Supplemental Digital Content is available in the text
Keywords: cadmium, meta-analysis, systematic review, urolithiasis
Abstract
Background:
We performed a meta-analysis to determine whether a consistent relationship exists between cadmium exposure and urolithiasis in humans. Accordingly, we summarized and reviewed previously published quantitative studies.
Methods:
Eligible studies with reference lists published before June 1, 2017 were obtained from searching several databases. Random effects models were used to summary the overall estimate of the multivariate adjusted odds ratios (ORs) with 95% confidence intervals (CIs).
Results:
Six observational studies involving 88,045 participants were identified and stratified into the following categories according to cadmium assessment results: occupational (n = 4) and dietary (n = 2). The findings of the meta-analysis suggested that the risk of urolithiasis increases significantly by 1.32 times at higher cadmium exposure (OR = 1.32; 95% CI = 1.08–1.62; for highest vs lowest category urinary cadmium values). The summary OR in occupational exposure (OR = 1.56; 95% CI = 1.13–2.14) increased at the same condition. Meanwhile, no association was observed between cadmium exposure and urolithiasis risk in dietary exposure (OR = 1.13; 95% CI = 0.87–1.47). A significant association remained consistent, as indicated by subgroup analyses and sensitivity analyses.
Conclusions:
The meta-analysis indicated that increased risk of urolithiasis is associated with high cadmium exposure, and this association is higher in occupational exposure than in dietary exposure. Nevertheless, well-designed observational studies with different ethnic populations are still needed.
1. Introduction
Urolithiasis has recently attracted considerable attention worldwide because of its increasing morbidity and recurrence rates, seriously affecting the quality of life of affected individuals and increasing the economic burden on societies globally.[1,2] In China, 7.54% of the general population is at risk of developing urolithiasis; in Western countries, the risk ranges from 0.1% to 14.8%.[3–5] However, the prevalence of urolithiasis, including renal colic, urinary tract infection, and decreased renal function, has doubled in men (1988–1994: 6.3%; 2000–2010: 10.3%) and women (1988–1994: 4.1%; 2000–2010: 7.1%) in the United States over the past 15 years.[6,7] Moreover, economic burden caused by urolithiasis accounted for $2.1 billion of medical expenses in the United States alone in 2000.[8]
In the public health context, cadmium is a widespread occupational and environmental contaminant with known toxic effects, including fracture and prostate cancer. Thus, cadmium exposure is of concern,[9,10] especially that occupational workers employed in alloy and battery manufacturing and metal smelting industries are exposed to high cadmium levels.[11,12] Meanwhile, the general population is exposed to cadmium in environments containing cadmium generated by several industries. Other possible sources of chronic cadmium toxicity are cigarette smoking and food (eg, bread, cereals, and vegetables).[13,14]
Studies have reported inconsistent results with regard to the association between urolithiasis risk and cadmium exposure. Particularly, some epidemiological studies reported elevated urolithiasis risk among the general and occupational populations,[15–18] whereas some studies reported null associations.[19,20] Meanwhile, to the best of our knowledge, no recent systematic review or meta-analysis has synthesized evidence from epidemiological studies regarding this association. Thus, we performed a meta-analysis on published studies to shed light on these inconsistent results and evaluate the association between cadmium exposure and urolithiasis risk.
2. Methods
2.1. Search strategies
Meta-analysis was performed in accordance with the Cochrane Collaboration criterion.[21] Moreover, we reported our meta-analysis on the basis of the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).[22] All analyses were based on previous published studies; thus, no ethical approval and patient consent are required.
