An early Cambrian greenhouse climate constrained by combining oxygen isotope and climate modeling data approaches.
Abstract
The oceans of the early Cambrian (~541 to 509 million years ago) were the setting for a marked diversification of animal life. However, sea temperatures—a key component of the early Cambrian marine environment—remain unconstrained, in part because of a substantial time gap in the stable oxygen isotope (δ18O) record before the evolution of euconodonts. We show that previously overlooked sources of fossil biogenic phosphate have the potential to fill this gap. Pristine phosphatic microfossils from the Comley Limestones, UK, yield a robust δ18O signature, suggesting sea surface temperatures of 20° to 25°C at high southern paleolatitudes (~65°S to 70°S) between ~514 and 509 million years ago. These sea temperatures are consistent with the distribution of coeval evaporite and calcrete deposits, peak continental weathering rates, and also our climate model simulations for this interval. Our results support an early Cambrian greenhouse climate comparable to those of the late Mesozoic and early Cenozoic, offering a framework for exploring the interplay between biotic and environmental controls on Cambrian animal diversification.
INTRODUCTION
The oxygen isotope (δ18O) composition of fossil biominerals is one of the most widely used proxies for investigating ancient environments and is particularly useful as a deep time paleothermometer (1). The most widespread δ18O records come from analyses of calcium carbonate (2–4) and calcium phosphate (5–7) marine shelly fossils. However, although a near-continuous δ18O record exists back to the Early Ordovician epoch, 485 million years (Ma) ago (1, 6), there is a substantial early Phanerozoic data gap that covers the interval of the Cambrian explosion, when the oldest identifiable fossils of most of the animal phyla appear in the rock record (8). This gap exists because phosphatic euconodonts with diagenetically robust hyaline crown tissues (9) are absent below uppermost Cambrian strata, whereas most calcareous fossils in these rocks have been demonstrably diagenetically altered (1, 4). An alternative source of Cambrian oxygen isotope data is therefore needed to quantitatively assess the state of the early Cambrian climate, which has so far only been qualitatively interpreted as a greenhouse interval (8, 10, 11). We propose that “small shelly fossils” (SSFs) are a potential new source of δ18O data that could help constrain Cambrian marine environments.
SSFs comprise the skeletal elements of a diverse range of early biomineralizing animals that produced siliceous, calcareous, or phosphatic skeletons. Although many SSFs are preserved in secondary (diagenetic) phosphate (12), some are suggested to retain an original biophosphatic composition (13). To avoid sampling secondarily phosphatized specimens, we targeted fossils from the Comley Limestones, Shropshire, UK (fig. S1). These rocks are notable for yielding arthropods with limited (and very early) secondary phosphatization of soft anatomy but lack evidence for phosphatic replacement of original shell material (14). The Comley Limestones were deposited under normal marine conditions beneath the well-mixed waters of a shallow sea (15), approximately 65°S to 70°S on the peri-Gondwanan microcontinent Avalonia (16) between 514.45 ± 0.36 and 509.10 ± 0.22 Ma ago (17).
To ensure that isotope data recovered from the phosphatic SSFs reflect a Cambrian paleoenvironmental signal rather than later diagenetic conditions, we restricted our analyses to taxa known to have produced phosphatic skeletons based on phylogenetic and geological criteria (13). These included linguliformean brachiopods, which have well-known extant relatives, and the robust, thick-walled benthic tubular microfossil Torellella. We assembled taxon-specific samples for bulk isotope analysis comprising tens to a few hundred individual specimens and separated them according to their visual quality of preservation (fig. S2). Representative specimens from each bulk subset were subjected to a rigorous protocol to assess the preservation of biogenic phosphate. We could consistently distinguish subsets of pristine and altered SSFs using optical microscopy, confirmed by combining high-resolution scanning electron microscopy (SEM) and energy dispersive x-ray (EDX) spectroscopy to assess micro- and ultrastructural preservation and the distribution of diagenetically sensitive elements (see Materials and Methods). In particular, brachiopod specimens taken from pristine subsets were found to have compact laminae comprising phosphate spherules tens of nanometres in diameter, whereas the compact laminae of specimens taken from altered subsets were recrystallized as micrometer-scale phosphate prisms (Fig. 1). To help define a δ18O diagenetic gradient, we also analyzed an early diagenetic phosphate hardground, which likely formed at or close to the sediment-water interface, soon after deposition, from pore waters in communication with the overlying ocean (see Materials and Methods).
Fig. 1. Preservation of linguliformean brachiopods and Torellella from the Comley Limestones.
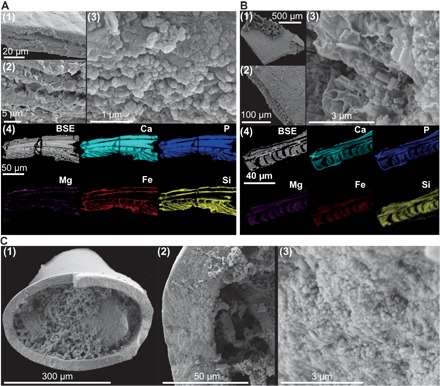
(A) SEM and EDX analyses of pristine brachiopods preserve alternating compact and porous phosphatic laminae [(1) and (2)]; compact laminae comprise densely packed phosphatic spherules (3). Diagenetically sensitive elements, particularly Fe and Mg, are restricted to porous laminae (4). BSE, backscattered electron image. (B) SEM and EDX analyses of altered brachiopods may preserve laminar microstructure, but compact laminae phosphate has recrystallized to micrometer-scale prismatic crystals (3). Diagenetically sensitive elements indicative of alteration are found throughout altered specimens (4). (C) Pristine Torellella specimens comprise densely packed phosphatic spherules a few tens of nanometers in diameter (3).
RESULTS AND DISCUSSION
Bulk oxygen isotope analyses (see Materials and Methods and data S1) of pristine phosphatic microfossils (five samples of linguliformean brachiopods and two of Torellella) yielded δ18Ophos values of +13.9 to +15.2 per mil (‰) Vienna standard mean ocean water (VSMOW). In contrast, three linguliformean brachiopod samples, identified a priori as being affected by diagenetic alteration, yielded lighter values of +13.3 to +14.3‰ (Fig. 2). Samples of sedimentary phosphate from an early diagenetic phosphatic hardground, in situ and as a rip-up clast, yielded still lighter δ18Ophos values ranging from +12.8 to +14.0‰.
Fig. 2. Early Cambrian δ18Ophos data from SSFs from the Comley Limestones.

(A) In bulk analyses, altered subsets are isotopically lighter than the pristine counterparts from the same sample. Sample labels follow Table 1; error bars of 1 SD; δ18Ophos indicates trisilver phosphate analysis in which only phosphate oxygen isotopes were measured. (B) In situ (SIMS) data from linguliformean brachiopod specimens (sample Ad) show that porous laminae are consistently isotopically lighter than compact laminae. See data S1 and fig. S3. Box plots display the median and first and third quartiles, with the whiskers extending up to 1.5 times the interquartile range. All isotope data are reported relative to VSMOW.
In paired analyses of linguliformean brachiopods from the same sample, identified during our preanalysis screening as preserving either pristine or demonstrably altered phosphate, the pristine brachiopods consistently yielded heavier δ18Ophos than their diagenetically altered counterparts, by +0.3 to +1.0‰. The close correspondence of the δ18Ophos of diagenetically altered brachiopods (+13.3 to +14.3‰) to those of the phosphate hardground (+12.8 to +14.0‰) suggests that these altered SSFs may record early diagenetic conditions or at least have followed a similar diagenetic pathway as the hardground. In contrast, pristine Torellella samples yielded δ18Ophos values of 14.6 to 14.7‰, comparable to the pristine brachiopods (+13.9 to +15.2‰) and heavier values than those from the diagenetically altered brachiopods and the hardground samples. Most Torellella specimens were identified as pristine, and altered Torellella specimens were too scarce to assemble samples for bulk analysis (see Materials and Methods).
In situ secondary ion mass spectrometry (SIMS) analysis shows that oxygen isotopic preservation varies in the same manner as bulk elemental composition within individual brachiopod specimens (see Materials and Methods, fig. S3, and data S2). SIMS analysis consistently distinguishes between the δ18O values of compact and porous laminae, with compact laminae consistently heavier, by at least approximately 2‰, than porous laminae in the same specimen (Fig. 2). Bulk δ18Ophos values approximate to those of the compact laminae, likely because of the proportionately small contribution of the phosphate in the porous laminae to the total volume of shelly material analyzed (18, 19). The systematic offset between the heavier values in the bulk δ18Ophos and lighter values from in situ SIMS analyses (Fig. 1) is likely due to the incorporation of all oxygen isotope species in a SIMS analysis spot [including the high proportion of structural carbonate in biogenic apatite (19)], compared with only phosphate-bound oxygen (PO43−) measured by the trisilver bulk phosphate method. Because phosphate-oxygen temperature equations are based on trisilver δ18Ophos analyses (7, 20–22), bulk δ18Ophos analyses are likely the most robust paleoenvironmental data from SSFs, with SIMS analyses providing critical information on intrasample variability and preservation.
