Abstract
Background
The objective of this study was to investigate the predictive power of a high-sensitivity cardiac troponin I (hs-cTnI) assay for cardiovascular events and mortality in a large population of older community dwellers.
Methods
Blood was collected from 5764 individuals (age 66–98 years) during the period of 2002–2006 and the outcome as to all-cause death and incidence of cardiovascular disease (CVD) and coronary heart disease (CHD) followed up to 10 years. hs-cTnI (Abbott) was measured in serum to assess the association of this marker with CVD, CHD and death and finally to compare the results with conventional risk factors by multivariable statistical analysis.
Results
The median (IQR) concentrations of hs-cTnI were 8.4 ng/L (5.6–14.2 ng/L) and 5.3 ng/L (3.8–8.1 ng/L) in men (2416) and women (3275), respectively, and the concentrations increased linearly with age. Outcomes as to all-cause death and incidence of CVD and CHD were significantly associated with increasing concentrations of hs-cTnI beginning well below the 99th percentile concentrations. The associations to outcome remained after adjustments for conventional risk factors and were similar in men and women.
Conclusions
Our findings suggest that hs-cTnI reflects the status of the myocardium even in seemingly healthy individuals and that the measurements of hs-cTnI may be useful for primary prediction of heart disease; this should form the basis for future prospective clinical trials for determining whether measuring hs-cTnI can be used in the prevention of CVD/CHD.
Keywords: Cardio-vascular disease, Coronary heart disease, Biomarker, Troponin, Prediction
Introduction
The measurement of cardiac troponins in blood is central in the diagnosis of acute coronary syndromes. The 99th percentile upper reference limits of these biomarkers are key elements of the Third Universal Definition of Myocardial Infarction (MI)(1). Cardiac troponins are increased in serum not only due to acute myocardial injuries, but also in non-cardiac conditions such as pulmonary embolism, sepsis, chronic renal failure, ischemic stroke and vigorous exercise (2). The high-sensitivity cardiac troponin I (hs-cTnI) assays recently introduced allow for the measurement of cTnI in almost all healthy individuals (3). The increased sensitivity of the assays also should allow for the earlier detection of myocardial injury in patients with acute MI as well as the identification of patients at risk of major adverse cardiac events and premature deaths. Cardiac troponins were shown to have additional value beyond the traditional risk factors for prediction of mortality, heart failure and cardiovascular events in community-based cohorts. cTnT was measured in most of these studies (4–6), whereas there are fewer studies on cTnI (3;7).
The objectives of the present study of a large cohort of older community dwelling individuals were to assess the additional prognostic value of hs-cTnI for major adverse cardiac events and mortality above traditional risk factors.
Methods
Participants/study design
The Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-Reykjavik) cohort is a community based prospective study and has been described earlier(8). Physical, physiological and questionnaire examinations were conducted at three visits for each individual. An extensive data collection of various biological aspects, medical history, as well as recording of lifestyle factors was performed. The first wave of AGES-Reykjavik study was conducted during the years 2002–2006. The 5764 individuals included in the AGES-Reykjavik study were born between 1907 and 1935 and were selected randomly from the 11,549 survivors of the Reykjavik Study cohort, which was initiated in 1967 by the Icelandic Heart Association (9). The aim of the original study was to investigate prospectively risk factors for cardiovascular disease in the Icelandic population. 5706 individuals gave informed consent for access to hospital records and 5691 had valid hs-cTnI results and were included in this study. The AGES-Reykjavik study was approved by the National Bioethics Committee in Iceland that acts as the Institutional Review Board for the Icelandic Heart Association (approval number: VSN-00-063), by the National Institute on Aging Intramural Institutional Review Board and the Data Protection Authority in Iceland. Informed consent was obtained from all study participants.
Biochemical analysis, blood sampling/baseline examination
Blood samples were drawn after overnight fasting and centrifuged within 2h at room temperature. The serum samples were divided into aliquots and immediately frozen and stored at −80°C. The serum samples were shipped on dry ice to Uppsala in Sweden before analysis.
In the AGES-Reykjavik study, lipids, creatinine, high sensitive C-reactive protein (CRP) and glucose were analysed in serum using a Hitachi 912 analyzer (Roche Diagnostics) with reagents from Roche Diagnostics and following the manufacturer’s instructions. cTnI was measured using the cTnI assay from Abbott Diagnostics, ARCHITECT STAT high-sensitivity Troponin. The limit of detection was reported to be 1.2 ng/L. A 10% coefficient of variation was reported at a concentration of 8 ng/L and a 20% coefficient of variation at 2 ng/L, as confirmed in a recent study(10). Concentrations lower than the detection limit were assigned the value 1.2 ng/L. The 99th percentile concentrations of healthy men and women as indicated by the manufacturer were 34.2 and 15.6 ng/L, respectively. In the SWISCH cohort of healthy Scandinavian individuals the 99th percentile concentrations were found to be 24.2 (men) and 15.2 (women) ng/L(11).
