2014 (Appendix S3). Ambient temperatures exceeded 48 °C at numerous locations during the 2014 event (Anonymous 2014b). Large numbers of flying foxes died during presumably lesser heat spells in 1790 and during the early 1900s (Appendix S3), and many thousands of grey-headed and black flying foxes Pteropus alecto died during 2000-2007, presumably from excessive heat (Appendix S3; Welbergen et al. 2008). The main cause of these MMEs is hyperthermia. Welbergen et al. (2008) found that ambient temperatures ≥ 42 ⁰C were associated with lethal hyperthermia; up to 13% of the black flying foxes in one large, well-studied colony died from hyperthermia, the majority being dependent young. These MMEs took place during summer when bats are in optimal body condition. MMEs from hyperthermia are expected to increase in number and severity with climate change (Welbergen et al. 2008). Droughts and resulting bush fires often accompany extreme heat, and also have killed ‘many’ flying foxes through smoke inhalation, heat exposure, or immolation (Hall and Richards 2000). Droughts also reduce food supplies and cause MMEs in grey-headed flying foxes through starvation (Appendix S3). At the other abiotic extreme, over 1000 flying foxes (species unspecified) died in northeastern New South Wales and southeastern Queensland in 1990 and 1991 during wet, windy, cold winter weather when food supplies were low (Hall & Richards 2000).
Islands
Major storms (hurricanes, typhoons, cyclones; Appendix S3) have caused MMEs in bats on islands. These MMEs primarily involved pteropodid bats, including Pacific flying foxes Pteropus tonganus on the Vava’u islands of Tonga in 2001, Pacific flying foxes and and Solomons flying foxes Pteropus rayneri on the Solomon Islands in 1986,, Pacific flying foxes and Samoan flying foxes Pteropus samoensis on Tutuila, American Samoa in 1990 and 1991, Rodrigues flying foxes Pteropus rodricensis on Rodrigues, Mauritius in 1979, and greater Mascarene flying foxes on Réunion during 1960 and 1979 (Appendix S3). In many of these cases, some of the mortality following severe storms was delayed and attributable to lack of food caused by winds stripping vegetation, and to opportunistic hunting by people (e.g. Cheke & Dahl 1981, Appendix S3). Such MMEs are exacerbated by increased vulnerability to hunting due to lack of concealing vegetation, and by bats being forced to forage in daylight on the ground where they also are vulnerable to predation by dogs, cats, and pigs (Pierson & Rainey 1992; Stinson et al. 1992).
One report confirmed storms causing an MME of insectivorous bats on islands: flooding from Cyclone Hyacinthe in 1980 drowned over 3000 Mauritian little mastiff bats Mormopterus acetabulosus in a cave on Réunion (Cheke & Dahl 1981). Hurricanes on Puerto Rico in 1989 and 1994 preceded dramatic declines in populations of the red fig-eating bat Stenoderma rufum, but no carcasses were reported (Gannon & Willig, 1994). Similarly, marked decreases and changes in relative abundance among frugivorous species of bats followed the 1989 hurricane on Montserrat in the Caribbean (Pedersen et al. 2009).
Eruptions have caused MMEs in bats on volcanic islands. Direct mortality of multiple individuals of Seychelles flying foxes Pteropus seychellensis was observed on Grande Comore in the Comoros Islands in the Indian Ocean (Appendix S3). Volcanic activity may also have caused MMEs in pteropodid bats on some Pacific islands (Lemke 1992). Beginning in 1995, volcanic activity on Monserrat in the Caribbean “reduced the eastern and western flanks of the volcano to an ecological wasteland and have buried much of the southern half of the island under varying amounts of volcanic ash” (Pedersen et al. 2009). Volcanic action destroyed roosts on Monserrat, and frugivorous bat abundance decreased; although no mortality was directly observed and no species were lost, survivors showed signs of sublethal pathologies (Pedersen et al. 2009, 2012).
North America
Flooding of caves during unusually rainy weather or spring runoff has caused MMEs in bats in North America. Floods in the Ohio River valley, USA, in 1964 led to declines in four species of bats at one hibernaculum in Kentucky, which dropped from about 6000 bats in February to about 500 in March after the flood; numerous carcasses were found trapped among flood debris and mud after the waters receded (Appendix S3). Similarly, about 6500 dead southeastern myotis Myotis austroriparius were observed awash in one Florida cave in 1989 following a summer downpour, an estimated 57000 individual southeastern myotis died in a second cave during record high water in 1990, and flooding in 1994 killed 85000 individual southeastern myotis in Snead’s Cave, Florida (Appendix S3). An estimated 10000 grey bats Myotis grisescens were found dead after flooding in a Tennessee cave in 1970 (Appendix S3). Based on skeletal deposits, an estimated 300000 Indiana bats (Myotis sodalis) died from past flooding at Bat Cave, Kentucky, thought by some to be a major flood in 1937 (Appendix S3). MMEs in Indiana bats caused by flooding at other caves involved 3000 individuals in 1997, and hundreds at two caves in 1996 (Appendix S3).
Despite the thermal stability of caves and mines used by bats for hibernation, extremely cold weather has caused MMEs at hibernacula in North America. Subfreezing temperatures killed 200 individual Indiana bats at a hibernaculum in Indiana in 1977, and “large numbers” at a hibernaculum in Missouri (Appendix S3). Over 100 big brown bats Eptesicus fuscus were found dead in snow drifts formed by an early winter storm that blocked access to hibernacula in Minnesota in 1940 (Appendix S3). Hundreds of little brown bats Myotis lucifugus were found dead or dying in the streets of a Wisconsin town in autumn 1936, and were thought to have become exhausted while migrating in association with a cold front (Appendix S3). In the Mojave Desert of California, over 40 Brazilian free-tailed bats attempting to drink in a normally ice-free pond were entrapped and frozen at the surface during an unusual cold snap in 1930 (Appendix S3).
