Abstract
Objectives
Neural stimulation is well-accepted as an effective therapy for a wide range of neurological disorders. While the scale of clinical devices is relatively large, translational and pilot clinical applications are underway for microelectrode-based systems. Microelectrodes have the advantage of stimulating a relatively small tissue volume which may improve selectivity of therapeutic stimuli. Current microelectrode technology is associated with chronic tissue response which limits utility of these devices for neural recording and stimulation. One approach for addressing the tissue response problem may be to reduce physical dimensions of the device. “Thinking small” is a trend for the electronics industry, and for implantable neural interfaces, the result may be a device that can evade the foreign body response.
Materials and Methods
This review paper surveys our current understanding pertaining to the relationship between implant size and tissue response and the state-of-the-art in ultra-small microelectrodes. A comprehensive literature search was performed using PubMed, Web of Science (Clarivate Analytics), and Google Scholar.
Results
The literature review shows recent efforts to create microelectrodes that are extremely thin appear to reduce or even eliminate the chronic tissue response. With high charge capacity coatings, ultra-microelectrodes fabricated from emerging polymers and amorphous silicon carbide appear promising for neurostimulation applications.
Conclusion
We envision the emergence of robust and manufacturable ultra-microelectrodes that leverage advanced materials where the small cross-sectional geometry enables compliance within tissue. Nevertheless, future testing under in vivo conditions is particularly important for assessing the stability of thin film devices under chronic stimulation.
INTRODUCTION
Neural stimulation is well-accepted as an effective therapy for a wide range of neurological disorders. Deep brain stimulation significantly reduces motor symptoms associated with movement disorders such as essential tremor (1,2) and Parkinson’s Disease (3,4), and has shown promising results in a range of other disorders (5,6,7). Driven by one or more implantable pulse generators, DBS electrodes consist of four cylindrical platinum/iridium contacts on the order of 1.27 mm in diameter and often operate as voltage-controlled devices to deliver amplitudes from 1-3V to stimulate estimated volumes from 100–250 mm2 of tissue for therapeutic ends (8). Assuming an electrode impedance of 1000–2000 Ω, stimulation currents on the order of 1–3 mA per pulse can result (8). Recent developments have shown the feasibility of horizontal current steering with the use of segmented electrodes (9,10).
While the scale of clinical devices is relatively large, translational and pilot clinical applications are underway for microelectrode-based systems. Microelectrodes have the advantage of stimulating a relatively small tissue volume which may improve selectivity of therapeutic stimuli (11). Arrays of microelectrodes, which are capable of recording neural signals and microstimulation, are shown in Fig. 1. In principal, all electrodes are fundamentally capable of either recording or stimulation. What limits the utility of microelectrodes for stimulation is whether or not the electrode-electrolyte interface exhibits sufficient charge injection capacity to induce excitation without surpassing safe levels, and the extent that the material is chemically and physically stable with pulsing in vivo. The Utah (or Blackrock) devices consist of multiple shanks of length 0.5-1.5 mm and cross-sectional area of 1500–3500 μm2 decreasing in dimension approaching the conical tip which consists of either a platinum or iridium oxide coating of 2000 μm2. The Michigan (or Neuronexus) array commonly consists of a single shank of length 3–5 mm with a thickness of 15–50 μm and multiple substrate integrated microelectrode contacts of 121–1250 μm2. Both of these array structures have been used for neural stimulation applications (12,13). For example, the Utah array has been used to explore restoration of tactile and proprioceptive feedback where patterned stimuli are provided as current pulses of 20–80 μA to the human sensory cortex (14). Furthermore, the Utah slant array, consisting of shanks of decreasing length to allow access to fascicular structures, has emerged as one of several strategies for peripheral nerve stimulation (15). These arrays have feature sizes an order of magnitude smaller than those of DBS electrodes and offer enhanced specificity for more precise and selective delivery of stimulation. Nevertheless, implantation of both the Utah and Michigan arrays, as well as arrays of microwires, triggers a chronic tissue response which is associated with a loss of neural cell bodies and processes proximal to the microelectrode sites (16,17). The details associated with the neuroinflammatory response have been well-documented and described previously. In short, this biotic mechanism is believed to originate from the chronic foreign body response (FBR) following initial implantation, and downstream processes that have features consistent with neurotrauma and neurodegeneration (18–23).
Figure 1.
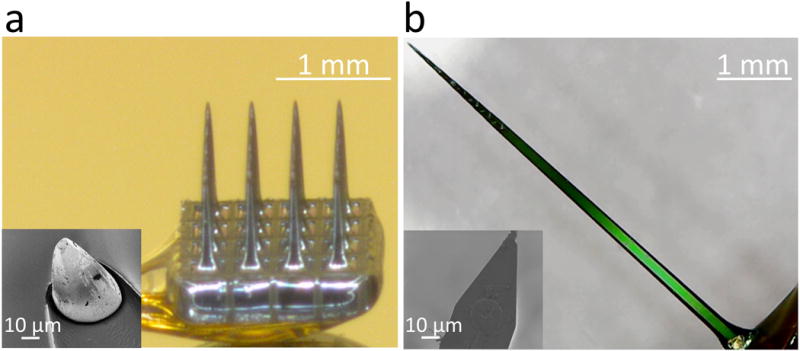
a, An optical micrograph of a commercial, 4×4 cortical microelectrode array produced by Blackrock Microsystems. Using semiconductor processing techniques, a single piece of silicon is fabricated into three dimensional, conical needles. Insulators like Parylene C provide insulation around the individual needles. The conductive tips, consisting of platinum, iridium, or iridium oxide, have a geometric surface area of approximately 2000 μm2; inset shows a scanning electron micrograph of the tip. b, An optical micrograph of an A16 planar Michigan style microelectrode array produced by NeuroNexus. The array is produced in silicon substrates through the addition of multiple conductive and insulating thin films, resulting in an overall device thickness of either 15 or 50 μm. Unlike the Utah style array, the standard singular planar “shank” hosts multiple electrodes, in either linear columns, down the edge of the electrode, or arrayed in sets of four known as tetrodes. The SEM inset shows that limitations from both the planar, two dimensional surface, and real estate lost to route the electrode traces down the shaft of the implant, lead to the NeuroNexus electrodes possessing a smaller geometric surface area of approximately 176 μm2.
Evidence suggests that mechanical mismatch between an implanted device and the surrounding brain tissue plays a significant role in the tissue response. Fig. 2 compares the elastic or Young’s modulus of various materials used in brain implants and surrounding tissue. Brain tissue exhibits a Young’s modulus on the order of 1–10 kilopascals (kPa), whereas Young’s modulus for silicon, a common implantable device material, is 130–170 gigapascals (GPa) (27). There is a substantial literature concerning probes consisting of materials which have a relatively low degree of stiffness, i.e. nearing 10–20 MPa, that appear to elicit decreased tissue response in comparison to their stiffer counterparts (34). The central argument is that soft implantable probes blunt the micromotion induced strain believed to be the source of chronic inflammation (35).
