Abstract
Most diseases, especially cancer, would significantly benefit from precision medicine where treatment is shaped for the individual. The concept of theragnostics or theranostics emerged around 2002 to describe the incorporation of diagnostic assays into the selection of therapy for this purpose. Increasingly, theranostics has been used for strategies that combine noninvasive imaging-based diagnostics with therapy. Within the past decade theranostic imaging has transformed into a rapidly expanding field that is located at the interface of diagnosis and therapy.
A critical need in cancer treatment is to minimize damage to normal tissue. Molecular imaging can be applied to identify targets specific to cancer with imaging, design agents against these targets to visualize their delivery, and monitor response to treatment, with the overall purpose of minimizing collateral damage. Genomic and proteomic profiling can provide an extensive ‘fingerprint’ of each tumor. With this cancer fingerprint, theranostic agents can be designed to personalize treatment for precision medicine of cancer, and minimize damage to normal tissue. Here, for the first time, we have introduced the term ‘metabolotheranostics’ to describe strategies where disease-based alterations in metabolic pathways detected by MRS are specifically targeted with image-guided delivery platforms to achieve disease-specific therapy. The versatility of MRI and MRS in molecular and functional imaging makes these technologies especially important in theranostic MRI and ‘metabolotheranostics’. Our purpose here is to provide insights into the capabilities and applications of this exciting new field in cancer treatment with a focus on MRI and MRS.
Graphical abstract

Introduction
Achieving cancer cure, or even control, continues to be a major unanswered challenge in health care despite the remarkable technological advances of the 21st century. Advances in multi-modal noninvasive molecular and functional imaging are providing unique opportunities to expand our understanding of cancer, an understanding that is critical to, and that can be integrated with, effective and cancer specific treatment. The field of theranostic imaging can be broadly categorized based on different imaging modalities and the different aspects of cancer being targeted. Since theranostic imaging requires the delivery of therapeutic cargo to cancer-specific targets that can be noninvasively imaged, receptors and antigens expressed specifically by cancer cells provide the most direct targets (Figure 1). Many tumors do not express cancer cell specific receptors and antigens, creating a critical need to mine other aspects of the tumor such as metabolism, angiogenesis, inflammation, the tumor microenvironment (TME), and stromal cell receptors for theranostics. Unlike most other diseases where pathologies arise from cell death, cancer is a disease of cell survival and tissue colonization. In strategies to survive, cancer cells co-opt the TME that consists of stromal cells such as cancer associated fibroblasts (CAFs), adipocytes, immune cells, endothelial cells and the extracellular matrix (ECM). The abnormal vasculature in tumors result in hypoxia, extracellular acidosis, and an abnormal metabolism that is superimposed on the intrinsically altered metabolism of cancer cells. These complexities of the tumor ecosystem can be visualized by imaging and transformed into opportunities for theranostic imaging for precision medicine in cancer [1] (Figure 1). Theranostic probes are administered intravenously, extravasate through leaky vasculature in tumors, and bind to a specific target to release a therapeutic cargo. In those cancer cells that do not express cancer-cell specific antigens or receptors, probes enter cells through endocytosis or phagocytosis (Figure 1).
Figure 1.
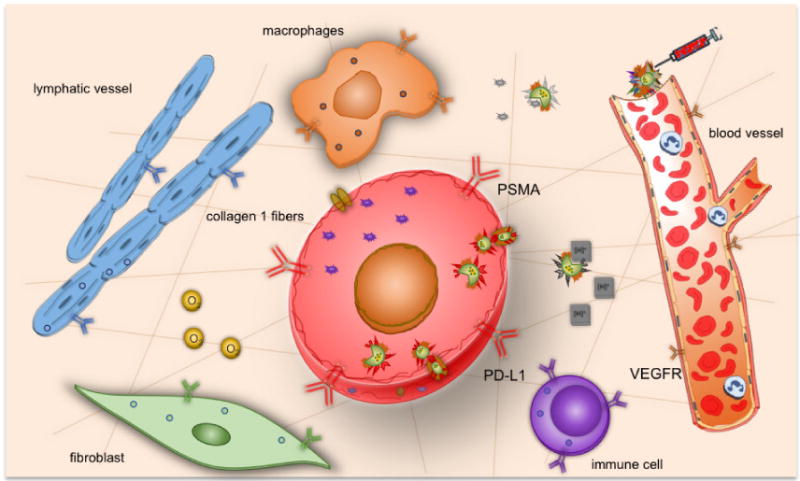
Schematic display of the tumor ecosystem that can be incorporated into strategies for theranostic imaging.
