Abstract
Background
In many children with unilateral spastic cerebral palsy (USCP), the corticospinal tract to the affected hand atypically originates in the hemisphere ipsilateral to the affected hand. Such ipsilateral connectivity is on average a predictor of poor hand function. However, there is high variability in hand function in these children, which might be explained by the complexity of motor representations of both hands in the contralesional hemisphere.
Objective
To measure the link between hand function and the size and excitability of motor representations of both hands, and their overlap, in the contralesional hemisphere children with USCP.
Methods
We used single-pulse transcranial magnetic stimulation to measure the size and excitability of motor representations of both hands, and their overlap, in the contralesional hemisphere of 50 children with USCP. We correlated these measures with manual dexterity of the affected hand, bimanual performance, and mirror movement strength.
Results
The main and novel findings were (1) the large overlap in contralesional motor representations of the two hands and (2) the moderate positive associations of the size and excitability of such shared-site representations with hand function. Such functional associations were not present for overall size and excitability of representations of the affected hand.
Conclusions
Greater relative overlap of the affected hand representation with the less-affected hand representation within the contralesional hemisphere was associated with better hand function. This association suggests that overlapping representations might be adaptively “yoked” such that cortical control of the child’s less-affected hand supports that of the affected hand.
Keywords: hemiparesis, corticospinal tract, hand function, transcranial magnetic stimulation
Introduction
Unilateral spastic cerebral palsy (USCP) is typically characterized by motor skill impairments on the side of the body contralateral to the lesion. However, the range of severity in impairment is large. Many factors potentially contribute to impaired hand function in children with USCP. The type of corticospinal tract (CST) pattern, and the timing, location, and size of brain lesions, predict upper limb function in USCP (reviews1,2). In many children with USCP, the CST to the affected hand does not originate in the hemisphere contralateral to the affected hand, as it does in typically developing children. Very early in development, the CST of each hemisphere projects bilaterally to the spinal cord3. In typical development, ipsilateral (same-sided) CST projections are pruned, while contralateral CST projections are strengthened4. In USCP, a loss of activity from the lesioned hemisphere have been shown to contribute to a loss of the CST from the lesioned hemisphere and maintained CST projections to each hand from the contralesional hemisphere5,6. The presence of same-sided (ipsilateral) CST connections to the affected hand is associated with poorer hand function than preserved crossed CST connections to the affected hand2,7–10. However, even among children with this ipsilateral CST pattern, there is high variability in hand function9. The source of this variability remains unknown.
Motor map organization might explain some variability in hand function in children with USCP. Changes in the areal size and total excitability of motor representations of an affected limb following injury and intervention have been landmarked in a range of contexts (e.g. animal models11–14, adult stroke15, amputation16, spinal cord injury17) and in healthy adults (e.g. 12). Although our group found an association between changes in map size and improved hand function after intensive bimanual training in children with USCP, we found no correlation between excitability of motor representations and hand function18. Also in USCP, but not in the context of therapeutic intervention, Kesar et al.19 found no link between motor function and the excitability of motor representations. Since sample sizes in both studies were small (n=1018, n=719), associations might be identified in a larger study.
Healthy animal models and those of cerebral palsy have suggested that the relative overlap of primary motor cortex (M1) motor representations, or so-called ‘dual-response representations’, is more predictive of hand skill than the absolute size and excitability of such representations. A study with healthy squirrel monkeys showed an increase in dual-response representations of movement combinations were more frequently used following digit skill training20. The authors suggested that the temporal correlation of movements drove changes in cortical motor reorganization. Similarly, increases in dual-response representations have been shown following activity-dependent use in cats21. In contrast, damage to M1 (by means of muscimol inactivation) have been shown to dramatically decrease dual-response representations22. Thus, dual-response representations in the context of damage to a primary motor structure and in the context of motor-skill intervention suggest at least some functional importance in their relative existence, at least with regards to contralateral representations of multiple joints within a given limb. In USCP, representations of both hands are often predominantly found in the contralesional hemisphere. Similar to the convergence of within-limb representations, convergence of between-limb representations in USCP might be related to function. Whether such representations are overlapping, and at all related to function, is currently unknown. It is conceivable that such representations overlap to some extent such that the cortical control of the unaffected hand can assist or be adaptively yoked to the advantage of the affected hand. On the other hand, unlike the convergence of within-limb representations, the convergence of between-limb representations might be negatively related to function, as that which has been shown in focal hand dystonia23.
We investigated the relationship between hand function and the size and excitability of contralesional motor representations of the affected hand, and the overlap of these representations with that of the less-affected hand, using single-pulse transcranial magnetic stimulation (TMS). We focused on children with contralesional (i.e. ipsilateral) CST connections to the affected hand because (1) most children with USCP have such CST patterns (either predominantly, called an ipsilateral CST pattern, or combined with contralateral connections, called a bilateral CST pattern)9,24; (2) the source of variability in hand function in children with contralesional CST patterns is unknown; and (3) overlap in motor representations of the two hands can only be studied in children whose maps are both located in one hemisphere. We hypothesized that hand function would be positively associated with overall size and excitability of affected hand motor representations, and their overlap with less-affected hand representations.
Methods
Participants
Fifty children with USCP participated. Table 1 shows demographics and clinical scores for children included in the final analyses. Some functional and motor mapping data have been used: n=23 and n=17 of this study’s participants were included in10 and 18, respectively. Pre-intervention unimanual and bimanual measures for these participants are in10 and 18. The measure of total map size for these participants is used in their comparison with post-intervention scores in18. However, neither publications addressed the hypotheses presented here.10,18
Table 1.
Demographic and clinical characteristics of participants, stratified by CST pattern
| Ipsilateral (n=30) |
Bilateral (n=14) |
|
|---|---|---|
| Age in years, months M (SD) | 10,0 (2,11) | 10,7 (3,10) |
| Gender | ||
| Male | 17 (57%) | 10 (71%) |
| Female | 13 (43%) | 4 (29%) |
| Lesion side (type a,b,c)* | ||
| Left | 13 (0, 7, 6) | 10 (0, 4, 5) |
| Right | 17 (2, 9, 6) | 4 (0, 3, 1) |
| MACS level* | ||
| I | 5 (17%) | 3 (21%) |
| II | 19 (63%) | 5 (36%) |
| III | 6 (20%) | 6 (43%) |
Lesion type:
Brain malformation –note, all children with this lesion type were within 2 standard deviations of the mean of their respective CST subgroups on all measures of interest.