Eligible studies published before June 1, 2017 were included. These studies investigated the association between cadmium exposure and urolithiasis risk. A literature search was performed on PubMed, EMBASE, Cochrane Library, Scopus, and China National Knowledge Infrastructure and relevant reports and reference lists were obtained. The search method was not restricted to regions, publication status, or languages, and various combinations of Medical Subject Headings (MeSH) and non-MeSH terms (ie, search terms) were used. For instance, “urolithiasis,” “kidney stone,” or “nephrolithiasis” was combined with “cadmium” (supplementary material). Manual search techniques were also employed for the identification of appropriate studies. The main search was completed independently by 2 investigators (ZG and JW). For studies with insufficient information, we contacted the primary authors to acquire and verify the data. Any discrepancy was resolved by consulting an investigator not involved in the initial procedure.
2.2. Eligibility criteria and study selection
The inclusion criteria were as follows: outcome was urolithiasis; the general or occupational populations were exposed to cadmium; study design included case-control, retrospective, and prospective cohorts, and cross-sectional studies; the odds ratio (OR), relative risk (RR), or hazard risk (HR) of urolithiasis related to cadmium exposure were reported, and crude HR, OR, or RR with corresponding 95% confidence intervals (CIs) were calculated. Any disagreements were resolved through discussion.
2.3. Data extraction and methodological quality assessment
Data from the included studies were extracted and summarized independently by the 2 of the authors. Disagreements were settled through discussions. The following data were extracted into a standardized evidence table: first author, publication year, country, study design, study period, participant characteristics (ie, mean age, sex distribution, and sample size), cadmium exposure type, and adjusted or non-adjusted covariates (OR, RR, and HR). For studies with insufficient information, the reviewers contacted the primary authors to acquire and verify the data.
The methodological qualities of the case-control and cohort studies were assessed through the original Newcastle-Ottawa scale (NOS),[23] which consists of 3 factors, namely, patient selection, comparability of the study groups, and assessment of outcome. A score of 0–9 (allocated as stars) was allocated to each study. All the included studies with eight or more stars were considered to be of high quality. The Agency for Healthcare Research and Quality (AHRQ) recommend 11 items for assessing the quality of cross-sectional studies. Disagreements were also settled through discussion.
2.4. Statistical analysis