Several imaging modalities with a bench to bedside span such as magnetic resonance imaging/spectroscopy (MRI/S), positron emission tomography (PET), single photon emission computerized tomography (SPECT), ultrasound (US), as well as optical imaging that is increasingly being explored for intra-operative imaging, are available for theranostic imaging (Figure 2). MRI and MRS perform very well with structural and functional as well as molecular applications, but do not have the sensitivity of nuclear or optical imaging. The development of rapid imaging techniques that allow almost on-line visualization [2], and strategies to significantly improve sensitivity of detection such as chemical exchange saturation transfer (CEST) [3–5] and hyperpolarized 13C MRSI [6, 7] have greatly increased the scope of MRI and MRS for molecular and functional imaging. The metabolic imaging capabilities of MRSI create new opportunities in cancer for ‘metabolotheranostics’ where cancer-specific metabolic alterations detected by MRSI can be normalized by delivering siRNA or cDNA or drugs delivered under image-guidance to tumors to downregulate or express enzymes in these pathways, and detect their effectiveness with MRSI.
Figure 2.

Overview of the strengths of different imaging modalities for structural, functional and molecular imaging, and schematic of a probe used for theranostics.
There is a rapid expansion of innovative nanoscale probes for theranostics that are being developed based on the imaging modality, the therapeutic cargo, and the target [8]. These probes are typically liposomes, nanoparticles, micelles and viral vectors that are decorated with imaging reporters and deliver conventional therapy or molecularly targeted medicine such as complementary DNA (cDNA) or small interfering RNA (siRNA). There is an increasing trend to combine imaging modalities and, as a result, the probes are decorated with multi-modal imaging reporters (Figure 2).
Most MR based probes are of the order of 5 nm – 200 nm in size. Circulating probes of this size are cleared through the reticuloendothelial system (RES) organs such as the liver and spleen rather than by renal clearance [9]. Design of these probes includes incorporating physicochemical properties to increase circulation times [10]. With this size of probes, the enhanced permeability of tumors provides a first layer of tumor specific delivery that allows the probes to leak out easily into the tumor interstitium but not normal tissue. Surface targeting ligands, either on the cancer cell or on stromal cells or immune cells in the TME, can provide a second layer of specificity to minimize damage to normal tissue. Stimuli-responsive probes that release a therapeutic cargo under conditions of hypoxia or acidic pH are another class of probes that are being developed for tumor specific therapeutic delivery [11, 12]. Light responsive systems, and hyperthermia or ultrasound triggered delivery are other options that are available for tumor specific release [13–16].
The major goal of theranostics is to achieve minimal toxicity in normal tissues by cancer specific targeting. While developing these theranostic probes it is therefore essential to characterize probe biodistribution, pharmacodynamics, stability, and toxicity. Some nanoplatforms may induce an inflammatory response in cancer cells [17]. Since most of the probes are cleared through the RES, toxicity in RES organs such as the liver and spleen should be carefully evaluated.
In this review we have briefly described novel strategies that incorporate MRI and MRS for theranostics, and have provided our perspective of new areas where MRI and MRS are likely to have the most impact for cancer theranostics.
Targeted theranostics
In targeted theranostics the nanoplatform has a targeting moiety to attach the platform to a target on the cell surface. An example of a target that is being exploited for theranostic imaging is prostate-specific membrane antigen (PSMA), a type II integral membrane protein that is abundantly expressed on the surface of androgen-independent, advanced prostate cancer [18–20].
PSMA-targeted nanoplexes carrying multimodality imaging reporters together with siRNA or cDNA and a prodrug enzyme for cancer theranostic imaging have been described [20, 21]. The prodrug enzyme is a drug-activating enzyme that converts a non-toxic prodrug to a chemotherapy agent. The ability to image the delivery of the prodrug enzyme can be exploited to time prodrug administration to minimize damage to normal tissue. The PSMA targeted nanoplex incorporated a low molecular weight PSMA binding agent to target the nanoplex to PSMA expressing prostate cancer cells, a prodrug enzyme cytosine deaminase (CD), and siRNA or cDNA [22]. CD converted a non-toxic prodrug 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU) that was detected by 19F MRS [23],[21]. Aberrant choline metabolism, characterized by increased phosphocholine (PCho) and total choline-containing compounds (tCho), is a characteristic of most cancers [24]. Phosphocholine, obtained from the phosphorylation of choline by choline kinase (Chk), is both a precursor and a breakdown product of phosphatidylcholine (PtdCho), a major component of the lipid bilayer of cell membranes [24]. Since choline kinase (Chk) is significantly upregulated in aggressive cancer cells, siRNA against Chk was delivered by the nanoplex. Chk downregulation was detected using 1H MRS. The nanoplex was detected with SPECT imaging of In-111, since the sensitivity of MRI was not sufficient to detect the PSMA bound nanoplex (Figure 3). Delivery of siRNA downregulating a metabolic enzyme such as Chk provides a proof-of-principle example of metabolotheranostics, using cancer-cell specific image-guided delivery of the theranostic probe and detecting the metabolic effects on the target enzyme. Targeting metabolic pathways with siRNA or cDNA delivering probes and detecting their metabolic outcome with MRS provides new opportunities for ‘metabolotheranostic’ strategies. The ability to downregulate or overexpress genes using nanocarrier based delivery systems brings every gene within reach for cancer treatment strategies, including down-regulating oncogenic pathways, multi-drug resistance pathways, or repair enzymes, to increase the efficiency of chemo- or radiation therapy and expressing tumor suppressor genes or pro-apoptotic genes. Advances in 13C MRSI hyperpolarized probes [25] combined with image-guided cancer specific delivery of metabolic targeting molecular reagents or pharmacological agents should expand future metabolotheranostic strategies.