Abnormality of periventricular white matter.
Cortical/subcortical lesion (note one subject with a bilateral CST pattern did not receive an MRI, so we were unable to identify their lesion type); MACS: Manual Ability Classification System.
Participants were recruited from clinics, online communities, ClinicalTrials.gov (NCT00305006), and our website (http://www.tc.edu/centers/cit/) as part of a larger intervention study; data presented here were pre-intervention measures. Inclusion criteria were (1) congenital USCP, (2) ability to lift arms 15 cm above a surface and grasp light objects, (3) mainstreamed in school (n=2 received special education services), and (4) ability to follow instructions. Exclusion criteria were (1) medical illness that would interfere with participation, (2) seizure history after age 2 or using anti-seizure medications, (3) uncorrected visual problems, (4) severe spasticity (Ashworth ≥ 3), (5) affected hand surgery in last year, (6) botulinum toxin in upper extremity in last six months, and (7) non-removable metal in body. All study procedures were approved by Institutional Review Boards where testing was conducted (Teachers College, Columbia University, Burke Rehabilitation Hospital, Université Catholique de Louvain, and New York State Psychiatric Institute and Weill Cornell Medical College). Participants gave written informed assent. Parents gave written informed consent.
Materials and Procedures
Hand Function
We measured manual dexterity, bimanual performance, and the strength of mirror movements (involuntary movement of the less-affected hand during voluntary movement of the affected hand). Manual dexterity of the affected hand was measured using the Jebsen-Taylor Test of Hand Function25 (JTTHF). The JTTHF measures the duration (in seconds) of one hand to perform functional movements. The total time of six subtests was used (flipping cards, manipulating small objects, simulated eating, checker stacking, and manipulating empty and full cans). The maximum time to perform each subtest was 180s. A higher score indicates poorer dexterity. Bimanual performance was measured using the Assisting Hand Assessment (AHA)26,27, which quantifies the quality with which the child uses their affected hand as an assisting hand during bimanual play-based activities. The AHA has excellent validity and reliability26,27, with a higher score (AHA units) indicating better bimanual performance.
Mirror movements were measured using electromyographic (EMG) signal recorded from the first dorsal interosseous (FDI) muscles bilaterally using surface bipolar electrodes (Coviden, Mansfield, MA) in a belly-tendon configuration with a ground electrode on the wrist styloid process. The EMG signal was sampled (4000 Hz), amplified (gain 600 V/V), bandpass filtered (10–400 Hz), and notch filtered (55–60Hz) to minimize movement artifacts. With both forearms and hands relaxed and supported comfortably on cushions, participants were visually cued to pinch with the thumb and index finger of one hand only. Pinches were held for 5s, followed by a 5s rest period. EMG activity was monitored in real-time to ensure instructions were followed appropriately. Ten trials were completed using the less-affected hand, then the affected hand. We are currently investigating the relationship between EMG- and clinically-derived measures of mirror movements28 in a larger separate study. At this time, we only have clinical ratings of mirror movements in a small subset of children included in this study (n=14 out of 30 in the ipsilateral CST group, and n=7 out of 14). In this small subset for which we have clinical data, correlations between clinically-derived and EMG-derived mirror movements are moderate and positive, but not significant – we suspect the associations to become significant with more data points. We decided to use the EMG-derived measures of mirror movements over the clinical-derived measures because (1) we have double the amount of data for the former than the latter, and (2) the former is a more objective measure of the two and is not subject to rater reliability.
Motor Mapping
We used single-pulse TMS to map the motor representation of the relaxed FDI muscle in each hand, using the same EMG protocol described above.
Single pulses were delivered by a Magstim 2002(Magstim Co., Whitland, Dyfed, UK) through a figure-of-eight coil with a 7-cm diameter. The coil handle was oriented at 45° to the mid-sagittal line to induce a current in a posterior to anterior direction, approximately perpendicular to the central sulcus. Before TMS testing, we acquired a T-1 weighted image using a 3T MRI scanner (except for four participants who, with their caregivers, did not consent to receiving an MRI). Using Brainsight frameless stereotaxy (Rogue Research, Montreal, Canada), TMS coil location was recorded and superimposed onto the child’s T1-weighted image. For those without an MRI, the TMS coil location was recorded and superimposed onto a single-subject “model” MRI. Our measures were concerned with the size, excitability, and overlap of motor representations, none of which are affected by the absolute relation to an anatomical image.
We first searched for the region at which TMS produced the largest MEP in the affected FDI (the motor ‘hotspot’). Single pulses were delivered to the lesioned hemisphere (interstimulus interval: 8-10s). If no MEP responses for the affected FDI was found in the lesioned hemisphere, single pulses were then delivered to the contralesional hemisphere.
After the hotspot was found, resting motor threshold (rMT) of the affected FDI was measured by delivering pulses over the hotspot and adjusting the stimulator output in 2%-increments until an MEP with an amplitude of >50μV and response latency <40ms was elicited by 6 of 10 pulses at a given stimulator output. Note, the hotspot of the affected and less-affected FDIs were in most cases in different locations (in 34 of 44 participants). Responses were excluded if baseline EMG activity was >50μV. The mean rMT of the affected FDI was 63% (SD = 13) of maximum stimulator output and the mean rMT of the less-affected FDI was 61% (SD = 11), with no significant difference (t(32)=2.03, p>0.05) between these in the 33 children for which we have both values (missing data are due to time and cooperativity issues, and not collecting both measures in the first few children we tested).
We next derived a complete motor map of the affected FDI. In Brainsight (Rogue Research, Montreal, Canada), a circular grid of 81 sites, spaced 1-cm apart in five concentric rings, centered around the hotspot, was superimposed onto the child’s cortical model. The mapping procedure was ceased once a responsive site was surrounded with 1-cm border of sites that did not elicit MEPs. Single pulses were delivered at 110% rMT of the affected FDI at each grid site, starting over the hotspot and moving concentrically along each ring. Three to six pulses were delivered at sites that elicited an MEP (to capture three clear trials without noise contamination i.e. EMG activity before TMS onset). One to two pulses were delivered at sites that did not elicit an MEP: if the non-responsive site neighbored a responsive site, two pulses were delivered.
