Abstract
Introduction
Lamin proteins confer nuclear integrity and relay external mechanical cues that drive changes in gene expression. However, the influence these lamins have on whole-cell mechanical properties is unknown. We hypothesized that protein expression of lamins A, B1, and C would depend on the integrity of the actin cytoskeleton and correlate with cellular elasticity and viscoelasticity.
Methods
To test these hypotheses, we examined the protein expression of lamins A, B1, and C across five different cell lines with varied mechanical properties. Additionally, we treated representative “soft/stiff” cell types with cytochalasin D and LMNA siRNA to determine the effect of a more compliant whole-cell phenotype on lamin A, B1 and C protein expression.
Results
A positive, linear correlation existed between lamin C protein expression and average cell moduli/apparent viscosity. Though moderate correlations existed between lamin A/B1 protein expression and whole-cell mechanical properties, they were statistically insignificant. Inhibition of actin polymerization, via cytochalasin D treatment, resulted in reduced cell elasticity, viscoelasticity, and lamin A and C protein expression in “stiff” MG-63 cells. In “soft” HEK-293T cells, this treatment reduced cell elasticity and viscoelasticity but did not affect lamin B1 or C protein expression. Additionally, LMNA siRNA treatment of MG-63 cells decreased whole-cell elasticity and viscoelasticity.
Conclusion
These findings suggest that lamin C protein expression is strongly associated with whole-cell mechanical properties and could potentially serve as a biomarker for mechanophenotype.
Electronic supplementary material
The online version of this article (10.1007/s12195-018-0518-y) contains supplementary material, which is available to authorized users.
Keywords: Mechanophenotype, Elasticity, Viscoelasticity, Atomic force microscopy, Cytoskeleton, Mechanical biomarkers
Introduction
Whole-cell mechanical properties have emerged as important phenotypic traits that can identify specific cell types, differentiation states, and disease progression.8,15 Cellular mechanophenotypes are defined by an amalgam of both elastic and viscous components that give cells a characteristic, inherent mechanical property, as reviewed elsewhere.43 These components are responsible for relaying physical cues from their surrounding microenvironment, across the cytoskeletal network, and into the nucleus via nucleocytoskeletal protein complexes known as linker of the nucleus to cytoskeleton (LINC).33 Previous studies have shown that altering the mechanical properties of tissue-specific cell types results not only in structural and morphological changes but also in genetic modifications that may lead to different phenotypic traits.10,25,46
At the heart of the mechanotransduction cascade, nuclear envelope lamin proteins are responsible for receiving these mechanical cues from the LINC complex and contributing to chromatin rearrangements that influence gene expression.2,18 Lamins are intermediate filament proteins that exist in most mammalian cells and include lamins A and C, splice variants of the LMNA gene,31 and lamins B1 and B2, encoded by genes LMNB1 and LMNB2, respectively.38,48 Specifically, lamins A and C have been shown to affect nuclear and cellular deformability, mechanotransduction, cell polarization, and migration.17,24,26,27,29,34 Gene and protein expression for lamin A/C is affected by changes in the physical and chemical microenvironment.44 Most importantly, lamin A and C protein expression vary across tissues known to exhibit different mechanical properties.45 Consequently, changes in lamin A/C expression may indicate changes in cellular phenotype that affect biological processes of clinical relevance, such as stem cell differentiation,1,4,7 development of laminopathies,28,36,40 and cancer progression.20
The role of lamins B1 and B2 in mechanotransduction are less apparent. Previous studies indicate that these lamins are not significant contributors to nuclear mechanical properties or cellular mechanotransduction.26 However, these results have been observed mostly in fibroblastic cell types where lamin A/C seems to predominantly influence cellular mechanophenotype. In other studies, lamin B1 has been found to be important in maintaining nuclear structural integrity and anchoring the nucleus to the LINC protein complex.22,47 This work suggests a potential connection between lamin B1 and the mechanophenotype of compliant cells, such as neuronal and hematopoietic cell types, which express low levels of lamin A compared to B1 and B2.45
Given the differential expression of lamin proteins in cells from different tissue sources, stages of differentiation, and inherent mechanophenotype, we hypothesized that lamin A, B1, and C protein levels would correlate with cellular elasticity and viscoelasticity and act as a biomarker of whole-cell mechanical properties. To test this hypothesis, we examined lamin protein expression for five different cell types exhibiting differences in their elastic and viscoelastic properties. The human cell lines used in this study were normal human fibroblasts (NHF), osteosarcoma cells (MG-63), ovarian granulose cells (KGN), embryonic kidney cells (HEK-293T), and neuroblastoma cells (SH-SY5Y). Atomic force microscopy (AFM)-based single-cell indentation tests were conducted to determine cellular elastic and viscoelastic properties. Protein expression were evaluated using western blots. Correlation analyses between cellular mechanical properties and lamin protein levels illustrated the potential roles of lamin A, B1, and C as biomarkers for mechanophenotype. Furthermore, the interdependency of the actin cytoskeleton, lamin A/C gene expression, and whole-cell mechanophenotype was explored using cytochalasin D (CytoD) and siRNA treatments.
Methods
Cell Culture
Passage 7 NHF, MG-63, KGN, HEK293T, and SH-SY5Y cells were cultured until 80-90% confluence before being used for experiments. NHF and KGN cells were cultured in High glucose, Dulbecco Modified Eagle’s Medium (hg DMEM, Hyclone, GE Healthcare, UT) containing 10% fetal bovine serum (FBS, Zen-Bio, NC), and 1% penicillin and streptomycin (P/S, Hyclone). MG-63 and HEK293T cells were cultured in Minimum Essential Medium Eagle (MEM 1X, Cellgro, VA) containing 10% FBS, and 1% P/S. SH-SY5Y cells were cultured in hg DMEM, 10% FBS, 1% P/S, and 1% glutamine. For whole-cell mechanical tests, 2.5 × 104 cells were seeded on square, glass coverslips (18 mm x 18 mm) placed in 50 mm culture dishes, given cell type-specific media, and allowed to attach and spread at 37 °C for 2 days prior to testing.
Mechanical Testing
AFM-based, single cell indentation tests were used to measure whole-cell mechanical properties (MFP-3D-Bio, Asylum Research, CA). Spherically tipped cantilevers (diameter = 5 µm, Novascan Technologies, IA) had a nominal stiffness of k = 0.03 N/m. Cells were indented over the perinuclear region at a constant indentation velocity of 10 µm/s using a force trigger of 1 nN, yielding cell strains < 10%. Stress relaxation experiments maintained an approximately constant indentation for 30 s prior to retracting. Indentation and force data were used to determine cellular elasticity (Eelastic) and viscoelasticity (relaxed modulus, ER; and apparent viscosity, µapp) using a modified Hertz contact model and thin-layer stress relaxation model (Fig. S1).11,12,41 For CytoD (Enzo Life Sciences, NY) experiments, representative “stiff” (MG-63) and “soft” (HEK-293T) cells were treated with growth medium (no treatment, NT), growth medium with 0.05% dimethyl sulfoxide (DMSO, Acros Chemicals, China) in distilled water, or growth medium with 0.05% DMSO and 2 µM CytoD one hour before testing. For LMNA gene knockdown experiments, “stiff” MG-63 cells were treated with either 50 nM LMNA siRNA (siLMNA, s8221, 4390824, sense: 5′-CCAAAAAGCGCAAACUGGATT-3′, antisense: 5′-UCCAGUUUGCGCUUUUUGGTG-3′, LMNA Silencer Select Validated siRNA, Ambion, Thermo Fisher Scientific) or 50 nM Scramble siRNA (siScramble, 4390843, Silencer Select Negative Control #1 siRNA, Ambion, Thermo Fisher Scientific) for 72 h before mechanical testing. Sample sizes for mechanical characterization of all cell types are as follows: NHF (n = 49), MG-63 (n = 55, n = 43–55 for CytoD, n = 25–46 for siRNA), KGN (n = 67), HEK-293T (n = 67, n = 32–67 for CytoD), and SH-SY5Y (n = 56). Mechanical measurements, for all conditions and cell types, were repeated across three separate sessions to account for any systematic errors.