Figure 2.
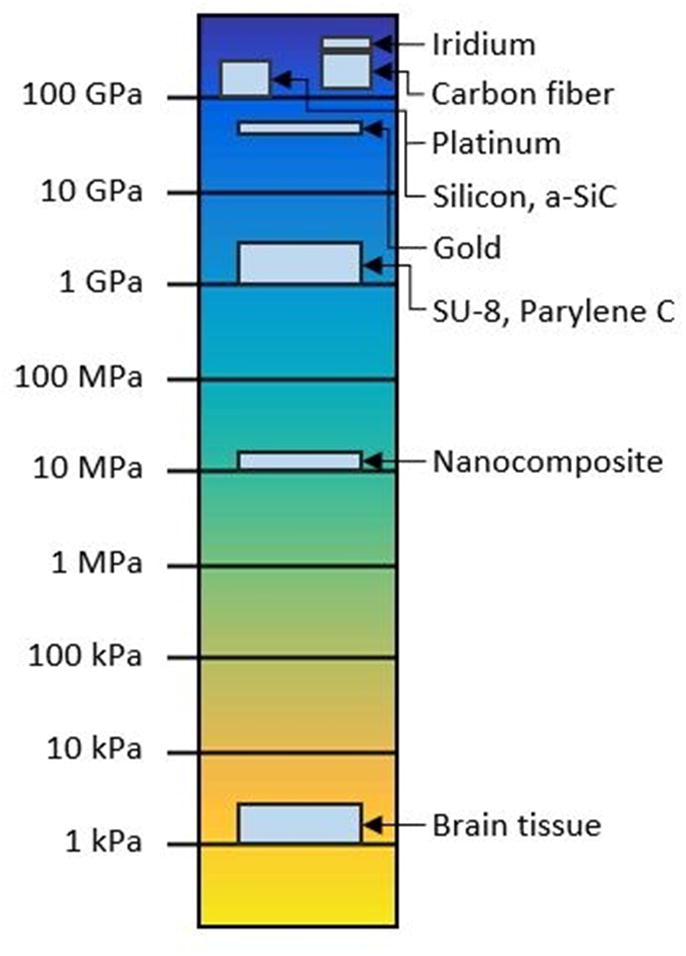
Log scale plot comparing Young’s modulus of commonly used implant materials. Implantable electrodes are usually fabricated from high modulus materials such as silicon, which exhibits a modulus 6–7 orders of magnitude higher than that of brain tissue. Softer, polymeric materials such as SU-8 and bio-inspired nanocomposites may decrease this gap by 3–4 orders of magnitude. Values for moduli of each substrate were taken from published literature: Iridium (24), carbon fiber (25,26), platinum (24), silicon (27), a-SiC (28), gold (29), SU-8 (30), Parylene C (31), nanocomposite (32), and human brain tissue (33).
A seemingly different approach to dealing with the tissue response problem may be to reduce physical dimensions of the device. Interestingly, the evolution of electronics can be characterized, at least in part, with the reduction in feature size of components and devices within integrated circuits (36), and the future progress of neurotechnology may also follow this path. While for electronics, “thinking small” results in improvements in circuit speed and efficiency, for implantable neural interfaces, the result may be a device that can evade the FBR. Quite recently, there have been several studies demonstrating that ultra-small microelectrodes offer an apparent benefit with regard to the tissue response (37,38). These devices are fabricated of materials that inherently exhibit a high modulus of elasticity, but the small cross-sectional areas make them flexible within the brain. Small, compliant devices may decrease the volumetric displacement of brain tissue and minimize the risk of disrupting vascular structures such that there is a significant reduction or elimination of neuroinflammation. The purpose of this paper is to review our current understanding pertaining to the relationship between implant size and tissue response, the state-of-the-art in ultra-small microelectrodes, discuss the advantages and limitations, and identify future research and development opportunities.
Biomechanics of flexible devices
Broadly speaking, stiffness (or compliance) connotes a resistance (or susceptibility) to mechanical deformation. In general, the stiffness of a mechanical structure such as a brain implant depends upon both its geometry and material composition. Stiffness can be measured experimentally by subjecting the structure to a sequence of increasing mechanical loads. The magnitudes of these loads can then be plotted with respect to some measure of deformation (e.g., elongation, deflection). For small deformations, the “stiffness” is taken to be the proportionality constant between these quantities. One can make an estimate of a brain implant’s susceptibility to bending by modeling as a cantilevered beam subjected to transverse mechanical loading (Fig. 3). The stiffness of the beam or, in our case, the implant, is a function of both the area moment of inertia of the cross-section, I, and the elastic (or Young’s) modulus of the material, E. For a given end force, F, an implant of length L will exhibit a deflection, δ. Since the area moment of inertia, I, is related to the geometry of the cross-section, compliant implants can be created using either ultra-thin geometries, which reduce the moment of inertia, or extremely soft materials. Reducing cross sectional dimensions is particularly effective in increasing the compliance of an electrode since the moment of inertia is proportional to the fourth power of the device radius for a circular cross section or the third power of thickness in the bending direction for a rectangular cross section. For purposes of this review, we focus on state-of-the-art ultra-small microelectrode arrays which achieve flexibility through small dimensions.
Figure 3.
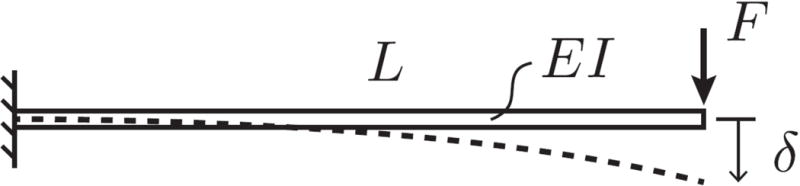
Cartoon depiction of the stiffness of a cantilevered beam subjected to transverse mechanical loads as a representation for a brain implant of length L. I is the moment of inertia, E is the inherent stiffness or modulus of the material, and F is the force required for a deflection, δ.
