Science-based, multinational management of the Baltic Sea offers lessons on amelioration of highly disturbed marine ecosystems.
Abstract
Coastal global oceans are expected to undergo drastic changes driven by climate change and increasing anthropogenic pressures in coming decades. Predicting specific future conditions and assessing the best management strategies to maintain ecosystem integrity and sustainable resource use are difficult, because of multiple interacting pressures, uncertain projections, and a lack of test cases for management. We argue that the Baltic Sea can serve as a time machine to study consequences and mitigation of future coastal perturbations, due to its unique combination of an early history of multistressor disturbance and ecosystem deterioration and early implementation of cross-border environmental management to address these problems. The Baltic Sea also stands out in providing a strong scientific foundation and accessibility to long-term data series that provide a unique opportunity to assess the efficacy of management actions to address the breakdown of ecosystem functions. Trend reversals such as the return of top predators, recovering fish stocks, and reduced input of nutrient and harmful substances could be achieved only by implementing an international, cooperative governance structure transcending its complex multistate policy setting, with integrated management of watershed and sea. The Baltic Sea also demonstrates how rapidly progressing global pressures, particularly warming of Baltic waters and the surrounding catchment area, can offset the efficacy of current management approaches. This situation calls for management that is (i) conservative to provide a buffer against regionally unmanageable global perturbations, (ii) adaptive to react to new management challenges, and, ultimately, (iii) multisectorial and integrative to address conflicts associated with economic trade-offs.
INTRODUCTION
Climate change and anthropogenic pressures are increasingly affecting all ecosystems on Earth (1, 2). With >4 × 109 people soon to be living close to the coastline, managing the ecological integrity of marine waters and the ecosystem services they provide is becoming a prime objective of environmental policy. Highlighting the fact that “life below water” is of global and urgent concern, marine ecosystems were assigned a separate Sustainable Development Goal (SDG), SDG 14, among the 17 global goals recently adopted by the United Nations (UN) (3). It has been argued that we must understand the oceans of the past to understand present-day ecological perturbations (4). Here, we extend this idea and posit that highly perturbed present-day seas can serve as time machines for other marine areas that are on a slower trajectory of anthropogenic perturbation [see also the study of Lejeusne et al. (5)]. We further argue that the Baltic Sea, a semi-enclosed water body surrounded by nine developed and industrialized countries and five more belonging to the catchment area (Box 1), representing 85 million inhabitants, is a particularly well-suited marine “time machine.”
Box 1. The Baltic Sea.
The Baltic Sea, a semi-enclosed postglacial sea with a surface of 415,000 km2 and a volume of 21,700 km3 (Fig. 1A), is characterized by a strong salinity gradient from marine salinity (30 g kg−1) in the entrance to near freshwater (2 g kg−1) in the innermost parts (6). Along this gradient, marine species drop out according to their tolerances for low salinities to be progressively replaced by freshwater species (62). Preceded by a freshwater lake, the marine Baltic Sea is only 8000 years old (6). Given its young age, an average water depth of only 58 m, and a low rate of exchange with North Atlantic waters, the Baltic Sea is extreme for shelf seas let alone the open ocean. The key notion of our review is that these particular features are also precisely the prerequisites that have led to the present-day multistressor situation (eutrophication, warming, oxygen, and acidification status), making the Baltic Sea a large-scale, real-world analog for other coastal regions only in the future. Owing to its young age, the Baltic Sea is populated by few endemic species, with a few notable exceptions of macroalgal and fish species (100, 101). Notwithstanding, many populations within the Baltic have evolved to locally adapted populations that show enhanced resilience toward ocean acidification or lower salinity (102).
The evolutionarily young age of this sea in combination with the predominant brackish conditions results in naturally low species diversity, facilitating analyses of community changes in response to both anthropogenic pressures and implemented countermeasures via management. Although the Baltic is species-poor, it is a productive marine ecosystem that, despite its small area (0.11% of the total ocean area), contributes 1.2% to global capture fisheries. Currently, 140 and counting nonindigenous species (NIS) have been recorded in the Baltic Sea (41), some of which have caused restructuring and changed functioning of both pelagic and benthic ecosystems. This simplified ecosystem is one reason why the Baltic Sea ecosystem provides an ideal test case for marine ecosystem management. Owing to its young age, extreme conditions, and limited habitat size, Baltic populations often have less genetic diversity than their counterparts in the open NE Atlantic (103). Considering that a general reduction in genetic diversity is also predicted for many species/populations globally under future ocean climate change scenarios, Baltic populations can serve as a test case as to how adaptive evolution may play out under reduced genetic diversity (104).
Today, the Baltic Sea ecosystem is affected by levels of warming, acidification, nutrient pollution, and deoxygenation that most coastal areas will experience only in the future (Fig. 1, A to D; Table 1; and table S1A and references therein) (6, 7). The Baltic Sea region is also one of the most intensely studied coastal areas with high data density and many long-term data series. Accordingly, our understanding of its ecosystem structure and processes is relatively advanced, and it has been used as a model region in the past, for example, to understand major connections between pelagic and benthic subsystems (8) or to understand processes leading to oxygen depletion (9). Both system understanding and systematic long-term monitoring have, in many cases, allowed an informed science-based management approach that started earlier (1970s) than in many other regions worldwide (Table 2 and table S1B). At the same time, its governance can constitute an example for other coastal and marine systems that face the problem of implementing international governance systems, such as the Mediterranean Sea and the Black Sea, as well as the Arctic seas. Compared to many other ocean regions, the environmental challenges in the Baltic Sea are major but also relatively high on political agendas; the region has been an institutional forerunner with a long record of international cooperation, extensive scientific research, and a well-developed governance structure (10). Although many coastal areas in the world display a better ecological condition on an absolute scale, Baltic Sea management has been able to reverse several detrimental trends. Thus, the region is an ideal illustration of a complex governance setting in which environmental management has to operate (Fig. 1A).
Fig. 1. The Baltic Sea time machine.
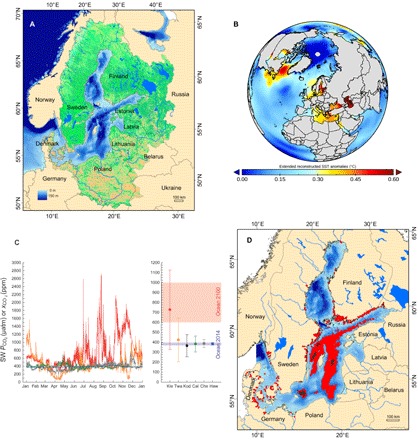
(A) The Baltic Sea, its neighboring countries, and the catchment area. (B) Sea surface temperature (SST) change per decade since 1980. The Baltic Sea is at the center of the map. (C) Left: High-resolution surface seawater CO2 variability in 2014 at a coastal Baltic Sea site (Kiel Fjord Time Series, 54.2°N, 10.9°E; red symbols) in comparison to an oceanic site close to Hawai’i (Woods Hole—Hawaii Ocean Time-series Site, 22.7°N, 157.9°W; blue symbols) and coastal sites in Florida (Cheeca Rocks, 24.9°N, 80.6°W), California [California Current Ecosystem Mooring 2 (CCE2), 34.3°N, 120.8°W; green symbols], Alaska (Kodiak, 57.7°N, 152.3°W; black symbols), and Washington (Twanoh, 47.4°N, 123°W; orange symbols). Right: Mean Pco2/xco2 values and SD for 2014 data from selected time series stations (see above). All data are seawater (SW) Pco2 (in microatmospheres) except for station Twanoh [xco2, in parts per million (ppm), dry]. Kie, Kiel; Twa, Twanoh, Kod, Kodiak; Cal, CCE2; Che, Cheeca Rocks; Haw, Hawai’i. (D) Expansion of hypoxic zones in the Baltic Sea during 115 years of monitoring. Black shading shows the situation for the period 1900–1910, whereas red shading indicates the period 2001–2010. Coastal hypoxia is depicted by red dots. For data sources, see data S3.
Table 1. Summary of abiotic and biotic changes in the Baltic Sea in comparison to other coastal areas worldwide.
Red coloration in the heat map depicts drivers that are above average in severity/impact; yellow, average; and green, below average. Gray: No assessment possible. NIS, nonindigenous species. For full documentation on how scores for each system and parameter were obtained, including all references underlying the assessment, see data S1.
| System |
Warming of surface water |
Increased nutrient load |
Oxygen depletion in bottom waters |
Shipping intensity | Proportion of NIS |
Organochlorines in organisms |
Status of marine fish stocks |
| Baltic Sea | |||||||
| North Sea | |||||||
| Mediterranean Sea | |||||||
| Black Sea | |||||||
| Gulf of Mexico | |||||||
| East China Sea | |||||||
| Barents Sea |
Table 2. Summary of data availability, system understanding, and management/governance regime in the Baltic Sea compared to other coastal areas worldwide.
Green coloration in the heat map indicates good scientific knowledge/effective management/governance structures, red denotes the opposite, and yellow indicates intermediate. For full documentation on how scores for each system and parameter were obtained, including all references underlying the assessment, see data S1.
| System | Research activities | Monitoring activities |
Data availability for fish stock assessments |
Governance structure |
| Baltic Sea | ||||
| North Sea | ||||
| Mediterranean Sea | ||||
| Black Sea | ||||
| Gulf of Mexico | ||||
| East China Sea | ||||
| Barents Sea |
Our synthesis has four objectives. First, we review the status of major anthropogenic pressures in the Baltic Sea to show that, in combination, they are ahead of time (that is, worse) compared to many other regional seas and coastal areas. Second, we highlight how science-based management was imperative for developing management actions. Third, we describe how a functioning governance system was implemented, despite a complex multistate policy setting, and the lessons this history holds for other regions that need to overcome complex international or jurisdictional settings. Finally, in the light of management successes and failures, we also highlight the addition of major novel challenges that Baltic Sea environmental management is facing because global perturbations such as warming and enhanced precipitation patterns affect this region in fast-forward as well. Throughout, we attempt to outline the possible lessons from these present-day examples for other regional seas and, where applicable, for the coastal oceans of the future.
THE BALTIC AS A TIME MACHINE FOR COASTAL MARINE CHANGE
The onset of deterioration of coastal ecosystems predates the industrial age (4, 11, 12) and continues largely unabated today (13). Further drastic increases in major perturbations resulting from global climate change, such as temperature increase, acidification, and altered precipitation patterns, are expected by the year 2100 (1). These factors add to regional and local impacts, including eutrophication, habitat loss, overfishing, species translocation, and pollution. Here, we argue that, at present, the Baltic Sea already provides combinations of multiple pressures that mimic those expected for many coastal areas in the future (Fig. 1, A to D; Table 1; and table S1, A and B), making the Baltic a suitable time machine for the global coastal ocean. Anthropogenic perturbations have simultaneously, and often more severely than elsewhere, affected the Baltic Sea ecosystem. For example, warming trends of up to 0.6 K per decade exceed the global ocean average by a factor of ≈3, notwithstanding the fact that some polar regions are warming even faster (Fig. 1B) (14), and warming increases the vulnerability of coastal systems to nutrient loading (15). Parts of the Baltic water body stand out negatively for very high ocean acidification levels compared to other world coastal regions where such data series are available (Fig. 1C). Owing to local upwelling of carbon dioxide–enriched oxygen-deficient waters, as well as the low buffering capacity of the brackish Baltic Sea, surface Pco2 (partial pressure of CO2) can far exceed values predicted for carbon emission scenarios compatible with the 2°C goal (1, 16). High Pco2 values often coincide with low oxygen values, exacerbating the physiological stress on organisms and populations (17). Nutrient pollution due to intense agriculture in northern Europe and discharge from wastewater resulted in high waterborne nutrient load starting in the 1950s (18). Atmospheric reactive nitrogen deposition rates, in addition, are now among the highest worldwide for any marine area, including catchments (19). This nitrogen deposition, in turn, drives eutrophication and concomitant severe deep pelagic and benthic oxygen deficiencies (Fig. 1D). Oxygen-free “dead zones” are increasing worldwide but have shown a particularly drastic 10-fold increase during the past 115 years in the Baltic Sea (20), turning it into one of the ocean areas most severely affected by hypoxia (9).
The Baltic Sea is one of the most intensely fished marine areas, and fisheries have caused several fish populations to decline (21), which, in turn, contributed to the coastal and offshore ecosystem shifts (see sections on regime shifts) (22). Shipping is intense and increases risks for accidents, oil spills, and species translocations (23). Finally, contamination from land-based industries has led to high amounts of persistent organic micropollutants accumulated in sediments and biomagnified in top predators. Despite recent improvements, contamination remains a serious problem for human consumption of fat fish from the Baltic Sea (24).
Although there are ocean areas that have higher rates of perturbation for single variables (for example, the high Arctic for ocean warming), collectively, the interaction of perturbations that the Baltic ecosystem is subjected to is among the strongest for any marine region (Table 1 and table S1A). Recent experimental evidence suggests the synergistic interaction of cumulative pressures such as warming, deoxygenation, and acidification (25). Hence, the unique multistressor situation in the Baltic Sea resembles coastal processes increasingly expected in coastal zones of the future global ocean (13, 25), underscoring the role of the Baltic Sea as a suitable time machine.
BALTIC SEA ECOSYSTEM CHANGES AND THEIR ECOLOGICAL-ECONOMIC CONSEQUENCES
Ecosystems worldwide are undergoing dramatic changes that can occur not only gradually (1, 26, 27) but also abruptly (“regime shifts”) (28) under mounting anthropogenic pressure. In the Baltic Sea, the good availability of abiotic and biotic time series enabled marine ecologists not only to monitor trajectories in anthropogenic pressures and regime shifts but also to measure improvements in key indicator variables such as nutrients, pollutants, and oxygen, as well as phytoplankton, zooplankton species, fish stocks, and large predators (Fig. 2, A to J). Upon adopting the Helsinki Convention on the Protection of the Marine Environment of the Baltic Sea and establishing its governing body [the Helsinki Commission (HELCOM)], and later adding European Union (EU) legislature and directives, member states have agreed to monitor basic physicochemical and ecological data in a systematic way. In some cases, ecological deterioration only became apparent when temporal analyses are extended >100 years, as is the case in the expansion of anoxic areas (20). We focus here primarily on the increasing pressure levels since the beginning of the last century, but note that anthropogenic pressures on coastal ecosystems date back much longer (centuries), which can lead to erroneous characterization of “pristine” baselines (“shifting baseline problem”) (12, 27, 29).