Height and weight were measured, and BMI was calculated as kg/m2. Diabetes mellitus was defined as a fasting plasma glucose >126 mg/dL (>7.0 mmol/L) or self-reported history of diabetes or the use of insulin or oral glucose lowering drugs. Hypertension was defined as current hypertension with resting blood pressure >140/90 mmHg as the mean value of two consecutive blood pressure measurements with a mercury sphygmomanometer and a large cuff, or treatment with anti-hypertensive drugs. Smoking status was defined as non-smoker, previous smoker or current smoker and obtained from a questionnaire. Previous cardiovascular disease was defined as history of myocardial infarction, coronary heart disease, stroke, percutaneous transluminal coronary angioplasty (PTCA) or coronary artery bypass grafting (CABG) derived from hospital records, MI according to ECG, and bypass surgery determined from questionnaire, all occurring before entry to AGES.
Outcomes/follow up evaluation
The outcomes in this study included: all-cause death, incident coronary heart disease and incident cardiovascular disease. Information on all-cause death was recorded from a complete adjudicated registry of deaths available from the national mortality register of Iceland. Incident coronary heart disease was defined as myocardial infarction, coronary revascularization by either CABG surgery or PTCA intervention according to hospital records or death from coronary heart disease in participants with no previous history of cardiovascular diseases (International Classification of Diseases 10, ICD-10 codes I21-I25).
Incident cardiovascular disease was defined as coronary heart disease and stroke according to hospital records (International Classification of Diseases 10, ICD-10 codes I21–I25 and I610–639) or death from cardiovascular disease in participants with no previous history of cardiovascular diseases.
Follow-up of all-cause death, incident coronary heart disease and incident cardiovascular disease was until December 31st 2013. Follow-up time was calculated as the number of days between baseline and death/event or end of follow-up, whichever happened first. The median follow-up for all-cause death was 8.9 years (interquartile ranges 6.6 – 9.9 years). The median follow-up for incident cardiovascular disease was 8.2 years (interquartile ranges 3.8–9.6 years) and for incident coronary heart disease 8.4 years (interquartile ranges 4.1–9.6 years).
Statistical analysis
Descriptive data are presented as percentages or median and interquartile ranges (25th – 75th percentiles) or mean and standard deviation. Specific quartiles of hs-cTnI were calculated. We tested the proportional hazard assumption for the hs-cTnI I variable. This was done both formally, using a test of interaction of the hs-cTnI variable with log(time), and visually, by inspecting scaled Shoenfeld residuals as a function of time.
Highly skewed variables (hs-cTnI, CRP, triglycerides and creatinine) were logarithmically transformed to normalize the distribution. The non-parametric Mann-Whitney U-test was used for comparison between two groups and the Kruskal-Wallis test was used to compare baseline characteristics and outcomes across the different quartiles of hs-cTnI. P-values for trend were estimated by chi-square analysis. The Pearson correlation coefficient was used to evaluate the relation between hs-cTnI concentrations and age.
Survival by quartiles of hs-cTnI was estimated by Kaplan-Meier survival analysis.
The relationship of hs-cTnI to death from all causes, incident cardiovascular disease and incident coronary heart disease were investigated separately for men and women after dividing the whole group into hs-cTnI quartiles with the use of Cox proportional-hazards regression in 2 models. These models elaborated separately for men and women were adjusted for established risk factors for cardiovascular disease as follows: model a) adjusted for age; model b) adjusted for variable in model a) plus diabetes, BMI, hypertension medication, systolic blood pressure, smoking, log CRP, log creatinine, cholesterol, high density cholesterol, log triglycerides, statin medication.
The optimal cutpoints of hs-TnI for predicting all cause death and cardiovascular event were determined from the Youden index.
Predictive ability of the models with and without hs-cTnI were quantified by estimating the area under the receiver operating curve (AUC) and by the net reclassification improvement (NRI) using log(hs-cTnI) with the variables as in model b). The AUC was estimated using the R-package timeROC(12) and the NRI was estimated using the R-package nricens.
A P value < 0.05 was considered statistically significant.
Statistica for Windows v. 12.0 (Statsoft Inc.), MedCalc v 12.7 (MedCalc Software), STATA (StataCorp. 2011. Stata Statistical Software: Release 12) and R were used for the statistical analyses.
Results
Baseline characteristics
The concentration of hs-cTnI was measured in 5691 individuals in the AGES-Reykjavik cohort; 2416 men, mean age 77 years (SD 5.7 years) and 3275 women, mean age 77 years (SD 6 years) collected during the years 2002–2006 (Supplementary Table 1).