Data Analyses
Mirror Movement Quantification (n=41)
The EMG data for the cued hand was epoched (4-5s), with movement onset and offset identified manually. Mirror movement epochs were obtained from corresponding epochs of the other hand. Trials were excluded if EMG remained <1mV for at least 4s after cue onset (mean number of included trials per child n=7.8±2.1). The epoched data was rectified, and the root mean square (RMS) was estimated. The relative mirror movement strength was calculated as the mean ratio of mirror to voluntary RMS.
Classification of CST Pattern
Participants were classified as having an ipsilateral CST pattern when 100% of MEPs recorded in the affected FDI resulted from stimulation of the contralesional hemisphere. Participants were classified as having a bilateral CST pattern when MEPs in the affected FDI resulted from stimulation of both hemispheres. Participants were classified as having a contralateral CST pattern when 100% of MEPs in the affected FDI resulted from stimulation of the lesioned hemisphere.
Motor Representation Quantification
We characterized the areal size and excitability of the contralesional motor representation of the affected FDI. Size (reflective of the spread of the FDI representation) was quantified as per previous motor mapping work in USCP18 as the number of grid sites at which stimulation evoked an MEP. To quantify excitability (reflective of the strength of the FDI representation), we first calculated the mean peak-to-peak amplitude of MEPs of the affected FDI at each site. We normalized the mean MEP amplitude at each site by dividing it by the largest mean MEP amplitude recorded in the map. The excitability of the affected FDI motor representation was quantified as the total sum of normalized MEP amplitudes; we quantified map excitability in this way to match the quantification of map excitability used in the Kesar et al. study19. Finally, we rationalized our measures of overlap as the number of sites (and their excitability) that gave dual-responses in both hands (as that which has been done in previous animal work). We calculated the proportion of stimulated sites that elicited an MEP in the affected FDI, which also elicited an MEP in the less-affected FDI . We quantified the relative excitability of these shared sites as the sum of normalized MEPs of these ‘shared’ sites recorded in the affected FDI relative to the total sum of normalized MEPs recorded in the affected FDI .
Results
Statistical Analyses
More than half of the data were not normally distributed (4 of our 7 measures did not meet the normality assumption, as tested by the Shapiro Wilk test). Thus, we report results from non-parametric tests (however, the results remain the same with parametric tests). We used Mann-Whitney U tests to compare ipsilateral versus bilateral CST subgroups on manual dexterity (JTTHF affected hand score), bimanual performance (AHA units), mirror movement strength (voluntary to mirror RMS ratio), size and excitability of contralesional motor representations of the affected FDI, and its overlap with motor representations of the less-affected FDI. We report Spearman’s Rank correlations (rs) and 95% confidence intervals in square parentheses as measures of the association between TMS and functional measures. Alpha-level was 0.05.
CST Pattern Characterization
In 30 children, only stimulation of the non-lesioned hemisphere evoked MEPs in the affected FDI (i.e., ipsilateral affected CST pattern). In 14 participants, stimulation of both hemispheres evoked MEPs of the affected FDI (i.e., bilateral affected CST pattern). In six children, only stimulation of the lesioned hemisphere evoked MEPs in the contralateral, affected FDI (i.e., contralateral affected CST pattern); as stated above, these children were excluded from analyses.
No Association Between Hand Function and CST Pattern
We found no significant difference between ipsilateral and bilateral CST subgroups on measures of unimanual dexterity (U=177.00, p>0.05), bimanual performance (U=192.00, p>0.05), and mirror movement strength (U=86.00, p>0.05) – see Figure 1. We explored whether size and excitability of contralesional motor representations of the affected hand, and their overlap with the less-affected hand, might be related to these functional measures in CST subgroups. Since the subgroups did not differ on any measures, we combined them for correlational analyses.
Figure 1.
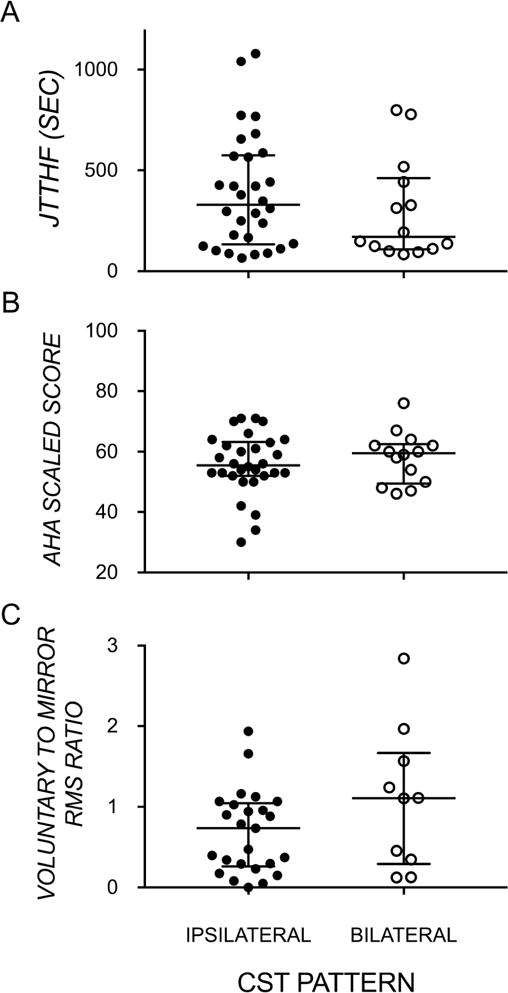
Median and interquartile range of (A) manual dexterity of the affected hand, (B) bimanual performance, by ipsilateral and bilateral CST pattern, and (C) strength of mirror movements. (CST – corticospinal tract; JTTHF – Jebsen-Taylor Test of Hand Function; AHA – Assisting Hand Assessment; RMS – root mean square)
No Association Between Size and Excitability of Contralesional Motor Representations and Hand Function
Representative maps of stimulated sites, and their excitability, in a child with an ipsilateral CST and a child with a bilateral CST are shown in Figure 2. The number of stimulated sites (size), and their overall excitability evoking MEP responses in the affected FDI were similar in both children. As shown in Figure 3 across all participants, there were no significant differences in size (U=190.50, p>0.05) or excitability (U=167.00, p>0.05) of MEP responses between CST subgroups. Nevertheless, these measures of size and excitability of MEP responses in the affected FDI were not associated with manual dexterity (size: rs=0.03[−0.28, 0.33]; p>0.05; excitability: rs=0.10[−0.21, 0.40]; p>0.05), bimanual performance (size: rs= −0.17[−0.45, 0.14]; p>0.05; excitability: rs= −0.17[−0.45, 0.15]; p>0.05), or mirror movements (size: rs=0.15[−0.21, 0.47]; p>0.05; excitability: rs=0.13[−0.22, 0.45]; p>0.05). Together, this suggests that the magnitude of unimanual and bimanual impairments and mirror movement strength in these children is not explained by the absolute size or excitability of the contralesional motor representation of the affected hand.
