Abstract
Background:
This study aimed to explore the efficacy and safety of recombinant human erythropoietin (RHE) for the treatment of severe traumatic brain injury (STBI).
Methods:
One hundred and twenty eligible patients with STBI were randomly divided into an intervention group or a control group equally. Patients in the intervention group received RHE. The participants in the control group received 0.9% saline. The outcome measurements included the Glasgow Outcome Scale (GOS) scores, mortality, and any adverse events.
Results:
At the end of 10-week follow-up after treatment, RHE neither showed greater efficacy in GOS scores (1–2, P = .43; 3–4, P = .25; 5–6, P = .58; 7–8, P = .23), nor the lower mortality in the intervention group than those in the control group (P = .47). In addition, both groups had similar safety profile.
Conclusion:
This study found that RHE did not improve the neurological outcomes in patients with STBI.
Keywords: efficacy, recombinant human erythropoietin, safety, traumatic brain injury
1. Introduction
Traumatic brain injury (TBI) is often associated with substantial mortality and lifelong disability, especially in patients with severe TBI (STBI).[1–3] It has been reported that about 30% of STBI patients die from this condition, and 50% of them become moderately disabled.[4–7] Although its treatment involves intensive care and guidelines of standard care, its morbidity and mortality remain high.
The management of STBI remains a challenge for practitioners, because few pharmacological and surgical interventions can reverse the primary brain damage that occurs following STBI.[8–10] The most important intervention for treating patients with STBI in the early stage is to protect the nerve cells of the brain from secondary injury from ischemia and hypoxia. It has been reported that recombinant human erythropoietin (RHE) can promote neurogenesis and angiogenesis following brain injury in animal studies.[11–13] Previous study has focused on patients with STBI, and found that RHE enhances functional recovery.[14] However, a study conducted in Australia reported that erythropoietin does not improve patients’ neurological status or the incidence of deep venous thrombosis of the lower limbs.[15]
The inconsistent results in the existing randomized controlled trials have tested the efficacy of RHE in patients with STBI. Thus, this trial further compares the efficacy and safety of RHE to a placebo in patients with STBI in China. We tested the hypothesis that RHE would improve neurological outcomes in patients with STBI.
2. Methods
This study was approved by the Medical Ethical Committee of The People's Hospital of Yan’an and was conducted at this hospital from April 2013 to March 2016. One hundred and twenty patients with STBI were included and were randomly divided into an intervention group or a control group equally. The severity of each participant was evaluated by the Acute Physiology and Chronic Health Evaluation (APACHE II),[16] in which higher values indicate more severe TBI.[16]
In this study, all patients with STBI were diagnosed and confirmed according to the patient's history, clinical examination, computerized tomography (CT) or magnetic resonance imaging (MRI) scan, and a Glasgow Coma Scale (GCS) score of <8. Additionally, patients also met the following inclusion criteria: <24 hours since brain injury; age 18 to 70 years; and ability to provide signed informed consent from either themselves or their closest family member. Patients were excluded if they had other major organ injuries or a history of thromboembolic events such as deep vein thrombosis, pulmonary embolism, and other conditions; died in the first 48 hours; pregnancy; or received erythropoientin within 1 month before the present study.
All participants were randomly assigned to an intervention group or a control group at 1:1 ratio by the stratified block randomization method with a computer-generated randomization schedule (generated by Software SAS, version 8.3, SAS Institute, Cary, NC). All investigators, patients, outcome assessors, and data analyst were blind to the treatment allocation.
All patients received either 6000 IU REH (E-Hua Biotech, Shandong, China) or placebo (0.9% saline) by a subcutaneous injection within 2 hours of admission. This schedule was also adhered to on 3rd, 5th, 10th, and 15 days after admission.
The primary outcome was neurological outcome, as measured by Glasgow Outcome Scale (GOS) scores. The secondary outcomes were measured by mortality and adverse events (AEs). All outcome measurements were evaluated at 10 weeks post-treatment.
