Abstract
Hemangiosarcoma (HSA) is a highly malignant tumor with aggressive biological behavior. HSAs are more common in dogs than other domestic animals. The median survival time of dogs with HSA remains short, even with chemotherapy and surgery. Therefore, there is a critical need to improve the adjuvant chemotherapeutic regimens to improve clinical outcomes in dogs with HSA. Resveratrol has been shown to possess strong anti-proliferative and/or pro-apoptotic properties in human cancer cell lines. Nevertheless, the potential anticancer effects of resveratrol have not been reported in canine HSAs. The objective of this study is to determine the growth inhibitory effects of resveratrol in HSA cells when used alone or in combination with doxorubicin, a commonly used chemotherapeutic agent. Frog and DD-1 canine HSA cell lines were treated with varying concentrations of resveratrol with and without doxorubicin. Cell viability was measured by the MTT assay. The expression of apoptotic proteins, activation of p38 mitogen-activated protein kinase (MAPK), AMP-activated protein kinase (AMPK), and extracellular signal-regulated kinase1/2 (ERK1/2) were assessed by western blotting. Similar to human cancer cell lines, resveratrol markedly inhibited the growth and induced apoptosis in both HSA cell lines. Mechanistically, resveratrol activated p38 MAPK, but did not affect the AMPK or the ERK1/2 pathways. Additional experiments showed that resveratrol augmented the growth-inhibitory and apoptotic effects of doxorubicin in both HSA cell lines. These findings suggest that resveratrol has pro-apoptotic effects in canine HSA cells; therefore, its use as a potential adjunct therapy in canine HSA patients warrants further investigation.
Introduction
Hemangiosarcoma (HSA) is a highly malignant tumor of vascular endothelial or hemangioblastic origin that exhibits aggressive biological behavior.1, 2 HSAs are more common in dogs than other domestic species and occur mainly in older animals with higher relative risk in certain breeds such as the German Shepherd, Golden Retriever, Labrador Retriever, Boxer, Bernese Mountain dog, German Shorthaired Pointer, and Flat Coated Retriever.3 Generally, canine HSAs have poor prognosis. In dogs treated with surgery alone, the median survival time (MST) is 19 days to 1.6 months.4, 5 Adjuvant treatment with chemotherapy, especially anthracycline-based regimens such as doxorubicin, can result in modest improvement of survival, with MSTs of less than 4 months.4 Unfortunately, the originally reported survival times of dogs with HSA treated with surgery and chemotherapy as reported in 1985 (117 days) has not significantly improved 30 years later in similarly treated dogs (103 days).4, 6 Therefore, there is an urgent need to improve the adjuvant chemotherapeutic regimens to achieve better clinical outcomes in dogs with HSA.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a non-flavonoid polyphenolic compound found naturally in red wine, grapes, a variety of berries, and peanuts.7 It has demonstrated anticancer properties in human cancer cell lines including those of breast,8 colorectal, 9 and lung origins.10 There are several proposed mechanisms to explain the anticancer effects of resveratrol including: apoptosis through downregulation of the AKT and extracellular signal-regulated kinase (ERK) pathways,11–13 inhibition of cell proliferation through induction of AMP-activated protein kinase (AMPK),14 and induction of autophagy through activating the p38 MAPK pathway.12 However, the potential anticancer effects of resveratrol have rarely been investigated in canine cancer cells. To the best of our knowledge, there is only one study that reported the growth inhibitory effect of resveratrol and its synthetic oligomers against canine glioblastoma and histiocytic sarcoma cell lines.15 The potential anticancer effects of resveratrol have not been determined in canine HSAs. Therefore, the objective of this study is to determine the growth inhibitory effects of resveratrol on HSA cell lines.
In the current work, resveratrol markedly inhibited the growth and induced apoptosis in two canine HSA cell lines, Frog and DD-1. Since doxorubicin is the standard-of-care anticancer for canine HSA,16 we also determined the growth inhibitory effect of resveratrol when combined with doxorubicin. Interestingly, resveratrol significantly enhanced the growth inhibitory effect of doxorubicin when used in combination. These findings suggest that resveratrol may have anticancer effects against canine HSA and could be a potential adjunct therapy to the standard-of-care anticancer drugs used in canine HSA patients.
Materials and Methods
Materials
Ham’s F12 medium and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Corning Cellgro, Mediatech (Manassas, VA), Penicillin-streptomycin and HEPES buffer were purchased from Gibco, Life Technologies (Grand Island, NY), fetal bovine serum was purchased from EMD Millipore (Temecula, CA), endothelial cell growth supplement (ECGS) from Corning (Bedford, MA), heparin and protease inhibitor cocktail from Sigma (St Louis, MO), resveratrol from EMD Millipore (Billerica, MA), and doxorubicin was purchased from APP Pharmaceuticals (Lake Zurich, IL). The primary antibodies (anti-cleaved caspase-3 catalog# 9664, anti-Total Caspase-3 catalog# 9665, anti-phospho-p38 catalog# 4511, anti-total-p38 catalog# 8690, anti-phospho-ERK1/2 catalog# 4370, and anti-total-ERK1/2 catalog# 4695) and the secondary antibody (goat anti-rabbit IgG, HRP-linked catalog# 7074) were purchased from Cell Signaling Technology (Danvers, MA). The primary antibodies (anti-phospho-AMPKα (Thr-172) catalog# 07-681 and anti-total-AMPKα catalog# 07-363) were purchased from EMD Millipore (Billerica, MA). Chemiluminescence western blotting detection reagents, MemCode Reversible Protein Stain Kit, and BCA protein assay were purchased from Thermo Pierce (Rockford, IL). Nitrocellulose membrane was purchased from Bio-Rad Laboratories (Hercules, CA).
Cell culture
The canine HSA cell lines Frog and DD-1 were obtained as a generous gift from the laboratory of Dr. Jaime Modiano, College of Veterinary Medicine, University of Minnesota. Both cell lines have been used extensively in previous studies of canine HSA biology and treatment.1, 17, 18 Frog and DD-1 cells were maintained in Ham’s F12 medium supplemented with 10% fetal bovine serum, 0.05 mg/ml ECGS, 0.01 mg/ml heparin, 10 mM HEPES Buffer, 100 IU/ml penicillin, and 10 μg/ml streptomycin. The cardiac-derived H9c2 cells were purchased from ATCC and were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 IU/ml penicillin, and10 μg/ml streptomycin. Cells were grown in 75-cm2 tissue culture flasks at 37°C in a 5% CO2 humidified incubator. Cells were sub-cultured when they reached 80% confluency; every 2 days for Frog and H9c2 cells and 6 days for DD-1 cells.
