Abstract
Little has been published on blood management in total hip and knee arthroplasty (THA and TKA, respectively) patients focusing on both hematopoiesis and hemostasis. Our aim was to explore the effectiveness and safety of an optimized blood management program in THA and TKA patients in a large, single-center, retrospective study.
We retrospectively reviewed consecutive primary unilateral THA and TKA patients’ data at our institution through the National Health Database. They were divided into 3 groups according to an optimized blood management program: group A—combined use of intravenous and topical tranexamic acid (TXA); group B—use of recombinant human erythropoietin (rHuEPO) and iron supplements in addition to treatments in group A; group C—use of additional multiple boluses of TXA in addition to treatments in group B. The primary outcomes were hemoglobin (Hb) drop and calculated total blood loss (TBL). Other outcome measurements such as transfusion rate, postoperative length of stay (PLOS), venous thromboembolism (VTE), and mortality were also compared.
From 2014 to 2016, a total of 1907 unilateral THA (986 in group A, 745 in group B, and 176 in group C) and 1505 unilateral TKA (795 in group A, 556 in group B, and 154 in group C) procedures were conducted at our institution. The Hb drop, calculated TBL, and PLOS in group C were significantly lower than those in groups A and B for THA and TKA patients. The transfusion rate in group C was also significantly less than in groups A and B for THA patients, while it was similar in groups A and B for TKA patients. No patients in group C received a transfusion. A significant difference was not detected in the incidence of deep vein thrombosis. No episode of symptomatic pulmonary embolism or all-cause mortality occurred within 30 days postoperatively.
The current retrospective study suggests that for patients receiving primary unilateral THA or TKA, multiple boluses of intravenous TXA combined with topical TXA, rHuEPO, and iron supplements can reduce the calculated TBL, Hb drop, transfusion rate, and PLOS without increasing the incidence of VTE or mortality.
Keywords: blood management, iron supplements, recombinant human erythropoietin, total hip arthroplasty, total knee arthroplasty, tranexamic acid
1. Introduction
Total hip and knee arthroplasty (THA and TKA, respectively) are efficient treatments that can relieve pain, improve knee function, and correct deformity in patients with end-stage hip and knee osteoarthropathy.[1,2] However, the substantial perioperative blood loss and subsequent acute anemia and need for transfusion are major concerns for joint surgeons. The total perioperative blood loss ranges from 700 to 2000 mL,[3,4] and the percentage of hidden blood loss (HBL) can be 29% to 60% in THA and 45% to 60% in TKA.[5–7] Postoperative anemia may hinder the recovery of patients after THA and TKA and increase the incidence of complications and mortality, and blood transfusions may increase the risk of anaphylaxis, transfusion-related acute lung injury, and virus transmission.[7–9] Therefore, an ideal blood management program for THA and TKA patients should include an attempt to minimize perioperative blood loss and correct perioperative anemia.
Preoperative anemia (hemoglobin [Hb] <120 g/L for females and <130 g/L for males)[10] is common (prevalence of 24%) in older patients presenting for elective THA or TKA.[11] Preoperative anemia increases the severity and rate of postoperative anemia, and increases the need for transfusions.[4] Erythropoietin (EPO) and iron administration now appear to be an alternative choice for correcting perioperative anemia.[4,5] Several studies have shown that intravenous iron administration is more rapid and effective, and is better tolerated than oral iron in various populations.[12] However, there is a lack of strong evidence to prove the clinical benefits of EPO and iron supplement application in THA and TKA patients in the perioperative period.
Furthermore, hyperfibrinolysis is one of the most important causes of perioperative blood loss,[13] and the application of anti-fibrinolytic drugs seems to be an effective treatment to reduce perioperative blood loss. As an analogue of the amino acid lysine, tranexamic acid (TXA) can competitively inhibit plasminogen activation and plasmin binding to fibrin to achieve the effect of anti-fibrinolysis,[14] and thus reduce perioperative blood loss and transfusion rate.[9,15] There are 3 common methods of TXA administration in THA and TKA patients: intravenous,[16] topical,[17] and combined application.[6] Many studies have shown that combined application is the most effective regimen.[6,12,18] In most studies, a single dose of intravenous TXA (IV-TXA) was administrated preoperatively. However, fibrinolysis peaked at 6 hours after surgery and lasted for 18 hours, according to a laboratory follow-up study,[19] so a single dose of TXA was not adequate to inhibit fibrinolysis completely.[13] In a previous randomized controlled trial, it was reported that multiple boluses of TXA can effectively reduce HBL after primary TKA and lead to a smaller decline in Hb.[13] However, the sample size was relatively small. Therefore, more studies with larger sample sizes are necessary to prove the clinical effectiveness of multiple boluses of TXA.