Concentrations of hs-cTnI at or above the limit of detection of the assay were observed in 5682 patients (99.9 %). The distribution of hs-cTnI concentrations by sex is shown in Figure 1, with higher values in men. The median concentration of hs-cTnI was 6.3 ng/L (interquartile ranges: 4.4–10.5 ng/L) in the whole cohort. hs-cTnI concentrations were significantly higher in men than in women (P<0.001). In men the median concentration was 8.4 ng/L (5.6–14.2 ng/L) and in women 5.3 ng/L (3.8–8.1 ng/L). hs-cTnI concentrations correlated significantly with age (r=0.32, P<0.001) in the whole cohort (Supplementary Figure 1) and in men (r=0.30 P<0.001) and women (r=0.36, P<0.001). Linear relationships between hs-cTnI concentrations and age were seen in both genders irrespective of a history of previous cardiovascular disease. For men the linear regression relationship was: Log10(y) = −0.5497 + 0.02005 x, and for women the equation was: Log10 (y) = −0.6558 + 0.01867 x, where y was hs-cTnI and x was age in years. 310 (12.8%) men had hs-cTnI concentrations above the 99th percentile (24.2 ng/L for men) and 296 (9%) women had hs-cTnI concentrations above the 99th percentile (15.2 ng/L for women). After elimination of individuals with previous cardiovascular events, diabetes mellitus, medication for hypertension, statin medication and serum creatinine > 1.3 mg/dL (115 μmol/L), 70 (2.9%) men and 148 (4.5%) women had concentrations above the respective 99th percentiles
Figure 1.
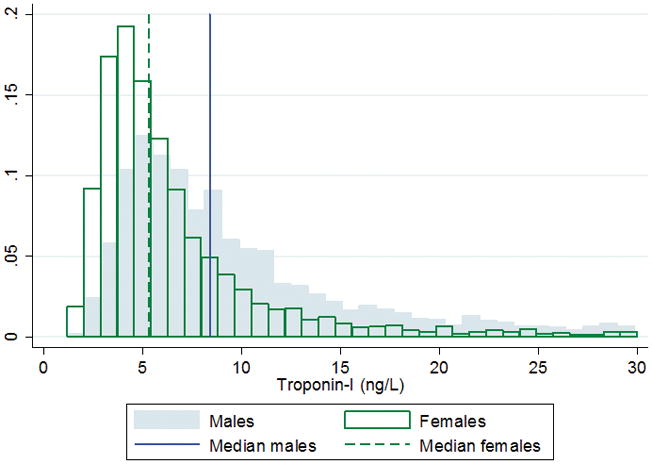
Distributions of high-sensitive cardiac troponin I by sex, presented for a maxium value of 30 ng/L
The relation of hs-cTnI to baseline variables, cardiovascular disease risk factors and outcome
As summarized in Supplementary Table 1, significant gender differences were seen for most baseline variables. Notably, 39% of men and 21% of women had previous cardiovascular disease, P<0.001. The prevalence of diabetes mellitus was higher among men (16.5%) as compared to women (11.2%), P<0.001. More than 60% were on treatment with anti-hypertensive drugs, but with no difference between genders. Deaths or incidences of coronary heart disease and cardiovascular disease were all higher among men than among women. All-cause death during the follow-up period was 48% in men as opposed to 37% in women, P<0.001. Incidences of cardiovascular disease versus coronary heart disease during this period were 18% vs 16% or 14% vs 12% for men and women, respectively, P<0.001.
Supplementary Table 2 shows baseline variables relative to sex and quartiles of hs-cTnI. Major gender differences were seen in that results from women comprised 80 % of the results of quartile 1 and only 36 % of the results of quartile 4, P<0.001. For most other variables we saw increased concentrations or proportions with increasing quartiles of hs-cTnI with the exceptions of total and HDL cholesterol in both sexes and triglycerides in men.
The numbers of individuals who suffered all-cause death and incidence of cardiovascular disease and coronary heart disease per quartile of hs-cTnI are shown in Table 1 and separated by gender. The number of adverse events increased significantly in both men and women by increasing quartiles. Thus, all-cause death was increased almost two-fold comparing the first with the second quartiles, i.e. from 20–22% to more than 35%. No significant differences in proportions of all-cause death at the different quartiles of hs-cTnI were seen between the genders whereas the incidences of cardiovascular diseases and coronary heart disease were higher among men, P<0.001. A more detailed analysis of the relationship between all-cause death and hs-cTnI is shown in Figure 2. The figure shows a very consistent linear association of hs-cTnI with all-cause death in men and women with 4 fold increase in all-cause death between the lowest and highest values for hs-cTnI. Similarly, the incidences of cardiovascular disease and coronary heart disease increased linearly with increasing hs-cTnI concentrations (Supplementary Figures 2 and 3).
Table 1.