Figure 3.

(A) Structure of a PSMA theranostic agent that carries a prodrug enzyme to convert 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU) that is detected by 19F MRS and siRNA to downregulate choline kinase (Chk) that results in a decrease of total choline detected by 1H MRSI. (B) Increased retention of the theranostic agent in a PSMA expressing tumor compared to a non-PSMA expressing tumor. (C) Functional changes in tumor metabolism detected by 1H MRSI and the formation of the cytotoxic drug 5-FU from 5-FC in the tumor detected by 19F MRS. Adapted with permission from [21].
The human epidermal growth factor receptor 2 (Her-2) expressed by approximately 20–30% of breast cancers is another example of a receptor that can be used for theranostics. Trastuzumab, a humanized monoclonal antibody directed against the extracellular domain of Her-2 has provided a breakthrough in the treatment of Her-2 positive metastatic breast cancer [26]. Patients with Her-2/neu overexpressing cancers respond well to antibody targeting of Her-2/neu with trastuzumab or with the dual tyrosine kinase inhibitor lapatinib that inhibits EGFR/ErbB1 and Her-2/ErbB2, but a percentage of patients develop resistance due to adaptation of signaling pathways [27]. Her-2 is therefore an attractive target for theranostic imaging of antibody resistant tumors. In a different strategy, a two-component, two-step, pre-targeting drug delivery system was developed to deliver therapeutic cargo under image-guidance [28]. In this strategy, Her-2 receptors were pre-labeled with a functionalized trastuzumab antibody followed by the delivery of drug-loaded nanocarriers. Both components were cross-linked by multiple bioorthogonal click reactions in situ on the surface of the target cell, and internalized as nanoclusters [29]. A trastuzumab-based component was initially delivered to label the Her-2(+) tumor cells. This was followed by administration of a paclitaxel-loaded albumin carrier as the cytotoxic treatment component. The localization of the pre-targeting component was determined with noninvasive imaging to guide the administration and timing of the cytotoxic drug carrier component to minimize side effects. This theranostic strategy achieved high therapeutic efficacy with no significant toxicity in a Her-2 positive human breast cancer xenograft. The feasibility of such a strategy using MRI is presented in Figure 4. Briefly, the delivery and retention of the albumin carrier in a tumor that was pretargeted with functionalized mAbs were monitored by T1-weighted imaging at 9.4T using albumin conjugated with GdDTPA groups.
Figure 4.

Bioorthogonal pretargeting theranostics. (A) Model illustrating the strategy of the target-specific, two-component, two-step drug delivery driven by bioorthogonal trans-cyclooctene (TCO)/tetrazine (Tt) click chemistry in Her-2(+) cancers. Briefly, in this strategy, Her-2(+) cancer cells are prelabeled with TCO conjugated trastuzumab. Drug-loaded albumin conjugated with Tt is delivered subsequently. Multiple click reactions between two components form nanoclusters enhancing the internalization. Adapted with permission from [28]. (B) 3D reconstructed MR images of pretargeted theranostics in tumors showing contrast enhancement in the BT-474 tumor treated with specific mAb and albumin-GdDTPA (right) and control tumor treated with non-reactive components (left). Images were acquired at ~2h after administration of albumin-GdDTPA on a Bruker 9.4T Biospec system using a mouse volume coil and 3D gradient echo T1-weighted sequence with parameters TE/TR= 2/100ms and RF pulse flip angle of 60-degree.
Antibodies are used for cancer cell-specific targeting but their size presents limitations that can be overcome by using either antibody fragments or peptides. Even fully humanized antibodies, can induce an immune response through their Fc domain, and their relatively large size may limit tissue penetration [10]. Because of their smaller size, fragment antibodies have improved tissue penetration, while lack of the Fc domain results in decreased immunogenicity.
Other multifunctional nanoparticles targeting Her-2 have been described for cancer detection and treatment [30]. A laser activated multimodal polymeric nanoparticle has been recently developed to image and treat Her2-positive breast cancer [30]. The particles combined MR, photoacoustic (PA), US, and near-infrared fluorescence (NIRF) imaging abilities with photothermal capabilities. When irradiated by a NIR laser, the liquid perfluoropentane encapsulated in the PLGA polymer shell vaporized to a gas, rapidly expelling the contents and damaging surrounding tissues. The resulting micro-sized bubbles were visualized with US. The treated mice had a nearly complete response to treatment [30].
Peptides are also used as targeting agents in theranostic strategies. Peptides present multiple advantages including a smaller size, reduced immunogenicity, high multivalency, deep tumor penetration, ease of synthesis and lower production cost [31]. Lung cancer-binding peptides were identified using phage display biopanning of H460 lung cancer cells. Three targeting phages (HPC1, HPC2, and HPC4) and their respective displayed peptides (HSP1, HSP2, and HSP4) bound to small cell lung cancer (SCLC) and non small cell lung cancer (NSCLC) cell lines, and to in vivo human lung cancer tissue, but not to normal lung tissues. While optical and MR imaging studies revealed that HSP1 was the most favorable probe for multimodal molecular imaging (Figure 5), HSP4 was better endocytosed. Liposomal doxorubicin (LD) conjugated to HSP1, HSP2, or HSP4 was tested and showed significantly greater therapeutic efficacy compared to non-targeted liposomal drugs in NSCLC animal models [31].
