Abstract
Type 2 diabetes (T2D) imposes a heavy public health burden in both developed and developing countries. It is necessary to understand the effect of T2D in different settings and population groups. This report aimed to present baseline characteristics of study participants in the demonstration area for the “Type 2 Diabetes Prevention in Barranquilla and Juan Mina” (DEMOJUAN) project after randomization and to compare their fasting and 2-hour glucose levels according to lifestyle and T2D risk factor levels.
The DEMOJUAN project is a randomized controlled field trial. Study participants were recruited from study sites using population-wide screening using the Finnish Diabetes Risk Score (FINDRISC) questionnaire. All volunteers with FINDRISC of ≥13 points were invited to undergo an oral glucose tolerance test (OGTT). Participant inclusion criteria for the upcoming field trial were either FINDRISC of ≥13 points and 2-hour post-challenge glucose level of 7.0 to 11.0 mmol/L or FINDRISC of ≥13 points and fasting plasma glucose level of 6.1 to 6.9 mmol/L. Lifestyle habits and risk factors for T2D were assessed by trained interviewers using a validated questionnaire.
Among the 14,193 participants who completed the FINDRISC questionnaire, 35% (n = 4915) had a FINDRISC score of ≥13 points and 47% (n = 2306) agreed to undergo the OGTT. Approximately, 33% (n = 772) of participants underwent the OGTT and met the entry criteria; these participants were randomized into 3 groups. There were no statistically significant differences found in anthropometric or lifestyle risk factors, distribution of the glucose metabolism categories, or other diabetes risk factors between the 3 groups (P > .05). Women with a past history of hyperglycaemia had significantly higher fasting glucose levels than those without previous hyperglycaemia (103 vs 99 mg/dL; P < .05).
Lifestyle habits and risk factors were evenly distributed among the 3 study groups. No differences were found in fasting or 2-hour glucose levels among different lifestyle or risk factor categories with the exception of body mass index, past history of hyperglycaemia, and age of ≥64 years in women.
Trial registration: NCT01296100 (2/12/2011; Clinical trials.gov).
Keywords: Colombia, nutrition, physical activity, primary healthcare
1. Introduction
Type 2 diabetes (T2D) imposes a heavy public health burden in both developed and developing countries.[1] The growing prevalence of T2D with its high morbidity and mortality will impose a heavy burden on healthcare systems.[2] The predicted number of diagnosed T2D patients will increase in Latin America and a large number of asymptomatic cases of T2D will remain undiagnosed. This is problematic, as asymptomatic T2D is associated with 2-fold and impaired glucose tolerance (IGT) with 1.4-fold increased mortality.[3]
People with a positive family history of diabetes mellitus have a higher likelihood of developing T2D once exposed to a lifestyle that enhances obesity (unhealthy diet and physical inactivity).[4–6] The treatment of T2D is difficult and regardless of pharmacological treatment, blood glucose levels have been shown to increase over time.[7] Therefore, primary T2D prevention strategies may be more efficient than secondary prevention approaches. In addition, the most common T2D complications (e.g., cardiovascular diseases) may be postponed by preventing the development of T2D, highlighting the importance of early T2D prevention in the susceptible population.
There is a growing body of evidence suggesting that T2D can be prevented or at least delayed. Early studies in Finland and the USA revealed that nutritional and physical activity interventions can decrease the relative risk of T2D up to 58% in people with IGT.[8,9]
While these findings offer a compelling evidence base, it is necessary to understand how the prevention of T2D works in different settings and population groups. In addition, it is important to determine the extent to which each component (e.g., nutrition, physical activity, nutrition/physical activity) of lifestyle interventions works best in the prevention of T2D in Latin America, and especially in the Caribbean region. For instance, it has been clearly shown that each component (nutrition, physical activity) of lifestyle interventions either separately[10] or combined[8–13] has successfully decreased the risk of T2D in people with IGT. Thus far, such trials have not been conducted in the Caribbean region and it is unclear whether these lifestyle changes are effective in a hot, humid climate. This is particularly true for the physical activity component. The aim of this report is to present baseline characteristics of study participants in the demonstration area for the “Type 2 Diabetes Prevention in Barranquilla and Juan Mina” (DEMOJUAN) project after randomization to groups and to compare their fasting and 2-hour glucose levels according to lifestyle and T2D risk factor levels.
