Abstract
Malignant melanoma (MM) is a highly malignant melanocytic tumor, it occurs mostly in the skin, the mucous membrane close to the skin, but also in the tunicae rhagoides and the pia mater. The Uyghur is the largest ethnic group living in the Xinjiang Uyghur Autonomous Region of China, accounting for 46% of the total population of 20 million. Large-scale studies on MMs in Asian countries are limited. This study aimed to investigate BRAF mRNA expression and mutations in Chinese Uyghur patients with MMs and to identify the clinical features associated with these parameters.
Formalin-fixed, paraffin wax-embedded tumor sections from 60 MMs were analyzed for BRAF expression using reverse transcription polymerase chain reaction (RT-PCR). Exons 11 and 15 of BRAF were analyzed for the presence of mutations using PCR and DNA sequencing. Sixty MMs were followed by mobile phone for survival analysis.
BRAF mRNA expression was higher in MMs than in pigmented moles and normal skin tissues. Fourteen of 60 MMs had BRAF mutations. The frequency of BRAF mutations was significantly higher in patients younger than 60 years (10/28, 4/32, P = .02). A significant difference was observed in the frequency of BRAF mutations among specimens of mucosal, acral, chronic sun-induced damage (CSD), and non-CSD MMs (2/10, 3/19, 8/25, 1/6, P = .002). No significant association was found among BRAF mutations, sex, ulceration, or lymph node metastasis. MMs lymph node metastasis (hazard ratio 2.54 [95% confidence interval 1.062 – 6.066], P = .01) affected survival.
This study indicated that BRAF mutations and expression might serve as independent adverse prognostic factors in melanoma.
Keywords: BRAF, Chinese Uyghur, melanoma, mRNA expression, mutation
1. Introduction
Malignant melanoma (MM), a common type of skin cancer, originates in melanocytes. It has the clinical features of high metastatic rate, rapid development, poor prognosis, and high mortality rate. Its incidence is also rising globally. Based on the anatomic location and degree of sun exposure, melanomas can be classified into 4 subtypes: melanomas that occur on skin without chronic sun-induced damage (non-CSD); melanomas on skin with chronic sun-induced damage (CSD); mucosal melanomas; and acral melanomas.[1,2] Clinical and histological subtypes of MM also vary among different ethnicities.[3,4] For instance, Caucasians are often afflicted with superficial spreading MM and nodular MM,[5] but acral lentiginous MMs are often found in Asian patients.[6] Our hospital is one of large general hospitals in Xinjiang, China. In our hospital most of the patients who we receive are Uygur patients. In daily clinical practice, we can receive Uygur melanoma patients. A recent study[7] found that Chinese Han patients were different from Chinese Uyghur patients; CSD MM is the most prevalent MM among Chinese Uyghur patients, whereas acral and mucosal MMs are the most prevalent in Chinese Han patients.
BRAF aberrations in MM were discovered by Davies et al[8] who performed the first kinome mutation screen of melanoma. BRAF mutations are reported in up to 70% of melanoma cell lines. BRAF mutation have been correlated with clinicopathological features and prognosis of melanomas.[9–13] However, some studies revealed a BRAF mutation rate of about 25% in Asian countries, which was significantly lower than that in European and American countries and suggested racial differences.[14–16] Xinjiang, a territory located in the far west of China, is an important pathway connecting East Asia with Central Asia and Europe. About half of the total population in Xinjiang are Uyghurs (>9.4 million). They demonstrate an array of mixed European and Asian anthropological features. So far limited research has been conducted on the ethic population of Chinese Uyghurs in Xinjiang. Because of the differences, it is necessary to explore the involvement of BRAF in the mechanism underlying the development of MM in Xinjiang.