Fig. 2. Examples of long-term time series available for the Baltic Sea.
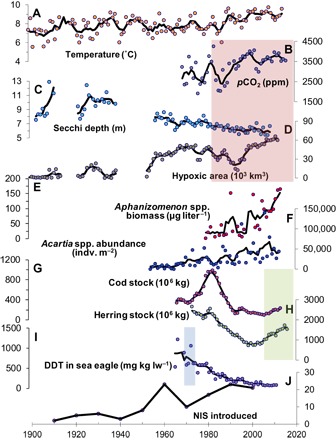
(A) Temperature (0 to 10 m). (B) Pco2 in the bottom waters (>150 m) for station BY15 in the central Gotland Basin. (C) Secchi depths after Baltic Sea Environmental Proceedings no. 133. (D) Benthic area with anoxic conditions (<2 mg O2 liter−1). (E) Abundance of cyanobacteria in the Gulf of Finland. (F) Abundance of zooplankton (Acartia spp.) in Pärnu Bay, Estonia. (G) Eastern Baltic cod total spawning stock biomass. (H) Herring total spawning stock biomass data. (I) DDT concentration in liver of sea eagles. (J) Counts of NIS. Green-, red-, and blue-colored areas indicate the time period when policies for fisheries management, the reduction of nutrients, and the ban of DDT were implemented, respectively. For data sources, see data S3.
Offshore regime shifts
The pelagic zone of the Baltic Sea underwent multiple ecosystem-level shifts in the past century that were triggered by human activity, first by reducing the population size of top predators in the 1950s and 1960s (seals) and, subsequently, by increasing nutrient inputs and, thus, primary productivity (30). The most recent regime shift in the pelagic ecosystem of the central Baltic Sea occurred in the late 1980s to mid-1990s and was triggered by the combination of overfishing of the key fish predator, Atlantic cod (Gadus morhua), and eutrophication-driven deterioration of its spawning grounds, in combination with decadal climatic changes (31, 32). Within the fish community, a previously cod-dominated system flipped therefore to domination by small pelagic fishes, namely, sprat and herring (33). Zooplankton species composition shifted to more warm-temperate species as a result of trophic cascades and hydrological changes at a much faster rate than in the adjacent North Sea and North Atlantic (32, 34). Fish community changes were partially self-enforcing by the consumption of cod eggs by its prey species sprat and herring and by the competition for zooplankton between cod larvae and its prey species (22, 33) and, hence, match the definition of alternative stable states (28). Other important pelagic regime shifts driven by nutrient inputs are the increased frequency and spatial distribution of potentially toxic cyanobacteria blooms during the past 35 years (Fig. 2E), with negative socioeconomic impacts on water quality and, hence, recreational use of the sea area and the coastline (35).
Coastal regime shifts
The Baltic has also witnessed drastic coastal ecosystem shifts, most notably, a strong decline in the distribution of macrophytes (perennial algae and seagrasses) during the past 50 years, driven by coastal eutrophication and concomitant decreases in water clarity and an increase in filamentous algal aggregations and epiphytes, as well as increased mussel bed abundance (36, 37). Exacerbating these bottom-up driven changes, the removal of large predatory fish has led to cascading effects that create additional stresses on macrophytes via the following causal chain: increase in small-sized fishes→decrease in grazing invertebrates→increase in epiphytic algae (38, 39). The increase in three-spined stickleback (39) has been linked to a decrease in their coastal predators, including cod from off-shore regions (40), highlighting the existence of trophic cascades across systems mediated by the seasonal migrations of key species.
NIS-driven regime shifts
Although NIS are ubiquitous to coastal seas, the Baltic example provides good documentation on invasion trajectories and expansions after arrival (41) and an opportunity for thorough before-after comparisons owing to good baseline ecosystem composition and functioning information. Large-scale habitat transformations have been observed throughout the Baltic Sea upon establishment of nonnative habitat engineering species (42). Habitat-modifying species such as the polychaete Marenzelleria spp. have been shown to mitigate benthic hypoxia and decrease P release from the sediments (43). Several nonnative species have added nodes and complexity and have increased intraspecific competition in coastal areas, for example, the invasive mud crab (Rhithropanopeus harrisii) that induced trophic cascades in the rocky shore habitat (44). Another recent invader with strong ecological impact in the Baltic Sea is the round goby Neogobius melanostomus (23) with documented effects on physical habitat and food web structure (45). Pelagic NIS with pronounced grazing impact such as the comb jelly Mnemiopsis leidyi have added new functional niches and can lead to food web cascades (46).
MARINE COASTAL GOVERNANCE IN A COMPLEX INTERNATIONAL SETTING
The cross-border nature of many global change–related pressures greatly complicates successful management, requiring nested institutions and multinational governance solutions (47). A priori, the prerequisites for efficient management of a sea surrounded by nine nations and further five states belonging to the catchment (Fig. 1A) would therefore be considered difficult. Governance of the Baltic Sea region faces serious challenges (48); the Baltic Sea is a common resource that is subject to high demands, from diverse user groups, and geographically shared among nine nation-states (49). Nevertheless, environmental regulation via international governing bodies and treaties of the entire drainage basin started already in the 1970s and places the Baltic among the most intensely managed marine regions in the world. The governance in the Baltic Sea region is polycentric and represents a multilevel system (50) encompassing global conventions (such as the Convention of Biological Diversity), regional conventions such as the Helsinki Convention (with the governing body HELCOM), the European Union (EU), national and subnational authorities, non-government organizations, and the civil society (10).
Internationally, the Baltic Sea stands out through the Helsinki Convention that was formed in 1974 as a response to the mounting environmental problems (10). With all surrounding countries of the Baltic Sea participating, the Helsinki Convention was the first regional sea convention worldwide and became a model for others (51). Upon its foundation, member states rapidly agreed on certain industrial hotspots, so that point-source pollution to the Baltic Sea was significantly reduced over a relatively short period of time. Although all states bordering the Baltic Sea are part of the Helsinki Convention, membership in the EU is “nested” within that convention because Russia is not an EU member. Together, the two organizations constitute the core of the Baltic Sea governance system. Subsequently, the enlargement of, and policy development within, the EU has influenced Baltic Sea governance significantly. Although the Helsinki Convention, as an international convention, is run by unanimous decisions, the EU has supranational elements and sanctioning mechanisms. In 2007, all HELCOM member states and the EU adopted the Baltic Sea Action Plan (BSAP), an ambitious policy agenda outlining (for EU countries binding) environmental targets to be reached by 2021, incorporating the latest scientific findings and a number of novel management instruments (52).
Another forerunner to the current intergovernmental management structure was the International Baltic Sea Fisheries Commission that was established 1 year before HELCOM in 1973 and which formed the basis of fisheries management in the Baltic Sea. Thus, both environment and fisheries management have a similar long history in the Baltic.
The division of authority among different governance bodies and levels in the system is dependent on the type of policy problem, which is illustrated for the three important management areas: fisheries, nutrients, and pollutants (Fig. 3, A to C) (53). In comparison with other shared regional seas, the Baltic stands out because of the institutionalized link between natural science and management and the institutional capacity to formulate and implement policy. The long-standing research collaboration in the region has been of paramount importance and is centered in the International Council for the Exploration of the Sea (ICES), one of the first international scientific organizations (founded in 1902). Devoted to the sustainable use of all North Atlantic waters, ICES gained convention status in 1964 and has since played a critical role in providing policy-makers and managers with scientific data and advice (54). The coherence of Baltic research agendas in a multinational setting has further increased since 2009 through the implementation of the EU policy–driven macroregional “Joint Baltic Sea Research and Development Programme BONUS” (55). Baltic Sea governance increasingly tries to adopt a comprehensive ecosystem-based approach (56), and its management policies have been partially successful, which we review in the next section (Boxes 2 and 3 and data S2) (53, 57).
Fig. 3. Governance structure in the Baltic Sea region.
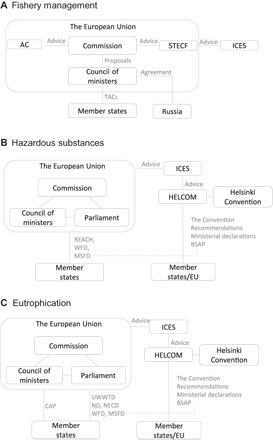
(A) Baltic fisheries management is an exclusive EU competence under the Common Fisheries Policy (2013). Fishing is based on the maximum sustainable yield principle resulting in total allowable catches (TACs) and national quotas. TACs are developed in a process involving the following steps: Advice from stakeholder groups is collected by Advisory Councils (ACs), and scientific advice is provided by ICES and communicated to the EU Commission by the EU Scientific, Technical, and Economic Committee for Fisheries (STECF). The EU Commission suggests TACs to the EU Council of Ministers that makes the final decisions. On the basis of the TACs, national quotas are distributed, implemented, and monitored by member states. Bilateral agreements integrate Russia into the EU environmental management. (B) In the management of hazardous substances, HELCOM carries a significant role for monitoring, assessing, and agenda setting, whereas the EU provides legal basis and enforcement. HELCOM works through its recommendations, the BSAP, and ministerial declarations. The EU has addressed the issue via, for example, the Registration, Evaluation, Authorization and restriction of Chemicals (REACH) regulation, the Marine Strategy Framework Directive (MSFD), and the Water Framework Directive (WFD). The EU Commission initiates and proposes new legislation to be approved by both the Council of Ministers and the European Parliament. The EU and HELCOM closely interact. For example, the BSAP was initiated in 2007 following the EU MSFD. ICES provides scientific data to HELCOM and was involved in the development of the MSFD. (C) Governance of eutrophication. HELCOM targets the sources of eutrophication via several recommendations (for example, Rec 28E/4 on measures to hinder land-based pollution) and the BSAP with reduction targets for emissions of nitrogen and phosphorus. EU has adopted several directives to deal specifically with eutrophication including the Urban Waste Water Treatment Directive (UWWTD), the Nitrate Directive (ND), and the National Emission Ceilings Directive (NECD). The EU Common Agricultural Policy (CAP) strongly influences nutrient management. Within the CAP, member states implement specific agricultural measures targeted at nutrient reduction from agriculture that (partly) reflect measures recommended by HELCOM. For detailed references and sources, see data S3.
Box 2. The return of top predators.
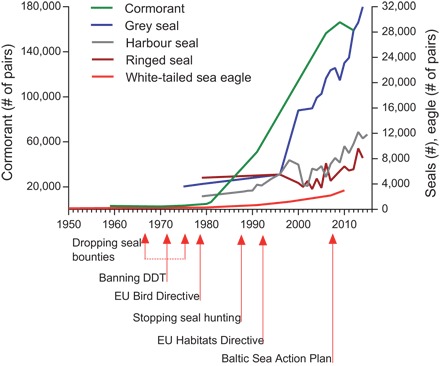
The fast collapse of several marine predators in the Baltic Sea is a poster-child example of similar losses in other world regions following industrialization. Declines were observed in charismatic marine mammals such as grey seal (Halichoerus grypus), ringed seal (Phoca hispida), harbor seal (Phoca vitulina), and harbor porpoise (Phocoena phocoena), as well as birds, the fish-eating great cormorant (Phalacrocorax carbo), and the white-tailed sea eagle (Haliaeetus albicilla). The pressures underlying population collapses were hunting and persecution, exacerbated later on through bioaccumulation of anthropogenic hazardous substances, mainly DDT and polychlorinated biphenyl (PCB), with their detrimental effects on reproduction. In contrast to many other areas in the world, many of the Baltic Sea top predators have seen a recent recovery. Seals, for example, were historically highly abundant in the Baltic Sea, with population estimates of ringed seal, grey seal, and harbor seal up to 220,000, 100,000, and more than 20,000 individuals in the late 19th to early 20th century, respectively, but were then hunted to near extinction. Hunting regulation along with a ban on DDT (105, 106) led to a trend reversal and allowed their recovery (Fig. 4). Several bird species showed a similar recovery, for similar reasons (107, 108). PCB levels in eggs of white-tailed sea eagle and fish-eating birds drastically decreased from those in early 1970s (109), and the consequent higher reproductive success allowed their fast population growth (Fig. 4). Not all species have recovered. Harbor porpoise (P. phocoena) remains red-listed, with one subpopulation of few hundred individuals and another one about an order of magnitude larger (110). This shows that management actions and recovery may be more difficult if causes such as hunting, toxins, by-catch, noise pollution, prey depletion, desertion of hypoxic benthic feeding grounds, and habitat deterioration (111) are more complex and interconnected. The Baltic Sea shows that if a conservation-prone public attitude can be reinforced, and if hazardous substances can be efficiently limited, many top predators can recover. To enable recovery, it is worth to preserve (often genetically unique) populations even if individual numbers become low. The Baltic example also highlights the importance of monitoring bioaccumulation of both conventional and novel toxic substances. Furthermore, with the recovery of seals and cormorants, “new old” interactions are resurfacing: for example, conflicts with fisheries—which were the motivation for the original persecution—are now rearing their head and require practical management (59).
Time courses in the abundance of Baltic top predators. Counts for all species represent breeding pairs. For data sources, see data S3.
Box 3. Fisheries management meets global change.