Figure 2.
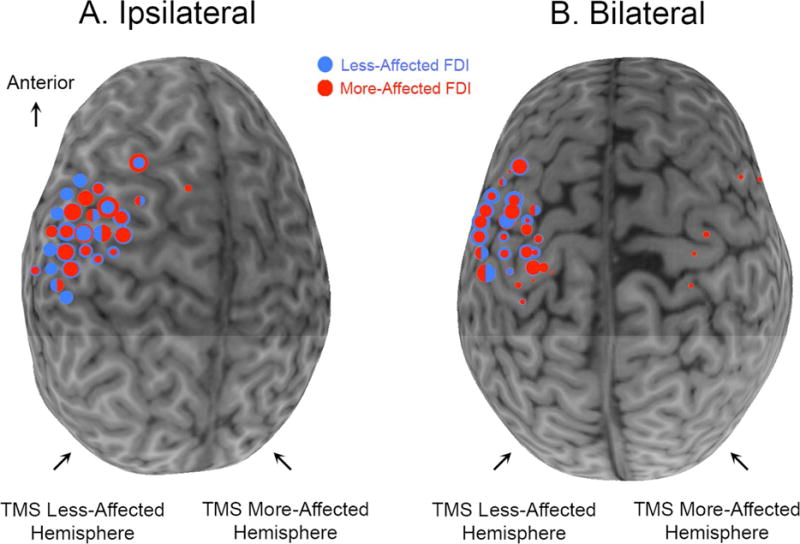
Individual representative maps of children with ipsilateral (A) or bilateral (B) control of the affected FDI. Circles are superimposed onto each child’s MRI. Red circles denote sites at which a TMS pulse evoked an MEP in the affected FDI. Blue circles denote sites at which a TMS pulse evoked an MEP in the less-affected FDI. The size of the circle represents the excitability of the MEP response at that site. At many sites, MEPs were evoked in both FDIs. When one MEP was stronger than the other, different sized concentric circles represent the sizes of the two MEPs. If MEP size was similar for both FDIs at one spot (less than 0.20 difference in normalized MEP), the circle is half red, half blue.
Figure 3.
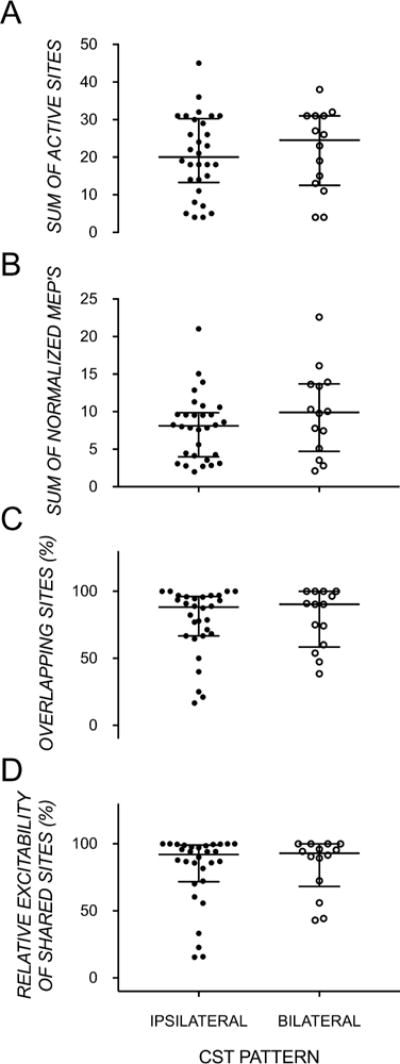
Medians and interquartiles range showing no group differences in ipsilateral and bilateral CST types, but large within-group variation, in (A) the number of stimulated sites that evoked an MEP in the affected FDI, (B) the sum of normalized MEPs in the affected FDI, (C) proportion of stimulated sites that elicited an MEP in the affected FDI, which also elicited an MEP in the less-affected FDI, and (D) the proportion of the sum of normalized MEPs of ‘shared’ sites (sites evoking bilateral MEPs) recorded in the affected FDI relative to the total sum of normalized MEPs recorded in the affected FDI.
Association Between Overlap in Motor Representations and Hand Function
In most children, the maps of the affected and less-affected FDIs were highly overlapping (ipsilateral: 77.50±24.56%; bilateral: 79.77±21.71%) and contributed substantially to the summed excitability from all sites eliciting MEPs in the affected FDI (ipsilateral: 80.64±26.30%; bilateral: 83.78±20.96%). On average, 4 sites elicited MEPs in only the more affected FDI, 8 sites elicited MEPs in only the less-affected FDI, and 17 sites (i.e. ~80% of responses in affected FDI) elicited MEPs in both FDIs. There were no significant differences in the amount of overlap (U=196.00, p>0.05) and the excitability of overlapping sites (U=188.00, p>0.05) between CST subgroups.
We found significant moderate correlations between manual dexterity of the affected hand (rs= −0.51[−0.70, −0.24]; p<0.001) and bimanual performance (rs=0.44[0.15, 0.65]; p<0.01) with the amount of overlap of the maps of the two FDIs (Figure 4). Furthermore, the relative excitability of these shared sites also correlated with manual dexterity (rs= −0.48[−0.68, −0.20]; p<0.01) and bimanual performance (rs=0.39[0.10, 0.62]; p<0.01). These findings suggest that the size and excitability of overlapping shared representations of the two hands, rather than the overall size or excitability of representations of the affected hand, is related to manual dexterity and bimanual hand use in children with contralesional CST patterns.
Figure 4.