Figure 5.

(A) T2-weighted MR images subjected to pseudo-color mapping to reveal signal changes in tumor tissues showing signal reduction in blue, corresponding to roughly 4/5, 3/4, and 1/4 of tumor area at 6 h post-contrast with HSP1-, HSP2-, and HSP4-Dex-Fe3O4 nanoparticles, respectively. (B) Histological analyses of H460 tumor tissue specimens acquired 24 h after injection of HSP1-, HSP2-, HSP4-, or Ctrl P-Dex-Fe3O4 nanoparticles. Sections stained with Prussian blue to detect Fe deposition, and counterstained with Nuclear Fast Red. Yellow arrows indicate blood vessels of tumor tissue. (C) Quantification of Prussian blue reaction products from the representative tumor sections. **, P<0.01; * * *, P<0.001 compared with Ctrl P-Dex-Fe3O4 group. Adapted with permission from [31].
The RGD peptide is used for vascular targeting because it binds to αvβ3 integrins expressed by tumor vasculature. Gold nanoparticles (AuNPs) have been gaining interest as potential radiosensitizers in tumor therapy. A novel cyclic c(RGDyC)-peptide-conjugated with Gd- and 99mTc-labeled AuNPs (RGD@AuNPs-Gd99 mTc) probe was developed and evaluated for radiosensitization therapy (Figure 6) [32]. The probe presented a high specificity for αvβ3 integrin positive cells and high cellular uptake. In vivo MR and SPECT/CT imaging showed specific targeting to the tumors. While the 80 nm RGD@AuNPs-Gd99 mTc probe exhibited the greatest effects in vitro, the 29 nm RGD@AuNPs-Gd99 mTc probes were the most efficient in vivo. RGD@AuNPs-Gd99 mTc probes have the potential of guiding and enhancing radiation therapy of tumors [32].
Figure 6.

(A) schematic illustrations of RGD@AuNPs-Gd99 mTc targeting tumors for theranostics, (B) SPECT/CT imaging of mice bearing H1299 tumors after intravenous injection with the RGD@AuNPs-Gd99 mTc (RGD) probe, (C) T1-weighted MR imaging of H1299 tumor after intravenous injection with the 29 nm RGD@AuNPs-GdTc (RGD) probe. Adapted with permission from [32].
Natural killer (NK) cells play a critical role as part of the innate immune system, and several therapeutic approaches based on their adoptive transfer are being developed. The efficacy of their transfer depends on their ability to recognize and target the tumors. Iron oxide nanoparticles (IONPs) are endocytosed or phagocytosed by cells, especially immune cells such as macrophages and natural killer (NK) cells, providing the ability to track these cells with MRI through changes in T2 and T2* induced by these particles [33, 34]. Low dose focused ultrasound ldb with microbubbles (FUS) was used to facilitate the targeting and accumulation of NK cells in a mouse xenograft model of human colorectal cancer [15]. Human NK cells were labeled with ultra small iron oxide particle (USPIO) for MRI detection. Simultaneously with the intravenous injection of microbubbles, focused ultrasound was applied to the tumor. Significant accumulation of NK cells was detected with MRI up to 24 h in the tumor when ensonified with 0.50 MPa peak acoustic pressure, whereas tumors treated with 0.25 MPa showed no detectable NK cell accumulation (Figure 7). These clinically translatable results show that ldbFUS of the tumor mass can potentiate tumor homing of NK cells that can be evaluated non-invasively using MRI.
Figure 7.

(A) Representative parametric R2* images (axial slices) of an NSG mouse bearing bilateral flank LS-174T tumors before (pre), 1 h, 6 h, and 24 h post ldbFUS/Fe-NK administration. Yellow outlines show tumor regions of interest. Note that at some imaging time points, left and right tumors were not in the same field of view–thus left & right sides (dashed line) may be from slightly different axial slices. Units in sec−1. (B) Histogram showing relative frequency R2* distributions of whole tumors from (A). The histogram shifts to increasing values of R2* with time in (+)ldbFUS tumor. Adapted with permission from [15].
Macrophages are a critical component of the innate immune system. Multiple studies have been performed to track the recruitment of tumor-associated macrophages (TAMs) in tumors by labeling them with IONPs [33, 35]. Recently the effects of IONPs on inhibiting tumor growth by inducing pro-inflammatory macrophage polarization in tumor tissues were reported [36].
IONPs can be also used to therapeutically target cancer cells, as shown in a glioblastoma (GBM) model [37, 38]. Epidermal growth factor receptor (EGFR) is frequently overexpressed in GBM. IONPs conjugated to an EGFR inhibitor, cetuximab, allowed cancer cell detection by MRI and targeted therapy of a GBM model in vitro and in vivo. Treatment with cetuximab-EGFR IONPs resulted in an antitumor effect greater than that with cetuximab alone due to cellular targeting and uptake [37]. More recently cetuximab-IONPs were found to increase the radiosensitivity of radioresistant GBM [38].