Kinase inhibitors for BRAF, especially the BRAF V600E-specific inhibitors PLX4032 and GSK2118436, which have been demonstrated to be effective in clinical trials on Caucasian populations.[17–19] Identification of mutations in BRAF may be a great translational relevance for future clinical practice. Therefore, all ongoing trials require documenting any BRAF mutation in the tumor tissue before a patient is treated. However, these correlations and translations of melanomas are currently conducted in Caucasian populations. Thus, it is clinically significant to detect whether same aberrations of BRAF and clinical features might be present in Chinese populations, particularly the unique ethic group of Uyghur.
The aim of the present study was to investigate the clinicopathology of melanomas in Chinese Uyghur patients in Xinjiang, and to analyze BRAF mutations and mRNA expression in these patients to determine whether either of them correlated with the clinical features of melanoma, to discuss whether the BRAF mutation is associated with the survival time of melanoma patients.
2. Methods
The present study was approved by the ethics committee of the People's Hospital of the Xinjiang Uyghur Autonomous Region (PHXUAR) and conducted according to the principles of the Declaration of Helsinki. All patients provided written informed consent.
2.1. Patient selection
In total, 60 patients (31 men, 29 women) with histologically confirmed diagnosis of MM, 20 patients (7 face, 7 acrals, 6 trunks, 10 men, 10 women) with histologically confirmed diagnosis of pigmented nevi, the median age of the patients was (60 ± 10.2) years, and 10 patients (5 prepuces, 5 traumas, 5 men, 5 women) with normal skin, the median age of the patients was (61 ± 10.7) years at PHXUAR between January 2011 and December 2015 were enrolled. All patients were Chinese Uyghur. The demographic and clinicopathological characteristics included age, sex, MM subtype, ulceration, and regional lymph node metastasis.
2.2. BRAF mRNA expression
Stored samples of formalin-fixed, paraffin wax-embedded tumors were obtained from the Departments of Dermatology and Pathology, People's Hospital of Xinjiang. The tissue was cut into serial 5-μm-thick sections, and then 10 sections of tumor-rich areas were collected. Total RNA was isolated from a subset of primary melanomas using RNeasy Mini kit and RNA FFPE kit (Qiagen GmbH, Germany) after xylene treatment according to the manufacturer's protocol. To avoid possible contamination of genomic DNA, each RNA sample was treated with DNase I (Qiagen GmbH, Germany) prior to subsequent analysis. RNA concentrations were measured using NanoDrop ND-1000 UV-Vis Spectrophotometer (NanoDrop Technologies, DE). The integrity of the RNA was determined on the Agilent 2100 Bioanalyser using an RNA 6000 Nano Kit (Agilent Technologies, CA). All samples used for gene expression analysis had a 28S/18S ribosomal RNA ratio of >1.5. cDNAs were synthesized from 1 μg of extracted RNA using a reverse transcription polymerase chain reaction (RT-PCR) kit (Takara Bio Inc. Tokyo, Japan) and further purified using QIAquick PCR Purification Kits (Qiagen, The transcripts of BRAF gene were quantified using aCA, USA). The primer sequences: BRAF For 5′-GAACACCACCCAATACCACAG-3′, BRAF Rev 5′-GGATTTTGAAGGAGACGGACT-3′. The Length is 132 bp. β-actin For 5′-CTGCC CTGAG GCACT CTT-3′, β-actin Rev 5′-TGTGT TGGCG TACAG GTCTT T-3′. The length is 124 bp.
Light Cycler 1.5 Real-Time PCR machine (Roche, IN). Each PCR reaction mixture contained 2 μL of purified cDNA, 4 mL of MgCl2, 0.5 μL of each of the primers, and 2 μL of FastStart DNA Master mix (Roche). The results were presented as means from independent experiments using the same cDNA preparation. To compare expression profiles between specimens, normalization based on β-actin gene was used to correct for differences arising from variability in RNA quality and total quantity of RNA in each assay. The relative quantification of each transcript was referred to the Cronin work.