The perioperative blood management program in THA and TKA patients has been developed over the last 3 years at our institution and can be divided into 3 different groups according to time periods. From January 2014, combined use of intravenous and topical TXA began to be administered to THA and TKA patients (group A). Starting in April 2015, we also included treatments of recombinant human erythropoietin (rHuEPO) and iron supplements (group B). Then, starting in February 2016, we added multiple boluses of TXA to the treatment regimen (group C). We conducted this large, single-center, retrospective study to evaluate the following: whether applying rHuEPO and iron supplements can improve the postoperative Hb level and reduce the transfusion rate when added to the combined use of intravenous and topical TXA; whether adding multiple boluses of TXA can reduce blood loss when added to treatment with rHuEPO, iron supplements, and intravenous and topical TXA; and whether the optimized blood management program was safe.
2. Materials and methods
2.1. Ethical approval
The study protocol was approved by the Institutional Review Board (IRB) of West China Medical Center of Sichuan University (2012-268). For this type of study formal consent is not required.
2.2. Patient selection
All the data were derived from the National Health Database of China. The server for this database was set up at our institution, and our center was in charge of the management of the database, ensuring the standardization and accuracy of the data entry. This study included consecutive patients undergoing primary unilateral THA or TKA from January 2014 to April 2016 in our department. The inclusion criteria were as follows: patients receiving primary unilateral THA or TKA; normality of preoperative platelet and coagulation function. The exclusion criteria were as follows: history of venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE); cardiovascular problems or cerebrovascular conditions (history of myocardial infarction, angina, atrial fibrillation, or previous stroke); serious liver or kidney dysfunction; known allergy to TXA, rHuEPO, or iron supplements; discontinuation of oral antiplatelet agents or aspirin <1 week before surgery; or preoperative Hb level <100 g/L.
2.3. Study design and blood management
The patients were divided into 3 groups according to the optimized blood management program (Table 1). The details of each strategy are described below.
Table 1.
Optimized blood management program of the 3 groups.

Combined use of intravenous and topical TXA: a single bolus of 20 mg/kg of IV-TXA (Chongqing Lummy Pharmaceutical Co., Ltd., Chongqing, China) was administered 5 to 10 minutes before skin incision, and 1 g of topical TXA was injected through the drainage tube after closing the deep fascia, as described in our previous study.[12] The drain tube was kept clamped for 2 hours, then the tube was fully opened and was removed by the next morning.
RHuEPO and iron supplements: patients in group A with perioperative anemia were only given 150 to 300 mg/d of oral iron treatment (Shanghai UCB Trading Co., Ltd., Shanghai, China), and the detailed regimen of rHuEPO and iron supplement therapy for patients in groups B and C is shown in Figure 1. Subcutaneous rHuEPO (Shenyang 3SBio Inc., Shenyang, China), intravenous iron supplements (Shanghai UCB Trading Co., Ltd), and oral iron supplements (Shanghai UCB Trading Co., Ltd.) were given in this regimen. The indication to stop treatment with rHuEPO and iron supplements was Hb >130 g/L for males and Hb >120 g/L for females.
Figure 1.