Number and rates of incident event by sex and quartiles of high-sensitivity cardiac troponin I
| Endpoint | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-value** | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||||
| n | % | Rate* | n | % | Rate* | n | % | Rate* | n | % | Rate* | |||
| Males | All-cause death | 63 | (22.3) | 2557 | 190 | (37.3) | 4465 | 316 | (43.8) | 5529 | 578 | (64.1) | 9625 | <0.001 |
| Incident CVD | 39 | (18.8) | 2325 | 93 | (25.7) | 3483 | 130 | (28.3) | 4116 | 163 | (37.2) | 6312 | <0.001 | |
| Incident CHD | 27 | (13) | 1581 | 73 | (20.2) | 2684 | 107 | (23.3) | 3262 | 125 | (28.5) | 4625 | <0.001 | |
| Females | All-cause death | 236 | (20.6) | 2302 | 328 | (36.1) | 4302 | 318 | (45.4) | 5698 | 314 | (60.6) | 8916 | <0.001 |
| Incident CVD | 129 | (13.1) | 1549 | 139 | (19) | 2416 | 157 | (29.5) | 4090 | 107 | (31.6) | 4921 | <0.001 | |
| Incident CHD | 92 | (9.3) | 1089 | 95 | (13) | 1615 | 113 | (21.2) | 2853 | 84 | (24.8) | 3780 | <0.001 | |
Values are number (percent). CVD = Cardiovascular disease; CHD = Coronary heart disease
Values for high-sensitive cardiac troponin I. Quartile 1: 1.2 – 4.4 ng/L; Quartile 2: 4.5 – 6.3 ng/L; Quartile 3: 6.4 – 10.5; Quartile 4: 10.6–1691 ng/L.
Rate per 100.000 person years
P-value for trend from log rank test
Figure 2.
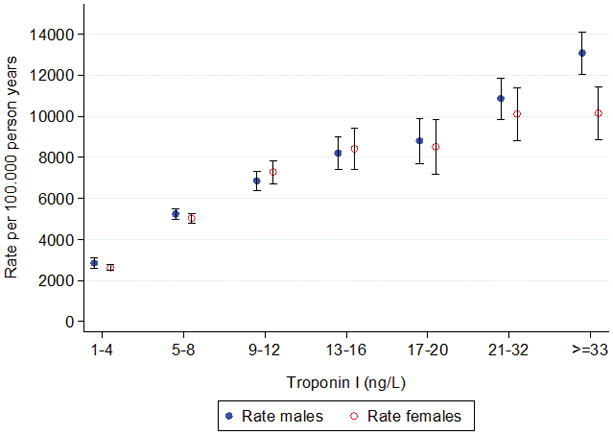
All-cause death, rates per 100,000 person years in men vs women, stratified by categories of high-sensitivity cardiac troponin I (hs-cTnI). Values for hs-cTnI in ng/L. Bars are standard error
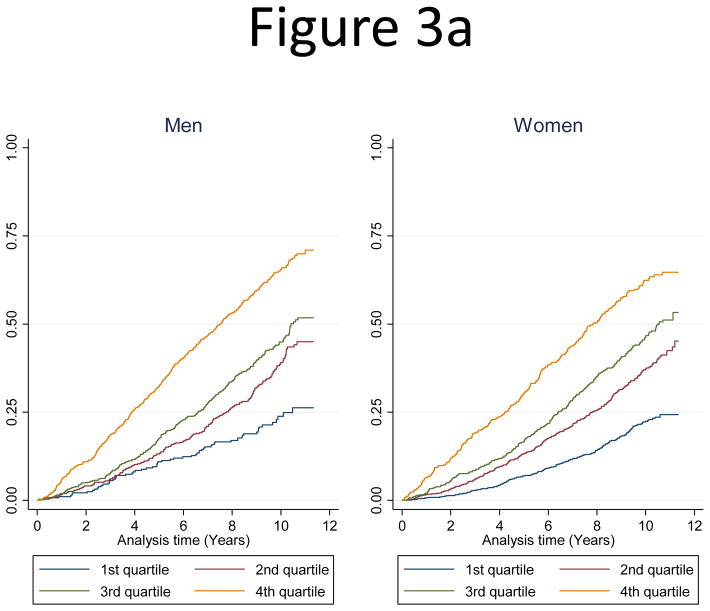
Adjusted hazard ratios for age and sex in the whole cohort are presented in Table 2. Adjusted hazard ratios for all-cause death, incident cardiovascular events and incident coronary heart disease were 2.31, 2.52 and 2.8 respectively. Adjusted hazard ratios for age were 2.27 for all-cause death, 2.53 for incident cardiovascular events and 2.88 for incident coronary heart events for men in the highest quartile of hs-cTnI (> 10.6 ng/L) (Supplementary Table 3a). Similar results were seen in women (Supplementary Table 3b). By Cox proportional hazard models adjusting for traditional risk factors including age, diabetes, BMI, hypertension medication, systolic blood pressure, smoking, log CRP, log creatinine, cholesterol, HDL cholesterol, log triglycerides and statin medication the hazard ratios remained increased and highly significant over quartiles 2 to 4 in the whole cohort and separately in both men and women. A violation of the the proportional hazard assumption was not detected. In the whole study cohort considering baseline hs-cTnI concentrations as a continuous variable there was a strong and graded association between concentrations of hs-cTnI and all cause death, incidence of cardiovascular diseases and coronary heart diseases. After adjustment for: age, sex, diabetes, BMI, hypertension medication, systolic blood pressure, smoking, log CRP, log creatinine, cholesterol, HDL cholesterol, log triglycerides and statins medication, the association remained significant. Hazard ratio was 1.24 per one SD log baseline hs-cTnI (95% CI 1.19–1.30) for all cause death; 1.25 per one SD log hs-cTnI (95% CI 1.21–1.34) for incidence of cardiovascular diseases and 1.20 per one SD log hs-cTnI (95% CI 1.08–1.33) for incidence of coronary heart diseases. Similar results were seen in men and women separately (Supplementary Table 4). As shown in Figure 3 by cumulative incidence graphs there were significant linear relationships over time between all-cause death (Figure 3a), incidence of cardiovascular disease (Figure 3b) and incidence of coronary heart disease (Figure 3c) and hs-cTnI quartiles. Similar figures based on sex-specific quartiles are shown in Supplementary Figures 4 a–c.
Table 2.
Hazards ratios (HR) for all-cause death and cardiovascular outcomes in relation to quartiles of high-sensitive cardiac troponin I in the whole cohort
| HR (95% CI), adjusteda | HR (95% CI), adjustedb | |
|---|---|---|
| All-cause death | ||
| Quartile 1 | Referent | Referent |
| Quartile 2 | 1.41 (1.22–1.63)** | 1.40 (1.20–1.62)** |
| Quartile 3 | 1.62 (1.41–1.87)** | 1.55 (1.33–1.79)** |
| Quartile 4 | 2.31 (2.01–2.67)** | 2.07 (1.78–2.40)** |
| Incidence of cardiovascular diseases | ||
| Quartile 1 | Referent | Referent |
| Quartile 2 | 1.44 (1.18–1.77)** | 1.32 (1.07–1.61) |
| Quartile 3 | 1.99 (1.63–2.43)** | 1.66 (1.35–2.04)** |
| Quartile 4 | 2.52 (2.04–3.11)** | 2.05 (1.65–2.56)** |
| Incidence of coronary heart disease | ||
| Quartile 1 | Referent | Referent |
| Quartile 2 | 1.50 (1.18–1.90)** | 1.34 (1.05–1.71)* |
| Quartile 3 | 2.17 (1.72–2.74)** | 1.76 (1.38–2.24)** |
| Quartile 4 | 2.8 (2.20–3.60)** | 2.22 (1.72–2.87)** |
Values were calculated with the use of multivariable Cox regression analysis
Data were adjusted for:
age, sex;
age, sex, diabetes, BMI, hypertension medication, systolic blood pressure, smoking, log CRP, log creatinine, cholesterol, HDL cholesterol, log triglycerides, statins medication
p<0.05;
p<0.001
Values for high-sensitive cardiac troponin I. Quartile 1; (1.2–4.4 ng/L); Quartile 2; (4.5–6.3 ng/L); Quartile 3; (6.4–10.5 ng/L); Quartile 4; (10.6–1692 ng/L)
Figure 3.
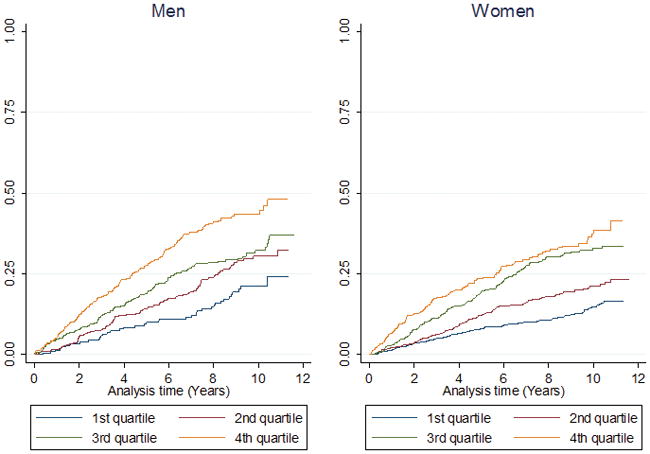
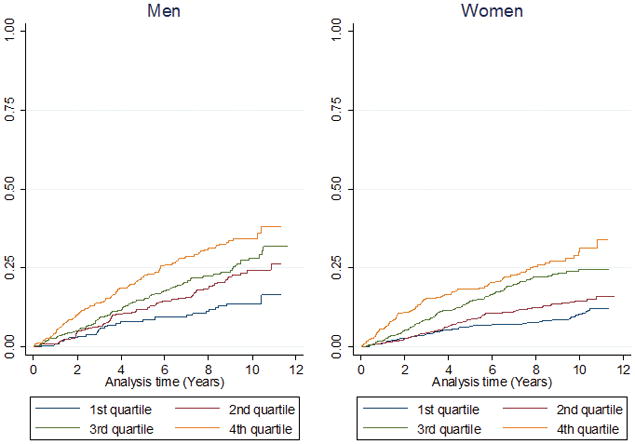
Quartile analysis of hs-cTnI by gender showing probability of: A) all-cause death, B) cardiovascular diseases, C) coronary heart disease
Values for high-sensitivity cardiac troponin I. Quartile 1; (1.2–4.4 ng/L); Quartile 2; (4.5–6.3 ng/L); Quartile 3; (6.4–10.5 ng/L); Quartile 4; (10.6–1692 ng/L)
The interaction terms of sex on the associations of hs-cTnI with all three outcomes were not significant.