2.3. Mutational analysis
Stored samples of formalin-fixed, paraffin wax-embedded tumors were obtained from the Departments of Dermatology and Pathology, People's Hospital of Xinjiang. The tissue was cut into serial 5-μm-thick sections, and then 10 sections of tumor-rich areas were collected. Genomic DNA was extracted from the samples using a QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. The genomic DNA was used as template for separate PCR amplifications of the DNA sequences, encompassing exons 11 and 15 of BRAF. The primers used for the PCR amplification are shown here (Exon 11 For 5′-AGGTAATGTACTTAGGGTGAA-3′, Exon 11 Rev 5′-TGTTAGAAACTTTTGGAGGAG-3′, The Length is 350 bp. Exon 15 For 5′-TCATAATGCTTGCTCTGATAGGA-3′, Exon 15 Rev 5′-GGCCAAAAATTTAATCAGTGGA-3′. The Length is 224 bp). PCR was performed using 2 μL of genomic DNA, 1 μL of each primer (20 μmol/L), and 25 μL of Master Mix (TaKaRa, Shiga, Japan) in a total volume of 50 μL. Thermal cycling for the amplification of exon 11 was performed at 95 °C for 5 minutes, followed by 40 cycles of 95 °C for 40 seconds, 58 °C for 40 seconds, and 72 °C for 40 seconds, with a final extension at 72 °C for 10 minutes. Conditions for amplification of exon 15 were 95 °C for 5 minutes, followed by 40 cycles of 95 °C for 40 seconds, 57 °C for 40 seconds, and 72 °C for 40 seconds, with a final extension at 72 °C for 10 minutes. The PCR products were stored at 4 °C. The PCR products were separated by electrophoresis in a 1.5% agarose gel (Sangon Biotech, Shanghai, China), and the PCR products were confirmed by DNA sequencing (Sangon).
2.4. Follow-up
The patients were followed by mobile phone from January 2011 to December 2015. No patients were lost at follow-up and all deaths were tumor related in our patients.
2.5. Statistical analysis
Statistical analysis was performed using the SPSS software (v17.0; IBM, NY). The level of BRAF mRNA expression was evaluated by the 2−ΔΔct method. The measurement data were expressed as mean ± standard deviation, and the 2 groups were compared by using the t test. The single factor analysis of variance was used. Associations between the demographic and clinicopathological characteristics and the BRAF mutation status were evaluated using the Chi-squared test or Fisher exact test. The analysis provided descriptive statistics estimated with a 95% confidence interval (CI). Median survival times (MST) was estimated using the Kaplan–Meier product limit estimator. Overall survival times were stratified according to the clinical variables that potentially affect survival. Log-rank tests were used to assess the significance of the differences between the groups. Hazard ratios were estimated using a proportional hazard Cox regression model. A P value <.05 was considered statistically significant.
3. Results
3.1. Demographic and clinicopathological characteristics
The patients’ demographic and clinicopathological characteristics were listed in Table 1. The median age of the Uyghur patients was (62 ± 11.8) years. Ulceration was present in 24 patients, while 22 patients (37.1%) had regional lymph node metastases. As shown in Fig. 1, CSD MMs were the most common tumor subtype (n = 25), followed by acral (n = 19), mucosal (n = 10), and non-CSD (n = 6) MMs.
Table 1.
Clinicopathological features of the patients with MMs having BRAF mutations.
Figure 1.