2. Methods
2.1. Design and sample size calculations
The study design of the DEMOJUAN project is a randomized controlled field trial. The main objective of the DEMOJUAN project is to investigate to what extent it is possible to reach normal glucose metabolism and optimal cardiovascular disease risk factor levels with early lifestyle interventions in people at high risk of T2D compared with those who receive standard therapy (usual care) only. Finally, this project will examine the effect of these interventions, for the first time, in people of low socio-economic levels living in a Caribbean environment. This study may provide important information and experiences for policy making and planning of primary prevention activities not only in the local healthcare system but in the entire Caribbean region. The current report presents the results of the baseline assessment and participant recruitment process using a cross-sectional design.
The study was designed to have 90% power to detect a 20% unit difference in recovery from IGT (comparing 70% vs 50% recovery rates) between the treatment groups at a 5% significance level. Assuming a loss to follow-up of 30% at the end of the 24-month intervention, a total of 200 participants were needed in each treatment group (i.e., total sample size of 600 individuals). The drop-out rate of 30% was estimated according to the results and experiences of previous randomized controlled diabetes trials in translational research.[14,15] In addition, this sample size provides >90% power to detect a 6-mmHg difference in change in systolic blood pressure (standard deviation [SD] = 14 mmHg) between the treatment groups with a 5% significance level.
2.2. Screening for study participants
Study participants were recruited from the study sites (Juan Mina and Barranquilla) by population-wide screening using the Finnish Diabetes Risk Score (FINDRISC).[14–16] The study sites have approximately 1,000,000 inhabitants. FINDRISC is based on easily attainable information using 8 parameters with categorized answers. The total risk score ranges from 0 to 26. FINDRISC was shown to predict the 10-year risk of drug-treated T2D with a sensitivity of 78% to 81% and a specificity of 76% to 77%.[14] Furthermore, it also detects reasonably prevalent asymptomatic T2D and other disorders of glucose metabolism.[17] FINDRISC has been validated in many populations with good results[18–23] and has been successfully applied in primary care in Barcelona, Spain.[21] It is recommended as a screening tool for T2D by the International Diabetes Federation and in the guidelines of the European Society for the Study of Diabetes and the European Society of Cardiology.[22,24] In the current study, all volunteers with a FINDRISC score of ≥13 were invited to undergo an oral glucose tolerance test (OGTT). Participant inclusion criteria for the upcoming field trial were either FINDRISC of ≥13 points and 2-hour post-challenge glucose level of 7.0 to 11.0 mmol/L or FINDRISC of ≥13 points and fasting plasma glucose level of 6.1 to 6.9 mmol/L.
If a study participant met the inclusion criteria and agreed to participate, he/she was randomized into 1 of 3 groups (A, B, or C). Sequences for the random allocation groups were generated by IBM SPSS Statistics for Windows (Version 19.0. Armonk, NY: IBM Corp.). This study followed the Good Clinical Practice guidelines and the guidelines of the Helsinki Declaration. All data have been collected using previously tested questionnaires and methods as much as possible. Besides blood samples, no invasive methods were used. The study protocol was approved by the Research Ethics Committee of the University Pontificia Javeriana, Bogota, Colombia. All participants gave their written informed consent prior to participation to the study.
3. Non-invasive measurements
Lifestyle habits and risk factors for T2D were assessed by an interview using a questionnaire consisting of information regarding sociodemographic factors, history of T2D, medical history, tobacco consumption, hypertension, and nutritional and physical activity habits. The instruments applied were designed based on FINDRISC, the STEPwise approach to surveillance and the International Physical Activity Questionnaire (IPAQ),[25–30] all of which have been previously successfully validated in large international studies.[25–31] Physical activity measured with the questionnaire used herein (IPAQ) shows a high correlation with physical fitness, measured by maximal oxygen uptake.[32,33] Dietary habits were assessed via 16 questions (e.g., dietary pattern, quality and quantity of dietary fat, consumption of fruit and vegetables, grain, milk, and meat products, desserts, sweets, and alcoholic beverages). In addition, 6 questions were related to perceived need and intentions to make dietary changes. Scientific validation of the dietary questionnaire is ongoing in the National Institute for Health and Welfare in Helsinki, Finland.