The required sample size for the present study was estimated to be 60 patients in each group, with α = 0.05 (2-sided) and β = 0.20. All outcome data were analyzed by an intention to treat approach. Chi-square tests and t tests were used to analyze the categorical and the continuous data, respectively, with 95% confidence intervals. A P value <.05 was considered to have a significant statistical difference.
3. Results
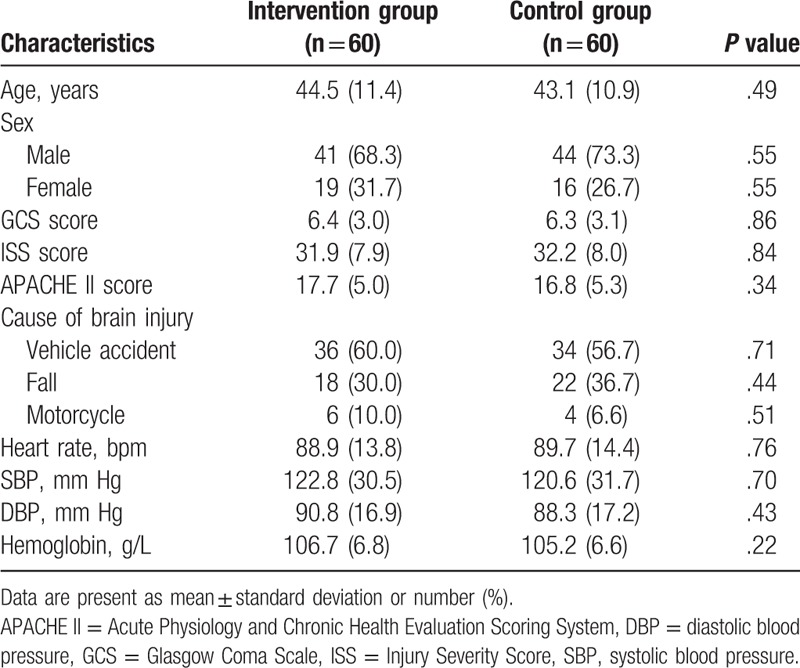
A total of 187 patients with STBI were initially screened for inclusion in the study (Fig. 1). Sixty-seven patients were excluded because they did not meet the inclusion criteria (n = 43), or declined to enroll in the study (n = 24). Thus, 120 patients were included. Of those, 17 patients withdrew from the study because of the lacking of follow-up (n = 5), consent withdrawal (n = 4), or death (n = 8) (Fig. 1). The baseline characteristics of all patients in both groups are listed in Table 1. There were no significant differences of all baseline characteristics were found between the 2 groups (Table 1).
Figure 1.
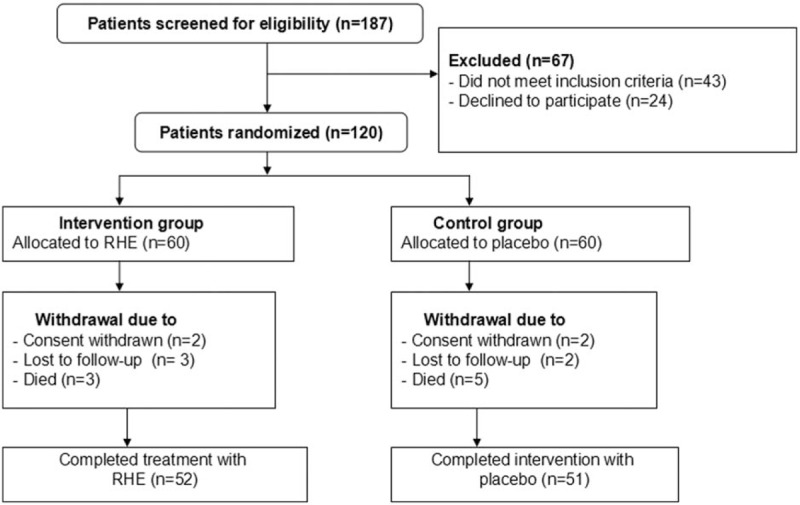
Flowchart of patients selection.
Table 1.
Patients characteristics at baseline.