Protein Expression
Western blot assays were conducted in triplicate to investigate lamin A, B1, and C protein expression in NHF, MG-63, KGN, HEK-293T and SH-SY5Y cells as well as in MG-63 and HEK-293T cells treated with CytoD. For blots used to investigate differential protein expression across mechanically distinct cells, nearly confluent cell cultures were lysed on ice for 30 min using either a sodium dodecyl sulfate (SDS)/urea buffer or a radioimmunoprecipitation (RIPA) lysis buffer. The SDS/urea buffer consisted of 2 M urea (Sigma Aldrich, MO), 34 mM sodium dodecyl sulfate (SDS, ThermoFisher Scientific), and 50 mM Tris–HCl (ThermoFisher Scientific) at pH 8.0 in triplicate. The RIPA buffer (sc-24948, Santa Cruz Biotechnologies, CA) consisted of 1X lysis buffer (1X tris buffer saline, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.004% sodium azide, pH 7.4), 2 mM phenylmethylsulfonyl fluoride in DMSO, 10 µL of a proprietary protein inhibitor cocktail in DMSO, and 1 mM sodium orthovanadate. For blots used to investigate CytoD effects on lamin protein expression, nearly confluent MG-63 and HEK-293T cultures were treated with CytoD for 1 h and lysed using RIPA lysis buffer as instructed by manufacturer specifications. Total protein concentrations from protein lysates, extracted either using SDS/urea or RIPA lysis buffer, were determined using a bicinchoninic acid (BCA) Assay (Pierce™, ThermoFisher Scientific) prior to gel loading. These two lysis buffers were examined to account for the possibility of different protein solubilities, with RIPA being standard in the field and SDS/urea being potentially more suitable for extracting hydrophobic proteins like lamins and β-tubulin.21 For all lysates, 10 µg of protein were loaded and separated on SDS-PAGE Any KD gels (Bio-Rad, CA) and transferred onto Immobilon™-FL polyvinylidene fluoride membranes (Millipore, MA). Membranes detected via fluorescence were blocked in non-mammalian Odyssey® Blocking Buffer (LiCOR, NE) for 1 h at room temperature to limit interference with the IRDye™ secondary antibodies. Following blocking, the membranes were incubated with rabbit anti-human lamin A/C (1:500 dilution, 2032S, Cell Signaling Technology, MA), polyclonal goat anti-human lamin B1 (1:250 dilution, sc-6217, Santa Cruz Biotechnologies), and mouse anti-human β-tubulin (1:1000 dilution, E7-s, Developmental Studies Hybridoma Bank, IA) primary antibodies overnight at 4 °C. Membranes were washed three times at 15-minute intervals in 1X Tris Buffer Saline Tween (TBST, ThermoFisher Scientific) and then incubated separately with infrared fluorophore-labeled donkey anti-rabbit IRDye® 680RD (1:5000 dilution, 925-68073, LiCOR), donkey anti-goat IRDye® 800CW (1:5000 dilution, 926-32214, LiCOR), and goat anti-mouse IRDye® 800CW (1:5000 dilution, 925-32210, LiCOR) secondary antibodies for 1 h each. Membranes were washed three more times at 15-minute intervals in 1X TBST between secondary antibody incubations. Membranes treated with all IRDye® secondary antibodies were visualized using the Odyssey CLx near-infrared scanner (LiCOR). For all western blots, densitometry analyses were done using ImageJ version 1.51d. Protein expression data were normalized to β-tubulin expression.
Gene Expression
Lamin gene expression was assessed by qPCR. mRNA was extracted from three sample replicates for each cell type using QuickRNA Miniprep Kits (Zymo Research, CA), as instructed by manufacturer guidelines. Reverse transcription of RNA was accomplished using a SuperScript III First Strand cDNA Synthesis Kit (0.5–1 μg/reaction, Life Technologies, MA). TaqMan Gene Expression Assay human primers (Life Technologies) for genes of interest LMNA (Hs00153462_m1) and LMNB1 (Hs01059210_m1) and reference gene GAPDH (Hs03929097_g1) were used for all qPCR runs. Fluorescence levels were measured using an ABI 7900HT Fast Real-Time PCR Detection Instrument (Life Technologies) and analyzed using the inverse ∆Ct method. Relative expression of LMNA and LMNB1 was calculated by normalizing expression to GAPDH. All qPCR runs were done in technical triplicate.
Immunofluorescent Staining
Fixed, untreated and CytoD-treated MG-63 and HEK-293T cells were labeled for lamin A/C protein expression using 4 µg/mL of mouse IgG1 anti-lamin A/C monoclonal primary antibody (E-1, sc-376248, SCBT) and 1 µg/mL of Alexa Fluor 488-conjugated goat anti-mouse IgG1 secondary antibody (A-21121, Thermo Fischer Scientific), respectively, using standard immunostaining procedures. Actin cytoskeleton was labeled with 0.1 µg/mL Alexa Fluor 647-conjugated phalloidin toxin (Invitrogen). Lamin A/C and actin cytoskeleton staining was determined visually at ×20 magnification using a Nikon Eclipse Ti-U epifluorescence camera (Nikon Instruments, Melville, NY). Images were captured using a QiCAM 12-bit digital camera (QImaging, Surrey, BC, Canada).
Statistical Analysis
Data normality was assessed using a Shapiro-Wilks test. Mechanical property and protein expression data used for correlation analyses (from five cell types) were non-normal. Therefore, statistically significant comparisons among groups were determined using two-sided Kruskal–Wallis tests with Dunn’s post hoc test. Mechanical property data for CytoD experiments followed a log-normal distribution, and following transformation, were analyzed using two-sided, one-way ANOVA with Tukey post hoc tests. Protein expression data for CytoD experiments were normally distributed and analyzed similarly. Correlation analyses between mechanical property data and protein expression were determined by calculating Pearson’s r coefficient for each set of properties. All experiments were done in triplicate. Statistical analyses were performed using SigmaPlot software.
Results
Whole-Cell Mechanical Properties of Lineage-Specific Cell Types
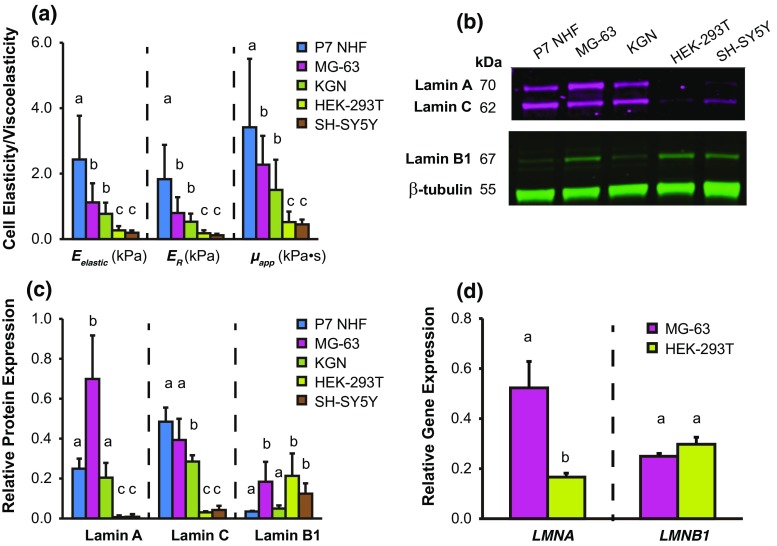
Single-cell indentation tests were conducted via AFM to characterize the elastic (Eelastic) and viscoelastic (ER, µapp) properties of five lineage-specific cell types (Fig. 1a and Table S1). Specifically, the tested cells NHF, MG-63, KGN, HEK-293T, and SH-SY5Y were chosen based on their resemblance to primary cells derived from connective tissue, bone, epithelium, kidney, and brain, respectively, since these tissues are mechanically distinct from one another. The cells tested showed a consistent trend in their Eelastic, ER, and µapp values: NHF > MG-63 > KGN > HEK-293T ~ SH-SY5Y. Stiffer cell types had higher Eelastic, ER, and µapp values. Comparisons of mechanical properties between individual cell types were statistically significant (p < 0.05), with the exceptions of comparisons between MG-63 vs. KGN cells and HEK-293T vs. SH-SY5Y cells (p > 0.05). These results show that lineage-specific cells exhibit distinct elastic and viscoelastic properties.
Figure 1.
Mechanical properties and lamina protein levels for five, mechanically distinct cell types. (a) Cellular elastic and viscoelastic properties for NHF, MG-63, KGN, HEK-293T, and SH-SY5Y cells. Cellular elasticity was determined from elastic modulus (Eelastic); cellular viscoelasticity was determined by relaxed modulus (ER) and apparent viscosity (µapp). (b and c) Lamin A, C, and B1 representative protein expression. Densitometry values normalized to β-tubulin expression. (d) LMNA and LMNB1 gene expression associated with “stiff” MG-63 and “soft” HEK-293T cells. Mechanical property and gene expression data represented as arithmetic mean ± SD. Statistical significance (p < 0.05) was determined using Kruskal–Wallis non-parametric tests with Tukey post hoc tests. Groups with different letters exhibit statistically significant differences.
Lamin Protein and Gene Expression
BCA assays were used to determine total protein concentration from lysates extracted from the five cell types, using either SDS/urea or RIPA lysis buffer (Fig. S2). Results showed that SDS/urea extracted more total protein than RIPA, suggesting improved solubilization and potentially better representation of the proteins present. Thus, data presented in the main text focused on SDS/urea-extracted samples, with data from RIPA-extracted samples being presented in Supplemental Materials. Trends among cell types were largely conserved between the two extraction methods, suggesting that while less protein was solubilized with RIPA, the relative composition of proteins was similar to SDS/urea samples.
Western blot was used to assess representative lamin protein expression for five cell types (Figs. 1b and 1c, Fig. S2). A consistent relationship was observed for lamin A protein expression: MG-63 > NHF ~ KGN > HEK-293T ~ SH-SY5Y. A similar relationship was observed for lamin C protein expression: MG-63 ~ NHF > KGN > HEK-293T ~ SH-SY5Y. No particular relationship was observed for lamin B1 protein expression: NHF ~ KGN < MG-63 ~ HEK-293T ~ SH-SY5Y. qPCR was used to examine LMNA and LMNB1 gene expression for representative stiff, MG-63 and soft, HEK-293T cells (Fig. 1d). Results showed that LMNA expression was 3-times greater in MG-63 than HEK-293T cells (p < 0.05). LMNB1 was not significantly different between cell types.
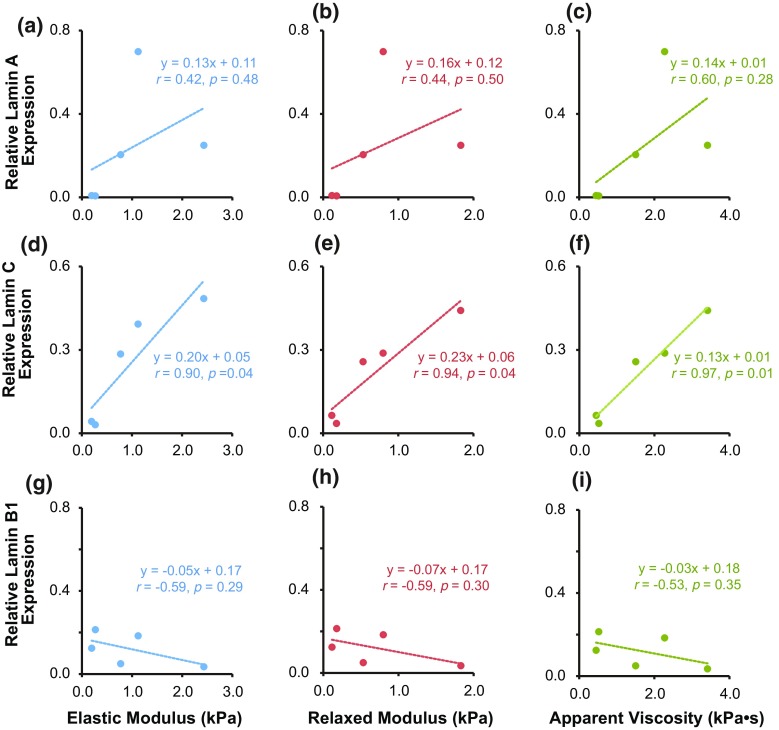
Mechanical Property-Lamin Protein Correlations
Correlation analyses were conducted to determine the relationship between elastic/viscoelastic properties and lamin protein expression (Fig. 2). Lamin C protein expression was positively correlated with Eelastic, ER, and µapp, as evidenced by their respective Pearson’s r coefficients: rEelastic = 0.90 (p < 0.05), = 0.94 (p < 0.05), and = 0.97 (p < 0.05). Lamin A protein expression was moderately correlated with Eelastic, ER, and µapp in a positive fashion but not to the level of statistical significance: rEelastic = 0.42 (p = 0.44); = 0.44 (p = 0.41) and = 0.60 (p = 0.23). Lamin B1 expression was negatively correlated with Eelastic, ER, and µapp, but again, not to the level of statistical significance: rEelastic = − 0.59 (p = 0.24); = − 0.59 (p = 0.23) and = − 0.53 (p = 0.30). Correlation analyses were also run for data obtained using RIPA buffer lysates, detected using either fluorescence or chemiluminescence methods (Figs. S3 and S4). RIPA samples detected via fluorescence (N = 1) exhibited similar positive/negative trends for mechanical properties vs. lamins A/B1/C to SDS/urea samples detected the same way, although the shape of the fits varied slightly (Fig. S3). However, RIPA samples detected via chemiluminescence (N = 3) exhibited different trends from RIPA samples detected via fluorescence, with lamin A exhibiting the strongest relationship with mechanical properties rather than lamin C (Fig. S4). Additional analyses showed that lamin A and C protein correlations were moderately strong but not statistically significant (rA–C = 0.72, p = 0.11), whereas correlations between lamin A and B1 and lamin C and B1 were weak and not statistically significant (Fig. S5, rA–B1 = 0.09, p = 0.88; rC–B1 = 0.54, p = 0.30, respectively).
Figure 2.
Relative lamin A, C, and B1 expression correlate with cellular elasticity and viscoelasticity across the five cell types tested. Correlations were examined between mechanical properties and lamin A (a–c), lamin C (d–e), or lamin B1 (g and h) expression from protein samples extracted using SDS/urea lysis buffer. Pearson’s r correlation coefficients are reported for the linear and inverse exponential fits associated with the data. Each data point corresponds to the median values of elastic modulus, relaxed modulus, or apparent viscosity measured for each cell type, plotted against the associated, average lamina protein expression.
Actin Cytoskeleton Disruption and LMNA Silencing
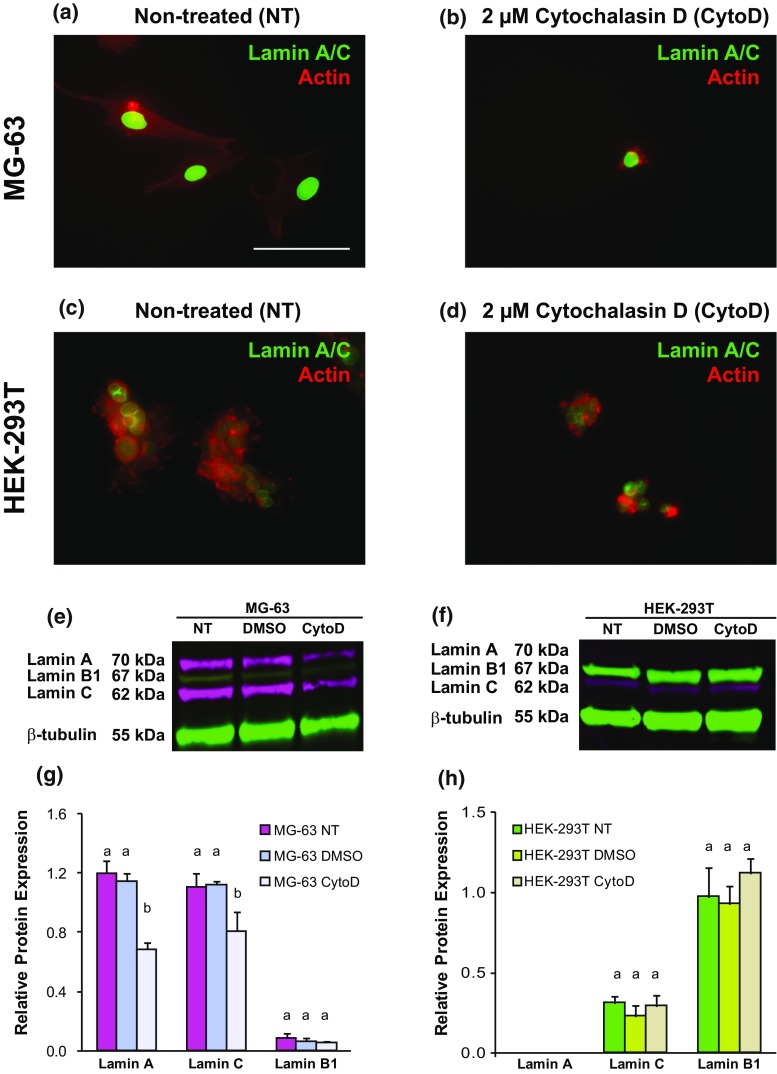
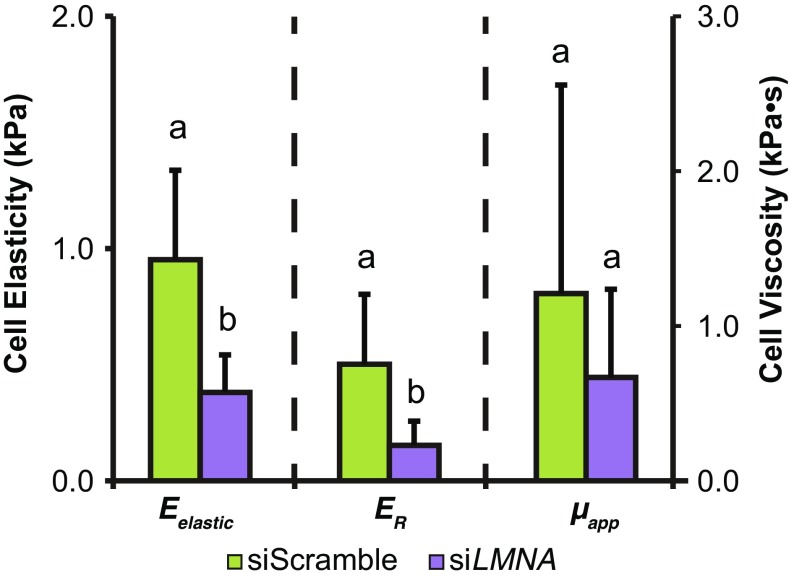
Representative “stiff” MG-63 and “soft” HEK-293T cells were treated with CytoD to determine whether elastic/viscoelastic properties and lamin A, B1, and C protein expression were affected by disrupting the actin cytoskeleton. As expected, the elastic and viscoelastic properties of CytoD-treated MG-63 cells were substantially lower than untreated cells, with a concurrent increase in cell height (Fig. 3a, p < 0.05). Immunostaining of lamin A and C proteins, along with actin cytoskeleton, in untreated and CytoD-treated cells showed lower lamin fluorescence intensity in CytoD-treated MG-63 cells than in untreated groups (Figs. 4a and 4b). In HEK-293T cells, changes in actin cytoskeleton organization were observed between untreated and CytoD-treated cells, but no qualitative difference in lamin A/C intensity was observed between the groups (Figs. 4c and 4d). Representative lamin A and C protein levels by western blot were, respectively, ~ 70 and ~ 60% lower in CytoD-treated MG-63 cells compared to untreated cells (Figs. 4e and 4f). Lamin B1 protein expression was negligible in both groups. “Soft” HEK-293T cells treated with CytoD also exhibited a substantial reduction in elastic and viscoelastic properties (Fig. 3b, p < 0.05). Lamin A protein expression was negligible in both non-treated and CytoD-treated HEK-293T cells while lamin B1 and C expression levels remained unchanged in all HEK-293T cell groups (Figs. 4g and 4h). Similar to what was observed in actin disruption experiments, MG-63 cells treated with 50 nM siLMNA for three days were significantly softer than cells treated with 50 nM siScramble (Fig. 5, p < 0.05). Specifically, Eelastic and ER parameters, but not µapp (p = 0.06), were significantly decreased between siLMNA- and siScramble-treated MG-63 cells.