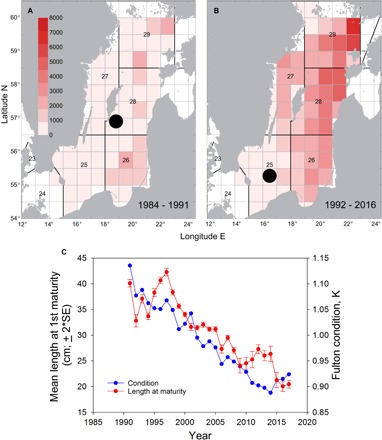
As in other world regions, changes in productivity of Baltic fish stocks have occurred both gradually and abruptly (“regime shifts”) during the 20th century. The underlying drivers have also changed in relative importance over time, for example, for the Eastern Baltic cod stock from seal predation to climate-induced hydrographic conditions in combination with eutrophication and overfishing (112). Some of the management reference points for Baltic fish stocks have been guided by documented changes in past productivity (113, 114), and a few “experimental” multispecies assessments have been conducted to explore impacts of species interactions on sustainable fishing levels (90). Although these efforts are important first steps toward ecosystem-based fishery management, the rapidly changing environment in the Baltic Sea highlights some of the difficulties that marine fisheries management will increasingly face. A case in point is large temporal changes in the spatial distributions of sprat to the colder and more productive Northern Baltic, resulting in a spatial mismatch with its predator cod (Box 3 figure, panels A and B) (65, 115). Spatially explicit management plans for the Baltic sprat fisheries have been suggested, although not yet developed, with the goal of increasing the abundance of the key prey species for cod in the area currently occupied by cod (116). The Baltic also witnesses in fast-forward how deteriorating environmental conditions interfere with classical management of fish stocks. The Eastern Baltic cod stock has experienced drastic declines in individual condition (88, 117) and length at maturity over the last 20 years (Box 3 figure, panel C) (118) that are likely driven both by prey distribution moving north and by a reduction of benthic prey availability in response to worsening oxygen conditions (117). The reduction in length at maturity by almost half may be a drastic example of fisheries-induced evolution, but reduced growth related to the condition decline cannot be excluded, because Eastern Baltic cod cannot be aged because of methodological difficulties (119). Finally, other management actions addressing different members of the food web can interact as well. This is illustrated by the recent increase in seal abundance, otherwise considered a successful management story (Box 2) but which has led to increased infestation of cod with parasites, possibly contributing to the decline in cod condition (Box 3 figure, panel C) (88, 117).
(A and B) Spatial disconnect of predator and prey driven by deoxygenation and SST warming. Sprat abundance (S. sprattus) (that is, cod prey) is compared among the time intervals 1984–1991 (A) and 1992–2016 (B) in 106 individuals km−3. Sprat distribution moved northward, driven by SST increase, whereas cod (G. morhua), its predator, shifted distribution southward because of deteriorating oxygen conditions. The circles correspond to the mean center of gravity of Eastern Baltic cod. Data are from autumn acoustic surveys in SDs 25 to 29 [see the study of Casini et al. (115)].
(C) Condition of Eastern Baltic cod stock (G. morhua). Decreasing length at maturity (in centimeters) and deteriorating condition (Fulton’s condition index in the Eastern Baltic cod stock over time). Data on length at maturity were obtained from the study of ICES (120).
THE BALTIC AS A SCIENCE-BASED MARINE MANAGEMENT LABORATORY
Despite multiple pressures impinging upon the Baltic owing to 85 million inhabitants in its watershed, for all environmental issues that can be managed at a macroregional level, positive trend reversals could be observed. From a low point in the 1970s/1980s, an overall improvement in the ecosystem status of the Baltic Sea could be observed (compare Boxes 2 and 3; Fig. 2 and data S2). This applies to the return of top predators, some successes in the sustainable management of fish stocks, and the reduction of nutrient pollution and concomitant eutrophication effects. Although, ideally, ecosystem deterioration should be prevented from the onset by suitable management, one lesson from the Baltic Sea for other regional seas facing severe environmental perturbations (for example, the Black Sea) and for many coastal areas for which perturbations are mounting is that science-based management was able to reverse the decline of a severely degraded system. However, all of these examples also highlight additional (and often initially unexpected) management complexity, including intersectorial conflict and consequences of global change, hampering management success.
Top predators
A particularly successful example of biological conservation in the past decades was the return of top predators such as seals, cormorants, and eagles (Box 2). Because these species accumulate many toxic pollutants, a reduction in organic contaminants (Fig. 3B) (58) was critical for their recovery, along with direct habitat protection, the regulation of hunting, and the process of enlightening attitudes toward large predators. At the same time, novel or reinstated interactions such as conflicts between seals and potential fisheries yields are now resurfacing and require new management responses (see also Box 3) (59).
Fisheries management
For Baltic Sea fish stocks, the message is a more mixed one. Baltic stocks are, on average, better managed than those in other European regional seas, as indicated by a recent analysis of the status of Europe’s major commercially exploited stocks (60, 61). However, it has to be kept in mind that these assessments exclude stocks and species that formerly were commercially important but that now are locally or commercially extinct, such as many fall-spawning herring stocks and sturgeon (62). This comparatively good status is due to a combination of several factors, particularly the high level of regional cooperation, coordination, and transparency regarding fisheries data collection/monitoring, management, control, and enforcement (Fig. 3A). The cooperative management approach originated several decades ago, and has continued to incorporate improvements (21), including implementation of EU management plans, initially for cod, and recently for all major fish stocks (cod G. morhua, sprat Sprattus sprattus, and herring Clupea harengus) in the Baltic Sea (63). The status of the stocks is assessed annually using standardized survey methods, data collection procedures, and, for some stocks, fisheries population models—all of which are coordinated, reviewed, and approved by scientific experts (64). These data and model outputs provide a scientific basis for advice on fishing quotas. In the Baltic Sea, as part of the EU Common Fisheries Policy, a major reduction in illegal, unreported, and unregulated (IUU) fishing has also recently been achieved (64, 65), as well as a discard ban (66).
The relatively good management status of fish stocks is now in danger due to accumulating additional pressures such as warming, deoxygenation, and the disruption of food web links in higher-order interactions (Box 3), which means that currently used assessment models and management frameworks that worked in the past may be ecologically too simplistic for the future. A case in point is the long-term sustainability of the eastern cod stock, which is severely threatened under future scenarios of climate change and nutrient loading (67). The Baltic Sea provides a compelling example as to how intensities of sustainable exploitation can become unsustainable under new (worsened) ecological circumstances, including in particular, desalination via increased precipitation and run-off from land in combination with warming and deoxygenation.
In contrast to the status of Baltic cod, the improved management of anadromous Atlantic salmon (Salmo salar) populations throughout the eastern and northern Baltic Sea is a success story (data S2). Early population genetics research showed that the Baltic salmon population is strongly structured; historically, each river harbored at least one genetically unique population. Upon salmon decline in the 1950s, large-scale hatchery breeding and release of young salmon were carried out without taking genetic issues into account. Too few breeders, combined with the use of fish from nonnative rivers, resulted in elevated levels of inbreeding and loss of genetic variation. In 2011, the EU Commission recommended phasing out of compensatory releases within 7 years, followed by two multinational management strategies following scientific genetic advice. First, fishing activity moved from open sea fisheries, where multiple populations are harvested in a mixed fishery, to separate river fisheries, reducing the risk of overexploiting separate populations. Second, restoration efforts are performed in several rivers using original or genetically close populations (data S2). Although not yet on the level of near real-time management as accomplished in Pacific salmon stocks (68), it is one of the few cases worldwide where genetic-level differentiation and diversity of fish stocks have been recognized and implemented as one key management parameter. As such, these efforts highlight how critical scientific knowledge, here of genetic stock structure and natal homing, was indispensible for deriving appropriate management measures (69).
Given rapid community changes and species invasions, the development of new fisheries may open up new opportunities. One example is the invasion of the round goby (N. melanostomus), which is a potential threat to Baltic ecosystems and commercial fishes but has also led to new and expanding commercial fisheries (45). This is a key example where new adaptive management concepts with moving targets and faster response times of all players, including the stakeholders in fisheries, are essential for changing fishing habits and traditions (59).
Baltic lessons for nutrient management—Successful trend reversal
Nutrient pollution is considered one of the key future threats for global coastal systems, related to the increase in coastal populations, lack of human and animal waste treatment, and increase in intensive agriculture (70). The Baltic provides one of the rare examples where successful management has led to a macroregional trend reversal in this bottom-up pressure. Biogeochemical fluxes of nitrogen (N) and phosphorus (P) are currently considered to breach planetary boundaries (2), that is, they exceed the environmental limits within which human societies can safely operate. In the Baltic Sea, long-term data series show that N and P loads increased between 1950 and 1980 to peak around 1990 but then decreased substantially before reaching a plateau in recent years (Fig. 4) (18). Compared to other similar regions where eutrophication is recognized as a major problem, the increase in nutrient loads, the introduction of management policies to reduce loads, and the trend reversal with decreasing loads took place earlier in the Baltic Sea Region than, for example, in the Black Sea (71) and the Great Barrier Reef (72) (Fig. 3C, table S1A, and data S1). The early onset of countermeasures was catalyzed by the 1972 Stockholm UN Conference on the Human Environment. The first initiatives responding to local and national problems, including a major improvement in wastewater treatment, resulted in a 50 and 70% decrease in N and P loads from coastal point sources between 1985 and 1995 (73). This decrease took place one to two decades earlier than existing nutrient management plans, for example, those in other well-managed regions such as the Great Barrier Reef region (72). With the enlargement of the EU, the existing environmental directives have led to further improvement in wastewater treatment in the new member states. Similarly, significant collaborative investments in wastewater treatment have taken place since the mid-2000s in Russia and Belarus.
Fig. 4. Nutrient input into the Baltic Sea.
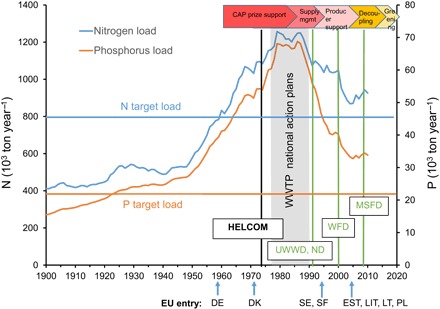
Five-year moving average values of N and P loads (in 1000 metric tons per year) to the Baltic Sea together with the BSAP targets. Along the x axis, the timing of countries joining the EU and the introduction of key EU environmental legislation are shown. WWTP, wastewater treatment plans; HELCOM, signing of the Helsinki Convention; UWWD, urban wastewater directive. Key developments of the EU CAP are indicated by arrows at top of the diagram. Supply mgmt, supply management; DE, Germany; DK, Denmark; SE, Sweden; FI, Finland; EST, Estonia; LIT, Lithuania; LT, Latvia; PL, Poland. For detailed references and sources, see data S3.
HELCOM, and later on the EU, successfully promoted systematic monitoring, data sharing, awareness raising, and modeling including the entire catchment area. This knowledge was critical for the identification of scientifically based nutrient reduction targets (74) and for the formulation of the HELCOM Baltic Sea Action Plan (BSAP) (52, 75). This scientific information was also used to define the ecological targets for the EU Water Framework Directive river basin management plans (Fig. 3C). Ecological targets were also formulated for transitional and coastal waters that include threshold values for groundwater according to the EU Groundwater Directive, highlighting the interaction of the land-sea interface (76).
To fully meet the targets outlined under the BSAP, current N and P loads need to be reduced a further 13 and 41%, respectively (75), whereas, for many coastal water bodies, the requirements for complete compliance with target loads are even higher. Unfortunately, nutrients are maintained in the ecosystem on decadal or even centennial scales because of large pools stored in the sediments. P release from sediments, in particular, will continue for several decades after the load reduction (18), which is likely to be a problem also for other world regions, particularly in coastal brackish areas. Second, it appears that increased N fixation by cyanobacteria stimulated by P release specifically in hypoxic areas counteracts the decreasing nutrient inputs (77). In addition, climate warming will make the ecosystem more vulnerable to nutrient loads (15) and could even result in increased N loads from agricultural areas due to increased run-off from land under altered precipitation patterns.
The accumulated natural scientific knowledge about the drivers of nutrient pollution and the costs, capacity, and effectiveness of candidate abatement measures have allowed economics researchers to develop cost-efficient programs of measures to reduce nutrient loading (78). The overall benefits of alleviating eutrophication in the open sea (€3 billion to €4 billion annually; table S2) along with environmental side benefits of the measures outweigh the costs of reaching the corresponding nutrient abatement targets (€1 billion to €4 billion) (78, 79). On the other hand, country-wise targets are based on proportional reductions and lead to uneven distribution of the costs and benefits, and a truly cost-effective plan would require still closer international collaboration (80). To conclude, substantial improvement was accomplished from relatively straightforward measures; however, the plateau that has been reached also highlights that further improvement will only be possible through much more costly actions that are in partial conflict, for example, with other policy targets such as the EU Common Agricultural Policy.
Nutrients and conflicting environmental policy targets
Past management in the Baltic, as almost anywhere in the world, largely dealt with environmental targets in isolation from other conservation targets or policy goals. Baltic Sea nutrient management is highly illustrative as to how sectorial targets in regional policy are currently in conflict with each other, such as food security and environmental protection. Although the focus in the EU CAP has shifted over the years from price support until 1980 toward greening in recent years (arrows in Fig. 4), several EU environmental directives are at odds with the CAP that still subsidizes intensive agriculture (81). Hence, a particular challenge is the reduction of nutrient loads from agriculture. Currently, agricultural sources contribute to two-thirds of diffuse nutrient losses (N and P) that reach the Baltic (82), and the nutrient load from agriculture only marginally decreased since 1980. This decrease can mostly be attributed to a collapse in the agricultural system in former communist states and only secondarily to improvements in agricultural practices in some countries (83), rather than to dedicated agricultural policies. Further reductions in Baltic Sea nutrient loads will entail much higher costs and restrictions on agriculture, and the required intersectorial policy conflict (that is, ecosystem protection versus provisioning of affordable food) will make implementation difficult. Because the Baltic Sea experiences above-average rates of climate change, even ambitious nutrient reduction goals may now be offset by increased freshwater run-off, enhanced nutrient remineralization, and water stratification due to ocean warming (84). Accordingly, in all but the most drastic nutrient reduction schemes, the oxygen-free zones in the Baltic Sea will expand (9, 84). It is therefore imperative to develop policies, as has recently been done for the Baltic Sea region, that simultaneously address nutrient reduction and mitigation of climate change (85).
MANAGEMENT LESSONS FROM THE BALTIC TIME MACHINE
Marine coastal areas are under increasing, multifactorial pressures, with the Baltic Sea being one of the regional seas suffering from an exceptional combination of multiple stressors (Fig. 1, B to D, and Table 1). However, as summarized here, in the Baltic Sea, some negative trends caused by major regional pressures have now peaked and have been partly reversed (Fig. 2 and Boxes 2 and 3), a progression that has led to the improvement of overall ecosystem status. Examples of positive developments include substantial decrease of hazardous substances (Fig. 2I), the return of top predators (Box 2), the partial recovery of fish stocks (Fig. 2, G and H; Box 3; and data S2), and a reduction in nutrient input from the post-industrialization peak load (Fig. 4) and, hence, of some eutrophication symptoms.