Cell treatments
Treatments were performed in serum-free media supplemented as required for each cell line. Frog or DD-1 cells were grown in 6- or 96-well cell culture plates and incubated with increasing concentrations of resveratrol (10 – 100 μM) for 24 or 48 h, respectively. A longer incubation time was used in DD-1 cells because of their very slow growth rate. Concentrations of resveratrol were chosen to parallel previous experiments conducted in human cancer cells.8, 19, 20 Stock resveratrol solutions were prepared by dissolving resveratrol in sterile 100% ethanol. Control wells were treated with equal volume of the vehicle (sterile 100% ethanol), and the volume used in all experiments did not exceed 1% of the medium. In another set of experiments, Frog, DD-1, and H9c2 cells were pre-treated with increasing concentrations of resveratrol for 2 h prior to the addition of 1 μM doxorubicin for 22 h, 46 h, or 22 h, respectively. The 1 μM concentration of doxorubicin is based on previous in vitro studies,21 and it corresponds to the peak plasma concentration of doxorubicin in canine cancer patients.22 To determine the effect of resveratrol on HSA cells viability when combined with a wide range of doxorubicin concentrations, Frog cells were pre-treated with 50 μM resveratrol for 2 h prior to the addition of 0.1 – 2 μM doxorubicin for an additional 22 h. Stock doxorubicin solutions were prepared by dissolving doxorubicin in the appropriate serum free medium.
Cell viability assay
Cell viability was determined by measuring the capacity of reducing enzymes present in viable cells to convert 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) to formazan crystals as described previously.23 Briefly, following drug treatment, the media were removed and 100 μl of serum-free media containing 1.2 mM MTT was added to each cell culture well. The plate was then incubated at 37 °C for 1 h. The media were decanted off and 100 μl of isopropyl alcohol was added to each well followed by shaking for 1 h to dissolve the formed formazan crystals. The color intensity in each well was measured at a wavelength of 550 nm. The percentage of cell viability was calculated relative to control wells, which were designated as 100% viable cells.
Protein extraction and western blotting
Cells were grown in 6-well plates and subjected to the treatments outlined above. Cells were harvested in lysis buffer containing 20 mm Tris, 5 mM EDTA, 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 1% NP-40, and supplemented with protease and phosphatase inhibitor cocktails. The total cell homogenate was prepared and the protein concentration was measured by the Pierce ® BCA assay as described previously 24. The cell homogenates (equivalent to 25 μg protein) were prepared in 6X sample buffer, boiled for 5 min, separated by 15% SDS-polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane. In order to confirm equal loading, membranes were stained for total protein using the MemCode Reversible Protein Stain Kit. Membranes were scanned and the protein stain was removed from the membranes as described in the kit instructions. Protein blots were blocked for 1 h at room temperature in blocking buffer containing 5% skim milk powder and 0.05% (v/v) Tween 20 in Tris-buffered saline solution (0.15 M sodium chloride, 3 mM potassium chloride, 25 mM Tris-base). After blocking, the blots were incubated with the specified primary antibody overnight at 4°C. Thereafter, the membranes were washed and incubated with a peroxidase-conjugated secondary antibody in blocking buffer for 1 h at room temperature. The immune complex was visualized using the Amersham Imager 600 (GE Healthcare Life Sciences). Thereafter, the blots were stripped for 45 min in stripping buffer (62.5 mM Tris, 2% SDS, 18mM 2-mercaptoethanol, pH 6.7) at 50 °C, washed, then blocked and probed as previously described. The intensity of protein bands was quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Data were checked for normality using the Shapiro-Wilk normality test. Comparisons between control and treatments were performed using a one-way analysis of variance (ANOVA), with a Bonferroni post hoc test. A probability value of <0.05 is considered significant.
Results
The effect of resveratrol on viability of HSA cells
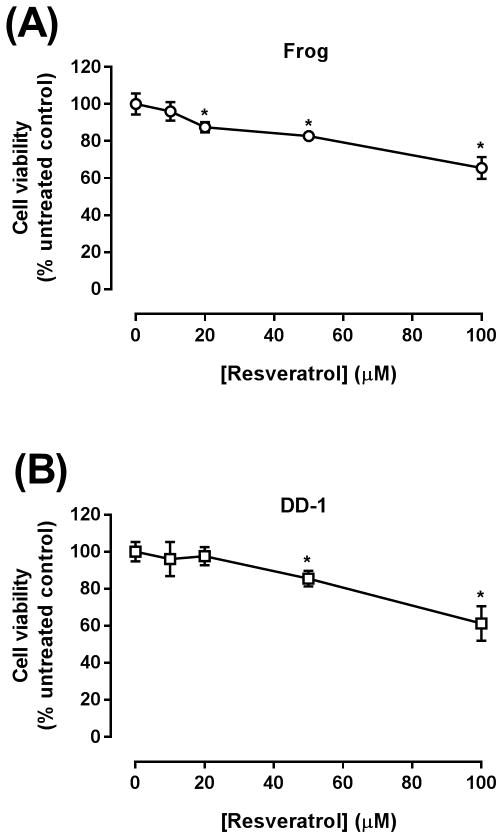
Cell viability of HSA cells was determined by the MTT viability assay following incubation with resveratrol for 24 h with Frog cells or 48 h with DD-1 cells, as described in the Methods section. In both Frog and DD-1 cells, a marked concentration-dependent inhibition of cell viability was observed following treatment with resveratrol. In Frog cells, cell viability was inhibited by 14, 18, and 35% at 20, 50, and 100μM resveratrol concentrations, respectively (Fig. 1A). Similarly, DD-1 cell viability was reduced by 15 and 30% at 50 and 100μM resveratrol concentrations, respectively (Fig. 1B).
Figure 1. Resveratrol inhibits the growth of Frog (A) and DD-1 cells (B).
Canine HSA cells were incubated with increasing concentrations of resveratrol (10–100 μM) for 24 h in Frog cells and for 48 h in DD-1 cells. Cell viability was determined by the MTT assay. Results are shown as Mean +/− SEM (n = 8). * p < 0.05 as compared to control values by one-way ANOVA.