MMEs due to exposure to environmental contaminants
Chemical contaminants caused MMEs in bats in Australia, Europe, New Zealand and North America, and at a global scale are suspected of causing far more mortality than has been clearly documented. We compiled 43 accounts of MMEs caused by chemical contaminants in 16 species of bats since 1952; organochlorine pesticides such as DDT were the most prevalent agents (Appendix S4). Worldwide use of many organochlorines markedly declined in recent decades due to legislative action and international treaties. Impacts of substitute chemicals on bats have not been adequately investigated (e.g. O’Shea and Johnston 2009).
Organochlorine insecticides and their metabolites can persist in the environment and may become concentrated in fatty tissues of insects and the bats that eat them. Organochlorines may reside in the bodies of bats with no overt effects until stored fats are metabolised, whereupon the chemicals increase in brains until they reach lethal, neurotoxic concentrations. Research has established threshold concentrations in bat brains that are diagnostic of lethality, and correlated concentrations measured in carcasses or guano also indicate lethal exposure (Clark & Shore 2001, O’Shea & Johnston 2009). Although circumstantial evidence and simple presence of chemical residues in carcasses are not truly diagnostic of this cause of death, some die-offs due to unexplained causes (Appendix S9) were very likely to have been caused by chemical contaminants. MMEs described below and in Appendix S4 pertain to poisoning through the food-web or other environmental exposures, and to unintended consequences of pesticide treatments of timber at roosts in buildings. Cases where chemicals were applied directly to bats or their roosts for ‘pest control’ are summarised under intentional killing (Appendix S1). We found no conclusive evidence that chemical contaminants directly caused MMEs through food-chain exposures in Africa, Asia, or South America, although organochlorines were applied much more recently in these regions.
Australia
About 30% of the flying foxes (three species, Appendix S4) found dead in urban areas of Brisbane during the 1980s had histopathological lesions and concentrations in tissues indicating exposure to lethal amounts of lead, likely as an atmospheric pollutant. Investigators hypothesised that insecticide exposure caused die-offs of long-fingered bats in the 1960s, but reported no corroborating diagnostic evidence (Appendix S4).
Europe
Treating of timbers in buildings with organochlorine compounds (DDT, dieldrin, lindane, pentachlorophenol) as protection against wood-boring insects, which took place from about 1950 to the 1980s, is thought to have been “the most important factor in killing bats and reducing breeding success” in Europe (Stebbings & Griffiths 1986). First documented in the literature during the early 1970s (Appendix S4), timber treatment occurred on a massive scale; over 1500 private companies were directly involved in Britain alone during the 1980s (Mitchell-Jones et al. 1989). Many species of bats in Europe are highly dependent on wooden-frame buildings for roosts. Organochlorine compounds were used in hundreds of thousands of such buildings, and remained available for uptake by roosting bats through contact, ingestion while grooming, and inhalation of vapours for years after application (e.g. Shore et al. 1990). Captive bats experimentally exposed to treated timbers died (reviewed in Clark & Shore 2001), and such uptake has been implicated in killing nine species of bats in the wild (Appendix S4). We are unaware of reports of food-web exposure to environmental contaminants causing MMEs in Europe. Researchers detected potentially toxic concentrations of lead in pipistrelles Pipistrellus sp. in England, but not definitively in association with MMEs (Appendix S4).
Islands
The anti-coagulant rodenticide diphacinone caused the death of at least 115 New Zealand lesser short-tailed bats Mystacina tuberculata on North Island, New Zealand; presumably it was ingested from prey or while ground-foraging (Appendix S4)
North America
Dieldrin (or the parent compound aldrin) clearly caused MMEs through food-web exposure (including juveniles nursing contaminated milk) in grey bats in the USA during the 1970s (Appendix S4). Grey bats roost in caves where carcasses can be found easily. Although the full extent of organochlorine-caused die-offs is unknown, lethal concentrations of dieldrin, DDT, endrin, heptachlor, and their metabolites also have been found in Indiana bats (Appendix S4) and possibly Brazilian free-tailed bats (Reidinger 1976). Similarly, exposure to DDT and its metabolites have been linked to major declines in populations of Brazilian free-tailed bats in the southwestern USA (Clark 2001, Geluso et al. 1976), but these data were not obtained from carcasses found during MMEs. The role of other classes of insecticides in causing MMEs is more difficult to determine but has been long suspected. For example, Brazilian free-tailed bats were found dead in Arizona agricultural fields where organophosphate insecticides were applied near a large colony that suffered a major contemporaneous decline (Clark & Shore 2001; Appendix S9). Carbamate poisoning was recently reported in bats in Idaho (Appendix S4).
Mining operations that utilise cyanide for gold extraction and then store contaminated waters in ponds on site attract bats to drink. MMEs in bats at these ponds due to cyanide poisoning have been recorded in South Carolina, Arizona, and Nevada (Appendix S4), and are likely to be more prevalent globally than documented in the literature.
MMEs due to accidents
Since at least 1906, accidental deaths of multiple bats have been reported from Africa, Australia, Europe, the Seychelles Islands, and North America (Appendix S5), and stem from both natural and anthropogenic sources. We tabulated 66 MMEs involving 37 species of bats. ‘Natural’ accidental mortality includes entrapment of bats in buildings (e.g. Dietz et al. 2009), entrapment of lasiurine bats (Genus Lasiurus; not normally cavernicolous) that cannot find their way out of large caves in North America, first noted in 1907 (Appendix S5), and odd accidents such as being crushed by falling trees (Appendix S5). Accidental falls from roost ceilings probably caused mortality of juvenile bats of several species in North America (Appendix S5). Such MMEs involved about 13000 young in one season in dense populations of Brazilian free-tailed bats in Texas caves, and as few as 36 per season for grey bats in Kentucky (Appendix S5). This mortality is strongest in the first days of life (Foster et al. 1978, Hermanson & Wilkins 1986), and fallen young are sometimes eaten by scavengers. Some young bats may fall from weakness due to disease, malnutrition, or pesticide poisoning, but ultimate causes are generally unknown.