We here attempt to identify the foundation of these successes and, where possible, to formulate resulting lessons that may hold for other areas. We acknowledge that these lessons may not be applicable everywhere and that there are examples of coastal areas that have successfully managed to curb pressures even before serious degradation could result. Nevertheless, we posit that experiences gained in the unique interplay of strong perturbations, complex management scenario, good scientific underpinning, and successful trend reversals are worth to be shared and considered at the global coastal level.
As one prerequisite, a comparatively good scientific underpinning had been imperative for bending the pressure curves. The understanding of the ecological processes within the Baltic is high, partly explained by relatively small number of species in this geologically very young Baltic Sea and partly by dedicated collaborative, macroregional-scale research programs, such as EU BONUS (55), that further enhance collaboration at the science-management interface. However, science alone would have failed completely without important science policy interventions. For example, breaking the negative trends was only possible in a multinational setting because there were science-based international and legally binding agreements (most importantly, several EU directives, including the MSFD and the EU Common Fisheries Policy; Fig. 3, A to C). Long-term data series providing baselines against which to measure environmental deterioration and the success of management measures have been particularly valuable also in the communication of the scientific data to policy-makers and other stakeholders (Fig. 2).
Increasingly, research in the Baltic Sea area addresses the direct economic benefits of management options, including reducing the risk of oil spills, reducing the frequency of the establishment of new harmful alien species, and alleviating eutrophication, for which economic benefits or costs have been quantified and measured. Regarding the latter, it has been estimated that the total economic benefits provided by the Baltic Sea–based recreation, estimated at €14.8 billion per year, could be more than €1 billion higher if the environmental status of the sea improved (table S2 and references therein) (86). Although this research field is still developing, it already demonstrates how the accumulation of economic evidence on benefits and costs of different environmental policy goals for the Baltic Sea provides stimuli for policy-makers and demonstrates the need for actions.
Lessons from the Baltic Sea region highlight the importance of setting up macroregional policy frameworks to consistently implement at least less costly measures (for example, wastewater treatment, banning of IUU fishing, curbing contaminants, and regulating hunting) to improve the ecological status. However, insufficient coordination and integration between sectorial policies due to imbalanced power relations and opposing agendas remain a constraint for the effectiveness of existing policy strategies, regulations, and directives (87). Implementation of such frameworks and governance mechanisms can proceed incrementally (instead of waiting to achieve a complete transition) as local authorities acquire knowledge and experience to set them up and as they identify expected future ecological changes and the policy needs to address such changes.
BEYOND TRADITIONAL MANAGEMENT
The Baltic Sea is pushed rapidly into a zone where traditional management is at its limits. Cases in point are starving cod populations due to warming-induced distribution shifts in prey but not predator in combination with the loss of benthic prey with the spread of oxygen-free zones (Box 3) (8, 88), the stagnation of N and P loading curves due to variability in run-off from land (Fig. 4), and increasing frequency of cyanobacteria blooms owing to warming despite measurable nutrient reductions (Fig. 2E) (35). These are key examples on how global pressures that cannot be addressed via regional collective management efforts, such as ocean warming and acidification, are increasingly affecting Baltic ecosystems (Fig. 1, B and C). As such, they constitute new boundary conditions that seriously challenge “traditional” sectorial management interventions that lack a dedicated ecosystem management approach. Although the idea of ecosystem-based management is not at all new (89), its implementation is still in its infancy (57).
The Baltic Sea Action Plan (BSAP) (52) is a major first attempt to integrate diverse management measures. There are also some first attempts in the Baltic Sea multispecies fisheries management (90). Hence, the Baltic Sea is also developing into a region where novel management tools are being developed, tested, and partially already implemented (91). Given that rates of change in pressures and ecosystem responses are above average (Table 1), the need to overcome sectorial environmental management by adaptive management under the conceptual umbrella of marine stewardship is particularly timely in the Baltic Sea (92). One issue that may need reconsideration relates to the arrival of NIS and, possibly, novel ecosystem functions. Although we agree that such invasions should be prevented by curbing introduction pathways, because NIS have the potential to drastically change local species composition and cause regime shifts, with often as yet unclear long-term consequences [see, for example, the study of Ojaveer et al. 41)], it is also becoming increasingly clear that some novel species may actually improve ecosystem functioning (93). Moreover, eradication is impossible in most cases such that any sensible management needs to face the reality of new food webs that are composed of a mix of resident and novel taxa, once the latter have established (94).
Adaptive management approaches are a projection of the shifting-baseline concept into the future and acknowledge that ecosystems are changing rapidly, with the consequence that conservation targets need to move as well to stay operational and feasible (“Ecosystem Stewardship concept”) (92). As one major step forward, in the Baltic Sea region, a framework for sustainable stewardship management that considers rapidly increasing external pressures and intends to enhance the resilience of ecosystem functions has been developed (95).
Another area of rapid development at the policy-science interface in the Baltic Sea is the development of participatory approaches that are critical to improving horizontal policy integration across sectors (96). More research is needed to understand how to successfully implement more inclusive governance arrangements based on stakeholder participation and social learning, which support recognition and awareness of different perceptions, values, interests, and objectives across sectors, increasing (in the long term) the acceptance of implementing more costly but also more effective measures in the Baltic Sea region. For example, the Kosterhavet marine protected area (Kattegat region) was implemented in a participatory process involving all stakeholders and resulted in regulations such as changed fishing gear with measurable effects (97). Other recent examples include participatory approaches to regional management of nutrient inputs in Swedish water councils (98). By and large, countries neighboring the Baltic Sea have taken leading roles in these new, promising approaches to regionalized management (99).
CONCLUSIONS
We have argued that regional seas are potential time machines where impacts of regional and global change are accumulating faster than in the coastal oceans. We show that the Baltic Sea, because of the unique combination of strong multistressor perturbations ahead of most other regional seas and coastal areas, good data availability, and the implementation of an advanced multinational governance and management structure, is particularly suitable as a time machine to study consequences and mitigation of coastal perturbations. Key lessons to consider in other regional seas and future coastal oceans include the following: (i) the implementation of effective intergovernmental policies that served as an essential foundation in reversing the status of a highly disturbed regional sea such as the Baltic Sea with its exemplary cross-border nature of regional perturbations; (ii) the early and maintained implementation of monitoring and long-term data series is priceless, to understand problems and evaluate and guide management actions; (iii) tackling the “low hanging fruit” (for example, dealing with point sources of nutrients or contaminants first), if addressed consistently on a macroregional scale, is an important first step and can lead to significant environmental improvements; (iv) global change interacting with regional perturbations is increasingly jeopardizing initial management successes, which calls for a revised management that is both conservative and adaptive; and (v) for further improvement, more inclusive governance arrangements based on stakeholder participation and cross-sector collaboration are imperative.
Supplementary Material
Acknowledgments
We thank the participants of the 2015 BONUS BIO-C3/BAMBI/INSPIRE/COCOA summer school in Glückstadt, Germany, as well as C. E. Lee, for input on the time machine idea. Funding: This synthesis was a joint effort of the EU BONUS projects BIO-C3 (Biodiversity changes—Causes, consequences, and management implications), BALTICAPP (Wellbeing from the Baltic Sea—Applications combining natural science and economics), BAMBI (BAltic Sea Marine BIodiversity), COCOA (Nutrient COcktails in COAstal zones of the Baltic Sea), GO4BALTIC (Coherent policies and governance of the Baltic Sea ecosystems), INSPIRE (INtegrating SPatIal pRocesses into Ecosystem models for sustainable utilisation of fish resources), MIRACLE (Mediating integrated actions for sustainable ecosystem services in a changing climate), and SOILS2SEA (Reducing nutrient loadings from agricultural soils to the Baltic SEA via groundwater and streams), supported by BONUS (Article 185), funded jointly by the EU and the following national funding institutions: Innovation Fund Denmark (Denmark), the Estonian Research Council (Estonia), the Academy of Finland (Finland), the German Federal Ministry of Education and Research (BMBF, Germany), the National Centre for Research and Development (Poland), the Swedish Research Council (FORMAS, Sweden), and the Swedish Environmental Protection Agency (Sweden). Additional support came from the German Cluster of Excellence “The Future Ocean”. Author contributions: T.B.H.R. and J.D. derived the time machine concept paper idea and initiated the collaboration, T.B.H.R., J.C., and J.D. coordinated the time machine writing workshops, and all authors further developed the idea, contributed expertise from their area and sections and data to the manuscript, and edited previous versions of the work. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/5/eaar8195/DC1
data S1. Methods and references used for the assembly of Tables 1 and 2 of the main text.
table S1A. Detailed assessment of the qualitative status of environmental pressures/drivers in the Baltic Sea region compared to other worldwide coastal seas.
table S1B. Detailed assessment of scientific knowledge, management regimes, and governance structures in the Baltic Sea region compared to other worldwide coastal seas/areas.
table S2. Valuation of benefits of environmental intervention or management measurements in the Baltic Sea area.
data S2. Box: Genetic knowledge in marine management—The recovery of Baltic salmon.
data S3. Data sources for figures.
REFERENCES AND NOTES
- 1.IPCC, “Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change,” Core Writing Team, R. K. Pachauri and L. A. Meyer, Eds. (IPCC, 2014), 151 pp. [Google Scholar]
- 2.Steffen W., Richardson K., Rockström J., Cornell S. E., Fetzer I., Bennett E. M., Biggs R., Carpenter S. R., de Vries W., de Wit C. A., Folke C., Gerten D., Heinke J., Mace G. M., Persson L. M., Ramanathan V., Reyers B., Sörlin S., Planetary boundaries: Guiding human development on a changing planet. Science 347, 1259855 (2015). [DOI] [PubMed] [Google Scholar]
- 3.UN General Assembly, Transforming our world: The 2030 Agenda for Sustainable Development, 21 October 2015, A/RES/70/1, (2015); www.refworld.org/docid/57b6e3e44.html.
- 4.J. B. C. Jackson, K. E. Alexander, E. Sala, Shifting Baselines—The Past and The Future of Ocean Fisheries (Island Press, 2011), 312 pp. [Google Scholar]
- 5.Lejeusne C., Chevaldonné P., Pergent-Martini C., Boudouresque C.-F., Pérez T., Climate change effects on a miniature ocean: The highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 25, 250–260 (2010). [DOI] [PubMed] [Google Scholar]
- 6.P. Snoeijs-Leijonmalm, H. Schubert, T. Radziejewska, Biological Oceanography of the Baltic Sea (Springer Netherlands, 2017), 683 pp. [Google Scholar]
- 7.The BACC II Author Team, Second Assessment of Climate Change for the Baltic Sea Basin (Springer International Publishing, 2015), 501 pp. [Google Scholar]
- 8.Griffiths J. R., Kadin M., Nascimento F. J. A., Tamelander T., Törnroos A., Bonaglia S., Bonsdorff E., Brüchert V., Gårdmark A., Järnström M., Kotta J., Lindegren M., Nordström M. C., Norkko A., Olsson J., Weigel B., Žydelis R., Blenckner T., Niiranen S., Winder M., The importance of benthic–pelagic coupling for marine ecosystem functioning in a changing world. Glob. Chang. Biol. 23, 2179–2196 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Breitburg D., Levin L. A., Oschlies A., Grégoire M., Chavez F. P., Conley D. J., Garçon V., Gilbert D., Gutiérrez D., Isensee K., Jacinto G. S., Limburg K. E., Montes I., Naqvi S. W. A., Pitcher G. C., Rabalais N. N., Roman M. R., Rose K. A., Seibel B. A., Telszewski M., Yasuhara M., Zhang J., Declining oxygen in the global ocean and coastal waters. Science 359, eaam7240 (2018). [DOI] [PubMed] [Google Scholar]
- 10.VanDeveer S. D., Networked Baltic environmental cooperation. J. Balt. Stud. 42, 37–55 (2011). [Google Scholar]