The effect of resveratrol on apoptotic markers in HSA cells
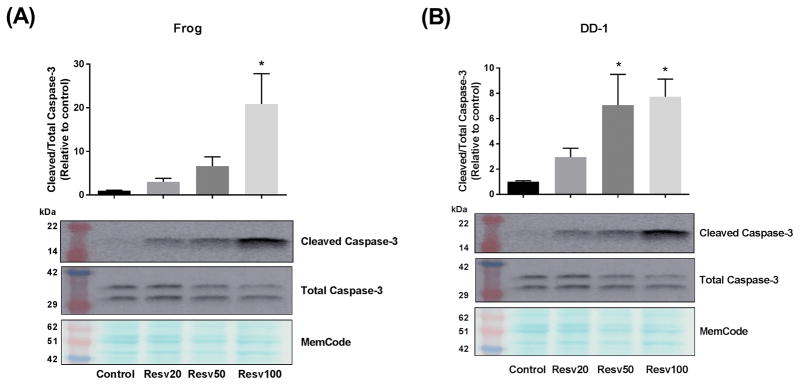
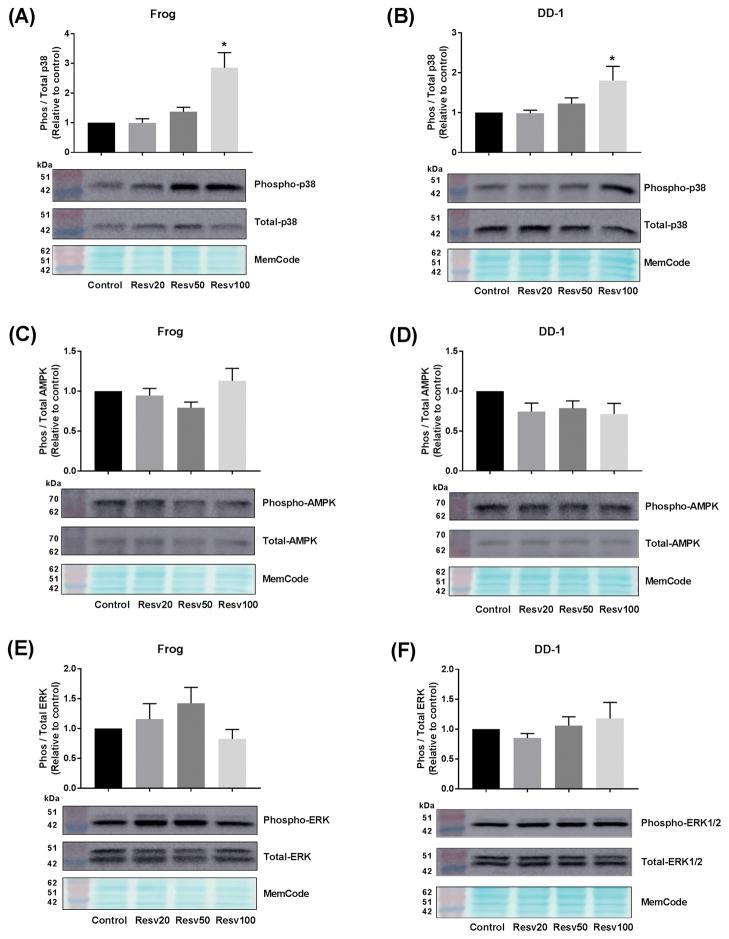
To determine the role of apoptosis in the growth inhibitory effect of resveratrol in HSA cells, the protein expression of the apoptotic marker, cleaved caspase-3, was determined by western blotting. Frog and DD-1 cells were incubated with increasing concentrations of resveratrol for 24 h as outlined in the Methods section. In agreement with the cell viability experiments, there was a concentration-dependent increase in the expression of cleaved caspase-3 in Frog (Fig. 2A) and DD-1 cells (Fig. 2B). To elucidate the mechanism of the observed apoptotic effect of resveratrol, we evaluated the effect of resveratrol on the p38 MAPK, AMPK and the ERK1/2 pathways, since they have been shown to mediate the apoptotic effect of resveratrol in human cancer cells.12–14 In Frog and DD-1 cells, resveratrol 100 μM caused 3- and 2-fold increase in phospho-p38/total-p38, respectively (Fig. 3A and 3B). Conversely, resveratrol did not change the expression of phospho-AMPK (Fig. 3C and 3D) or phospho-ERK1/2 (Fig. 3E and 3F) in either Frog or DD-1 cells.
Figure 2. Resveratrol (Resv) induces apoptosis in Frog (A) and DD-1 cells (B).
Canine HSA cells were incubated with increasing concentrations of resveratrol (20, 50, and 100 μM) for 24 h. Induction of apoptosis was assessed by measuring the cleaved caspase-3/total caspase-3 by western blotting. Results are shown as Mean +/− SEM (n = 6) from three independent experiments (duplicate of wells on three independent times). * p < 0.05 as compared to control values by one-way ANOVA.
Figure 3. Resveratrol (Resv) activates p38 MAPK (A and B), but does not affect AMPK (C and D) or ERK1/2 (E and F) pathways.
Canine HSA cells were incubated with increasing concentrations of resveratrol (20, 50, and 100 μM) for 24 h. p38 MAPK activation was assessed by measuring the phospho p38 MAPK/total p38 MAPK in Frog (A) or DD-1 (B). AMPK activation was assessed by measuring the phospho-AMPK/total AMPK in Frog (C) or DD-1 (D) cells. Likewise, ERK1/2 activation was assessed by measuring the phospho-ERK1/2/total ERK1/2 in Frog (E) or DD-1 (F) cells. Results are shown as Mean +/− SEM (n = 6) from three independent experiments (duplicate of wells on three independent times). * p < 0.05 as compared to control values by one-way ANOVA.