Human-caused MMEs from accidents (Appendix S5) include electrocution of flying foxes on utility wires in Australia and on islands, incidental demolition of buildings with roosts (Europe), collisions with aircraft (Australia), and collisions with motor vehicles on roadways (Europe, North America). Collisions with motor vehicles may be a large but widely unrecognised mortality factor for bats. Globally there are over 35 million km of roadways (Anonymous 2015); most reported MMEs on roads have been from studies in Europe that covered only about 150 km of roadway in total, yet revealed deaths in 26 species of bats (Appendix S5).
MMEs due to collisions with wind turbines
A recent and unexpected form of MMEs in bats is associated with the global expansion of industrial wind energy production. Multiple fatalities have been reported from wind turbines in North America, Europe, South America, Africa, and Australia, most during the past decade (Appendix S6). Wind turbines are increasing globally and MMEs in bats are likely to occur at most facilities, but the majority of available reports are limited to Europe and North America. We tabulated 281 MMEs involving 41 species; some carcass counts numbered in the hundreds (Table 1 and Appendix S6). This cause of MMEs is very recent (Fig. 1 and 3), widespread, and growing rapidly. Numbers of deaths vary among sites for unknown reasons (Arnett et al. 2008). However, estimates that include bias corrections (see Appendix S6) range to thousands of bat deaths annually at some facilities (Appendix S6). Cumulative deaths of bats at turbines tabulated for Europe for the period 2003-2013 involved 5626 bats of 27 species in 18 countries (Rodrigues et al. 2014), only a fraction of the likely mortality. Most deaths of bats at wind turbines in temperate latitudes occur during late summer and autumn, and disproportionately affect migratory species that roost in trees (Cryan & Barclay 2009, Arnett & Baerwald 2013). In some regions, deaths of some species at wind turbines far exceed other known sources of mortality (Cryan 2011). Causes of susceptibility to wind turbines are not fully understood, but some bats seem attracted to them (Cryan et al. 2014).
MMEs attributable to or strongly suggestive of viral or bacterial diseases
Despite the high number of viruses known to infect bats (e.g. Calisher et al. 2006, Luis et al. 2013), MMEs attributable to infectious viral or bacterial diseases are rarely reported. The absence of epizootics in bats has been noted as remarkable by field researchers over many years. In his review of Australian pteropodid ecology, Ratcliffe (1932) stated: “No reliable evidence of the occurrence of epidemics among the fruit-bat population was discovered.” In studies of the southeastern myotis Myotis austroriparius, Rice (1957) remarked “Disease is apparently unimportant … During the course of this study, which involved observations on over a million bats in every known cave colony in Florida, I have never found a dead bat, and have seen only one which appeared diseased.” In extensive research on cavernicolous bats in North America, Twente (1955) concluded “it would not seem probable that disease is an important limiting factor.” Similarly, in ecological studies of Brazilian free-tailed bats in Texas, which form the largest aggregations of mammals on Earth, Davis et al. (1962) observed that “Better conditions for epizootic spread of disease can hardly be imagined, yet we did not observe anything that looked like epizootic disease”.
We found 25 MMEs in 14 species of bats (Table 1) attributable to or suggestive of disease other than WNS, many without confirmatory evidence of a specific agent or with unexplained components (Appendix S7). MMEs in this category involved maximum numbers in the thousands, included some of the earliest (1839) reported die-offs of bats, and occurred in Africa, Asia, Australia, Europe, North America, and on islands (Appendix S7). Nine MMEs involved pteropodid bats (six on islands). Brazilian free-tailed bats accounted for seven of the 13 cases in North America (Appendix S7). One event in North America provided supporting evidence (bacterial isolation and pathology) for a bacterial agent (Pasteurella multocida) killing about 100 individual big brown bats during a four-week period at a roost in Wisconsin, USA (Blehert et al. 2014). With the exception of rabies, few of the other MMEs had strong confirmatory evidence of a causal organism; only three non-rabies reports suggested a specific agent. A Bunyavirus was implicated in deaths of wrinkle-lipped free-tailed bats Chaerephon plicatus in Cambodia, Lagos bat virus (a rabies-like lyssavirus) was found in about 10-15% of several hundred dead Wahlberg’s epauletted fruit bats Epomophorus wahlbergi examined in South Africa (Appendix S7), and the recently identified Lloviu filovirus was found in carcasses of Schreiber’s long-fingered bat Miniopterus schreibersii that were sampled during MMEs involving tens to hundreds of bats at two locations in Spain (Appendix S7). In this latter case the finding of the filovirus is possibly incidental and the responsible cause remains unconfirmed (Olival and Hayman 2014). Multiple roosts in Spain, France, and Portugal were also subject to MMEs contemporaneously, but these populations were not extensively sampled diagnostically, and pathological and virological results were inconclusive (Appendix S9).
Rabies is enzootic in populations of Brazilian free-tailed bats, and given the huge size of some of these colonies it is not unexpected that deaths of 10 or more bats from rabies have been reported within a season at roosts (Appendix S7). These deaths from rabies, however, are not of epizootic proportions (e.g. Davis et al. 2012 in Appendix S7). MMEs in Brazilian free-tailed bats during the 1950s at Carlsbad Caverns were equivocal: although > 10 individuals had died of rabies each year, others may have died of pesticide poisoning (Clark 2001), whereas inclement weather and abnormally cool conditions may have been associated with many of the deaths in 1955 and 1956, but not in 1957 (Appendix S7 and references therein). Constantine (1967) postulated that inclement, cool weather during periods of migratory stress contributed to or perhaps caused the MMEs at Carlsbad Caverns, as well as some unexplained MMEs in this highly migratory species elsewhere (Appendix S9). The Old World Schreiber’s long-fingered bat is also a migrant that gathers in large colonies, and similarly has been reported to suffer mortality that is unexplained or suspected to be due to pesticides, migratory stress, or inclement weather (Appendices S3, S9).