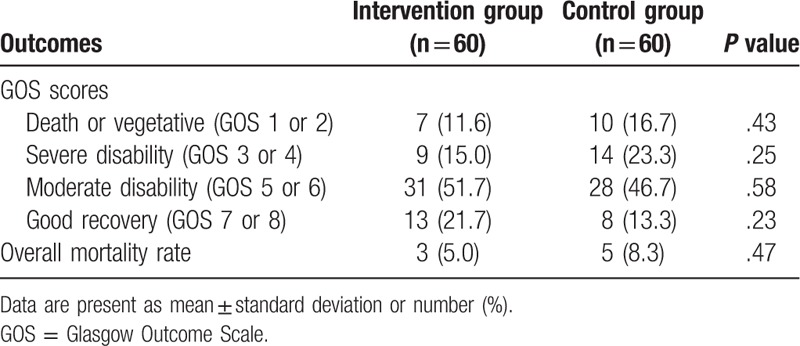
The outcome measurements are listed in Table 2. There were no significant differences in GOS scores between the 2 groups at the 10-week follow-up (GOS; 1–2, P = .43; 3–4, P = .25; 5–6, P = .58; 7–8, P = .23). In addition, no significant difference was found in terms of mortality at the 10-week follow-up (P = .47, Table 2).
Table 2.
Outcome measurements in both groups at 10 weeks after the treatment.
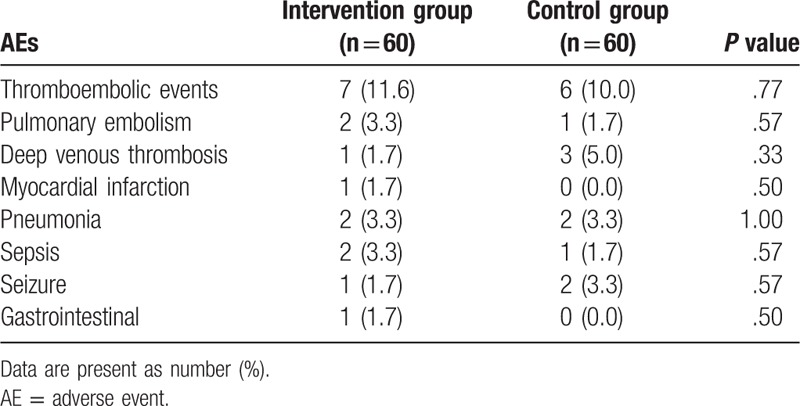
The AEs in both groups are listed in Table 3. No significant differences in any kinds of AEs were found between the 2 groups at the 10-week follow-up. The most common AEs were thromboembolic events, with 7 (11.6%) in the intervention group and 6 (10.0%) in the control group (Table 3).
Table 3.
AEs at 10-week follow-up after the treatment.
4. Discussion
Previous studies have reported that erythropoietin can be used to treat patients with TBI.[14–15] Li et al[14] investigated the short-term effect of RHE for treating patients with STBI. They found that RHE can improve patients’ functional recovery, and does not increase the risk for thromboembolic events or severe infections. Conversely, Nichol et al[15] also explored the effect of erythropoietin on the neurological recovery, mortality, and venous thrombotic events in patients with TBI, and found that erythropoietin does not decrease the number of patients with severe neurological dysfunction, and also does not increase venous thrombosis in lower limbs. Moreover, the effect of erythropoietin on mortality remains unclear. The findings of the present study are partly consistent with the Nichol's study in showing that short-term intervention with RHE does not improve neurological function for patients with STBI. The present study also did not find that RHE can reduce mortality for patients with STBI.
In this study, the patients with STBI in the intervention group did not show significantly higher neurological function improvement, as measured by GOS scores (P >.05) or lower mortality (P = .47) than that observed in patients in the control group, suggesting that RHE neither enhances neurological function, nor decreases mortality in patients with STBI during the period of the treatment and 10-week follow-up.