Silicon devices with smaller cross-sectional areas reduce tissue response
With respect to tissue response, Ratner’s group has studied and reviewed the relationship between implant size and FBR, especially for subcutaneous sensors (39,40). In general, the smaller the sensor size, the less the inflammation due to initial disruption to the surrounding tissue and sustained inflammation from the continued presence of the implant (39). Just how small does an implant need to be to avert a tissue response? The lore among neural engineers suggests that feature sizes below 6 μm should eliminate the tissue response. This threshold appears to be inspired by findings that the single polymer fibers with diameters below that level, implanted subcutaneously parallel to the surface of the skin, evoke little or no response (41). It was hypothesized that disruption of the extracellular matrix oriented parallel to the skin was the basis for the FBR such that thicker implants would augment the disruption and consequently increase the tissue response (39,42). Even though neural interfaces are implanted perpendicular to the surface into highly vascularized tissue, studies suggest some consistency with this sub-10 μm threshold. In one of relatively few studies, Seymour and Kipke examined the tissue response as a function of feature size of the implantable probe (43). The test structure consisted of a single shank containing a major architectural feature consisting of a regular rectangular lattice design, composed of structures with 4, 10, and 30 μm side walls. Lattice dimensions below 5 μm seem to have attenuated cell attachment to its surface, resulting in diminished astrocyte activation associated with the tissue response and concomitant retention of neurons proximal to the sites. In addition, Stice and colleagues compared the tissue response evoked by 12 μm and 25 μm diameter thin microelectrodes showing statistically significant reductions, although not elimination, in activated astrocyte staining for smaller diameter probes after 4 weeks implantation (44). Stice and co-workers suggested three possible reasons for this attenuated tissue response: 1) less CNS tissue displacement with smaller dimension probes; 2) less surface area for adhesion of cells necessary to trigger inflammatory processes; 3) enhanced flexibility of probes with smaller cross-sectional areas. Another possibility put forth by Skousen and colleagues is that the FBR is due to the persistence of activated macrophages and subsequent release of neurotoxic biomolecules; the larger surface area causes concentration elevations proximal to the biotic-abiotic interface (45). It is noteworthy that relatively large implants that become flexible by softening within the brain also show decreased tissue response (34). While both mechanical and geometric factors are important to the tissue response (46), there appears to be a geometric threshold at or below ~10 μm diameter or a cross-sectional area of under ~100 μm2.
While it may seem straightforward to simply create thinner silicon structures as neural implants, material limitations become evident at small scales. Silicon is brittle, will dissolve over time in vivo, and for 1.5-2 mm long probes of thickness of 15 μm, buckling of the probe during insertion and fracture can occur (47). It is also important to recognize that with a reduction in the size of an implant, the microelectrode sites themselves become smaller posing significant challenges at the electrode-electrolyte interface.
Electrical interface challenges for small microelectrodes
Neural stimulation and recording works by a remarkable electrochemical reaction which occurs at the electrode-electrolyte interface. Electrical devices including amplifiers and stimulators operate with the flow of electrons but, physiologically, charge movement is carried by hydrated anions and cations. Conversion occurs at the electrode-electrolyte interface where the larger the surface area of an electrode, the more readily the conversion between electronic and ionic charge can proceed. Decreasing the surface area of any electrode raises the measured impedance of the electrode-electrolyte interface, thereby increasing the thermal, or Johnson, noise and compromising the ability to transfer electrical charge between the electrode and the tissue. Thermal noise at an electrode-electrolyte interface is proportional to the square root of the real component of the impedance, large impedances make it difficult to separate small extracellular signals from baseline noise. For electrical stimulation, it is important to avoid faradaic reactions that may result in nonreversible, toxic interactions with the surrounding tissue (48). In addition, current injection should produce potentials at the electrode that fall within the “water window”. Exceeding these thresholds, which depend on the particular electrode material, will trigger the electrolysis of water to liberate oxygen; alternatively, if the voltage falls below a negative threshold, hydrogen is produced. Clearly, these irreversible reactions are deleterious to the electrode and surrounding tissue. The amount of charge that can be reversibly injected during a stimulation pulse while the potential remains within the water window is called the charge injection capacity, a parameter that is a related to the electrode material and the surface area. When microelectrode sites become too small, they may not provide a sufficient level of charge per stimulation phase to evoke neural activity (49). For example, gold microelectrodes with a surface area of only 100 μm2 have a charge injection capacity below 0.067 mC/cm2. If at least 1–2 nC/phase for a 200 μsec pulse is required to trigger cortical excitation (11,50), then such small microelectrodes fall short. Instead, a charge injection capacity of at least 1–2 mC/cm2 is necessary for effective neural stimulation with microelectrode of this size.
So how do we achieve low impedance and high charge capacity for a small microelectrode? The answer: by coating microelectrode sites with materials that increase reduction-oxygen capacity and effective electrochemical surface area. There are a number of approaches for coating microelectrodes with ceramics, conductive polymers, or metallic oxides. Non-polymeric approaches include sputtered iridium oxide (SIROF), titanium nitride (TiN) (51), or electrodeposited iridium oxide (EIROF) (52) which provide high charge injection capacity and low impedance coatings for electrodes. For example, sputtered iridium oxide can produce a maximum charge injection capacity between 1–5 mC/cm2, which is 10–100 times greater than Pt or Pt/Ir (11). With respect to conductive polymers, the most commonly used coating strategy is poly(3,4-ethylenedioxythiophene) or PEDOT, which is often deposited through oxidative polymerization of ethylenedioxythiophene in the presence of poly(styrenesulfonate) or PSS onto microelectrode sites (53). Cui and Martin demonstrated that application of PEDOT-PSS to microelectrodes decreased the impedance by almost two orders of magnitude (54). PEDOT-PSS also displayed a 15-fold improvement in charge-injection capacity of ~15 mC/cm2 (55). The problem for PEDOT-PSS is durability, and while electrodeposition of PEDOT with a tetrafluoroborate (56,57) or perhaps inclusion of appropriately functionalized carbon nanotubes (58) improves electrochemical performance and stability, long term robustness especially for neural stimulation remains elusive. Instead, metallic/oxide-based coatings are more likely to demonstrate long term stability in vivo for neural stimulation applications. Recently, ultra-small microelectrodes have emerged that leverage conductive materials such as carbon that enable small size for minimizing tissue response.
Ultra-small carbon-fiber bundles for neurostimulation and recording
The use of carbon fiber electrodes for the voltammetric/amperometric detection and quantification of neurochemicals such as dopamine is well known (59,60). For neural recording or stimulation, carbon fibers are electrically conductive and their small cross-sectional area makes them attractive due to their minimal invasiveness during implantation, minimal risk of blood-brain-barrier disruption, and reduced tissue displacement (37,61,62). Carbon fibers have a typical radii of about 4–7 μm and are mechanically stiff with a Young’s modulus of 241 - 380 GPa (25,26). The small cross-sectional area allows the fibers to be mechanically compliant within neuronal tissues (62). Implanted carbon fiber microelectrodes have provided high quality single and multi-unit recordings in acute and chronic experiments (25,26,62). Carbon fiber microelectrodes have been shown to record at high signal-to-noise from a variety of neuronal cell types, across a variety of brain regions at varying depth in different animal models including songbirds (26) and rat cortex (25,62). As shown in Fig. 4, carbon fibers, inserted as bundles that agglomerate when wet to allow insertion without buckling, appear to splay within the tissue to behave as 3D arrays that sample from a volume of neural tissue (26). Splaying dynamics, however, may rely on a delicate balance between the Van der Waal’s force that induces bundling and the tissue mechanics. While penetration and splaying occurs in songbird cortex with carbon fiber bundles, it may or may not work reliably in other species of brain structures that have differing mechanical characteristics.