Figure 3.
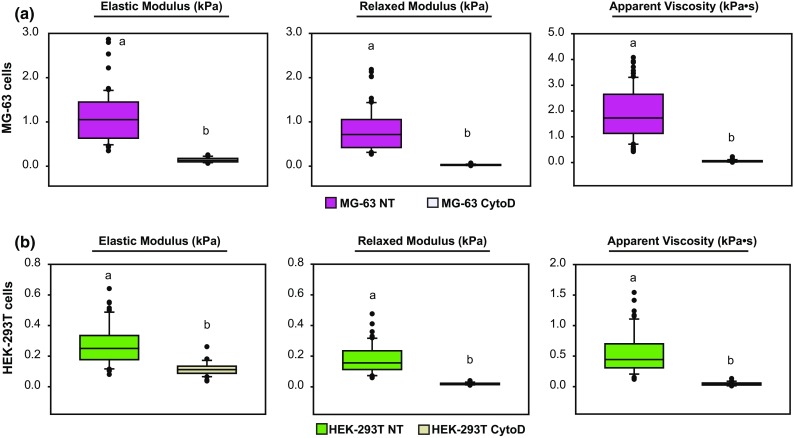
Disruption of actin cytoskeleton affects the mechanophenotype of stiff and soft cells similarly. (a) Elastic moduli, relaxed moduli, and apparent viscosities for “stiff” MG-63 cells exposed to either no treatment (NT) or 2 µM cytochalasin D (CytoD). (b) Elastic moduli, relaxed moduli, apparent viscosities, and cell heights for NT or CytoD, “soft” HEK-293T cells. Statistical significance (p < 0.05) was determined using one-way ANOVA with Tukey post hoc tests. Groups with different letters exhibit statistically significant differences.
Figure 4.
Robust lamin A/C protein expression in stiff MG-63 cells is dependent on actin cytoskeletal organization, whereas weak expression in soft HEK-293T cells is not. (a) Non-treated and (b) CytoD-treated MG-63 cells exhibited a qualitative decrease in lamin A/C levels and dramatic changes in actin organization. (c) Non-treated and (d) CytoD-treated HEK-293T cells both exhibited similar lamin A/C levels, regardless of cytoskeletal disruption, that were much lower than MG-63 cells. (e) Lamin protein expression from MG-63 cells exposed to no treatment (NT), 0.05% DMSO for 1 h (DMSO), or 0.05% DMSO and 2 µM CytoD for 1 h (CytoD). (f) Representative relative lamin protein expression quantification for NT-, DMSO- and CytoD-treated MG-63 cells. (h) Lamin protein expression from HEK-293T cells exposed to the same conditions as described in (e). (h) Representative relative lamin protein expression quantification for NT-, DMSO- and CytoD-treated HEK-293T cells. Lamin A (70 kDa) and C (62 kDa) bands are shown in magenta fluorescence while lamin B1 (67 kDa) and β-tubulin (55 kDa) bands are shown in green fluorescence. Relative protein expression was determined by normalizing all protein expression to β-tubulin (loading control). Statistical significance (p < 0.05) was determined using one-way ANOVA with Tukey post hoc tests. Groups with different letters exhibited statistically significant differences.
Figure 5.
Lamin A/C knockdown via siRNA treatment reduces elastic and viscoelastic properties in stiff cell types. MG-63 cells were treated with either 50 nM siLMNA or 50 nM siScramble for a three-day period before mechanical testing. Statistical significance was determined using Student’s t test (p < 0.05). Groups with different letters exhibited statistically significant differences.
Discussion
Nuclear lamina proteins are known to play critical roles in cellular mechanotransduction machinery and maintenance of nuclear structural integrity.9 Therefore, we hypothesized that lamin A, B1, and C protein expression would strongly correlate to whole-cell elastic and viscoelastic properties. Because of their involvement in mechanotransduction and nuclear-cytoskeletal anchorage, we also hypothesized that nuclear lamin A, B1, and C protein expression were dependent on the integrity of the actin cytoskeleton. Findings showed that cells derived from different lineages exhibited distinct mechanical properties and lamin protein expression profiles. Specifically, stiffer and more viscous cell types exhibited higher lamin A and C protein expression than “soft” cell types, which predominantly expressed lamin B1. Correlations between individual lamin A, B1, and C protein expression and cellular elastic/viscoelastic properties showed that lamin C protein exhibited the strongest linear correlation to cellular mechanophenotype. Meanwhile, lamin A and B1 protein expression exhibited moderate but statistically insignificant correlations to these mechanical properties. Disruption of the actin cytoskeleton via CytoD decreased elasticity, viscoelasticity, and lamin A and C protein expression in stiff cells. Similarly, lamin A/C knockdown also reduced elasticity and viscoelasticity in stiff cells. Altogether, these results validate the importance of lamin proteins in the mechanotransductive linkage between actin cytoskeleton and nuclear lamina.
The current work showed that lamin C protein is a strong indicator of whole-cell mechanophenotype. Therefore, lamin C could represent an important, yet under-investigated, mechanical biomarker. Previous studies have demonstrated that both lamin A and C proteins can influence cellular mechanotransduction,29 nuclear stiffness,26 ECM elasticity sensing,44 and tissue microelasticity,45 suggesting the isotypes share functions that are connected to cellular mechanical characteristics. Lamins A and C are highly similar in their amino acid (aa) primary structure and composition of their N-terminus, being different only in length (664 and 572 aa).13 They share two protein domains, 1B and 2B, that are responsible for imparting the characteristic elastic properties of both lamins.3 The functional redundancy of these lamin isotypes has been reported for nuclear deformation characteristics in cells expressing both lamin A and C compared to lamin C alone.14,26 That said, lamins A and C have been shown to contribute differently to microtissue elasticity.45 Both isotypes correlate highly, but lamin A scales more strongly than lamin C. In the current work, correlation to whole-cell mechanical properties was investigated as opposed to tissue microelasticity. For this comparison, lamin C was the isotype that correlated more strongly to mechanical properties rather than lamin A. Our study also showed that lamin A and C expression levels were different across cell types of different stiffness, with lamin A being expressed only in the stiffest of cell types while lamin C was expressed in cell types regardless of their stiffness. This result suggests that lamin A and C could have different mechanical roles that are connected to inherent cellular mechanical properties and the tissue microenvironment. It is possible such differences could contribute to the discrepancies between lamins A and C vs. mechanophenotype observed in our study compared to previous work.
A secondary finding of our work was that protein extraction techniques could potentially bias resulting data due to variations in protein solubilization. This will be discussed further below but is a possible contributor to the discrepancies between our work and previously published studies. Regardless of extraction technique, we hypothesize that the small differences in amino acid sequence and consequent supramolecular assembly could account for differences in scaling between lamin A and C. These differences are further compounded by cell-specific expression of lamin A/C proteins within given microenvironments, all of which merits further study.
The functional redundancy of lamin A and C proteins could be important for a given phenotype because not all cells express the proteins to the same degree. This is especially true if one isotype is not expressed at all, making the other lamin a better biomarker given the biological context. For example, lamin A expression in murine brain has been shown to be limited to endothelial and meningeal cells, whereas neurons and glia only express lamin C.23 In the current study, we found that NHF and KGN cells had higher lamin C than A protein expression but MG-63 cells showed the reverse. This differential expression between lamin A and C composition across evaluated cell types could be lineage-specific and has been observed previously in tissues of varying stiffness.45 Low compliance tissues like bone and cartilage expressed higher levels of lamin A protein than C while softer tissues such kidney, liver, and heart expressed higher levels of lamin C than A.45 Additionally, these lamin expression differences could be dependent on environmental signals that favor the expression of one lamin protein over another. Previous work showed that altering the expression of spliceosome proteins such as serine/arginine-rich splicing factors (SRSFs) changed lamin A to C protein ratios.30 Specifically, silencing the expression of SRSF2 resulted in an increase in lamin C and a reduction in lamin A by preventing prelamin A synthesis. The expression of these splicing factors can be altered by inducing changes in physical cues such as substrate stiffness,5 which is a well-known physical cue that modulates cell phenotype and fate. Altogether, these findings contribute to understanding how different lamin A and C compositions affect whole-cell mechanical properties in different cells types and how different environments could lead to shifts in cellular lamina composition.
Results related to lamin B1 protein expression agreed with previous reports indicating that lamin B1, as well as lamin B2, play no significant role in nuclear- and tissue-level mechanical properties.26,45 In fact, lamin B1 has been reported to scale very weakly with tissue microelasticity, while lamin B2 does not scale at all.45 In our study, LMNB1 gene expression was similar in “soft” HEK-293T cells and “stiff” MG-63 cells. However, lamin B1 protein expression was variable across tested cells. This phenomenon is attributed to lineage-specific, post-transcriptional regulation of lamin B1.
Actin is the cytoskeletal element most responsible for determining cellular elastic and viscoelastic properties.37,39 From a mechanotransduction standpoint, actin microfilaments bind to LINC complex proteins that ultimately relay mechanical cues, such as cellular tension, to the nuclear lamina.42 Therefore, it was hypothesized that disrupting the actin microfilament network via CytoD treatment would not only decrease elastic and viscoelastic properties but also affect lamin A, C, and B1 protein expression. CytoD treatment for 1 h significantly reduced elastic and viscoelastic properties in stiff and soft cell types, confirming the importance of the actin cytoskeleton to whole-cell mechanical properties. Since the CytoD treatment regimen was successful in depolymerizing actin filaments and inducing a sharp reduction in cellular mechanical properties, we hypothesized CytoD would also lower lamin A/C expression. Despite lamin A/C being a very stable protein in undisturbed conditions, previous reports suggest that disruption of the actomyosin cytoskeleton triggers changes in lamin-A dependent nuclear behavior that is concomitant with intracellular tension loss.19,32 Specifically, perturbations of the actomyosin cytoskeleton result in loss of intracellular tension sufficient to increase fluidity and dynamics of nuclear heterochromatin in only 30 min after CytoD treatment 32 and alter nuclear envelope lamin A/C polarization and size by 1 h after CytoD treatment,19 thus showing that the lamin A/C protein meshwork and its structural functionality are rapidly compromised after Cyto D treatment. Another study used a 2-h treatment with blebbistatin, which inhibits myosin, to disrupt intracellular tension and likewise induce rapid lamin A/C protein changes.6 Lamin turnover was shown to occur in only 10 min after attached cells were uplifted, which results in a drastic cytoskeletal rearrangement (i.e., from spread to spherical morphology) that is accompanied by loss of intracellular tension. These reports, combined with the present findings, provide evidence that lamin A/C expression and mechanical properties can respond quickly and dramatically to perturbations of the actomyosin cytoskeleton, serving as responsive biomarkers.
Additionally, transient silencing of the LMNA gene in stiff MG-63 cells resulted in a significant reduction in elastic and viscoelastic properties, which further demonstrates the connection between lamin A/C expression and cellular mechanophenotype. In cells predominantly expressing lamin A and C (stiff cells), a significant decrease in these proteins was observed, which was anticipated since cytoskeletal disruption has previously been reported to affect nuclear compliance and promote lamin A and C protein turnover.6,16 Interestingly, in cells predominantly expressing lamin B1 (soft cells), negligible changes were observed, suggesting that this protein is neither affected by the disruption of actin cytoskeletal structures nor involved in actin-mediated mechanotransduction processes. It is important to clarify that while CytoD-treated cells are significantly softer than untreated cells, the amount of reduction in elastic properties is very different for MG-63 vs. HEK-293T cell types. Specifically, stiff cells like MG-63 cells lose most of their profuse cytoskeletal connections and the entire actin-dependent mechanotransduction machinery.39 As such, the connection between actin-mediated mechanotransduction and lamin A/C force transduction is severed and this loss of intracellular tension is accompanied by downregulation of lamin A/C protein,6 which is consistent with our finding that siLMNA-treated MG-63 cells were significantly softer than their siScramble-treated counterparts. However, HEK-293T cells have a predominantly cortical actin cytoskeleton that is not robustly linked to the nucleus and employ entirely different mechanotransduction machinery that appears to depend very little on lamin A/C expression, as observed in the CytoD-treatment experiments. Thus, lamin B1 protein was disregarded as a contributor to whole-cell mechanophenotype.
One major distinction between the current study and others is the special attention given to how specific protein extraction methods can potentially bias lamin protein expression experiments. Specifically, the work presented here used an SDS/urea-based lysis buffer to maximize the solubilization of lamin proteins rather than a RIPA-based lysis buffer that is routinely used in other studies.45 RIPA lysis buffers have been found to be suboptimal for solubilizing hydrophobic proteins such as lamins and, therefore, could result in underestimation of actual protein concentration by 20–30%.21 Therefore, correlative and comparative lamin A/C studies that used RIPA buffers for protein extraction may have reported lamin concentrations that are lower than in actuality and, as result, arrived at inaccurate conclusions. For direct comparison purposes, we also included experiments in the current study that used cell lysates extracted with RIPA buffer. Total protein concentrations for these samples were substantially lower than SDS/urea lysates, indicating that fewer proteins, including lamin and β-tubulin proteins, were extracted (Fig. S2). However, correlative analyses between lamin proteins and whole-cell mechanical properties showed similar findings regardless of lysis extraction buffer, with lamin C being the strongest predictor of whole-cell mechanical properties, although for RIPA samples, this was not to the same level of statistical significance (Fig. S3). Qualitatively, when cells were lysed with RIPA buffer, a supernatant and an insoluble pellet were generated. The supernatant was used for protein expression analyses while the insoluble pellet, which could include a disproportionally higher concentration of lamin proteins, was discarded. When cells were lysed with SDS/urea buffer, no insoluble pellet remained, as observed by us and others,35 suggesting that a much greater proportion of the material was successfully solubilized. This conclusion was supported by the presence of more intense lamin and β-tubulin protein bands for SDS/urea vs. RIPA samples in western blot experiments. The extraction efficiency of the two buffers for lamins A/B1/C, β-tubulin, and potentially other proteins of interest could dramatically influence resulting data and should be carefully considered for studies involving nucleocytoskeletal components. One experimental approach that did result in replication of previously reported findings was to use a less sensitive western blot imaging system. RIPA-extracted samples assessed using chemiluminescent detection showed lamin A as the strongest predictor of whole-cell properties (Fig. S4). However, use of a highly sensitive fluorescence-based detection system, along with increased solubilization of proteins should provide a more accurate representation of these lamin-mechanophenotype relationships. While lamin A does correlate with whole-cell properties, using a more appropriate protein extraction method showed that lamin C was actually the strongest predictor. That said, the current study includes a relatively modest number of points to establish these correlations. While increasing this sample set could potentially alter the shape of the trendlines (e.g., linear to exponential) or even change which lamin isotype has the strongest relationship with whole-cell mechanophenotype, the protein extraction method is still expected to be a key factor in any discrepancies between the current work and other reported correlations.