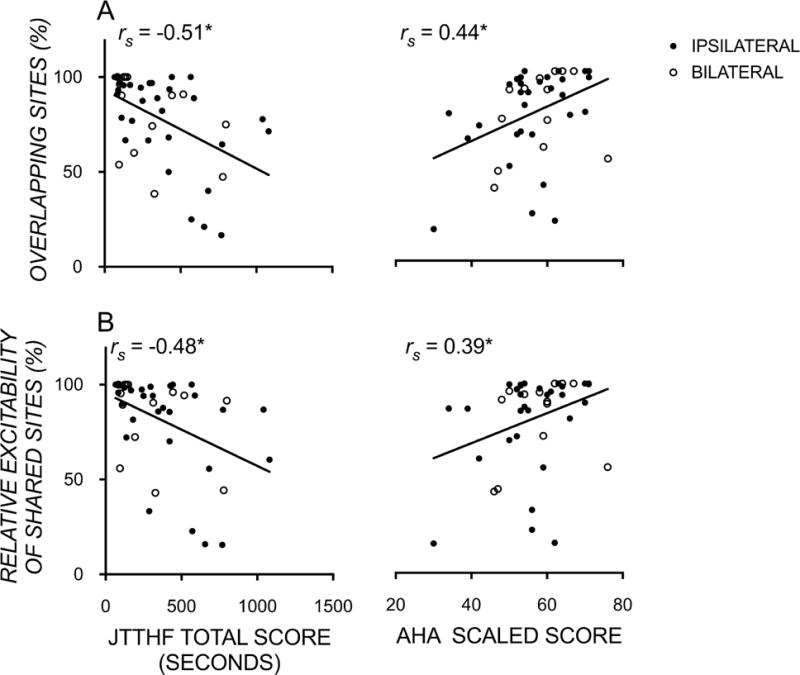
Scatterplots showing significant moderate correlations between unimanual function (JTTHF), bimanual function (AHA logit score) and the proportion of (A) sites and (B) their strength/excitability, that were ipsilateral to the more affected hand and evoked bilateral motor evoked potentials recorded in both the more and less-affected FDI muscles. * p<0.05.
Finally, we found moderate correlations between mirror movement strength and the size (rs=0.54; [0.24, 0.74]; p<0.001) and excitability (rs=0.58; [0.30, 0.77]; p<0.001) of overlapping maps of the two FDIs (Figure 5). Of note, there was no significant association between mirror movement strength and unimanual dexterity (rs= −0.07[−0.40, 0.28]; p>0.05) or bimanual performance (rs= −0.009[−0.33, 0.35]; p>0.05). This indicates that a greater map overlap has the functional consequence of coactivating both hands during voluntary movement of the less-affected hand, the latter of which does not help nor hinder unimanual or bimanual performance.
Figure 5.
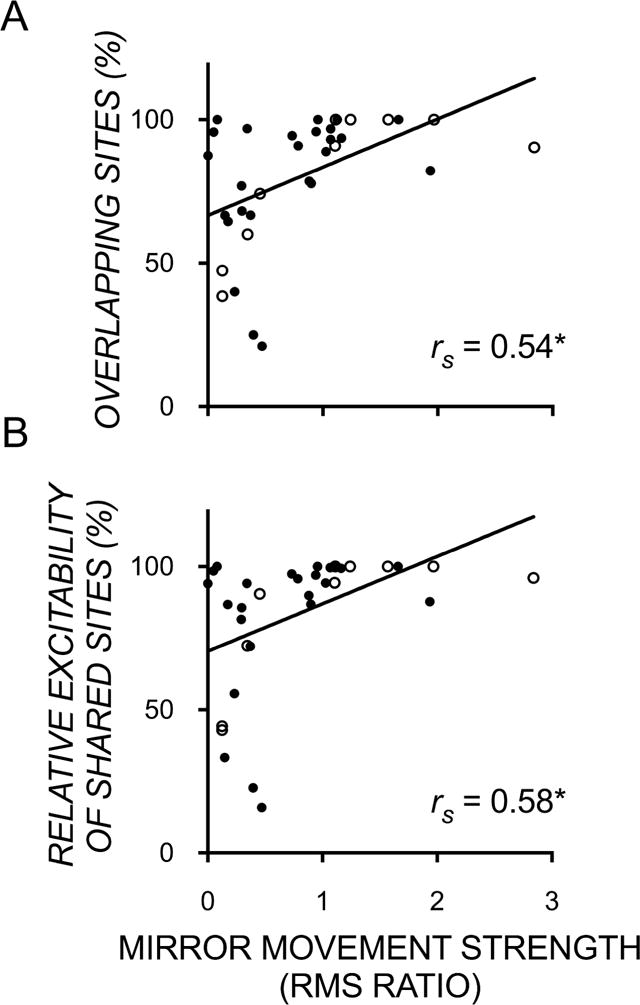
Scatterplots showing significant moderate correlations between mirror movement strength (ratio of mirror to voluntary root mean square) and the proportion of (A) sites and (B) their excitability, that were ipsilateral to the more affected hand and evoked bilateral motor evoked potentials recorded in both the more and less-affected FDI muscles. (RMS – root mean square) * p<0.001.
Discussion
Here we investigated the link between hand function and the size and total excitability of motor representations of the affected hand, and their overlap with motor representations of the less-affected hand, in the contralesional hemisphere of children with USCP. The first main and novel finding is the large amount of overlap in contralesional motor representations of the affected hand with that of the less-affected hand. Second, and as we hypothesized, we found that the size and excitability of such shared-site representations were moderately associated with manual dexterity of the affected hand, and the effectiveness to which this hand is incorporated in bimanual play. We also found the size and excitability of such shared-site representations were moderately associated with the strength of mirror movements. Contrary to our hypothesis, such associations were not present when we correlated these behavioral measures with overall size and excitability of motor representations of the affected hand (i.e. including representations from sites that were not shared). To our knowledge, this is the first study to have examined the overlap between affected and less-affected motor representations in the contralesional hemisphere in children with USCP.
Substantial Overlapping Motor Representations
We found that the affected hand’s contralesional motor representation was largely overlapping with the less-affected hand’s contralateral motor representation. This has not yet been demonstrated in children with USCP with ipsilateral CST patterns, though overlap of “multi-joint” representations has been demonstrated in healthy adults12 and animal models11,20–22 Also, the center of gravity of less-affected and affected motor maps have been shown to overlap in a small subset of children (n=6) with congenital hemiplegia29.
