Abstract
This study aimed to explore the efficacy and toxicity of image-guided stereotactic body radiotherapy (IGSBR) by helical tomotherapy in patients with lung cancer among Chinese Han population.
A total of 21 patients with stage I lung cancer were included. They received a total of 60 Gy factions IGSBR. The outcomes included complete response (CR), partial response (PR), stable disease (SD), progress disease (PD), overall response rate (ORR), and overall survival (OS). In addition, toxicities were also recorded in this study.
Three-year CR, PR, SD, PD, ORR, and OS were 47.6%, 38.1%, 9.5%, 4.8%, 85.7%, and 48.0 months, respectively. Additionally, mild toxicities were found in this study.
This study demonstrated that IGSBR is efficacious for patients with stage I lung cancer with mild toxicities among Chinese Han population.
Keywords: efficacy, lung cancer, stereotactic body radiotherapy, toxicity
1. Introduction
Lung cancer is one of the most common causes of cancer-related death in the world.[1,2] Its early detection has been improved by using the examinations of computed tomography, and positron emission tomography.[3] It has been reported that patients with lung cancer at early stage after the surgery often have encouraging effect with more than 90% local control rate, and 50% of 5-year survival.[4] However, some patients are not available to have surgery, and they receive the management with radiotherapy.[5–7] Previous studies have reported that conventional radiotherapy cannot control the primary lung cancer in more than 60% patient's population.[8] In addition, more than 50% patients died from its progression ultimately with such kind of management.[8]
Image-guided stereotactic body radiotherapy (IGSBR) is an advanced radiotherapy based on the conventional radiotherapy.[9–13] It utilized advanced imaging techniques to deliver a targeted radiation dose to a tumor with a high degree of precision and steep dose gradients.[9–10] Additionally, it can also minimize the dose to normal tissues, which can help preserve the healthy tissue for patients with lung cancer. It has been reported that IGSBR for the treatment of lung cancer achieved excellent effect of local control rates for primary tumors, and metastatic malignancies.[14–17]
Presently, limited data of IGSBR for the treatment of patients in lung cancer at the early stage among Chinese population are available. In this study, we retrospectively analyzed the efficacy and toxicity of IGSBR in patients with lung cancer at the early stage among the Chinese population.
2. Patients and methods
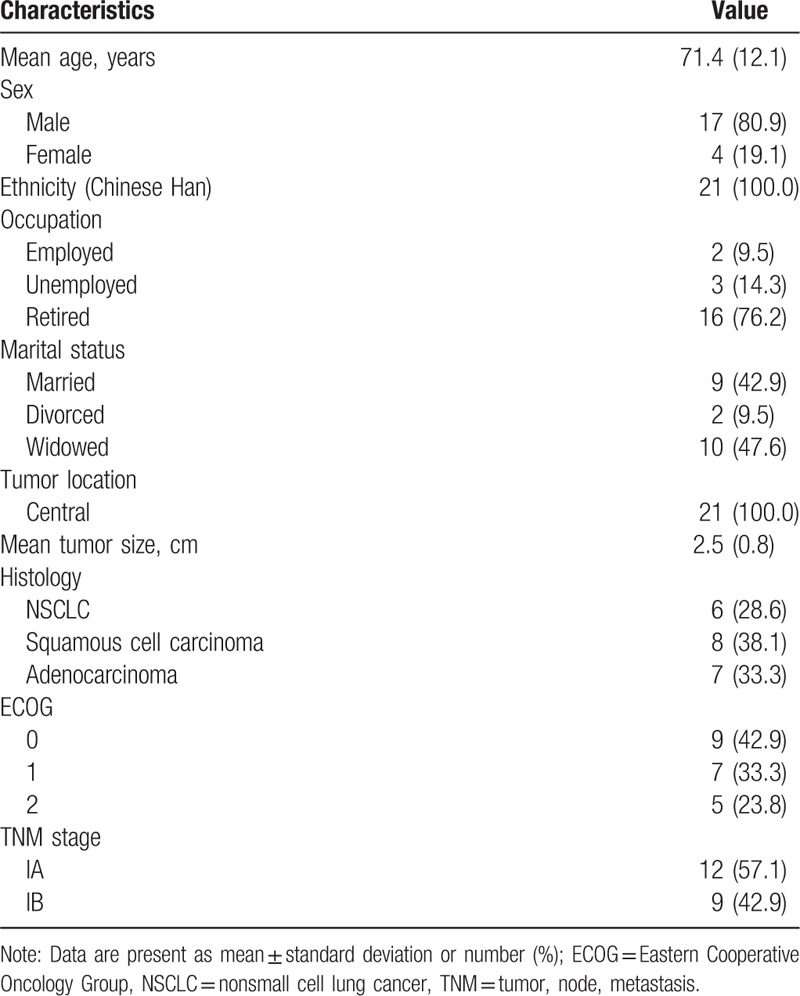