Participant height and weight were measured without shoes and wearing light clothing. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Waist circumference (to the nearest cm) was measured at the approximate midpoint between the lower margin of the last palpable rib and the top of the iliac crest. Blood pressure (precision = 2 mmHg) was recorded twice using a mercury sphygmomanometer while study participants were in a seated position.
4. Biochemical measurements
All participants underwent an OGTT that was conducted according to the World Health Organization recommendations.[34] The test was conducted after fasting for 12 hours, and fasting and 2-hour blood samples were obtained after oral ingestion of a water solution with 75 g of anhydrous glucose. Glucose tolerance status was classified according to the criteria of the American Diabetes Association.[35] Participants with a fasting plasma glucose (FPG) level of ≥126 mg/dL or a 2-hour plasma glucose (2hPG) level of ≥200 mg/dL were classified as having T2D. Those with 2hPG of ≥140 mg/dL but <200 mg/dL and FPG of <100 mg/dL were classified as having isolated IGT. Isolated impaired fasting glucose (IFG) was defined as FPG of ≥100 but <126 mg/dL and 2hPG of <140 mg/dL. Participants with 2hPG of ≥140 mg/dL but <200 mg/dL and FPG of ≥100 but <126 mg/dL were defined as having combined IGT and IFG. Finally, those with T2D, IGT, or IFG were classified as having impaired glucose regulation.
5. Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows. Variables were checked for normality using Kolmogorov–Smirnoff tests. The chi-squared test was used to test for differences in the distribution between categorized variables. The independent t test for normally distributed variables and the Mann–Whitney U test for non-normally distributed variables, respectively, were used to test for differences in continuous variables between men and women. Differences in continuous variables between ≥3 groups were assessed using analysis of variance. Results are expressed as percentages, means and standard errors or SDs. The threshold for statistical significance was set at P < .05.
6. Results
Figure 1 presents the flow chart of participant recruitment of the DEMOJUAN project. In total, 14,193 participants completed the FINDRISC questionnaire during participant screening activities at the study sites. Among these, 35% (n = 4915) had a FINDRISC of ≥13 points and 47% (n = 2306) agreed to undergo the OGTT. Approximately, 33% (n = 772) of participants underwent the OGTT and met the entry criteria; these participants were then randomized into 3 groups. The control group (A) comprised of 246 participants, the nutritional/physical activity intervention group (B) comprised of 261 participants, and the physical activity/nutrition group (C) comprised of 265 participants.
Figure 1.
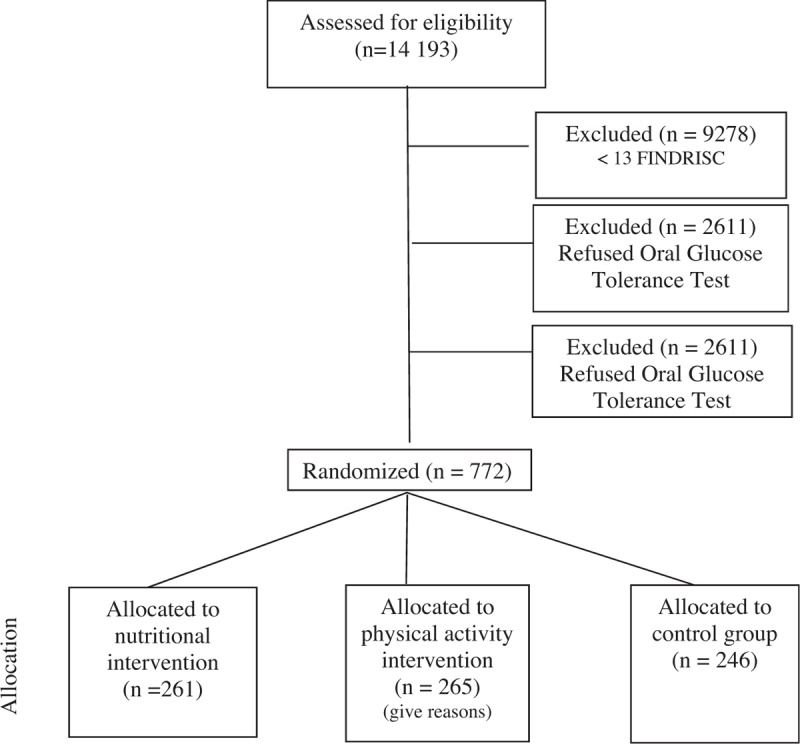
Flow chart of the participant recruitment for the DEMOJUAN project.