Isotopic composition of Cambrian seas
Selected SSFs from the Comley Limestones, screened to identify and exclude specimens unduly affected by diagenetic alteration, give δ18Ophos values that reflect shallow marine conditions on early Cambrian Avalonia. The δ18Ophos data incorporate signals from the oxygen isotopic composition of contemporaneous sea water (δ18Osw), the temperature of the water in which the animal lived, and biological fractionation (“vital effects”). The convergence of phosphate oxygen isotope temperature equations based on both marine invertebrate and nonmammalian marine vertebrate biominerals indicates that phosphate oxygen vital effects are small in comparison with analytical uncertainty (21). The impact of any vital effects in the phosphate oxygen isotope system is incorporated within the uncertainty of the empirically derived phosphate oxygen temperature (Eq. 1) (22)
| (1) |
However, an estimate of δ18Osw is still required. Local δ18Osw is a function of the δ18Osw of the global ocean average value, the local influence of freshwater input, and the regional precipitation-evaporation (P-E) balance (23). Secular variability of the global ocean δ18O reservoir, other than ice volume effects of ~1‰, is often disregarded in deep time paleoclimate studies (5, 6) because of the potential buffering effect of balanced hot and cold hydrothermal alteration processes (24)—a view that has found some support from the emerging field of carbonate clumped isotopes (25–28). However, an invariant global ocean δ18O reservoir is at odds with the long-term carbonate, phosphate, and silica records, which all show comparable nonlinear secular trends in δ18O values (2, 4, 5, 29, 30). Over the Phanerozoic eon, this trend is thought to have shifted the δ18O value of the global ocean reservoir from approximately −6‰ in the early Cambrian to the heavier modern values of −1 to 0‰ (2, 4, 30). The δ18Osw secular trend is further supported by similar magnitude trends derived from numerical modeling, with a substantial amount of this shift occurring in the early part of the Phanerozoic eon (31, 32).
Our new data support a secular trend in the global ocean δ18O reservoir and extend the biomineral isotope evidence for this trend back into the early Cambrian (Fig. 3 and data S3). We therefore suggest that paleoenvironmental δ18O studies in deep time should detrend raw data before making paleoenvironmental interpretations of temperature or ice volume change to account for this secular variation. To avoid the circularity of inferring both δ18Osw and temperature from our data, we use preexisting whole-rock data (4) and geochemical modeling (31, 32) to infer that global average early Cambrian δ18Osw was approximately −6‰.
Fig. 3. The Paleozoic phosphate δ18O record.

Conodonts, blue squares; well-preserved linguliformean brachiopods, dark blue triangles; well-preserved Torellella samples, yellow circles; phosphate hardground data, purple hexagons). This trend is most pronounced in the earlier part of the Paleozoic. Data span a range of paleolatitudes and water depths. Our Cambrian data are essentially contemporaneous at approximately 513 Ma ago; for clarity, Torellella and phosphate hardground points have been shifted slightly on the age axis. All values are relative to VSMOW. See data S3.
In addition to secular variability, we account for latitudinal P-E effects on local δ18Osw in our temperature calculations—an important factor in paleoclimate research on more recent intervals but which is not usually considered in Paleozoic studies (26–28, 33). Latitudinal P-E effects in modern oceans can be substantial, with surface δ18Osw values ranging from −7.7 to +2.5‰ (34), although most of the global ocean is within ±1.5‰ of the global average (23, 34, 35). Isotope-enabled climate models for the early Cenozoic greenhouse climate suggest that the δ18Osw distribution was similar to that of modern oceans, with perhaps slightly increased variability driven by an enhanced hydrological cycle (36, 37). Given the well-connected position of Avalonia to the global ocean in the early Paleozoic (see Materials and Methods and fig. S1) and the range of δ18Osw at comparable high southern latitudes in both the current icehouse and early Cenozoic greenhouse climate states (34–37), we use a conservative estimate of a −0.5‰ deviation from the Cambrian global average of −6‰ to give a δ18Osw value of −6.5‰.
Cambrian sea surface temperatures
Because the Comely Limestones were deposited in a shallow marine setting (15), we can interpret our isotope data as reflecting sea surface, rather than deep marine, conditions. Using the phosphate oxygen temperature (Eq. 1) (22) with a δ18Osw value of −6.5‰, we reconstruct sea surface temperatures (SSTs) of 20° to 25°C for the Comley Limestones (Table 1). This is within the range of high-latitude temperatures of more recent greenhouse intervals in Earth’s history (Fig. 4 and data S4), such as those of the late Mesozoic and early Cenozoic (38–41).
Table 1. Bulk (trisilver phosphate) δ18Ophos data from the Comley Limestones and SSTs exploring the influence of different values for δ18Osw.
Temperatures calculated from Eq. 1 (22). T1 δ18Osw = −6.5‰, the most reasonable value; T2 δ18Osw = −6‰, assumes no latitudinal P-E effects; T3 δ18Osw = −1‰, the most commonly used Paleozoic value; T4 δ18Osw = −8‰, incorporating the maximum likely latitudinal P-E effect of −2‰. Temperature uncertainty (2 SD T1) calculated from δ18O measurement SDs, assuming that δ18Osw = −6.5‰. See data S1.
| Sample | Triplicate mean δ18Ophos (‰ VSMOW) | Triplicate 1 SD (‰ VSMOW) | T1 (°C) | T2 (°C) | T3 (°C) | T4 (°C) | 2 SD T1 (°C) |
| Pristine brachiopods | |||||||
| Aa-Br-L | 15.17 | 0.18 | 20 | 22 | 45 | 13 | 1.6 |
| Ad-Br-L | 15.06 | 0.03 | 20 | 23 | 45 | 14 | 0.3 |
| La2-Br-L | 13.93 | 0.22 | 25 | 28 | 50 | 19 | 2.0 |
| Lb-Br-L | 14.63 | 0.14 | 22 | 25 | 47 | 16 | 1.3 |
| Sa-Br-L | 14.62 | 0.15 | 22 | 25 | 47 | 16 | 1.3 |
| Pristine Torellella | |||||||
| Lb-To-To | 14.70 | 0.14 | 22 | 24 | 47 | 15 | 1.3 |
| Sa-To-To | 14.59 | 0.07 | 22 | 25 | 47 | 16 | 0.6 |
| Altered brachiopods | |||||||
| La2-Br-D | 13.33 | 0.17 | 28 | 30 | 53 | 21 | 1.5 |
| Lb-Br-D | 14.31 | 0.23 | 24 | 26 | 49 | 17 | 2.1 |
| Sa-Br-D | 13.94 | 0.18 | 25 | 28 | 50 | 19 | 1.7 |
| Sedimentary phosphate | |||||||
| HG-A | 13.95 | 0.05 | 25 | 28 | 50 | 19 | 0.5 |
| HG-A-DC | 14.07 | 0.21 | 25 | 27 | 50 | 18 | 1.9 |
| HG-B | 12.77 | 0.09 | 31 | 33 | 55 | 24 | 0.8 |
| HG-B-DC | 12.78 | 0.20 | 31 | 33 | 55 | 24 | 1.8 |
Fig. 4. Cambrian isotopic SSTs in the context of Mesozoic and early Cenozoic greenhouse climate states.

Cambrian data (black diamonds) from the δ18Ophos values of pristine SSF samples from the Comley Limestones. Data are plotted in 10° bins of the modulus of paleolatitude to illustrate latitudinal temperature variation irrespective of paleocontinental configuration. Plotted point data summarized in box plots displaying the median and first and third quartiles, with the whiskers extending up to 1.5 times the interquartile range. Cretaceous data, Cenomanian to Turonian; Paleogene data, Paleocene to Eocene. For references and literature data, see data S4. The modern latitudinal mean (black line) and range (gray envelope) data from the 2013 World Ocean Atlas 1° resolution data set (60).
Repeating our calculations assuming the ice-free Cenozoic value of −1‰ for δ18Osw typically used in Paleozoic paleoclimate studies (5, 6), rather than −6.5‰, our isotopic temperatures would shift from ~45° to 50°C (Table 1). This is unrealistic for high-latitude SSTs because they approach or exceed the lethal temperature limits for many marine animals, including brachiopods (42). We note that our δ18Ophos values are similar to those derived from well-preserved conodonts from the Tremadocian (Early Ordovician) of northern Gondwana (5) and Laurentia (6), which provide SST estimates of 40° to 44°C, assuming a δ18Osw value of −1‰, albeit for lower latitudes. However, a recent paleoclimate modeling study encompassing the Early Ordovician epoch predicts much lower sea temperatures for the paleogeographic positions of these conodont δ18O data (43). Sea temperature estimates of 27° to 30°C, in much closer agreement with general circulation model (GCM) estimates (43), are obtained when a detrended Early Ordovician global ocean average δ18Osw value of −4‰ (32) is used. Detrending the conodont δ18Ophos record to account for the observed δ18O secular trend (2, 4, 29) improves data-model comparison in early Paleozoic climate studies (43), providing further confidence in our δ18Osw value.