We calculated the overall estimate to assess the association between cadmium exposure and urolithiasis risk in the general and occupational populations. For consistent definitions, OR with corresponding 95% CI was used as common measure in all studies because cadmium-caused urolithiasis was considered as a rare event. The HR and RR values in the observational studies were considered as approximations of OR. The aggregated results and 95% CIs for effect size were calculated through inverse-variance weighted random-effect meta-analysis. Then, we performed the I square (I2) test to assess heterogeneity across the studies, and I2 values of 0%, 25%, 50%, and 75% represented no, low, moderate, and high heterogeneity, respectively. Forest plots were visually inspected to assess heterogeneity. Furthermore, statistical significance was set at P < .05. Sensitivity analysis was conducted by examining the exclusion of each study in a step-wise manner for the evaluation of the quality and consistency of the results. Subgroup analyses were performed according to geographical region, study design, sex, and exposure type. A meta-regression analysis was conducted to investigate possible sources of heterogeneity on 5 variables. The restricted maximum likelihood method was used for the analysis. Nevertheless, the use of Egger's regression asymmetry test was limited because of the small number of studies evaluated.[21,24]
3. Results
3.1. Study characteristics and methodological quality
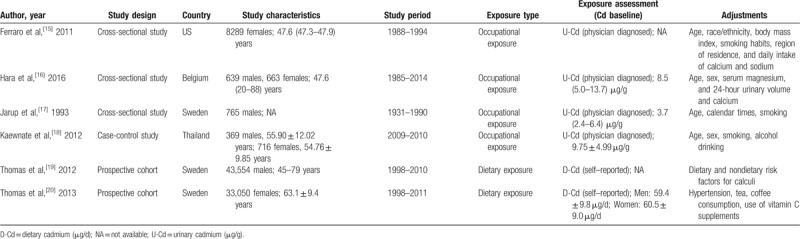
Figure 1 shows the flowchart depicting the search process and study selection, and Table 1 summarized the basic characteristics of the included studies. These studies (3 cross-sectional studies,[15–17] 2 cohort studies,[19,20] and 1 case-control study[18]) were published between 1993 and 2016, comprising 88,045 participants and sample sizes with the number of participants ranging between 765 and 43,554. Three studies were based in Sweden,[17,19,20] 1 in America,[15] 1 in Belgium,[16] and 1 in Thailand.[18] Two studies were designed to calculate OR,[15,18] 3 to calculate HR,[16,19,20] and 1 to calculate RR.[17] Two studies reported results for the association between cadmium exposure and urolithiasis risk in both males and females,[16,18] 2 investigated males,[17,19] and 2 investigated females.[15,20] Occupational cadmium exposure was measured with urine specimens among the included studies.[15–18] Moreover, daily cadmium intake was estimated by multiplying the frequency of consumption of each food type by its specific content by using age-specific portion sizes; the result was adjusted to the mean energy intake through the residual method.[20,21] Various confounding factors in urolithiasis, particularly age, ethnicity, body mass index, sex, and smoking, were adjusted in all studies; confounding factors were not comprehensively examined in 1 study.[19] The association between occupational cadmium exposure and urolithiasis risk was reported in four studies,[15–18] and dietary cadmium exposure was investigated in 2 studies.[19,20]
Figure 1.
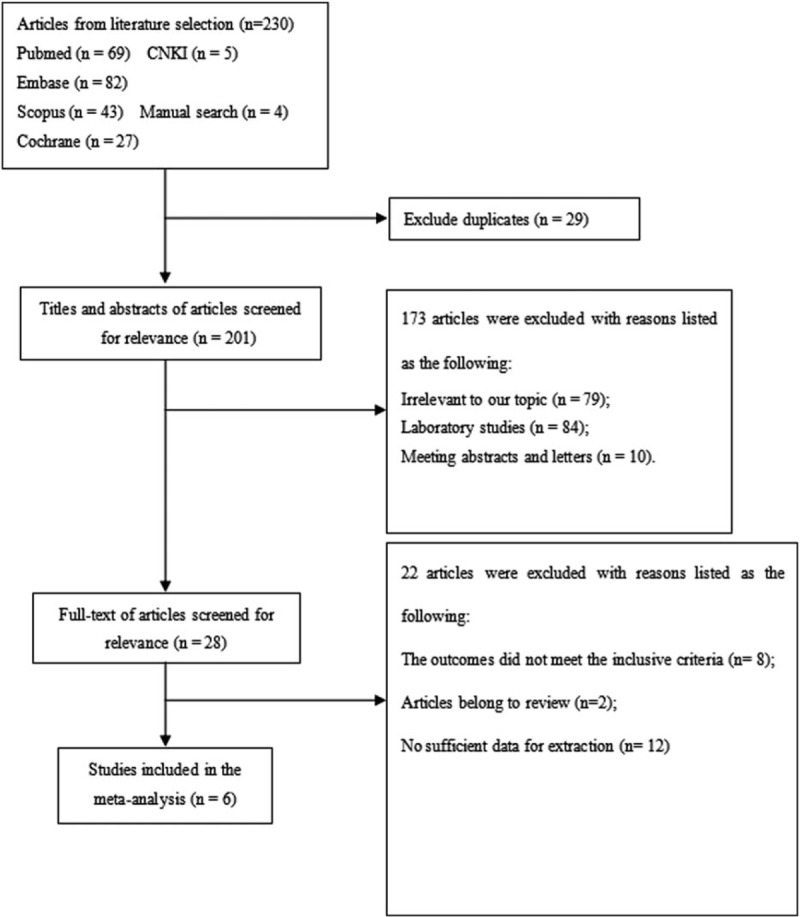
Flow diagram of study selection.
Table 1.
Characteristic of studies included in the meta–analysis.