Figure 4.
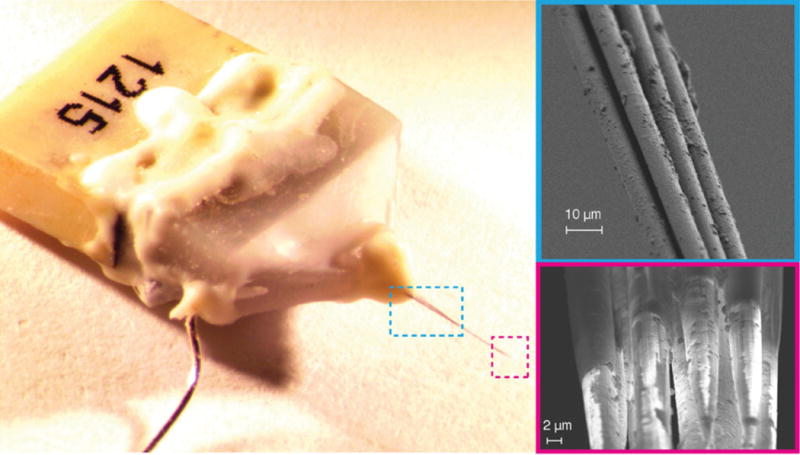
Bundled 16-channel carbon fiber electrode array. When drawn from water bath, the individual shanks are held by weak van der Waals attraction forming an electrode bundle about 26 μm in diameter (upper right). The fire-sharpened process de-insulates the Parylene C coatings creating an exposed electrode tip whose geometric surface area depends largely on the length of the de-insulated fiber (bottom right). From Guitchounts et al. (26) with permission.
There are three major disadvantages of carbon fiber microelectrodes that limit widespread use for neural stimulation applications: 1) The electrochemical characteristics of bare carbon in physiological saline are poorly suited for neural recording and stimulation. For an exposed geometric surface area of 500 μm2, carbon fibers show impedance levels of greater than 1 MΩ at 1 kHz and a charge-injection limit below 0.05 mC/cm2. The majority of prior work with carbon fiber tips has relied on coatings with PEDOT-PSS or PEDOT-PSS with carbon nanotubes (58). Unfortunately, as previously noted, PEDOT is unlikely to provide a stable electrochemical interface for chronic neural stimulation. An alternative to PEDOT is coating with electrodeposited iridium oxide films (EIROF) which have been demonstrated to create stable interfaces for neural stimulation (52,63,64). Coating the tips of carbon fibers with EIROF produced a highly nodular electrode surface (Fig. 5) which reduced the electrode impedance by 10-fold and increased the charge injection capacity to 17 mC/cm2 with appropriate biasing. 2) Regardless of coatings, the fiber-based microelectrodes are fabricated by hand. The tips of carbon fibers, which are coated with a thin layer of Parylene C for insulation, are opened at the distal end either mechanically by using surgical scissors or razor blade to expose the active microelectrode site or by thermal ablation usually with a flame (26). Manual fabrication limits the ability to create reproducible structures for wide spread use and commercial dissemination.
Figure 5.
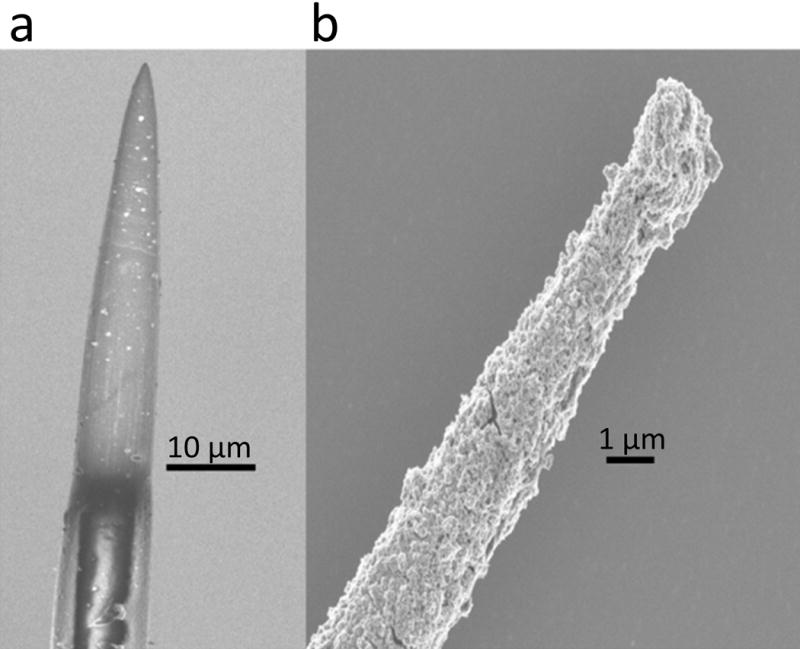
Scanning electron micrograph of a fire-sharpened carbon fiber electrode tip before (left) and after (right) electrodeposited iridium oxide film (EIROF) coatings. EIROF coatings improved the electrochemical properties of the electrode. The nodular surface morphology of EIROF creates a higher electrochemical surface area for charge transfer. With appropriate positive biasing, EIROF coated carbon fiber can readily injected 4 nC/ph in a 200 μs and 400 μs pulses without exceeding water electrolysis limits.
Future opportunities for reproducible and scalable fabrication of ultra-small microelectrode arrays
The key to creating reproducible microscale devices is by leveraging state-of-the-art photolithography and microfabrication techniques. Very recently, Luan et al. reported the development and demonstration of ultra-thin linear arrays of microelectrodes (38). Composed of SU-8, which is conventionally used in microfabrication laboratories as a photo-resist, two types of multilayer probes have been described that are 1 μm in thickness and possess microelectrode widths of either 10 or 50 μm. With such small cross-sectional areas, these highly flexible devices exhibit little or no tissue response after months of implantation in mice where single unit neuronal activity could be readily resolved. While the electrode sites are either Pt or Au, coatings including EIROF or SIROF would likely enable microscale neural stimulation applications. While SU-8 has a relatively positive literature supporting its in vitro and in vivo biocompatibility (65), it is not typically found in implantable biomedical devices and providers (e.g., MicroChem) may be hesitant to grant use for medical applications. Nevertheless, we remain optimistic that polymers, including those with shape memory thermomechanical characteristics (66,67), offer a solution for implantable microscale devices. We note that polymeric materials including silicone, parylene, and polyamide, are all components of the Medtronic DBS Lead Model 3387.