Association Between Hand Function and Overlapping Motor Representations
Consistent with a previous report9, we found large variation in affected hand function in children with ipsilateral or bilateral CST patterns. We examined whether features of contralesional motor representations might explain the source of this variation. Although hand function was unrelated to the size and excitability of motor representations of the affected FDI, we found that the size and excitability of overlapping motor representations was related to hand function. Greater and stronger overlap was associated with better unimanual and bimanual performance, as well as stronger mirror movements.
The links between manual dexterity and bimanual performance and overlapping motor representations has not been documented in USCP. However, in animal models, motor learning or post-injury motor recovery is associated with increased multi-joint representations21,22. In humans, motor representations of fingers that are often used together (e.g. ring and middle fingers)30, and muscles within one limb12,31,32 often overlap. There are important differences between that shown in previous work in healthy adults, in the animal work and in children with USCP. The healthy adult and animal work show relationships between function and overlap of motor representations of multiple muscles/movements of one limb in one hemisphere. Here we show relationships between function and overlap in motor representations of muscles from two limbs in one hemisphere. In both cases, their relationships to functional measures (e.g. hand motor skill in animal work, unimanual and bimanual motor performance, and mirror movement in USCP) raises the possibility that such convergence of representations (of muscles within the hand, and between hands) is to some extent functionally useful. In the animal work, it has been suggested that multi-joint sites might encode simple motor synergies and, at least in the cat and monkey, increase in frequency in skill training that encourages such synergies20,33. The role of overlapping representations of the two hands within the contralesional hemisphere might be different to that of overlapping representations of multiple joints of one limb in the contralesional hemisphere. Particularly, overlapping motor representations within the contralesional hemisphere might be adaptively “yoked,” such that cortical control of the less-affected hand assists or supports that of the affected hand. Although speculative, children with contralesional CST patterns might benefit more from bilateral hand therapeutic interventions, if such interventions increase overlapping motor representations.
The links between the strength of mirror movements and overlapping representations has also not been reported in USCP. However, in a mouse model, conditional forebrain ablation of the Ephrin A4 gene, which is involved in preventing spinal axons from crossing the midline, resulted in sustained bilateral CST connections34. Intracortical microstimulation of M1 unilaterally evoked movement of both forelimbs, suggesting overlapping motor representations to be a possible neural mechanism underlying mirror movements. Greater map overlap, and its excitability, has the functional consequence of coactivating both hands during voluntary movement of one hand. Interestingly, coactivating both hands during voluntary movement of one hand does not appear to help nor hinder unimanual or bimanual performance.
We found that mirror movements were not associated with unimanual or bimanual performance. This suggests that the presence of mirror movements does not help nor hinder hand function, at least as measured in our sample. The association between mirror movements and upper limb function is unclear: while some studies find no associations9,35, others show weak to moderate associations36,37. Varying findings are likely due to varying measures of mirror movements and hand function. For example, it could be argued that our assessments did not measure the ability to precisely individuate digits, or the ability to perform different actions with each hand, skills that might be impaired by mirror movements. Therefore, mirror movement or other functional assessments that specifically measure digit individuation might show an opposite relationship to overlapping motor representations, which would suggest negative consequences of such overlapping representations. We did not specifically measure how overlapping motor representations in the contralesional hemisphere might affect digit individuation, within or between hands. It is possible that overlapping motor representations might show a different functional association, depending on how function is measured.
It is notable that overlapping motor representations have also been associated with maladaptive processes, suggesting that overlap of motor representations can also have negative functional consequences. The best-characterized symptom arising from overlapping cortical representations is focal hand dystonia (review38). In focal dystonia, repetitive hand use and co-activation of sensory receptors can result in the convergence of sensory and motor representations of different muscles. This maladaptive plasticity results in painful, repetitive, uncontrolled movements. The neurophysiological underpinnings of focal dystonia include not only the overlap of different representations, but also altered inhibitory control (e.g. intracortical inhibition, surround inhibition) and sensorimotor integration23,38. Children with USCP with ipsilateral CST patterns showed reduced short-interval intracortical inhibition compared to those with contralateral CST patterns39. The interactions between motor representation overlap, inhibitory control, and hand function need further exploration in children with USCP.
No Association Between Hand Function and Size and Excitability of Contralesional Motor Representations of the Affected Hand
Unlike the demonstrated functional importance of overlapping hand representations in the contralesional hemisphere, there were no significant associations between hand function and the size and excitability of affected hand motor representations. This result was unsurprising, since many have shown that intervention-based changes in map size18,20,22 or overlap of maps11,22 rather than absolute size or excitability of maps, are most meaningfully related to function. These findings are consistent with Kesar et al.19 who showed no link between total map excitability and motor function in USCP.
Limitations and Future Directions
Some limitations constrain the applicability and interpretation of our findings. First, the less-affected FDI’s motor representation might have been under- or overestimated since the rMT of the affected FDI was used to calculate the mapping intensity (110% rMT). Thus, it is likely that we have not reliably captured the less-affected FDI motor representation. Ideally, contralesional motor maps would be mapped twice, at two stimulus intensities, based on the rMT of each FDI. The rMT of the less-affected FDI was typically lower or equal to the rMT of the affected FDI, meaning that the less-affected FDI representation was often derived using a stimulation intensity greater than 110% rMT. Thus, we could not compare the size and excitability of the less-affected FDI representation with that of the affected FDI.
Second, our results were derived from children with mild to moderate impairment severity. Therefore, our findings cannot be generalized to children with more severe impairments. Children with more severe impairments are more likely to have ipsilateral CST patterns7,8. Thus, including children with more severe impairments might show hand function differences between different CST subgroups. Furthermore, larger sample sizes (particularly of the bilateral CST group) might also show between-group differences in functional and TMS measures.
Third, a study by Vandermeeren et al.40 has identified in a sub-sample of children with congenital hemiplegia the presence of distinct clusters of long-latency MEPs (>40ms) in addition to the presence of the more commonly studied short-latency MEP (<40ms). In the current study, we excluded any MEPs with latencies longer than 40ms because we found it difficult to objectively discriminate such responses from muscular and/or auditory startle responses. As Vandermeeren et al. noted, these longer-latency MEPs might have mechanisms that are distinct from short-latency ones, and unique functional associations. Thus, it is conceivable for motor maps derived from longer-latency MEPs to have a different functional association than that derived from short-latency MEPs as was used here.