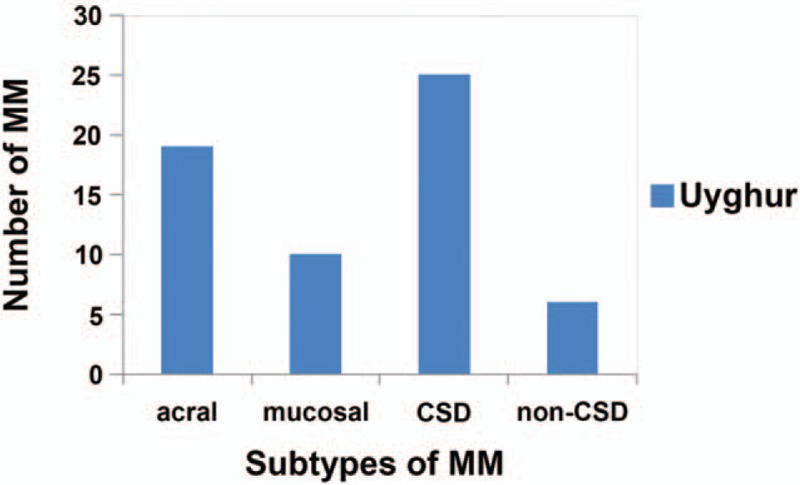
Distribution of malignant melanomas in Uyghur patients. CSD = chronic sun-induced damage.
3.2. BRAF mutation status
Of the 60 patients, 14 with BRAF mutations and 46 with wide-type BRAF, giving an overall mutation rate of 23.2%. Ten of the patients with BRAF mutations were aged ≥60 years at diagnosis, and the other 4 were younger than 60 years. The male:female ratio was 0.75 (6 men, 8 women) (Table 2). Of the 14 patients, 12 had exon 15 mutations (10 with V600E mutations, 1 with an L597Q mutation, and 1 with a G593C mutation), and 2 had exon 11 mutations (1 with an S465F mutation and 1 with a P453H mutation) (Fig. 2). BRAF mutations were more common in CSD (n = 7) and non-CSD MMs (n = 3).
Table 2.
BRAF gene mutations in melanoma with clinicopathological characteristics.
Figure 2.
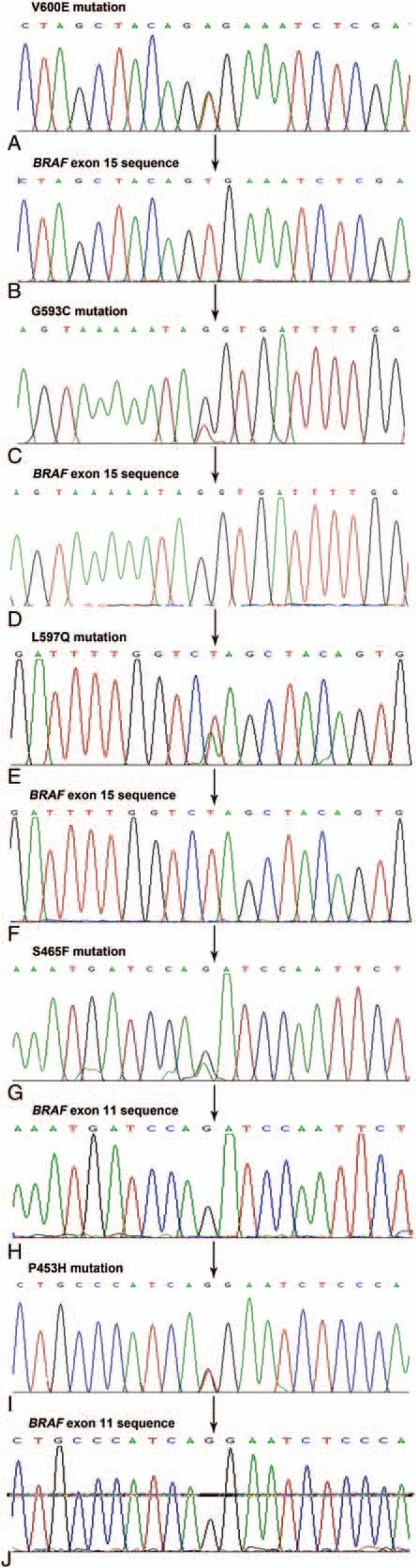
(A) Mutation corresponding to V600E. (B) Wild-type sequence of BRAF exon 15. (C) Mutation corresponding to G593C. (D) Wild-type sequence of BRAF exon 15. (E) Mutation corresponding to L597Q. (F) Wild-type sequence of BRAF exon 15. (G) Mutation corresponding to S465F. (H) Wild-type sequence of BRAF exon 11. (I) Mutation corresponding to P453H. (J) Wild-type sequence of BRAF exon 11.