Nontargeted theranostics
Nontargeted theranostic probes rely on the permeability of tumor vasculature to extravasate into tumor tissue but not into normal tissues. Probes with a size of 5–200 nm can effectively extravasate within the tumor interstitium, although as the size of the probe increases movement through the ECM may be limited. The ECM is consists of fiber proteins and glycosaminoglycans [39] that form an interlocking network that may act as a barrier to the diffusion of probes through the tumor interstitium. However, recent work suggests that collagen 1 fibers can in fact mediate transport [40, 41]. Improved delivery and diffusion properties have also been observed for elongated probes such as nanorods, nanotubes, or linear polymer chains due to easier extravasation because of their ‘string-like’ structures and potentially faster diffusion through the ECM [42].
A “one-for-all” nanocrystal combining three imaging capabilities with image-guided photothermal therapy (PTT) and oxygen regulation was recently described [13]. The study was performed with an experimental model of breast cancer using Prussian blue (PB)/manganese dioxide (MnO2) hybrid nanoparticles (PBMn) smaller than 50 nm. MnO2 controlled the particle size, the optical properties, and the transverse relaxation rate of PB. It also enhanced the catalytic efficacy of PB to H2O2 for oxygen generation. PBMn was used for photoacoustic imaging (PAI) and as a longitudinal relaxation (T1) MRI contrast. The particle also regulated the oxygen partial pressure of tumor tissue and modified the ratio of oxygenated to deoxygenated hemoglobin, modifying the T2-weighted imaging signal intensity. This trimodal particle inhibited the growth of MCF-7 human breast cancer xenografts following PTT [13].
Tumor microenvironment and stimuli based theranostics
Physiological environments such as hypoxia and acidic extracellular pH have been exploited in innovative strategies to detect these environments and deliver cargo exclusively within these environments. As an example, pH-responsive micelles that show pH-dependent demicellization at pH below 6.5 have been developed [43].
Transition metal dichalcogenides (TMDCs), such as MoS2, WS2, Bi2Se3 and TiS2 nanosheets have recently emerged as promising nanostructures for multimodal imaging, drug delivery, and cancer therapy due to their high surface area, and absorbance in the NIR region [11]. Certain types of TMDCs, such as Bi2Se3 and WS2, contain high-Z elements that can be used for enhanced radiotherapy (RT) by depositing radiation energy in the tumor [11]. A WS2 based nanocomposite that combined IONPs with silica and MnO2 was recently developed [11]. The particle was highly sensitive to pH, enabling tumor pH-responsive MRI with IONPs acting as a pH-inert T2 contrast probe, and MnO2 as the pH-sensitive T1 contrast probe. Using this probe, synergistic tumor therapy that combined NIR light and X-ray absorbance of WS2 for PTT and enhanced cancer radiotherapy (RT), respectively, as well as the ability of MnO2 to decompose tumor endogenous H2O2 and relieve tumor hypoxia to further overcome hypoxia-associated radiotherapy resistance was achieved. The efficacy of this novel therapeutic approach was demonstrated in a 4T1 breast tumor model [11].
pH-responsive nanoparticles have been developed to specifically deliver therapeutic agents to the tumor and limit damage to normal tissue. Results from a novel core-shell PB@MIL-100(Fe) dual metal-organic-frameworks (d-MOFs) nanoparticle with theranostic properties is shown in Figure 8 [44]. The d-MOFs nanoparticles were characterized by a T1-T2 dual-modal MRI contrast as well as fluorescent properties arising from the presence of inner PB MOFs and outer MIL-100(Fe) MOFs [44]. Artemisinin, an anti-malarial agent with anticancer activity [45], was used as the therapeutic agent. The anticancer properties of Artemisinin have been linked to free radical formation in cancer cells. Artemisinin was released from the d-MOFs upon tumor cell endocytosis due to the pH-responsive degradation of the outer MOFs in low pH lysosomes after delivery of the particle through the enhanced permeability and retention (EPR) effect. The inner PB MOFs allowed PTT due to a strong absorbance in the NIR region. This novel theranostic nanoagent, characterized by multimodal imaging capabilities, and synergistic therapy capabilities through combined chemotherapy and PTT, could lead to a promising next generation of nanomedicine for efficient and safe cancer therapy [44].
Figure 8.

(A) Schematic illustrations of d-MOFs targeting tumors for combined therapy, loading and delivery of d-MOFs in tumors through EPR effect and pH-responsive degradation of outer MOFs for drug release and dual-modal fluorescence and MRI-guided cancer therapy. (B) T1-(top) and T2*-weighted (bottom) in vivo MR images of tumors before, 10 min, 30 min and 24 h after intravenous injection of d-MOFs. T1-weighted in vivo MR images (coronal planes) of (top) lung circled with dashed line, (middle) liver circled with dashed line, and (bottom) kidney circled with dashed line before, 30 min and 24 h after intravenous injection of d-MOFs. T2*-weighted in vivo MR images (coronal planes) of (top) lung circled with dashed line, (middle) liver circled with dashed line, and (bottom) tumor circled with dashed line before, 30 min and 24 h after intravenous injection of d-MOFs. Adapted with permission from [44].