Paleoclimate implications
The characteristically warm high-latitude temperatures of greenhouse climate intervals, particularly noticeable in Mesozoic and Cenozoic paleotemperature proxy records (38–41), are consistent with our reconstructed SSTs (Fig. 4). Early Cambrian geological data generally support interpretations of a greenhouse climate state lacking permanent polar ice sheets, with evaporite and calcrete deposits spanning a wide paleolatitudinal range (10), the deposition of tropical soils (laterites) at high paleolatitudes (44), and a maximum of continental weathering rates over the past 900 Ma (11).
However, there may be some evidence for glaciation at high paleolatitudes (Avalonia) or even mid-paleolatitudes (Baltica) during the early Cambrian (45, 46). Imprecise age constraints have hindered integration of these possible cold-climate deposits into the international stratigraphic framework, with some age estimates ranging from late Neoproterozoic to Early Ordovician. However, the likelihood is that these deposits (45, 46) are of earliest (pretrilobitic) Cambrian or late Neoproterozoic age. Because our data from Cambrian Age 4 suggest climatic conditions that preclude even polar land ice at low altitude, it seems likely that any glacial activity was restricted to the earliest Cambrian or to short duration icehouse intervals.
To further investigate the viability of our temperature estimates, we ran new GCM simulations of the early Cambrian climate (Fig. 5 and fig. S4) using the Fast Ocean Atmosphere Model (FOAM) (47)—a coupled ocean-atmosphere GCM that has recently been applied to interrogate other questions about early Paleozoic climates (43, 48). The GCM simulations found good agreement with our new data for CO2-equivalent forcing of 32 times preindustrial atmospheric levels (PALs; 280 parts per million). This greenhouse gas forcing is in line with Cambrian pCO2 (partial pressure of CO2) estimates from GEOCARB suite of models (49). Both the data- and GCM-derived temperatures are comparable to late Mesozoic and early Cenozoic greenhouse conditions (Fig. 4) (38–41).
Fig. 5. Early Cambrian mean annual SSTs, modeled by the FOAM GCM.

The simulation was run under present-day orbital configurations with a CO2-equivalent greenhouse gas forcing of 32 PAL (see Materials and Methods and fig. S4). Black spot marks the position of our δ18Ophos data on Avalonia.
Overall, our new data provide the first quantitative constraints on early Cambrian climate, corroborating qualitative geological data and geochemical arguments that also support interpretations of this as a greenhouse world. Our new data fill an extensive time gap in the paleotemperature record of the beginning of the Phanerozoic eon and provide environmental context to a time when the animal-rich marine ecosystems of the Phanerozoic were first evolving. Using quantitative data to inform environmental and climate models will enable more rigorous interrogation of first-order hypotheses surrounding the ecological revolutions of the Cambrian Period, and these data can be recovered from globally widespread phosphatic Cambrian microfossils.
MATERIALS AND METHODS
Materials
The Comley Limestones
The Comley Limestones were deposited in a shallow sea (15) at ~65°S to 70°S on the peri-Gondwanan microcontinent Avalonia (16) between 514.45 ± 0.36 and 509.10 ± 0.22 Ma ago (fig. S1) (17). This fossiliferous and highly condensed limestone unit is less than 2 m thick, with five lithostratigraphic units (Ac2 to Ac5 and Ad) recognized on the basis of their petrographic and paleontological characteristics and separated by erosional disconformities that represent depositional hiatuses (15). Glauconite clasts and laminated iron-manganese nodules are common throughout the succession. The horizons are all more-or-less-sandy limestones, with detrital mineral abundance and faunal composition varying throughout the succession. The abundance of glauconite, laminated iron-manganese nodules, evidence for erosion and condensation, and the typically stenohaline trilobitic fauna indicates that there was no major freshwater (riverine) influence. There was deposition characteristic of normal marine conditions in the Welsh Basin throughout much of the Cambrian, Ordovician, and Silurian (50), and its peri-Gondwanan position close to the passive margin of the Iapetus Ocean during the Cambrian Period (16) suggests that it was well connected to the global ocean. The Comley Limestones are considered to have been deposited in an energetic shallow marine environment (15) that was unlikely to have been subjected to a seasonal thermocline.
Microfossil processing
Blocks of the Comley Limestones were macerated in buffered (10 to 15%) acetic acid using a standard extraction protocol, modified after Jeppsson et al. (51), that is not known to affect δ18O values (9). Heavy liquid separation, which is commonly used to concentrate microfossils within an acid residue but is known to affect δ18Ophos values (9), was not used. Acetic acid residues were collected between 1000- and 125-μm sieves and thoroughly rinsed with deionized water before being gently dried in an oven (T < 50°C). The residues were subsequently examined using a binocular microscope, and fossil specimens were picked out with a brush and deionized water. SSFs were assembled into 30-mg taxon-specific bulk samples, with each sample comprising several tens to a few hundred individual specimens.
Specimens were assembled into “pristine” and “altered” samples based on their appearance under optical microscopy. Pristine brachiopod specimens had a translucent light-brown appearance under reflected light, whereas altered specimens were opaque and appeared very dark brown to black (fig. S2). Specimens intermediate between these end-member states were excluded from further analysis. The vast majority of Torellella specimens were deemed pristine: light blue-gray in color, thick-walled, and with visible growth structures both within the tube walls and on the exterior surface. Although altered Torellella specimens exist (bleached white or black in color and with tube walls typically thin with no internal or external differentiation), no lithological sample yielded sufficient of these for bulk isotope analysis. The actual preservation state of samples was confirmed following the protocol outlined below.
Microfossil preservation
Because it was not possible to assess the preservation of every fossil in a bulk isotope sample, individual representative specimens were taken from bulk samples and examined to determine the typical preservation of each sample. Fractured surfaces of individual specimens were examined using high-magnification SEM to investigate the extent of recrystallization or overgrowth by diagenetic phosphate. Polished cross sections of individual specimens embedded in epoxy resin were examined using EDX spectroscopy to investigate the extent of chemical alteration and particularly the distribution of any such alteration within individual specimens.
The shells of modern linguliformean brachiopods comprised alternating dense layers of biophosphate (compact laminae) and organic-rich layers with much less biophosphate (porous laminae of various morphologies). The compact laminae, which constituted most of the biomineralized material, were composed of densely packed calcium phosphate spherules measuring from tens to a few hundred nanometers in diameter (18). We identified these submicrometer-scale phosphatic spherules in the compact laminae of specimens taken from pristine bulk samples (Fig. 1 and fig. S1). We also found these submicrometer-scale phosphatic spherules in specimens taken from pristine samples of the tubular SSF Torellella (Fig. 1). Specimens from samples identified as diagenetically altered showed recrystallization of these spherules, forming micrometer-scale phosphatic prisms (Fig. 1). These very small crystallite sizes, where microstructures are otherwise well preserved, may simply reflect solid-state recrystallization with no or minimal isotopic exchange because phosphate oxygen isotopes are known to be robust to low-temperature non–microbially mediated recrystallization (52). Secondary phosphatization was restricted to apatite overgrowths protruding into the porous laminae of specimens taken from samples of altered brachiopod samples. Overgrowths were also observed in some Torellella specimens, although where present, this was apparent under optical microscopy as bulges formed on the surfaces of these specimens wherever overgrowths had formed. Torellella specimens with these bulges were excluded from isotope analyses. In the most severely altered brachiopod specimens, all internal microstructure was lost, with the specimens being preserved as crude (blocky) outlines only.
Individual specimens from pristine and altered samples were embedded in epoxy resin and cured under pressure (approximately 2 bar) before being ground and polished with silicon carbide paper, diamond paste, and γ-alumina, before being thoroughly rinsed with ethanol and deionized water. EDX analyses of these specimens enabled us to map element distributions across different biological domains (compact and porous laminae). In brachiopod specimens taken from pristine samples, we found that elements indicative of alteration (most commonly, Fe, Mg, and Si) were restricted to porous laminae and were excluded from compact laminae (Fig. 1). In EDX analyses of specimens taken from altered samples, we found that these elements pervade the brachiopod compact laminae, where we had already observed prismatic phosphate recrystallization (Fig. 1). Having confirmed these preservation states with detailed SEM-EDX investigations, we determined that it was possible to separate pristine and altered specimens into bulk isotope samples using optical microscopy (fig. S2).
Phosphatic hardground
Sedimentary phosphate samples were also taken for bulk isotope analysis to provide a δ18O diagenetic gradient. These were acquired by microdrilling approximately 30 mg of powder from an irregular phosphatic hardground (HG-A) horizon in the lower part of the Comley Limestones and from a phosphatic pebble (HG-B) a few centimeters above the hardground. The in situ hardground is up to 3 cm deep, with an angular upper surface, and separates two distinct lithologies: an underlying trilobite-rich sandy limestone and an overlying glauconite-rich sandy limestone with few fossils. The overlying glauconite-rich unit also contains subrounded to angular phosphatic pebble-sized clasts. In places, the uppermost part of the hardground includes laminae of glauconite and quartz clasts.