3.3. Analysis of BRAF mRNA expression
The level of BRAF mRNA expression was measured by quantitative real-time PCR in samples with high-quality RNA. No difference was found in groups with BRAF mutation and with wide-type BRAF in MMs (P = .0903). In pigmented nevi, it had no difference between groups with BRAF mutation and with wide-type BRAF (P = .6275). No difference was found in terms of patient's age or sex for both nevi and MM samples, but BRAF mRNA expression levels were significantly higher in MM (0.377 ± 0.167) than in pigmented nevi (0.159 ± 0.167; P = .0093). In addition, the BRAF mRNA expression levels were also significantly higher in MM tissues than in their corresponding normal skin tissues (0.134 ± 0.050; P = .0087) (Fig. 3).
Figure 3.
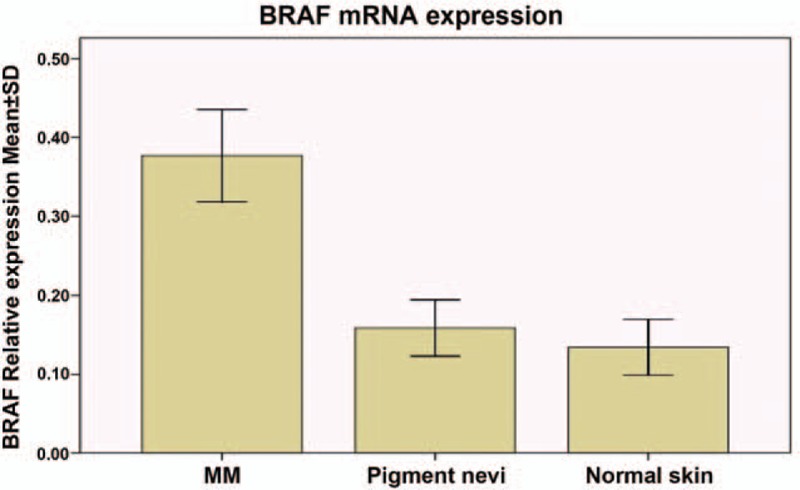
Two groups were compared using the t test, and the single factor analysis of variance was used.
3.4. Follow-up status and survival analysis
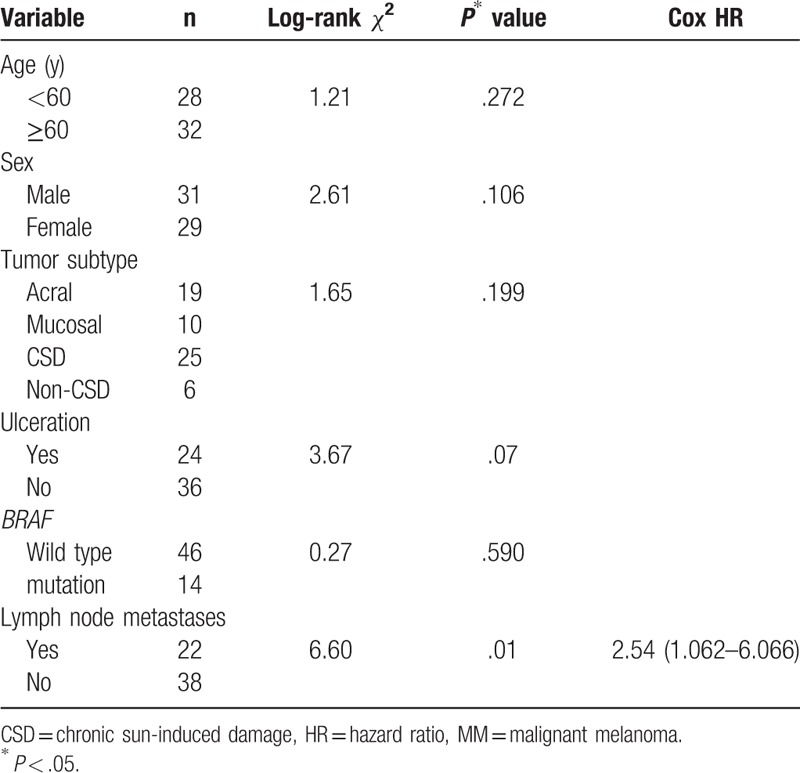
The median follow-up duration was 36 months (range, 10–47 months), 14 of 60 patients died because of melanoma. The median survival time was 32 months (range, 11–38 months). The 1-year, 3-year, and 5-year survival rates were 70.3%, 22.7%, and 9.2%, respectively. MMs regional lymph node metastasis (hazard ratio 2.54 [95% CI 1.062–6.066], P = .01) affected survival, whereas age, sex, MM subtype, ulceration, and BRAF status did not (Table 3).
Table 3.
Survival according to age, sex, MM subtype, ulceration, BRAF status, regional lymph node metastasis.
4. Discussion
CSD has been documented as the major subtype of MM in Caucasian populations.[1,2,20,21] Acral and mucosal types only account for a small proportion of MM, but these 2 are the most common subtypes in Asian populations, especially in Chinese.[3,22] Chinese Han patients are different from Chinese Uyghur patients. CSD MM is the most prevalent MM among Chinese Uyghur patients, whereas acral and mucosal MMs are the most prevalent in Chinese Han patients.[7] CSD (25/60) was found to be the most common type of MM in Uyghur patients in Xinjiang, which was reported by a previous study.[7] The incidence of BRAF mutations was only 32% (8/25). The present study was the first to examine the Uyghur patients with MM. Thus, this study is of significance to understand melanoma tumorigenesis.
Approximately, 90% of BRAF mutations occur at V600E, which is located in the activation domain of BRAF kinase.[22,23] Consistent with previous studies, this study further confirmed that BRAF mutations concentrated in exons 11 and 15. Twelve cases of mutations were found in exon 15 (rate 80%), of which 10 were V600E heterozygous missense mutations and the other 2 were L597Q and G593C mutations; S465F and P453H mutations were found in exon 11. A most recent report, which examined the BRAF mutational status in a Chinese Han population, suggested that 15.0% of MM harbored the BRAF V600E mutation while BRAF mutation might not be related to the melanocyte transformation.[24] The present study found that the frequency of BRAF V600E mutation in Uyghur patients with MM (23.2%) was slightly higher. But the BRAF V600E mutation was lower than that (25.5%) reported by Long et al[14] who studied with Chinese Han patients. Previous studies have found correlations of BRAF mutations with age, sex, tumor, ulceration, and lymph node metastases at diagnosis.[25] These notions were further confirmed in this study, which found that patients with CSD MMs had higher BRAF mutation rates compared with other subtypes. But in Chinese Han patients who with non-CSD MMs had higher BRAF mutation rates. And they found others BRAF mutations which we did not find.[14] The frequency of BRAF mutations was significantly higher in patients younger than 60 years than in those older than 60 years. However, this study did not find any relevance of BRAF mutations to patient's sex and ulceration. But Long et al found it has a relevance of BRAF mutations to patient's ulceration in Chinese Han patients. The tissues from the metastatic sites were excluded in this study, indicating that the present results might be more relevant to the primary melanomas.