Resveratrol increases the growth inhibitory effect of doxorubicin in HSA cells
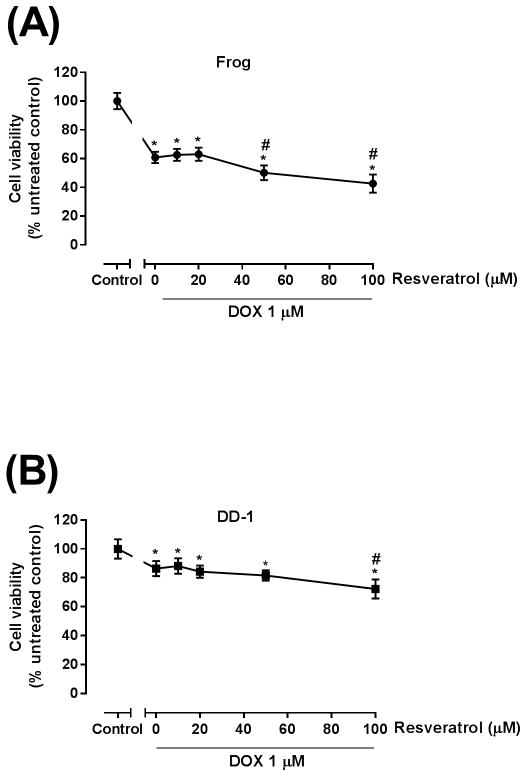
Further experiments assessed the effect of co-administration of resveratrol with doxorubicin on HSA cell viability. Frog and DD-1 cells were cultured in the presence or absence of increasing resveratrol concentrations for 2 h; thereafter, the cells were co-treated with 1 μM doxorubicin for an additional 22 h in Frog cells, or 46 h in DD-1 cells. Cell viability was then measured by the MTT assay as described in the Methods section. Treatment with 1 μM doxorubicin resulted in a 40% inhibition of cell viability in Frog cells (Fig. 4A) and 20% inhibition in DD-1 cells (Fig. 4B). Interestingly, resveratrol increased the growth inhibitory effect of doxorubicin in Frog cells to 50 and 60% of control at concentrations of 50 and 100 μM, respectively (Fig. 4A), and in DD-1 cells to 30% of control at the 100 μM concentration (Fig. 4B).
Figure 4. Resveratrol increased the growth inhibitory effect of DOX in Frog (A) and DD-1 cells (B).
Canine HSA cells were incubated with 1 μM DOX in the absence or presence of increasing concentrations of resveratrol (10–100 μM) for 24 h in Frog cells and for 48 h in DD-1 cells. Cell viability was determined by the MTT assay. Results are shown as Mean +/− SEM (n = 8). * p < 0.05 as compared to control, # p < 0.05 as compared to doxorubicin-treated cells by one-way ANOVA.
Resveratrol increases the apoptotic effect of doxorubicin in HSA cells
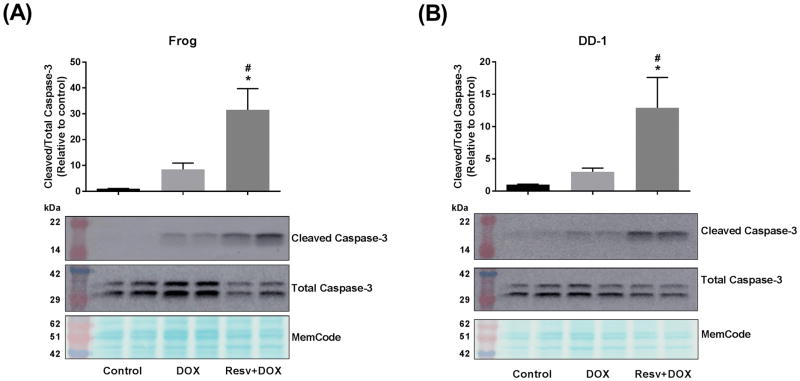
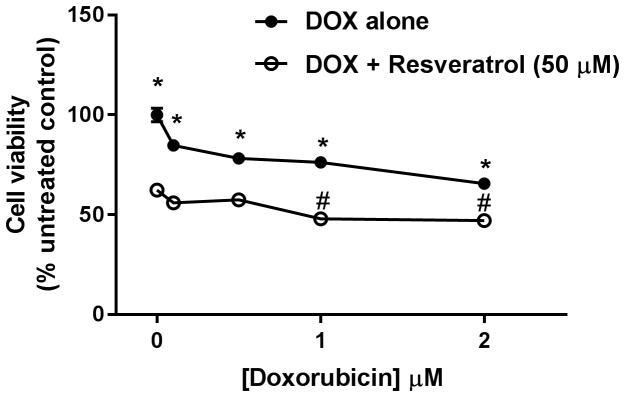
To elucidate the mechanisms by which resveratrol increased the growth inhibitory effect of doxorubicin in HSA cells, Frog and DD-1 cells were treated with 100 μM resveratrol for 2 h; thereafter, the cells were co-treated with 1 μM doxorubicin for an additional 22 h in Frog and DD-1 cells. In Frog cells, treatment with doxorubicin increased cleaved caspase-3 expression 7-fold over control. Co-treatment with resveratrol increased cleaved caspase-3 expression 30- and 3-fold relative to control and doxorubicin-treated cells, respectively (Fig. 5A). Similarly, in DD-1 cells, doxorubicin treatment resulted in a 2-fold increase in cleaved caspase-3 expression. Resveratrol increased cleaved caspase-3 expression 10- and 3-fold relative to control and doxorubicin-treated cells, respectively (Fig. 5B). In order to ascertain the effect of resveratrol on cell viability when combined with a broader range of doxorubicin concentrations, Frog cells were incubated with 50 μM resveratrol and increasing doxorubicin concentrations (0.1 – 2 μM). Figure 6 shows that the growth inhibitory effect of the combination (resveratrol + doxorubicin) is significantly higher than doxorubicin alone at all the tested concentrations. Interestingly, the IC50 was only reached when Frog cells were treated with 1 – 2 μM doxorubicin in the presence of 50 μM resveratrol.
Figure 5. Resveratrol (Resv) augments doxorubicin (DOX)-induced apoptosis in Frog (A) and DD-1 cells (B).
Canine HSA cells were incubated with 100 μM resveratrol for 2 h, then treated with 1 μM DOX for an additional 22 h. Induction of apoptosis was assessed by measuring the cleaved caspase-3/total caspase-3 by western blotting. Results are shown as Mean +/− SEM (n = 6) from three independent experiments (duplicate of wells on three independent times). * p < 0.05 as compared to control values, # p < 0.05 as compared to cells treated by doxorubicin only by one-way ANOVA.
Figure 6. Resveratrol increased the growth inhibitory effect of a broad range of doxorubicin (DOX) concentrations in Frog cells.
Canine HSA Frog cells were incubated with 0.1 – 2 μM DOX in the absence or presence of 50 μM resveratrol for 24 h. Cell viability was determined by the MTT assay. Results are shown as Mean +/− SEM (n = 8). * p < 0.05 as compared to resveratrol-treated cells, # p < 0.05 as compared to cells treated by resveratrol only by one-way ANOVA.