Tumor acidity-responsive NPs have been developed for optical and MRI guided photodynamic therapy (PDT) [46]. PDT allows site-specific therapy using low optical power densities limiting damage to normal tissues. The particle contained a distinct three-layer nanostructure with tumor acidity-responsiveness that encapsulated the photosensitizer chlorin e6 (Ce6) and chelated Gd3+. While the outer PEG layer of the particle significantly prolonged circulation time, the inner poly(ε-caprolactone) (PCL) core allowed the encapsulation of Ce6. The middle layer of the particle acted as a molecular fence to keep Ce6 contained in the hydrophobic core during circulation. After accumulation in the tumor tissue, this pH sensitive layer was dismantled, following which the PEG shell was deshielded. Deshielding of the PEG shell from the particle within the tumor improved cell uptake, increased MR signal intensity, rapidly released Ce6 within tumor cells, and elevated PDT efficacy [46].
Redox responsive particles have also been developed for NIR and MR imaging guided PDT [47]. To improve the therapeutic efficiency of PDT, a multifunctional nanoparticle system consisting of Fe3O4 loaded in redox-responsive Ce6 conjugated dextran NPs was used for NIR and MRI imaging as well as magnetic targeting. The Ce6 fluorescence signal was visible after self-quenching in a reductive intracellular environment, while the iron present in the NP was visualized by T2-weighted MR imaging. In vivo MRI showed accumulation of the particles in tumors under a magnetic field. These novel NPs could be a promising theranostic system for both NIR/MR imaging-guided PDT precision therapy [47].
The TME is characterized by the presence of proteases that remodel the ECM, such as matrix metalloproteinases (MMPs) [48]. A novel strategy to improve glioblastoma (GBM) treatment strategy based on tumor enzyme-activatable theranostic nanoparticles was recently described [49]. This theranostic cross-linked iron oxide nanoparticle (CLIO) was conjugated to a highly potent vascular disrupting agent (ICT, azademethylcolchicine) and secured with a MMP-14 cleavable peptide. As shown in Figure 9, negative contrast in T2 weighted images could be observed in vitro and in vivo after delivery of CLIO-ICT into the tumors due to the presence of iron. Treatment with CLIO-ICT disrupted tumor vasculature of MMP-14-expressing GBM, inducing GBM cell apoptosis, and significant reduction of tumor growth. Treatment with CLIO-ICT plus temozolomide achieved tumor remission and significantly increased survival of tumor-bearing mice by more than 2-fold compared to temozolomide alone [49].
Figure 9.

(A) Schematic demonstration of CLIO-ICT activation in presence of tumor enzyme MMP-14, to release the active drug, azademethylcolchicine. Azademethylcolchicine targets tubulin to induce apoptosis in tumor cells. (B) Representative T2-weighted MR images of CLIO-ICT and ferumoxytol at different dilutions. T2 MSME sequences were used to generate R2 relaxivities. R2 relaxivities of CLIO-ICT (blue line) and ferumoxytol (red line). (C) Representative T2-weighted MR images of mouse brain. Nanoparticle and theranostic nanoparticle delivery is demonstrated by T2 darkening or negative enhancement in CLIO and CLIO-ICT–treated animals, respectively. On day 14, the tumor periphery is marked by dotted yellow line. (D) Quantification of T2 darkening. CLIO and CLIO-ICT–treated tumors demonstrated shorter T2 values corresponding to T2 darkening or negative enhancement. Adapted with permission from [49].
High-intensity focused ultrasound (HIFU) is a noninvasive technique that can be used for thermal ablation of target volumes deep within the body [14]. Imaging during treatment is crucial to localize the target area. MRI offers multiple advantages including excellent anatomical imaging for treatment planning, real-time temperature monitoring during therapy, and direct evaluation of treatment results [14]. An initial study of tumor ablation in 10 breast cancer patients using an MR-HIFU breast platform specifically designed for breast tumor ablation was recently reported [14]. In this dedicated platform, the breast was targeted laterally, reducing the risk of heating the heart and lungs. The wide transducer aperture used decreased local energy density in the skin during ablation. In this study, larger and more homogeneous ablation volumes were achieved together with a reduction in treatment duration. The safety and feasibility of tumor ablation in breast cancer patients was evaluated using this novel platform, and showed that MR-HIFU ablation with a dedicated breast system was safe and resulted in histopathologically proven tumor necrosis [14].
Challenges and future directions
Cancer immunotherapy using CAR-T cells and the inhibition of immune checkpoints has generated significant excitement and hope in cancer treatment [50, 51]. MRI and MRSI will play valuable roles in these treatments to track immune cells and gain new insights into the metabolism of these cells to improve treatment outcome. As novel MR probes that target specific aspects of cancer such as collagen 1 fibers [52], fibroblasts [53] or molecules such as extradomain B Fibronectin [54] present in the tumor stroma are being developed, these probes will lead to new theranostic strategies of the TME.