This is the first study to demonstrate strong correlation between lamin C protein and whole-cell, elastic and viscoelastic properties. Due to this link, lamin C can potentially be used as a tool to characterize cellular mechanophenotype. Additionally, future studies exploring involvement of lamin C in mechanotransduction pathways could provide further insight into the specific roles it plays in determining the mechanophenotype of cells in a variety of biological scenarios of clinical relevance, such as stem cell differentiation, cancer progression, and a plethora of laminopathies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank Dr. Jeffrey Morgan for his gift of NHF cells. We would also like to acknowledge the Brown Genomics Core Facility and Dr. Christoph Schorl for assistance with fluorescence-based western blot detection. This work by supported by NIH Grants R01 AR063642 and P20 GM104937 (EMD) and R25 GM083270 (RDGC) and NSF CAREER Award CBET 1253189 (EMD).
Funding
This work by supported by NIH Grants R01 AR063642 and P20 GM104937 (EMD) and R25 GM083270 (RDGC) and NSF CAREER Award CBET 1253189 (EMD).
Conflict of interest
Authors RDGC, JSS, VCF, and EMD have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author Contributions
R.D.G.C. and E.M.D. designed all experiments. R.D.G.C. conducted all AFM experiments. R.D.G.C. and V.C.F. conducted all western blot experiments. J.S.S. conduced all qPCR experiments. R.D.G.C. and E.M.D. analyzed the data and wrote the manuscript.
References
- 1.Akter R, Rivas D, Geneau G, Drissi H, Duque G. Effect of lamin A/C knockdown on osteoblast differentiation and function. J. Bone Miner. Res. 2009;24(2):283–293. doi: 10.1359/jbmr.081010. [DOI] [PubMed] [Google Scholar]
- 2.Alam SG, Zhang Q, Prasad N, Li Y, Chamala S, Kuchibhotla R, Kc B, Aggarwal V, Shrestha S, Jones AL, Levy SE, Roux KJ, Nickerson JA, Lele TP. The mammalian linc complex regulates genome transcriptional responses to substrate rigidity. Sci. Rep. 2016;6:38063. doi: 10.1038/srep38063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bera M, Ainavarapu SR, Sengupta K. Significance of 1b and 2b domains in modulating elastic properties of lamin A. Sci. Rep. 2016;6:27879. doi: 10.1038/srep27879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermeo S, Vidal C, Zhou H, Duque G. Lamin a/c acts as an essential factor in mesenchymal stem cell differentiation through the regulation of the dynamics of the wnt/beta-catenin pathway. J. Cell Biochem. 2015;116(10):2344–2353. doi: 10.1002/jcb.25185. [DOI] [PubMed] [Google Scholar]
- 5.Bordeleau F, Califano JP, Negron Abril YL, Mason BN, LaValley DJ, Shin SJ, Weiss RS, Reinhart-King CA. Tissue stiffness regulates serine/arginine-rich protein-mediated splicing of the extra domain B-fibronectin isoform in tumors. Proc. Natl. Acad. Sci. USA. 2015;112(27):8314–8319. doi: 10.1073/pnas.1505421112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buxboim A, Swift J, Irianto J, Spinler KR, Dingal PC, Athirasala A, Kao YR, Cho S, Harada T, Shin JW, Discher DE. Matrix elasticity regulates lamin-A, C phosphorylation and turnover with feedback to actomyosin. Curr. Biol. 2014;24(16):1909–1917. doi: 10.1016/j.cub.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. 2006;24(1):177–185. doi: 10.1634/stemcells.2004-0159. [DOI] [PubMed] [Google Scholar]
- 8.Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007;2(12):780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- 9.Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ. Res. 2008;102(11):1307–1318. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darling EM, Topel M, Zauscher S, Vail TP, Guilak F. Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. J. Biomech. 2008;41(2):454–464. doi: 10.1016/j.jbiomech.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darling EM, Zauscher S, Guilak F. Viscoelastic properties of zonal articular chondrocytes measured by atomic force microscopy. Osteoarthr. Cartil. 2006;14(6):571–579. doi: 10.1016/j.joca.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Dimitriadis EK, Horkay F, Maresca J, Kachar B, Chadwick RS. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys. J. 2002;82(5):2798–2810. doi: 10.1016/S0006-3495(02)75620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher DZ, Chaudhary N, Blobel G. Cdna sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc. Natl. Acad. Sci. USA. 1986;83(17):6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fong LG, Ng JK, Lammerding J, Vickers TA, Meta M, Cote N, Gavino B, Qiao X, Chang SY, Young SR, Yang SH, Stewart CL, Lee RT, Bennett CF, Bergo MO, Young SG. Prelamin A and lamin A appear to be dispensable in the nuclear lamin A. J. Clin. Invest. 2006;116(3):743–752. doi: 10.1172/JCI27125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Cruz RD, Fonseca VC, Darling EM. Cellular mechanical properties reflect the differentiation potential of adipose-derived mesenchymal stem cells. Proc. Natl. Acad. Sci. USA. 2012;109(24):E1523–E1529. doi: 10.1073/pnas.1120349109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hale CM, Shrestha AL, Khatau SB, Stewart-Hutchinson PJ, Hernandez L, Stewart CL, Hodzic D, Wirtz D. Dysfunctional connections between the nucleus and the actin and microtubule networks in laminopathic models. Biophys. J. 2008;95(11):5462–5475. doi: 10.1529/biophysj.108.139428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada T, Swift J, Irianto J, Shin JW, Spinler KR, Athirasala A, Diegmiller R, Dingal PC, Ivanovska IL, Discher DE. Nuclear lamin stiffness is a barrier to 3d migration, but softness can limit survival. J. Cell Biol. 2014;204(5):669–682. doi: 10.1083/jcb.201308029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho CY, Lammerding J. Lamins at a glance. J. Cell Sci. 2012;125(Pt 9):2087–2093. doi: 10.1242/jcs.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ihalainen TO, Aires L, Herzog FA, Schwartlander R, Moeller J, Vogel V. Differential basal-to-apical accessibility of lamin A/C epitopes in the nuclear lamina regulated by changes in cytoskeletal tension. Nat. Mater. 2015;14(12):1252–1261. doi: 10.1038/nmat4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irianto J, Pfeifer CR, Ivanovska IL, Swift J, Discher DE. Nuclear lamins in cancer. Cell Mol. Bioeng. 2016;9(2):258–267. doi: 10.1007/s12195-016-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janes KA. An analysis of critical factors for quantitative immunoblotting. Sci. Signal. 2015;8(371):rs2. doi: 10.1126/scisignal.2005966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji JY, Lee RT, Vergnes L, Fong LG, Stewart CL, Reue K, Young SG, Zhang Q, Shanahan CM, Lammerding J. Cell nuclei spin in the absence of lamin B1. J. Biol. Chem. 2007;282(27):20015–20026. doi: 10.1074/jbc.M611094200. [DOI] [PubMed] [Google Scholar]