Receiver operating characteristics (ROC) analysis of hs-cTnI
The c-index for the prediction of mortality, incidence of cardiovascular and coronary heart diseases was not significantly different between men and women. Table 3 shows the area under the ROC-curves (AUC) with the inclusion of a number of risk factors and the increment in the AUC by the inclusion of hs-cTnI in the model. As can be seen there were significant increments in the AUCs of both all-cause death and incidences of cardiovascular and coronary heart disease by adding hs-cTnI to the model for men and of all-cause death for women. However, a significant increase in the predictive ability of hs-cTnl beyond the use of traditional and novel risk factors was observed for incidence of cardiovascular disease and coronary heart disease as measured by the net reclassification improvement in both men and women.
Table 3.
Receiver operating characteristics (ROC) and net reclassification analyses
| Endpoint | AUC (%) with 95% CI without hs-cTnI | AUC (%) with 95% CI with hs-cTnI | P-value for difference | The continuous NRI with 95% CI | |
|---|---|---|---|---|---|
| Men | All-cause death | 73.2 (70.5,75.8) | 74.2 (71.5,76.8) | 0.0014 | 0.24 (0.14,0.35) |
| Incidence of CVD | 63.5 (59.6,67.3) | 65.9 (62.1,69.7) | 0.0049 | 0.27 (0.08,0.40) | |
| Incidence of CHD | 62.7 (58.5,66.9) | 65.0 (60.8,69.1) | 0.010 | 0.26 (0.05,0.40) | |
| Women | All-cause death | 73.5 (70.9,76.1) | 74.4 (71.8,77.0) | 0.0064 | 0.24 (0.11,0.36) |
| Incidence of CVD | 68.7 (65.6,71.8) | 69.6 (66.5,72.7) | 0.072 | 0.20 (0.02,0.29) | |
| Incidence of CHD | 68.5 (65.0,72.0) | 69.7 (66.2,73.2) | 0.087 | 0.24 (0.05,0.34) | |
| All | All-cause death | 73.9 (72.1,75.8) | 74.8 (73.0,76.6) | <0.0001 | 0.26 (0.18,0.33) |
| Incidence of CVD | 67.6 (65.2,69.9) | 69.0 (66.6,71.3) | 0.0007 | 0.21 (0.11,0.29) | |
| Incidence of CHD | 67.1 (64.5,69.7) | 68.8 (66.3,71.4) | 0.0010 | 0.23 (0.12,0.32) |
ROC analyses included the following variables: age, diabetes, BMI, hypertension medication, systolic blood pressure, smoking, log CRP, log creatinine, cholesterol, HDL cholesterol, log triglycerides and statin medication. Hs-cTnI = high-sensitive cardiac troponin I; CI: confidence interval; NRI: net reclassification improvement; CVD = Cardiovascular disease; CHD = Coronary heart disease
The optimal cutpoints of hs-TnI for predicting all cause death and cardiovascular diseases were 8.8 ng/L vs 7.0 ng/L or 9.4 ng/L vs 5.8 ng/L for men and women, respectively (Supplementary Figure 5a and 5b).
Discussion
This report appears to be the first study of the predictive power of hs-cTnI assay (Abbott Diagnostics) in a large and older community-based population. The hs-cTnI assay used in this study measures very low concentrations and virtually all studied individuals (>99%) had concentrations above the level of detection. Four important conclusions were drawn from the study. First, was the finding that hs-cTnI concentrations even within the normal range, below the 99th percentile limits, were predictive of adverse events as to mortality and incidence in cardiovascular disease. Second, the predicted rates of mortality and major adverse cardiac events increased with increasing concentrations of hs-cTnI. Third, there was a strong association of hs-cTnI with age. The concentrations increased linearly by age up to the age of 98 years in both men and women irrespective of any history of cardiovascular disease. Fourth, hs-cTnI offered incremental predictive power over traditional risk factors for prediction of coronary heart disease and cardiovascular disease.