Petrographic observations, including the lithological differences between the underlying and overlying units, the irregular upper surface, the preservation of calcareous fossils between the phosphate groundmass, and the fine glauconite and quartz laminae near the top of the horizon, suggested an early diagenetic origin of the phosphate, with occasional interruption of phosphate precipitation at the sediment-water interface and, ultimately, exposure of the hardground on the sea bed. Phosphate clasts were incorporated into the horizon above the hardground and were interpreted as deriving from the hardground during its exposure on the sea bed after its formation.
Methods
Bulk isotope analyses
All δ18O values were reported with respect to VSMOW. Bulk isotope data were obtained from 30-mg fossil samples, with each sample comprising several tens of individual specimens, treated to solubilize PO4 anions and precipitated as silver phosphate [adapted after O’Neil et al. (53)] at the Natural Environment Research Council (NERC) Isotope Geoscience Facilities (NIGF). Samples of microfossils were crushed using a glass rod, cleaned in concentrated hydrogen peroxide for 24 hours to remove organic material, and subsequently evaporated to dryness. The samples were then dissolved in 2 M HNO3 and transferred to clean polypropylene test tubes. Each sample was then treated with 2 M KOH for neutralization and 2 M HF to remove calcium from the solution by precipitation of calcium fluoride. The samples were then centrifuged, and the supernatant was added to beakers containing ammoniacal silver nitrate solution and heated gently to precipitate silver phosphate. The silver phosphate was filtered, rinsed, dried, and weighed into silver capsules for analysis. Oxygen isotope measurements on each sample were analyzed in triplicate by continuous flow isotope ratio mass spectrometry (54). Analysis was via a high-temperature conversion elemental analyzer coupled to a Delta Plus XL isotope ratio mass spectrometer via a ConFlo III interface (Thermo Finnigan). The reference material B2207 (silver phosphate, Elemental Microanalysis) has an accepted value of 21.70‰, and the reproducibility of B2207 during this set of analyses was better than ±0.15 (1σ). All δ18O analyses were performed in triplicate, and the average SD of the triplicates was ±0.15‰.
Ion microprobe analyses
In situ SIMS analyses were conducted at the Edinburgh Ion Microprobe Facility (EIMF). For SIMS analysis, specimens were embedded under pressure (2 bar) in epoxy resin within 5 mm of the center of the block and around a central Durango apatite standard. Sample blocks were prepared using diamond grinding compounds, followed by diamond and alumina polishing compounds. Sample blocks were treated for 24 hours with H2O2 to remove organic matter from the embedded fossils. Surface reimpregnation, with minimal regrinding and polishing, was used to ensure a smooth and flat surface before gold coating for SIMS analysis.
SIMS analyses were made using the CAMECA IMS-1270 ion microprobe. A primary beam of Cs+ ions at ~5 nA was focused to a 30-μm-diameter spot on the sample block surface. Secondary ions were extracted at −10 kV, with 16O [~2 × 109 counts/s (cps)] and 18O (~4 × 106 cps) monitored simultaneously on dual Faraday cups (L’2 and H’2). Each analysis began with 50-s presputtering time, followed by automatic secondary beam and entrance slit centring, before data collection in two 10-cycle blocks. Each SIMS run began with 10 Durango analyses, followed by alternating analysis sets of five unknowns (samples) and five standards (Durango apatites), dropping to three standards when beam stability was good. Linear regressions were applied to each analysis run to correct for instrument drift. Mean external precision was derived from the SD of Durango analyses following linear regression corrections for long-term (session duration) drift. This value was reported for each unknown (sample) analysis and ranged from ±0.11 to 0.41‰. The Durango apatite standards were fragments of a larger crystal, supplied by EIMF, whose isotopic composition (δ18Ophos, +8.7‰) was independently verified by trisilver phosphate analysis at NIGF before this project began.
Climate modeling
We used the three-dimensionally coupled ocean-atmosphere FOAM version 1.5 (47) that was widely applied to deep time paleoclimate studies (43, 48, 55, 56). The atmospheric module is a parallelized version of the National Center for Atmospheric Research Community Climate Model 2 (CCM2), upgraded to include radiative and hydrologic physics from CCM3 version 3.2. We ran the atmospheric module with R15 spectral resolution (4.5° × 7.5°) and 18 vertical levels. The ocean module was the higher-resolution Ocean Model version 3, a 24-level z-coordinate ocean GCM giving 1.4° × 2.8° resolution on a regular longitude-latitude grid. The coupled model had no flux corrections, and its short turnaround time allowed millennial-scale integration.
We used the Cambrian continental configuration from BugPlates for 510 Ma ago (57). In the absence of land plants, the land surface was defined as a rocky desert (albedo, 0.24; modified by snow, if present). The solar luminosity was decreased by 4.3 % compared to its present value (1368 W m−2) (58), and orbital parameters were maintained constant to the present-day configuration. The pCO2 was fixed at 32 PALs (59), and concentrations of other greenhouse gases were kept to the present-day level so that the imposed radiative forcing must be considered a CO2 equivalent that may include some contribution of other greenhouse gases such as methane. The simulation was initialized using a warm ice-free ocean and a uniform salinity of 35‰. We integrated the model for 2000 years to reach deep-ocean equilibrium. During the last 100 years of the simulation, there was no apparent drift in the upper ocean and <0.01°C change in deep ocean (−3700 m) temperature. The last 50 years of the model run were used to build the climatology files used for analysis.
Supplementary Material
Acknowledgments
We are indebted to J. Craven and M. Hall (EIMF) for the assistance with SIMS sample preparation and analysis. We thank L. Neep and K. Johnson [British Geological Survey (BGS)] for the assistance with microfossil processing. P.R.W. publishes by permission of the Director of the BGS. We thank A. Sluijs and an anonymous reviewer for the constructive comments that improved the clarity of this manuscript. Funding: T.W.H. is funded by NERC studentship NE/L0022493/1 within the Central England NERC Training Alliance (CENTA) consortium, with CASE partnership funding from the BGS (BUFI S266). Oxygen isotope analyses were supported by the NIGF (IP-1530-0515 and IP-1667-1116) and the EIMF (IMF 567-1015). Climate modeling was supported by the Le Commissariat à l’énergie atomique et aux énergies alternatives/Centre de calcul pour la recherche et la technologie (CEA/CCRT) who provided access to the high-performance computing resources of Très Grand Centre de calcul du CEA [TGCC; allocation 2014-012212 by Grand Équipement National de Calcul Intensif (GENCI)]. Author contributions: M.W., T.H.P.H., P.R.W., and T.W.H. designed the study. A.L.L. and T.W.H. conducted the bulk isotope analyses. T.W.H. conducted the SIMS analyses. T.W.H. made the base maps for the climate model simulations. T.W.H., M.W., A.P., and Y.D. discussed the model boundary conditions. A.P. ran the climate simulations and extracted the results. T.W.H., T.H.P.H., and M.W. wrote the manuscript, with input from all authors. All authors discussed the data. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/5/eaar5690/DC1
fig. S1. Paleogeographic and stratigraphic setting of the Comley Limestones (Avalonia, Cambrian Series 2).
fig. S2. Examples of pristine and altered brachiopod and Torellella specimens.
fig. S3. Box plots of ion microprobe (SIMS) data collected from pristine linguliformean brachiopods by tissue sampled.
fig. S4. Global SST contour plots produced by early Cambrian FOAM GCM simulations for CO2-equivalent forcing of 32 PALs (see Materials and Methods).
data S1. Triplicate trisilver phosphate isotope measurements.
data S2. Processed ion microprobe (SIMS) data.
data S3. Paleozoic phosphate δ18O data used to produce Fig. 3.
data S4. Paleotemperature data used to produce Fig. 4.
REFERENCES AND NOTES
- 1.E. L. Grossman, Oxygen isotope stratigraphy, in The Geologic Time Scale, F. M. Gradstein, J. G. Ogg, M. Schmitz, Eds. (Elsevier, 2012), pp. 181–206. [Google Scholar]
- 2.Veizer J. , Ala D. , Azmy K. , Bruckschen P. , Buhl D. , Bruhn F. , Carden G. A. F. , Diener A. , Ebneth S. , Godderis Y. , Jasper T. , Korte C. , Pawellek F. , Podlaha O. G. , Strauss H. , 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem. Geol. 161, 59–88 (1999). [Google Scholar]
- 3.Zachos J. , Pagani M. , Sloan L. , Thomas E. , Billups K. , Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Veizer J. , Prokoph A. , Temperatures and oxygen isotopic composition of Phanerozoic oceans. Earth Sci. Rev. 146, 92–104 (2015). [Google Scholar]
- 5.Bassett D. , Macleod K. G. , Miller J. F. , Ethington R. L. , Oxygen isotopic composition of biogenic phosphate and the temperature of Early Ordovician seawater. PALAIOS 22, 98–103 (2007). [Google Scholar]
- 6.Trotter J. A. , Williams I. S. , Barnes C. R. , Lécuyer C. , Nicoll R. S. , Did cooling oceans trigger Ordovician biodiversification? Evidence from conodont thermometry. Science 321, 550–554 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Pucéat E. , Joachimski M. M. , Bouilloux A. , Monna F. , Bonin A. , Motreuil S. , Morinière P. , Hénard S. , Mourin J. , Dera G. , Quesne D. , Revised phosphate–water fractionation equation reassessing paleotemperatures derived from biogenic apatite. Earth Planet. Sci. Lett. 298, 135–142 (2010). [Google Scholar]
- 8.D. H. Erwin, J. W. Valentine,The Cambrian Explosion: The Reconstruction of Animal Biodiversity (Roberts & Co., ed. 1, 2013). [Google Scholar]
- 9.Wheeley J. R. , Smith M. P. , Boomer I. , Oxygen isotope variability in conodonts: Implications for reconstructing Palaeozoic palaeoclimates and palaeoceanography. J. Geol. Soc. 169, 239–250 (2012). [Google Scholar]
- 10.A. J. Boucot, C. Xu, C. R. Scotese, R. J. Morley, SEPM Concepts in Sedimentology and Paleontology. Phanerozoic Paleoclimate: An Atlas of Lithologic Indicators of Climate (Society for Sedimentary Geology, ed. 1, 2013). [Google Scholar]
- 11.Peters S. E. , Gaines R. R. , Formation of the ‘Great Unconformity’ as a trigger for the Cambrian explosion. Nature 484, 363–366 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Porter S. M. , Closing the phosphatization window: Testing for the influence of taphonomic megabias on the pattern of small shelly fossil decline. PALAIOS 19, 178–183 (2004). [Google Scholar]
- 13.Brasier M. D. , Phosphogenic events and skeletal preservation across the Precambrian-Cambrian boundary interval. Geol. Soc. Lond. Spec. Publ. 52, 289–303 (1990). [Google Scholar]
- 14.Siveter D. J. , Williams M. , Waloszek D. , A phosphatocopid crustacean with appendages from the Lower Cambrian. Science 293, 474–479 (2001). [DOI] [PubMed] [Google Scholar]
- 15.A. W. A. Rushton, The Cambrian of Wales and England, in Cambrian of the British Isles, Norden and Spitzbergen, C. H. Holland, Ed. (John Wiley & Sons Ltd, 1974), pp. 43–122. [Google Scholar]
- 16.T. H. Torsvik, L. R. M. Cocks,Earth History and Palaeogeography (Cambridge Univ. Press, 2016). [Google Scholar]
- 17.Harvey T. H. P. , Williams M. , Condon D. J. , Wilby P. R. , Siveter D. J. , Rushton A. W. A. , Leng M. J. , Gabbott S. E. , A refined chronology for the Cambrian succession of southern Britain. J. Geol. Soc. 168, 705–716 (2011). [Google Scholar]
- 18.Schmahl W. W. , Griesshaber E. , Merkel C. , Kelm K. , Deuschle J. , Neuser R. D. , Göetz A. J. , Sehrbrock A. , Mader W. , Hierarchical fibre composite structure and micromechanical properties of phosphatic and calcitic brachiopod shell biomaterials—An overview. Mineral. Mag. 72, 541–562 (2008). [Google Scholar]
- 19.A. Williams, Shell structure, inTreatise on Invertebrate Paleontology. Part H. Brachiopoda (Revised 1: Introduction), R. L. Kaesler, Ed. (Paleontological Institute, 1997), vol. 1. [Google Scholar]
- 20.Kolodny Y. , Luz B. , Navon O. , Oxygen isotope variations in phosphate of biogenic apatites, I. Fish bone apatite—Rechecking the rules of the game. Earth Planet. Sci. Lett. 64, 398–404 (1983). [Google Scholar]
- 21.Lécuyer C. , Grandjean P. , Emig C. C. , Determination of oxygen isotope fractionation between water and phosphate from living lingulids: Potential application to palaeoenvironmental studies. Palaeogeogr. Palaeoclim. Palaeoecol. 126, 101–108 (1996). [Google Scholar]
- 22.Lécuyer C. , Amiot R. , Touzeau A. , Trotter J. , Calibration of the phosphate δ18O thermometer with carbonate–water oxygen isotope fractionation equations. Chem. Geol. 347, 217–226 (2013). [Google Scholar]
- 23.P. N. Pearson, Oxygen isotopes in foraminifera: Overview and historical review, in Reconstructing Earth’s Deep Time Climate—The State of the Art in 2012, Paleontological Society Short Course, November 3, 2012The Paleontological Society Papers (The Paleontological Society, 2012), vol. 18, pp. 1–38. [Google Scholar]
- 24.Gregory R. T. , Taylor H. P. Jr., An oxygen isotope profile in a section of Cretaceous oceanic crust, Samail Ophiolite, Oman: Evidence for δ18O buffering of the oceans by deep (>5 km) seawater-hydrothermal circulation at mid-ocean ridges. J. Geophys. Res. Solid Earth 86, 2737–2755 (1981). [Google Scholar]
- 25.Came R. E. , Eiler J. M. , Veizer J. , Azmy K. , Brand U. , Weidman C. R. , Coupling of surface temperatures and atmospheric CO2 concentrations during the Palaeozoic era. Nature 449, 198–201 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Finnegan S. , Bergmann K. , Eiler J. M. , Jones D. S. , Fike D. A. , Eisenman I. , Hughes N. C. , Tripati A. K. , Fischer W. W. , The magnitude and duration of late Ordovician–early silurian glaciation. Science 331, 903–906 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Cummins R. C. , Finnegan S. , Fike D. A. , Eiler J. M. , Fischer W. W. , Carbonate clumped isotope constraints on Silurian ocean temperature and seawater δ18O. Geochim. Cosmochim. Acta 140, 241–258 (2014). [Google Scholar]
- 28.Bergmann K. D. , Finnegan S. , Creel R. , Eiler J. M. , Hughes N. C. , Popov L. E. , A paired apatite and calcite clumped isotope thermometry approach to estimating Cambro-Ordovician seawater temperatures and isotopic composition. Geochim. Cosmochim. Acta 224, 18–41 (2018). [Google Scholar]
- 29.Prokoph A. , Shields G. A. , Veizer J. , Compilation and time-series analysis of a marine carbonate δ18O, δ13C,87Sr/86Sr and δ34S database through Earth history. Earth Sci. Rev. 87, 113–133 (2008). [Google Scholar]
- 30.Giles P. S. , Low-latitude Ordovician to Triassic brachiopod habitat temperatures (BHTs) determined from δ18O[brachiopod calcite]: A cold hard look at ice-house tropical oceans. Palaeogeogr. Palaeoclim. Palaeoecol. 317–318, 134–152 (2012). [Google Scholar]
- 31.Kasting J. F. , Howard M. T. , Wallmann K. , Veizer J. , Shields G. , Jaffrés J. , Paleoclimates, ocean depth, and the oxygen isotopic composition of seawater. Earth Planet. Sci. Lett. 252, 82–93 (2006). [Google Scholar]
- 32.Jaffrés J. B. D. , Shields G. A. , Wallmann K. , The oxygen isotope evolution of seawater: A critical review of a long-standing controversy and an improved geological water cycle model for the past 3.4 billion years. Earth Sci. Rev. 83, 83–122 (2007). [Google Scholar]
- 33.Joachimski M. M. , Breisig S. , Buggisch W. , Talent J. A. , Mawson R. , Gereke M. , Morrow J. R. , Day J. , Weddige K. , Devonian climate and reef evolution: Insights from oxygen isotopes in apatite. Earth Planet. Sci. Lett. 284, 599–609 (2009). [Google Scholar]
- 34.LeGrande A. N. , Schmidt G. A. , Global gridded data set of the oxygen isotopic composition in seawater. Geophys. Res. Lett. 33, L12604 (2006). [Google Scholar]
- 35.G. A. Schmidt, G. R. Bigg, E. J. Rohling, Global Seawater Oxygen-18 Database - v1.21 (1999);https://data.giss.nasa.gov/o18data/.