Furthermore, the methodological quality of the 3 studies was considered to be of high quality,[15,16,20] and 3[17–19] were regarded to be of low quality on the basis of NOS and AHRQ. The main deficiency of low-quality study are as followings: Järup et al[17] did not indicate that study controls were comparable for age, sex, and all additional factors reported; Kaewnate et al[18] and Thomas et al[19] did not indicate that assessment of exposure was from a secure record or the nonresponse rate was similar in both groups.
3.2. Overall meta-analysis
The meta-analysis results indicated that a high cadmium exposure significantly increases urolithiasis risk by 1.32 times (OR = 1.32; 95% CI = 1.08–1.62; for highest vs lowest category of cadmium exposure), with moderate heterogeneity (I2 = 58.1%, P = .036; Fig. 2). The OR in occupational exposure (OR = 1.56; 95% CI = 1.13–2.14) was higher whereas cadmium exposure was not associated with urolithiasis risk in dietary exposure (OR = 1.13; 95% CI = 0.87–1.47; Fig. 3).
Figure 2.
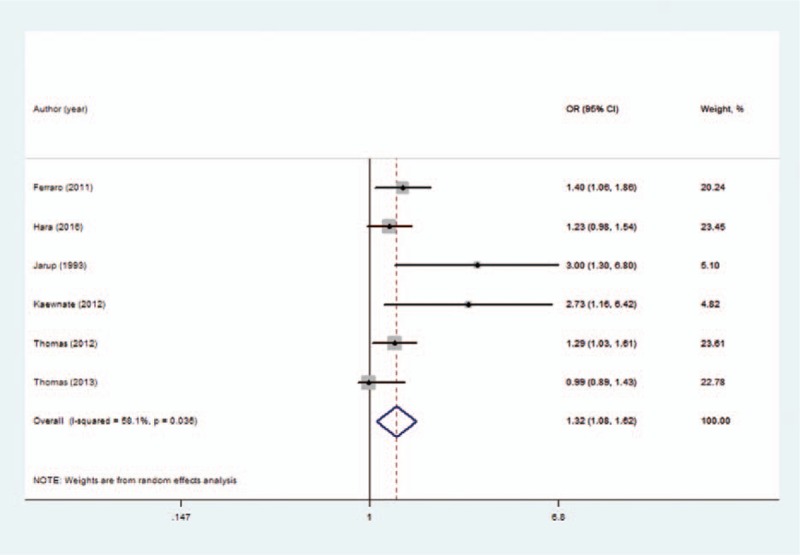
Forest plot depicting the risk estimates from included studies on the association between cadmium exposure and risk of urolithiasis.
Figure 3.
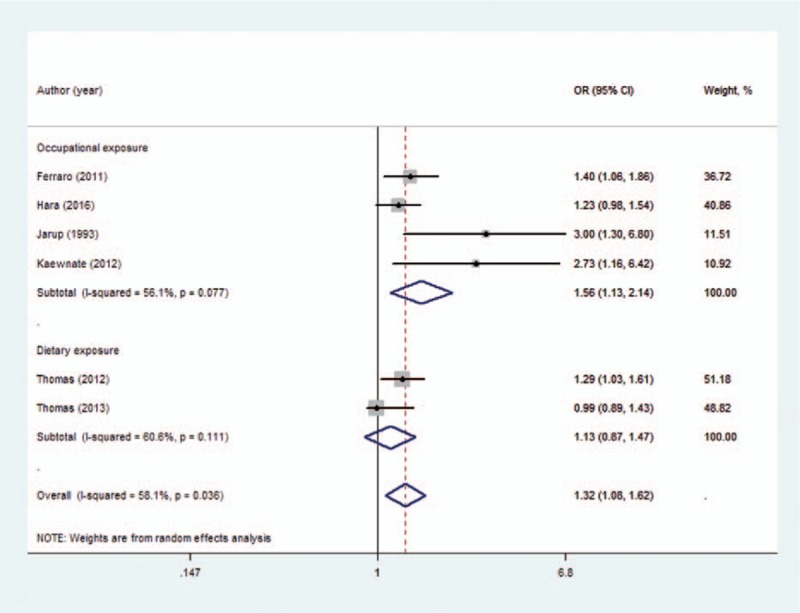
Forest plot depicting the risk estimates from included studies for different cadmium exposure groups.