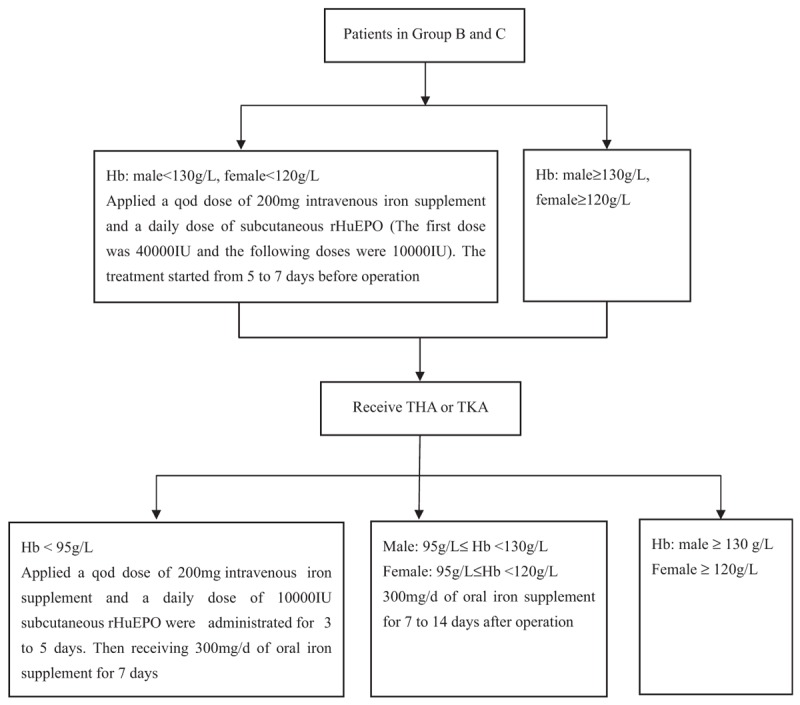
The regimen of rHuEPO and iron supplement therapy for patients in group B and C. Hb = hemoglobin, rHuEPO = recombinant human erythropoietin, THA = total hip arthroplasty, TKA = total knee arthroplasty.
Additional multiple boluses of IV-TXA: 10 mg/kg of IV-TXA was administered 3 and 6 hours after the first dose of 20 mg/kg of IV-TXA, as described in our previous studies.[12,13]
2.4. Surgical procedure
All of the operations were performed by 5 senior surgeons in our department, 1 of whom was in charge of the joint replacement institution, and the others who had surgical training under his guidance. All of the operations were performed under general anesthesia or spinal anesthesia in 1 of 2 operating rooms with laminar airflow. The posterolateral approach and cementless cups and stems were used in the THA procedures. A midline skin incision, medial parapatellar approach, measured resection technique, and cemented posterior-stabilized prosthetic designs were used in the TKA procedures. All patients received a drainage catheter at the end of the operation. In groups A and B, a tourniquet was applied to all patients receiving TKA with the strategy of inflating before incision and deflating after compressing the lower limb with 2 elastic bandages at 100 mmHg above systolic pressure. In group C, a tourniquet was not applied routinely.
2.5. Postoperative care protocol
A combination of mechanical and chemical thromboprophylaxis was adopted to prevent VTE for all patients. As mechanical prophylaxis, an intermittent foot slope pump system was used before walking. As chemical prophylaxis, a half-dose of low-molecular-weight heparin (LMWH; 2000 IU in 0.2 mL; Clexane; Sanofi-Aventis, Paris, France) was subcutaneously administered at 6 hours postoperatively, and repeated at 24-hour intervals with a full dose (4000 IU in 0.4 mL). As prophylaxis after discharge, patients were instructed to take 10 mg of Rivaroxaban (Xarelto; Bayer, Leverkusen, Germany) orally once a day for 10 to 14 days if no bleeding events occurred. Doppler ultrasound was used routinely to detect DVT at the time of discharge and at 30-day follow-up, or when there was a clinically suspected DVT. PE was diagnosed by clinical symptoms and enhanced chest computed tomography scan. The criterion of blood transfusion was set as an Hb level of <70 or 70–100 g/L with symptomatic anemia (defined as tachycardia, pallor and lethargy, poor appetite, and fatigue) according to the guidelines by the National Ministry of Health.
2.6. Outcome measurements
All measurements were derived from the National Health Database of China, including demographic data (gender, age, height, weight, and body mass index), diagnosis, comorbidities, American Society of Anesthesiologists (ASA) grade, duration of surgery, intraoperative fluid volume, Hb and hematocrit (Hct) level preoperatively and on postoperative day 1 (POD1) and postoperative day 3 (POD3). The primary outcomes were Hb drop and calculated total blood loss (TBL). Hb drop = Hbpre − Hbpost (Hbpost: Hb level on POD1 or POD3). TBL was calculated according to the Nadler et al[20] and Gross formula[21]: TBL = patient's blood volume (PBV) × (Hctpre − Hctpost)/(Hctpre + Hctpost) × 2 (Hctpre: initial preoperative Hct level, Hctpost: Hct on POD1 or POD3). The PBV was assessed according to the formula of Nadler et al[20]: PBV = k1 × height (m)3 + k2 × weight (kg) + k3 (male: k1 = 0.3669, k2 = 0.03219, k3 = 0.6041; female: k1 = 0.3561, k2 = 0.03308, k3 = 0.1833). If either reinfusion or allogeneic transfusion was performed, the TBL was equal to the loss calculated from the Gross formula plus the volume transfused. Other outcome measurements such as transfusion rate and amount, postoperative length of stay (PLOS), and complications were also compared carefully.