In this retrospective study, 21 patients with the diagnosis of stage I lung cancer by computed tomography, or positron emission tomography were included, and were treated with IGSBR by helical tomotherapy. It was formally approved by the Medical Ethical Committee of The Affiliated Hongqi Hospital of Mudanjiang Medical University, and the informed consent was obtained from all patients. It was conducted at The Affiliated Hongqi Hospital of Mudanjiang Medical University from January 2013 to December 2015. The clinical characteristics are shown in Table 1. All patients were unavailable or declined to receive surgery. All patients aged from 40 to 82 years, with median age of 69 years. The mean tumor size was 2.5 ± 0.8 cm. All patients had central tumors. The histology included nonsmall cell lung cancer, squamous cell carcinoma, and adenocarcinoma. The status of Eastern Cooperative Oncology Group ranged from 0 to 2. The tumor, node, metastasis stage consisted of IA and IB.
Table 1.
Characteristics of included patients.
Before the IGSBR treatment, all patients were positioned with supine to make sure that they were in a stereotactic body frame. The IGSBR was performed by using Elekta Synergy S linear machine (Elekta, Stockholm, Sweden). Real-time tumor tracking was conducted to track the tumors motion, and other internal organs. The slices thickness was 3 mm. The target lesion was delineated as the gross tumor volume (GTV) on the planning computed tomography slices using a standardized computed tomography lung level setting. It was equal to the clinical planning target volume. If it is necessary, 18F-fluorodeoxyglucose positron-emission tomography/x-ray computed tomography was also used as optional. The attacked tumor regions were scanned daily. After that, the planning computed tomography scans and contours of each patient were transferred by Elekta x-ray volumetric imaging (XVI) software for treatment planning. The dose of risk-adapted fractionation scheme was applied. 60 Gy/10 fractions were delivered once daily, 5 days weekly.
In this study, all included patients were monitored and recorded daily for the acute and late treatment related toxicity. The standard of Response Evaluation Criteria in Solid Tumors 1.1 was used to measure each tumor size. In addition, complete response (CR) was defined as the total tumor disappearance. Partial response (PR) was defined as a ≥30%decrease in the longest diameter of tumors. Stable disease (SD) was defined as a <30% decrease or a ≤20% increase of the longest tumor diameter. Progress disease (PD) was defined as a >20% increase in the longest tumor diameter, or with the new lesion. Overall response rate consisted of CR and PR. Overall survival (OS) was calculated at the beginning of IGSBR applied to the date of death with any reasons. OS were evaluated by the Kaplan–Meier method. Toxicities were assessed by using the Common Toxicity Criteria for Adverse Events (V4.0). The toxicity was defined from the first day to the 90th day from the beginning of the IGSBR, and late toxicity was recorded after the 90th day. The log-rank test was conducted to perform univariate analysis. All data were analyzed by using SPSS software (SPSS Version 17.0, IBM Corp., Armonk, NY).
3. Results
The CR, PR, CD, PD, and ORR of the 3-year follow-up were 47.6%, 38.1%, 9.5%, 4.8%, and 85.7%, respectively (Table 2). The median OS was 48.0 months (95% confidence interval: 36.9–59.1 months) (Fig. 1).
Table 2.
Response rate of all included patients.

Figure 1.