- 11.Jackson J. B. C., What was natural in the coastal oceans? Proc. Natl. Acad. Sci. U.S.A. 98, 5411–5418 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotze H. K., Lenihan H. S., Bourque B. J., Bradbury R. H., Cooke R. G., Kay M. C., Kidwell S. M., Kirby M. X., Peterson C. H., Jackson J. B. C., Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312, 1806–1809 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Harley C. D. G., Randall Hughes A., Hultgren K. M., Miner B. G., Sorte C. J. B., Thornber C. S., Rodriguez L. F., Tomanek L., Williams S. L., The impacts of climate change in coastal marine systems. Ecol. Lett. 9, 228–241 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Rutgersson A., Jaagus J., Schenk F., Stendel M., Observed changes and variability of atmospheric parameters in the Baltic Sea region during the last 200 years. Climate Res. 61, 177–190 (2014). [Google Scholar]
- 15.Glibert P. M., Icarus Allen J., Artioli Y., Beusen A., Bouwman L., Harle J., Holmes R., Holt J., Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution in response to climate change: Projections based on model analysis. Glob. Chang. Biol. 20, 3845–3858 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Melzner F., Thomsen J., Koeve W., Oschlies A., Gutowska M. A., Bange H. W., Hansen H. P., Körtzinger A., Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 160, 1875–1888 (2013). [Google Scholar]
- 17.Jansson A., Norkko J., Dupont S., Norkko A., Growth and survival in a changing environment: Combined effects of moderate hypoxia and low pH on juvenile bivalve Macoma balthica. J. Sea Res. 102, 41–47 (2015). [Google Scholar]
- 18.Gustafsson B. G., Schenk F., Blenckner T., Eilola K., Meier H. E. M., Müller-Karulis B., Neumann T., Ruoho-Airola T., Savchuk O. P., Zorita E., Reconstructing the development of Baltic Sea eutrophication 1850–2006. Ambio 41, 534–548 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phoenix G. K., Hicks W. K., Cinderby S., Kuylenstierna J. C. I., Stock W. D., Dentener F. J., Giller K. E., Austin A. T., Lefroy R. D. B., Gimeno B. S., Ashmore M. R., Ineson P., Atmospheric nitrogen deposition in world biodiversity hotspots: The need for a greater global perspective in assessing N deposition impacts. Glob. Chang. Biol. 12, 470–476 (2006). [Google Scholar]
- 20.Carstensen J., Andersen J. H., Gustafsson B. G., Conley D. J., Deoxygenation of the Baltic Sea during the last century. Proc. Natl. Acad. Sci. U.S.A. 111, 5628–5633 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aps R., Lassen H., Recovery of depleted Baltic Sea fish stocks: A review. ICES J. Mar. Sci. 67, 1856–1860 (2010). [Google Scholar]
- 22.Casini M., Hjelm J., Molinero J.-C., Lövgren J., Cardinale M., Bartolino V., Belgrano A., Kornilovs G., Trophic cascades promote threshold-like shifts in pelagic marine ecosystems. Proc. Natl. Acad. Sci. U.S.A. 106, 197–202 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotta J., Nurkse K., Puntila R., Ojaveer H., Shipping and natural environmental conditions determine the distribution of the invasive non-indigenous round goby Neogobius melanostomus in a regional sea. Estuar. Coast. Shelf Sci. 169, 15–24 (2016). [Google Scholar]
- 24.Pandelova M., Henkelmann B., Roots O., Simm M., Järv L., Benfenati E., Schramm K.-W., Levels of PCDD/F and dioxin-like PCB in Baltic fish of different age and gender. Chemosphere 71, 369–378 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Gunderson A. R., Armstrong E. J., Stillman J. H., Multiple stressors in a changing world: The need for an improved perspective on physiological responses to the dynamic marine environment. Ann. Rev. Mar. Sci. 8, 357–378 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Vitousek P. M., Beyond global warming: Ecology and global change. Ecology 75, 1861–1876 (1994). [Google Scholar]
- 27.Jackson J. B. C., Kirby M. X., Berger W. H., Bjorndal K. A., Botsford L. W., Bourque B. J., Bradbury R. H., Cooke R., Erlandson J., Estes J. A., Hughes T. P., Kidwell S., Lange C. B., Lenihan H. S., Pandolfi J. M., Peterson C. H., Steneck R. S., Tegner M. J., Warner R. R., Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Scheffer M., Carpenter S., Foley J. A., Folke C., Walker B., Catastrophic shifts in ecosystems. Nature 413, 591–596 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Pauly D., Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 10, 430 (1995). [DOI] [PubMed] [Google Scholar]
- 30.Österblom H., Hansson S., Larsson U., Hjerne O., Wulff F., Elmgren R., Folke C., Human-induced trophic cascades and ecological regime shifts in the Baltic Sea. Ecosystems 10, 877–889 (2007). [Google Scholar]
- 31.Möllmann C., Diekmann R., Müller-Karulis B., Kornilovs G., Plikshs M., Axe P., Reorganization of a large marine ecosystem due to atmospheric and anthropogenic pressure: A discontinuous regime shift in the Central Baltic Sea. Glob. Chang. Biol. 15, 1377–1393 (2009). [Google Scholar]
- 32.Alheit J., Möllmann C., Dutz J., Kornilovs G., Loewe P., Mohrholz V., Wasmund N., Synchronous ecological regime shifts in the central Baltic and the North Sea in the late 1980s. ICES J. Mar. Sci. 62, 1205–1215 (2005). [Google Scholar]
- 33.Köster F. W., Möllmann C., Hinrichsen H.-H., Wieland K., Tomkiewicz J., Kraus G., Voss R., Makarchouk A., MacKenzie B. R., St. John Dietrich Schnack M. A., Rohlf N., Linkowski T., Beyer J. E., Baltic cod recruitment—The impact of climate variability on key processes. ICES J. Mar. Sci. 62, 1408–1425 (2005). [Google Scholar]
- 34.Casini M., Lövgren J., Hjelm J., Cardinale M., Molinero J.-C., Kornilovs G., Multi-level trophic cascades in a heavily exploited open marine ecosystem. Proc. R. Soc. B. Biol. Sci. 275, 1793–1801 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahru M., Elmgren R., Multidecadal time series of satellite-detected accumulations of cyanobacteria in the Baltic Sea. Biogeosciences 11, 3619–3633 (2014). [Google Scholar]
- 36.Torn K., Krause-Jensen D., Martin G., Present and past depth distribution of bladderwrack (Fucus vesiculosus) in the Baltic Sea. Aquat. Bot. 84, 53–62 (2006). [Google Scholar]
- 37.Boström C., Baden S., Bockelmann A.-C., Dromph K., Fredriksen S., Gustafsson C., Krause-Jensen D., Möller T., Nielsen S. L., Olesen B., Olsen J., Pihl L., Rinde E., Distribution, structure and function of Nordic eelgrass (Zostera marina) ecosystems: Implications for coastal management and conservation. Aquat. Conserv. 24, 410–434 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moksnes P.-O., Gullström M., Tryman K., Baden S., Trophic cascades in a temperate seagrass community. Oikos 117, 763–777 (2008). [Google Scholar]
- 39.Eriksson B. K., Sieben K., Eklöf J., Ljunggren L., Olsson J., Casini M., Bergström U., Effects of altered offshore food webs on coastal ecosystems emphasize the need for cross-ecosystem management. Ambio 40, 786–797 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergström U., Olsson J., Casini M., Eriksson B. K., Fredriksson R., Wennhage H., Appelberg M., Stickleback increase in the Baltic Sea—A thorny issue for coastal predatory fish. Estuar. Coast. Shelf Sci. 163, 134–142 (2015). [Google Scholar]
- 41.Ojaveer H., Olenin S., Narščius A., Florin A.-B., Ezhova E., Gollasch S., Jensen K. R., Lehtiniemi M., Minchin D., Normant-Saremba M., Strāke S., Dynamics of biological invasions and pathways over time: A case study of a temperate coastal sea. Biol. Invasions 19, 799–813 (2017). [Google Scholar]
- 42.Olenin S., Leppäkoski E., Non-native animals in the Baltic Sea: Alteration of benthic habitats in coastal inlets and lagoons. Hydrobiologia 393, 233–243 (1999). [Google Scholar]
- 43.Norkko J., Reed D. C., Timmermann K., Norkko A., Gustafsson B. G., Bonsdorff E., Slomp C. P., Carstensen J., Conley D. J., A welcome can of worms? Hypoxia mitigation by an invasive species. Glob. Chang. Biol. 18, 422–434 (2012). [Google Scholar]
- 44.Jormalainen V., Gagnon K., Sjöroos J., Rothäusler E., The invasive mud crab enforces a major shift in a rocky littoral invertebrate community of the Baltic Sea. Biol. Invasions 18, 1409–1419 (2016). [Google Scholar]
- 45.Ojaveer H., Galil B. S., Lehtiniemi M., Christoffersen M., Clink S., Florin A.-B., Gruszka P., Puntila R., Behrens J. W., Twenty five years of invasion: Management of the round goby Neogobius melanostomus in the Baltic Sea. Manag. Biol. Invasion 6, 329–339 (2015). [Google Scholar]
- 46.Tiselius P., Møller L. F., Community cascades in a marine pelagic food web controlled by the non-visual apex predator Mnemiopsis leidyi. J. Plankton Res. 39, 271–279 (2017). [Google Scholar]
- 47.Dietz T., Ostrom E., Stern P. C., The struggle to govern the commons. Science 302, 1907–1912 (2003). [DOI] [PubMed] [Google Scholar]
- 48.O. Young, International Governance: Protecting the Environment in a Stateless Society (Cornell University Press, 1994). [Google Scholar]
- 49.Tynkkynen N., The challenge of environmental governance in the network society: The case of the Baltic Sea. Environ. Policy Gov. 23, 395–406 (2013). [Google Scholar]
- 50.Eckerberg K., Joas M., Multi-level environmental governance: A concept under stress? Local Environ. 9, 405–412 (2004). [Google Scholar]
- 51.Kern K., Governance for sustainable development in the Baltic Sea region. J. Balt. Stud. 42, 21–35 (2011). [Google Scholar]
- 52.HELCOM, Baltic Sea Action Plan. HELCOM ministerial meeting, Krakow, Poland, 15 November 2007 (H. Commission, 2007).
- 53.M. Gilek, M. Karlsson, S. Linke, K. Smolarz, Environmental Governance of the Baltic Sea (MARE Publication Series 10, Springer, 2016), 253 pp. [Google Scholar]
- 54.H. M. Rozwadowski, The Sea Knows No Boundaries—A Century of Marine Science Under ICES (International Council for the Exploration of the Sea, 2002). [Google Scholar]
- 55.Snoeijs-Leijonmalm P., Barnard S., Elliott M., Andrusaitis A., Kononen K., Sirola M., Towards better integration of environmental science in society: Lessons from BONUS, the joint Baltic Sea environmental research and development programme. Environ. Sci. Policy 78, 193–209 (2017). [Google Scholar]
- 56.M. Joas, D. Jahn, K. Kern, Governing a Common Sea. Environmental Policies in the Baltic Sea Region (Earthscan, 2008). [Google Scholar]
- 57.Elmgren R., Blenckner T., Andersson A., Baltic Sea management: Successes and failures. Ambio 44, 335–344 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nyberg E., Faxneld S., Danielsson S., Eriksson U., Miller A., Bignert A., Temporal and spatial trends of PCBs, DDTs, HCHs, and HCB in Swedish marine biota 1969–2012. Ambio 44, 484–497 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansson S., Bergström U., Bonsdorff E., Härkönen T., Jepsen N., Kautsky L., Lundström K., Lunneryd S.-G., Ovegård M., Salmi J., Sendek D., Vetemaa M., Hunsicker M., Competition for the fish–fish extraction from the Baltic Sea by humans, aquatic mammals, and birds. ICES J. Mar. Sci. 2017, fsx207 (2017). [Google Scholar]
- 60.Fernandes P. G., Ralph G. M., Nieto A., García Criado M., Vasilakopoulos P., Maravelias C. D., Cook R. M., Pollom R. A., Kovačić M., Pollard D., Farrell E. D., Florin A.-B., Polidoro B. A., Lawson J. M., Lorance P., Uiblein F., Craig M., Allen D. J., Fowler S. L., Walls R. H. L., Comeros-Raynal M. T., Harvey M. S., Dureuil M., Biscoito M., Pollock C., McCully Phillips S. R., Ellis J. R., Papaconstantinou C., Soldo A., Keskin Ç., Knudsen S. W., de Sola L. G., Serena F., Collette B. B., Nedreaas K., Stump E., Russell B. C., Garcia S., Afonso P., Jung A. B. J., Alvarez H., Delgado J., Dulvy N. K., Carpenter K. E., Coherent assessments of Europe’s marine fishes show regional divergence and megafauna loss. Nat. Ecol. Evol. 1, 0170 (2017). [Google Scholar]
- 61.EEA, “Status of Marine Fish Stocks. World wide web electronic publication,” downloaded 28 September 2017 (2017); www.eea.europa.eu/data-and-maps/indicators/status-of-marine-fish-stocks-2/assessment.
- 62.Ojaveer H., Jaanus A., MacKenzie B. R., Martin G., Olenin S., Radziejewska T., Telesh I., Zettler M. L., Zaiko A., Status of biodiversity in the Baltic Sea. PLOS ONE 5, e12467 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.EU , Regulation (EU) 2016/1139 of the European Parliament and of the Council of 6 July 2016 establishing a multiannual plan for the stocks of cod, herring and sprat in the Baltic Sea and the fisheries exploiting those stocks, amending Council Regulation (EC) N. Official Journal of the European Union L 191/1, 1–15 (2016). [Google Scholar]
- 64.ICES, Report of the Baltic Fisheries Assessment Working Group (WGBFAS), (ICES CM 2017/ACOM:11, 2017), 810 pp.
- 65.Eero M., Vinther M., Haslob H., Huwer B., Casini M., Storr-Paulsen M., Köster F., Spatial management of marine resources can enhance the recovery of predators and avoid local depletion of forage fish. Conserv. Lett. 5, 486–492 (2012). [Google Scholar]
- 66.Sardà F., Coll M., Heymans J. J., Stergiou K. I., Overlooked impacts and challenges of the new European discard ban. Fish Fish. 16, 175–180 (2015). [Google Scholar]
- 67.Gårdmark A., Lindegren M., Neuenfeldt S., Blenckner T., Heikinheimo O., Müller-Karulis B., Niiranen S., Tomczak M. T., Aro E., Wikström A., Möllmann C., Biological ensemble modeling to evaluate potential futures of living marine resources. Ecol. Appl. 23, 742–754 (2013). [DOI] [PubMed] [Google Scholar]
- 68.Beacham T. D., Lapointe M., Candy J. R., Miller K. M., Withler R. E., DNA in action: Rapid application of DNA variation to sockeye salmon fisheries management. Conserv. Genet. 5, 411–416 (2004). [Google Scholar]
- 69.Wennerström L., Jansson E., Laikre L., Baltic Sea genetic biodiversity: Current knowledge relating to conservation management. Aquat. Conserv. 27, 1069–1090 (2017). [Google Scholar]
- 70.Rabalais N. N., Turner R. E., Díaz R. J., Justić D., Global change and eutrophication of coastal waters. ICES J. Mar. Sci. 66, 1528–1537 (2009). [Google Scholar]
- 71.Strokal M., Kroeze C., Nitrogen and phosphorus inputs to the Black Sea in 1970–2050. Reg. Environ. Change 13, 179–192 (2013). [Google Scholar]
- 72.Kroon F. J., Thorburn P., Schaffelke B., Whitten S., Towards protecting the Great Barrier Reef from land-based pollution. Glob. Chang. Biol. 22, 1985–2002 (2016). [DOI] [PubMed] [Google Scholar]
- 73.O. P. Savchuk, B. G. Gustafsson, M. R. Medina, A. V. Sokolov, F. V. Wulff, “External nutrient loads to the Baltic Sea, 1970-2006” (Technical Report No. 5, Baltic Nest Institute, Stockholm Resilience Centre, Stockholm University, 2012).
- 74.Wulff F., Savchuk O. P., Sokolov A., Humborg C., Mörth C.-M., Management options and effects on a marine ecosystem: Assessing the future of the Baltic. Ambio 36, 243–249 (2007). [DOI] [PubMed] [Google Scholar]
- 75.HELCOM, “HELCOM Copenhagen Declaration "Taking Further Action to Implement the Baltic Sea Action Plan—Reaching Good Environmental Status for a healthy Baltic Sea.” Adopted 3 October 2013 (H. Commission, 2013).