Participant characteristics at baseline are shown in Table 1. There was no statistically significant difference found in anthropometric, lifestyle, or other diabetes risk factors between the 3 groups (P > .05 for all). The mean FINDRISC was 16 points, with no statistically significant differences between groups (P = .663).
Table 1.
Baseline characteristics of the study participants.
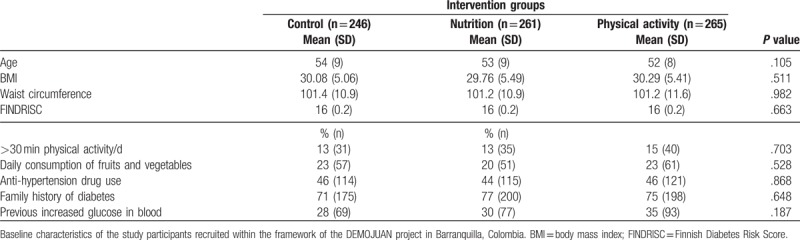
Table 2 shows the prevalence of glucose metabolism disorders between the 3 groups. No statistically significant difference in the distribution of glucose metabolism categories was observed between groups in the bivariate analysis (P = .069).
Table 2.
Categories of disturbances of glucose metabolism in participants in 2011 according to the intervention group.
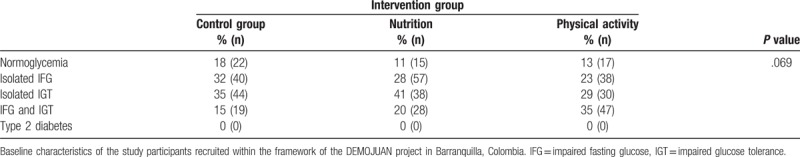
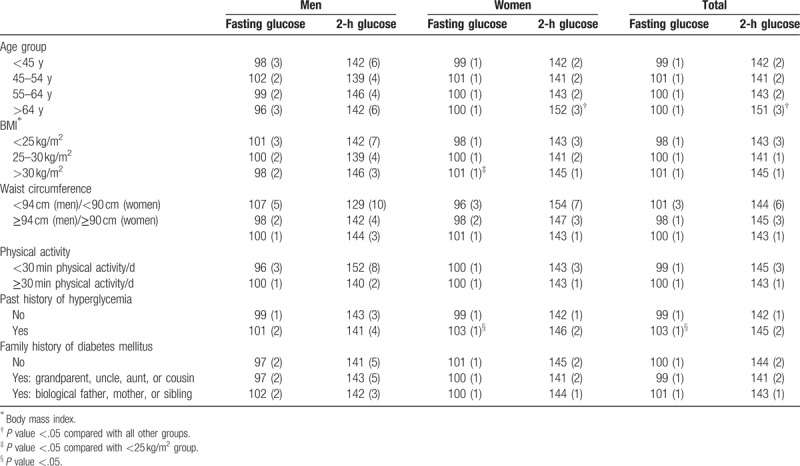
Table 3 presents participants’ mean fasting and 2-hour glucose levels according to lifestyle habits and risk factors of T2D. Women with a past history of hyperglycaemia had significantly higher fasting glucose levels compared with those without previous hyperglycaemia (mean = 103 vs 99 mg/dL; P < .05). In addition, women with a BMI of >30 kg/m2 had higher fasting glucose levels compared with those with a normal BMI. Finally, women in the oldest age group (>64 years) had significantly higher 2-hour glucose levels (mean = 152 mg/dL) compared with younger age groups (P < .05). No statistically significant differences were found in men in regard to fasting or 2-hour glucose levels between different categories of lifestyle habits or risk factors of T2D.
Table 3.
Mean fasting and 2-hour glucose levels according to lifestyle habits and risk factors of type 2 diabetes in the study participants.