Theranostics is a young field that, as it matures, will have to address several issues such as reproducibility of synthesis, side-effects of the probes used such as the induction of an inflammatory response, and translation to human applications. Despite these challenges, it is clear that the most exciting advances in cancer precision medicine will occur at the interface of chemistry, molecular biology, and imaging applications in theranostics.
Theranostic imaging is a rapidly expanding aspect of molecular imaging that is located at the interface of diagnosis and therapy.
A critical need in cancer treatment is to minimize damage to normal tissue. MR molecular imaging can be applied to identify targets specific to cancer with imaging, design agents against targets and visualize their delivery, monitor response to treatment, and minimize collateral damage to normal tissue.
Because of the complex characteristics of cancer, genomic and proteomic profiling can provide an extensive ‘fingerprint’ of each tumor.
With this cancer fingerprint, theranostic agents can be designed to personalize treatment for precision medicine of cancer, and minimize damage to normal tissue.
Acknowledgments
Support from NIH R01 CA82337, R01 CA136576, R01 CA193365 and R35 CA209960 is gratefully acknowledged. We gratefully acknowledge Dr. Ackerman’s kind support and valuable discussions over the past decade.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of References
- 1.Stasinopoulos I, Penet MF, Chen Z, Kakkad S, Glunde K, Bhujwalla ZM. Exploiting the tumor microenvironment for theranostic imaging. NMR Biomed. 2011;24(6):636–47. doi: 10.1002/nbm.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng L, Benkert T, Block KT, Sodickson DK, Otazo R, Chandarana H. Compressed sensing for body MRI. J Magn Reson Imaging. 2017;45(4):966–87. doi: 10.1002/jmri.25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Chen H, Xu J, Yadav NN, Chan KW, Luo L, McMahon MT, Vogelstein B, van Zijl PC, Zhou S, Liu G. CEST theranostics: label-free MR imaging of anticancer drugs. Oncotarget. 2016;7(6):6369–78. doi: 10.18632/oncotarget.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivlin M, Navon G. CEST MRI of 3-O-methyl-D-glucose on different breast cancer models. Magn Reson Med. 2017 doi: 10.1002/mrm.26752. [DOI] [PubMed] [Google Scholar]
- 5.Xu X, Yadav NN, Knutsson L, Hua J, Kalyani R, Hall E, Laterra J, Blakeley J, Strowd R, Pomper M, Barker P, Chan K, Liu G, McMahon MT, Stevens RD, van Zijl PC. Dynamic Glucose-Enhanced (DGE) MRI: Translation to Human Scanning and First Results in Glioma Patients. Tomography. 2015;1(2):105–14. doi: 10.18383/j.tom.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timm KN, Hu DE, Williams M, Wright AJ, Kettunen MI, Kennedy BW, Larkin TJ, Dzien P, Marco-Rius I, Bohndiek SE, Brindle KM. Assessing Oxidative Stress in Tumors by Measuring the Rate of Hyperpolarized [1–13C]Dehydroascorbic Acid Reduction Using 13C Magnetic Resonance Spectroscopy. J Biol Chem. 2017;292(5):1737–48. doi: 10.1074/jbc.M116.761536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal R, Vigneron DB, Kurhanewicz J. Hyperpolarized 1-[(13)C]-Pyruvate Magnetic Resonance Imaging Detects an Early Metabolic Response to Androgen Ablation Therapy in Prostate Cancer. Eur Urol. 2017;72(6):1028–9. doi: 10.1016/j.eururo.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaittanis C, Shaffer TM, Thorek DL, Grimm J. Dawn of advanced molecular medicine: nanotechnological advancements in cancer imaging and therapy. Critical reviews in oncogenesis. 2014;19(3–4):143–76. doi: 10.1615/critrevoncog.2014011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond) 2008;3(5):703–17. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cormode DP, Skajaa T, Fayad ZA, Mulder WJ. Nanotechnology in medical imaging: probe design and applications. Arterioscler Thromb Vasc Biol. 2009;29(7):992–1000. doi: 10.1161/ATVBAHA.108.165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang G, Zhang R, Liang C, Zhao H, Yi X, Shen S, Yang K, Cheng L, Liu Z. Manganese Dioxide Coated WS2 @Fe3 O4 /sSiO2 Nanocomposites for pH-Responsive MR Imaging and Oxygen-Elevated Synergetic Therapy. Small. 2017 doi: 10.1002/smll.201702664. [DOI] [PubMed] [Google Scholar]
- 12.Wright CM, Wright RC, Eshleman JR, Ostermeier M. A protein therapeutic modality founded on molecular regulation. Proc Natl Acad Sci U S A. 2011;108(39):16206–11. doi: 10.1073/pnas.1102803108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng J, Dong M, Ran B, Li W, Hao Y, Yang Q, Tan L, Shi K, Qian Z. “One-for-All”-Type, Biodegradable Prussian Blue/Manganese Dioxide Hybrid Nanocrystal for Trimodal Imaging-Guided Photothermal Therapy and Oxygen Regulation of Breast Cancer. ACS Appl Mater Interfaces. 2017;9(16):13875–86. doi: 10.1021/acsami.7b01365. [DOI] [PubMed] [Google Scholar]