Alternatively, amorphous silicon carbide (a-SiC) has emerged as a candidate encapsulation material for next generation brain implants (68–71). Created through plasma enhanced chemical vapor deposition, a-SiC films exhibit robust long-term stability, high electronic resistivity, and resistance to corrosion (68,72,73). Moreover, a-SiC has an established track record as a biomedical device material, specifically as a coronary stent coating (74). Prototype arrays of microelectrodes designed for intracortical stimulation and recording that utilize a-SiC as a structural and insulating material have been developed by the Cogan group and are shown in Fig. 6. These devices consist of individual shanks <10 μm wide yielding a shank cross-sectional area of <60 μm2. These small dimensions and mechanical robustness of the film enable overall device flexibility. In contrast to carbon fibers, a-SiC processing is amenable to thin-film fabrication processes and photolithography. In addition, a-SiC devices can be readily customized and reliably reproduced at scales necessary for translation. To achieve suitable charge injection capacity, microelectrode sites can be coated with sputtered iridium oxide (SIROF) or porous titanium nitride (TiN) to produce low impedance coatings resulting in 100 μm2 sites with charge injection capacities >3 mC/cm2. These devices have the potential to achieve highly spatially selective neural stimulation under chronic conditions where the tissue response is minimized due to the small physical dimensions.
Figure 6.
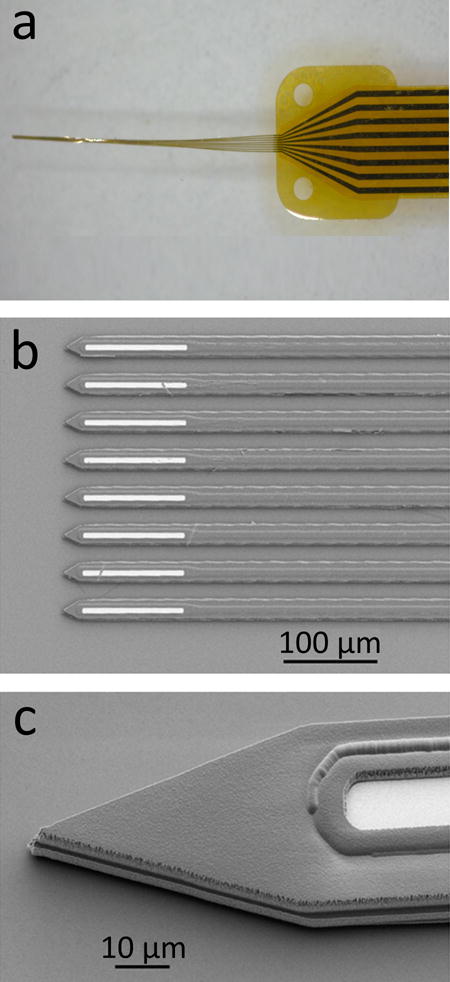
An 8-channel a-SiC microelectrode array (MEA) developed using standard semiconductor fabrication processes. The MEAs are fabricated on a thin layer of polyimide which is spin-coated on a silicon carrier wafer. After fabrication, the carrier wafer is soaked in deionized water to release the devices. a, When withdrawn from deionized water, the shanks of the a-SiC MEA forms a bundle. b, optical micrograph showing the electrode sites at the distal end of the array. Electrode openings are created by reactive ion etching. c, scanning electron micrograph showing the tip profile with a near-vertical sidewall created using an inductively coupled plasma etching system.
Practical considerations for clinical use of arrays of ultra-small microelectrodes
After fabrication issues are solved, there will be a challenge with respect to in vivo imaging of the ultra-small microelectrodes to verify the exact position in the brain after implantation. The value of spatial resolution enabled by ultra-small microelectrodes will be limited if devices are readily dislodged or experience migration. Experiments in rodents with 7T magnetic resonance imaging (MRI) demonstrated imaging of 50 μm diameter Tungsten microwires (75,76), however the limited resolution of clinical MRI will be problematic for imaging ultrasmall microelectrodes. Furthermore, a consequence of microscale neural stimulation strategies will likely be the challenge of programming a larger number of electrode sites. For DBS, programming is typically performed empirically by surveying the effects of monopolar stimulation to determine an optimal stimulation configuration. It may not be practical to survey the vast parameter space associated with potentially many ultra-small microelectrodes. Model based optimization approaches may facilitate discovery of optimal settings (77), but it is also entirely likely that there will be only marginal improvement in therapy for certain disorders treated with DBS given the volume of tissue required for activation to achieve results (78). Instead, the benefits of spatial precision enabled by microscale neural stimulation are more likely to be realized for emergent neuroprosthetic applications such as restoration of proprioceptive/tactile sensation or visual perception through cortical stimulation. In these applications, the topographic organization of the brain cortices and relationship to sensation will aid in the initial programming stage, however the large neurostimulation parameter space will remain a challenge.
Conclusion
A natural evolution for electronic devices, including those of biomedical use, involves the decrease in size. For chronically implantable neural interfaces, “thinking small” results in size scales approaching the dimensions of cells within the brain, offering the possibility of more selective spatial stimulation and minimization of the tissue reactions by effectively evading the FBR. This tissue response likely involves both the inherent stiffness of the materials comprising the device and the physical dimensions of the implant. Extremely flexible devices of sufficiently small cross-sectional areas can be fabricated from inherently stiff and physically robust materials. For any ultra-small microelectrode technology to become reproducible and ultimately translatable to the clinic, it is vital that devices can be created at scale and are comprised of robust materials. In spite of its widespread use, Parylene C is well known to have significant limitations with respect to robustness (79) necessitating the exploration of alternative materials such as liquid crystal polymers, which are chemically inert and have very low permeability (80). Alternatively, a-SiC has advantages as an extremely robust structural and insulating material that can be coupled with state-of-the-art microelectrode coatings such as SIROF to enable a wide range of microscale neural stimulation applications. Future studies will be necessary to compare the relative merits of probes of differing materials including a-SiC with respect to tissue response and device longevity. Testing under in vivo conditions is particularly important, since the rigors of in vivo stimulation can stress thin-film devices and could lead to dissolution of metal contact pads or their coatings, as well as other modes of device failure such as delamination.
Acknowledgments
Source(s) of financial support:
This work was sponsored by the Defense Advanced Research Projects Agency (DARPA) BTO under the auspices of Dr. Douglas Weber through the Space and Naval Warfare Systems Center, Pacific Grant/Contract No. HR0011-15-2-0017 to SFC and a NIH grant U01NS090454-01 to Dr. Timothy Gardner.