2.7. Statistical analysis
All the data analyses were performed using SPSS version 22.0 (SPSS Inc., IBM Corp., New York). The Pearson χ2 test and the Fisher exact test were performed to analyze the qualitative variables. One-way analysis of variance and Tukey's post-hoc tests were performed to analyze the parametric samples while the Kruskal–Wallis H test and Mann–Whitney U test were used for nonparametric data. The threshold of significance was defined as P < .05, and the corrected significance level for post-hoc correction was P′ < .017.
3. Results
There were 1907 cases of THA, with 986 cases in group A, 745 in group B, and 176 in group C. There were 1505 cases of TKA, with 795 cases in group A, 556 in group B, and 154 in group C. The baseline characteristics of the 3 groups were comparable (Table 2).
Table 2.
Preoperative and intraoperative characteristics of patients receiving total hip or knee arthroplasty.

The levels of Hb and drop in Hb in THA patients are shown in Figures 2A and 3A. The mean Hb in group C (116.32 ± 16.36 g/L) and group B (115.45 ± 15.91 g/L) were significantly higher than in group A (112.40 ± 17.15 g/L, P < .001, P < .001, respectively). However, there was no significant difference between groups B and C (P = .68) on POD1. On POD3, the mean Hb in group C (108.31 ± 16.41 g/L) was still higher than those in group B (105.33 ± 15.53 g/L, P = .02) and group A (101.35 ± 16.46 g/L, P < .001), and it was higher in group B than in group A (P < .001). The mean Hb drop in group C (15.66 ± 9.90 g/L) was significantly lower than in group B (17.61 ± 9.94 g/L, P = .03) and group A (21.45 ± 9.68 g/L, P < .001), and it was lower in group B than in group A (P < .001) on POD1. On POD3, The Hb drop in group C (23.67 ± 9.66 g/L) was still lower than in group B (27.72 ± 10.68 g/L, P < .001) and group A (32.51 ± 11.74 g/L, P < .001), and it was lower in group B than in group A (P < .001).
Figure 2.
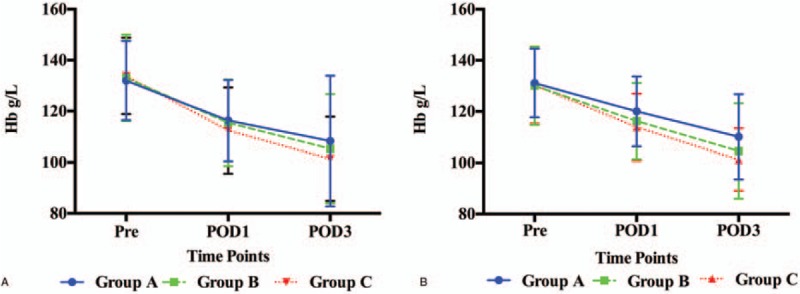
The level of Hb in THA (A) and TKA patients (B). Hb = hemoglobin, POD1 = postoperative day 1, POD3 = postoperative day 3, Pre = preoperatively, THA = total hip arthroplasty, TKA = total knee arthroplasty.
Figure 3.
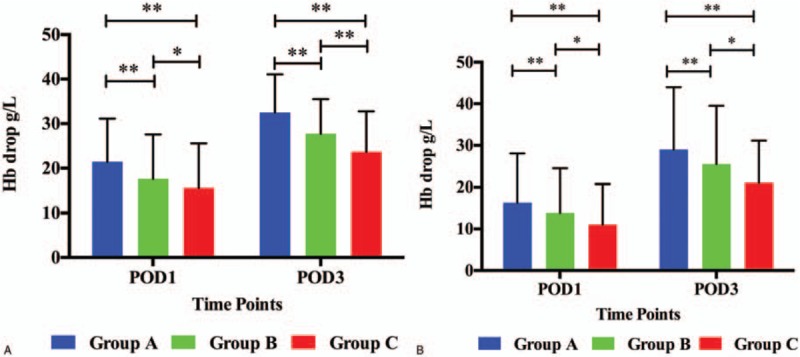
The Hb drop in THA (A) and TKA patients (B). Hb = hemoglobin, POD1 = postoperative day 1, POD3 = postoperative day 3, THA = total hip arthroplasty, TKA = total knee arthroplasty. ∗Means P value <0.05, ∗∗means P value <0.01.