7. Discussion
7.1. Screening and baseline characteristics of study participants
Up-to-date information regarding compliance with instructions to undergo an OGTT after a positive screening test is scant. Although Davies and Day[36] reported compliance of 93% following a positive screening test in Great Britain, in our project, only approximately half of high-risk individuals invited to an OGTT appeared at the laboratory.[37] Furthermore, in the European Diabetes Prevention Study (EDIPS), only 66% of participants invited to be screened using an OGTT appeared at the laboratory for blood extractions.[38] One possible reason for lower compliance may be related to time constraints, as the OGTT requires a visit to the laboratory in the morning and lasts at least 2 hours, which may be difficult to arrange for people working during the daytime. A possible explanation for the difference in the findings between our and the British study may be accessibility to a laboratory and the fact that access to/availability of public transportation surely differs between Barranquilla (Colombia) and Western Europe. Therefore, a future challenge will be to develop strategies to motivate individuals identified as being at a high risk of T2D to attend the laboratory test to confirm their diagnosis. Implementing simpler ways to test blood glucose levels, such as the recently developed home-based OGTT test, may also offer solutions to improve treatment compliance.[39]
The baseline characteristics of our study sample in regard to fasting and 2-hour glucose levels were different compared with previous randomized clinical trials in individuals with glucose intolerance.[10,11,13,38,40–42] The 2-hour glucose levels reported in previous studies were approximately 160 mg/dL,[10,11,13,34,40–42] whereas the post-prandial glucose levels in our study participants were almost 15 mg/dL lower. Moreover, fasting glucose levels of participants at baseline in previous clinical trials were higher than those in our participants, with the exception of the Da Qing study population.[10] Further, whereas study participants of lifestyle intervention trials conducted in Asian populations[10,11,42] had a lower baseline waist circumference and BMI, those of our study participants were similar to those of the 2 European studies.[13,41] The participants of the US Diabetes Prevention Program and EDIPS-Newcastle[38,40] had a remarkably higher BMI and waist circumference at baseline than those observed in our study. In addition, whereas 7 out of 10 participants in our study had a positive family history of diabetes, only 1 out of 4 had family members with diabetes in a Japanese trial.[42] Finally, the Asian studies included participants with a lower mean age than did most other previous clinical lifestyle intervention trials.[10,11,42]
7.2. Description of upcoming lifestyle interventions
During the following 24 months, the control group (A) will receive standard treatment (usual care: lifestyle advice prescribed by his/her physician). The 2 intervention groups (B and C) will receive early, intensive lifestyle interventions. Group B will receive a 6-month nutritional intervention followed by a 6-month physical activity intervention and finally a 12-month combined nutritional and physical activity intervention. Group B will also receive individual advice about how to achieve the intervention goals, which are as follows: reduction in weight of ≥5%; total fat intake of <30% of energy consumed; saturated fat intake of <10% of energy consumed, and fruit or vegetable intake of ≥500 g per day. Group C will begin with the 6-month physical activity intervention followed by the 6-month nutritional intervention. The physical activity intervention consists of individual visits with a physical activity specialist and monthly group seminars. Specifically, each participant will have 6 individual visits with a physical activity specialist (4 times during the first year and twice in the second year) in which they will receive an individual physical activity prescription. The goal of the physical activity intervention is to practice moderate-intensity exercise for ≥30 min/d. In addition, Group B and C participants will attend group seminars in groups of 10 participants. Group seminars will be held monthly during the first 12 months of the intervention and then every second month during the second year of the intervention. Group seminars will be led by a nutritionist and physical activity specialist.
Finally, in order to reduce the possibility of bias due to the visit schedule, both intervention groups will undergo their group sessions and individual visits in parallel. Another possible bias that will be more difficult to control for is the Hawthorne (i.e., observer) effect. As participants in the control group will be aware that they are at increased risk of T2D and that they are taking part in a T2D prevention study, they may change their lifestyles due to the fact that they are under supervision. However, the Hawthorn effect is reduced with longer intervention programme duration. Our intervention will last for 24 months and may thus be long enough to reduce the bias of the Hawthorne effect.
8. Conclusions
This baseline assessment of participants in the DEMOJUAN project revealed that it is possible to recruit individuals at risk of T2D for a field trial using a simple screening tool in a population of a country in economic transition such as Colombia. The randomization process revealed that lifestyle habits and risk factors were distributed evenly among the 3 study groups. No differences were found in fasting or 2-hour glucose levels among the various lifestyle or risk factor categories, with the exception of BMI, past history of hyperglycaemia and age >64 years in women.
As the participants in our study had lower fasting and 2-hour glucose levels at baseline compared with those of the previous trials mentioned above, the upcoming interventions will show to what extent normoglycaemia can be achieved, even in individuals with a more favorable glucose profile.