BRAF mRNA expression was significantly higher in MM than in pigmented nevi and normal skin tissues (Fig. 2).[26] In the study, using immunohistochemistry, phosphorylated (active) MAPK and BRAF expression was studied in 24 common nevi, and 26 cutaneous melanomas. BRAF mutations at codon 600 were assessed by PCR-RFLP. Active MAPK was detected in 29% of common nevi, and 85% of cutaneous melanomas. In all, 23% of common nevi, and 93% of cutaneous melanomas with BRAF mutation have activated MAPK. BRAF mutation does not seem to be sufficient to produce MAPK activation in melanocytic nevi, and it is suggested that other events are needed to induce MAPK activation, that is, BRAF overexpression, inhibition of MAPK phosphatases, or suppression of RAF kinase inhibitors.[27] In melanoma, the gene amplification and mutation of BRAF can lead to overexpression of BRAF. The overexpression of BRAF can activate the MAPK pathway, then stimulate the growth of melanoma cells. It might be reasonable to speculate that BRAF mRNA expression is correlated with malignancy, regardless of its mutational status. Recent research also shows that BRAF mutations occur in a high proportion of nevi, indicating that measuring the mRNA expression may be more effective to identify MM, than detecting BRAF mutations. Using larger cohorts and collecting mRNA samples by fine-needle aspiration may be necessary in the future to further confirm the connection of BRAF mRNA expression with malignancy.
The 1-year, 3-year, and 5-year survival rates were 70.3%, 22.7%, and 9.2%, respectively. MMs regional lymph node metastasis affected survival, but BRAF mutation did not.[28] The 5-year survival rate is low. Stage III and VI melanoma affected survival. Stage III and VI melanoma always had lymph node metastasis. So in MMs lymph node metastasis may be one of the indicators of poor prognosis.
BRAF may be an important oncogene causing MM, which regulates proliferation, survival, and invasion/metastasis.[29] It has been established that BRAF is a valid and important therapeutic target. BRAF mutations may have a great clinical significance in identifying patients who may benefit from small-molecule inhibitors.
Selective BRAF inhibitors, such as PLX4032 and GSK2118436, have already been proved to be clinically promising, with the overall response rate of about 63% to 80%.[17–19] The prevalence of BRAF V600E mutation in Chinese patients with melanoma may indicate that clinical trials of PLX4032 or GSK2118436 may be reasonable and ideal in Asian patients with MM, particularly the Uyghur patients.
In conclusion, this study confirmed that CSD MM is the most prevalent subtype of melanoma in Uyghur patients. It indicated that BRAF mutations and expression might serve as independent adverse prognostic factors in melanoma. Future studies can improve the diagnosis and prognosis of melanoma, hence benefiting the design of personalized treatment for patients.
Footnotes
Abbreviations: CI = confidence interval, CSD = chronic sun-induced damage, HR = hazard ratio, MM = malignant melanoma, MST = median survival times, RT-PCR =reverse transcription polymerase chain reaction.
This work was funded by the International Science and Technology Cooperation Project of Xinjiang Uyghur Autonomous Region (grant no. 20146022) and the Natural Science Foundation of Xinjiang Uyghur Autonomous Region (grant no. 2016D01C101).
The authors state that they have no conflicts of interest.