- 76.Hinsby K., Markager S., Kronvang B., Windolf J., Sonnenborg T. O., Thorling L., Threshold values and management options for nutrients in a catchment of a temperate estuary with poor ecological status. Hydrol. Earth Syst. Sci. 16, 2663–2683 (2012). [Google Scholar]
- 77.Vahtera E., Conley D. J., Gustafsson B. G., Kuosa H., Pitkänen H., Savchuk O. P., Tamminen T., Viitasalo M., Voss M., Wasmund N., Wulff F., Internal ecosystem feedbacks enhance nitrogen-fixing cyanobacteria blooms and complicate management in the Baltic Sea. Ambio 36, 186–194 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Wulff F., Humborg C., Andersen H. E., Blicher-Mathiesen G., Czajkowski M., Elofsson K., Fonnesbech-Wulff A., Hasler B., Hong B., Jansons V., Mörth C.-M., Smart J. C. R., Smedberg E., Stålnacke P., Swaney D. P., Thodsen H., Was A., Żylicz T., Reduction of Baltic Sea nutrient inputs and allocation of abatement costs within the Baltic Sea catchment. Ambio 43, 11–25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahlvik L., Ekholm P., Hyytiäinen K., Pitkänen H., An economic–ecological model to evaluate impacts of nutrient abatement in the Baltic Sea. Environ. Model. Software 55, 164–175 (2014). [Google Scholar]
- 80.Elofsson K., Swedish nutrient reduction policies: An evaluation of cost-effectiveness. Reg. Environ. Change 12, 225–235 (2012). [Google Scholar]
- 81.Pe’er G., Dicks L. V., Visconti P., Arlettaz R., Báldi A., Benton T. G., Collins S., Dieterich M., Gregory R. D., Hartig F., Henle K., Hobson P. R., Kleijn D., Neumann R. K., Robijns T., Schmidt J., Shwartz A., Sutherland W. J., Turbé A., Wulf F., Scott A. V., EU agricultural reform fails on biodiversity. Science 344, 1090–1092 (2014). [DOI] [PubMed] [Google Scholar]
- 82.HELCOM, “The Fifth Baltic Sea Pollution Load Compilation (PLC-5)” (Baltic Sea Environment Proceedings No. 128, Helsinki Commission, 2011).
- 83.Dalgaard T., Hansen B., Hasler B., Hertel O., Hutchings N. J., Jacobsen B. H., Jensen L. S., Kronvang B., Olesen J. E., Schjørring J. K., Kristensen I. S., Graversgaard M., Termansen M., Vejre H., Policies for agricultural nitrogen management—Trends, challenges and prospects for improved efficiency in Denmark. Environ. Res. Lett. 6, 115002 (2014). [Google Scholar]
- 84.Meier H. E. M., Müller-Karulis B., Andersson H. C., Dieterich C., Eilola K., Gustafsson B. G., Höglund A., Hordoir R., Kuznetsov I., Neumann T., Ranjbar Z., Savchuk O. P., Schimanke S., Impact of climate change on ecological quality indicators and biogeochemical fluxes in the Baltic Sea: A multi-model ensemble study. Ambio 41, 558–573 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nainggolan D., Hasler B., Andersen H. E., Gyldenkærne S., Termansen M., Water quality management and climate change mitigation: Cost-effectiveness of joint implementation in the Baltic Sea region. Ecol. Econ. 144, 12–26 (2018). [Google Scholar]
- 86.Czajkowski M., Ahtiainen H., Artell J., Budzinski W., Hasler B., Hasselström L., Meyerhoff J., Nõmmann T., Semeniene D., Söderqvist T., Tuhkanen H., Lankia T., Vanags A., Zandersen M., Żylicz T., Hanley N., Valuing the commons: An international study on the recreational benefits of the Baltic Sea. J. Environ. Manage. 156, 209–217 (2015). [DOI] [PubMed] [Google Scholar]
- 87.E. De Santo, The marine strategy framework directive as a catalyst for maritime spatial planning: Internal dimensions and institutional tensions, in Governing Europe’s Marine Environment. Europeanization of Regional Seas or Regionalization of EU Policies? M. Gilek, K. Kern, Eds. (Ashgate Publishing, 2015). [Google Scholar]
- 88.Eero M., Hjelm J., Behrens J., Buchmann K., Cardinale M., Casini M., Gasyukov P., Holmgren N., Horbowy J., Hüssy K., Kirkegaard E., Kornilovs G., Krumme U., Köster F. W., Oeberst R., Plikshs M., Radtke K., Raid T., Schmidt J., Tomczak M. T., Vinther M., Zimmermann C., Storr-Paulsen M., Eastern Baltic cod in distress: Biological changes and challenges for stock assessment. ICES J. Mar. Sci. 72, 2180–2186 (2015). [Google Scholar]
- 89.Browman H. I., Stergiou K. I., Perspectives on ecosystem-based approaches to the management of marine resources. Mar. Ecol. Prog. Ser. 274, 269–303 (2004). [Google Scholar]
- 90.ICES, Report of the Benchmark Workshop on Baltic Multispecies Assessments (WKBALT 2013), Copenhagen, Denmark, 4 to 8 February 2013. [Google Scholar]
- 91.M. Boström, S. Grönholm, B. Hassler, in Environmental Governance of the Baltic Sea. M. Gilek, M. Karlsson, S. Linke, K. Smolarz, Eds. (MARE Publication Series, Springer, 2016), vol. 10. [Google Scholar]
- 92.Chapin F. S. III, Carpenter S. R., Kofinas G. P., Folke C., Abel N., Clark W. C., Olsson P., Smith D. M., Walker B., Young O. R., Berkes F., Biggs R., Grove J. M., Naylor R. L., Pinkerton E., Steffen W., Swanson F. J., Ecosystem stewardship: Sustainability strategies for a rapidly changing planet. Trends Ecol. Evol. 25, 241–249 (2010). [DOI] [PubMed] [Google Scholar]
- 93.Andersen J. H., Carstensen J., Conley D. J., Dromph K., Fleming-Lehtinen V., Gustafsson B. G., Josefson A. B., Norkko A., Villnäs A., Murray C., Long-term temporal and spatial trends in eutrophication status of the Baltic Sea. Biol. Rev. 92, 135–149 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Soule M. E., The onslaught of alien species, and other challenges in the coming decades. Conserv. Biol. 4, 233–239 (1990). [Google Scholar]
- 95.Scharin H., Ericsdotter S., Elliott M., Turner R. K., Niiranen S., Blenckner T., Hyytiäinen K., Ahlvik L., Ahtiainen H., Artell J., Hasselström L., Söderqvist T., Rockström J., Processes for the sustainable stewardship of marine environments. Ecol. Econ. 128, 55–67 (2016). [Google Scholar]
- 96.Schleyer C., Görg C., Hauck J., Winkler K. J., Opportunities and challenges for mainstreaming the ecosystem services concept in the multi-level policy-making within the EU. Ecosyst. Serv. 16, 174–181 (2015). [Google Scholar]
- 97.L. Píriz, “Sweden: SSF guidelines, prawns, parks and rights” (Samudra Report 76, 2017), pp. 16–19. [Google Scholar]
- 98.Matti S., Lundmark C., Ek K., Managing participation: Prospects for learning and legitimacy-creation in Swedish water management. Water Policy 19, 99–114 (2017). [Google Scholar]
- 99.Jager N. W., Challies E., Kochskämper E., Newig J., Benson D., Blackstock K., Collins K., Ernst A., Evers M., Feichtinger J., Fritsch O., Gooch G., Grund W., Hedelin B., Hernández-Mora N., Hüesker F., Huitema D., Irvine K., Klinke A., Lange L., Loupsans D., Lubell M., Maganda C., Matczak P., Parés M., Saarikoski H., Slavíková L., van der Arend S., von Korff Y., Transforming European water governance? Participation and river basin management under the EU Water Framework Directive in 13 member states. Water 8, 156 (2016). [Google Scholar]
- 100.Pereyra R. T., Bergström L., Kautsky L., Johannesson K., Rapid speciation in a newly opened postglacial marine environment, the Baltic Sea. BMC Evol. Biol. 9, 70 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Momigliano P., Jokinen H., Fraimout A., Florin A.-B., Norkko A., Merilä J., Extraordinarily rapid speciation in a marine fish. Proc. Natl. Acad. Sci. U.S.A. 114, 6074–6079 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.R. Väinölä, K. Johannesson, Genetic diversity and evolution, in Biological Oceanography of the Baltic Sea, P. Snoeijs-Leijonmalm, H. Schubert, T. Radziejewska, Eds. (Springer, 2017), pp. 233–253. [Google Scholar]
- 103.Johannesson K., André C., Life on the margin: Genetic isolation and diversity loss in a peripheral marine ecosystem, the Baltic Sea. Mol. Ecol. 15, 2013–2029 (2006). [DOI] [PubMed] [Google Scholar]
- 104.Reusch T. B. H., Climate change in the oceans: Evolutionary versus phenotypically plastic responses of marine animals and plants. Evol. Appl. 7, 104–122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harding K. C., Härkönen T., Helander B., Karlsson O., Status of Baltic grey seals: Population assessment and extinction risk. NAMMCO Sci. Publ. 6, 33–56 (2007). [Google Scholar]
- 106.Olsen M. T., Andersen S. M., Teilmann J., Dietz R., Edrén S. M. C., Linnet A., Status of the harbour seal (Phoca vitulina) in Southern Scandinavia. NAMMCO Sci. Publ. 8, 18 (2010). [Google Scholar]
- 107.C. Herrmann, T. Bregnballe, K. Larsson, K. Rattiste, “Population development of Baltic bird species: Great cormorant (Phalacrocorax carbo sinensis)” (HELCOM Baltic Sea Environment Fact Sheets, HELCOM, 2014); http://www.helcom.fi/baltic-sea-trends/environment-fact-sheets/biodiversity/population-development-of-great-cormorant.
- 108.C. Herrmann, O. Krone, T. Stjernberg, B. Helander, “Population development of white-tailed sea eagle” (HELCOM Baltic Sea Environment Fact Sheets, HELCOM, 2011); www.helcom.fi/baltic-sea-trends/environment-fact-sheets/biodiversity/population-development-of-white-tailed-sea-eagle.
- 109.European Environment Agency, “Environment and human health” (EEA, report no 5/2013, European Union, 2013).
- 110.HELCOM, “Red List of Baltic Sea species in danger of becoming extinct” (Baltic Sea Environment Proceedings No. 140, H. Commission, 2013).
- 111.Benke H., Bräger S., Dähne M., Gallus A., Hansen S., Honnef C. G., Jabbusch M., Koblitz J. C., Krügel K., Liebschner A., Narberhaus I., Verfuß U. K., Baltic Sea harbour porpoise populations: Status and conservation needs derived from recent survey results. Mar. Ecol. Prog. Ser. 495, 275–290 (2014). [Google Scholar]
- 112.Eero M., MacKenzie B. R., Köster F. W., Gislason H., Multi-decadal responses of a cod (Gadus morhua) population to human-induced trophic changes, fishing, and climate. Ecol. Appl. 21, 214–226 (2011). [DOI] [PubMed] [Google Scholar]
- 113.ICES, “Report of the Study Group on the further development of the precautionary approach to fisheries management, Copenhagen, Denmark” (ICES Document CM 2002/ACFM: 10. ICES, 2002). [Google Scholar]
- 114.Köster F. W., Vinther M., MacKenzie B. R., Eero M., Plikshs M., Environmental effects on recruitment and implications for biological reference points of eastern Baltic cod (Gadus morhua). J. Northw. Atl. Fish. Sci. 41, 205–220 (2009). [Google Scholar]
- 115.Casini M., Kornilovs G., Cardinale M., Möllmann C., Grygiel W., Jonsson P., Raid T., Flinkman J., Feldman V., Spatial and temporal density dependence regulates the condition of central Baltic Sea clupeids: Compelling evidence using an extensive international acoustic survey. Pop. Ecol. 53, 511–523 (2011). [Google Scholar]
- 116.ICES Advice, “Sprat (Sprattus sprattus) in subdivisions 22-32 (Baltic Sea)” (Report of the ICES Advisory Committee 2017, spr.27.22-32: 1–9, 2017).
- 117.Casini M., Käll F., Hansson M., Plikshs M., Baranova T., Karlsson O., Lundström K., Neuenfeldt S., Gårdmark A., Hjelm J., Hypoxic areas, density-dependence and food limitation drive the body condition of a heavily exploited marine fish predator. R. Soc. Open Sci. 3, 160416 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Köster F. W., Huwer B., Hinrichsen H.-H., Neumann V., Makarchouk A., Eero M., Dewitz B. V., Hüssy K., Tomkiewicz J., Margonski P., Temming A., Hermann J.-P., Oesterwind D., Dierking J., Kotterba P., Plikshs M., Browman H., Eastern Baltic cod recruitment revisited—Dynamics and impacting factors. ICES J. Mar. Sci. 74, 3–19 (2017). [Google Scholar]
- 119.Hüssy K., Radtke K., Plikshs M., Oeberst R., Baranova T., Krumme U., Sjöberg R., Walther Y., Mosegaard H., Challenging ICES age estimation protocols: Lessons learned from the eastern Baltic cod stock. ICES J. Mar. Sci. 73, 2138–2149 (2016). [Google Scholar]
- 120.ICES, “Report of the Workshop on Biological Input to Eastern Baltic Cod Assessment (WKBEBCA)” (ICES CM 2017/SSGEPD:19, 2017), 40 pp.
- 121.Huang B., Banzon V. F., Freeman E., Lawrimore J., Liu W., Peterson T. C., Smith T. M., Thorne P. W., Woodruff S. D., Zhang H.-M., Extended reconstructed sea surface temperature version 4 (ERSST.v4). Part I: Upgrades and intercomparisons. J. Climate 28, 911–930 (2015). [Google Scholar]
- 122.Liu W., Huang B., Thorne P. W., Banzon V. F., Zhang H.-M., Freeman E., Lawrimore J., Peterson T. C., Smith T. M., Woodruff S. D., Extended reconstructed sea surface temperature version 4 (ERSST.v4): Part II. Parametric and structural uncertainty estimations. J. Climate 28, 931–951 (2015). [Google Scholar]
- 123.K. Eilola, J. Hansen, H. E. M. Meier, K. Myrberg, V. A. Reabchenko, M. D. Skogen, Eutrophication Status Report of the North Sea, Skagerrak, Kattegat and the Baltic Sea: A Model Study Years 2001-2005 (Oceanografi 110, Swedish Meteorological and Hydrographical Institute, 2011).