The levels of Hb and drop in Hb in TKA patients are shown in Figures 2B and 3B. The mean Hb in group C (120.05 ± 13.05 g/L) was significantly higher than in group B (116.26 ± 13.67 g/L, P = .015) and group A (113.90 ± 14.91 g/L, P < .001), and it was lower in group B than in group A (P = .012) on POD1. On POD3, the mean Hb in group C (110.13 ± 12.15 g/L) was still higher than in group B (104.58 ± 16.65 g/L, P = .001) and group A (101.25 ± 18.62 g/L, P < .001), and it was higher in group B than in group A (P = .001). The mean Hb drop in group C (11.08 ± 9.11 g/L) was significantly lower than in group B (13.84 ± 7.79 g/L, P = .001) and group A (16.31 ± 8.57 g/L, P < .001), and it was lower in group B than in group A (P < .001) on POD1. On POD3, The Hb drop in group C (21.01 ± 10.14 g/L) was still lower than in group B (25.52 ± 13.98 g/L, P = .002) and group A (28.96 ± 15.02 g/L, P < .001), and it was lower in group B than in group A (P < .001).
Table 3 shows the details of calculated TBL, transfusion, and PLOS after THA or TKA among the 3 groups. The mean TBL in group C was significantly lower than in groups A and B, and it was lower in group B than in group A both for THA and TKA patients on POD1 and POD3 (P < .01). The transfusion rate was significantly less and the amount of transfusion was lower in group C than in groups A and B for THA patients (P < .01). However, for TKA patients, the transfusion rate and amount in groups A and B were similar (P > .05), and no patients in group C received a transfusion. There were favorable effects in shortening PLOS in both THA and TKA patients in group C compared with patients in groups A and B. Differences were also detected between groups A and B (P < .001).
Table 3.
Calculated total blood loss, transfusion details and postoperative length of stay after total hip or knee arthroplasty.

Table 4 shows the details of the postoperative complications in our study. No episodes of symptomatic PE or stroke occurred in any patient. The differences among the 3 groups for the incidence of DVT, intramuscular venous thrombosis, wound complications, and 30-day readmission were not significantly different (P > .05). Furthermore, no episodes of all-cause 30-day mortality occurred during the patients’ hospital stay or 30-day follow-up period.
Table 4.
Complications during the 30-day follow-up period.

4. Discussion
The most important finding of this large, single-center, retrospective study was that the application of rHuEPO and iron supplements can reduce the calculated TBL, Hb drop, transfusion rate, and PLOS, and improve the postoperative Hb level when added to the combined use of intravenous and topical TXA. Also, applying additional multiple boluses of TXA can further improve the effects in patients undergoing THA or TKA without increasing the incidence of VTE, wound complications, or mortality.
There are a considerable number of patients who have preoperative anemia, and socioeconomic status, customs, and religion can contribute to this. This is significant because preoperative anemia is associated with postoperative morbidity and mortality.[22] A preoperative Hb level of <120 g/L triples the risk of blood transfusion.[4] Thus, correcting preoperative anemia and raising preoperative Hb level to >120 g/L are important. However, this cannot be achieved with the use of TXA. Moreover, the majority of patients requiring THA or TKA are middle-aged or elderly people whose preoperative anemia is mainly iron deficiency anemia or anemia of chronic disease, and one-third of these patients have iron deficiency.[23] It has been shown that providing rHuEPO and iron supplements to patients with preoperative anemia can improve the Hb level and improve operative safety and reduce the transfusion requirement.[23,24] It is also effective for patients with postoperative anemia, which is caused by surgical bleeding.[8] In our study, applying rHuEPO and iron supplements in addition to the combined use of intravenous and topical TXA improved postoperative Hb level and reduced the transfusion rate, which was in accordance with previous studies.[22,24]
Recovery from blood loss requires a greatly enhanced supply of iron to support expanded erythropoiesis. After hemorrhage, suppression of the iron regulatory hormone hepcidin allows increased iron absorption and mobilization from stores. At the same time, EPO stimulates production of the Hb-containing red blood cells with adequate iron. In our study, we routinely added rHuEPO and iron supplements to the combined use of intravenous and topical TXA during the perioperative period for THA or TKA patients, which may have promoted hematopoietic function and recovery from acute surgical bleeding, whereas the HBL was gradually increased by postoperative hyperfibrinolysis.[13] Meanwhile, rHuEPO and iron supplements were associated with improved hematological parameters, especially Hb and Hct levels, and reduced the TBL as calculated by the Gross formula,[21] which is still the most commonly used formula that predicts blood loss accurately. However, arithmetical differences between preoperative and postoperative period may not fully reflect real perioperative blood loss. Thus, further prospective studies using a more exact method to confirm the effect are in demand.
The maximum Hb drop in group B was up to 25 g/L, and the TBL on POD3 was up to 800 mL, an important component of which was HBL. Thus, based on the results in group B, the key goal in the next step was to reduce HBL further. One of the main reasons for HBL is fibrinolysis, which peaks at 6 hours after surgery and lasts 18 hours.[19] In a previous study, D-dimer and fibrin(-ogen) degradation products were tested at 6, 12, 24, and 48 hours postoperatively, and it was found that fibrinolysis peaked at 6 hours postoperatively and lasted for at least 24 hours.[13] It is advantageous to achieve anti-fibrinolysis during the first 24 hours after THA or TKA, which provides the theoretical basis for the application of multiple boluses of TXA. According to pharmacokinetics, the half-life of TXA in plasma is about 3 hours. TXA can rapidly distribute into the joint fluid and synovium, and the concentration can reach the level of the plasma concentration, and the biological half time is also about 3 hours.[25] These distribution and metabolic features also provide the theoretical basis for the application of multiple boluses of TXA. Thus, administration of 10 mg/kg of IV-TXA was repeated at 3 and 6 hours after the first dose in our study. We found that the postoperative Hb level was increased, the Hb drop was decreased, and the TBL, transfusion rate, and PLOS were all reduced after additional multiple boluses of TXA were applied. The results were consistent with the previous study.[13]
Adverse effects from the short-term use of rHuEPO are rare based on the literature.[24] The maximum total dose of rHuEPO in our study was 110,000 to 150,000 IU (1833–2500 IU/kg) and the mean dose was 208 to 229 IU/kg/day (the mean weight was 60 kg). While in related study, the dose of rHuEPO was up to 300 IU/kg/day, the duration was 8 to 15 days,[26] which was higher than the dose in our study, and they did not observe any serious adverse effects. The risk of DVT was increased in patients on rHuEPO with a baseline Hb level >130 g/L, while the occurrence of DVT in patients with a baseline Hb level of 101 to 130 g/L was similar to that of patients receiving a placebo.[27] It was thought that iron depletion and associated relative thrombocytosis might contribute to increased VTE incidence when administering high rHuEPO doses.[28] Therefore, combining rHuEPO with iron can prevent functional iron deficiency and thrombocytosis when administering high rHuEPO.[28]
The postoperative VTE and mortality is the major concern, which hindered the wide adoption of TXA in the setting of THA or TKA. In a retrospective study conducted by Poeran et al,[29] a total of 872,416 patients (20,051 in TXA group) undergoing THA or TKA in 510 US hospitals at a 6-year period were included, the results showed no significant difference on postoperative DVT between TXA group and control group (0.4% vs 0.5%), and the rate of clinically significant PE was 0.2% in patients receiving TXA. TXA dose categories (vs no TXA use) were not significantly associated with the risk of thromboembolic complications (odds ratio 0.85–1.02) in the multilevel logistic regression model. Another retrospective study by Duncan et al,[30] a total of 13,262 elective TKA or THA procedures in 11,175 unique patients at a 5-year period were included, the result founded the incidence of postoperative DVT was 1.7% in TXA group whereas it was 2.0% in control group. It was also reported an all-cause 30-day mortality of 0.04% in patients undergoing THA or TKA when TXA was administered. Compared with previous studies, the reduction in the frequency of VTE and all-cause 30-day mortality in the current study is likely due to several factors as follows. First, postoperative screening for thrombus by ultrasound was a routine practice for every patients included. Second, a regular protocol of thrombosis prevention, including physical approaches and early rehabilitation activities, was implemented well by the patients even after the discharge. Third, we moved the usage of the LMWH ahead to 6 hours after wound closure. Last but not the least, all patients followed up regularly and timely in our outpatient department after discharge.
There were several limitations in this study. First, it was a retrospective study from a single tertiary-care teaching institution. A prospective study might better verify the effectiveness and safety of an optimized blood management program with the application of TXA, rHuEPO, and iron supplements. In addition, we did not use propensity-matched analysis in statistics, which is really a practical method for retrospective study to exclude a variety of confounding factors. However, this limitation is likely minimized in the data by 3 factors. First of all, the patients’ preoperative and intraoperative baseline characteristics of 3 groups were comparable in the current study. Then, there were 6 to 8 THA or TKA procedures were conducted by each senior surgeon every week, the amount and proportion of procedures by each senior surgeon in each group was the same. At last, no tourniquet in group C was applied, which make the surgical procedures were not standardized among groups. However, with the improvement of surgical techniques and the use of TXA, it is safe and feasible for patients undergoing TKA without using the tourniquet and even obtaining lower blood loss.[31] Second, this study solely focused on a short follow-up period, and it may have been too short to sufficiently assess the clinical efficacy and safety of these treatments. Third, the sample in group C was relatively small, and further studies with a larger sample size are necessary in the application of multiple boluses of TXA.
In conclusion, the current retrospective study suggests that for patients receiving primary unilateral THA or TKA, applying rHuEPO and iron supplements can reduce the calculated TBL, Hb drop, transfusion rate, and PLOS, and improve the postoperative Hb level when added to combined treatment using intravenous and topical TXA, and applying additional multiple boluses of TXA can further improve these effects in patients undergoing THA or TKA without increasing the incidence of VTE, wound complications, and mortality.
Acknowledgments
The authors sincerely acknowledge the entire staffs of the Department of Orthopaedics, West China Hospital, who offered assistance in the coursing of this study. The also thank Peter Mittwede, MD, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, DVT = deep vein thrombosis, EPO = erythropoietin, Hb = hemoglobin, HBL = hidden blood loss, Hct = hematocrit, IV-TXA = intravenous tranexamic acid, LMWH = low-molecular-weight heparin, PBV = patient's blood volume, PE = pulmonary embolism, PLOS = postoperative length of stay, POD1 = postoperative day 1, POD3 = postoperative day 3, rHuEPO = recombinant human erythropoietin, TBL = total blood loss, THA = total hip arthroplasty, TKA = total knee arthroplasty, TXA = tranexamic acid, VTE = venous thromboembolism.
SZ, QH, and BX contributed equally to this work and should be considered as cofirst authors.
This study was funded by the National Health and Family Planning Commission of the People's Republic of China (CN) program (201302007).
The authors report no conflicts of interest.
References
- [1].Kolk S, Minten MJ, van Bon GE, et al. Gait and gait-related activities of daily living after total hip arthroplasty: a systematic review. Clin Biomech (Bristol, Avon) 2014;29:705–18. [DOI] [PubMed] [Google Scholar]
- [2].Paxton RJ, Melanson EL, Stevens-Lapsley JE, et al. Physical activity after total knee arthroplasty: a critical review. World J Orthop 2015;6:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Haien Z, Yong J, Baoan M, et al. Post-operative auto-transfusion in total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. PLoS One 2013;8:e55073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee JH, Han SB. Patient blood management in hip replacement arthroplasty. Hip Pelvis 2015;27:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lemaire R. Strategies for blood management in orthopaedic and trauma surgery. J Bone Joint Surg Br 2008;90:1128–36. [DOI] [PubMed] [Google Scholar]
- [6].Lin SY, Chen CH, Fu YC, et al. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty 2015;30:776–80. [DOI] [PubMed] [Google Scholar]
- [7].Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br 2004;86:561–5. [PubMed] [Google Scholar]
- [8].Steuber TD, Howard ML, Nisly SA. Strategies for the management of postoperative anemia in elective orthopedic surgery. Ann Pharmacother 2016;50:578–85. [DOI] [PubMed] [Google Scholar]