- 36.Tindall J. , Flecker R. , Valdes P. , Schmidt D. N. , Markwick P. , Harris J. , Modelling the oxygen isotope distribution of ancient seawater using a coupled ocean–atmosphere GCM: Implications for reconstructing early Eocene climate. Earth Planet. Sci. Lett. 292, 265–273 (2010). [Google Scholar]
- 37.Roberts C. D. , LeGrande A. N. , Tripati A. K. , Sensitivity of seawater oxygen isotopes to climatic and tectonic boundary conditions in an early Paleogene simulation with GISS ModelE-R. Paleoceanography 26, PA4203 (2011). [Google Scholar]
- 38.Suan G. , Popescu S.-M. , Suc J.-P. , Schnyder J. , Fauquette S. , Baudin F. , Yoon D. , Piepjohn K. , Sobolev N. N. , Labrousse L. , Subtropical climate conditions and mangrove growth in Arctic Siberia during the early Eocene. Geology 45, 539–542 (2017). [Google Scholar]
- 39.O’Brien C. L. , Robinson S. A. , Pancost R. D. , Damsté J. S. S. , Schouten S. , Lunt D. J. , Alsenz H. , Bornemann A. , Bottini C. , Brassell S. C. , Farnsworth A. , Forster A. , Huber B. T. , Inglis G. N. , Jenkyns H. C. , Linnert C. , Littler K. , Markwick P. , Wrobel N. E. , Cretaceous sea-surface temperature evolution: Constraints from TEX86 and planktonic foraminiferal oxygen isotopes. Earth Sci. Rev. 172, 224–247 (2017). [Google Scholar]
- 40.Frieling J. , Gebhardt H. , Huber M. , Adekeye O. A. , Akande S. O. , Reichart G.-J. , Middelburg J. J. , Schouten S. , Sluijs A. , Extreme warmth and heat-stressed plankton in the tropics during the Paleocene-Eocene Thermal Maximum. Sci. Adv. 3, e1600891 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans D. , Sagoo N. , Renema W. , Cotton L. J. , Müller W. , Todd J. A. , Saraswati P. K. , Stassen P. , Ziegler M. , Pearson P. N. , Valdes P. J. , Affek H. P. , Eocene greenhouse climate revealed by coupled clumped isotope-Mg/Ca thermometry. Proc. Natl. Acad. Sci. U.S.A. 201714744 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen K. D. T. , Morley S. A. , Lai C.-H. , Clark M. S. , Tan K. S. , Bates A. E. , Peck L. S. , Upper temperature limits of tropical marine ectotherms: Global warming implications. PLOS ONE 6, e29340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nardin E. , Goddéris Y. , Donnadieu Y. , Le Hir G. , Blakey R. C. , Pucéat E. , Aretz M. , Modeling the early Paleozoic long-term climatic trend. Geol. Soc. Am. Bull. 123, 1181–1192 (2011). [Google Scholar]
- 44.Avigad D. , Sandler A. , Kolodner K. , Stern R. J. , McWilliams M. , Miller N. , Beyth M. , Mass-production of Cambro–Ordovician quartz-rich sandstone as a consequence of chemical weathering of Pan-African terranes: Environmental implications. Earth Planet. Sci. Lett. 240, 818–826 (2005). [Google Scholar]
- 45.Lindström M. , Cold age sediment in lower Cambrian of South Sweden. Geol. Paleontol. 6, 9–23 (1972). [Google Scholar]
- 46.Landing E. , MacGabhann B. A. , First evidence for Cambrian glaciation provided by sections in Avalonian New Brunswick and Ireland: Additional data for Avalon–Gondwana separation by the earliest Palaeozoic. Palaeogeogr. Palaeoclimatol. Palaeoecol. 285, 174–185 (2010). [Google Scholar]
- 47.R. L. Jacob, “Low frequency variability in a simulated atmosphere-ocean system,” thesis, The University of Wisconsin (1997), 170 pp. [Google Scholar]
- 48.Pohl A. , Donnadieu Y. , Le Hir G. , Buoncristiani J.-F. , Vennin E. , Effect of the Ordovician paleogeography on the (in)stability of the climate. Clim. Past 10, 2053–2066 (2014). [Google Scholar]
- 49.Royer D. L. , Donnadieu Y. , Park J. , Kowalczyk J. , Goddéris Y. , Error analysis of CO2 and O2 estimates from the long-term geochemical model GEOCARBSULF. Am. J. Sci. 314, 1259–1283 (2014). [Google Scholar]
- 50.P. J. Brenchley, A. W. A. Rushton, M. Howells, R. Cave, Cambrian and Ordovician: The early Palaeozoic tectonostratigraphic evolution of the Welsh Basin, Midland and Monian Terranes of Eastern Avalonia, in The Geology of England and Wales, P. J. Brenchley, P. F. Rawson, Eds. (The Geological Society, ed. 2, 2006), pp. 25–74. [Google Scholar]
- 51.Jeppsson L. , Anehus R. , Fredholm D. , The optimal acetate buffered acetic acid technique for extracting phosphatic fossils. J. Paleontol. 73, 964–972 (1999). [Google Scholar]
- 52.Blake R. E. , O’Neil J. R. , Garcia G. A. , Oxygen isotope systematics of biologically mediated reactions of phosphate: I. Microbial degradation of organophosphorus compounds. Geochim. Cosmochim. Acta 61, 4411–4422 (1997). [Google Scholar]
- 53.O’Neil J. R. , Roe L. J. , Reinhard E. , Blake R. E. , A rapid and precise method of oxygen isotope analysis of biogenic phosphate. Israel J. Earth Sci. 43, 203–212 (1994). [Google Scholar]
- 54.Vennemann T. W. , Fricke H. C. , Blake R. E. , O’Neil J. R. , Colman A. , Oxygen isotope analysis of phosphates: A comparison of techniques for analysis of Ag3PO4. Chem. Geol. 185, 321–336 (2002). [Google Scholar]
- 55.Poulsen C. J. , Jacob R. L. , Factors that inhibit snowball Earth simulation. Paleoceanography 19, PA4021 (2004). [Google Scholar]
- 56.Donnadieu Y. , Goddéris Y. , Bouttes N. , Exploring the climatic impact of the continental vegetation on the Mezosoic atmospheric CO2 and climate history. Clim. Past 5, 85–96 (2009). [Google Scholar]
- 57.T. H. Torsvik, L. R. M. Cocks, BugPlates: Linking Biogeography and Palaeogeography (Centre for Geodymanics, 2009); www.geodynamics.no.
- 58.Gough D. O. , Solar interior structure and luminosity variations. Sol. Phys. 74, 21–34 (1981). [Google Scholar]
- 59.Berner R. A. , Inclusion of the weathering of volcanic rocks in the GEOCARBSULF model. Am. J. Sci. 306, 295–302 (2006). [Google Scholar]
- 60.R. A. Locarnini, A. V. Mishonov, J. I. Antonov, T. P. Boyer, H. E. Garcia, O. K. Baranova, M. M. Zweng, C. R. Paver, J. R. Reagan, D. R. Johnson, M. Hamilton, D. Seidov, World Ocean Atlas 2013, Volume 1: Temperature (NOAA Atlas NESDIS, 2013). [Google Scholar]
- 61.M. R. Saltzman, E. Thomas, Carbon Isotope Stratigraphy, in The Geologic Time Scale, F. M. Gradstein, J. G. Ogg, M. D. Schmitz, G. M. Ogg, Eds. (Elsevier, 2012), pp. 207–232. [Google Scholar]
- 62.S. Peng, L. E. Babcock, R. A. Cooper, The Cambrian Period, in The Geologic Time Scale, F. M. Gradstein, J. G. Ogg, M. D. Schmitz, G. M. Ogg, Eds. (Elsevier, ed. 1, 2012), vol. 2, pp. 437–488. [Google Scholar]
- 63.Wenzel B. , Lécuyer C. , Joachimski M. M. , Comparing oxygen isotope records of silurian calcite and phosphate—δ18O compositions of brachiopods and conodonts. Geochim. Cosmochim. Acta 64, 1859–1872 (2000). [Google Scholar]
- 64.Y. Kolodny, B. Luz, Oxygen isotopes in phosphates of fossil fish; Devonian to Recent, in Stable Isotope Geochemistry: A Tribute to Samuel Epstein, H. P. Taylor Jr., J. R. O’Neil, I. R. Kaplan, Eds. (The Geochemical Society, Special Publication, 1991), pp. 105–119. [Google Scholar]
- 65.Barham M. , Murray J. , Joachimski M. M. , Williams D. M. , The onset of the Permo-Carboniferous glaciation: Reconciling global stratigraphic evidence with biogenic apatite δ18O records in the late Visean. J. Geol. Soc. 169, 119–122 (2012). [Google Scholar]
- 66.Chen B. , Joachimski M. M. , Wang X.-d. , Shen S.-z. , Qi Y.-p. , Qie W.-k. , Ice volume and paleoclimate history of the Late Paleozoic Ice Age from conodont apatite oxygen isotopes from Naqing (Guizhou, China). Palaeogeogr. Palaeoclimatol. Palaeoecol. 448, 151–161 (2016). [Google Scholar]
- 67.Joachimski M. M. , von Bitter P. H. , Buggisch W. , Constraints on Pennsylvanian glacioeustatic sea-level changes using oxygen isotopes of conodont apatite. Geology 34, 277–280 (2006). [Google Scholar]
- 68.Kaiser S. I. , Steuber T. , Becker R. T. , Joachimski M. M. , Geochemical evidence for major environmental change at the Devonian–Carboniferous boundary in the Carnic Alps and the Rhenish Massif. Palaeogeogr. Palaeoclimatol. Palaeoecol. 240, 146–160 (2006). [Google Scholar]
- 69.Korte C. , Kozur H. W. , Joachimski M. M. , Strauss H. , Veizer J. , Schwark L. , Carbon, sulfur, oxygen and strontium isotope records, organic geochemistry and biostratigraphy across the Permian/Triassic boundary in Abadeh, Iran. Int. J. Earth Sci. 93, 565–581 (2004). [Google Scholar]
- 70.Frieling J. , Reichart G.-J. , Middelburg J. J. , Röhl U. , Westerhold T. , Bohaty S. M. , Sluijs A. , Tropical Atlantic climate and ecosystem regime shifts during the Paleocene–Eocene Thermal Maximum. Clim. Past 14, 39–55 (2018). [Google Scholar]
- 71.Littler K. , Robinson S. A. , Bown P. R. , Nederbragt A. J. , Pancost R. D. , High sea-surface temperatures during the Early Cretaceous Epoch. Nat. Geosci. 4, 169–172 (2011). [Google Scholar]
- 72.Ando A. , Huber B. T. , MacLeod K. G. , Watkins D. K. , Early Cenomanian “hot greenhouse” revealed by oxygen isotope record of exceptionally well-preserved foraminifera from Tanzania. Paleoceanography 30, 1556–1572 (2015). [Google Scholar]
- 73.Ando A. , Huber B. T. , MacLeod K. G. , Ohta T. , Khim B.-K. , Blake Nose stable isotopic evidence against the mid-Cenomanian glaciation hypothesis. Geology 37, 451–454 (2009). [Google Scholar]
- 74.Aze T. , Pearson P. N. , Dickson A. J. , Badger M. P. S. , Bown P. R. , Pancost R. D. , Gibbs S. J. , Huber B. T. , Leng M. J. , Coe A. L. , Cohen A. S. , Foster G. L. , Extreme warming of tropical waters during the Paleocene–Eocene Thermal Maximum. Geology 42, 739–742 (2014). [Google Scholar]
- 75.Bemis B. E. , Spero H. J. , Bijma J. , Lea D. W. , Reevaluation of the oxygen isotopic composition of planktonic foraminifera: Experimental results and revised paleotemperature equations. Paleoceanography 13, 150–160 (1998). [Google Scholar]
- 76.K. L. Bice, R. D. Norris, Data Report: Stable Isotope Ratios of Foraminifers from ODP Leg 207, Sites 1257, 1258, and 1260, and a Cleaning Procedure for Foraminifers in Organic-Rich Shales (Ocean Drilling Program, 2005); www-odp.tamu.edu/publications/207_SR/104/104.htm.