MMEs suggested to be due to disease affected large numbers of pteropodid bats on islands (Appendix S7). Perhaps disease is more likely to kill pteropodids in immunologically naïve populations on islands, but corroborating evidence for any infectious agents remains slim. In two MMEs, disease was suspected because the die-off was contemporaneous with epidemics in humans (measles and dysentery). In a third case, subsequent authors suggested alternatively that invasive ants may have been a causal factor (Appendices S2 and S7). Two events involving mass abortions of pteropids in Australia (Appendix S7) may have been due to disease, but there was no confirmatory evidence, and other possible causes (malnutrition, weather, non-infectious agents) were not ruled out. However, as in other cases, availability and application of diagnostic tools for diseases of bats has been limited.
MMEs attributable to white-nose syndrome (WNS)
WNS is a fungal disease recognised in bats within the past decade in North America. WNS is the only epizootic disease known to cause widespread and high mortality in multiple species of bat over multiple years. First documented at a cave in New York, USA, during 2006, WNS has spread in subsequent winters to affect most species of hibernating bat in eastern North America (Turner et al. 2011). This ongoing epizootic has killed millions of bats and is affecting six or more species (Turner et al. 2011; Appendix S8); important populations are in serious decline due to WNS (Frick et al. 2010a, Thogmartin et al. 2013).
WNS is caused by the cold-growing fungus Pseudogymnoascus destructans, which severely infects the skin tissues of hibernating bats and catastrophically disrupts hibernation and physiology during winter (e.g. Lorch et al. 2011, Warnecke et al. 2012). The fungus also infects bats in Europe, where it is not known to cause MMEs (Puechmaille et al. 2011). Macroecological analysis supports a hypothesis that more severe WNS mortality in Europe may have occurred much earlier than in North America (Frick et al. 2015). WNS continues to spread across North America, and will probably continue to cause MMEs in additional species and regions.
Unexplained MMEs
Seventy-three MMEs in 20 species of bats in Australia, Europe, North America, and on islands lacked causal evidence (Tables 1 and Appendix S9). Nearly all of these MMEs involved species that depend strongly on caves as roosts. Two MMEs in Peter’s ghost-faced bat Mormoops megalophylla during the 1950s in Mexico and cave myotis Myotis velifer in the USA were mysterious; thousands of mummified remains covered the walls and floors of caves (Appendix S9). Other cases occurred in the 1960s during peak usage and impacts of organochlorine insecticides; pesticide poisoning may have caused some of these MMEs, as previous authors speculated (Appendix S9). Forty-one unexplained MMEs involved migratory species (Brazilian free-tailed bats, Schreiber’s long-fingered bat, mouse-eared myotis Myotis myotis); some researchers suggest migratory stress as a possible cause (Appendix S9).
Bat population dynamics, MMEs, and implications for the future
Recent studies indicate that population dynamics of bats may be particularly sensitive to mortality. These studies primarily involved temperate zone species, but similar dynamics are seen in large tropical pteropodids (Hayman et al. 2012). A recent brief review noted the similarity in the relative importance of life history traits of bats to those of many large mammals: for the size of a bat population to remain stable or to increase, annual survival of adults must be relatively high: > 75-80% (O’Shea et al. 2011a). Population growth rates of bats show greater sensitivity and elasticity to adult survival than to reproduction or juvenile survival. This suggests that, compared to that of other small mammals, the demography of bats is adapted to a narrow range of mortality drivers, such as the natural biotic and abiotic factors, disease, and natural accidents documented in this review. These natural factors (with the recent exception of WNS) may operate more diffusely than some anthropogenic factors. The magnitude of mortality due to past intentional killing, recent incidence of WNS, and increases in levels of accidental mortality from collisions with wind turbines are likely to be additive; we doubt that bat populations can sustain such additive mortality for long. Furthermore, future climate change may increase the frequency of MMEs through severe weather events, such as extreme droughts, more frequent storms, and flooding (examples in Appendix S3). Recent researchers (see Herring et al. 2014) have concluded that climate change drove the high temperatures in Australia in 2013 and 2014 that killed hundreds of thousands of flying foxes (Appendix S3). Population growth also can be impacted negatively through the suppressed reproduction and reduced juvenile survival of bats seen during periods of drought (Adams 2010, Frick et al 2010b, O’Shea et al 2010, 2011b).
CONCLUSIONS
Many MMEs in bats have been reported over the years. Of the nine potential causes that we differentiated, intentional killing by people caused the greatest proportion of MMEs prior to the year 2000. People killed bats because they were considered sources of zoonotic disease, nuisances (e.g. bats that roosted in buildings), or, in Australia and Asia, competitors for fruit crops. People still kill and eat both insectivorous and pteropodid bats in Asia, Africa, and on some islands. Efforts to control bovine rabies transmitted by common vampire bats in South America and southern North America led to indiscriminate killing of non-target cavernicolous bat species that continues to the present. Prior to the year 2000, about 11% of the reported MMEs were attributed to natural abiotic factors. Projected extreme weather due to continuing climate change (e.g. severe storms, flooding, and drought) may increase the number of abiotic MMEs in the future.
Two new causes of MMEs have taken precedence since around the year 2000: death due to collisions with wind turbines globally, and the fungal disease causing WNS in eastern North America. Reports of MMEs due to these two causes will probably soon outnumber all prior reports from all categories combined. Among all categories, MMEs due to viral or bacterial diseases were most rarely reported. Unexplained MMEs were not very common. This supports the hypothesis that many microparasitic infections of bats do not result in MMEs.
We believe that the life history attributes of bats historically allowed populations to compensate more easily for natural causes of mortality. Intentional killing by humans and very recent increases in mortality from other anthropogenic sources has put markedly greater pressures on many populations of bats. Bats globally could benefit from policy, education and conservation actions targeting human-caused mortality. Such actions are particularly important in the face of the new and increasing threats of the 21st Century.
Supplementary Material
Appendix S1. Reports of multiple bat deaths due to intentional killing by humans, including counts of dead bats taken for use as food.
Appendix S10. Literature cited for Appendices S1-S9.
Appendix S2. Reports of multiple bat deaths due to biotic factors other than infectious disease.
Appendix S3. Reports of multiple bat deaths due to natural abiotic factors.
Appendix S4. Reports of multiple bat deaths due to environmental contaminants, including pesticides.
Appendix S5. Reports of multiple bat deaths due to accidents other than collisions with wind turbines.