References
- [1].Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005;353:2135–47. [DOI] [PubMed] [Google Scholar]
- [2].Curtin JA, Busam K, Pinkel D, et al. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol 2006;24:4340–6. [DOI] [PubMed] [Google Scholar]
- [3].Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol 2007;6:10–6. [PubMed] [Google Scholar]
- [4].Ishihara K, Saida T, Otsuka F, et al. Statistical profiles of malignant melanoma and other skin cancers in Japan: 2007 update. Int J Clin Oncol 2008;13:33–41. [DOI] [PubMed] [Google Scholar]
- [5].Lang J, MacKie RM. Prevalence of exon 15 BRAF mutations in primary melanoma of the superficial spreading, nodular, acral, and lentigo maligna subtypes. J Invest Dermatol 2005;125:575–9. [DOI] [PubMed] [Google Scholar]
- [6].Sasaki Y, Niu C, Makino R, et al. BRAF point mutations in primary melanoma show different prevalences by subtype. J Invest Dermatol 2004;123:177–83. [DOI] [PubMed] [Google Scholar]
- [7].Kang XJ, Shi XH, Chen WJ, et al. Analysis of KIT mutations and c-KIT expression in Chinese Uyghur and Han patients with melanoma. Clin Exp Dermatol 2016;41:81–7. [DOI] [PubMed] [Google Scholar]
- [8].Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949–54. [DOI] [PubMed] [Google Scholar]
- [9].Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res 2002;62:6997–7000. [PubMed] [Google Scholar]
- [10].Omholt K, Karsberg S, Platz A, et al. Screening of N-ras codon 61 mutations in paired primary and metastatic cutaneous melanomas: mutations occur early and persist throughout tumor progression. Clin Cancer Res 2002;8:3468–74. [PubMed] [Google Scholar]
- [11].Kumar R, Angelini S, Czene K, et al. BRAF mutations in metastatic melanoma: a possible association with clinical outcome. Clin Cancer Res 2003;9:3362–8. [PubMed] [Google Scholar]
- [12].Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol 2007;127:900–5. [DOI] [PubMed] [Google Scholar]
- [13].Ellerhorst JA, Greene VR, Ekmekcioglu S, et al. Clinical correlates of NRAS and BRAF mutations in primary human melanoma. Clin Cancer Res 2011;17:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 2011;29:1239–46. [DOI] [PubMed] [Google Scholar]
- [15].Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer 2012;48:94–100. [DOI] [PubMed] [Google Scholar]
- [16].Goel VK, Lazar AJ, Warneke CL, et al. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J Invest Dermatol 2006;126:154–60. [DOI] [PubMed] [Google Scholar]
- [17].Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010;363:809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Falchook GS, Long GV, Kurzrock R, et al. Dose selection, pharmacokinetics, and pharmacodynamics of BRAF inhibitor dabrafenib (GSK2118436). Clin Cancer Res 2014;20:4449–58. [DOI] [PubMed] [Google Scholar]
- [20].Menzies AM, Ashworth MT, Swann S, et al. Characteristics of pyrexia in BRAFV600E/K metastatic melanoma patients treated with combined dabrafenib and trametinib in a phase I/II clinical trial. Ann Oncol 2015;26:415–21. [DOI] [PubMed] [Google Scholar]
- [21].Manola J, Atkins M, Ibrahim J, et al. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol 2000;18:3782–93. [DOI] [PubMed] [Google Scholar]
- [22].Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001;19:3635–48. [DOI] [PubMed] [Google Scholar]
- [23].Fecher LA, Cummings SD, Keefe MJ, et al. Toward a molecular classification of melanoma. J Clin Oncol 2007;25:1606–20. [DOI] [PubMed] [Google Scholar]
- [24].Qi RQ, He L, Zheng S, et al. BRAF exon 15 T1799A mutation is common in melanocytic nevi, but less prevalent in cutaneous malignant melanoma, in Chinese Han. J Invest Dermatol 2011;131:1129–38. [DOI] [PubMed] [Google Scholar]
- [25].Edlundh-Rose E, Egyhazi S, Omholt K, et al. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res 2006;16:471–8. [DOI] [PubMed] [Google Scholar]
- [26].Uribe P, Andrade L, Gonzalez S, et al. Lack of association between BRAF mutation and MAPK ERK activation in melanocytic nevi. J Invest Dermatol 2006;126:161–6. [DOI] [PubMed] [Google Scholar]
- [27].Tanami H, Imoto I, Hirasawa A, et al. Involvement of overexpressed wild-type BRAF in the growth of malignant melanoma cell lines. Oncogene 2004;23:8796–804. [DOI] [PubMed] [Google Scholar]
- [28].Stefano G, Giammaria F, Marco C, et al. MGMT methylation correlates with melphalan pelvic erfusion survival in stage III melanoma patients: a pilot study. Melanoma Research 2017;27:439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70. [DOI] [PubMed] [Google Scholar]