Fourth, we note the possibility of TMS-evoked responses to arrive via extrapyramidal tracts and not necessarily CST41. That extrapyramidal tracts are polysynaptic rather than monosynaptic in nature might result in TMS-evoked responses with longer latencies that are present after the 40-ms cut off we imposed on useable MEPs. Carr et al.24 has also shown in children with CP that ipsilateral MEPs are similar in latency to contralateral MEPs (unlike that seen in healthy adults, e.g. Wasserman et al. 1991), thereby adding to the likelihood that these MEPs are mediated via CST and not via other polysynaptic tracts. Finally, Williams and Martin42 recently showed that these extrapyramidal tracts compete with the corticospinal system after developmental corticospinal injury. They found that M1 inactivation in the cat reduced rubrospinal tracts, and increased CST (thus CST outcompeted rubrospinal tracts on the ipsilateral side), demonstrating that CST steer development and ultimately function of another. Thus, it is more likely for ipsilateral responses to be mediated by CST than rubrospinal tracts, following developmental CST injury.
Finally, although we found moderate correlations between hand function and contralesional map overlap, we suspect the contribution to a child’s impairment to be multifactorial, which likely include the timing and location of a child’s lesion1, and the amount of therapy received.
Conclusion
This is the first study in children with USCP to document the large amount overlap in contralesional motor representations of the two hands, which our results suggest, is a behaviorally favorable byproduct of neuroplasticity.
Acknowledgments
Funded by R01 HD076436-A01, NS062116 (KMF), Columbia Professional Schools Diversity Award (KMF), NIH CTSA Award (KMF) (KL2 RR024157, UL1 RR024156, TL1 RR024158), Fonds de recherche du Québec - Santé (Postdoctoral training award, VHF), Horace W. Goldsmith Foundation Grant (DG), and Endeavour Australia (Research Fellowship, MM). Research reported in this publication was supported by the National Center For Advancing Translational Science of the National Institute of Health Under Award Number UL1TR000457. We thank Muc Du, Doug Fallon (PhD), and Jonathan Dyke (PhD) for MRI acquisition, and Geoff Hammond (PhD) for his assistance with data interpretation. We also thank all study volunteers for their assistance with data collection and analyses, and the participants and their families for participating in the study.
References
- 1.Jaspers E, Byblow W, Feys H, Wenderoth N. The corticospinal tract: A biomarker to categorize upper limb functional potential in unilateral cerebral palsy. Frontiers in Pediatrics. 2016 Jan;3(9):1–10. doi: 10.3389/fped.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon AM. Progress in Motor Control. Springer; 2016. Impaired Voluntary Movement Control and Its Rehabilitation in Cerebral Palsy; pp. 291–311. [DOI] [PubMed] [Google Scholar]
- 3.Friel KM, Martin JH. Bilateral activity-dependent interactions in the developing corticospinal system. Journal of Neuroscience. 2007;27(41):11083–11090. doi: 10.1523/JNEUROSCI.2814-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friel KM, Chakrabarty S, Martin JH. Pathophysiological mechanisms of impaired limb use and repair strategies for motor systems after unilateral injury of the developing brain. Developmental Medicine & Child Neurology. 2013;55(s4):27–31. doi: 10.1111/dmcn.12303. [DOI] [PubMed] [Google Scholar]
- 5.Eyre JA, Smith M, Dabydeen L, et al. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Annals of Neurology. 2007;62(5):493–503. doi: 10.1002/ana.21108. [DOI] [PubMed] [Google Scholar]
- 6.Martin JH, Friel KM, Salimi I, Chakrabarty S. Activity-and use-dependent plasticity of the developing corticospinal system. Neuroscience & Biobehavioral Reviews. 2007;31(8):1125–1135. doi: 10.1016/j.neubiorev.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krägeloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis. Brain. 2002;125(10):2222–2237. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- 8.Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krägeloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Annals of Neurology. 2004;56(6):854–863. doi: 10.1002/ana.20297. [DOI] [PubMed] [Google Scholar]
- 9.Holmström L, Vollmer B, Tedroff K, et al. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Developmental Medicine & Child Neurology. 2010;52(2):145–152. doi: 10.1111/j.1469-8749.2009.03496.x. [DOI] [PubMed] [Google Scholar]
- 10.Smorenburg AR, Gordon AM, Kuo H-C, et al. Does Corticospinal Tract Connectivity Influence the Response to Intensive Bimanual Therapy in Children With Unilateral Cerebral Palsy? Neurorehabilitation and Neural Repair. 2016 doi: 10.1177/1545968316675427. 1545968316675427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. Journal of Neurophysiology. 1996;75(5):2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 12.Sanes JN, Donoghue JP, Thangaraj V, Edelman RR, Warach S. Shared neural substrates controlling hand movements in human motor cortex. Science. 1995;268(5218):1775. doi: 10.1126/science.7792606. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318(5853):1150–1155. doi: 10.1126/science.1147243. [DOI] [PubMed] [Google Scholar]
- 14.Milliken GW, Plautz EJ, Nudo RJ. Distal forelimb representations in primary motor cortex are redistributed after forelimb restriction: a longitudinal study in adult squirrel monkeys. Journal of Neurophysiology. 2013;109(5):1268–1282. doi: 10.1152/jn.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grefkes C, Ward NS. Cortical Reorganization After Stroke How Much and How Functional? The Neuroscientist. 2013 doi: 10.1177/1073858413491147. 1073858413491147. [DOI] [PubMed] [Google Scholar]
- 16.Ridding M, Rothwell J. Reorganisation in human motor cortex. Canadian Journal of Physiology and Pharmacology. 1995;73(2):218–222. doi: 10.1139/y95-032. [DOI] [PubMed] [Google Scholar]
- 17.Topka H, Cohen LG, Cole RA, Hallett M. Reorganization of corticospinal pathways following spinal cord injury. Neurology. 1991;41(8):1276–1276. doi: 10.1212/wnl.41.8.1276. [DOI] [PubMed] [Google Scholar]