Appendix S6. Reports of multiple deaths due to fatal interactions with the blades of industrial wind turbines.
Appendix S7. Reports of multiple bat deaths due to or suggestive of infectious viral or bacterial disease.
Appendix S8. Reports of multiple bat deaths due to the fungal agent of white-nose syndrome, Pseudogymnoascus destructans.
Appendix S9. Reports of multiple bat deaths due to unexplained causes.
Acknowledgments
We thank Peggy Eby, Alan Hicks, Lee McMichael, Danilo Russo, and anonymous reviewers for comments. This work is a product of the Small Mammals Working Group of the Research and Policy for Infectious Disease Dynamics (RAPIDD) program of the Science and Technology Directorate (US Department of Homeland Security) and the Fogarty International Center (National Institutes of Health, NIH). RKP was supported by NIH IDeA Program grants P20GM103474 and P30GM110732, P. Thye and the Commonwealth of Australia, the State of New South Wales, and the State of Queensland under the National Hendra Virus Research Program. Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site:
Contributor Information
Thomas J. O’SHEA, Fort Collins Science Center, United States Geological Survey (USGS), Fort Collins, Colorado 80526 USA.
Paul M. CRYAN, Fort Collins Science Center, United States Geological Survey (USGS), Fort Collins, Colorado 80526 USA
David T.S. HAYMAN, Molecular Epidemiology and Public Health Laboratory, Hopkirk Research Institute, Massey University, Private Bag 11222, Palmerston North 4442, New Zealand
Raina K. PLOWRIGHT, Department of Microbiology and Immunology, Montana State University, Bozeman, Montana 59717 USA
Daniel G. STREICKER, Institute of Biodiversity, Animal Health and Comparative Medicine, MRC-University of Glasgow Centre for Virus Research, University of Glasgow, G12 8QQ, Scotland, UK
References
- Adams RA. Bat reproduction declines when conditions mimic climate change projections for western North America. Ecology. 2010;91:2437–2445. doi: 10.1890/09-0091.1. [DOI] [PubMed] [Google Scholar]
- Aguiar LMS, Brito D, Machado RB. Do current vampire bat (Desmodus rotundus) population control practices pose a threat to Dekeyser’s nectar bat’s (Lonchophylla dekeyseri) long-term persistence in the Cerrado? Acta Chiropterologica. 2010;12:275–282. [Google Scholar]
- Aldred J. Conservationists urge Mauritius to halt cull of threatened fruit bat. The Guardian; 2015a. Nov 17, http://www.theguardian.com/environment/2015/nov/17/conservationists-urge-mauritius-to-halt-cull-of-threatened-fruit-bat. [Google Scholar]
- Anonymous. Proceedings of the IUCN Latin American Conference on the Conservation of Renewable Natural Resources. San Carlos Bariloche, Argentina: 1968. Apr 2, Resolution adopted by IUCN on vampire bats. [Google Scholar]
- Anonymous. Resolution on Vampire Bats from the 11th International Bat Research Conference; Pirenopolis, Brazil. 3-6 August.1998. [Google Scholar]
- Anonymous. Flying Foxes: damage mitigation permits for crop protection. Queensland Department of Environment and Heritage Protection; Brisbane, Australia: 2014a. http://www.ehp.qld.gov.au/wildlife/livingwith/flyingfoxes/damage-mitigation-permits.html#summary_of_dmps_issued_for. [Google Scholar]
- Anonymous. Special Climate Statement 47- An Intense Heatwave in Central and Eastern Australia. Australian Bureau of Meteorology; Melbourne: 2014b. http://www.bom.gov.au/climate/current/statements/scs47.pdf. [Google Scholar]
- Anonymous. Roads, total network (km) World Bank; Washington, DC: 2015. http://data.worldbank.org/indicator/IS.ROD.TOTL.KM. [Google Scholar]
- Arellano-Sota C. Biology, ecology, and control of the vampire bat. Reviews of Infectious Diseases. 1988;10:S615–S619. doi: 10.1093/clinids/10.supplement_4.s615. [DOI] [PubMed] [Google Scholar]
- Arnett EB, Baerwald EF. Impacts of wind energy development on bats: implications for conservation. In: Adams RA, Peterson SC, editors. Bat Evolution, Ecology, and Conservation. Springer Science Press; New York, USA: 2013. pp. 435–455. [Google Scholar]
- Arnett EB, Brown WK, Erickson WP, Fiedler JK, Hamilton BL, Henry TH, et al. Patterns of bat fatalities at wind energy facilities in North America. Journal of Wildlife Management. 2008;71:61–78. [Google Scholar]
- Baer GM. Vampire bat and bovine paralytic rabies. In: Baer GM, editor. The Natural History of Rabies. 2nd. CRC Press; Boca Raton, Florida, USA: 1991. pp. 389–403. [Google Scholar]
- Blackwood JC, Streicker DG, Altizer S, Rohani P. Resolving the roles of immunity, pathogenesis, and immigration for rabies persistence in vampire bats. Proceedings of the National Academy of Sciences, USA. 2013;110:20837–20842. doi: 10.1073/pnas.1308817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blehert DS, Maluping RP, Green DE, Berlowski-Zier BM, Ballmann AE, Langenberg JA. Acute pasteurellosis in wild big brown bats (Eptesicus fuscus) Journal of Wildlife Diseases. 2014;50:136–139. doi: 10.7589/2012-02-063. [DOI] [PubMed] [Google Scholar]
- Brown DE. Vampiro: the Vampire Bat in Fact and Fantasy. University of Utah Press; Salt Lake City, Utah, USA: 1994. [Google Scholar]
- Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: important reservoir hosts of emerging viruses. Clinical Microbiology Reviews. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheke AS, Dahl JF. The status of bats on western Indian Ocean islands, with special reference to Pteropus. Mammalia. 1981;45:205–238. [Google Scholar]