- 23.Jung HJ, Coffinier C, Choe Y, Beigneux AP, Davies BS, Yang SH, Barnes RH, 2nd, Hong J, Sun T, Pleasure SJ, Young SG, Fong LG. Regulation of prelamin a but not lamin C by mir-9, a brain-specific microrna. Proc. Natl. Acad. Sci. USA. 2012;109(7):E423–E431. doi: 10.1073/pnas.1111780109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolb T, Kraxner J, Skodzek K, Haug M, Crawford D, Maass KK, Aifantis KE, Whyte G. Optomechanical measurement of the role of lamins in whole cell deformability. J. Biophotonics. 2017;10(12):1657–1664. doi: 10.1002/jbio.201600198. [DOI] [PubMed] [Google Scholar]
- 25.Labriola NR, Darling EM. Temporal heterogeneity in single-cell gene expression and mechanical properties during adipogenic differentiation. J. Biomech. 2015;48(6):1058–1066. doi: 10.1016/j.jbiomech.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, Lee RT. Lamins A and C but not lamin B1 regulate nuclear mechanics. J. Biol. Chem. 2006;281(35):25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 27.Lammerding J, Lee RT. The nuclear membrane and mechanotransduction: Impaired nuclear mechanics and mechanotransduction in lamin A/C deficient cells. Novartis Found Symp. 2005;264:264–273. [PubMed] [Google Scholar]
- 28.Lanzicher T, Martinelli V, Puzzi L, Del Favero G, Codan B, Long CS, Mestroni L, Taylor MR, Sbaizero O. The cardiomyopathy lamin A/C d192 g mutation disrupts whole-cell biomechanics in cardiomyocytes as measured by atomic force microscopy loading-unloading curve analysis. Sci. Rep. 2015;5:13388. doi: 10.1038/srep13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JS, Hale CM, Panorchan P, Khatau SB, George JP, Tseng Y, Stewart CL, Hodzic D, Wirtz D. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys. J. 2007;93(7):2542–2552. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JM, Nobumori C, Tu Y, Choi C, Yang SH, Jung HJ, Vickers TA, Rigo F, Bennett CF, Young SG, Fong LG. Modulation of Lmna splicing as a strategy to treat prelamin A diseases. J. Clin. Invest. 2016;126(4):1592–1602. doi: 10.1172/JCI85908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J. Biol. Chem. 1993;268(22):16321–16326. [PubMed] [Google Scholar]
- 32.Makhija E, Jokhun DS, Shivashankar GV. Nuclear deformability and telomere dynamics are regulated by cell geometric constraints. Proc. Natl. Acad. Sci. USA. 2016;113(1):E32–E40. doi: 10.1073/pnas.1513189113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mellad JA, Warren DT, Shanahan CM. Nesprins linc the nucleus and cytoskeleton. Curr. Opin. Cell Biol. 2011;23(1):47–54. doi: 10.1016/j.ceb.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Neelam S, Chancellor TJ, Li Y, Nickerson JA, Roux KJ, Dickinson RB, Lele TP. Direct force probe reveals the mechanics of nuclear homeostasis in the mammalian cell. Proc. Natl. Acad. Sci. USA. 2015;112(18):5720–5725. doi: 10.1073/pnas.1502111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngoka LC. Sample prep for proteomics of breast cancer: proteomics and gene ontology reveal dramatic differences in protein solubilization preferences of radioimmunoprecipitation assay and urea lysis buffers. Proteome Sci. 2008;6:30. doi: 10.1186/1477-5956-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostlund C, Bonne G, Schwartz K, Worman HJ. Properties of lamin a mutants found in emery-dreifuss muscular dystrophy, cardiomyopathy and dunnigan-type partial lipodystrophy. J. Cell Sci. 2001;114(Pt 24):4435–4445. doi: 10.1242/jcs.114.24.4435. [DOI] [PubMed] [Google Scholar]
- 37.Pan W, Petersen E, Cai N, Ma G, Run Lee J, Feng Z, Liao K, Leong K. Viscoelastic properties of human mesenchymal stem cells. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2005;5:4854–4857. doi: 10.1109/IEMBS.2005.1615559. [DOI] [PubMed] [Google Scholar]
- 38.Peter M, Kitten GT, Lehner CF, Vorburger K, Bailer SM, Maridor G, Nigg EA. Cloning and sequencing of cdna clones encoding chicken lamins A and B1 and comparison of the primary structures of vertebrate A- and B-type lamins. J. Mol. Biol. 1989;208(3):393–404. doi: 10.1016/0022-2836(89)90504-4. [DOI] [PubMed] [Google Scholar]
- 39.Rotsch C, Radmacher M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophys. J. 2000;78(1):520–535. doi: 10.1016/S0006-3495(00)76614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat. Cell Biol. 2008;10(4):452–459. doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin JW, Spinler KR, Swift J, Chasis JA, Mohandas N, Discher DE. Lamins regulate cell trafficking and lineage maturation of adult human hematopoietic cells. Proc. Natl. Acad. Sci. USA. 2013;110(47):18892–18897. doi: 10.1073/pnas.1304996110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart-Hutchinson PJ, Hale CM, Wirtz D, Hodzic D. Structural requirements for the assembly of linc complexes and their function in cellular mechanical stiffness. Exp. Cell Res. 2008;314(8):1892–1905. doi: 10.1016/j.yexcr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007;3(4):413–438. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swift J, Discher DE. The nuclear lamina is mechano-responsive to ECM elasticity in mature tissue. J. Cell Sci. 2014;127(Pt 14):3005–3015. doi: 10.1242/jcs.149203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341(6149):1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Titushkin I, Cho M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys. J. 2007;93(10):3693–3702. doi: 10.1529/biophysj.107.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc. Natl. Acad. Sci. USA. 2004;101(28):10428–10433. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vorburger K, Kitten GT, Nigg EA. Modification of nuclear lamin proteins by a mevalonic acid derivative occurs in reticulocyte lysates and requires the cysteine residue of the C-terminal cxxm motif. EMBO J. 1989;8(13):4007–4013. doi: 10.1002/j.1460-2075.1989.tb08583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.