This study has several limitations. First, it was conducted at a single hospital, which may limit the generalization of our findings to other hospitals. Second, differences in the severity of the clinical symptoms of patients with STBI may also have affected the outcomes evaluation, although they did not have significant differences in the injury severity scores. Third, the injured area of brain and the psychiatric history may have also affected outcome measurements. Finally, this study only evaluated the effect and safety of RHE for treating patients with STBI in the short term. Thus, future clinical trials with longer intervention and follow-up periods are still needed.
5. Conclusion
The present study demonstrated that RHE neither enhanced neurological outcomes, nor reduced mortality for patients with STBI in the short term.
Footnotes
Abbreviations: AEs = adverse events, APACHE II = Acute Physiology and Chronic Health Evaluation, CT = computerized tomography, DBP= diastolic blood pressure, GCS = Glasgow Coma Scale, ISS = Injury Severity Score, MRI = magnetic resonance imaging, RHE = recombinant human erythropoietin, SBP = systolic blood pressure, STBI = severe traumatic brain injury, TBI = traumatic brain injury.
The authors have no conflicts of interest to disclose.
References
- [1].Han J, Yang S, Zhang C, et al. Impact of intracranial pressure monitoring on prognosis of patients with severe traumatic brain injury: a PRISMA systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jang SH, Park SM, Kwon HG. Relation between injury of the periaqueductal gray and central pain in patients with mild traumatic brain injury: observational study. Medicine (Baltimore) 2016;95:e4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Abou-Abbass H, Bahmad H, Ghandour H, et al. Epidemiology and clinical characteristics of traumatic brain injury in Lebanon: a systematic review. Medicine (Baltimore) 2016;95:e5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Myburgh JA, Cooper DJ, Finfer SR, et al. Epidemiology and 12-month outcomes from traumatic brain injury in Australia and New Zealand. J Trauma 2008;64:854–62. [DOI] [PubMed] [Google Scholar]
- [5].Hilario A, Ramos A, Millan JM, et al. Severe traumatic head injury: prognostic value of brain stem injuries detected at MRI. Am J Neuroradiol 2012;33:1925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mannion RJ, Cross J, Bradley P, et al. Hutchinson, Mechanism-based MRI classification of traumatic brainstem injury and its relationship to outcome. J Neurotrauma 2007;24:128–35. [DOI] [PubMed] [Google Scholar]
- [7].Thornhill S, Teasdale GM, Murray GD, et al. Disability in young people and adults one year after head injury: prospective cohort study. BMJ 2000;320:1631–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sashika H, Takada K, Kikuchi N. Rehabilitation needs and participation restriction in patients with cognitive disorder in the chronic phase of traumatic brain injury. Medicine (Baltimore) 2017;96:e5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang Y, Li JP, Song YL, et al. Intensive insulin therapy for preventing postoperative infection in patients with traumatic brain injury: a randomized controlled trial. Medicine (Baltimore) 2017;96:e6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang R, Li M, Gao WW, et al. Outcomes of early decompressive craniectomy versus conventional medical management after severe traumatic brain injury: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Juul S. Neuroprotective role of erythropoietin in neonates. J Matern Fetal Neonatal Med 2012;25:105–7. [DOI] [PubMed] [Google Scholar]
- [12].Kumral A, Tuzun F, Oner MG, et al. Erythropoietin in neonatal brain protection: the past, the present and the future. Brain Dev 2011;33:632–43. [DOI] [PubMed] [Google Scholar]
- [13].Wng L, Wang X, Su H, et al. Recombinant human erythropoietin improves the neurofunctional recovery of rats following traumatic brain injury via an increase in circulating endothelial progenitor cells. Translat Stroke Res 2015;6:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li ZM, Xiao YL, Zhu JX, et al. Recombinant human erythropoietin improves functional recovery in patients with severe traumatic brain injury: a randomized, double blind and controlled clinical trial. Clin Neurol Neurosurg 2016;150:80–3. [DOI] [PubMed] [Google Scholar]
- [15].Nichol A, French C, Little L, et al. Erythropoietin in traumatic brain injury (EPO-TBI): a double-blind randomised controlled trial. Lancet 2015;386:2499–506. [DOI] [PubMed] [Google Scholar]
- [16].Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]