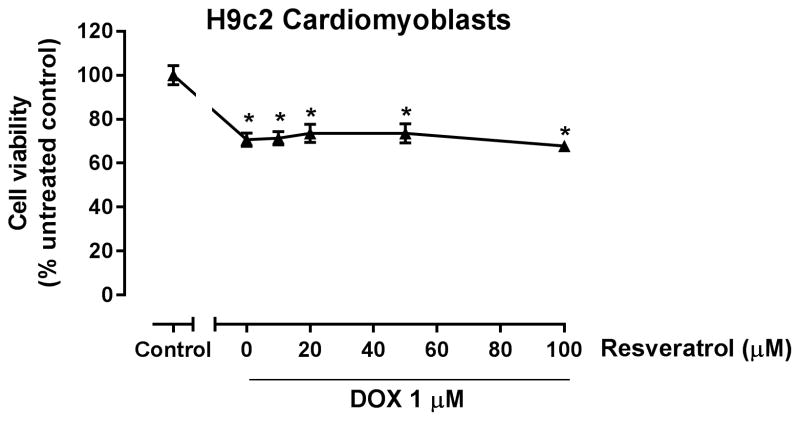
Resveratrol does not increase doxorubicin-induced toxicity in H9c2 cells
Since a main adverse effect of doxorubicin therapy is cardiotoxicity, we determined whether resveratrol alters the toxic effect of doxorubicin in the cardiac-derived H9c2 cells. H9c2 cells were cultured in the presence or absence of increasing resveratrol concentrations for 2 h; thereafter, the cells were co-treated with 1 μM doxorubicin for an additional 22 h. Cell viability was determined by the MTT assay as described in the Methods section. MTT assay has been widely used to assess doxorubicin-induced toxicity in H9c2 cardiomyoblasts. 25–28 In contrast to HSA cells, resveratrol did not increase the toxicity of doxorubicin in the cardiac-derived H9c2 cells (Fig. 7).
Figure 7. Resveratrol does not increase doxorubicin (DOX) toxicity in H9c2 cardiomyoblast cell line.
H9c2 cells were incubated with 1 μM DOX in the absence or presence of increasing concentrations of resveratrol (10–100 μM) for 24 h. Cell viability was determined by the MTT assay. Results are shown as Mean +/− SEM (n = 8). * p < 0.05 as compared to control by one-way ANOVA.
Discussion
Resveratrol is a non-flavonoid polyphenolic phytochemical which possesses a plethora of biological effects.7 A significant body of evidence suggests that resveratrol has anticancer and chemopreventive properties.29 It has been shown to have both anti-proliferative and apoptotic properties resulting in a strong growth-inhibitory effect in multiple human cancer cell lines in vitro.8, 9, 30 It has also been shown to inhibit tumor growth of several xenograft models of human cancers in vivo.31, 32 Nevertheless, its anticancer effects have rarely been investigated in canine cancers. In a single study, resveratrol and its synthetic oligomers induced apoptosis and inhibited the growth of canine glioblastoma D-GBM cells and canine histiocytic sarcoma DH82 cells.15 However, the growth inhibitory effect of resveratrol has never been determined in HSA cells. In the current work, we demonstrate for the first time the growth-inhibitory effect of resveratrol in two HSA cell lines, Frog and DD-1. Resveratrol concentrations as low as 20 and 50 μM, significantly reduced cell viability in Frog and DD-1 cells, respectively. These concentrations are clinically relevant, since plasma levels of 20–50 μM have been safely achieved with oral doses of 600–1200 mg/kg/day administered to beagle dogs,33 suggesting a translational potential of our current findings. In this safety study, no treatment-related mortality or clinical signs of toxicity were observed after 13 weeks of resveratrol administration with no changes in histopathology or organ weights. However, there was a significant decrease in body weight due to decreased food consumption, and an increase in inflammatory infiltrates in the urinary bladders and kidney in dogs receiving 1200 mg/day.33
To explore the mechanism of this growth-inhibitory effect, we measured the expression of the apoptotic marker, cleaved caspase-3. Similar to previous findings in human cancer cells,34 we demonstrate that resveratrol induces apoptosis in both Frog and DD-1 canine HSA cells, suggesting that apoptosis plays a significant role in mediating the growth-inhibitory effect of resveratrol in these cells. A limitation of the current study is the dependence on cleaved caspase-3 as the only apoptotic marker. Additional assays could have been used to verify the late stages of apoptosis e.g. TUNEL assay. In an attempt to elucidate the molecular mechanisms of the observed pro-apoptotic effect, we determined the effect of resveratrol on the key signaling pathways, p38 MAPK, AMPK and ERK1/2. The p38 MAPK is a key player in the regulation of apoptosis, cell cycle arrest, and growth inhibition.35 The anticancer effects of resveratrol have been shown to be mediated through p38 MAPK activation in several human cancer cell lines including: colon cancer,32 chronic myelogenous leukemia,36 and acute lymphoblastic leukemia cells.12 Nevertheless, the effect of resveratrol on p38 MAPK signaling has never been previously demonstrated in canine cancer cells. In the current work, we show, for the first time, that resveratrol activates p38 MAPK in both Frog and DD-1 HSA cell lines. Resveratrol-induced activation of p38 MAPK may contribute, at least in part, to the observed apoptotic effects of resveratrol in these cell lines.
AMPK is an energy-sensing kinase that has been shown to have anti-proliferative 37 and pro-apoptotic 38 effects in human cancer cells. Similar to p38 MAPK, AMPK activation has been shown to mediate the effects of resveratrol in several human cancer cell lines.14, 39–41 Nevertheless, it is unknown whether resveratrol exerts its pro-apoptotic effects through AMPK activation in canine cancer cells in general, or in HSA cells in particular. In contrast to earlier studies conducted in human cancer cells, resveratrol did not activate AMPK in the two HSA cell lines, Frog and DD-1, in the current study. It warrants further investigations to determine the effect of resveratrol on AMPK in other HSA cells as well as in other canine cancer cells. In contrast to p38 MAPK and AMPK, the ERK1/2 MAPK pathway has been shown to mediate survival in human cancer cells.37, 42, 43 While some studies demonstrated that resveratrol inhibits ERK1/2 in Panc-1 human pancreatic cancer cells 44, 45 and SW620 human colon cancer cells,46 another study showed that resveratrol activates ERK1/2 in MDA-231 breast cancer cells.47 Interestingly, Hogg et al. showed a biphasic effect of resveratrol on ERK1/2, wherein a low resveratrol concentration (10 μM) activated ERK1/2, whereas higher concentrations (50 – 100 μM) inhibited it.48 In the current study, resveratrol (10 – 100 μM) had no effect on ERK1/2 in HSA cells, suggesting that the effect of resveratrol on ERK1/2 is concentration and cell type-dependent.