Footnotes
Drs. Joseph Pancrazio and Stuart Cogan organized and interpreted the core ideas and contributed the main text of the manuscript. Dr. Victor Varner contributed significant concepts on biomechanics of implantable probes. Felix Deku and Dr. Timothy Gardner contributed ideas on amorphous silicon carbide based implantable devices and carbon fiber based devices. Atefeh Ghazavi and Rashed Rihani provided a survey of electrical properties of novel coatings. Allison M. Stiller contributed a survey of material properties associated with implantable devices. Dr. Christopher L. Frewin contributed ideas associated with the tissue response to implantable devices.
Conflict of Interest Statement: The authors have no relevant conflicts of interest to disclose.
References
- 1.Benabid AL, Pollak P, Gao DM, Hoffmann D, Limousin P, Gay E, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84:203–214. doi: 10.3171/jns.1996.84.2.0203. [DOI] [PubMed] [Google Scholar]
- 2.Flora ED, Perera CL, Cameron AL, Maddern GJ. Deep brain stimulation for essential tremor: A systematic review. Mov Disord. 2010;25:1550–1559. doi: 10.1002/mds.23195. [DOI] [PubMed] [Google Scholar]
- 3.Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wichmann T, DeLong MR. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron. 2006;52:197–204. doi: 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57:510–516. doi: 10.1016/j.biopsych.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry. 2008;79:136–142. doi: 10.1136/jnnp.2006.104067. [DOI] [PubMed] [Google Scholar]
- 8.Butson CR, Maks CB, McIntyre CC. Sources and effects of electrode impedance during deep brain stimulation. Clin Neurophysiol. 2005;117:447–454. doi: 10.1016/j.clinph.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contarino MF, Bour LJ, Verhagen R, Lourens MA, de Bie RM, van den Munckhof P, et al. Directional steering: A novel approach to deep brain stimulation. Neurology. 2014;83:1163–1169. doi: 10.1212/WNL.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 10.Steigerwald F, Müller L, Johannes S, Matthies C, Volkmann J. Directional deep brain stimulation of the subthalamic nucleus: A pilot study using a novel neurostimulation device. Mov Disord. 2016;31:1240–1243. doi: 10.1002/mds.26669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cogan SF. Neural stimulation and recording electrodes. Annu Rev Biomed Eng. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- 12.Torab K, Davis TS, Warren DJ, House PA, Normann RA, Greger B. Multiple factors may influence the performance of a visual prosthesis based on intracortical microstimulation: nonhuman primate behavioural experimentation. J Neural Eng. 2011;8:035001. doi: 10.1088/1741-2560/8/3/035001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost SB, Dunham CL, Barbay S, Krizsan-Agbas D, Winter MK, Guggenmos DJ, Nudo RJ. Output properties of the cortical hindlimb motor area in spinal cord-injured rats. J Neurotrauma. 2015;32:1666–1673. doi: 10.1089/neu.2015.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flesher SN, Collinger JL, Foldes ST, Weiss JM, Downey JE, Tyler-Kabara EC, et al. Intracortical microstimulation of human somatosensory cortex. Sci Transl Med. 2016;8:361ra141. doi: 10.1126/scitranslmed.aaf8083. [DOI] [PubMed] [Google Scholar]
- 15.Davis TS, Wark HAC, Hutchinson DT, Warren DJ, O’Neill K, Scheinblum T, et al. Restoring motor control and sensory feedback in people with upper extremity amputations using arrays of 96 microelectrodes implanted in the median and ulnar nerves. J Neural Eng. 2016(13):036001. doi: 10.1088/1741-2560/13/3/036001. [DOI] [PubMed] [Google Scholar]
- 16.Ward MP, Rajdev P, Ellison C, Irazoqui PP. Toward a comparison of microelectrodes for acute and chronic recordings. Brain Res. 2009;1282:183–200. doi: 10.1016/j.brainres.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 17.Jorfi M, Skousen JL, Weder C, Capadona JR. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J Neural Eng. 2015;12:011001. doi: 10.1088/1741-2560/12/1/011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rousche PJ, Normann RA. Chronic recording capability of the Utah intracortical electrode array in cat sensory cortex. J Neurosci Meth. 1998;82:1–15. doi: 10.1016/s0165-0270(98)00031-4. [DOI] [PubMed] [Google Scholar]
- 19.Turner JN, Shain W, Szarowski DH, Andersen M, Martins S, Isaacson M, et al. (1999) Cerebral astrocyte response to micromachined silicon implants. Exp Neurol. 1999;156:33–49. doi: 10.1006/exnr.1998.6983. [DOI] [PubMed] [Google Scholar]
- 20.Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, et al. Brain responses to micro-machined silicon devices. Brain Res. 2003;983:23–35. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- 21.Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195:115–126. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Meth. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Grill WM, Norman SE, Bellamkonda RV. Implanted neural interfaces: Biochallenges and engineered solutions. Annu Rev Biomed Eng. 2009;11:1–24. doi: 10.1146/annurev-bioeng-061008-124927. [DOI] [PubMed] [Google Scholar]
- 24.Merker J, Lupton D, Töpfer M, Knake H. High temperature mechanical properties of the platinum group metals. Platinum Metals Rev. 2001;45:74–82. [Google Scholar]
- 25.Patel PR, Na K, Zhang H, Kozai TD, Kotov NA, Yoon E, et al. Insertion of linear 8.4 μm diameter 16 channel carbon fiber electrode arrays for single unit recordings. J Neural Eng. 2015;12:046009. doi: 10.1088/1741-2560/12/4/046009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guitchounts G, Markowitz JE, Liberti WA, Gardner TJ. A carbon-fiber electrode array for long-term neural recording. J Neural Eng. 2013;10:046016. doi: 10.1088/1741-2560/10/4/046016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho CH. Characterization of Young’s modulus of silicon versus temperature using a “beam deflection” method with a four-point bending fixture. Curr Appl Physics. 2009;9:538–545. [Google Scholar]
- 28.Kulikovsky V, Vorlíček V, Boháč P, Stranyánek M, Čtvrtlík R, Kurdyumov A, et al. Hardness and elastic modulus of amorphous and nanocrystalline SiC and Si films. Surf Coat Technol. 2008;202:1738–1745. [Google Scholar]
- 29.Salvadori MC, Brown IG, Vaz AR, Melo LL, Cattani M. Measurement of the elastic modulus of nanostructured gold and platinum thin films. Phys Rev B. 2003;67:153404. [Google Scholar]