3.3. Subgroup analysis
Subgroup analysis was conducted, and the results are shown in Table 2. A statistically significant association between cadmium exposure and urolithiasis risk was observed in the cross-sectional studies (OR = 1.43; 95% CI = 1.06–1.92) and case-control study (OR = 2.73; 95% CI = 1.16–6.42) but not in the cohort studies (OR = 1.13; 95% CI = 0.87–1.47). When the studies were stratified by different exposure types, the association among occupational exposure populations was significant (OR = 1.56; 95% CI = 1.13–2.14). However, the dietary exposure populations showed insufficient significance (OR = 1.13; 95% CI = 0.87–1.47). A significant association was observed among the studies performed in North America and Asia but not among those conducted in Europe. In addition, no significant association was observed among the studies with respect to sex. Moreover, a significant association was observed among low-quality studies but not in high-quality studies. However, all the subgroups had no considerable contributions to heterogeneity.
Table 2.
Results of overall subgroup analysis.
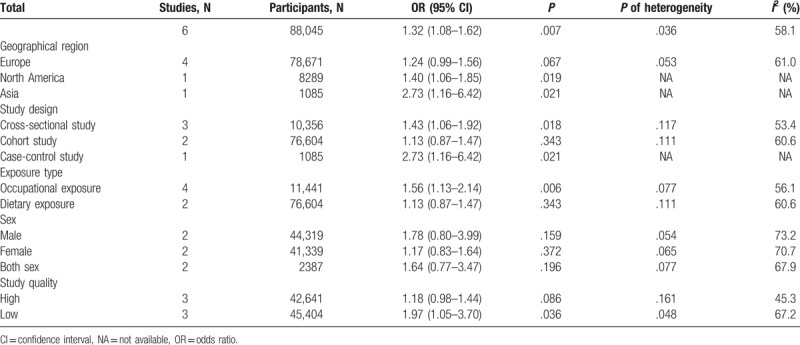
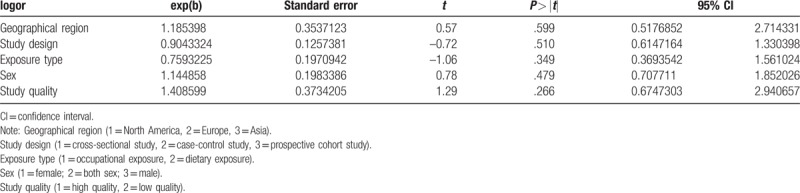
3.4. Meta-regression analysis
The results indicated that none of the covariates (P > .05) resulted in heterogeneity among 6 studies, and the adjusted R-square in the between-study variance was unavailable (Table 3).
Table 3.
Results of meta-regression.
3.5. Sensitivity analysis
We evaluated the effect of each study on the summary results by sequentially excluding a single study (Fig. 4). The omission of any single study did not prominently affect the overall combined OR, which ranged from 1.28 (95% CI = 1.09 –1.49) to 1.42 (95% CI = 1.08–1.86). The rationality and reliability of our meta-analysis was validated through sensitivity analysis.
Figure 4.