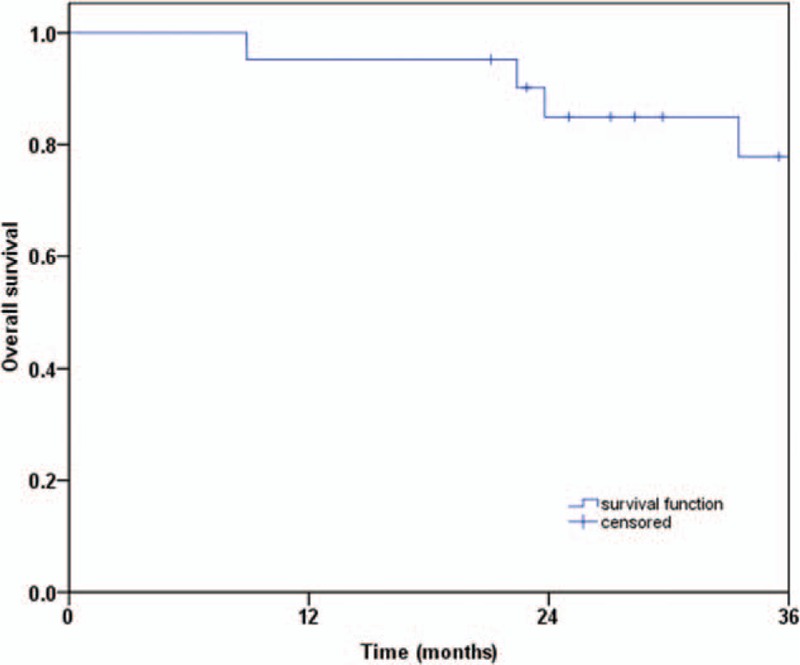
Overall survival.
The toxicity-related-treatment is shown in Table 3. The total toxicities were mild in this study, and all occurred in grade 1 and 2. No grade 3 or higher toxicities were recorded in this study. As for acute toxicities, five (23.8%) patients were found to have acute fatigue; 3 patients had dyspnea (14.3%); and 2 patients occurred radiation esophagitis (9.5%), and pain (9.5%). As for late toxicities, 7 (33.4%) patients were recorded pneumonitis; and 1 (4.8%) patient was found chest pain. No patients were reported for rib fractures, as well as hematololgy toxicity.
Table 3.
Toxicities after IGSBR treatment.

4. Discussion
Previous studies have explored the efficacy and safety of IGSBR in patients with lung cancer, and achieved promising outcome results. Two studies assessed the toxicity and efficacy of IGSBR in a high-risk population of patients with early stage but medically inoperable lung cancer (IOLC).[18,19] One concluded that patients with nonsmall cell IOLC received SBRT had a survival rate of 55.8% at 3 years.[18] In addition, it also had high rates of local tumor control, and moderate treatment-related morbidity. The results of the other one demonstrated that IGSBR allows for real-time tumor tracking and also the risk-adapted fractionation achieves satisfactory local control and low toxicity rates in patients with IOLC at early stage.[19] The other study compared the effects between central and peripheral stage I lung cancer using IGSBR by helical tomotherapy.[20] It is found that helical IGSBR for the treatment of central stage I lung cancer is safe and effective, when it is compared with the patients in peripheral stage I lung cancer.[20] The another study analyzed the inter- and intrafractional changes in tumor volume with respect to both spatial and volumetric parameters among patients treated by IGSBR for lung cancer.[21] Its results demonstrated that interfractional IGSBR is necessary for lung cancer.
The results of our study are consistent with the previous studies.[18,20] Our study found that the efficacy and safe of image guidance radiotherapy via helical tomotherapy in patients with stage I lung cancer is encouraging. The ORR of 3-year follow-up was 85.7%. In addition, the median OS was 48.0 months. The total toxicities were mild without sever toxicities. The most frequencies toxicities were acute fatigue (23.8%), and late pneumonitis (33.4%).
This study has 3 limitations. First, this study had a relative small number of patients with lung cancer, which may be affect the results of this study. Second, this study only explored the central lung cancer, thus patients with peripheral lung cancer still need to be explored. Third, all included patients are ethnicity of Chinese Han. Thus, the efficacy and safety of IGSBR in patients of other ethnicities of Chinese population should also be focused in the future.
5. Conclusion
The results of this study demonstrated that IGSBR is effective for patients with stage I lung cancer with mild toxicities among Chinese Han population.
Footnotes
Abbreviations: CR = complete response, GTV = gross tumor volume, IGSBR = image-guided stereotactic body radiotherapy, IOLC = inoperable lung cancer, ORR = overall response rate, OS = overall survival, PD = progress disease, PR = partial response, SD = stable disease, XVI = x-ray volumetric imaging.
The authors have no conflicts of interest to disclose.