- Clark DR., Jr DDT and the decline of free-tailed bats (Tadarida brasiliensis) at Carlsbad Cavern, New Mexico. Archives of Environmental Contamination and Toxicology. 2001;40:537–543. doi: 10.1007/s002440010207. [DOI] [PubMed] [Google Scholar]
- Clark DR, Jr, Shore RF. Chiroptera. In: Shore RF, Rattner BA, editors. Ecotoxicology of Wild Mammals. John Wiley & Sons; Chichester, West Sussex, UK: 2001. pp. 159–214. [Google Scholar]
- Collins C, Kays R. Causes of mortality in North American populations of large and medium-sized mammals. Animal Conservation. 2011;14:474–483. [Google Scholar]
- Constantine DG. Bats in relation to the health, welfare, and economy of man. In: Wimsatt WA, editor. Biology of Bats. II. Academic Press; New York, USA: 1970. pp. 319–449. [Google Scholar]
- Constantine DG. Activity patterns of the Mexican free-tailed bat. University of New Mexico Publications in Biology. 1967;7:1–79. [Google Scholar]
- Corrêa-Scheffer K, Iamamoto K, Miyuki Asano K, Mori E, Estevez Garcia AI, Achkar SM, Oliveira Fahl W. Murciélagos hematófagos como reservorios de la rabia. Revista Peruana de Medicina Experimental y Salud Publica. 2014;31:302–309. [PubMed] [Google Scholar]
- Cryan PM. Wind turbines as landscape impediments to the migratory connectivity of bats. Environmental Law. 2011;41:355–370. [Google Scholar]
- Cryan PM, Barclay RMR. Causes of bat fatalities at wind turbines: hypotheses and predictions. Journal of Mammalogy. 2009;90:1330–1340. [Google Scholar]
- Cryan PM, Gorresen PM, Hein CD, Schirmacher MR, Diehl RH, Huso MM, et al. Behavior of bats at wind turbines. Proceedings of the National Academy of Sciences USA. 2014;111:15126–15131. doi: 10.1073/pnas.1406672111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A, Gordy P, Rudd R, Jarvis JA, Bowen RA. Naturally acquired rabies virus infections in wild-caught bats. Vector-Borne and Zoonotic Diseases. 2012;12:55–60. doi: 10.1089/vbz.2011.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RB, Herreid CF, Short HL. Mexican free-tailed bats in Texas. Ecological Monographs. 1962;32:311–346. [Google Scholar]
- Dietz C, von Helversen O, Nill D. Bats of Britain, Europe and Northwest Africa. A & C Black; London, UK: 2009. [Google Scholar]
- Eby P, Lunney D, editors. Managing the Grey-Headed Flying-Fox as a Threatened Species in NSW. Royal Zoological Society of New South Wales, Southwood Press; Marrickville, New South Wales, Australia: 2002. [Google Scholar]
- Fenton MB, Simmons NB. Bats: A World of Science and Mystery. University of Chicago Press; Chicago, USA: 2014. [Google Scholar]
- Fey SB, Siepielski AM, Nusslé S, Cervantes-Yoshida K, Hwan JL, Huber ER, Fey MJ, Catenazzi A, Carlson SM. Recent shifts in the occurrence, cause, and magnitude of animal mass mortality events. Proceedings of the National Academy of Sciences, USA. 2015;112:1083–1088. doi: 10.1073/pnas.1414894112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Crespo R, Arellano-Sota C. Biology and control of the vampire bat. In: Baer GM, editor. The Natural History of Rabies. 2nd. CRC Press; Boca Raton, Florida, USA: 1991. pp. 461–476. [Google Scholar]
- Foster GW, Humphrey SR, Humphrey PP. Survival rate of young southeastern brown bats, Myotis austroriparius, in Florida. Journal of Mammalogy. 1978;59:299–304. [Google Scholar]
- Frick WF, Pollock JF, Hicks AC, Langwig KE, Reynolds DS, Turner GR, Butchkoski CM, Kunz TH. An emerging disease causes regional population collapse of a common North American bat species. Science. 2010a;329:679–682. doi: 10.1126/science.1188594. [DOI] [PubMed] [Google Scholar]
- Frick WF, Reynolds DS, Kunz TH. Influence of climate and reproductive timing on demography of little brown myotis Myotis lucifugus. Journal of Animal Ecology. 2010b;79:128–136. doi: 10.1111/j.1365-2656.2009.01615.x. [DOI] [PubMed] [Google Scholar]
- Frick WF, Puechmaille SJ, Hoyt JR, Nickel BA, Langwig KE, Foster JT, et al. Disease alters macroecological patterns of North American bats. Global Ecology and Biogeography. 2015;24:741–749. [Google Scholar]
- Gannon MR, Willig MR. The effects of Hurricane Hugo on bats of the Luquillo Experimental Forest of Puerto Rico. Biotropica. 1994;26:320–331. [Google Scholar]
- Geluso KN, Altenbach JS, Wilson DE. Bat mortality: pesticide poisoning and migratory stress. Science. 1976;194:194–186. doi: 10.1126/science.959845. [DOI] [PubMed] [Google Scholar]
- Gillette DD, Kimbrough JD. Chiropteran mortality. In: Slaughter BH, Walton DW, editors. About Bats: a Chiropteran Symposium. Southern Methodist: University Press; Dallas, Texas, USA: 1970. pp. 262–283. [Google Scholar]
- Goodman SM. Hunting of Microchiroptera in south-western Madagascar. Oryx. 2006;40:225–228. [Google Scholar]
- Greenhall AM, Joermann G, Schmidt U. Desmodus rotundus. Mammalian Species. 1983;202:1–6. [Google Scholar]
- Greenhall AM, Schmidt U, editors. Natural History of Vampire Bats: Desmodus, Diaemus, Diphylla. CRC Press; Boca Raton, Florida, USA: 1988. [Google Scholar]
- Hall L, Richards G. Flying Foxes: Fruit and Blossom Bats of Australia. Krieger Publishing Company; Malabar, Florida, USA: 2000. [Google Scholar]
- Hayman DTS, McCrea R, Restif O, Suu-Ire R, Fooks AR, Wood JLN, Cunningham AA, Rowcliffe JM. Demography of straw-colored fruit bats in Ghana. Journal of Mammalogy. 2012;93:1393–1404. doi: 10.1644/11-MAMM-A-270.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanson JW, Wilkins KT. Pre-weaning mortality in a Florida maternity roost of Myotis austroriparius and Tadarida brasiliensis. Journal of Mammalogy. 1986;67:751–754. [Google Scholar]
- Herring SC, Hoerling MP, Peterson TC, Stott PA. Explaining extreme events of 2013 from a climate perspective. Bulletin of the American Meteorological Society Special Supplement. 2014;95:S1–S96. [Google Scholar]
- Hutson AM, Mickleburgh, Racey PA. Global Status Survey and Conservation Action Plan: Microchiropteran Bats. IUCN/SSC Chiroptera Specialist Group; IUCN, Gland, Switzerland: 2001. [Google Scholar]
- Johnson N, Aréchiga-Ceballos N, Aguilar-Setien Vampire bat rabies: ecology, epidemiology and control. Viruses. 2014;6:1911–1928. doi: 10.3390/v6051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuti M, Paploski AD, Silva MDCP, de Oliveira EA, da Silva AWC, Biondo AW. Prevention educational program of human rabies transmitted by bats in rain forest preserved area of southern Brazilian coast. Zoonoses and Public Health. 2011;58:529–532. doi: 10.1111/j.1863-2378.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- Lang H, Chapin JP. Notes on the distribution and ecology of central African Chiroptera. Bulletin of the American Museum of Natural History. 1917a;37:479–496. [Google Scholar]
- Lang H, Chapin JP. Field notes on African Chiroptera. Bulletin of the American Museum of Natural History. 1917b;37:497–563. [Google Scholar]
- Lemke TO. Status of the Marianas fruit bat (Pteropus mariannus) in the Northern Mariana Islands north of Saipan. In: Wilson DE, Graham GL, editors. Pacific Flying Foxes - Proceedings of an International Conservation Conference. Washington DC, USA: 1992. pp. 68–73. (U.S. Fish and Wildlife Service Biological Report 90). [Google Scholar]
- Lorch JM, Meteyer CU, Behr MJ, Boyles JG, Cryan PM, Hicks AC, et al. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature. 2011;480:376–378. doi: 10.1038/nature10590. [DOI] [PubMed] [Google Scholar]
- Luis AD, Hayman DTS, O’Shea TJ, Cryan PM, Gilbert AT, Pulliam JRC, et al. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proceedings of the Royal Society B. 2013;280:20122753. doi: 10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin D, Mendelsshon H. The fruit bat issue in Israel. Bat News. 1987;11:4–5. [Google Scholar]
- Martin L. Is the fruit you eat flying-fox friendly? The effects of orchard electrocution grids on Australian flying-foxes (Pteropus spp. Megachiroptera) In: Law B, Eby P, Lunney D, Lumsden L, editors. The Biology and Conservation of AustralAsian Bats. Royal Zoological Society of New South Wales; Australia: 2011. pp. 380–390. [Google Scholar]
- Mayen F. Haematophagous bats in Brazil, their role in rabies transmission, impact on public health, livestock industry and alternatives to an indiscriminate reduction of bat population. Journal of Veterinary Medicine B Infectious Diseases and Veterinary Public Health. 2003;50:469–472. doi: 10.1046/j.1439-0450.2003.00713.x. [DOI] [PubMed] [Google Scholar]
- Messenger SL, Rupprecht CE, Smith JS. Bats, emerging virus infections, and the rabies paradigm. In: Kunz TH, Fenton MB, editors. Bat Ecology. University of Chicago Press; Chicago, Illinois, USA: 2003. pp. 622–679. [Google Scholar]
- Mickleburgh S, Waylen K, Racey P. Bats as bushmeat: a global review. Oryx. 2009;43:217–234. [Google Scholar]
- Mickleburgh SP, Hutson AM, Racey PA. Old World Fruit Bats: An Action Plan for their Conservation. IUCN/SSC Chiroptera Specialist Group. International Union for the Conservation of Nature; Gland, Switzerland: 1992. [Google Scholar]
- Mikula P, Morelli F, Lučan RK, Jones D, Tryjanowski P. Bats as prey of diurnal birds: a global perspective. Mammal Review (In press) [Google Scholar]
- Mitchell-Jones AJ, Cooke AS, Boyd IL, Stebbings RE. Bats and remedial timber treatment chemicals – a review. Mammal Review. 1989;19:93–110. [Google Scholar]
- Olival KJ, Hayman DTS. Filoviruses in bats: current knowledge and future directions. Viruses. 2014;6:1759–1788. doi: 10.3390/v6041759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea TJ, Johnston JJ. Environmental contaminants and bats: investigating exposure and effects. In: Kunz TH, Parsons S, editors. Ecological and Behavioral Methods for the Study of Bats. Johns Hopkins University Press; Baltimore, Maryland USA: 2009. pp. 500–528. [Google Scholar]
- O’Shea TJ, Cryan PM, Cunningham AA, Fooks AR, Hayman DTS, Luis AD, Peel AJ, Plowright RK, Wood JLN. Bat flight and zoonotic viruses. Emerging Infectious Diseases. 2014;20:741–745. doi: 10.3201/eid2005.130539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea TJ, Ellison LE, Stanley TR. Adult survival and population growth rate in Colorado big brown bats. Journal of Mammalogy. 2011a;92:433–443. [Google Scholar]
- O’Shea TJ, Cryan PM, Snider EA, Valdez EW, Ellison LE, Neubaum DJ. Bats of Mesa Verde National Park, Colorado: composition, reproduction, and roosting habits. Monographs of the Western North American Naturalist. 2011b;5:1–19. [Google Scholar]
- O’Shea TJ, Ellison LE, Neubaum DJ, Neubaum MA, Reynolds CR, Bowen RA. Recruitment in a Colorado population of big brown bats: breeding probabilities, litter size, and first-year survival. Journal of Mammalogy. 2010;91:418–428. [Google Scholar]
- Pedersen SC, Kwiecinski GG, Larsen PA, Morton MN, Adams RA, Genoways HH, Swier VJ. Bats of Montserrat: population fluctuation and response to hurricanes and volcanoes, 1978-2005. In: Fleming TH, Racey PA, editors. Island Bats: Evolution, Ecology, and Conservation. University of Chicago Press; Chicago, USA: 2009. pp. 302–340. [Google Scholar]