9. Authors’ contributions
TA, NCB, and JT planned the study, analyzed the data and wrote the report. AA and CR reviewed the manuscript and assisted in statistical analysis and results interpretation.
Footnotes
Abbreviations: FPG = fasting plasma glucose, IFG = impaired fasting glucose, IGT = impaired glucose tolerance, OGTT = oral glucose tolerance test, T2D = type 2 diabetes.
Consent for publication: Not applicable.
Availability of data and materials: The datasets analyzed during the current study are available upon request.
Funding: This project is supported by a BRIDGES Grant from the Global Diabetes Foundation. BRIDGES, a Global Diabetes Foundation project, is supported by an educational grant from Lilly Diabetes.
The authors declare that they have no competing interests.
References
- [1].King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998;21:1414–31. [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. WHO Technical Report Series. 2000;Geneva, Switzerland: World Health Organization, 894. [PubMed] [Google Scholar]
- [3].The DECODE Study Group on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet 1999;354:617–21. [PubMed] [Google Scholar]
- [4].Knowler W, Narayan K, Hanson R, et al. Perspectives in diabetes. Preventing non-insulin-dependent diabetes. Diabetes 1995;44:483–8. [DOI] [PubMed] [Google Scholar]
- [5].The DECODA Study Group. Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care 2003;26:1770–80. [DOI] [PubMed] [Google Scholar]
- [6].The DECODE Study Group. Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care 2003;26:61–9. [DOI] [PubMed] [Google Scholar]
- [7].Haffner SM, Stern MP, Hazuda HP, et al. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA 1990;263:2893–8. [DOI] [PubMed] [Google Scholar]
- [8].The DECODE Study Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med 2001;161:397–405. [DOI] [PubMed] [Google Scholar]
- [9].Qiao Q, Jousilahti P, Eriksson J, et al. Predictive properties of impaired glucose tolerance for cardiovascular risk are not explained by the development of overt diabetes during follow-up. Diabetes Care 2003;26:2910–4. [DOI] [PubMed] [Google Scholar]
- [10].Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–44. [DOI] [PubMed] [Google Scholar]
- [11].Ramachandran A, Snehalatha C, Mary S, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–97. [DOI] [PubMed] [Google Scholar]
- [12].Turner RC, Cull CA, Frighi V, et al. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus. Progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–12. [DOI] [PubMed] [Google Scholar]
- [13].Tuomilehto J, Lindström J, Eriksson J, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50. [DOI] [PubMed] [Google Scholar]
- [14].Adams R, Hebert CJ, Mcvey L, et al. Implementation of the YMCA Diabetes Prevention Program throughout an Integrated Health System: a Translational Study. Perm J 2016;20:82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Costa B, Barrio F, Cabré JJ, et al. DE-PLAN-CAT Research Group. Delaying progression to type 2 diabetes among high-risk Spanish individuals is feasible in real-life primary healthcare settings using intensive lifestyle intervention. Diabetologia 2012;55:1319–28. [DOI] [PubMed] [Google Scholar]
- [16].Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725–31. [DOI] [PubMed] [Google Scholar]
- [17].Saaristo T, Peltonen M, Lindström J, et al. Cross-sectional evaluation of the Finnish Diabetes Risk Score: a tool to identify undetected type 2 diabetes, abnormal glucose tolerance and metabolic syndrome. Diab Vasc Dis Res 2005;2:67–72. [DOI] [PubMed] [Google Scholar]
- [18].Franciosi M, De Berardis G, Rossi MC, et al. Use of the diabetes risk score for opportunistic screening of undiagnosed diabetes and impaired glucose tolerance: the IGLOO (Impaired Glucose Tolerance and Long-Term Outcomes Observational) study. Diabetes Care 2005;28:1187–94. [DOI] [PubMed] [Google Scholar]
- [19].Makrilakis K, Liatis S, Grammatikou S, et al. Validation of the Finnish diabetes risk score (FINDRISC) questionnaire for screening for undiagnosed type 2 diabetes, dysglycaemia and the metabolic syndrome in Greece. Diabetes Metab 2011;37:144–51. [DOI] [PubMed] [Google Scholar]