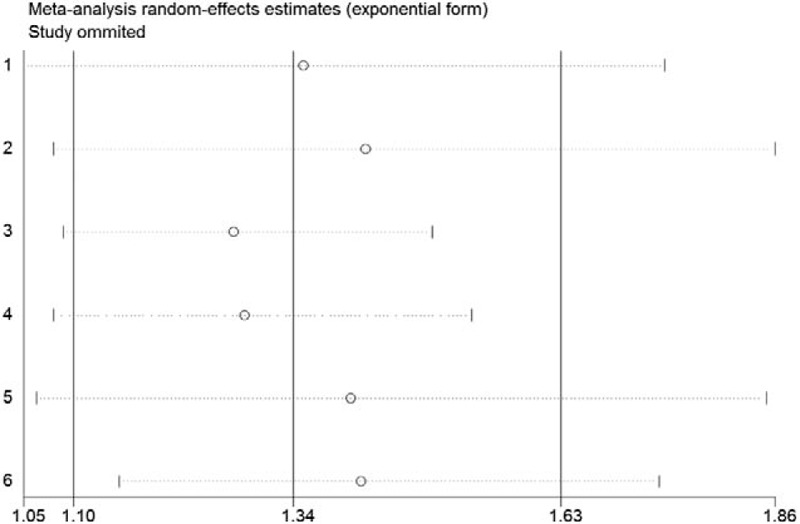
Plot showing the influence of excluding each individual study on the summary estimate on the association between cadmium exposure and risk of urolithiasis.
4. Discussion
The association between cadmium exposure and urolithiasis risk was analyzed through meta-analysis. As far as we know, this is the first study that provided comprehensive insights into this association through meta-analysis. Our main results indicated that cadmium exposure is significantly correlated with urolithiasis risk. The summary OR in occupational exposure was high, and cadmium exposure had no association with urolithiasis risk in dietary exposure. Notably, the subgroup and sensitivity analyses validated the reliability of our meta-analysis. However, publication bias was not performed because of the limited number of included studies.
Recently, studies on urolithiasis and its potential risk factors have been increasing, although the proposed preventive measures in these studies require further evaluation.[25,26] Urolithiasis is common in workers employed in alloy and battery manufacturing and metal smelting industries because they are frequently exposed to high levels of cadmium.[1,11,12] Patients with this disease suffer agonizing pain and substantial economic loss. Moreover, although urolithiasis is a multifactorial disease related primarily to several dietary and genetic factors, a high prevalence of urolithiasis has been detected in subjects occupationally exposed to cadmium.[27–29]
The mechanisms underlying the association between urolithiasis formation and cadmium exposure at levels observed in the current study must be explored further. Cadmium-related toxic effects may be elicited at exposure levels that are considerably lower than those previously reported. Some researches indicated that the effect of dietary cadmium exposure on increased bone resorption in the populations might translate into increased urinary calcium excretion.[30] Several mechanisms have been assumed to explain bone anomalies in relation to cadmium exposure, and these anomalies included direct toxic effects on renal tubular cells, particularly hypercalciuria and reduced intestinal calcium absorption.[31]
Determining whether these findings are applicable to different levels of cadmium exposure and geographical location is invaluable. Thus, the possible influence of differences in ethnicity on the association between urolithiasis and cadmium exposure must be explored. We found that house dust and contaminated soils were the important possible sources of exposure to cadmium.[32] Thus, the government should strengthen sanitization of contaminated soils after we showed association between urolithiasis and exposure to cadmium especially in the developing world. Moreover, the most important thing is reducing exposure to pollutants. Furthermore, nephrolithiasis is preventable by increasing diuresis and dietary measures.[33] It is within this context, that the clinical implications of our study should be gauged. Moreover, clinicians and other healthcare providers working with patients with urolithiasis should be aware of the risks and effects of cadmium exposure.[13,34]
In general, our study exhibited strengths in several aspects. First, this study was the first to explore a potential association between cadmium exposure and urolithiasis risk in general and occupational populations through meta-analysis. Second, the overall combined estimates were based on a large sample size, and thus the rationality and reliability of our meta-analysis results was apparently improved. Third, confounding factors that might influence cadmium exposure levels were minimized because multivariable-adjusted risk estimates were applied. All the results accurately reflected the association between cadmium exposure and urolithiasis risk and resulted in well-founded conclusions.