- 77.Bice K. L. , Huber B. T. , Norris R. D. , Extreme polar warmth during the Cretaceous greenhouse? Paradox of the late Turonian δ18O record at Deep Sea Drilling Project Site 511. Paleoceanography 18, 1031 (2003). [Google Scholar]
- 78.Bijl P. K. , Schouten S. , Sluijs A. , Reichart G.-J. , Zachos J. C. , Brinkhuis H. , Early Palaeogene temperature evolution of the southwest Pacific Ocean. Nature 461, 776–779 (2009). [DOI] [PubMed] [Google Scholar]
- 79.Bornemann A. , Norris R. D. , Friedrich O. , Beckmann B. , Schouten S. , Damsté J. S. , Vogel J. , Hofmann P. , Wagner T. , Isotopic evidence for glaciation during the cretaceous supergreenhouse. Science 319, 189–192 (2008). [DOI] [PubMed] [Google Scholar]
- 80.Bown P. , Pearson P. , Calcareous plankton evolution and the Paleocene/Eocene thermal maximum event: New evidence from Tanzania. Mar. Micropaleontol. 71, 60–70 (2009). [Google Scholar]
- 81.Douglas P. M. J. , Affek H. P. , Ivany L. C. , Houben A. J. P. , Sijp W. P. , Sluijs A. , Schouten S. , Pagani M. , Pronounced zonal heterogeneity in Eocene southern high-latitude sea surface temperatures. Proc. Natl. Acad. Sci. U.S.A. 111, 6582–6587 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dunkley Jones T. , Lunt D. J. , Schmidt D. N. , Ridgwell A. , Sluijs A. , Valdes P. J. , Maslin M. , Climate model and proxy data constraints on ocean warming across the Paleocene–Eocene Thermal Maximum. Earth Sci. Rev. 125, 123–145 (2013). [Google Scholar]
- 83.Falzoni F. , Petrizzo M. R. , Clarke L. J. , MacLeod K. G. , Jenkyns H. C. , Long-term Late Cretaceous oxygen- and carbon-isotope trends and planktonic foraminiferal turnover: A new record from the southern midlatitudes. Geol. Soc. Am. Bull. 128, 1725–1735 (2016). [Google Scholar]
- 84.Fauquette S. , Guiot J. , Suc J.-P. , A method for climatic reconstruction of the Mediterranean Pliocene using pollen data. Palaeogeogr. Palaeoclimatol. Palaeoecol. 144, 183–201 (1998). [Google Scholar]
- 85.Forster A. , Schouten S. , Baas M. , Sinninghe Damsté J. S. , Mid-Cretaceous (Albian–Santonian) sea surface temperature record of the tropical Atlantic Ocean. Geology 35, 919–922 (2007). [Google Scholar]
- 86.Forster A. , Schouten S. , Moriya K. , Wilson P. A. , Sinninghe Damsté J. S. , Tropical warming and intermittent cooling during the Cenomanian/Turonian oceanic anoxic event 2: Sea surface temperature records from the equatorial Atlantic. Paleoceanography 22, PA1219 (2007). [Google Scholar]
- 87.Friedrich O. , Erbacher J. , Moriya K. , Wilson P. A. , Kuhnert H. , Warm saline intermediate waters in the Cretaceous tropical Atlantic Ocean. Nat. Geosci. 1, 453–457 (2008). [Google Scholar]
- 88.Frieling J. , Iakovleva A. I. , Reichart G.-J. , Aleksandrova G. N. , Gnibidenko Z. N. , Schouten S. , Sluijs A. , Paleocene–Eocene warming and biotic response in the epicontinental West Siberian Sea. Geology 42, 767–770 (2014). [Google Scholar]
- 89.Hollis C. J. , Handley L. , Crouch E. M. , Morgans H. E. G. , Baker J. A. , Creech J. , Collins K. S. , Gibbs S. J. , Huber M. , Schouten S. , Zachos J. C. , Pancost R. D. , Tropical sea temperatures in the high-latitude South Pacific during the Eocene. Geology 37, 99–102 (2009). [Google Scholar]
- 90.Hollis C. J. , Hines B. R. , Littler K. , Villasante-Marcos V. , Kulhanek D. K. , Strong C. P. , Zachos J. C. , Eggins S. M. , Northcote L. , Phillips A. , The Paleocene–Eocene Thermal Maximum at DSDP Site 277, Campbell Plateau, southern Pacific Ocean. Clim. Past 11, 1009–1025 (2015). [Google Scholar]
- 91.Hollis C. J. , Taylor K. W. R. , Handley L. , Pancost R. D. , Huber M. , Creech J. B. , Hines B. R. , Crouch E. M. , Morgans H. E. G. , Crampton J. S. , Gibbs S. , Pearson P. N. , Zachos J. C. , Early Paleogene temperature history of the Southwest Pacific Ocean: Reconciling proxies and models. Earth Planet. Sci. Lett. 349–350, 53–66 (2012). [Google Scholar]
- 92.Huber B. T. , Hodell D. A. , Hamilton C. P. , Middle–Late Cretaceous climate of the southern high latitudes: Stable isotopic evidence for minimal equator-to-pole thermal gradients. Geol. Soc. Am. Bull. 107, 1164–1191 (1995). [Google Scholar]
- 93.Huber B. T. , Norris R. D. , MacLeod K. G. , Deep-sea paleotemperature record of extreme warmth during the Cretaceous. Geology 30, 123–126 (2002). [Google Scholar]
- 94.Jenkyns H. C. , Schouten-Huibers L. , Schouten S. , Sinninghe Damsté J. S. , Warm Middle Jurassic–Early Cretaceous high-latitude sea-surface temperatures from the Southern Ocean. Clim. Past 8, 215–226 (2012). [Google Scholar]
- 95.John C. M. , Bohaty S. M. , Zachos J. C. , Sluijs A. , Gibbs S. , Brinkhuis H. , Bralower T. J. , North American continental margin records of the Paleocene-Eocene thermal maximum: Implications for global carbon and hydrological cycling. Paleoceanography 23, PA2217 (2008). [Google Scholar]
- 96.Keating-Bitonti C. R. , Ivany L. C. , Affek H. P. , Douglas P. , Samson S. D. , Warm, not super-hot, temperatures in the early Eocene subtropics. Geology 39, 771–774 (2011). [Google Scholar]
- 97.Kelly D. C. , Bralower T. J. , Zachos J. C. , Evolutionary consequences of the latest Paleocene thermal maximum for tropical planktonic foraminifera. Palaeogeogr. Palaeoclimatol. Palaeoecol. 141, 139–161 (1998). [Google Scholar]
- 98.Kelly D. C. , Bralower T. J. , Zachos J. C. , Silva I. P. , Thomas E. , Rapid diversification of planktonic foraminifera in the tropical Pacific (ODP Site 865) during the late Paleocene thermal maximum. Geology 24, 423–426 (1996). [Google Scholar]
- 99.Kennett J. P. , Stott L. D. , Abrupt deep-sea warming, palaeoceanographic changes and benthic extinctions at the end of the Palaeocene. Nature 353, 225–229 (1991). [Google Scholar]
- 100.Kim J.-H. , van der Meer J. , Schouten S. , Helmke P. , Willmott V. , Sangiorgi F. , Koç N. , Hopmans E. C. , Sinninghe Damsté J. S. , New indices and calibrations derived from the distribution of crenarchaeal isoprenoid tetraether lipids: Implications for past sea surface temperature reconstructions. Geochim. Cosmochim. Acta 74, 4639–4654 (2010). [Google Scholar]
- 101.Kim S.-T. , O’Neil J. R. , Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates. Geochim. Cosmochim. Acta 61, 3461–3475 (1997). [Google Scholar]
- 102.Kozdon R. , Kelly D. C. , Kita N. T. , Fournelle J. H. , Valley J. W. , Planktonic foraminiferal oxygen isotope analysis by ion microprobe technique suggests warm tropical sea surface temperatures during the Early Paleogene. Paleoceanography 26, PA3206 (2011). [Google Scholar]