- [9].Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood 2009;113:3406–17. [DOI] [PubMed] [Google Scholar]
- [10].World Health Organization. Iron Deficiency Anaemia: Assessment Prevention And Control. A Guide for Program Managers. Vol 21. 2001;Geneva, Switzerland: World Health Organization, 42. [Google Scholar]
- [11].Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology 2010;113:482–95. [DOI] [PubMed] [Google Scholar]
- [12].Huang Z, Ma J, Shen B, et al. Combination of intravenous and topical application of tranexamic acid in primary total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty 2014;29:2342–6. [DOI] [PubMed] [Google Scholar]
- [13].Xie J, Ma J, Yao H, et al. Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss after primary total knee arthroplasty without tourniquet: a randomized clinical trial. J Arthroplasty 2016;31:2458–64. [DOI] [PubMed] [Google Scholar]
- [14].Mahdy AM, Webster NR. Perioperative systemic haemostatic agents. Br J Anaesth 2004;93:842–58. [DOI] [PubMed] [Google Scholar]
- [15].Jiang X, Ma XL, Ma JX. Efficiency and safety of intravenous tranexamic acid in simultaneous bilateral total knee arthroplasty: a systematic review and meta-analysis. Orthop Surg 2016;8:285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, et al. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am 2014;96:1937–44. [DOI] [PubMed] [Google Scholar]
- [17].Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am 2013;95:1961–8. [DOI] [PubMed] [Google Scholar]
- [18].Yi Z, Bin S, Jing Y, et al. Tranexamic acid administration in primary total hip arthroplasty: a randomized controlled trial of intravenous combined with topical versus single-dose intravenous administration. J Bone Joint Surg Am 2016;98:983–91. [DOI] [PubMed] [Google Scholar]
- [19].Blanie A, Bellamy L, Rhayem Y, et al. Duration of postoperative fibrinolysis after total hip or knee replacement: a laboratory follow-up study. Thromb Res 2013;131:e6–11. [DOI] [PubMed] [Google Scholar]
- [20].Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery 1962;51:224–32. [PubMed] [Google Scholar]
- [21].Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology 1983;58:277–80. [DOI] [PubMed] [Google Scholar]
- [22].Salido JA, Marin LA, Gomez LA, et al. Preoperative hemoglobin levels and the need for transfusion after prosthetic hip and knee surgery: analysis of predictive factors. J Bone Joint Surg Am 2002;84-A:216–20. [DOI] [PubMed] [Google Scholar]
- [23].Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth 2011;106:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Voorn VM, van der Hout A, So-Osman C, et al. Erythropoietin to reduce allogeneic red blood cell transfusion in patients undergoing total hip or knee arthroplasty. Vox Sang 2016;111:219–25. [DOI] [PubMed] [Google Scholar]
- [25].Andersson L, Eriksson O, Hedlund PO, et al. Special considerations with regard to the dosage of tranexamic acid in patients with chronic renal diseases. Urol Res 1978;6:83–8. [DOI] [PubMed] [Google Scholar]
- [26].Dubois RW, Lim D, Hebert P, et al. The development of indications for the preoperative use of recombinant erythropoietin. Can J Surg 1998;41:351–65. [PMC free article] [PubMed] [Google Scholar]
- [27].de Andrade JR, Jove M, Landon G, et al. Baseline hemoglobin as a predictor of risk of transfusion and response to Epoetin alfa in orthopedic surgery patients. Am J Orthop (Belle Mead NJ) 1996;25:533–42. [PubMed] [Google Scholar]
- [28].Garcia-Erce JA, Cuenca J, Haman-Alcober S, et al. Efficacy of preoperative recombinant human erythropoietin administration for reducing transfusion requirements in patients undergoing surgery for hip fracture repair. An observational cohort study. Vox Sang 2009;97:260–7. [DOI] [PubMed] [Google Scholar]
- [29].Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ 2014;349(aug12 8):g4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Duncan CM, Gillette BP, Jacob AK, et al. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty 2015;30:272–6. [DOI] [PubMed] [Google Scholar]
- [31].Schnettler T, Papillon N, Rees H. Use of a tourniquet in total knee arthroplasty causes a paradoxical increase in total blood loss. J Bone Joint Surg Am 2017;99:1331–6. [DOI] [PubMed] [Google Scholar]