However, our study is limited in some aspects. First, despite our rigorous methodology, the number of studies included in the meta-analysis was limited, especially hospital-based studies and studies that used the same range of cadmium exposure level. This limitation may contribute to moderate heterogeneity. Second, to the best our knowledge, a case-control study is the most appropriate design for toxicity exposure (eg, occupational, dietary, or environmental) that causes rare health events. However, this design has some selective and recall bias. Third, confounding factors, such as coexposure to other toxic factors (eg, tobacco and alcohol consumption), were difficult to control in the meta-analysis. Fourth, the values of cadmium exposure were measured with urine specimens in occupational exposure. These values were also estimated by multiplying the frequency of consumption of each food type by its specific content by using age-specific portion sizes. The estimation was conducted through the residual method in dietary exposure. This approaches resulted in moderate heterogeneity. Finally, the included studies were only distributed in Europe, North America, and Asia. Therefore, further study should explore the association between cadmium exposure and urolithiasis risk among African and Caucasian populations. The dose-response relationship between cadmium exposure and urolithiasis risk was limited because of insufficient data from the included studies. Therefore, well-designed studies conducted among additional regions on other continents with different doses of cadmium exposure are necessary.
5. Conclusions
Our meta-analysis indicates that high cadmium exposure is associated with increased risk of urolithiasis in occupational populations. Hence, human studies in occupational and general populations should provide further information on the dose-effect and dose-response curves relating cadmium exposure to urolithiasis incidence, and potential biological mechanisms involved in this potential association must be explored.
Supplementary Material
Acknowledgment
The authors would like to thank KGSupport for editing the language of this study.
Footnotes
Abbreviations: CIs = confidence intervals, HR = hazard ratio, MeSH = Medical Subject Headings, NOS = Newcastle-Ottawa scale, OR = odds ratio, RR= risk ratio.
Author contributions: S.W. conceived the study idea. ZG and JW performed the literature search, study selection, and data extraction. LG performed the statistical analyses and interpretation of the corresponding results. The article was drafted by ZG and JW and finalized by SW, SG, and CG. SW, SG, and CG had the primary responsibility for the final content. All authors made critical comments for the initial article.
The authors declare no conflicts of interest.
This study was supported by the social development project of the Guangdong Provincial Department of Science and Technology with Grant No. 2014A020212501. Trial registration number: PROSPERO 2017: CRD 42017057872.
Supplemental Digital Content is available for this article.
References
- [1].Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol 2010;12:e86–96. [PMC free article] [PubMed] [Google Scholar]
- [2].Worcester EM, Coe FL. Clinical practice. Calcium kidney stones. N Engl J Med 2010;363:954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang W, Fan J, Huang G, et al. Prevalence of kidney stones in mainland China: a systematic review. Sci Rep 2017;7:41630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang PX, Li HT, Zhang L, et al. The clinical profile and prognosis of Chinese children with melamine-induced kidney disease: a systematic review and meta-analysis. Biomed Res Int 2013;2013:868202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pearle MS, Calhoun EA, Curhan GC. Urologic diseases in America project: urolithiasis. J Urol 2005;173:848–57. [DOI] [PubMed] [Google Scholar]
- [6].Kirkali Z, Rasooly R, Star RA, et al. Urinary stone disease: progress, status, and needs. Urology 2015;86:651–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Scales CD, Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol 2012;62:160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Parr JM, Desai D, Winkle D. Natural history and quality of life in patients with cystine urolithiasis: a single centre study. BJU Int 2015;116:31–5. [DOI] [PubMed] [Google Scholar]
- [9].Järup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 2009;238:201–8. [DOI] [PubMed] [Google Scholar]