- 18.Friel KM, Kuo H-C, Fuller J, et al. Skilled Bimanual Training Drives Motor Cortex Plasticity in Children With Unilateral Cerebral Palsy. Neurorehabilitation and Neural Repair. 2016 doi: 10.1177/1545968315625838. 1545968315625838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesar T, Sawaki L, Burdette J, et al. Motor cortical functional geometry in cerebral palsy and its relationship to disability. Clinical Neurophysiology. 2012;123(7):1383–1390. doi: 10.1016/j.clinph.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nudo RJ, Milliken G, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. Journal of Neuroscience. 1996;16(2):785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friel K, Chakrabarty S, Kuo H-C, Martin J. Using motor behavior during an early critical period to restore skilled limb movement after damage to the corticospinal system during development. The Journal of Neuroscience. 2012;32(27):9265–9276. doi: 10.1523/JNEUROSCI.1198-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakrabarty S, Friel KM, Martin JH. Activity-dependent plasticity improves M1 motor representation and corticospinal tract connectivity. Journal of Neurophysiology. 2009;101(3):1283–1293. doi: 10.1152/jn.91026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zittel S, Helmich R, Demiralay C, Münchau A, Bäumer T. Normalization of sensorimotor integration by repetitive transcranial magnetic stimulation in cervical dystonia. Journal of neurology. 2015;262(8):1883–1889. doi: 10.1007/s00415-015-7789-1. [DOI] [PubMed] [Google Scholar]
- 24.Carr L, Harrison L, Evans A, Stephens J. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116(5):1223–1247. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- 25.Jebsen RH, Taylor N, Trieschmann R, Trotter MJ, Howard LA. An objective and standardized test of hand function. Archives of Physical Medicine and Rehabilitation. 1969;50(6):311. [PubMed] [Google Scholar]
- 26.Holmefur M, Krumlinde-Sundholm L, Eliasson A-C. Interrater and intrarater reliability of the Assisting Hand Assessment. American Journal of Occupational Therapy. 2007;61(1):79–84. doi: 10.5014/ajot.61.1.79. [DOI] [PubMed] [Google Scholar]
- 27.Krumlinde-Sundholm L, Holmefur M, Kottorp A, Eliasson AC. The Assisting Hand Assessment: current evidence of validity, reliability, and responsiveness to change. Developmental Medicine & Child Neurology. 2007;49(4):259–264. doi: 10.1111/j.1469-8749.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 28.Woods BT, Teuber H-L. Mirror movements after childhood hemiparesis. Neurology. 1978;28(11):1152–1152. doi: 10.1212/wnl.28.11.1152. [DOI] [PubMed] [Google Scholar]
- 29.Vandermeeren Y, Davare M, Duque J, Olivier E. Reorganization of cortical hand representation in congenital hemiplegia. European Journal of Neuroscience. 2009;29(4):845–854. doi: 10.1111/j.1460-9568.2009.06619.x. [DOI] [PubMed] [Google Scholar]
- 30.Ejaz N, Hamada M, Diedrichsen J. Hand use predicts the structure of representations in sensorimotor cortex. Nat Neurosci. 2015;18(7):1034–1040. doi: 10.1038/nn.4038. [DOI] [PubMed] [Google Scholar]
- 31.Meier JD, Aflalo TN, Kastner S, Graziano MS. Complex organization of human primary motor cortex: a high-resolution fMRI study. Journal of neurophysiology. 2008;100(4):1800–1812. doi: 10.1152/jn.90531.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strother L, Medendorp WP, Coros AM, Vilis T. Double representation of the wrist and elbow in human motor cortex. European Journal of Neuroscience. 2012;36(9):3291–3298. doi: 10.1111/j.1460-9568.2012.08241.x. [DOI] [PubMed] [Google Scholar]
- 33.Martin JH, Engber D, Meng Z. Effect of forelimb use on postnatal development of the forelimb motor representation in primary motor cortex of the cat. Journal of neurophysiology. 2005;93(5):2822–2831. doi: 10.1152/jn.01060.2004. [DOI] [PubMed] [Google Scholar]
- 34.Serradj N, Paixão S, Sobocki T, et al. EphA4-mediated ipsilateral corticospinal tract misprojections are necessary for bilateral voluntary movements but not bilateral stereotypic locomotion. Journal of Neuroscience. 2014;34(15):5211–5221. doi: 10.1523/JNEUROSCI.4848-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Islam M, Gordon AM, SKöLD A, Forssberg H, ELIASSON AC. Grip force coordination during bimanual tasks in unilateral cerebral palsy. Developmental Medicine & Child Neurology. 2011;53(10):920–926. doi: 10.1111/j.1469-8749.2011.04040.x. [DOI] [PubMed] [Google Scholar]
- 36.Klingels K, Jaspers E, Staudt M, et al. Do mirror movements relate to hand function and timing of the brain lesion in children with unilateral cerebral palsy? Developmental Medicine & Child Neurology. 2016;58(7):735–742. doi: 10.1111/dmcn.12977. [DOI] [PubMed] [Google Scholar]
- 37.Adler C, Berweck S, Lidzba K, Becher T, Staudt M. Mirror movements in unilateral spastic cerebral palsy: Specific negative impact on bimanual activities of daily living. European journal of paediatric neurology. 2015;19(5):504–509. doi: 10.1016/j.ejpn.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Furuya S, Hanakawa T. The curse of motor expertise: Use-dependent focal dystonia as a manifestation of maladaptive changes in body representation. Neuroscience research. 2016;104:112–119. doi: 10.1016/j.neures.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Zewdie E, Damji O, Ciechanski P, Seeger T, Kirton A. Contralesional Corticomotor Neurophysiology in Hemiparetic Children With Perinatal Stroke Developmental Plasticity and Clinical Function. Neurorehabilitation and Neural Repair. 2016 doi: 10.1177/1545968316680485. 1545968316680485. [DOI] [PubMed] [Google Scholar]
- 40.Vandermeeren Y, Bastings E, Fadiga L, Olivier E. Long-latency motor evoked potentials in congenital hemiplegia. Clinical neurophysiology. 2003;114(10):1808–1818. doi: 10.1016/s1388-2457(03)00161-5. [DOI] [PubMed] [Google Scholar]
- 41.Fisher KM, Zaaimi B, Baker SN. Reticular formation responses to magnetic brain stimulation of primary motor cortex. The Journal of Physiology. 2012;590(Pt 16):4045–4060. doi: 10.1113/jphysiol.2011.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams PTJA, Martin JH. Motor Cortex Activity Organizes the Developing Rubrospinal System. The Journal of Neuroscience. 2015;35(39):13363–13374. doi: 10.1523/JNEUROSCI.1719-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