References
- [1].Yan X, Chen X, Li G, et al. Two-portal versus three-port video-assist thoracoscopic surgery for early stage nonsmall cell lung cancer: a retrospective study. Medicine (Baltimore) 2017;96:e7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cao CL, Duan PY, Zhang WJ, et al. Acute pancreatitis induced by etoposide–lobaplatin combination chemotherapy used for the treatment of lung cancer: a case report and literature review. Medicine (Baltimore) 2017;96:e7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen X, Xu Y, Duan J, et al. Correlation of iodine uptake and perfusion parameters between dual-energy CT imaging and first-pass dual-input perfusion CT in lung cancer. Medicine (Baltimore) 2017;96:e7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg 2009;15:4–9. [PubMed] [Google Scholar]
- [5].Falkson CB, Vella ET, Yu E, et al. Radiotherapy with curative intent in patients with early-stage, medically inoperable, non-small-cell lung cancer: a systematic review. Clin Lung Cancer 2017;18:105–21. [DOI] [PubMed] [Google Scholar]
- [6].Falkson CB, Vella ET, Yu E, et al. Guideline for radiotherapy with curative intent in patients with early-stage medically inoperable non-small-cell lung cancer. Curr Oncol 2017;24:e44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Frelinghuysen M, Fest J, Van der Voort Van Zyp NC, et al. Consequences of referral time and volume doubling time in inoperable patients with early stage lung cancer. Clin Lung Cancer 2017;18:e403–9. [DOI] [PubMed] [Google Scholar]
- [8].McGarry RC, Song G, des Rosiers P, et al. Observation-only management of early stage, medically inoperable lung cancer: poor outcome. Chest 2002;121:1155–8. [DOI] [PubMed] [Google Scholar]
- [9].Wang P, Zhang D, Guo XG, et al. A propensity-matched analysis of surgery and stereotactic body radiotherapy for early stage non-small cell lung cancer in the elderly. Medicine (Baltimore) 2016;95:e5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nguyen NP, Godinez J, Shen W, et al. Is surgery indicated for elderly patients with early stage nonsmall cell lung cancer, in the era of stereotactic body radiotherapy? Medicine (Baltimore) 2016;95:e5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Okabe T, Kimura T, Nagata Y. Stereotactic body radiotherapy. Gan To Kagaku Ryoho 2014;41:2543–5. [PubMed] [Google Scholar]
- [12].Tree AC, Khoo VS, Eeles RA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol 2013;14:e28–37. [DOI] [PubMed] [Google Scholar]
- [13].Tree AC, Ostler P, Hoskin P, et al. Prostate stereotactic body radiotherapy—first UK experience. Clin Oncol (R Coll Radiol) 2014;26:757–61. [DOI] [PubMed] [Google Scholar]
- [14].Higgins KA, Pillai RN, Chen Z, et al. Concomitant chemotherapy and radiotherapy with SBRT Boost for Unresectable, Stage III Non-small Cell Lung Cancer: a phase I Study. J Thorac Oncol 2017;12:1687–95. [DOI] [PubMed] [Google Scholar]
- [15].Hörner-Rieber J, Bernhardt D, Dern J, et al. Histology of non-small cell lung cancer predicts the response to stereotactic body radiotherapy. Radiother Oncol 2017;125:317–24. [DOI] [PubMed] [Google Scholar]
- [16].Tsurugai Y, Takeda A, Sanuki N, et al. Stereotactic body radiotherapy for lung cancer patients with idiopathic interstitial pneumonias. Radiother Oncol 2017;125:310–6. [DOI] [PubMed] [Google Scholar]
- [17].Flores RM. Lung cancer randomized controlled trials should compare stereotactic body radiation therapy with observation, NOT surgery. J Thorac Cardiovasc Surg 2017;S0022-5223:31820–2. [DOI] [PubMed] [Google Scholar]
- [18].Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Temming S, Kocher M, Stoelben E, et al. Risk-adapted robotic stereotactic body radiation therapy for inoperable early-stage non-small-cell lung cancer. Strahlenther Onkol 2017;doi: 10.1007/s00066-017-1194-x. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [20].He J, Huang Y, Shi S, et al. Comparison of effects between central and peripheral stage I lung cancer using image-guided stereotactic body radiotherapy via helical tomotherapy. Technol Cancer Res Treat 2015;14:701–7. [DOI] [PubMed] [Google Scholar]
- [21].Yi BS, Perks J, Houston R, et al. Changes in position and volume of lung cancer target volumes during stereotactic body radiotherapy (SBRT): is image guidance necessary? Technol Cancer Res Treat 2011;10:495–504. [DOI] [PubMed] [Google Scholar]