- 124.Rabalais N. N., Cai W.-J., Carstensen J., Conley D. J., Fry B., Hu X., Quinones-Rivera Z., Rosenburg R., Rutger S., Caroline P., Turner R. E., Eutrophication-driven deoxygenation in the coastal ocean. Oceanography 27, 172–183 (2014). [Google Scholar]
- 125.Vermaat J. E., McQuatters-Gollop A., Eleveld M. A., Gilbert A. J., Past, present and future nutrient loads of the North Sea: Causes and consequences. Estuar. Coast. Shelf Sci. 80, 53–59 (2008). [Google Scholar]
- 126.Karydis M., Kitsiou D., Eutrophication and environmental policy in the Mediterranean Sea: A review. Environ. Monit. Assess. 184, 4931–4984 (2012). [DOI] [PubMed] [Google Scholar]
- 127.Ludwig W., Dumont E., Meybeck M., Heussner S., River discharges of water and nutrients to the Mediterranean and Black Sea: Major drivers for ecosystem changes during past and future decades? Prog. Oceanogr. 80, 199–217 (2009). [Google Scholar]
- 128.Mee L. D., The Black Sea in crisis: A need for concerted international action. Ambio 21, 278–286 (1992). [Google Scholar]
- 129.Alexander R. B., Smith R. A., Schwarz G. E., Boyer E. W., Nolan J. V., Brakebill J. W., Differences in phosphorus and nitrogen delivery to The Gulf of Mexico from the Mississippi River Basin. Environ. Sci. Technol. 42, 822–830 (2008). [DOI] [PubMed] [Google Scholar]
- 130.Chen C.-C., Gong G.-C., Shiah F.-K., Hypoxia in the East China Sea: One of the largest coastal low-oxygen areas in the world. Mar. Environ. Res. 64, 399–408 (2007). [DOI] [PubMed] [Google Scholar]
- 131.Xing Q., Gao M., Gao X., Tosi L., Schmitt F. G., Zhang Y., Shi P., Wei J., Luo Y., Progressive eutrophication behind the world-largest super floating macroalgal blooms in the Yellow Sea. Biogeosciences 7029–7054 (2014). [Google Scholar]
- 132.Duarte C. M., Agustí S., Wassmann P., Arrieta J. M., Alcaraz M., Coello A., N. MarbàIris, Hendriks I. E., Holding J., García-Zarandona I., Kritzberg E., Vaqué D., Tipping elements in the arctic marine ecosystem. Ambio 41, 44–55 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.MarineTraffic (2017); accessible at www.marinetraffic.com.
- 134.AquaNIS Editorial Board, Information system on aquatic non-Indigenous and cryptogenic species (World Wide Web electronic publication, version 2.36+, 2015); www.corpi.ku.lt/databases/aquanis [accessed 2 October 2017].
- 135.P. W. Fofonoff, G. M. Ruiz, B. Steves, A. H. Hines, J. T. Carlton, National Exotic Marine and Estuarine Species Information System, accessible at http://invasions.si.edu/nemesis/ (NEMESIS) (Smithsonian Environmental Research Center, 2003).
- 136.H. G. Hansson, NEAT (North East Atlantic Taxa) database on marine invertebrates (1998); www.tmbl.gu.se/libdb/taxon/taxa.html [accessed December 2017].
- 137.Bartsch I., Kuhlenkamp R., The marine macroalgae of Helgoland (North Sea): An annotated list of records between 1845 and 1999. Helgoland Mar. Res. 54, 60–189 (2000). [Google Scholar]
- 138.Heip C. H. R., Benthic studies: Summary and conclusions. Mar. Ecol. Prog. Ser. 91, 265–269 (1992). [Google Scholar]
- 139.Coll M., Piroddi C., Steenbeek J., Kaschner K., Ben Rais Lasram F., Aguzzi J., Ballesteros E., Bianchi C. N., Corbera J., Dailianis T., Danovaro R., Estrada M., Froglia C., Galil B. S., Gasol J. M., Gertwagen R., Gil J., Guilhaumon F., Kesner-Reyes K., Kitsos M. S., Koukouras A., Lampadariou N., Laxamana E., López-Fé de la Cuadra C. M., Lotze H. K., Martin D., Mouillot D., Oro D., Raicevich S., Rius-Barile J., Saiz-Salinas J. I., San Vicente C., Somot S., Templado J., Turon X., Vafidis D., Villanueva R., Voultsiadou E., The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLOS ONE 5, e11842 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.D. L. Felder, D. K. Camp, Gulf of Mexico-Origins, Waters, and Biota. Biodiversity (Texas A&M University Press, 2009), vol. 1, 1393 pp. [Google Scholar]
- 141.Liu J. Y., Status of marine biodiversity of the China seas. PLOS ONE 8, e50719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Szlinder-Richert J., Usydus Z., Pelczarski W., Organochlorine pollutants in European eel (Anguilla anguilla L.) from Poland. Chemosphere 80, 93–99 (2010). [DOI] [PubMed] [Google Scholar]
- 143.Imazaki P. H., Brose F., Jauniaux T., Das K., Muller M., Scippo M.-L., Estrogenic evaluation and organochlorine identification in blubber of North Sea Harbour Porpoise (Phocoena phocoena) stranded on the North Sea Coast. Biomed. Res. Int. 2015, 438295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nicolaus E. E. M., Wright S. R., Bolam T. P. C., Barber J. L., Bignell J. P., Lyons B. P., Spatial and temporal analysis of the risks posed by polychlorinated biphenyl and metal contaminants in dab (Limanda limanda) collected from waters around England and Wales. Mar. Pollut. Bull. 112, 399–405 (2016). [DOI] [PubMed] [Google Scholar]
- 145.Storelli M. M., Losada S., Marcotrigiano G. O., Roosens L., Barone G., Neels H., Covaci A., Polychlorinated biphenyl and organochlorine pesticide contamination signatures in deep-sea fish from the Mediterranean Sea. Environ. Res. 109, 851–856 (2009). [DOI] [PubMed] [Google Scholar]
- 146.Cakirogullari G. C., Secer S., Seasonal variation of organochlorine contaminants in bonito (Sarda sarda L. 1758) and anchovy (Engraulis encrasicolus L. 1758) in Black Sea region, Turkey. Chemosphere 85, 1713–1718 (2011). [DOI] [PubMed] [Google Scholar]
- 147.Stancheva M., Georgieva S., Makedonski L., Polychlorinated biphenyls in fish from Black Sea. Food Control 72, 205–210 (2017). [Google Scholar]
- 148.Harvey J., Harwell L., Summers J. K., Contaminant concentrations in whole-body fish and shellfish from US estuaries. Environ. Monit. Assess. 137, 403–412 (2007). [DOI] [PubMed] [Google Scholar]
- 149.Yin G., Asplund L., Qiu Y., Zhou Y., Wang H., Yao Z., Jiang J., Bergmann Å., Chlorinated and brominated organic pollutants in shellfish from the Yellow Sea and East China Sea. Environ. Sci. Pollut. Res. 22, 1713–1722 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhou S., Tong L., Tang Q., Gu X., Xue B., Liu W., Residues, sources and tissue distributions of organochlorine pesticides in dog sharks (Mustelus griseus) from Zhoushan Fishing Ground, China. Mar. Pollut. Bull. 73, 374–380 (2013). [DOI] [PubMed] [Google Scholar]
- 151.Routti H., Lydersen C., Hanssen L., Kovacs K. M., Contaminant levels in the world’s northernmost harbor seals (Phoca vitulina). Mar. Pollut. Bull. 87, 140–146 (2014). [DOI] [PubMed] [Google Scholar]
- 152.Sobek A., McLachlan M. S., Borga K., Asplund L., Lundstedt-Enkel K., Polder A., Gustafsson O., A comparison of PCB bioaccumulation factors between an arctic and a temperate marine food web. Sci. Total Environ. 408, 2753–2760 (2010). [DOI] [PubMed] [Google Scholar]
- 153.EU, European Parliament and Council Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (2008).
- 154.M. Karnauskas, M. J. Schirripa, C. R. Kelble, G. S. Cook, J. K. Craig, “2013. Ecosystem status report for the Gulf of Mexico” (NOAA Technical Memorandum NMFS- SEFSC-653, 2013), 52 pp.
- 155.National Marine Fisheries Service, “Annual Report to Congress on the Status of U.S. Fisheries-2010” (US Dept. Commerce, Natl. Mar. Fish. Serv., 2011).
- 156.S. Heileman, Q. Tang, in The UNEP Large Marine Ecosystem Report: A Perspective on Changing Conditions in LMEs of the World’s Regional Seas, K. Sherman, G. Hempel, Eds. (UNEP Regional Seas Report and Studies, 2008), vol. 182, pp. 383–392. [Google Scholar]
- 157.Fisheries and Fishery Administration of the Ministry of Agriculture, China Fishery Statistical Yearbook (Agricultural Press of China, 1979–2014). [Google Scholar]
- 158.Liang C., Pauly D., Fisheries impacts on China’s coastal ecosystems: Unmasking a pervasive ‘fishing down’ effect. PLOS ONE 12, e0173296 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reusch T. B. H., Ehlers A., Hämmerli A., Worm B., Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc. Natl. Acad. Sci. U.S.A. 102, 2826–2831 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kahru M., Elmgren R., Savchuk O. P., Changing seasonality of the Baltic Sea. Biogeosciences 13, 1009–1018 (2016). [Google Scholar]
- 161.A. Stigebrand, Physical oceanography of the Baltic Sea, in A Systems Analysis of the Baltic Sea. Ecological Studies (Analysis and Synthesis), F. V. Wulff, L. A. Rahm, P. Larsson, Eds. (Springer Berlin, Heidelberg, 2001), vol. 148. [Google Scholar]
- 162.Johansson D., Pereyra R. T., Rafajlović M., Johannesson K., Reciprocal transplants support a plasticity-first scenario during colonisation of a large hyposaline basin by a marine macro alga. BMC Ecol. 17, 14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nissling A., Westin L., Hjerne O., Reproductive success in relation to salinity for three flatfish species, dab (Limanda limanda), plaice (Pleuronectes platessa), and flounder (Pleuronectes flesus), in the brackish water Baltic Sea. ICES J. Mar. Sci. 59, 93–108 (2002). [Google Scholar]
- 164.Thomsen J., Casties I., Pansch C., Körtzinger A., Melzner F., Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: Laboratory and field experiments. Glob. Chang. Biol. 19, 1017–1027 (2013). [DOI] [PubMed] [Google Scholar]
- 165.Piepho M., Assessing maximum depth distribution, vegetated area, and production of submerged macrophytes in shallow, turbid coastal lagoons of the southern Baltic Sea. Hydrobiologia 794, 303–316 (2017). [Google Scholar]
- 166.Lindegren M., Blenckner T., Stenseth N. C., Nutrient reduction and climate change cause a potential shift from pelagic to benthic pathways in a eutrophic marine ecosystem. Glob. Chang. Biol. 18, 3491–3503 (2012). [Google Scholar]
- 167.Carstensen J., Conley D. J., Bonsdorff E., Gustafsson B. G., Hietanen S., Janas U., Jilbert T., Maximov A., Norkko A., Norkko J., Reed D. C., Slomp C. P., Timmermann K., Voss M., Hypoxia in the Baltic Sea: Biogeochemical cycles, benthic fauna, and management. Ambio 43, 26–36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lehmann A., Hinrichsen H.-H., Getzlaff K., Myrberg K., Quantifying the heterogeneity of hypoxic and anoxic areas in the Baltic Sea by a simplified coupled hydrodynamic-oxygen consumption model approach. J. Mar. Syst. 134, 20–28 (2014). [Google Scholar]
- 169.Conley D. J., Carstensen J., Ærtebjerg G., Christensen P. B., Dalsgaard T., Hansen J. L. S., Josefson A. B., Long-term changes and impacts of hypoxia in Danish coastal waters. Ecol. Appl. 17, S165–S184 (2007). [Google Scholar]
- 170.Villnäs A., Norkko J., Hietanen S., Josefson A. B., Lukkari K., Norkko A., The role of recurrent disturbances for ecosystem multifunctionality. Ecology 94, 2275–2287 (2013). [DOI] [PubMed] [Google Scholar]
- 171.Sundqvist L., Harkonen T., Svensson C. J., Harding K. C., Linking climate trends to population dynamics in the Baltic ringed seal: Impacts of historical and future winter temperatures. Ambio 41, 865–872 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stål J., Paulsen S., Pihl L., Rönnbäck P., Söderqvist T., Wennhage H., Coastal habitat support to fish and fisheries in Sweden: Integrating ecosystem functions into fisheries management. Ocean Coastal Manag. 51, 594–600 (2008). [Google Scholar]
- 173.Oesterwind D., Bock C., Förster A., Gabel M., Henseler C., Kotterba P., Kotterba P., Menge M., Myts D., Winkler H. M., Predator and prey: The role of the round goby Neogobius melanostomus in the western Baltic. Mar. Biol. Res. 13, 188–197 (2017). [Google Scholar]
- 174.Maximov A. A., Eremina T. R., Lange E. K., Litvinchuk L. F., Maximova O. B., Regime shift in the ecosystem of the eastern Gulf of Finland caused by the invasion of the polychaete Marenzelleria arctia. Oceanology 54, 46–53 (2014). [Google Scholar]
- 175.Sieben K., Rippen A. D., Eriksson B. K., Cascading effects from predator removal depend on resource availability in a benthic food web. Mar. Biol. 158, 391–400 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lade S. J., Niiranen S., Hentati-Sundberg J., Blenckner T., Boonstra W. J., Orach K., Quaas M. F., Österblom H., Schlüter M., An empirical model of the Baltic Sea reveals the importance of social dynamics for ecological regime shifts. Proc. Natl. Acad. Sci. U.S.A. 112, 11120–11125 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ahlvik L., Hyytiäinen K., Value of adaptation in water protection—Economic impacts of uncertain climate change in the Baltic Sea. Ecol. Econ. 116, 231–240 (2015). [Google Scholar]