- 30.Lorenz H, Despont M, Fahrni N, LaBianca N, Renaud P, Vettiger P. SU-8: a low-cost negative resist for MEMS. J Micromech Microeng. 1997;7:121. [Google Scholar]
- 31.Hassler C, von Metzen RP, Ruther P, Stieglitz T. Characterization of parylene C as an encapsulation material for implanted neural prostheses. J Biomed Mater Res B Appl Biomater. 2010;93:266–274. doi: 10.1002/jbm.b.31584. [DOI] [PubMed] [Google Scholar]
- 32.Shanmuganathan K, Capadona JR, Rowan SJ, Weder C. Stimuli-responsive mechanically adaptive polymer nanocomposites. ACS Appl Mater Interfaces. 2010;2:165–174. doi: 10.1021/am9006337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budday S, Nay R, de Rooij R, Steinmann P, Wyrobek T, Ovaert TC, et al. Mechanical properties of gray and white matter brain tissue by indentation. J Mech Behav Biomed Mater. 2015;46:318–330. doi: 10.1016/j.jmbbm.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen JK, Park DJ, Skousen JL, Hess-Dunning AE, Tyler DJ, Rowan SJ, et al. Mechanically-compliant intracortical implants reduce the neuroinflammatory response. J Neural Eng. 2014;11:056014. doi: 10.1088/1741-2560/11/5/056014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sridharan A, Nguyen JK, Capadona JR, Muthuswamy J. Compliant intracortical implants reduce strains and strain rates in brain tissue in vivo. J Neural Eng. 2015;12:036002. doi: 10.1088/1741-2560/12/3/036002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizal B, Merlo JM, Burns MJ, Chiles TC, Naughton MJ. Nanocoaxes for optical and electronic devices. Analyst. 2015;140:39–58. doi: 10.1039/c4an01447b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozai TD, Marzullo TC, Hooi F, Langhals NB, Majewska AK, Brown EB, et al. Reduction of neurovascular damage resulting from microelectrode insertion into the cerebral cortex using in vivo two-photon mapping. J Neural Eng. 2010;7:046011. doi: 10.1088/1741-2560/7/4/046011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luan L, Wei X, Zhao Z, Siegel JJ, Potnis O, Tuppen CA, et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci Adv. 2017;3:e1601966. doi: 10.1126/sciadv.1601966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helton KL, Ratner BD, Wisniewski NA. Biomechanics of the sensor-tissue interface-effects of motion, pressure, and design on sensor performance and the foreign body response-part I: theoretical framework. J Diabetes Sci Technol. 2011;5:632–646. doi: 10.1177/193229681100500317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helton KL, Ratner BD, Wisniewski NA. Biomechanics of the sensor-tissue interface-effects of motion, pressure, and design on sensor performance and foreign body response-part II: examples and application. J Diabetes Sci Technol. 2011;5:647–656. doi: 10.1177/193229681100500318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanders JE, Stiles CE, Hayes CL. Tissue response to single-polymer fibers of varying diameters: evaluation of fibrous encapsulation and macrophage density. J Biomed Mater Res. 2000;52:231–237. doi: 10.1002/1097-4636(200010)52:1<231::aid-jbm29>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 42.Sanders JE, Cassisi DV, Neumann T, Golledge SL, Zachariah SG, Ratner BD, et al. Relative influence of polymer fiber diameter and surface charge on fibrous capsule thickness and vessel density for single-fiber implants. J Biomed Mater Res A. 2003;65:462–467. doi: 10.1002/jbm.a.10525. [DOI] [PubMed] [Google Scholar]
- 43.Seymour JP, Kipke DR. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials. 2007;28:3594–3607. doi: 10.1016/j.biomaterials.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 44.Stice P, Gilletti A, Panitch A, Muthuswamy J. Thin microelectrodes reduce GFAP expression in the implant site in rodent somatosensory cortex. J Neural Eng. 2007;4:42–53. doi: 10.1088/1741-2560/4/2/005. [DOI] [PubMed] [Google Scholar]
- 45.Skousen JL, Merriam SM, Srivannavit O, Perlin G, Wise KD, Tresco PA. Reducing surface area while maintaining implant penetrating profile lowers the brain foreign body response to chronically implanted planar silicon microelectrode arrays. Prog Brain Res. 2011;194:167–180. doi: 10.1016/B978-0-444-53815-4.00009-1. [DOI] [PubMed] [Google Scholar]
- 46.Spencer KC, Sy JC, Ramadi KB, Graybiel AM, Langer R, Cima MJ. Characterization of mechanically matched hydrogel coatings to improve the biocompatibility of neural implants. Sci Rep. 2017;7:1952. doi: 10.1038/s41598-017-02107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weltman A, Yoo J, Meng E. Flexible, penetrating brain probes enabled by advances in polymer microfabrication. Micromachines. 2016;2016(7):180. doi: 10.3390/mi7100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merrill DR, Bikson M, Jefferys JG. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Meth. 2005;141:171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 49.Cogan SF, Ehrlich J, Plante TD, Smirnov A, Shire DB, Gingerich M, et al. Sputtered iridium oxide films for neural stimulation electrodes. J Biomed Mater Res B Appl Biomater. 2009;89:353–361. doi: 10.1002/jbm.b.31223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCreery DB. Cochlear nucleus auditory prostheses. Hear Res. 2008;242:64–73. doi: 10.1016/j.heares.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 51.Weiland JD, Anderson DJ, Humayun MS. In vitro electrical properties for iridium oxide versus titanium nitride stimulating electrodes. IEEE Trans Biomed Eng. 2002;49:1574–1579. doi: 10.1109/TBME.2002.805487. [DOI] [PubMed] [Google Scholar]
- 52.Meyer RD, Cogan SF, Nguyen TH, Rauh RD. Electrodeposited iridium oxide for neural stimulation and recording electrodes. IEEE Trans Neural Syst Rehabil Eng. 2001;9:2–11. doi: 10.1109/7333.918271. [DOI] [PubMed] [Google Scholar]
- 53.Forcelli PA, Sweeney CT, Kammerich AD, Lee BC, Rubinson LH, Kayinamura YP, et al. Histocompatibility and in vivo signal throughput for PEDOT, PEDOP, P3MT, and polycarbazole electrodes. J Biomed Mater Res A. 2012;100:3455–3462. doi: 10.1002/jbm.a.34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cui X, Martin DC. Electrochemical deposition and characterization of poly (3,4-ethylenedioxythiophene) on neural microelectrode arrays. Sens Actuators B Chem. 2003;89:92–102. [Google Scholar]