Doxorubicin-based chemotherapy is the most effective adjuvant therapy for canine HSAs.2 Of interest, resveratrol has been shown to augment the chemotherapeutic effect of doxorubicin in human cancer cell lines in vitro 49 and in mouse xenografts of human tumor in vivo.50 However, the combined effect of resveratrol and doxorubicin in canine cancer cells has never been determined. Therefore, in the current work, we determined the growth inhibitory effect of resveratrol co-treatment with doxorubicin in canine HSA cells. We demonstrate that resveratrol enhances doxorubicin-induced cytotoxicity in both Frog and DD-1 cells. We also demonstrate that resveratrol markedly increases doxorubicin-induced apoptosis in these cells. It is interesting to note that in human breast cancer cells, resveratrol has been shown to down-regulate the expression of P-glycoprotein and multi-drug resistance protein, thus increasing doxorubicin accumulation in cancer cells.50, 51 Further research is required to explore the possible role of these proteins in mediating the effect of resveratrol in canine HSA cells.
Since cardiotoxicity is a major adverse effect of doxorubicin,52 we tested whether combining resveratrol with doxorubicin would alter its cardiotoxic effects. We used the rat-derived H9c2 cardiomyoblasts to determine the effect of resveratrol on doxorubicin-induced cardiotoxicity. H9c2 cell line is a common in vitro model to study the cardiotoxic effects of doxorubicin.53 While resveratrol has been shown to increase doxorubicin-induced cytotoxicity in cancer cells, it has been demonstrated to protect against doxorubicin-induced cardiotoxicity both in vitro 54 and in vivo.55 In the current work, we demonstrate that resveratrol did not increase the cardiotoxic effect of doxorubicin in the cardiac-derived H9c2 cells, suggesting that co-administration of resveratrol with doxorubicin adds to the cytotoxic effect of doxorubicin against cancer cells, without increasing its toxic effect in cardiac cells. Further research is required to determine the effect of resveratrol alone or resveratrol/doxorubicin combination on canine normal cells e.g. endothelial cells or fibroblasts.
In conclusion, resveratrol has a marked growth-inhibitory effect on two HSA cell lines, Frog and DD-1, mediated, at least in part, through its pro-apoptotic properties. In addition, resveratrol increases the cytotoxic effect of doxorubicin in these cell lines, without increasing its toxic effect on the cardiac-derived H9c2 cells. Taking into account the demonstrated relative safety of resveratrol in dogs,56 resveratrol can be a plausible additive therapy to doxorubicin-based regimens in the treatment of canine HSA.
Acknowledgments
We would like to thank Dr. J. Modiano, Department of Veterinary Clinical Sciences, College of Veterinary Medicine, University of Minnesota, for providing the HSA cells used in this publication. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. BZ is the recipient of a Pre-K Career Development Award and KA is funded by the Pathway to Research Program, Clinical and Translational Research Institute, University of Minnesota. AC is funded by the University of Minnesota College of Veterinary Medicine Summer Scholars Program and Skadron Family Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Lamerato-Kozicki AR, Helm KM, Jubala CM, Cutter GC, Modiano JF. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Exp Hematol. 2006;34:870–8. doi: 10.1016/j.exphem.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Finotello R, Stefanello D, Zini E, Marconato L. Comparison of doxorubicin-cyclophosphamide with doxorubicin-dacarbazine for the adjuvant treatment of canine hemangiosarcoma. Vet Comp Oncol. 2017;15:25–35. doi: 10.1111/vco.12139. [DOI] [PubMed] [Google Scholar]
- 3.Goritz M, Muller K, Krastel D, et al. Canine splenic haemangiosarcoma: influence of metastases, chemotherapy and growth pattern on post-splenectomy survival and expression of angiogenic factors. J Comp Pathol. 2013;149:30–9. doi: 10.1016/j.jcpa.2012.11.234. [DOI] [PubMed] [Google Scholar]
- 4.Wendelburg KM, Price LL, Burgess KE, et al. Survival time of dogs with splenic hemangiosarcoma treated by splenectomy with or without adjuvant chemotherapy: 208 cases (2001–2012) J Am Vet Med Assoc. 2015;247:393–403. doi: 10.2460/javma.247.4.393. [DOI] [PubMed] [Google Scholar]
- 5.Wood CA, Moore AS, Gliatto JM, et al. Prognosis for dogs with stage I or II splenic hemangiosarcoma treated by splenectomy alone: 32 cases (1991–1993) J Am Anim Hosp Assoc. 1998;34:417–21. doi: 10.5326/15473317-34-5-417. [DOI] [PubMed] [Google Scholar]
- 6.Brown NO, Patnaik AK, MacEwen EG. Canine hemangiosarcoma: retrospective analysis of 104 cases. J Am Vet Med Assoc. 1985;186:56–8. [PubMed] [Google Scholar]
- 7.Zordoky BNM, Robertson IM, Dyck JRB. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Bba-Mol Basis Dis. 2015;1852:1155–1177. doi: 10.1016/j.bbadis.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Deus CM, Serafim TL, Magalhaes-Novais S, et al. Sirtuin 1-dependent resveratrol cytotoxicity and pro-differentiation activity on breast cancer cells. Arch Toxicol. 2017;91:1261–78. doi: 10.1007/s00204-016-1784-x. [DOI] [PubMed] [Google Scholar]
- 9.Ji Q, Liu X, Fu X, et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/beta-catenin signal pathway. PLoS One. 2013;8:e78700. doi: 10.1371/journal.pone.0078700. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Wang X, Wang D, Zhao Y. Effect and Mechanism of Resveratrol on the Apoptosis of Lung Adenocarcinoma Cell Line A549. Cell Biochem Biophys. 2015;73:527–31. doi: 10.1007/s12013-015-0696-3. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Kim MJ, Sung B, et al. Resveratrol analogue, HS-1793, induces apoptotic cell death and cell cycle arrest through downregulation of AKT in human colon cancer cells. Oncol Rep. 2017;37:281–288. doi: 10.3892/or.2016.5219. [DOI] [PubMed] [Google Scholar]