- 103.MacLeod K. G. , Huber B. T. , Berrocoso Á. J. , Wendler I. , A stable and hot Turonian without glacial δ18O excursions is indicated by exquisitely preserved Tanzanian foraminifera. Geology 41, 1083–1086 (2013). [Google Scholar]
- 104.Moriya K. , Wilson P. A. , Friedrich O. , Erbacher J. , Kawahata H. , Testing for ice sheets during the mid-Cretaceous greenhouse using glassy foraminiferal calcite from the mid-Cenomanian tropics on Demerara Rise. Geology 35, 615–618 (2007). [Google Scholar]
- 105.Pearson P. N. , van Dongen B. E. , Nicholas C. J. , Pancost R. D. , Schouten S. , Singano J. M. , Wade B. S. , Stable warm tropical climate through the Eocene Epoch. Geology 35, 211–214 (2007). [Google Scholar]
- 106.Petersen S. V. , Schrag D. P. , Antarctic ice growth before and after the Eocene-Oligocene transition: New estimates from clumped isotope paleothermometry. Paleoceanography 30, 1305–1317 (2015). [Google Scholar]
- 107.Petrizzo M. R. , Huber B. T. , Wilson P. A. , MacLeod K. G. , Late Albian paleoceanography of the western subtropical North Atlantic. Paleoceanography 23, PA1213 (2008). [Google Scholar]
- 108.Schoon P. L. , Heilmann-Clausen C. , Schultz B. P. , Sinninghe Damsté J. S. , Schouten S. , Warming and environmental changes in the eastern North Sea Basin during the Palaeocene–Eocene Thermal Maximum as revealed by biomarker lipids. Org. Geochem. 78, 79–88 (2015). [Google Scholar]
- 109.Schouten S. , Hopmans E. C. , Forster A. , van Breugel Y. , Kuypers M. M. M. , Sinninghe Damsté J. S. , Extremely high sea-surface temperatures at low latitudes during the middle Cretaceous as revealed by archaeal membrane lipids. Geology 31, 1069–1072 (2003). [Google Scholar]
- 110.Sinninghe Damsté J. S. , van Bentum E. C. , Reichart G.-J. , Pross J. , Schouten S. , A CO2 decrease-driven cooling and increased latitudinal temperature gradient during the mid-Cretaceous Oceanic Anoxic Event 2. Earth Planet. Sci. Lett. 293, 97–103 (2010). [Google Scholar]
- 111.Sluijs A. , Bijl P. K. , Schouten S. , Röhl U. , Reichart G.-J. , Brinkhuis H. , Southern ocean warming, sea level and hydrological change during the Paleocene-Eocene thermal maximum. Clim. Past 7, 47–61 (2011). [Google Scholar]
- 112.Sluijs A. , Brinkhuis H. , Schouten S. , Bohaty S. M. , John C. M. , Zachos J. C. , Reichart G.-J. , Sinninghe Damsté J. S. , Crouch E. M. , Dickens G. R. , Environmental precursors to rapid light carbon injection at the Palaeocene/Eocene boundary. Nature 450, 1218–1221 (2007). [DOI] [PubMed] [Google Scholar]
- 113.Sluijs A. , Schouten S. , Pagani M. , Woltering M. , Brinkhuis H. , Sinninghe Damsté J. S. , Dickens G. R. , Huber M. , Reichart G.-J. , Stein R. , Matthiessen J. , Lourens L. J. , Pedentchouk N. , Backman J. , Moran K. Expedition 302 Scientists , Subtropical Arctic Ocean temperatures during the Palaeocene/Eocene thermal maximum. Nature 441, 610–613 (2006). [DOI] [PubMed] [Google Scholar]
- 114.Sluijs A. , Schouten S. , Donders T. H. , Schoon P. L. , Röhl U. , Reichart G.-J. , Sangiorgi F. , Kim J.-H. , Sinninghe Damsté J. S. , Brinkhuis H. , Warm and wet conditions in the Arctic region during Eocene Thermal Maximum 2. Nat. Geosci. 2, 777–780 (2009). [Google Scholar]
- 115.Thomas D. J. , Bralower T. J. , Zachos J. C. , New evidence for subtropical warming during the Late Paleocene thermal maximum: Stable isotopes from Deep Sea Drilling Project Site 527, Walvis Ridge. Paleoceanography 14, 561–570 (1999). [Google Scholar]
- 116.Thomas D. J. , Zachos J. C. , Bralower T. J. , Thomas E. , Bohaty S. , Warming the fuel for the fire: Evidence for the thermal dissociation of methane hydrate during the Paleocene-Eocene thermal maximum. Geology 30, 1067–1070 (2002). [Google Scholar]
- 117.Tripati A. K. , Elderfield H. , Abrupt hydrographic changes in the equatorial Pacific and subtropical Atlantic from foraminiferal Mg/Ca indicate greenhouse origin for the thermal maximum at the Paleocene-Eocene Boundary. Geochem. Geophys. Geosyst. 5, Q02006 (2004). [Google Scholar]
- 118.van Helmond N. A. G. M. , Sluijs A. , Reichart G.-J. , Sinninghe Damsté J. S. , Slomp C. P. , Brinkhuis H. , A perturbed hydrological cycle during Oceanic Anoxic Event 2. Geology 42, 123–126 (2014). [Google Scholar]
- 119.van Helmond N. A. G. M. , Sluijs A. , Sinninghe Damsté J. S. , Reichart G.-J. , Voigt S. , Erbacher J. , Pross J. , Brinkhuis H. , Freshwater discharge controlled deposition of Cenomanian–Turonian black shales on the NW European epicontinental shelf (Wunstorf, northern Germany). Clim. Past 11, 495–508 (2015). [Google Scholar]
- 120.Yamaguchi T. , Norris R. D. , No place to retreat: Heavy extinction and delayed recovery on a Pacific guyot during the Paleocene–Eocene Thermal Maximum. Geology 43, 443–446 (2015). [Google Scholar]
- 121.Zaarur S. , Affek H. P. , Brandon M. T. , A revised calibration of the clumped isotope thermometer. Earth Planet. Sci. Lett. 382, 47–57 (2013). [Google Scholar]
- 122.Zachos J. C. , Schouten S. , Bohaty S. , Quattlebaum T. , Sluijs A. , Brinkhuis H. , Gibbs S. J. , Bralower T. J. , Extreme warming of mid-latitude coastal ocean during the Paleocene-Eocene Thermal Maximum: Inferences from TEX86 and isotope data. Geology 34, 737–740 (2006). [Google Scholar]
- 123.Zachos J. C. , Wara M. W. , Bohaty S. , Delaney M. L. , Petrizzo M. R. , Brill A. , Bralower T. J. , Premoli-Silva I. , A transient rise in tropical sea surface temperature during the paleocene-eocene thermal maximum. Science 302, 1551–1554 (2003). [DOI] [PubMed] [Google Scholar]
- 124.Zachos J. C. , Stott L. D. , Lohmann K. C. , Evolution of Early Cenozoic marine temperatures. Paleoceanography 9, 353–387 (1994). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/5/eaar5690/DC1
fig. S1. Paleogeographic and stratigraphic setting of the Comley Limestones (Avalonia, Cambrian Series 2).
fig. S2. Examples of pristine and altered brachiopod and Torellella specimens.
fig. S3. Box plots of ion microprobe (SIMS) data collected from pristine linguliformean brachiopods by tissue sampled.
fig. S4. Global SST contour plots produced by early Cambrian FOAM GCM simulations for CO2-equivalent forcing of 32 PALs (see Materials and Methods).
data S1. Triplicate trisilver phosphate isotope measurements.
data S2. Processed ion microprobe (SIMS) data.
data S3. Paleozoic phosphate δ18O data used to produce Fig. 3.
data S4. Paleotemperature data used to produce Fig. 4.