- Pedersen SC, Popowics TE, Kwiecinski GG, Knudsen DE. Sublethal pathology in bats associated with stress and volcanic activity on Monserrat, West Indies. Journal of Mammalogy. 2012;93:1380–1392. [Google Scholar]
- Pierson EP, Rainey WE. The biology of flying foxes of the genus Pteropus: a review. In: Wilson DE, Graham GL, editors. Pacific Flying Foxes - Proceedings of an International Conservation Conference. Washington DC, USA: 1992. pp. 1–17. (U.S. Fish and Wildlife Service Biological Report 90). [Google Scholar]
- Puechmaille SJ, Frick WF, Kunz TH, Racey PA, Voigt CC, Wibbelt G, Teeling EC. White-nose syndrome: is this emerging disease a threat to European bats? Trends in Ecology and Evolution. 2011;26:570–576. doi: 10.1016/j.tree.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Ratcliffe F. Notes on the fruit bats (Pteropus spp.) of Australia. Journal of Animal Ecology. 1932;1:32–57. [Google Scholar]
- Reidinger RF., Jr Organochlorine residues in adults of six southwestern bat species. Journal of Wildlife Management. 1976;40:677–680. [Google Scholar]
- Rice DW. Life history and ecology of Myotis austroriparius in Florida. Journal of Mammalogy. 1957;38:15–32. [Google Scholar]
- Rodrigues L, Bach L, Dubourg-Savage MJ, Karapandža B, Kovač D, Kervyn T, et al. Draft Guidelines for Consideration of Bats in Wind Farm Projects – Revision 2014. EUROBATS Publication Series UNEP/EUROBATS Secretariat; Bonn, Germany: 2014. http://www.eurobats.org/sites/default/files/documents/pdf/Meeting_of_Parties/Doc.MoP7_.13.Annex_incl_annexes_0.pdf. [Google Scholar]
- Shore RF, Boyd IL, Leach DV, Stebbings RE, Myhill DG. Organochlorine residues in roof timbers and possible implications for bats. Environmental Pollution. 1990;64:179–188. doi: 10.1016/0269-7491(90)90114-r. [DOI] [PubMed] [Google Scholar]
- Speakman JR. The impact of predation by birds on bat populations in the British Isles. Mammal Review. 1991;21:123–142. [Google Scholar]
- Stebbings RE. Conservation of European bats. Christopher Helm Publishers; London, UK: 1988. [Google Scholar]
- Stebbings RE, Griffith F. Distribution and Status of Bats in Europe. Institute of Terrestrial Ecology, Monks Wood Experimental Station; Abbots Ripton, Huntingdon, UK: 1986. [Google Scholar]
- Stinson DW, Glass PO, Taisacan EM. Declines and trade in fruit bats on Saipan, Tinian, Aguijan, and Rota. In: Wilson DE, Graham GL, editors. Pacific Flying Foxes - Proceedings of an International Conservation Conference. Washington DC, USA: 1992. pp. 61–67. (U.S. Fish and Wildlife Service Biological Report 90). [Google Scholar]
- Streicker DG, Recuenco S, Valderrama W, Benavides JG, Vargas I, Pacheco V, et al. Ecological and anthropogenic drivers of rabies exposure in vampire bats: implications for transmission and control. Proceedings of the Royal Society B. 2012;279:3384–3392. doi: 10.1098/rspb.2012.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thogmartin WE, Sanders-Reed CA, Szymanski JA, McKann PC, Pruitt L, King RA, Runge MC, Russell RE. White-nose syndrome is likely to extirpate the endangered Indiana bat over large parts of its range. Biological Conservation. 2013;160:162–172. [Google Scholar]
- Turner GG, Reeder DM, Coleman JTH. A five-year assessment of mortality and geographic spread of white-nose syndrome in North American bats and a look to the future. Bat Research News. 2011;52:13–27. [Google Scholar]
- Twente JW., Jr Aspects of a population study of cavern-dwelling bats. Journal of Mammalogy. 1955;36:379–390. [Google Scholar]
- Vincenot CE, Koyama L, Russo D. Near threatened? First report of unsuspected human-driven decline factors in the Ryukyu flying fox (Pteropus dasymallus) in Japan. Mammalian Biology. 2015;80:273–277. [Google Scholar]
- Warnecke L, Turner JM, Bollinger TK, Lorch JM, Misra V, Cryan PM, Wibbelt G, Blehert DS, Willis CKR. Inoculation of a North American bat with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proceedings of the National Academy of Sciences, USA. 2012;109:6999–7003. doi: 10.1073/pnas.1200374109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbergen JA, Klose SM, Markus N, Eby P. Climate change and the effects of temperature extremes on Australian flying-foxes. Proceedings of the Royal Society B. 2008;275:419–425. doi: 10.1098/rspb.2007.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Reports of multiple bat deaths due to intentional killing by humans, including counts of dead bats taken for use as food.
Appendix S10. Literature cited for Appendices S1-S9.
Appendix S2. Reports of multiple bat deaths due to biotic factors other than infectious disease.
Appendix S3. Reports of multiple bat deaths due to natural abiotic factors.
Appendix S4. Reports of multiple bat deaths due to environmental contaminants, including pesticides.
Appendix S5. Reports of multiple bat deaths due to accidents other than collisions with wind turbines.
Appendix S6. Reports of multiple deaths due to fatal interactions with the blades of industrial wind turbines.
Appendix S7. Reports of multiple bat deaths due to or suggestive of infectious viral or bacterial disease.
Appendix S8. Reports of multiple bat deaths due to the fungal agent of white-nose syndrome, Pseudogymnoascus destructans.
Appendix S9. Reports of multiple bat deaths due to unexplained causes.