- [10].Wu H, Liao Q, Chillrud SN, et al. Environmental exposure to cadmium: health risk assessment and its associations with hypertension and impaired kidney function. Sci Rep 2016;6:29989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Agency for Toxic Substances and Disease Registry. Cadmium and Cadmium Compounds. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. 2011:80. [Google Scholar]
- [12].Agency for Toxic Substances and Disease Registry. Potential for human exposure. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. 2011: 277-331. [Google Scholar]
- [13].Amzal B, Julin B, Vahter M, et al. Population toxicokinetic modeling of cadmium for health risk assessment. Environ Health Perspect 2009;117:1293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen C, Xun P, Nishijo M, et al. Cadmium exposure and risk of prostate cancer: a meta-analysis of cohort and case-control studies among the general and occupational populations. Sci Rep 2016;6:25814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ferraro PM, Bonello M, Frigo AC, et al. Cadmium exposure and kidney stone formation in the general population–-an analysis of the National Health and Nutrition Examination Survey III data. J Endourol 2011;25:875–80. [DOI] [PubMed] [Google Scholar]
- [16].Järup L, Elinder CG. Incidence of renal stones among cadmium exposed battery workers. Br J Ind Med 1993;50:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kaewnate Y, Niyomtam S, Tangvarasittichai O, et al. Association of elevated urinary cadmium with urinary stone, hypercalciuria and renal tubular dysfunction in the population of cadmium-contaminated area. Bull Environ Contam Toxicol 2012;89:1120–4. [DOI] [PubMed] [Google Scholar]
- [18].Thomas L, Elinder CG, Wolk A, et al. Dietary cadmium intake and urinary calculi incidence among men. Toxicol Lett 2012;211:S99. [Google Scholar]
- [19].Hara A, Yang WY, Petit T, et al. Incidence of nephrolithiasis in relation to environmental exposure to lead and cadmium in a population study. Environ Res 2016;145:1–8. [DOI] [PubMed] [Google Scholar]
- [20].Thomas LD, Elinder CG, Tiselius HG, et al. Dietary cadmium exposure and kidney stone incidence: a population-based prospective cohort study of men & women. Environ Int 2013;59:148–51. [DOI] [PubMed] [Google Scholar]
- [21].Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1. 0 [updated March 2011]. The Cochrane Collaboration. Available at: http://handbook.cochrane.org. 2011. [Google Scholar]
- [22].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [23].Hartling L, Milne A, Hamm MP, et al. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J Clin Epidemiol 2013;66:982–93. [DOI] [PubMed] [Google Scholar]
- [24].Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cappuccio FP, Kalaitzidis R, Duneclift S, et al. Unravelling the links between calcium excretion, salt intake, hypertension, kidney stones, and bone metabolism. J Nephrol 2000;13:169–77. [PubMed] [Google Scholar]
- [26].Edwards JR, Prozialeck WC. Cadmium diabetes and chronic kidney disease. Toxicol Appl Pharmacol 2009;238:289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Friberg L. Health hazards in the manufacture of alkaline accumulators with special reference to chronic cadmium poisoning; a clinical and experimental study. Acta Med Scand Suppl 1950;240:1–24. [PubMed] [Google Scholar]
- [28].Elinder CG, Edling C, Lindberg E, et al. Assessment of renal function in workers previously exposed to cadmium. Br J Ind Med 1985;42:754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Adams RG, Harrison JF, Scott P. The development of cadmium-induced proteinuria, impaired renal function, and osteomalacia in alkaline battery workers. Q J Med 1969;38:425–43. [PubMed] [Google Scholar]
- [30].Navas-Acien A, Tellez-Plaza M, Guallar E, et al. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol 2009;170:1156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shinichi S, Kiyoshi N. Effects of vitamin D on calcium and bone metabolism. Clin Calcium 2003;13:863–8. [PubMed] [Google Scholar]
- [32].Hogervorst J, Plusquin M, Vangronsveld J, et al. House dust as possible route of environmental exposure to cadmium and lead in the adult general population. Environ Res 2007;103:30–7. [DOI] [PubMed] [Google Scholar]
- [33].Lotan Y, Buendia Jiménez I, Lenoir-Wijnkoop I, et al. Primary prevention of nephrolithiasis is cost-effective for a national healthcare system. BJU Int 2012;110:1060–7. [DOI] [PubMed] [Google Scholar]
- [34].Akesson A, Lundh T, Vahter MA, et al. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect 2005;113:1627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