- 12.Ge J, Liu Y, Li Q, et al. Resveratrol induces apoptosis and autophagy in T-cell acute lymphoblastic leukemia cells by inhibiting Akt/mTOR and activating p38-MAPK. Biomed Environ Sci. 2013;26:902–11. doi: 10.3967/bes2013.019. [DOI] [PubMed] [Google Scholar]
- 13.Vergara D, Simeone P, Toraldo D, et al. Resveratrol downregulates Akt/GSK and ERK signalling pathways in OVCAR-3 ovarian cancer cells. Mol Biosyst. 2012;8:1078–87. doi: 10.1039/c2mb05486h. [DOI] [PubMed] [Google Scholar]
- 14.Cai Y, Zhao L, Qin Y, Zhang M, He Y. Resveratrol inhibits proliferation and induces apoptosis of nasopharyngeal carcinoma cell line C666-1 through AMPK activation. Pharmazie. 2015;70:399–403. [PubMed] [Google Scholar]
- 15.Empl MT, Macke S, Winterhalter P, et al. The growth of the canine glioblastoma cell line D-GBM and the canine histiocytic sarcoma cell line DH82 is inhibited by the resveratrol oligomers hopeaphenol and r2-viniferin. Vet Comp Oncol. 2014;12:149–59. doi: 10.1111/j.1476-5829.2012.00349.x. [DOI] [PubMed] [Google Scholar]
- 16.Sorenmo K, Duda L, Barber L, et al. Canine hemangiosarcoma treated with standard chemotherapy and minocycline. J Vet Intern Med. 2000;14:395–8. doi: 10.1892/0891-6640(2000)014<0395:chtwsc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Schappa JT, Frantz AM, Gorden BH, et al. Hemangiosarcoma and its cancer stem cell subpopulation are effectively killed by a toxin targeted through epidermal growth factor and urokinase receptors. Int J Cancer. 2013;133:1936–44. doi: 10.1002/ijc.28187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez AM, Graef AJ, LeVine DN, et al. Association of Sphingosine-1-phosphate (S1P)/S1P Receptor-1 Pathway with Cell Proliferation and Survival in Canine Hemangiosarcoma. J Vet Intern Med. 2015;29:1088–97. doi: 10.1111/jvim.13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nivelle L, Hubert J, Courot E, et al. Anti-Cancer Activity of Resveratrol and Derivatives Produced by Grapevine Cell Suspensions in a 14 L Stirred Bioreactor. Molecules. 2017:22. doi: 10.3390/molecules22030474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh SK, Banerjee S, Acosta EP, Lillard JW, Singh R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/ p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget. 2017;8:17216–26. doi: 10.18632/oncotarget.15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smolensky D, Rathore K, Bourn J, Cekanova M. Inhibition of the PI3K/AKT Pathway Sensitizes Oral Squamous Cell Carcinoma Cells to Anthracycline-Based Chemotherapy In Vitro. J Cell Biochem. 2017;118:2615–24. doi: 10.1002/jcb.25747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnard RA, Wittenburg LA, Amaravadi RK, et al. Phase I clinical trial and pharmacodynamic evaluation of combination hydroxychloroquine and doxorubicin treatment in pet dogs treated for spontaneously occurring lymphoma. Autophagy. 2014;10:1415–25. doi: 10.4161/auto.29165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korashy HM, El-Kadi AO. Differential effects of mercury, lead and copper on the constitutive and inducible expression of aryl hydrocarbon receptor (AHR)-regulated genes in cultured hepatoma Hepa 1c1c7 cells. Toxicology. 2004;201:153–72. doi: 10.1016/j.tox.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Anwar-Mohamed A, Klotz LO, El-Kadi AO. Inhibition of heme oxygenase-1 partially reverses the arsenite-mediated decrease of CYP1A1, CYP1A2, CYP3A23, and CYP3A2 catalytic activity in isolated rat hepatocytes. Drug Metab Dispos. 2012;40:504–14. doi: 10.1124/dmd.111.042564. [DOI] [PubMed] [Google Scholar]
- 25.Wang WC, Uen YH, Chang ML, et al. Protective effect of guggulsterone against cardiomyocyte injury induced by doxorubicin in vitro. BMC Complement Altern Med. 2012;12:138. doi: 10.1186/1472-6882-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao J, Chen T, Zhao D, Zheng J, Liu Z. Ginkgolide B Exerts Cardioprotective Properties against Doxorubicin-Induced Cardiotoxicity by Regulating Reactive Oxygen Species, Akt and Calcium Signaling Pathways In Vitro and In Vivo. PLoS One. 2016;11:e0168219. doi: 10.1371/journal.pone.0168219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han X, Ren D, Fan P, Shen T, Lou H. Protective effects of naringenin-7-O-glucoside on doxorubicin-induced apoptosis in H9C2 cells. Eur J Pharmacol. 2008;581:47–53. doi: 10.1016/j.ejphar.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 28.Dong Q, Chen L, Lu Q, et al. Quercetin attenuates doxorubicin cardiotoxicity by modulating Bmi-1 expression. Br J Pharmacol. 2014;171:4440–54. doi: 10.1111/bph.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varoni EM, Lo Faro AF, Sharifi-Rad J, Iriti M. Anticancer Molecular Mechanisms of Resveratrol. Front Nutr. 2016;3:8. doi: 10.3389/fnut.2016.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taniguchi T, Iizumi Y, Watanabe M, et al. Resveratrol directly targets DDX5 resulting in suppression of the mTORC1 pathway in prostate cancer. Cell Death Dis. 2016;7:e2211. doi: 10.1038/cddis.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MH, Choi BY, Kundu JK, et al. Resveratrol suppresses growth of human ovarian cancer cells in culture and in a murine xenograft model: eukaryotic elongation factor 1A2 as a potential target. Cancer Res. 2009;69:7449–58. doi: 10.1158/0008-5472.CAN-09-1266. [DOI] [PubMed] [Google Scholar]
- 32.Yuan SX, Wang DX, Wu QX, et al. BMP9/p38 MAPK is essential for the antiproliferative effect of resveratrol on human colon cancer. Oncol Rep. 2016;35:939–47. doi: 10.3892/or.2015.4407. [DOI] [PubMed] [Google Scholar]