The analytical sensitivity of cTnI assays has increased substantially over the last decade with very few previous assays actually being able to accurately define the 99th percentile of a healthy population(13). In this study we show that the median concentrations of hs-cTnI in a community-based older population is as low as one fourth to one third of the 99th percentile of 25 ng/L and that the fourth quartile starts at the concentration of about 10 ng/L. In a previous study on a large cohort of children (14) the median concentrations using the same hs-cTnI assay were about half those found in our cohort. We speculate, therefore, that cTnI might leak out from older myocardial cells more readily than in younger persons. This finding was clearly supported by the progressive increase in cTnI with age, irrespective of gender and history of myocardial disease. A positive relation of hs-cTnI with age was previously found using the Beckman Coulter hs-cTnI prototype assay (15) and also the high-sensitivity cTnT assay (16); however, with the hs-cTnI Beckman Coulter assay no gender differences were seen as opposed to the findings with the cTnT assay. This discrepancy is not readily explained, but there has been further evidence for the presence of gender differences recently (17). Thus it seems safe to assume both gender and age differences in circulating concentrations of cardiac troponins, which should be taken into consideration when defining limits such as the 99th percentile.
Several previous studies have shown the powerful predictive value of cardiac troponins of adverse cardiovascular events and death (4;5;7;18–22). The extraordinary findings in this large study on older individuals were the facts that strong associations to death and cardiovascular events were seen already at normal concentrations and increased linearly from these low to high concentrations of hs-cTnI. Thus, the hazard ratios for adverse events in individuals having hs-cTnI concentrations in the second quartile, which started close to the median concentrations of presumably healthy individuals, were significantly higher than the hazard ratios of those with concentrations in the first quartile. The hazard ratios increased even further for individuals in the 3rd and 4th quartiles. The exciting question is whether these relationships reflect genetics or pathophysiological processes or a combination of both. One author was involved in a previous study on 70-year old men (the Uppsala Longitudinal Study of Adult Men (ULSAM) cohort) where strong associations were found between slightly higher cTnI concentrations and adverse events even among the healthy individuals (23). This finding was interpreted to reflect subclinical myocardial disease. In the PIVUS (Prospective Investigation of the Vasculature in Uppsala Seniors) cohort of 70 year old men and women hs-cTnI concentrations were independently predictive of death in those who had cTnI concentrations in the upper quartile (3). However, further stratification was not possible owing to the low frequency of events and therefore the associations to adverse outcome in the lower quartiles could not be determined.
One limitation of the current study is that only Caucasians were studied. However, in a similar study utilizing the hs- cTnT assay, deFilippi et al(19) concluded that hazard ratios did not depend on ethnicity which may support the generalizability of our study. Thus, the employment of hs-cTn assays reveals the powerful prediction of adverse outcomes that is associated to very low and subnormal concentrations of these biomarkers.
In a recent report from the Nord-Trøndelag Health Study (HUNT) study(22), a large, general population study, found that the prognostic value of hs-cTnI (assay manufacturer?) was stronger in women than in men. In the thorough examination of the AGES cohort we could not discern any such differences. The reasons for this discrepancy are not quite clear but may be related to the large differences in age, since the average age of the HUNT population was 50 years as compared to 77 years of the AGES population. Our findings in the AGES cohort of no sex differences in the prognostic impact of hs-cTnI are similar to findings in the PIVUS cohort(24). One speculation was that the results and discrepancies were biased by lower event rates in women with low hs-cTnI as compared to men. We also found much higher optimal cut-points for the prediction of death and cardiovascular disease in the AGES cohort as compared to the cut-points found in the HUNT study. Again a fact that may relate to the large differences in age between the two studies.
In conclusion in our study of older individuals, hs-cTnI as measured by the Abbott assay was highly predictive of death and major adverse cardiac events and these events were predicted at blood concentrations far below the discrimination limit of the diagnosis of acute events of MI i.e. the 99th percentiles and equally in both genders. These findings show that the measurements of hs-cTnI, in addition to being used as a diagnostic means for acute myocardial injury, can be used to monitor and risk assess individuals for death and/or incidence of major adverse cardiac events. These data support previous findings that the measurements of hs-cTnI may be used for primary prevention of heart disease and should form the basis for future prospective clinical trials for determining whether measuring hs-cTnI can be applied in the prevention of cardiovascular disease.
Supplementary Material
Acknowledgments
We thank all the participants of the AGES-Reykjavik Study and the clinic staff at the Icelandic Heart Association for their invaluable contribution.
Funding Statement
This study was funded by the National Institutes of Health, USA contract N01- AG-12100, and the National Institute on Aging Intramural Research Program, the National Eye Institute USA (Z01-EY000401), National Institutes of Health, Hjartavernd (The Icelandic Heart Association), and Althingi (Icelandic Parliament). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The assay of hs-cTnI was supported by a generous grant from Abbott Diagnostics.