- 55.Venkatraman S, Hendricks J, King ZA, Sereno AJ, Richardson-Burns S, Martin D, et al. In vitro and in vivo evaluation of PEDOT microelectrodes for neural stimulation and recording. IEEE Trans Neural Syst Rehabil Eng. 2011;19:307–316. doi: 10.1109/TNSRE.2011.2109399. [DOI] [PubMed] [Google Scholar]
- 56.Mandal HS, Kastee JS, McHail DG, Rubinson JF, Pancrazio JJ, Dumas TC. Improved poly(3,4-ethylenedioxythiophene) (PEDOT) for neural stimulation. Neuromodulation. 2015;18:657–663. doi: 10.1111/ner.12285. [DOI] [PubMed] [Google Scholar]
- 57.Mandal HS, Knaack GL, Charkhkar H, McHail DG, Kastee JS, Dumas TC, et al. Improving the performance of poly(3,4-ethylenedioxythiophene) for brain-machine interface applications. Acta Biomater. 2014;10:2446–2454. doi: 10.1016/j.actbio.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 58.Kozai TD, Catt K, Du Z, Na K, Srivannavit O, Haque RU, et al. Chronic in vivo evaluation of PEDOT/CNT for stable neural recordings. IEEE Trans Biomed Eng. 2016;63:111–119. doi: 10.1109/TBME.2015.2445713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang DA, Rand E, Marsh M, Andrews RJ, Lee KH, Meyyappan M, et al. Carbon nanofiber electrode for neurochemical monitoring. Mol Neurobiol. 2013;48:380–385. doi: 10.1007/s12035-013-8531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodeberg NT, Sandberg SG, Johnson JA, Phillips PE, Wightman RM. Hitchhiker’s guide to voltammetry: acute and chronic electrodes for in vivo fast-scan cyclic voltammetry. ACS Chem Neurosci. 2017;8:221–234. doi: 10.1021/acschemneuro.6b00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bjornsson CS, Oh SJ, Al-Kofahi YA, Lim YJ, Smith KL, Turner JN, et al. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. J Neural Eng. 2006;3:196–207. doi: 10.1088/1741-2560/3/3/002. [DOI] [PubMed] [Google Scholar]
- 62.Kozai TDY, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat Mater. 2012;11:1065–1073. doi: 10.1038/nmat3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X, McCreery DB, Bullara LA, Agnew WF. Evaluation of the stability of intracortical microelectrode arrays. IEEE Trans Neural Syst Rehabil Eng. 2006;14:91–100. doi: 10.1109/TNSRE.2006.870495. [DOI] [PubMed] [Google Scholar]
- 64.Han M, Manoonkitiwongsa PS, Wang CX, McCreery DB. In vivo validation of custom-designed silicon-based microelectrode arrays for long-term neural recording and stimulation. IEEE Trans Biomed Eng. 2012;59:346–354. doi: 10.1109/TBME.2011.2172440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nemani KV, Moodie KL, Brennick JB, Su A, Gimi B. In vitro and in vivo evaluation of SU-8 biocompatibility. Mater Sci Eng C Mater Biol Appl. 2013;33:4453–4459. doi: 10.1016/j.msec.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ware T, Simon D, Liu C, Musa T, Vasudevan S, Sloan AM, et al. Thiol-ene/Acrylate substrates for softening intracortical electrodes. J Biomed Mater Res B Appl Biomater. 2014;102:1–11. doi: 10.1002/jbmb.32946. [DOI] [PubMed] [Google Scholar]
- 67.Simon DM, Charkhkar H, St John C, Rajendran S, Kang T, Reit R, et al. Design and demonstration of an intracortical probe technology with tunable modulus. J Biomed Mater Res A. 2017;105:159–168. doi: 10.1002/jbm.a.35896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cogan SF, Edell DJ, Guzelian AA, Ping Liu Y, Edell R. Plasma-enhanced chemical vapor deposited silicon carbide as an implantable dielectric coating. J Biomed Mater Res A. 2003;67:856–867. doi: 10.1002/jbm.a.10152. [DOI] [PubMed] [Google Scholar]
- 69.Hsu JM, Tathireddy P, Rieth L, Normann AR, Solzbacher F. Characterization of a-SiC(x):H thin films as an encapsulation material for integrated silicon based neural interface devices. Thin Solid Films. 2007;516:34–41. doi: 10.1016/j.tsf.2007.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knaack GL, McHail DG, Borda G, Koo B, Peixoto N, Cogan S, et al. In vivo characterization of amorphous silicon carbide as a biomaterial for chronic neural interfaces. Front Neurosci. 2016;10:301. doi: 10.3389/fnins.2016.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lei X, Kane S, Cogan S, Lorach H, Galambos L, Huie P, et al. SiC protective coating for photovoltaic retinal prosthesis. J Neural Eng. 2016;13:046016. doi: 10.1088/1741-2560/13/4/046016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Azevedo RG, Zhang J, Jones DG, Myers DR, Jog AV, Jamshidi B, et al. Silicon carbide coated MEMS strain sensor for harsh environment applications. 2007 IEEE 20th Intern Conf Micro Electro Mech Syst (MEMS) 2007:643–646. [Google Scholar]
- 73.Zorman CA. Silicon carbide as a material for biomedical microsystems. Symp Design, Test, Integr Pack MEMS/MOEMS. 2009:1–7. [Google Scholar]
- 74.Kalnins U, Erglis A, Dinne I, Kumsars I, Jegere S. Clinical outcomes of silicon carbide coated stents in patients with coronary artery disease. Med Sci Monit. 2002;8:PI16–20. [PubMed] [Google Scholar]
- 75.Neuberger T, Paralikar K, Zhang Z, Clement RS, Webb A. Monitoring brain dimpling and intracortical micro-electrode arrays with high resolution MRI in rats. Proc Intl Soc Mag Reson Med. 2007;15:2352. [Google Scholar]
- 76.Paralikar KJ, Neuberger T, Matsui JT, Barber AJ, Webb A, Clement RS. Feasibility and safety of longitudinal magnetic resonance imaging in a rodent model with intracortical microwire implants. J Neural Eng. 2009;2009(6):034001. doi: 10.1088/1741-2560/6/3/034001. [DOI] [PubMed] [Google Scholar]
- 77.Xiao Y, Peña E, Johnson MD. Theoretical optimization of stimulation strategies for a directionally segmented deep brain stimulation electrode array. IEEE Trans Biomed Eng. 2016;63:359–371. doi: 10.1109/TBME.2015.2457873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teplitzky BA, Zitella LM, Xiao Y, Johnson MD. Model-based comparison of deep brain stimulation array functionality with varying number of radial electrodes and machine learning feature sets. Front Comput Neurosci. 2016;10:58. doi: 10.3389/fncom.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Minnikanti S, Diao G, Pancrazio JJ, Xie X, Rieth L, Solzbacher F, et al. Lifetime assessment of atomic-layer-deposited Al2O3-Parylene C bilayer coating for neural interfaces using accelerated age testing and electrochemical characterization. Acta Biomater. 2014;10:960–967. doi: 10.1016/j.actbio.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 80.Fekete Z, Pongrácz A. Multifunctional soft implants to monitor and control neural activity in the central and peripheral nervous system: A review. Sens Actuators B Chem. 2017;243:1214–1223. [Google Scholar]