- 33.Johnson WD, Morrissey RL, Usborne AL, et al. Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity. Food Chem Toxicol. 2011;49:3319–27. doi: 10.1016/j.fct.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucas IK, Kolodziej H. Trans-Resveratrol Induces Apoptosis through ROS-Triggered Mitochondria-Dependent Pathways in A549 Human Lung Adenocarcinoma Epithelial Cells. Planta Med. 2015;81:1038–44. doi: 10.1055/s-0035-1546129. [DOI] [PubMed] [Google Scholar]
- 35.Sui X, Kong N, Ye L, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014;344:174–9. doi: 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Wu XP, Xiong M, Xu CS, et al. Resveratrol induces apoptosis of human chronic myelogenous leukemia cells in vitro through p38 and JNK-regulated H2AX phosphorylation. Acta Pharmacol Sin. 2015;36:353–61. doi: 10.1038/aps.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zordoky BN, Bark D, Soltys CL, Sung MM, Dyck JR. The anti-proliferative effect of metformin in triple-negative MDA-MB-231 breast cancer cells is highly dependent on glucose concentration: implications for cancer therapy and prevention. Biochim Biophys Acta. 2014;1840:1943–57. doi: 10.1016/j.bbagen.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 38.Morishita M, Kawamoto T, Hara H, et al. AICAR induces mitochondrial apoptosis in human osteosarcoma cells through an AMPK-dependent pathway. Int J Oncol. 2017;50:23–30. doi: 10.3892/ijo.2016.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lea MA, Pourat J, Patel R, desBordes C. Growth inhibition of colon cancer cells by compounds affecting AMPK activity. World J Gastrointest Oncol. 2014;6:244–52. doi: 10.4251/wjgo.v6.i7.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan GH, Wang ZM, Yang X, et al. Resveratrol inhibits oesophageal adenocarcinoma cell proliferation via AMP-activated protein kinase signaling. Asian Pac J Cancer Prev. 2014;15:677–82. doi: 10.7314/apjcp.2014.15.2.677. [DOI] [PubMed] [Google Scholar]
- 41.Lin JN, Lin VC, Rau KM, et al. Resveratrol modulates tumor cell proliferation and protein translation via SIRT1-dependent AMPK activation. J Agric Food Chem. 2010;58:1584–92. doi: 10.1021/jf9035782. [DOI] [PubMed] [Google Scholar]
- 42.Zhao S, Qiu ZX, Zhang L, Li WM. Prognostic values of ERK1/2 and p-ERK1/2 expressions for poor survival in non-small cell lung cancer. Tumour Biol. 2015;36:4143–50. doi: 10.1007/s13277-015-3048-4. [DOI] [PubMed] [Google Scholar]
- 43.Wiesenauer CA, Yip-Schneider MT, Wang Y, Schmidt CM. Multiple anticancer effects of blocking MEK-ERK signaling in hepatocellular carcinoma. J Am Coll Surg. 2004;198:410–21. doi: 10.1016/j.jamcollsurg.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Cao L, Chen X, Xiao X, Ma Q, Li W. Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and migration of pancreatic cancer cells via suppression of the ERK and p38 MAPK signaling pathways. Int J Oncol. 2016;49:735–43. doi: 10.3892/ijo.2016.3559. [DOI] [PubMed] [Google Scholar]
- 45.Roy SK, Chen Q, Fu J, Shankar S, Srivastava RK. Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PLoS One. 2011;6:e25166. doi: 10.1371/journal.pone.0025166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen H, Jin ZL, Xu H. MEK/ERK signaling pathway in apoptosis of SW620 cell line and inhibition effect of resveratrol. Asian Pac J Trop Med. 2016;9:49–53. doi: 10.1016/j.apjtm.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen TH, Mustafa FB, Pervaiz S, Ng FS, Lim LH. ERK1/2 activation is required for resveratrol-induced apoptosis in MDA-MB-231 cells. Int J Oncol. 2008;33:81–92. [PubMed] [Google Scholar]
- 48.Hogg SJ, Chitcholtan K, Hassan W, Sykes PH, Garrill A. Resveratrol, Acetyl-Resveratrol, and Polydatin Exhibit Antigrowth Activity against 3D Cell Aggregates of the SKOV-3 and OVCAR-8 Ovarian Cancer Cell Lines. Obstet Gynecol Int. 2015;2015:279591. doi: 10.1155/2015/279591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rai G, Mishra S, Suman S, Shukla Y. Resveratrol improves the anticancer effects of doxorubicin in vitro and in vivo models: A mechanistic insight. Phytomedicine. 2016;23:233–42. doi: 10.1016/j.phymed.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 50.Kim TH, Shin YJ, Won AJ, et al. Resveratrol enhances chemosensitivity of doxorubicin in multidrug-resistant human breast cancer cells via increased cellular influx of doxorubicin. Biochim Biophys Acta. 2014;1840:615–25. doi: 10.1016/j.bbagen.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 51.Huang F, Wu XN, Chen J, Wang WX, Lu ZF. Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp Ther Med. 2014;7:1611–1616. doi: 10.3892/etm.2014.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raj S, Franco VI, Lipshultz SE. Anthracycline-induced cardiotoxicity: a review of pathophysiology, diagnosis, and treatment. Curr Treat Options Cardiovasc Med. 2014;16:315. doi: 10.1007/s11936-014-0315-4. [DOI] [PubMed] [Google Scholar]
- 53.Zordoky BN, El-Kadi AO. Induction of several cytochrome P450 genes by doxorubicin in H9c2 cells. Vascul Pharmacol. 2008;49:166–72. doi: 10.1016/j.vph.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Cao Z, Li Y. Potent induction of cellular antioxidants and phase 2 enzymes by resveratrol in cardiomyocytes: protection against oxidative and electrophilic injury. Eur J Pharmacol. 2004;489:39–48. doi: 10.1016/j.ejphar.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 55.Dolinsky VW, Rogan KJ, Sung MM, et al. Both aerobic exercise and resveratrol supplementation attenuate doxorubicin-induced cardiac injury in mice. Am J Physiol Endocrinol Metab. 2013;305:E243–53. doi: 10.1152/ajpendo.00044.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kondratyuk TP, Adrian JA, Wright B, et al. Evidence supporting the conceptual framework of cancer chemoprevention in canines. Sci Rep. 2016;6:26500. doi: 10.1038/srep26500. [DOI] [PMC free article] [PubMed] [Google Scholar]