References
- 1.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e426–e579. doi: 10.1161/CIR.0b013e318212bb8b. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol. 2006;48:1–11. doi: 10.1016/j.jacc.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 3.Eggers KM, Venge P, Lindahl B, Lind L. Cardiac troponin I levels measured with a high-sensitive assay increase over time and are strong predictors of mortality in an elderly population. J Am Coll Cardiol. 2013;61:1906–13. doi: 10.1016/j.jacc.2012.12.048. [DOI] [PubMed] [Google Scholar]
- 4.De Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saunders JT, Nambi V, De Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folsom AR, Nambi V, Bell EJ, Oluleye OW, Gottesman RF, Lutsey PL, et al. Troponin T, N-terminal pro-B-type natriuretic peptide, and incidence of stroke: the atherosclerosis risk in communities study. Stroke. 2013;44:961–7. doi: 10.1161/STROKEAHA.111.000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–16. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 8.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigurdsson E, Thorgeirsson G, Sigvaldason H, Sigfusson N. Unrecognized myocardial infarction: epidemiology, clinical characteristics, and the prognostic role of angina pectoris. The Reykjavik Study. Ann Intern Med. 1995;122:96–102. doi: 10.7326/0003-4819-122-2-199501150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Eggers KM, Johnston N, James S, Lindahl B, Venge P. Cardiac troponin I levels in patients with non-ST-elevation acute coronary syndrome-the importance of gender. Am Heart J. 2014;168:317–24. doi: 10.1016/j.ahj.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Johnston N, Jernberg T, Lindahl B, Lindback J, Stridsberg M, Larsson A, et al. Biochemical indicators of cardiac and renal function in a healthy elderly population. Clin Biochem. 2004;37:210–6. doi: 10.1016/j.clinbiochem.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med. 2013;32:5381–97. doi: 10.1002/sim.5958. [DOI] [PubMed] [Google Scholar]
- 13.Thygesen K, Mair J, Giannitsis E, Mueller C, Lindahl B, Blankenberg S, et al. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J. 2012;33:2252–7. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 14.Koerbin G, Potter JM, Abhayaratna WP, Telford RD, Hickman PE. The distribution of cardiac troponin I in a population of healthy children: lessons for adults. Clin Chim Acta. 2013;417:54–6. doi: 10.1016/j.cca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Venge P, Johnston N, Lindahl B, James S. Normal plasma levels of cardiac troponin I measured by the high-sensitivity cardiac troponin I access prototype assay and the impact on the diagnosis of myocardial ischemia. J Am Coll Cardiol. 2009;54:1165–72. doi: 10.1016/j.jacc.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 16.Saenger AK, Beyrau R, Braun S, Cooray R, Dolci A, Freidank H, et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin Chim Acta. 2011;412:748–54. doi: 10.1016/j.cca.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 17.Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem. 2012;58:1574–81. doi: 10.1373/clinchem.2012.192716. [DOI] [PubMed] [Google Scholar]
- 18.Eggers KM, Al-Shakarchi J, Berglund L, Lindahl B, Siegbahn A, Wallentin L, Zethelius B. High-sensitive cardiac troponin T and its relations to cardiovascular risk factors, morbidity, and mortality in elderly men. Am Heart J. 2013;166:541–8. doi: 10.1016/j.ahj.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 19.deFilippi CR, De Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apple FS, Steffen LM, Pearce LA, Murakami MM, Luepker RV. Increased cardiac troponin I as measured by a high-sensitivity assay is associated with high odds of cardiovascular death: the Minnesota Heart Survey. Clin Chem. 2012;58:930–5. doi: 10.1373/clinchem.2011.179176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leistner DM, Klotsche J, Pieper L, Stalla GK, Lehnert H, Silber S, et al. Circulating troponin as measured by a sensitive assay for cardiovascular risk assessment in primary prevention. Clin Chem. 2012;58:200–8. doi: 10.1373/clinchem.2011.174292. [DOI] [PubMed] [Google Scholar]
- 22.Omland T, De Lemos JA, Holmen OL, Dalen H, Benth JS, Nygard S, et al. Impact of sex on the prognostic value of high-sensitivity cardiac troponin I in the general population: the HUNT study. Clin Chem. 2015;61:646–56. doi: 10.1373/clinchem.2014.234369. [DOI] [PubMed] [Google Scholar]
- 23.Zethelius B, Johnston N, Venge P. Troponin I as a predictor of coronary heart disease and mortality in 70-year-old men: a community-based cohort study. Circulation. 2006;113:1071–8. doi: 10.1161/CIRCULATIONAHA.105.570762. [DOI] [PubMed] [Google Scholar]
- 24.Eggers KM, Johnston N, Lind L, Venge P, Lindahl B. Cardiac troponin I levels in an elderly population from the community - The implications of sex. Clin Biochem. 2015;48:751–6. doi: 10.1016/j.clinbiochem.2015.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.