- 178.R. Elmgren, C. Hill, Ecosystem function at low biodiversity—The Baltic example, in Marine Biodiversity Patterns and Processes, R. F. G. Ormond, J. Gage, M. Angel, Eds. (Cambridge Univ. Press, 1997), pp. 319–336. [Google Scholar]
- 179.Larsson K., Hajdu S., Kilpi M., Larsson R., Leito A., Lyngs P., Effects of an extensive Prymnesium polylepis bloom on breeding eiders in the Baltic Sea. J. Sea Res. 88, 21–28 (2014). [Google Scholar]
- 180.Kenny A. J., Skjoldal H. R., Engelhard G. H., Kershaw P. J., Reid J. B., An integrated approach for assessing the relative significance of human pressures and environmental forcing on the status of Large Marine Ecosystems. Prog. Oceanogr. 81, 132–148 (2009). [Google Scholar]
- 181.Hordoir R., Dieterich C., Basu C., Dietze H., Meier H. E. M., Freshwater outflow of the Baltic Sea and transport in the Norwegian current: A statistical correlation analysis based on a numerical experiment. Cont. Shelf Res. 64, 1–9 (2013). [Google Scholar]
- 182.Jonsson P. R., Nilsson Jacobi M., Moksnes P.-O., How to select networks of marine protected areas for multiple species with different dispersal strategies. Divers. Distrib. 22, 161–173 (2016). [Google Scholar]
- 183.HELCOM, Convention on the Protection of the Marine Environment of the Baltic Sea Area (H. Commission, 1974). [Google Scholar]
- 184.Linke S., Bruckmeier K., Co-management in fisheries—Experiences and changing approaches in Europe. Ocean Coast. Manag. 104, 170–181 (2015). [Google Scholar]
- 185.Kononen K., Andrusaitis A., Sirola M., BONUS+ in support of the ecosystem approach to management in the Baltic Sea. Ambio 43, 1–123 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ahtiainen H., Artell J., Czajkowski M., Hasler B., Hasselström L., Huhtala A., Meyerhoff J., Smart J. C. R., Söderqvist T., Alemu M. H., Angeli D., Dahlbo K., Fleming-Lehtinen V., Hyytiäinen K., Karlõševa A., Khaleeva Y., Maar M., Martinsen L., Nõmmann T., Pakalniete K., Oskolokaite I., Semeniene D., Benefits of meeting nutrient reduction targets for the Baltic Sea—A contingent valuation study in the nine coastal states. J. Environ. Econ. Policy 3, 278–305 (2014). [Google Scholar]
- 187.Markowska A., Żylicz T., Costing an international public good: The case of the Baltic Sea. Ecol. Econ. 30, 301–316 (1999). [Google Scholar]
- 188.Turner R. K., Georgiou S., Gren I.-M., Wulff F., Barrett S., Söderqvist T., Bateman I. J., Folke C., Langaas S., Żylicz T., Mäler K.-G., Markowska A., Managing nutrient fluxes and pollution in the Baltic: An interdisciplinary simulation study. Ecol. Econ. 30, 333–352 (1999). [Google Scholar]
- 189.Tuhkanen H., Piirsalu E., Nõmmann T., Karlõševa A., Nõmmann S., Czajkowski M., Hanley N., Valuing the benefits of improved marine environmental quality under multiple stressors. Sci. Total Environ. 551–552, 367–375 (2016). [DOI] [PubMed] [Google Scholar]
- 190.Pakalniete K., Aigars J., Czajkowski M., Strake S., Zawojska E., Hanley N., Understanding the distribution of economic benefits from improving coastal and marine ecosystems. Sci. Total Environ. 584–585, 29–40 (2017). [DOI] [PubMed] [Google Scholar]
- 191.Eggert H., Olsson B., Valuing multi-attribute marine water quality. Mar. Policy 33, 201–206 (2009). [Google Scholar]
- 192.Kosenius A.-K., Heterogeneous preferences for water quality attributes: The case of eutrophication in the Gulf of Finland, the Baltic Sea. Ecol. Econ. 69, 528–538 (2010). [Google Scholar]
- 193.Kosenius A.-K., Markku O., Ecosystem benefits from coastal habitats—A three-country choice experiment. Mar. Policy 58, 15–27 (2015). [Google Scholar]
- 194.Karlõševa A., Nõmmann S., Nõmmann T., Urbel-Piirsalu E., Budziński W., Czajkowski M., Hanley N., Marine trade-offs: Comparing the benefits of off-shore wind farms and marine protected areas. Energy Econ. 55, 127–134 (2016). [Google Scholar]
- 195.Vesterinen J., Pouta E., Huhtala A., Neuvonen M., Impacts of changes in water quality on recreation behavior and benefits in Finland. J. Environ. Manage. 91, 984–994 (2010). [DOI] [PubMed] [Google Scholar]
- 196.Limburg K. E., Waldman J. R., Dramatic declines in North Atlantic diadromous fishes. BioScience 59, 955–965 (2009). [Google Scholar]
- 197.G. Ståhl, Genetic differentiation among natural populations of Atlantic salmon (Salmo salar) in northern Sweden, in Fish Gene Pools: Preservation of Genetic Resources in Relation to Wild Fish Stocks, N. Ryman Ed. (Ecological Bulletine, 1981), vol. 34, pp. 95–105. [Google Scholar]
- 198.G. Ståhl, Genetic population structure of Atlantic salmon, in Population Genetics and Fisheries Management, N. Ryman, F. Utter, Eds. (Washington Sea Grant Program, University of Washington Press, 1987). [Google Scholar]
- 199.Ståhl G., Differences in the amount and distribution of genetic variation between natural populations and hatchery stocks of Atlantic salmon. Aquaculture 33, 23–32 (1983). [Google Scholar]
- 200.Laikre L., Schwartz M. K., Waples R. S., Ryman N.; GeM Working Group , Compromising genetic diversity in the wild: Unmonitored large-scale release of plants and animals. Trends Ecol. Evol. 25, 520–529 (2010). [DOI] [PubMed] [Google Scholar]
- 201.A. Palmé, L. Wennerström, P. Guban, N. Ryman, L. Laikre, “Compromising Baltic salmon genetic diversity—Conservation genetic risks associated with compensatory releases of salmon in the Baltic Sea” (Report for the Swedish Agency for Marine and Water Management, 2012:18); www.havochvatten.se/download/18.13780b7613b461ffa9e1a83/1355996777952/rapport-2012-18-compromising-baltic-salmon-english.pdf).
- 202.Ryman N., An analysis of growth capability in full sib families of salmon (Salmo salar L.). Hereditas 70, 119–128 (1972). [DOI] [PubMed] [Google Scholar]
- 203.Vasemägi A., Gross R., Paaver T., Koljonen M.-L., Nilsson J., Extensive immigration from compensatory hatchery releases into wild Atlantic salmon population in the Baltic Sea: Spatio-temporal analysis over 18 years. Heredity 95, 76–83 (2005). [DOI] [PubMed] [Google Scholar]
- 204.ICES, “Report of the Baltic Salmon and Trout Assessment Working Group (WGBAST), Klaipeda, Lithuania, 30 March-6 April 2016” (ICES CM 2016/ACOM:09, 2016), 257 pp.
- 205.C. Hiebenthal, P. Fietzek, J. Thomsen, V. Saderne, F. Melzner, Kiel fjord pCO2 datasets between 2012 (July) and 2015 (January) measured using a HydroC® pCO2 sensor. GEOMAR - Helmholtz Centre for Ocean Research Kiel (PANGAEA, 2016); https://doi.org/10.1594/PANGAEA.859407.
- 206.A. Sutton, C. Sabine, C. Dietrich, S. Maenner, S. Musielewicz, R. Bott, J. Osborne, High-resolution ocean and atmosphere pCO2 time-series measurements from mooring WHOTS_158W_23N (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, 2010); http://cdiac.esd.ornl.gov/ftp/oceans/Moorings/WHOTS_158W_23N/. doi: 10.3334/CDIAC/OTG.TSM_WHOTS.
- 207.A. Sutton, C. Sabine, D. Manzello, S. Musielewicz, S. Maenner, C. Dietrich, R. Bott, J. Osborne, Partial pressure (or fugacity) of carbon dioxide, pH, salinity and other variables collected from time series observations using Bubble type equilibrator for autonomous carbon dioxide (CO2) measurement, Carbon dioxide (CO2) gas analyzer and other instruments from MOORING_CHEECA_80W_25N in the Coastal Waters of Florida, Florida Keys National Marine Sanctuary and North Atlantic Ocean from 2011-12-07 to 2015-03-22 (NCEI Accession 0157417). Version 3.3. NOAA National Centers for Environmental Information, 2016. Dataset. doi:10.3334/CDIAC/OTG.CHEECA_80W_25N.
- 208.A. Sutton, C. Sabine, U. Send, M. Ohman, S. Musielewicz, S. Maenner, C. Dietrich, R. Bott, J. Osborne, High-resolution ocean and atmosphere pCO2 time-series measurements from mooring CCE2_121W_34N (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, 2013); http://cdiac.esd.ornl.gov/ftp/oceans/Moorings/CCE2_121W_34N/. doi: 10.3334/CDIAC/OTG.TSM_CCE2_121W_34N.
- 209.A. Sutton, C. Sabine, J. Newton, J. Mickett, S. Musielewicz, S. Maenner, C. Dietrich, R. Bott, J. Osborne, High-resolution ocean and atmosphere pCO2 time-series measurements from mooring Twanoh_123W_47N (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, 2015); http://cdiac.esd.ornl.gov/ftp/oceans/Moorings/Twanoh_123W_47N/. doi: 10.3334/CDIAC/OTG.TSM_Twanoh_123W_47N.
- 210.J. Cross, J. Mathis, N. Monacci, S. Musielewicz, S. Maenner, J. Osborne, High-resolution ocean and atmosphere pCO2 time-series measurements from mooring Kodiak_152W_57N (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, 2014); http://cdiac.ornl.gov/ftp/oceans/Moorings/Kodiak_152W_57N/. doi:10.3334/CDIAC/OTG.TSM_KODIAK_152W_57N.
- 211.Cloern J. E., Abreu P. C., Carstensen J., Chauvaud L., Elmgren R., Grall J., Greening H., Johansson J. O., Kahru M., Sherwood E. T., Xu J., Yin K., Human activities and climate variability drive fast-paced change across the world’s estuarine–coastal ecosystems. Glob. Chang. Biol. 22, 513–529 (2015). [DOI] [PubMed] [Google Scholar]
- 212.HELCOM, “Approaches and methods for eutrophication target setting in the Baltic Sea region” (Baltic Sea Environmental Proceedings No. 133, H. Commission, 2013).
- 213.Kuosa H., Fleming-Lehtinen V., Lehtinen S., Lehtiniemi M., Nygård H., Raateoja M., Raitaniemi J., Tuimala J., Uusitalo L., Suikkanen S., A retrospective view of the development of the Gulf of Bothnia ecosystem. J. Mar. Syst. 167, 78–92 (2017). [Google Scholar]
- 214.Klais R., Otto S. A., Teder M., Simm M., Ojaveer H., Winter-spring climate effects on small-sized copepods in the coastal Baltic Sea. ICES J. Mar. Sci. 74, 1855–1864 (2017). [Google Scholar]
- 215.ICES, “Report of the Baltic Fisheries Assessment Working Group (WGBFAS)” (ICES CM 2013/ACOM:10, 2013), 772 pp.
- 216.ICES Advice, “Herring (Clupea harengus) in subdivisions 25-29 and 32, excluding the Gulf of Riga (central Baltic Sea)” (Report of the ICES Advisory Committee 2017, ICES publication 3392, ICES, 2017), 9 pp.; doi: 10.17895/ices.pub.3392.
- 217.Roos A. M., Bäcklin B.-M. V. M., Helander B. O., Rigét F. F., Eriksson U. C., Improved reproductive success in otters (Lutra lutra), grey seals (Halichoerus grypus) and sea eagles (Haliaeetus albicilla) from Sweden in relation to concentrations of organochlorine contaminants. Environ. Pollut. 170, 268–275 (2012). [DOI] [PubMed] [Google Scholar]
- 218.P. Sellke, M. Dreyer, S. Linke, in Environmental Governance of the Baltic Sea, M. Gilek, M. Karlsson, S. Linke, K. Smolarz, Eds. (MARE Publication Series, Springer, 2016), vol. 10, pp. 45–71. [Google Scholar]
- 219.K. Karlsson, M. Gilek, in Environmental Governance of the Baltic Sea, M. Gilek, M. Karlsson, S. Linke, K. Smolarz, Eds. (MARE Publication Series, Springer, 2016), vol. 10, pp. 97–123 [Google Scholar]
- 220.Nilsson A. K., Bohman B., Legal prerequisites for ecosystem-based management in the Baltic Sea Area: The example of eutrophication. Ambio 44, 370–380 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.K. Karlsson, M. Gilek, C. Lundberg, in Environmental Governance of the Baltic Sea, M. Gilek, M. Karlsson, S. Linke, K. Smolarz, Eds. (MARE Publication Series, Springer, 2016), vol. 10, pp. 21–44 [Google Scholar]
- 222.Harding K. C., Härkönen T. J., Development in the Baltic grey seal (Halichoerus grypus) and ringed seal (Phoca hispida) populations during the 20th century. Ambio 28, 619–627 (1999). [Google Scholar]
- 223.Härkönen T., Isakson E., Status of harbour seals (Phoca vitulina) in the Baltic proper. NAMMCO Sci. Pub. 8, 71–76 (2010). [Google Scholar]
- 224.Hiby L., Lundberg T., Karlsson O., Watkins J., Jüssi M., Jüssi I., Helander B., Estimates of the size of the Baltic grey seal population based on photo-identification data. NAMMCO Sci. Pub. 6, 163–175 (2007). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/5/eaar8195/DC1
data S1. Methods and references used for the assembly of Tables 1 and 2 of the main text.
table S1A. Detailed assessment of the qualitative status of environmental pressures/drivers in the Baltic Sea region compared to other worldwide coastal seas.
table S1B. Detailed assessment of scientific knowledge, management regimes, and governance structures in the Baltic Sea region compared to other worldwide coastal seas/areas.
table S2. Valuation of benefits of environmental intervention or management measurements in the Baltic Sea area.
data S2. Box: Genetic knowledge in marine management—The recovery of Baltic salmon.
data S3. Data sources for figures.