- [20].Schwarz PE, Lindström J, Kissimova-Scarbeck K, et al. DE-PLAN project. The European Perspective of Type 2 Diabetes Prevention: diabetes in Europe - prevention using lifestyle, physical activity and nutritional intervention (DE-PLAN) project. Exp Clin Endocrinol Diabetes 2008;116:167–72. [DOI] [PubMed] [Google Scholar]
- [21].Costa B, Barrio F, Cabré JJ, et al. Delaying progression to type 2 diabetes among high-risk Spanish individuals is feasible in real-life primary healthcare settings using intensive lifestyle intervention. Diabetologia 2012;55:1319–28. [DOI] [PubMed] [Google Scholar]
- [22].Rydén L, Standl E, Bartnik M, et al. Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD). Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J 2007;28:88–136. [DOI] [PubMed] [Google Scholar]
- [23].Barengo NC, Tamayo DC, Tono T, et al. A Colombian diabetes risk score for detecting undiagnosed diabetes and impaired glucose regulation. Prim Care Diabetes 2017;11:86–93. [DOI] [PubMed] [Google Scholar]
- [24].Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: a consensus on type 2 diabetes prevention. Diabet Med 2007;24:451–63. [DOI] [PubMed] [Google Scholar]
- [25].Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- [26].Ekelund U, Sepp H, Brage S, et al. Criterion-related validity of the last 7-day, short form of the International Physical Activity Questionnaire in Swedish adults. Public Health Nutr 2006;9:258–65. [DOI] [PubMed] [Google Scholar]
- [27].Bonita R, deCourten M, Dwyer T, et al. Surveillance of Risk Factors for Noncommunicable Diseases: The WHO STEP Wise Approach. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- [28].WHO. Chronic diseases and health promotion. STEPwise approach to chronic disease risk factor surveillance (STEPS); 2013. [Last cited on 2013 Apr 15]. Available at: http://www.who.int/chp/steps/riskfactor/en/index.html. Accessed March 15, 2017. [Google Scholar]
- [29].Hu G, Barengo NC, Tuomilehto J, et al. Relationship of physical activity and body mass index to the risk of hypertension: a prospective study in Finland. Hypertension 2004;43:25–30. [DOI] [PubMed] [Google Scholar]
- [30].Hu G, Qiao Q, Silventoinen K, et al. Occupational, commuting, and leisure-time physical activity in relation to risk for type 2 diabetes in middle-aged Finnish men and women. Diabetologia 2003;46:322–9. [DOI] [PubMed] [Google Scholar]
- [31].Keys A. Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease. Cambridge, MA: Harvard University Press; 1980. [Google Scholar]
- [32].Albanes D, Conway JM, Taylor PR, et al. Validation and comparison of eight physical activity questionnaires. Epidemiology 1990;1:65–71. [DOI] [PubMed] [Google Scholar]
- [33].Salonen JT, Slater JS, Tuomilehto J, et al. Leisure time and occupational physical activity: risk of death from ischemic heart disease. Am J Epidemiol 1988;127:87–94. [DOI] [PubMed] [Google Scholar]
- [34].WHO Consultation. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. 1999;Geneva: World Health Organisation, Report No.: Report No 99.2. [Google Scholar]
- [35].American Diabetes Association Position Statement. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33:S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Davies M, Day J. Screening for noninsulin dependent diabetes mellitus (NIDDM): how often should it be done? J Med Screen 1994;1:78–81. [DOI] [PubMed] [Google Scholar]
- [37].Barengo NC, Acosta T, Arrieta A, et al. Screening for people with glucose metabolism disorders within the framework of the DEMOJUAN project (DEMOnstration area for primary prevention of type 2 diabetes, JUAN Mina and Barranquilla, Colombia). Diabetes Metab Res Rev 2013;doi: 10.1002/dmrr.2462. [DOI] [PubMed] [Google Scholar]
- [38].Penn L, White M, Oldroyd J, et al. Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health 2009;9:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Facchinetti A, Sparacino G, Guerra S, et al. Real-time improvement of continuous glucose monitoring accuracy: the smart sensor concept. Diabetes Care 2013;36:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mensink M, Corpeleijn E, Feskens EJM, et al. Study on lifestyle-intervention and impaired glucose tolerance Maastricht (SLIM): design and screening results. Diabetes Res Clin Pract 2003;61:49–58. [DOI] [PubMed] [Google Scholar]
- [42].Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention. A Japanese trial in IGT males. Diabetes Res Clin Pract 2005;67:152–62. [DOI] [PubMed] [Google Scholar]