- 14.Merckel LG, Knuttel FM, Deckers R, van Dalen T, Schubert G, Peters NH, Weits T, van Diest PJ, Mali WP, Vaessen PH, van Gorp JM, Moonen CT, Bartels LW, van den Bosch MA. First clinical experience with a dedicated MRI-guided high-intensity focused ultrasound system for breast cancer ablation. Eur Radiol. 2016;26(11):4037–46. doi: 10.1007/s00330-016-4222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sta Maria NS, Barnes SR, Weist MR, Colcher D, Raubitschek AA, Jacobs RE. Low Dose Focused Ultrasound Induces Enhanced Tumor Accumulation of Natural Killer Cells. PLoS One. 2015;10(11):e0142767. doi: 10.1371/journal.pone.0142767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Xiong Z, Wu Y, Cai W, Tseng JR, Gambhir SS, Chen X. Quantitative PET imaging of tumor integrin alphavbeta3 expression with 18F-FRGD2. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2006;47(1):113–21. [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Krishnamachary B, Penet MF, Bhujwalla ZM. Acid-degradable Dextran as an Image Guided siRNA Carrier for COX-2 Downregulation. Theranostics. 2018;8(1):1–12. doi: 10.7150/thno.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulke N, Varlamova OA, Donovan GP, Ma D, Gardner JP, Morrissey DM, Arrigale RR, Zhan C, Chodera AJ, Surowitz KG, Maddon PJ, Heston WD, Olson WC. The homodimer of prostate-specific membrane antigen is a functional target for cancer therapy. Proc Natl Acad Sci U S A. 2003;100(22):12590–5. doi: 10.1073/pnas.1735443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X, Bennett M, Thorpe PE. Anti-tumor effects and lack of side effects in mice of an immunotoxin directed against human and mouse prostate-specific membrane antigen. Prostate. 2004;61(1):1–11. doi: 10.1002/pros.20074. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Penet MF, Krishnamachary B, Banerjee SR, Pomper MG, Bhujwalla ZM. PSMA-specific theranostic nanoplex for combination of TRAIL gene and 5-FC prodrug therapy of prostate cancer. Biomaterials. 2016;80:57–67. doi: 10.1016/j.biomaterials.2015.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z, Penet MF, Nimmagadda S, Li C, Banerjee SR, Winnard PT, Jr, Artemov D, Glunde K, Pomper MG, Bhujwalla ZM. PSMA-targeted theranostic nanoplex for prostate cancer therapy. ACS Nano. 2012;6(9):7752–62. doi: 10.1021/nn301725w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Winnard PT, Jr, Takagi T, Artemov D, Bhujwalla ZM. Multimodal image-guided enzyme/prodrug cancer therapy. J Am Chem Soc. 2006;128(47):15072–3. doi: 10.1021/ja066199i. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Penet MF, Wildes F, Takagi T, Chen Z, Winnard PT, Artemov D, Bhujwalla ZM. Nanoplex delivery of siRNA and prodrug enzyme for multimodality image-guided molecular pathway targeted cancer therapy. ACS Nano. 2010;4(11):6707–16. doi: 10.1021/nn102187v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat Rev Cancer. 2011;11(12):835–48. doi: 10.1038/nrc3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallagher FA, Kettunen MI, Day SE, Hu DE, Ardenkjaer-Larsen JH, Zandt R, Jensen PR, Karlsson M, Golman K, Lerche MH, Brindle KM. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature. 2008;453(7197):940–3. doi: 10.1038/nature07017. [DOI] [PubMed] [Google Scholar]
- 26.Metro G, Mottolese M, Fabi A. HER-2-positive metastatic breast cancer: trastuzumab and beyond. Expert Opin Pharmacother. 2008;9(15):2583–601. doi: 10.1517/14656566.9.15.2583. [DOI] [PubMed] [Google Scholar]
- 27.Freudenberg JA, Wang Q, Katsumata M, Drebin J, Nagatomo I, Greene MI. The role of HER2 in early breast cancer metastasis and the origins of resistance to HER2-targeted therapies. Exp Mol Pathol. 2009;87(1):1–11. doi: 10.1016/j.yexmp.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hapuarachchige S, Kato Y, Artemov D. Bioorthogonal two-component drug delivery in HER2(+) breast cancer mouse models. Sci Rep. 2016;6:24298. doi: 10.1038/srep24298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu W, Okollie B, Artemov D. Controlled internalization of Her-2/neu receptors by cross-linking for targeted delivery. Cancer Biol Ther. 2007;6(12):1960–6. doi: 10.4161/cbt.6.12.4979. [DOI] [PubMed] [Google Scholar]
- 30.Deng L, Cai X, Sheng D, Yang Y, Strohm EM, Wang Z, Ran H, Wang D, