Abstract
PAR-1/MARK kinases are conserved serine/threonine kinases that are essential regulators of cell polarity. PAR-1/MARK kinases localize and function in opposition to the Anterior PAR proteins to control the asymmetric distribution of factors in a wide variety polarized cells. In this review, we discuss the mechanisms that control the localization and activity of PAR-1/MARK kinases, including their antagonistic interactions with the Anterior PAR proteins. We focus on the role PAR-1 plays in the asymmetric division of the C. elegans zygote, in the establishment of the anterior/posterior axis in the Drosophila oocyte and in the control of microtubule dynamics in mammalian neurons. In addition to conserved aspects of PAR-1 biology, we highlight the unique ways in which PAR-1 acts in these distinct cell types to orchestrate their polarization. Finally, we review the connections between disruptions in PAR-1/MARK function and Alzheimer’s disease and cancer.
Keywords: PAR-1, MARK, cell polarity, C. elegans, Drosophila, PAR proteins, microtubule-associated protein
1. Introduction
PAR-1/MARK kinases are conserved serine/threonine kinases that regulate cellular organization in diverse processes including asymmetric cell division, neuronal differentiation and epithelial organization. PAR-1 commonly functions as a member of the PAR (PARtitioning defective) network of cell polarity regulators. PAR-1 and the Anterior PAR proteins (PAR-3, PAR-6, and aPKC kinase) concentrate in opposing cortical domains within polarized cells, and their asymmetric distribution underlies their ability to control cortical, cytoplasmic and cytoskeletal asymmetries along the polarity axis (Goldstein and Macara, 2007). PAR-1 was initially discovered along with most of the other PAR proteins in a classic genetic screen for genes required for the establishment of the anterior/posterior polarity axis in the early C. elegans embryo (Kemphues et al., 1988). Shortly thereafter, PAR-1 was shown to be essential for the establishment of the anterior/posterior axis in the Drosophila oocyte and for apical/basal polarity in epithelial cells (Cox et al., 2001a; Shulman et al., 2000). Mammalian PAR-1/MARK kinases were independently purified based their ability to phosphorylate and thereby regulate the microtubule association of the microtubule-associated proteins TAU, MAP2 and MAP4 (Drewes et al., 1997; Drewes et al., 1995). More recently, dysregulation of PAR-1/MARK kinases has been implicated in a number of pathological settings, including in tumorigenesis and in Alzheimer’s disease (Matenia and Mandelkow, 2009).
In this review, we focus on three systems in which PAR-1 function has been particularly well characterized: establishment of the anterior/posterior axis in the C. elegans zygote, establishment of the anterior/posterior axis in the Drosophila oocyte, and control of microtubule dynamics in mammalian neurons. We use these examples to illustrate both commonalities and important differences in PAR-1 biology in different cellular settings. For example, antagonistic interactions between the PAR-1 and the Anterior PAR proteins is a common mechanism by which their reciprocal localization patterns are established. However, PAR-1 generates downstream cellular asymmetries through fundamentally different mechanisms in each of these examples. In the C. elegans zygote, PAR-1 locally controls reaction/diffusion mechanisms to rapidly generate cytoplasmic asymmetries. In the Drosophila oocyte, PAR-1 polarizes the microtubule cytoskeleton by locally inhibiting non-centrosomal microtubules, thus providing a foundation for long range polarized transport. In neurons, PAR-1/MARK locally controls microtubule growth and stability, which contributes to differences in the axonal and dendritic microtubule cytoskeletons.
2. Structure and Regulation of PAR-1/MARK kinases
PAR-1/MARK serine/threonine kinases are large proteins (for example, MARK1 is 88 kDa) and are members of the AMPK family of CamKII kinases (Manning et al., 2002). The overall architecture of PAR-1/MARK kinases is shared with most other AMPK kinases and features a kinase domain and an adjacent non-canonical Ubiquitin-association (UBA) domain located near the N-terminus and a Kinase-Associated (KA1) membrane binding domain near the C-terminus (Figure 1) (Murphy et al., 2007; Panneerselvam et al., 2006; Sack et al., 2016). (Marx et al., 2010; Timm et al., 2008b). A large, variable and relatively uncharacterized spacer domain lies between the UBA domain and the KA1 domain. Mammals encode four PAR-1/MARK kinases: MARK1 (Par1c), MARK2 (Par1b/EMK), MARK3 (Par1a/C-TAK1) and MARK4 (MARKL1/Par1d) are broadly expressed in embryonic and adult tissues and contribute to a wide range of biological processes. Both C. elegans and Drosophila encode a single PAR-1 family member that is essential for embryonic development (Guo and Kemphues, 1995; Shulman et al., 2000). The diversity of PAR-1 proteins is increased by alternative splice isoforms with differing amino or carboxy termini, which in the Drosophila oocyte, have distinct localization patterns and functional roles (Doerflinger et al., 2006). For simplicity, in this review I will refer to PAR-1/MARK kinases collectively as PAR-1, and will use specific names to refer particular family members (for example, MARK1-4 for the mammalian proteins and dPar-1 for the Drosophila kinase).
Figure 1.
Schematic of PAR-1/MARK kinase. The kinase and UBA domains are positioned near the N-terminus and a relatively long spacer domain separates the UBA domain from the C-terminal KA1 membrane binding domain. PAR-1 kinase activity is stimulated by phosphorylation on Thr208 in the activation loop by LKB1/PAR-4 and MARKK/TAO-1 kinases. GSK3β phosphorylation on Ser212 in the activation loop inhibits PAR-1 kinase activity. aPKC phosphorylation at residue Thr595 results in the association of the 14-3-3 protein PAR-5 (not depicted) and the sequestration of PAR-1 in the cytoplasm. MARK2 residues are indicated.
MARK2, MARK3 and MARK4 knockout mice are viable (the phenotypes of MARK1 deficient mice have not been reported), but have defects in energy metabolism that result in reduced body weight and adipocity, although the spectrum of metabolic defects are unique for each mutant (Bessone et al., 1999; Hurov et al., 2001; Lennerz et al., 2010; Sun et al., 2012). While the basis for these metabolic defects have not been fully elucidated, MARK4 is expressed in adipocytes where it has been shown to regulate respiration, proliferation and apoptosis (Feng et al., 2014; Liu et al., 2016). MARK2 knockout mice have a number of other phenotypes, including dwarfism, infertility, reduced learning and memory and immune system dysfunction (Bessone et al., 1999; Hurov et al., 2001; Segu et al., 2008). Additionally, MARK2 contributes to myogenesis by controlling the asymmetric division of muscle stem cells (satellite cells), and dysregulation of MARK2 was recently implicated in the loss of muscle in Duchenne muscular dystrophy (Dumont et al., 2015). MARK2/MARK3 double mutants fail to complete embryonic development indicating that in the single knockout mice, redundancy masks essential developmental functions of the MARK kinases (Lennerz et al., 2010).
2.1 Structure of PAR-1 kinases
The PAR-1 kinase domain adopts a canonical bilobed structure that is typical of most kinases (Marx et al., 2006; Murphy et al., 2007; Panneerselvam et al., 2006; Sack et al., 2016). Catalytic activity is stimulated by phosphorylation of the kinase domain activation loop (residue Thr208 in MARK2), which causes the activation loop to swing out of the catalytic cleft and makes the catalytic site accessible to substrates (Drewes et al., 1997; Timm et al., 2008b). Phosphorylation of the PAR-1 activation loop is mediated by LKB1/PAR-4, a conserved, master regulatory kinase that phosphorylates the activation loop of all 14 members of the AMPK family of kinases (Lizcano et al., 2004). AMPK family kinases have diverse functions and it remains unclear how a single upstream kinase can specifically activate individual family members. MARK kinases can also be activated by the kinase MARKK/TAO-1, which phosphorylates the same activation loop residue as LKB1/PAR-4 (Timm et al., 2003). In addition to activating modifications, the activation loop is also a site for inhibitory phosphorylation. GSK3β kinase phosphorylates MARK2 at residue Ser212, which occludes substrate access to the catalytic site and renders the kinase inactive, even if it is also phosphorylated at Thr208 (Timm et al., 2008a; Timm et al., 2003).
2.2 Regulation of PAR-1 localization
PAR-1 is typically asymmetrically distributed at the cell cortex of polarized cells. There are two elements in the C-terminus of PAR-1 that play a central role in controlling PAR-1 localization. First, the KA1 (Kinase Associated) domain is a membrane-binding domain located at the C-terminus that binds acidic phospholipids such as the plasma-membrane enriched phospholipid phosphatidylserine (Leventis and Grinstein, 2010; Moravcevic et al., 2010). Studies in C. elegans and mammalian cells have shown that the KA1 domain is both necessary and sufficient for cortical recruitment, consistent with the idea that recruitment to the cell cortex is mediated, at least in part, by the direct interaction between the KA1 domain and the cytoplasmic face of the plasma membrane (Goransson et al., 2006; Moravcevic et al., 2010; Motegi et al., 2011). The asymmetric distribution of PAR-1 at the cell cortex is regulated by the Anterior PAR protein aPKC, which phosphorylates PAR-1 at a conserved residue near the KA1 domain (Thr595 in MARK2). Phosphorylation of PAR-1 at this residue results in the binding of the 14-3-3 protein PAR-5 to PAR-1, which sequesters PAR-1 in the cytoplasm. This mechanism prevents the association of PAR-1 with the region of the cell cortex occupied by the Anterior PARs (Hurov et al., 2004; Motegi et al., 2011; Suzuki et al., 2004). The interactions between the PAR proteins will be discussed in more detail below.
2.3 Regulation of PAR-1 activity
A number of mechanisms have been identified that control PAR-1 activity, including interactions with binding partners that either inhibit (for example, PAK5) or activate (for example, GAB1 or DAPK) PAR-1 kinase activity (reviewed in (Matenia et al., 2005; Matenia and Mandelkow, 2009; Wu et al., 2011). Additionally, intramolecular interactions between the kinase domain, UBA domain and KA1 domain appear to play important roles in regulating PAR-1 catalytic activity. Although it is named for its homology to the ubiquitin-binding UBA domain, the PAR-1 UBA domain possesses an extremely weak affinity for ubiquitin and does not bind ubiquitin in vivo (Marx et al., 2006; Murphy et al., 2007; Panneerselvam et al., 2006). Rather, the UBA domain binds to the back surface of the kinase domain, opposite the catalytic cleft, and plays both positive and negative roles in controlling kinase activity (Reviewed in (Marx et al., 2010). On one hand, the UBA domain functions as an autoinhibitory domain by holding the kinase in an inactive, “open” conformation (Marx et al., 2006; Panneerselvam et al., 2006). Similar autoinhibitory interactions between the UBA and kinase domain have been identified in other AMPK family members, although the positioning of the UBA domain on the kinase domain varies (Chen et al., 2009; Wu J.X., et al., 2015). On the other hand, the UBA domain is required for LKB1 phosphorylation of the activation loop, and therefore for kinase activation (Jaleel et al., 2006). Because it appears to participate both in kinase activation and inhibition, the UBA domain has been suggested to serve as a fulcrum point for the regulation of kinase activity (discussed in (Marx et al., 2010)).
Several lines of evidence suggest an autoinhibitory interaction between the KA1 domain and the kinase domain likely regulates PAR-1 kinase activity. Interactions between the C-terminus and N-terminus have been detected by co-immunoprecipitation (MARK2) and by yeast two hybrid (the budding yeast homologs of PAR-1, KIN1 and KIN2) and genetic analysis in budding yeast are consistent with an autoinhibitory interaction (Elbert et al., 2005; Yang et al., 2012). Additionally, interaction between MARK2 and the scaffolding protein GAB1 likely stimulates MARK2 kinase activity by preventing the interaction between the N and C terminus (Yang et al., 2012). Further support for a potential inhibitory interaction between the C-terminus and kinase domain comes from biochemical and structural studies of SAD kinase, an AMPK kinase family member that is related to PAR-1 kinase. The SAD kinase C-terminus, including the KA1 domain and surrounding sequences, inhibits kinase activity by folding back and strengthening the autoinhibitory interaction between the UBA domain and the kinase domain (Wu et al., 2015). Although the importance and mechanism of the C-terminus/KA1 domain regulation of PAR-1 kinase activity await further characterization, such a regulatory interaction would be of considerable interest because it could provide a means to couple control of PAR-1 kinase activity with control of its localization, for example through aPKC phosphorylation or PAR-1 membrane association.
3. Regulation of Cell Polarity by the PAR proteins
PAR-1 is an essential component of a network of cell polarity regulators, the PAR proteins, which collectively function to spatially organize most polarized animal cells including epithelia, neurons and asymmetrically dividing cells (reviewed in (Goldstein and Macara, 2007; Nance and Zallen, 2011; St Johnston and Ahringer, 2010)). A hallmark of the PAR proteins is that they localize to two distinct, opposing domains at the plasma membrane/cell cortex of polarized cells. One cortical domain is occupied by the Anterior PAR proteins, which consist of the PDZ proteins PAR-3 (Bazooka in Drosophila) and PAR-6 and the kinase aPKC (PKC-3 in C. elegans) (Etemad-Moghadam et al., 1995; Tabuse et al., 1998; Watts et al., 1996). The reciprocal cortical domain is occupied by PAR-1 and, in C. elegans, by the RING finger protein PAR-2 (Boyd et al., 1996; Guo and Kemphues, 1995). From these asymmetric domains, the PAR proteins control a wide range of cellular asymmetries including the polarization of the actomyosin and microtubule cytoskeletons and the partitioning of both cortical and cytoplasmic factors along the polarity axis (Goldstein and Macara, 2007; Nance and Zallen, 2011). Two additional PAR proteins, the 14-3-3 protein PAR-5 and the PAR-1 activating kinase LKB-1/PAR-4 (discussed above), are symmetrically distributed in the cytoplasm and at the cell cortex (Benton et al., 2002; Morton et al., 2002; Watts et al., 2000).
3.1 Mutual antagonism between the PAR proteins
The mechanisms that establish and maintain opposing cortical PAR domains are of great interest as they provide the foundation for the elaboration of downstream asymmetries. Studies in a number of systems have demonstrated that mutually antagonistic interactions between Anterior and Posterior PAR proteins provide a core means by which they concentrate in opposing cortical domains. These mechanisms have been reviewed recently (Hoege and Hyman, 2013; Motegi and Seydoux, 2013; Nance and Zallen, 2011), and will only be covered briefly here. aPKC phosphorylation of PAR-1 on a conserved residue (Thr595 in MARK2) restricts PAR-1 from concentrating in the anterior PAR cortical domain and has been observed to reduce PAR-1 kinase activity (Chen et al., 2006; Hurov et al., 2004; Motegi et al., 2011; Suzuki et al., 2004). In C. elegans, aPKC similarly phosphorylates the posterior PAR protein PAR-2, which prevents PAR-2 association with the anterior cortex (Hao et al., 2006). The phosphorylation of PAR-3 by PAR-1 restricts PAR-3 from concentrating in the PAR-1 cortical domain (Benton and St Johnston, 2003). These phosphorylation events result in binding of the 14-3-3 protein PAR-5, leading to the sequestration of the phosphorylated protein in the cytoplasm (Benton et al., 2002; Goransson et al., 2006; Hao et al., 2006; Riechmann and Ephrussi, 2004). In mammalian cells, it has been shown that the phosphorylation of PAR-3 by PAR-1 is enhanced by the scaffolding protein GAB1, which brings PAR-1 and PAR-3 together in a transient complex (Yang et al., 2012).
As discussed in the following sections, although mutual antagonism between the PAR proteins is conserved, distinct mechanisms contribute to the triggering, establishment and maintenance of PAR polarity in different polarized cells. Furthermore, PAR-1 can drive the spatial reorganization of cells through a range of different substrates and different mechanisms. For example, PAR-1 engages fundamentally different mechanisms to control the rapid polarization of C. elegans zygote and the relatively slow, long range polarization of the Drosophila oocyte. These distinct polarization mechanisms accord with the significantly different spatial and temporal scales at which asymmetries are generated in these two cells.
4. Asymmetric division of the C. elegans zygote
4.1 Overview of polarization of the C. elegans zygote
The first function of the PAR proteins in the C. elegans embryo is to orchestrate the polarization and asymmetric division of the zygote. The PAR proteins are maternally deposited in the embryo and are initially symmetrically distributed. Shortly following fertilization and the subsequent completion of meiosis, the PAR proteins redistribute to form opposing anterior (the Anterior PARs) and posterior (PAR-1 and PAR-2) cortical domains. From these domains, the PAR proteins establish a number of asymmetries along the anterior/posterior axis. As will be discussed below, PAR-1 functions downstream of the other PAR proteins to direct the redistribution of somatic and germline fate determinants to the anterior and posterior cytoplasm, respectively (Figure 2). As a result, these determinants are asymmetrically inherited by the two daughter cells, specifying the anterior daughter cell as a somatic blastomere and the posterior daughter cell as a germline blastomere (reviewed in (Rose and Kemphues, 1998)). In addition, the PAR proteins regulate asymmetric distribution of cortical microtubule pulling forces such that the position of the mitotic spindle is shifted to the posterior, thereby generating a smaller posterior cell. Control of spindle positioning by the PAR proteins is an essential process underlying asymmetric cell division and has been reviewed recently (Kotak and Gonczy, 2013; Lu and Johnston, 2013).
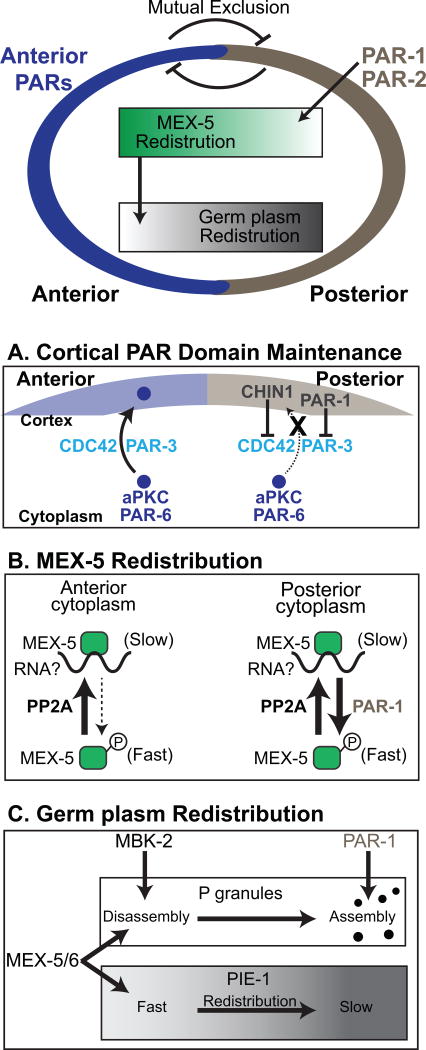
Figure 2.
Establishment of the Anterior/Posterior axis in the C. elegans zygote. In the polarized C. elegans zygote, the Anterior PARs (Blue) are enriched at the anterior cortex and PAR-1 and PAR-2 (brown) are enriched at the posterior cortex. Antagonistic interactions between the PAR proteins mediates their mutual exclusion. PAR-1 directs the redistribution of MEX-5 to the anterior cytoplasm and MEX-5 contributes to the redistribution of germ plasm proteins to the posterior cytoplasm. All of these factors are symmetrically distributed before symmetry breaking. A. Asymmetric PAR domains are maintained in part by the recruitment of cytoplasmic PAR-6 and aPKC to the anterior, but not to the posterior, cortex. PAR-1 and CHIN-1 restrict PAR-3 and active CDC42 from the posterior cortex, thereby preventing the recruitment of PAR-6 and aPKC from the cytoplasm to the posterior cortex. Other mechanisms that contribute to PAR domain maintenance are described in the text and not illustrated for simplicity. B. The redistribution of MEX-5 to the anterior cytoplasm is controlled by PAR-1. PAR-1 phosphorylates MEX-5 and increases MEX-5 mobility in the posterior cytoplasm while PP2A reverses this effect. As a result, MEX-5 mobility is relatively fast in the posterior cytoplasm and MEX-5 redistributes to the anterior cytoplasm. MEX-5 association with RNA is likely to contribute to its slow diffusion. C. As MEX-5 and MEX-6 accumulate in the anterior, germ plasm factors segregate to the posterior cytoplasm. P granules partition to the posterior cytoplasm because MEX-5/6 and MBK-2 promote their disassembly in the anterior cytoplasm and PAR-1 promotes their stability in the posterior cytoplasm. MEX-5/6 act to increase PIE-1 mobility in the anterior cytoplasm, thereby stimulating the redistribution of PIE-1 to the posterior cytoplasm.
4.2 Symmetry breaking and polarity establishment
Prior to the completion of meiosis, PAR-1 and PAR-2 are restricted to the cytoplasm by the Anterior PARs, which are uniformly distributed throughout the cell cortex (Boyd et al., 1996; Guo and Kemphues, 1995). Upon the completion of meiosis, this symmetry is broken by the maturation of the sperm-donated centrosome, which is located near the posterior cortex in association with the sperm pronucleus (Goldstein and Hird, 1996). The centrosome triggers two symmetry-breaking mechanisms. In one mechanism, an unknown cue from the centrosome inhibits contraction of the cortical actomyosin meshwork at the posterior cortex, thus initiating flow of the actomyosin cortex towards the anterior (Motegi and Sugimoto, 2006; Munro et al., 2004). These flows sweep the Anterior PARs from the posterior to the anterior, thus enabling PAR-1 and PAR-2 to associate with the posterior cortex (Cowan and Hyman, 2004; Goehring et al., 2011b; Munro et al., 2004). Because actomyosin flows do not depend on PAR-1 or PAR-2, the polarized Anterior PAR domain is established in par-1 and par-2 embryos (Boyd et al., 1996; Cuenca et al., 2003; Etemad-Moghadam et al., 1995). The second symmetry breaking mechanism depends on PAR-2, which binds to microtubules in a manner that protects PAR-2 from phosphorylation by aPKC (Motegi et al., 2011). Thus, microtubules that extend from the centrosome to the cortex enable PAR-2 to load onto the nearby posterior cortex despite the presence of aPKC. Cortical PAR-2 then acts to recruit its binding partner PAR-1 from the cytoplasm to the cortex. Once at the cortex, PAR-1 is able to phosphorylate PAR-3, thereby promoting the further growth of the posterior domain by locally excluding the Anterior PARs from the posterior cortex (Motegi et al., 2011). Either one of these symmetry breaking mechanisms is sufficient to break symmetry, although when only one mechanism is active polarization is either delayed or proceeds relatively slowly (Goehring et al., 2011b; Motegi et al., 2011). Therefore, the presence of two symmetry breaking pathways appears to ensure that establishment of the cortical PAR domains is both robust and rapid (Motegi and Seydoux, 2013).
After symmetry breaking, the establishment of cortical PAR polarity progresses as the Anterior PAR domain retracts and the Posterior PAR domain expands until, roughly 5 minutes later, the boundary between the two domains reaches the midpoint of the anterior/posterior axis (Boyd et al., 1996; Etemad-Moghadam et al., 1995; Guo and Kemphues, 1995; Tabuse et al., 1998; Watts et al., 1996). The expansion of the PAR-1 and PAR-2 posterior domain depletes the levels of these proteins in the cytoplasm, which eventually limits their cortical recruitment and the growth of the posterior domain (Goehring et al., 2011b). Following their establishment, the two PAR domains are maintained through cytokinesis, which occurs roughly 10 minutes later.
4.3 Polarity maintenance
The PAR domains are persistent in the polarized zygote even though individual PAR proteins undergo both lateral diffusion within the cortex and continual exchange between the cortex and the cytoplasm (Cheeks et al., 2004; Goehring et al., 2011a; Nakayama et al., 2009; Robin et al., 2014; Sailer et al., 2015). The maintenance of stable PAR domains depends on a combination of mechanisms that control where they are recruited to the cell cortex and that counteract their lateral diffusion within the cortex. During polarity maintenance, the recruitment of PAR-6/aPKC from the cytoplasm to the cortex depends on interactions with both PAR-3 and the active form of the small GTPase, CDC42 (Aceto et al., 2006; Gotta et al., 2001; Kay and Hunter, 2001). Both PAR-3 and active CDC42 are concentrated at the anterior cortex, resulting in PAR-6 cortical recruitment rates that are ~9 times higher in the anterior than the posterior cortex (Sailer et al., 2015). This dramatic asymmetry in cortical PAR-6 recruitment depends on two mechanisms that prevent PAR-3 and active CDC42 from localizing to the posterior cortex (Figure 2A). One mechanism relies on the phosphorylation of PAR-3 by PAR-1, which excludes PAR-3 from the posterior cortex (discussed above) (Sailer et al., 2015). The second mechanism depends on CHIN-1, a posteriorly enriched CDC42 GAP that inactivates CDC42 in the posterior (Beatty et al., 2013; Kumfer et al., 2010; Sailer et al., 2015). The presence of either one of these mechanisms is sufficient to prevent PAR-6 recruitment to the posterior cortex. However, in par-1;chin-1 double mutant embryos, PAR-6 is efficiently recruited to the posterior cortex, resulting in the uniform cortical distribution of the Anterior PARs (Sailer et al., 2015). By independently inhibiting PAR-3 and active CDC42 in the posterior, the combined activities of PAR-1 and CHIN-1 provide a robust and reliable mechanism by which to stabilize the polarized Anterior PAR domain. The cortical association rates of PAR-1 and PAR-2 have not been reported, and it will be interesting to learn whether analogous mechanisms act to prevent their recruitment from the cytoplasm to the anterior cortex. Lateral diffusion within the cortex leads to mixing of the Anterior and Posterior PAR proteins at the interface of their two domains. The PAR domain boundary is maintained by antagonistic interactions between the anterior and posterior PAR proteins (discussed above) that cause them to mutually exclude each other form the cortex (Goehring et al., 2011a; Hoege and Hyman, 2013).
4.4 Establishment of cytoplasmic asymmetries
A primary output of the PAR system in the C. elegans zygote is the partitioning of maternally-deposited cytoplasmic cell fate determinants along the anterior/posterior axis. In response to cues from the PAR proteins, the highly similar RNA-binding proteins MEX-5 and MEX-6 (MEX-5/6 hereafter) redistribute towards the anterior cytoplasm, leading to their preferential inheritance by the anterior daughter cell (Schubert et al., 2000). At the same time, a mixture of cytoplasmic RNAs and proteins that specify the germline lineage, collectively called the germ plasm, redistribute to the posterior cytoplasm and are therefore inherited preferentially by the posterior daughter cell. Germ plasm components include non-membranous RNA/protein assemblages called P granules and more diffusely concentrated RNA-binding proteins such as PIE-1 (Mello et al., 1996; Strome and Wood, 1983; Tenenhaus et al., 1998; Updike and Strome, 2010). The asymmetric inheritance of these factors causes the anterior daughter cell to adopt a somatic fate and the posterior daughter cell to adopt a germline fate (Rose and Kemphues, 1998; Wang and Seydoux, 2013).
The partitioning of cytoplasmic somatic and germline determinants along the zygotic anterior/posterior axis is remarkable in several respects. These cytoplasmic determinants are symmetrically distributed in the zygote prior to the symmetry breaking, and their segregation is largely completed during the ~5 minutes it takes to establish the cortical PAR domains. Segregation does not depend on new protein synthesis, local protein degradation or directed transport (Griffin, 2015; Hoege and Hyman, 2013). Rather, segregation results from mechanisms that locally increase protein diffusivity, generating gradients in protein mobility along the anterior/posterior axis that cause proteins to concentrate in the region of low mobility (Daniels et al., 2009; Griffin et al., 2011; Lipkow and Odde, 2008; Tenlen et al., 2008). In par-1 mutants, there is a complete failure to generate cytoplasmic asymmetries despite the fact the cortical Anterior PAR domain is established, which indicates PAR-1 plays a central role in transducing cortical polarity cues to the cytoplasm (Cuenca et al., 2003; Guo and Kemphues, 1995). PAR-1 does so by controlling the segregation of MEX-5/6 to the anterior cytoplasm, which, in turn drive the segregation of germline factors to the posterior cytoplasm (Griffin et al., 2011; Schubert et al., 2000; Tenlen et al., 2008).
In the polarized zygote, MEX-5 forms a 3-fold, anterior-rich concentration gradient that spans the 50 micron anterior/posterior axis of the cell (Daniels et al., 2010; Griffin et al., 2011; Schubert et al., 2000; Tenlen et al., 2008). During gradient formation, MEX-5 mobility is increased in the posterior cytoplasm by PAR-1 phosphorylation, which likely prevents the formation of slow-diffusing MEX-5/RNA complexes (Griffin et al., 2011; Tenlen et al., 2008). The uniformly distributed cytoplasmic phosphatase PP2A counteracts PAR-1 by acting to decrease MEX-5 mobility, resulting in the formation of an anterior-slow, posterior-fast MEX-5 mobility gradient (Figure 2B) (Griffin et al., 2011; Schlaitz et al., 2007). As a consequence of this mobility gradient, MEX-5 is preferentially retained in the anterior cytoplasm (Griffin et al., 2011). Epistasis analysis demonstrated that PAR-1 functions downstream of the Anterior PARs to control MEX-5 mobility, indicating the primary role of the Anterior PARs in MEX-5 segregation is to restrict PAR-1 to the posterior (Griffin et al., 2011; Tenlen et al., 2008). Interestingly, in addition to the enrichment of PAR-1 at the posterior cortex, there is a slight enrichment of PAR-1 in the posterior cytoplasm. Analysis of par-2 mutants, in which PAR-1 does not load onto the cortex but still concentrates in the posterior cytoplasm, indicates that the asymmetric activity of PAR-1 in the cytoplasm is sufficient to drive MEX-5 segregation (Boyd et al., 1996; Griffin et al., 2011; Labbé et al., 2006).
4.5 Germ plasm segregation
As MEX-5/6 accumulate in the anterior cytoplasm, they simultaneously stimulate the segregation of germ plasm components to the posterior cytoplasm (Figure 2C) (Schubert et al., 2000). MEX-5/6 act downstream of the PAR proteins and through an unknown mechanism to increase the mobility of the RNA-binding protein PIE-1 in the anterior cytoplasm, resulting in the formation of an anterior-fast, posterior-slow PIE-1 mobility gradient (Wu et al., 2015b). As a result of its mobility gradient, PIE-1 is preferentially retained in the posterior cytoplasm (Daniels et al., 2009; Wu Y, et al., 2015). The ability of MEX-5/6 to increase PIE-1 mobility is concentration-dependent, suggesting there is a direct coupling between the formation of the MEX-5/6 gradients and the PIE-1 mobility gradient (Wu Y, et al., 2015).
P granules are non-membranous organelles composed of RNA and of dozens of RNA-binding proteins, many of which contain intrinsically disordered domains (Updike and Strome, 2010). P granules are highly dynamic and behave like phase-separated liquid droplets (Brangwynne et al., 2009; Weber and Brangwynne, 2012). During the asymmetric division of the zygote, P granules become dramatically enriched in the posterior cytoplasm as a result of mechanisms that promote their disassembly in the anterior cytoplasm and their stabilization and growth in the posterior cytoplasm (Brangwynne et al., 2009; Gallo et al., 2010). PAR-1 regulates P granule segregation indirectly by controlling the segregation of MEX-5/6, which promote P granule disassembly in the anterior cytoplasm (Brangwynne et al., 2009; Gallo et al., 2010; Schubert et al., 2000). In vitro studies with the P granule protein PGL-3 indicate that PGL-3 and MEX-5 compete for binding to mRNA, and that mRNA-binding promotes PGL-3 phase separation (Saha et al., 2016). These findings support a model in which the high concentrations of MEX-5/6 in the anterior cytoplasm deplete the pool of mRNA available to participate in P granule assembly, thereby locally shifting the balance between assembly and disassembly towards disassembly. PAR-1 also acts independently of MEX-5/6 to stabilize P granules in the posterior cytoplasm, raising the possibility that PAR-1 may contribute more directly P granule segregation (Cheeks et al., 2004; Gallo et al., 2010). Apart from regulation by MEX-5/6 and PAR-1, P granule dynamics are also controlled by MBK-2 kinase, which stimulates P granule disassembly through phosphorylation of the intrinsically disordered P granule proteins MEG-3 and MEG-4 (Wang et al., 2014).
In summary, PAR-1 functions at a critical juncture in the mechanisms that control the asymmetric division of the C. elegans zygote. PAR-1 functions downstream of the Anterior PAR proteins to control the segregation of MEX-5/6 to the anterior cytoplasm. In turn, MEX-5/6 stimulate the segregation of the germ plasm to the posterior cytoplasm. These asymmetries are generated through reactions that control the redistribution of diffusive proteins, which enables the efficient partitioning of factors during the rapid cell divisions of the early embryo.
5. Establishment of the Anterior/Posterior axis during Drosophila oogenesis
5.1 Overview of Drosophila oogenesis
The development of the Drosophila oocyte begins with a single cell, called a cystoblast, that undergoes four rounds of incomplete cell division, giving rise to a 16 cell cyst. One of these 16 cystoblasts will differentiate into the oocyte while the other 15 become nurse cells (reviewed in (Roth and Lynch, 2009)). The nurse cells remain connected to the oocyte through cytoplasmic bridges called ring canals through which they transport cytoplasmic contents into the oocyte, fueling the extensive growth of the oocyte and providing factors that are essential for the patterning of the oocyte and the initial development of the fertilized embryo. The maturing oocyte and its nurse cells are encased within a single layer of somatic epithelial cells called the follicle cells. During mid-oogenesis, signaling between the oocyte and the follicle cells initiate the polarization of the oocyte microtubule cytoskeleton, which provides the basis for the transport of bicoid and oskar mRNA to the anterior and posterior poles, respectively (Januschke et al., 2002; Zimyanin et al., 2008). Oskar is subsequently translated and concentrated at the posterior pole where it organizes the formation of the germ plasm (reviewed in (Huynh and St Johnston, 2004; Lehmann, 2016)). Bicoid is translated at the anterior pole following fertilization where it functions as a morphogen to specify anterior embryonic tissues (Driever and Nusslein-Volhard, 1988; Frohnhofer and Nusslein-Volhard, 1986). In the following sections, we focus on the key roles played by dPar-1 at several stages of oocyte development, including the initial stage of oocyte specification, the polarization of the microtubule cytoskeleton and the organization of the germ plasm in the posterior. In addition to the functions discussed below, dPar-1 also regulates bicoid mRNA transport (Riechmann and Ephrussi, 2004), apical/basal polarity of the follicle cells (Cox et al., 2001a; Doerflinger, 2003) asymmetric stem cell divisions in the male germline (Inaba et al., 2015; Yuan et al., 2012) and the migration of a subset of the follicle cells (the border cells) through the nurse cells (Mcdonald et al., 2008).
5.2 Oocyte specification
During the initial cystoblast divisions, dPar-1 concentrates on an ER-like cytoplasmic organelle called the fusome, which traverses the cytoplasmic bridges that connect the cystoblast cells (Cox et al., 2001a; Shulman et al., 2000; Tomancak et al., 2000). Specification of the future oocyte is marked by the concentration of a number of factors, including dPar-1, Par-3 and the oocyte determinants Orb, BicD and Egl, in one of the 16 cystoblast cells. Shortly thereafter, the oocyte is polarized in a process that depends on dPar-1 and the Anterior PARs such that Orb, BicD and Egl concentrate in the oocyte posterior (Benton et al., 2002; Cox et al., 2001a; Cox et al., 2001b; Huynh et al., 2001a; Huynh et al., 2001b; Vaccari and Ephrussi, 2002). In par mutants, Orb, BicD and Egl become enriched in the future oocyte, but these proteins fail to concentrate in the posterior of the cell and the oocyte dedifferentiates into a nurse cell (Benton et al., 2002; Cox et al., 2001a; Huynh et al., 2001b; Vaccari and Ephrussi, 2002). Therefore, the PAR proteins are not required for the initial specification of the oocyte, but rather for the subsequent maintenance of oocyte fate. Whether dedifferentiation is a consequence of the failure to asymmetrically localize Orb, BicD and Egl within the oocyte or represents a separate function of the PAR proteins is not known.
5.3 Anterior/posterior axis specification during mid-oogenesis
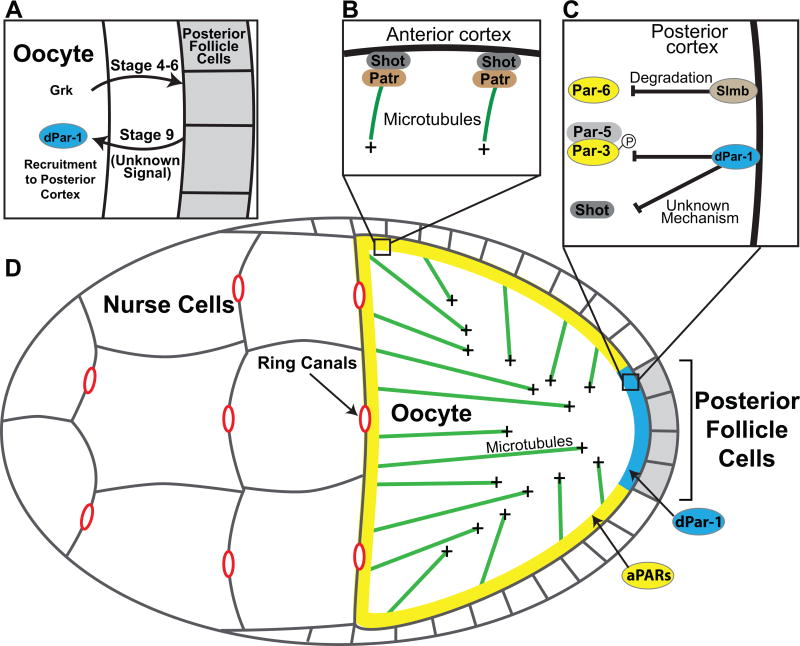
Several hours after its initial specification, the oocyte is positioned at the posterior end of the egg chamber where it sits adjacent to the posterior follicle cells. The establishment of the oocyte anterior/posterior axis begins early in oogenesis with the secretion of the EGF-like protein Gurken from the posterior end of the stage 4–6 oocyte (Chang et al., 2008; Peri et al., 1999). Gurken signals to the overlying posterior follicle cells, which subsequently signal back to the oocyte through an unknown mechanism (Figure 3A) (Gonzalez-Reyes et al., 1995; Roth and Lynch, 2009). These signaling events lead to the establishment of opposing cortical PAR domains: dPar-1 concentrates in a small cortical domain at the posterior end and the Anterior PARs occupy the anterior and lateral regions of the oocyte cortex (Benton and St Johnston, 2003; Doerflinger et al., 2006; Shulman et al., 2000; Tomancak et al., 2000). Although the final configuration of the PAR domains is similar to that in the C. elegans zygote, the dynamics by which these domains form are strikingly different. Whereas the PAR domains are established in ~5 minutes in C. elegans, it takes roughly 12 hours for these domains to mature between stage 7 and 9 fly oocytes. Furthermore, the mechanisms underlying the recruitment of dPar-1 to the oocyte posterior cortex differ from those that underlie the formation of the PAR-1 cortical domain in the C. elegans zygote. Recruitment of dPar-1 to the oocyte posterior does not involve cortical actomyosin flows or microtubules, and there is no clear PAR-2 homolog in Drosophila (Doerflinger et al., 2006). Rather, signaling from the posterior follicle cells initiates polarization of the oocyte by inducing the degradation of Par-6 and aPKC at the posterior cortex by the SCF E3 ubiquitin ligase Slimb (Figure 3C) (Morais-de-Sa et al., 2014). Slimb localizes to the posterior cortex and is required for the clearance of Par-6/aPKC, which allows for the recruitment of dPar-1 to the posterior cortex (Morais-de-Sa et al., 2014). Once dPar-1 is recruited to the posterior cortex, mutual antagonism between the PAR proteins stabilize their reciprocal localization patterns. dPar-1 phosphorylates Par-3 (Bazooka), leading to its clearance from the posterior cortex and aPKC phosphorylates dPar-1, thereby restricting dPar-1 from spreading into the lateral and anterior oocyte cortex (Doerflinger et al., 2010).
Figure 3.
Establishment of the Anterior/Posterior axis in the Drosophila oocyte. A. Gurken (Grk) is secreted from the oocyte and signals to the neighboring follicle cells. The posterior follicle cells subsequently signal back to the oocyte, leading to the recruitment of dPar-1 to the posterior cortex. The signal from the follicle cells to the oocyte is not known. B. Microtubules grow from the oocyte anterior cortex from foci containing Shot and Patronin, which binds microtubule minus ends. C. At the oocyte posterior cortex, Par-6 is removed from the cortex by the E3 ubiquitin ligase, Slimb. Par-3 is removed from the posterior cortex by dPar-1 phosphorylation, which leads to interaction with Par-5 and sequestration of Par-3 in the cytoplasm. dPar-1 also acts through an unknown mechnanism to restrict Shot from the posterior cortex D. The oocyte is positioned next to the posterior follicle cells and is connected to the nurse cells by ring canals. dPar-1 (blue) forms a posterior cortical domain that is reciprocal to the anterior/lateral Anterior PAR domain (aPAR, yellow). Microtubules (Green) are nucleated from the anterior and lateral cortex and not from the posterior cortex. As a result, microtubule density is higher in the anterior are microtubules tend to point towards the posterior.
The concentration of dPar-1 at the posterior cortex leads to a critical reorganization of the microtubule cytoskeleton along the anterior/posterior axis. During mid-oogenesis, non-centrosomal microtubules grow from the anterior and lateral cortex from foci containing the microtubule minus end binding protein Patronin. Patronin foci also contain spectraplakin (Shot in Drosophila), which is required to recruit Patronin to the oocyte cortex (Nashchekin et al., 2016). dPar-1 functions downstream of the Anterior PAR proteins to suppress microtubule growth from the posterior cortex by acting to exclude Shot from localizing to the posterior cortex (Figure 3B) (Nashchekin et al., 2016). It is not known whether Shot is a substrate of dPar-1 or if dPar-1 controls Shot localization more indirectly. As a result of the differences in cortical microtubule growth, there is both an anterior-high, posterior-low gradient in the density of microtubules and a weak bias in the orientation of the microtubules such that microtubule plus ends tend to be pointed towards the oocyte posterior (Khuc Trong et al., 2015; Parton et al., 2011b; Zimyanin et al., 2008). These asymmetries provide the basis for differential transport of anterior and posterior determinants. oskar mRNA is transported by the plus end-directed motor Kinesin with a slight bias towards the posterior, which is sufficient to concentrate oskar mRNA at the posterior cortex (Khuc Trong et al., 2015; Zimyanin et al., 2008). Similarly, the minus end-directed motor dynein is responsible for the transport of bicoid mRNA to the anterior (St Johnston, 2005). In dpar-1 mutant oocytes, microtubules are nucleated uniformly from the cortex with their plus ends oriented towards the center of the oocyte, resulting in the accumulation of oskar mRNA in a foci in the center of the oocyte (Figure 3D) (Doerflinger et al., 2006; Parton et al., 2011a; Shulman et al., 2000; Tomancak et al., 2000).
In addition to its role in directing the posterior transport of oskar mRNA through polarization of the microtubule cytoskeleton, dPar-1 also plays direct roles in the regulation of Oskar protein accumulation at the posterior pole. Oskar is translated in two forms, Long Oskar and Short Oskar. Long Oskar is required to anchor oskar mRNA at the posterior cortex during mid-oogenesis. Short Oskar does not accumulate to high levels until dPar-1 levels decrease during late oogenesis, at which point Short Oskar controls the organization of the germ plasm (reviewed in (Lehmann, 2016)). The delay in the accumulation of Short Oskar is controlled by its sequential phosphorylation by dPar-1 and Gsk-3, which target Short Oskar for degradation by the E3 ubiquitin ligase Slimb (Morais-De-Sa et al., 2013). dPar-1 has also been shown to stabilize Oskar through phosphorylation at a second site, which suggest dPar-1 may target different populations of Oskar for stabilization or degradation (Riechmann et al., 2002). Interestingly, overexpression of oskar mRNA results in the formation of an ectopic oskar mRNA dot in the cytoplasm that recruits dPar-1 (Zimyanin et al., 2007). This result suggests oskar mRNA and dPar-1 may participate in a positive feedback loop that promotes each other’s localization to the posterior cortex, thereby stabilizing the formation of the posterior domain.
5.4 Comparison of anterior/posterior polarization in worms and flies
Taken together, the studies described above have elucidated a collection of mechanisms by which the PAR proteins establish the anterior/posterior axis in flies and worms. In both cases, PAR-1 localizes and functions in opposition to the Anterior PAR proteins and PAR-1 activity is intimately associated with organization and partitioning germ line factors to germ cells. At the mechanistic level, however, PAR-1 plays a different role in the two systems. In the C. elegans zygote, the PAR-1 drives cytoplasmic asymmetries through modulation of MEX-5/6 mobility, which then propagates these signals to drive the segregation of germline factors. These mechanisms are capable of rapidly generating asymmetries over relatively short length scales. In the Drosophila oocyte, dPar-1 controls asymmetries in large part by restricting the growth of non-centrosomal microtubules to the anterior and lateral cortex, thereby biasing the orientation of the microtubule cytoskeleton. Polarization of the microtubule cytoskeleton, in turn, provides a foundation for the asymmetric transport of factors towards the anterior or posterior cytoplasm. This mechanism is particularly well-suited to the generation of long range asymmetries over relatively long time scales. Therefore, in different biological contexts, PAR-1 orchestrates polarities through mechanisms that are suited to the time scale and length scale of the cellular reorganization.
6. MARK kinases and neurogenesis
The mammalian MARKs (microtubule affinity-regulating kinase) were initially purified from porcine brain extracts based on their ability to phosphorylate the microtubule associated proteins TAU, MAP2 and MAP4 on conserved KXGS motifs present in their microtubule binding domains (Drewes et al., 1997). Microtubule associated proteins (MAPs) bind along the surface of microtubules and shift the equilibrium to microtubule polymerization by promoting microtubule nucleation, growth and stability (Penazzi et al., 2016). Phosphorylation by MARK kinases causes MAPs to dissociate from microtubules and thereby shifts the dynamics of microtubules towards depolymerization (Drewes et al., 1997; Schwalbe et al., 2013). Indeed, MARK overexpression in tissue culture cells results in destruction of the microtubule network (Drewes et al., 1997). Live imaging of microtubule dynamics demonstrated that MARK2 controls microtubule dynamics by decreasing the frequency of transitions from growth to depolymerization without altering the rate of plus end growth (Hayashi et al., 2011). In addition to regulating microtubule dynamics, MAPs can disrupt transport on microtubules by competing with motors for binding to microtubules (Penazzi et al., 2016). In this review, we will focus on the role of the MARK kinases in the initial polarization of neurons and in the morphogenesis of dendritic spines in mature neurons. Recent reviews have discussed the critical roles PAR-1/MARK kinases play in multiple steps of neurogenesis including the asymmetric division and migration of neural progenitors and the initial specification of axon/dendrite polarity (Knoblich, 2010; McDonald, 2014; Reiner and Sapir, 2013; Shelly and Poo, 2011).
6.1 Neuronal polarization
The antagonistic relationship between MARK kinases and the Anterior PARs is intimately involved in the initial polarization of neurons. During this process, the unpolarized neurons extend multiple neurites, one of which is selected as an axon while the remaining become dendrites. In cultured hippocampal neurons, MARK2 is active in nascent dendritic projections and inactive in axonal projections, which results in the accumulation of TAU specifically on axonal microtubules (Chen et al., 2006). Reduction of MARK2 expression results in the formation of multiple axons whereas the overexpression of MARK2 prevents axon formation, indicating that MARK2 plays an important role in the initial control of axon/dendrite asymmetry (Chen et al., 2006). The Anterior PAR proteins concentrate at the tip of the nascent axon and are required for its selection, at least in part because of their ability to inhibit MARK activity in the axon (reviewed in (Insolera et al., 2011). SAD kinases, which contain kinase domains similar to MARK kinases, also phosphorylate MAP protein at KXGS motifs, resulting in their dissociation from microtubules (Kishi et al., 2005). In vivo, SAD kinases are essential for neuronal polarization, likely due to their role in controlling the polarized accumulation of TAU and MAP2 axons and dendrites, respectively (Kishi et al., 2005).
In mature neurons, MARK1 and MARK2 concentrate in and are required for the morphogenesis of dendritic spines, which are postsynaptic protrusions through which dendrites receive synaptic inputs (Wu et al., 2012). MARK2 promotes the growth of microtubules and the associated transport of cargo into dendritic spines (Hayashi et al., 2011). MARK2 also phosphorylates PSD-95, a postsynaptic protein that scaffolds the assembly of proteins at the post-synaptic density and is required for spine morphogenesis (Wu et al., 2012). The role of MARK phosphorylation in PSD-95 function is not known, but may involve control of PSD-95 dynamics as dPar-1 phosphorylation of the Drosophila PSD-95 homologue Discs large (Dlg) stabilizes its postsynaptic localization at the neuromuscular junction (Zhang et al., 2007). Interestingly, the Anterior PAR proteins are also essential for dendritic spine morphogenesis through their control of the actin cytoskeleton, suggesting that the PAR polarity network is deployed within each dendritic spine to orchestrate their organization (Zhang, 2008; Zhang and Macara, 2006). It will be interesting to learn to what extent the principles and mechanisms underlying polarization at the cellular level are recapitulated in these small, subcellular compartments.
7. PAR-1 and disease
7.1 Alzheimer’s Disease
During the progression of Alzheimer’s disease, TAU becomes hyperphosphorylated, dissociates from microtubules, accumulates to abnormally high levels in the somatodendritic compartment, and forms paired helical filaments and neurofibrillary tangles (NFTs) (Iqbal et al., 2016). Phosphorylation by MARK is thought to contribute to disease progression by causing TAU to dissociate from microtubules, thereby increasing the cytoplasmic pool of TAU that is available to aggregate. During the preogression of Alzheimer’s, one of the earliest TAU phosphorylation sites to be upregulated is the MARK phosphorylation site Ser262, suggesting that MARK activity may play an early role in disease progression (Matenia and Mandelkow, 2009). In Drosophila, Tau phosphorylation by dPar-1 increases the rate of subsequent phosphorylation at other residues by Gsk-3 and Cdk5, consistent with the idea that PAR-1 phosphorylation primes TAU for hyperphosphorylation (Nishimura et al., 2004). Additionally, TAU phosphorylation also disrupts the sorting of TAU to the axonal compartment, leading to an increase in TAU levels in the somatodendritic compartments (Li et al., 2011). A later, more direct role for MARK in the formation of NFTs is suggested by the observation that MARK kinase is associated with NFTs (Chin et al., 2000). However, because TAU is subject to many different post-translational modifications, including phosphorylation by a large number of kinases, it has been challenging to parse the contribution of individual enzymes to disease progression (Iqbal et al., 2016).
7.2 Cancer
The connections between cell polarity mechanisms and tumorigenesis are of great interest. Loss of cell polarity is a hallmark of many tumors and may contribute to oncogenesis through a number of mechanisms including loss of epithelial polarity and junctions, increased metastasis and disrupted asymmetric progenitor cell divisions (Reviewed in (Halaoui and McCaffrey, 2015; Morrison and Kimble, 2006; Muthuswamy and Xue, 2012). Dysregulation of MARK kinases is associated with a number of tumors. MARK4 is amplified in glioblastoma, upregulated downstream of Wnt signaling in hepatocarcinoma and derepressed in breast and lung cancer cells (Beghini et al., 2003; Kato et al., 2001; Pardo et al., 2016). MARK2 is frequently upregulated in non-small cell lung carcinoma and its expression is correlated with malignant phenotypes (Hubaux et al., 2015). In addition, inherited mutations in LKB1 (PAR-4) kinase cause Peutz-Jeghers cancer syndrome and LKB1 is commonly inactivated in non-small cell lung carcinomas (reviewed in (Sanchez-Cespedes, 2007). As discussed above, LKB1 activates AMPK family kinases including AMPK and MARK kinases and it is likely that dysregulation of several of these kinases contributes to tumor progression (Shorning and Clarke, 2016). A recent study identified a pathway that connects LKB1 inactivation and the consequent loss of MARK1 and MARK4 activity to activation of the epithelial-to-mesenchymal transition. MARK1 and MARK4 phosphorylate the scaffolding protein DIXDC1 to drive its localization to focal adhesions. Inactivation of MARK1 and MARK4 delocalizes DIXDC1 from focal adhesions, which activates a downstream signaling cascade that results in the transcriptional upregulation of Snail, a potent activator of the epithelial-to-mesenchymal transition (Goodwin et al., 2014).
More than half the human population is chronically infected with Helicobactor pylori, which colonizes the stomach mucosa and can cause a variety of gastric diseases, including gastric cancer (Stein et al., 2013). H. pylori strains that express the cytotoxin CagA are associated with a higher risk of gastric cancer (Stein et al., 2013). CagA is injected by H. pylori into epithelial cells lining the stomach where it interacts with a several cellular proteins and causes disruption of epithelial contacts, loss of epithelial polarity and dramatic cell elongation (the “hummingbird” phenotype) that are thought to contribute to oncogenesis. One of the targets of CagA is MARK2, which is localized at the basolateral membrane in epithelial cells and is required to maintain apical/basal polarity (Saadat et al., 2007; Zeaiter et al., 2008). CagA inhibits MARK2 kinase activity via a short peptide that mimics MARK2 substrates and binds stably within the catalytic cleft (Nesić et al., 2010; Nishikawa et al., 2016). Inhibition of MARK2 causes defects in cell polarity and in the assembly of tight junctions that disrupts the architecture of the gastric epithelia and is thought to contribute to an epithelial-to-mesenchymal transition (Saadat et al., 2007; Zeaiter et al., 2008). CagA also targets the host oncoprotein SHP-2 through a different mechanism, and the combined inhibition of MARK2 and SHP-2 is thought to underlie the ability of CagA to trigger carcinogenesis (Stein et al., 2013).
8. Concluding Remarks
In this review, we have discussed how the localized activity of PAR-1 kinase and the Anterior PAR proteins underlies the establishment of cellular asymmetries in a few well characterized systems. While a conserved module of antagonistic interactions between the Anterior PAR proteins and PAR-1 provides a core means by which the activity of these proteins is localized, the mechanisms that trigger polarization and the speed and spatial scale at which the PAR domains are established are unique to different polarized cells. In addition, we have discussed how PAR-1 can drive the formation of downstream asymmetries through a variety of different mechanisms. As future studies of PAR-1 continue to extend beyond the well characterized systems described in this review, it will be critical to identify the relevant PAR-1 substrates, to characterize how phosphorylation modulates the function of these substrates and ultimately contributes to the organization of the cell. In addition, future studies are likely to further elucidate how PAR-1 kinase activity is controlled and in particular how kinase activity may be coordinated with PAR-1 localization. These studies will provide a foundation for understanding how cells establish and maintain polarity and for understanding how disruption of PAR-1 activity contributes to pathogenesis in a variety of contexts, including Alzheimer’s disease progression and tumorigenesis.
References
- Aceto D, Beers M, Kemphues KJ. Interaction of PAR-6 with CDC-42 is required for maintenance but not establishment of PAR asymmetry in C. elegans. Developmental Biology. 2006;299:386–397. doi: 10.1016/j.ydbio.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty A, Morton DG, Kemphues K. PAR-2, LGL-1 and the CDC-42 GAP CHIN-1 act in distinct pathways to maintain polarity in the C. elegans embryo. Development. 2013;140:2005–14. doi: 10.1242/dev.088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghini A, Magnani I, Roversi G, Piepoli T, Di Terlizzi S, Moroni RF, Pollo B, Fuhrman Conti AM, Cowell JK, Finocchiaro G, Larizza L. The neural progenitor-restricted isoform of the MARK4 gene in 19q13.2 is upregulated in human gliomas and overexpressed in a subset of glioblastoma cell lines. Oncogene. 2003;22:2581–2591. doi: 10.1038/sj.onc.1206336. [DOI] [PubMed] [Google Scholar]
- Benton R, Palacios IM, Johnston DS. Drosophila 14-3-3/PAR-5 Is an Essential Mediator of PAR-1 Function in Axis Formation. Developmental Cell. 2002;3:659–671. doi: 10.1016/s1534-5807(02)00320-9. [DOI] [PubMed] [Google Scholar]
- Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- Bessone S, Vidal F, Le Bouc Y, Epelbaum J, Bluet-Pajot MT, Darmon M. EMK protein kinase-null mice: dwarfism and hypofertility associated with alterations in the somatotrope and prolactin pathways. Developmental Biology. 1999;214:87–101. doi: 10.1006/dbio.1999.9379. [DOI] [PubMed] [Google Scholar]
- Boyd L, Guo S, Levitan D, Stinchcomb DT, Kemphues KJ. PAR-2 is asymmetrically distributed and promotes association of P granules and PAR-1 with the cortex in C. elegans embryos. Development. 1996;122:3075–3084. doi: 10.1242/dev.122.10.3075. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Jülicher F, Hyman AA. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- Chang WL, Liou W, Pen HC, Chou HY, Chang YW, Li WH, Chiang W, Pai LM. The gradient of Gurken, a long-range morphogen, is directly regulated by Cbl-mediated endocytosis. Development. 2008;135:1923–33. doi: 10.1242/dev.017103. [DOI] [PubMed] [Google Scholar]
- Cheeks RJ, Canman JC, Gabriel WN, Meyer N, Strome S, Goldstein B. C. elegans PAR proteins function by mobilizing and stabilizing asymmetrically localized protein complexes. Current Biology. 2004;14:851–862. doi: 10.1016/j.cub.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Chen L, Jiao ZH, Zheng LS, Zhang YY, Xie ST, Wang ZX, Wu JW. Structural insight into the autoinhibition mechanism of AMP-activated protein kinase. Nature. 2009;459:1146–9. doi: 10.1038/nature08075. [DOI] [PubMed] [Google Scholar]
- Chen YM, Wang QJ, Hu HS, Yu PC, Zhu J, Drewes G, Piwnica-Worms H, Luo ZG. Microtubule affinity-regulating kinase 2 functions downstream of the PAR-3/PAR-6/atypical PKC complex in regulating hippocampal neuronal polarity. Proceedings of the Natational Academy of Sciences of the United States of America. 2006;103:8534–9. doi: 10.1073/pnas.0509955103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin JY, Knowles RB, Schneider A, Drewes G, Mandelkow EM, Hyman BT. Microtubule-affinity regulating kinase (MARK) is tightly associated with neurofibrillary tangles in Alzheimer brain: a fluorescence resonance energy transfer study. Journal of Neuropathology and Experimental Neurology. 2000;59:966–71. doi: 10.1093/jnen/59.11.966. [DOI] [PubMed] [Google Scholar]
- Cowan CR, Hyman AA. Centrosomes direct cell polarity independently of microtubule assembly in C. elegans embryos. Nature. 2004;431:92–96. doi: 10.1038/nature02825. [DOI] [PubMed] [Google Scholar]
- Cox DN, Lu B, Sun TQ, Williams LT, Jan YN. Drosophila par-1 is required for oocyte differentiation and microtubule organization. Current Biology. 2001a;11:75–87. doi: 10.1016/s0960-9822(01)00027-6. [DOI] [PubMed] [Google Scholar]
- Cox DN, Seyfried SA, Jan LY, Jan YN. Bazooka and atypical protein kinase C are required to regulate oocyte differentiation in the Drosophila ovary. Proceedings of the Natational Academy of Sciences of the United States of America. 2001b;98:14475–80. doi: 10.1073/pnas.261565198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca AA, Schetter A, Aceto D, Kemphues K, Seydoux G. Polarization of the C. elegans zygote proceeds via distinct establishment and maintenance phases. Development. 2003;130:1255–1265. doi: 10.1242/dev.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels BR, Dobrowsky TM, Perkins EM, Sun SX, Wirtz D. MEX-5 enrichment in the C. elegans early embryo mediated by differential diffusion. Development. 2010;137:2579–2585. doi: 10.1242/dev.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels BR, Perkins EM, Dobrowsky TM, Sun SX, Wirtz D. Asymmetric enrichment of PIE-1 in the Caenorhabditis elegans zygote mediated by binary counterdiffusion. The Journal of Cell Biology. 2009;184:473–479. doi: 10.1083/jcb.200809077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger H, Benton R, Shulman JM, St Johnston D. The role of PAR-1 in regulating the polarised microtubule cytoskeleton in the Drosophila follicular epithelium. Development. 2003;130:3965–3975. doi: 10.1242/dev.00616. [DOI] [PubMed] [Google Scholar]
- Doerflinger H, Benton R, Torres I, Zwart M, Stjohnston D. Drosophila Anterior-Posterior Polarity Requires Actin-Dependent PAR-1 Recruitment to the Oocyte Posterior. Current Biology. 2006;16:1090–1095. doi: 10.1016/j.cub.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Doerflinger H, Vogt N, Torres IL, Mirouse V, Koch I, Nüsslein-Volhard C, St Johnston D. Bazooka is required for polarisation of the Drosophila anterior-posterior axis. Development. 2010;137:1765–1773. doi: 10.1242/dev.045807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Drewes G, Trinczek B, Illenberger S, Biernat J, Schmitt-Ulms G, Meyer HE, Mandelkow EM, Mandelkow E. Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. Journal of Biological Chemistry. 1995;270:7679–88. doi: 10.1074/jbc.270.13.7679. [DOI] [PubMed] [Google Scholar]
- Driever W, Nusslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988;54:83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Dumont NA, Wang YX, von Maltzahn J, Pasut A, Bentzinger CF, Brun CE, Rudnicki MA. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nature Medicine. 2015;21:1455–63. doi: 10.1038/nm.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert M, Rossi G, Brennwald P. The yeast par-1 homologs kin1 and kin2 show genetic and physical interactions with components of the exocytic machinery. Molecular Biology of the Cell. 2005;16:532–549. doi: 10.1091/mbc.E04-07-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemad-Moghadam B, Guo S, Kemphues KJ. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell. 1995;83:743–752. doi: 10.1016/0092-8674(95)90187-6. [DOI] [PubMed] [Google Scholar]
- Feng M, Tian L, Gan L, Liu Z, Sun C. Mark4 promotes adipogenesis and triggers apoptosis in 3T3-L1 adipocytes by activating JNK1 and inhibiting p38MAPK pathways. Biology of the Cell. 2014;106:294–307. doi: 10.1111/boc.201400004. [DOI] [PubMed] [Google Scholar]
- Frohnhofer HG, Nusslein-Volhard C. Organization of Anterior Pattern in the Drosophila Embryo by the Maternal Gene Bicoid. Nature. 1986;324:120–125. [Google Scholar]
- Gallo CM, Wang JT, Motegi F, Seydoux G. Cytoplasmic partitioning of P granule components is not required to specify the germline in C. elegans. Science. 2010;330:1685–1689. doi: 10.1126/science.1193697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring NW, Hoege C, Grill SW, Hyman AA. PAR proteins diffuse freely across the anterior-posterior boundary in polarized C. elegans embryos. The Journal of Cell Biology. 2011a;193:583–594. doi: 10.1083/jcb.201011094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring NW, Trong PK, Bois JS, Chowdhury D, Nicola EM, Hyman AA, Grill SW. Polarization of PAR Proteins by Advective Triggering of a Pattern-Forming System. Science. 2011b;334:1137–1141. doi: 10.1126/science.1208619. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Hird SN. Specification of the anteroposterior axis in Caenorhabditis elegans. Development. 1996;122:1467–1474. doi: 10.1242/dev.122.5.1467. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Developmental Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Reyes A, Elliott H, St Johnston D. Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature. 1995;375:654–8. doi: 10.1038/375654a0. [DOI] [PubMed] [Google Scholar]
- Goodwin JM, Svensson RU, Lou HJ, Winslow MM, Turk BE, Shaw RJ. An AMPK-independent signaling pathway downstream of the LKB1 tumor suppressor controls Snail1 and metastatic potential. Molecular Cell. 2014;55:436–50. doi: 10.1016/j.molcel.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goransson O, Deak M, Wullschleger S, Morrice NA, Prescott AR, Alessi DR. Regulation of the polarity kinases PAR-1/MARK by 14-3-3 interaction and phosphorylation. Journal of Cell Science. 2006;119:4059–70. doi: 10.1242/jcs.03097. [DOI] [PubMed] [Google Scholar]
- Gotta M, Abraham MC, Ahringer J. CDC-42 controls early cell polarity and spindle orientation in C. elegans. Current Biology. 2001;11:482–488. doi: 10.1016/s0960-9822(01)00142-7. [DOI] [PubMed] [Google Scholar]
- Griffin EE. Cytoplasmic localization and asymmetric division in the early embryo of Caenorhabditis elegans. Wiley Interdisciplinary Reviews. Developmental Biology. 2015;4:267–82. doi: 10.1002/wdev.177. [DOI] [PubMed] [Google Scholar]
- Griffin EE, Odde DJ, Seydoux G. Regulation of the MEX-5 Gradient by a Spatially Segregated Kinase/Phosphatase Cycle. Cell. 2011;146:955–968. doi: 10.1016/j.cell.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Kemphues KJ. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell. 1995;81:611–620. doi: 10.1016/0092-8674(95)90082-9. [DOI] [PubMed] [Google Scholar]
- Halaoui R, McCaffrey L. Rewiring cell polarity signaling in cancer. Oncogene. 2015;34:939–50. doi: 10.1038/onc.2014.59. [DOI] [PubMed] [Google Scholar]
- Hao Y, Boyd L, Seydoux G. Stabilization of cell polarity by the C. elegans RING protein PAR-2. Developmental Cell. 2006;10:199–208. doi: 10.1016/j.devcel.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Suzuki A, Hirai S, Kurihara Y, Hoogenraad CC, Ohno S. Maintenance of dendritic spine morphology by partitioning-defective 1b through regulation of microtubule growth. The Journal of Neuroscience. 2011;31:12094–12103. doi: 10.1523/JNEUROSCI.0751-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoege C, Hyman AA. Principles of PAR polarity in Caenorhabditis elegans embryos. Nature Reviews Molecular Cell Biology. 2013;14:315–322. doi: 10.1038/nrm3558. [DOI] [PubMed] [Google Scholar]
- Hubaux R, Thu KL, Vucic EA, Pikor LA, Kung SH, Martinez VD, Mosslemi M, Becker-Santos DD, Gazdar AF, Lam S, Lam WL. Microtubule affinity-regulating kinase 2 is associated with DNA damage response and cisplatin resistance in non-small cell lung cancer. International Journal of Cancer. 2015;137:2072–82. doi: 10.1002/ijc.29577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurov JB, Stappenbeck TS, Zmasek CM, White LS, Ranganath SH, Russell JH, Chan AC, Murphy KM, Piwnica-Worms H. Immune system dysfunction and autoimmune disease in mice lacking Emk (Par-1) protein kinase. Molecular Biology of the Cell. 2001;21:3206–3219. doi: 10.1128/MCB.21.9.3206-3219.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurov JB, Watkins JL, Piwnica-Worms H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Current Biology. 2004;14:736–741. doi: 10.1016/j.cub.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Huynh JR, Petronczki M, Knoblich JA, St Johnston D. Bazooka and PAR-6 are required with PAR-1 for the maintenance of oocyte fate in Drosophila. Current Biology. 2001a;11:901–6. doi: 10.1016/s0960-9822(01)00244-5. [DOI] [PubMed] [Google Scholar]
- Huynh JR, Shulman JM, Benton R, St Johnston D. PAR-1 is required for the maintenance of oocyte fate in Drosophila. Development. 2001b;128:1201–9. doi: 10.1242/dev.128.7.1201. [DOI] [PubMed] [Google Scholar]
- Huynh JR, St Johnston D. The origin of asymmetry: early polarisation of the Drosophila germline cyst and oocyte. Current Biology. 2004;14:R438–49. doi: 10.1016/j.cub.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Inaba M, Venkei ZG, Yamashita YM. The polarity protein Baz forms a platform for the centrosome orientation during asymmetric stem cell division in the Drosophila male germline. Elife. 2015;4:e04960. doi: 10.7554/eLife.04960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insolera R, Chen S, Shi S-H. Par proteins and neuronal polarity. Developmental Neurobiology. 2011;71:483–494. doi: 10.1002/dneu.20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX. Tau and neurodegenerative disease: the story so far. Nature Reviews Neurology. 2016;12:15–27. doi: 10.1038/nrneurol.2015.225. [DOI] [PubMed] [Google Scholar]
- Jaleel M, Villa F, Deak M, Toth R, Prescott AR, Van Aalten DM, Alessi DR. The ubiquitin-associated domain of AMPK-related kinases regulates conformation and LKB1-mediated phosphorylation and activation. The Biochemical Journal. 2006;394:545–55. doi: 10.1042/BJ20051844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januschke J, Gervais L, Dass S, Kaltschmidt JA, Lopez-Schier H, St Johnston D, Brand AH, Roth S, Guichet A. Polar transport in the Drosophila oocyte requires Dynein and Kinesin I cooperation. Current Biology. 2002;12:1971–81. doi: 10.1016/s0960-9822(02)01302-7. [DOI] [PubMed] [Google Scholar]
- Kato T, Satoh S, Okabe H, Kitahara O, Ono K, Kihara C, Tanaka T, Tsunoda T, Yamaoka Y, Nakamura Y, Furukawa Y. Isolation of a novel human gene, MARKL1, homologous to MARK3 and its involvement in hepatocellular carcinogenesis. Neoplasia. 2001;3:4–9. doi: 10.1038/sj.neo.7900132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AJ, Hunter CP. CDC-42 regulates PAR protein localization and function to control cellular and embryonic polarity in C. elegans. Current Biology. 2001;11:474–481. doi: 10.1016/s0960-9822(01)00141-5. [DOI] [PubMed] [Google Scholar]
- Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52:311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Khuc Trong P, Doerflinger H, Dunkel J, St Johnston D, Goldstein RE. Cortical microtubule nucleation can organise the cytoskeleton of Drosophila oocytes to define the anteroposterior axis. Elife. 2015;4:e06088. doi: 10.7554/eLife.06088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307:929–32. doi: 10.1126/science.1107403. [DOI] [PubMed] [Google Scholar]
- Knoblich JA. Asymmetric cell division: recent developments and their implications for tumour biology. Nature Reviews Molecular Cell Biology. 2010;11:849–860. doi: 10.1038/nrm3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak S, Gonczy P. Mechanisms of spindle positioning: cortical force generators in the limelight. Current Opinion in Cell Biology. 2013;25:741–8. doi: 10.1016/j.ceb.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Kumfer KT, Cook SJ, Squirrell JM, Eliceiri KW, Peel N, O'Connell KF, White JG. CGEF-1 and CHIN-1 regulate CDC-42 activity during asymmetric division in the Caenorhabditis elegans embryo. Molecular Biology of the Cell. 2010;21:266–277. doi: 10.1091/mbc.E09-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé J-C, Pacquelet A, Marty T, Gotta M. A genomewide screen for suppressors of par-2 uncovers potential regulators of PAR protein-dependent cell polarity in Caenorhabditis elegans. Genetics. 2006;174:285–295. doi: 10.1534/genetics.106.060517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann R. Germ Plasm Biogenesis--An Oskar-Centric Perspective. Current Topics in Developmental Biology. 2016;116:679–707. doi: 10.1016/bs.ctdb.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennerz JK, Hurov JB, White LS, Lewandowski KT, Prior JL, Planer GJ, Gereau RW, Piwnica-Worms D, Schmidt RE, Piwnica-Worms H. Loss of Par-1a/MARK3/C-TAK1 Kinase Leads to Reduced Adiposity, Resistance to Hepatic Steatosis, and Defective Gluconeogenesis. Molecular Biology of the Cell. 2010;30:5043–5056. doi: 10.1128/MCB.01472-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annual Reviews of Biophysics. 2010;39:407–27. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- Li X, Kumar Y, Zempel H, Mandelkow EM, Biernat J, Mandelkow E. Novel diffusion barrier for axonal retention of Tau in neurons and its failure in neurodegeneration. The EMBO Journal. 2011;30:4825–37. doi: 10.1038/emboj.2011.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkow K, Odde DJ. Model for Protein Concentration Gradients in the Cytoplasm. Cellular and Molecular Bioengineering. 2008;1:84–92. doi: 10.1007/s12195-008-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Gan L, Chen Y, Luo D, Zhang Z, Cao W, Zhou Z, Lin X, Sun C. Mark4 promotes oxidative stress and inflammation via binding to PPARgamma and activating NF-kappaB pathway in mice adipocytes. Science Reports. 2016;6:21382. doi: 10.1038/srep21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Mäkelä TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. The EMBO Journal. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MS, Johnston CA. Molecular pathways regulating mitotic spindle orientation in animal cells. Development. 2013;140:1843–56. doi: 10.1242/dev.087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Marx A, Nugoor C, Müller J, Panneerselvam S, Timm T, Bilang M, Mylonas E, Svergun DI, Mandelkow E-M, Mandelkow E. Structural variations in the catalytic and ubiquitin-associated domains of microtubule-associated protein/microtubule affinity regulating kinase (MARK) 1 and MARK2. The Journal of Biological Chemistry. 2006;281:27586–27599. doi: 10.1074/jbc.M604865200. [DOI] [PubMed] [Google Scholar]
- Marx A, Nugoor C, Panneerselvam S, Mandelkow E. Structure and function of polarity-inducing kinase family MARK/Par-1 within the branch of AMPK/Snf1-related kinases. The FASEB Journal. 2010;24:1637–1648. doi: 10.1096/fj.09-148064. [DOI] [PubMed] [Google Scholar]
- Matenia D, Griesshaber B, Li XY, Thiessen A, Johne C, Jiao J, Mandelkow E, Mandelkow EM. PAK5 kinase is an inhibitor of MARK/Par-1, which leads to stable microtubules and dynamic actin. Molecular Biology of the Cell. 2005;16:4410–22. doi: 10.1091/mbc.E05-01-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matenia D, Mandelkow E-M. The tau of MARK: a polarized view of the cytoskeleton. Trends in Biochemical Sciences. 2009;34:332–342. doi: 10.1016/j.tibs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Khodyakova A, Aranjuez G, Dudley C, Montell D. PAR-1 Kinase Regulates Epithelial Detachment and Directional Protrusion of Migrating Border Cells. Current Biology. 2008;18:1659–1667. doi: 10.1016/j.cub.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JA. Canonical and noncanonical roles of Par-1/MARK kinases in cell migration. International Review of Cell and Molecular Biology. 2014;312:169–99. doi: 10.1016/B978-0-12-800178-3.00006-3. [DOI] [PubMed] [Google Scholar]
- Mello CC, Schubert C, Draper B, Zhang W, Lobel R, Priess JR. The PIE-1 protein and germline specification in C. elegans embryos. Nature. 1996;382:710–712. doi: 10.1038/382710a0. [DOI] [PubMed] [Google Scholar]
- Morais-de-Sa E, Mukherjee A, Lowe N, St Johnston D. Slmb antagonises the aPKC/Par-6 complex to control oocyte and epithelial polarity. Development. 2014;141:2984–92. doi: 10.1242/dev.109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-De-Sa E, Vega-Rioja A, Trovisco V, St Johnston D. Oskar Is Targeted for Degradation by the Sequential Action of Par-1, GSK-3, and the SCF-Slimb Ubiquitin Ligase. Developmental Cell. 2013;26:303–314. doi: 10.1016/j.devcel.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moravcevic K, Mendrola JM, Schmitz KR, Wang Y-H, Slochower D, Janmey PA, Lemmon MA. Kinase associated-1 domains drive MARK/PAR1 kinases to membrane targets by binding acidic phospholipids. Cell. 2010;143:966–977. doi: 10.1016/j.cell.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- Morton DG, Shakes DC, Nugent S, Dichoso D, Wang W, Golden A, Kemphues KJ. The Caenorhabditis elegans par-5 gene encodes a 14-3-3 protein required for cellular asymmetry in the early embryo. Developmental Biology. 2002;241:47–58. doi: 10.1006/dbio.2001.0489. [DOI] [PubMed] [Google Scholar]
- Motegi F, Seydoux G. The PAR network: redundancy and robustness in a symmetry-breaking system. Philosophical Transactions of the Royal Society of London. Series B. Biological Sciences. 2013;368:20130010. doi: 10.1098/rstb.2013.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motegi F, Sugimoto A. Sequential functioning of the ECT-2 RhoGEF, RHO-1 and CDC-42 establishes cell polarity in Caenorhabditis elegans embryos. Nature Cell Biology. 2006:978–985. doi: 10.1038/ncb1459. [DOI] [PubMed] [Google Scholar]
- Motegi F, Zonies S, Hao Y, Cuenca AA, Griffin E, Seydoux G. Microtubules induce self-organization of polarized PAR domains in Caenorhabditis elegans zygotes. Nature Cell Biology. 2011;13:1361–7. doi: 10.1038/ncb2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Developmental Cell. 2004;7:413–424. doi: 10.1016/j.devcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Korzhnev DM, Ceccarelli DF, Briant DJ, Zarrine-Afsar A, Sicheri F, Kay LE, Pawson T. Conformational instability of the MARK3 UBA domain compromises ubiquitin recognition and promotes interaction with the adjacent kinase domain. Proceedings of the Natational Academy of Sciences of the United States of America. 2007;104:14336–14341. doi: 10.1073/pnas.0703012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy SK, Xue B. Cell polarity as a regulator of cancer cell behavior plasticity. Annual Review of Cell and Developmental Biology. 2012;28:599–625. doi: 10.1146/annurev-cellbio-092910-154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Y, Shivas JM, Poole DS, Squirrell JM, Kulkoski JM, Schleede JB, Skop AR. Dynamin participates in the maintenance of anterior polarity in the Caenorhabditis elegans embryo. Developmental Cell. 2009;16:889–900. doi: 10.1016/j.devcel.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J, Zallen JA. Elaborating polarity: PAR proteins and the cytoskeleton. Development. 2011;138:799–809. doi: 10.1242/dev.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashchekin D, Fernandes AR, St Johnston D. Patronin/Shot Cortical Foci Assemble the Noncentrosomal Microtubule Array that Specifies the Drosophila Anterior-Posterior Axis. Developmental Cell. 2016;38:61–72. doi: 10.1016/j.devcel.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesić D, Miller MC, Quinkert ZT, Stein M, Chait BT, Stebbins CE. Helicobacter pylori CagA inhibits PAR1-MARK family kinases by mimicking host substrates. Nature Structural and Molecular Biology. 2010;17:130–132. doi: 10.1038/nsmb.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa H, Hayashi T, Arisaka F, Senda T, Hatakeyama M. Impact of structural polymorphism for the Helicobacter pylori CagA oncoprotein on binding to polarity-regulating kinase PAR1b. Science Reports. 2016;6:30031. doi: 10.1038/srep30031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura I, Yang Y, Lu B. PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell. 2004;116:671–82. doi: 10.1016/s0092-8674(04)00170-9. [DOI] [PubMed] [Google Scholar]
- Panneerselvam S, Marx A, Mandelkow EM, Mandelkow E. Structure of the catalytic and ubiquitin-associated domains of the protein kinase MARK/Par-1. Structure. 2006;14:173–83. doi: 10.1016/j.str.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Pardo OE, Castellano L, Munro CE, Hu Y, Mauri F, Krell J, Lara R, Pinho FG, Choudhury T, Frampton AE, Pellegrino L, Pshezhetskiy D, Wang Y, Waxman J, Seckl MJ, Stebbing J. miR-515-5p controls cancer cell migration through MARK4 regulation. EMBO Reports. 2016;17:570–84. doi: 10.15252/embr.201540970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RM, Hamilton RS, Ball G, Yang L, Cullen CF, Lu W, Ohkura H, Davis I. A PAR-1-dependent orientation gradient of dynamic microtubules directs posterior cargo transport in the Drosophila oocyte. The Journal of Cell Biology. 2011a;194:121–35. doi: 10.1083/jcb.201103160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RM, Hamilton RS, Ball G, Yang L, Cullen CF, Lu W, Ohkura H, Davis I. A PAR-1-dependent orientation gradient of dynamic microtubules directs posterior cargo transport in the Drosophila oocyte. The Journal of Cell Biology. 2011b;194:121–135. doi: 10.1083/jcb.201103160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penazzi L, Bakota L, Brandt R. Microtubule Dynamics in Neuronal Development, Plasticity, and Neurodegeneration. International Review of Cell and Molecular Biology. 2016;321:89–169. doi: 10.1016/bs.ircmb.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Peri F, Bokel C, Roth S. Local Gurken signaling and dynamic MAPK activation during Drosophila oogenesis. Mechanisms of Development. 1999;81:75–88. doi: 10.1016/s0925-4773(98)00228-7. [DOI] [PubMed] [Google Scholar]
- Reiner O, Sapir T. Mark/Par-1 Marking the Polarity of Migrating Neurons. Advances in Experimental Medicine and Biology. 2013;800:97–111. doi: 10.1007/978-94-007-7687-6_6. [DOI] [PubMed] [Google Scholar]
- Riechmann V, Ephrussi A. Par-1 regulates bicoid mRNA localisation by phosphorylating Exuperantia. Development. 2004;131:5897–907. doi: 10.1242/dev.01515. [DOI] [PubMed] [Google Scholar]
- Riechmann V, Gutierrez GJ, Filardo P, Nebreda AR, Ephrussi A. Par-1 regulates stability of the posterior determinant Oskar by phosphorylation. Nature Cell Biology. 2002;4:337–342. doi: 10.1038/ncb782. [DOI] [PubMed] [Google Scholar]
- Robin FB, McFadden WM, Yao B, Munro EM. Single-molecule analysis of cell surface dynamics in Caenorhabditis elegans embryos. Nature Methods. 2014;11:677–82. doi: 10.1038/nmeth.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose LS, Kemphues KJ. Early patterning of the C. elegans embryo. Annual Review of Genetics. 1998;32:521–545. doi: 10.1146/annurev.genet.32.1.521. [DOI] [PubMed] [Google Scholar]
- Roth S, Lynch JA. Symmetry breaking during Drosophila oogenesis. Cold Spring Harbor Perspectives in Biology. 2009;1:a001891. doi: 10.1101/cshperspect.a001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat I, Higashi H, Obuse C, Umeda M, Murata-Kamiya N, Saito Y, Lu H, Ohnishi N, Azuma T, Suzuki A, Ohno S, Hatakeyama M. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330–333. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- Sack JS, Gao M, Kiefer SE, Myers JE, Jr, Newitt JA, Wu S, Yan C. Crystal structure of microtubule affinity-regulating kinase 4 catalytic domain in complex with a pyrazolopyrimidine inhibitor. Acta Crystallographica. Section F. Structural Biology Communications. 2016;72:129–34. doi: 10.1107/S2053230X15024747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Weber CA, Nousch M, Adame-Arana O, Hoege C, Hein MY, Osborne-Nishimura E, Mahamid J, Jahnel M, Jawerth L, Pozniakovski A, Eckmann CR, Julicher F, Hyman AA. Polar Positioning of Phase-Separated Liquid Compartments in Cells Regulated by an mRNA Competition Mechanism. Cell. 2016;166:1572–1584. doi: 10.1016/j.cell.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A, Anneken A, Li Y, Lee S, Munro E. Dynamic Opposition of Clustered Proteins Stabilizes Cortical Polarity in the C. elegans Zygote. Developmental Cell. 2015;35:131–42. doi: 10.1016/j.devcel.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene. 2007;26:7825–32. doi: 10.1038/sj.onc.1210594. [DOI] [PubMed] [Google Scholar]
- Schlaitz A-L, Srayko M, Dammermann A, Quintin S, Wielsch N, MacLeod I, de Robillard Q, Zinke A, Yates JR, Müller-Reichert T, Shevchenko A, Oegema K, Hyman AA. The C. elegans RSA complex localizes protein phosphatase 2A to centrosomes and regulates mitotic spindle assembly. Cell. 2007;128:115–127. doi: 10.1016/j.cell.2006.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CM, Lin R, de Vries CJ, Plasterk RH, Priess JR. MEX-5 and MEX-6 function to establish soma/germline asymmetry in early C. elegans embryos. Molecular Cell. 2000;5:671–682. doi: 10.1016/s1097-2765(00)80246-4. [DOI] [PubMed] [Google Scholar]
- Schwalbe M, Biernat J, Bibow S, Ozenne V, Jensen MR, Kadavath H, Blackledge M, Mandelkow E, Zweckstetter M. Phosphorylation of human Tau protein by microtubule affinity-regulating kinase 2. Biochemistry. 2013;52:9068–79. doi: 10.1021/bi401266n. [DOI] [PubMed] [Google Scholar]
- Segu L, Pascaud A, Costet P, Darmon M, Buhot MC. Impairment of spatial learning and memory in ELKL Motif Kinase1 (EMK1/MARK2) knockout mice. Neurobiology of Aging. 2008;29:231–40. doi: 10.1016/j.neurobiolaging.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Shelly M, Poo M-M. Role of LKB1-SAD/MARK pathway in neuronal polarization. Developmental Neurobiology. 2011;71:508–527. doi: 10.1002/dneu.20884. [DOI] [PubMed] [Google Scholar]
- Shorning BY, Clarke AR. Energy sensing and cancer: LKB1 function and lessons learnt from Peutz-Jeghers syndrome. Seminars in Cell and Developmental Biology. 2016;52:21–9. doi: 10.1016/j.semcdb.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Shulman JM, Benton R, St Johnston D. The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localization to the posterior pole. Cell. 2000;101:377–388. doi: 10.1016/s0092-8674(00)80848-x. [DOI] [PubMed] [Google Scholar]
- St Johnston D. Moving messages: the intracellular localization of mRNAs. Nature Reviews Molecular Cell Biology. 2005;6:363–75. doi: 10.1038/nrm1643. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141:757–774. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Stein M, Ruggiero P, Rappuoli R, Bagnoli F. Helicobacter pylori CagA: From Pathogenic Mechanisms to Its Use as an Anti-Cancer Vaccine. Frontiers in Immunology. 2013;4:328. doi: 10.3389/fimmu.2013.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome S, Wood WB. Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos. Cell. 1983;35:15–25. doi: 10.1016/0092-8674(83)90203-9. [DOI] [PubMed] [Google Scholar]
- Sun C, Tian L, Nie J, Zhang H, Han X, Shi Y. Inactivation of MARK4, an AMP-activated protein kinase (AMPK)-related kinase, leads to insulin hypersensitivity and resistance to diet-induced obesity. The Journal of Biological Chemistry. 2012;287:38305–15. doi: 10.1074/jbc.M112.388934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Kishikawa M, Hirose H, Amano Y, Izumi N. aPKC Acts Upstream of PAR-1b in Both the Establishment and Maintenance of Mammalian Epithelial Polarity. Current Biology. 2004;14:1425–1435. doi: 10.1016/j.cub.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Tabuse Y, Izumi Y, Piano F, Kemphues KJ, Miwa J, Ohno S. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development. 1998;125:3607–3614. doi: 10.1242/dev.125.18.3607. [DOI] [PubMed] [Google Scholar]
- Tenenhaus C, Schubert C, Seydoux G. Genetic requirements for PIE-1 localization and inhibition of gene expression in the embryonic germ lineage of Caenorhabditis elegans. Developmental Biology. 1998;200:212–224. doi: 10.1006/dbio.1998.8940. [DOI] [PubMed] [Google Scholar]
- Tenlen JR, Molk JN, London N, Page BD, Priess JR. MEX-5 asymmetry in one-cell C. elegans embryos requires PAR-4- and PAR-1-dependent phosphorylation. Development. 2008;135:3665–3675. doi: 10.1242/dev.027060. [DOI] [PubMed] [Google Scholar]
- Timm T, Balusamy K, Li X, Biernat J, Mandelkow E, Mandelkow EM. Glycogen synthase kinase (GSK) 3beta directly phosphorylates Serine 212 in the regulatory loop and inhibits microtubule affinity-regulating kinase (MARK) 2. The Journal of Biological Chemistry. 2008a;283:18873–82. doi: 10.1074/jbc.M706596200. [DOI] [PubMed] [Google Scholar]
- Timm T, Li XY, Biernat J, Jiao J, Mandelkow E, Vandekerckhove J, Mandelkow EM. MARKK, a Ste20-like kinase, activates the polarity-inducing kinase MARK/PAR-1. The EMBO Journal. 2003;22:5090–101. doi: 10.1093/emboj/cdg447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm T, Marx A, Panneerselvam S, Mandelkow E, Mandelkow E-M. Structure and regulation of MARK, a kinase involved in abnormal phosphorylation of Tau protein. BMC Neuroscience. 2008b;9:S9. doi: 10.1186/1471-2202-9-S2-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomancak P, Piano F, Riechmann V, Gunsalus KC, Kemphues KJ, Ephrussi A. A Drosophila melanogaster homologue of Caenorhabditis elegans par-1 acts at an early step in embryonic-axis formation. Nature Cell Biology. 2000;2:458–460. doi: 10.1038/35017101. [DOI] [PubMed] [Google Scholar]
- Updike D, Strome S. P granule assembly and function in Caenorhabditis elegans germ cells. Journal of Andrology. 2010;31:53–60. doi: 10.2164/jandrol.109.008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T, Ephrussi A. The fusome and microtubules enrich Par-1 in the oocyte, where it effects polarization in conjunction with Par-3, BicD, Egl, and dynein. Current Biology. 2002;12:1524–8. doi: 10.1016/s0960-9822(02)01079-5. [DOI] [PubMed] [Google Scholar]
- Wang JT, Seydoux G. Germ cell specification. Advances in Experimental Medicine and Biology. 2013;757:17–39. doi: 10.1007/978-1-4614-4015-4_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Smith J, Chen B-C, Schmidt H, Rasoloson D, AAlexandre P, Lambrus BG, Calidas D, Betzig E, Seydoux G. Regulation of RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in C. elegans. eLife. 2014;3:e04591. doi: 10.7554/eLife.04591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JL, Etemad-Moghadam B, Guo S, Boyd L, Draper BW, Mello CC, Priess JR, Kemphues KJ. par-6, a gene involved in the establishment of asymmetry in early C. elegans embryos, mediates the asymmetric localization of PAR-3. Development. 1996;122:3133–3140. doi: 10.1242/dev.122.10.3133. [DOI] [PubMed] [Google Scholar]
- Watts JL, Morton DG, Bestman J, Kemphues KJ. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development. 2000;127:1467–1475. doi: 10.1242/dev.127.7.1467. [DOI] [PubMed] [Google Scholar]
- Weber SC, Brangwynne CP. Getting RNA and Protein in Phase. Cell. 2012;149:1188–1191. doi: 10.1016/j.cell.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Wu JX, Cheng YS, Wang J, Chen L, Ding M, Wu JW. Structural insight into the mechanism of synergistic autoinhibition of SAD kinases. Nature Communications. 2015;6:8953. doi: 10.1038/ncomms9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P-R, Tsai P-I, Chen G-C, Chou H-J, Huang Y-P, Chen Y-H, Lin M-Y, Kimchi A, Chien C-T, Chen R-H. DAPK activates MARK1/2 to regulate microtubule assembly, neuronal differentiation, and tau toxicity. Cell Death and Differentiation. 2011;18:1507–1520. doi: 10.1038/cdd.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, DiBona VL, Bernard LP, Zhang H. The Polarity Protein Partitioning-defective 1 (PAR-1) Regulates Dendritic Spine Morphogenesis through Phosphorylating Postsynaptic Density Protein 95 (PSD-95) Journal of Biological Chemistry. 2012;287:30781–30788. doi: 10.1074/jbc.M112.351452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhang H, Griffin EE. Coupling between cytoplasmic concentration gradients through local control of protein mobility in the Caenorhabditis elegans zygote. Molecular Biology of the Cell. 2015;26:2963–70. doi: 10.1091/mbc.E15-05-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Xue B, Umitsu M, Ikura M, Muthuswamy SK, Neel BG. The signaling adaptor GAB1 regulates cell polarity by acting as a PAR protein scaffold. Molecular Cell. 2012;47:469–83. doi: 10.1016/j.molcel.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Chiang CYA, Cheng J, Salzmann V, Yamashita YM. Regulation of cyclin A localization downstream of Par-1 function is critical for the centrosome orientation checkpoint in Drosophila male germline stem cells. Developmental Biology. 2012;361:57–67. doi: 10.1016/j.ydbio.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeaiter Z, Cohen D, Musch A, Bagnoli F, Covacci A, Stein M. Analysis of detergent-resistant membranes of Helicobacter pylori infected gastric adenocarcinoma cells reveals a role for MARK2/Par1b in CagA-mediated disruption of cellular polarity. Cellular Microbiology. 2008;10:781–94. doi: 10.1111/j.1462-5822.2007.01084.x. [DOI] [PubMed] [Google Scholar]
- Zhang H. The PAR-6 Polarity Protein Regulates Dendritic Spine Morphogenesis through p190 RhoGAP and the Rho GTPase. Developmental Cell. 2008;14:216–226. doi: 10.1016/j.devcel.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Macara IG. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nature Cell Biology. 2006;8:227–37. doi: 10.1038/ncb1368. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guo H, Kwan H, Wang J, Kosek J, Lu B. PAR-1 Kinase Phosphorylates Dlg and Regulates Its Postsynaptic Targeting at the Drosophila Neuromuscular Junction. Neuron. 2007;53:201–215. doi: 10.1016/j.neuron.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimyanin V, Belaya K, Pecreaux J, Gilchrist M, Clark A, Davis I, Stjohnston D. In Vivo Imaging of oskar mRNA Transport Reveals the Mechanism of Posterior Localization. Cell. 2008;134:843–853. doi: 10.1016/j.cell.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimyanin V, Lowe N, St Johnston D. An oskar-dependent positive feedback loop maintains the polarity of the Drosophila oocyte. Current Biology. 2007;17:353–359. doi: 10.1016/j.cub.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]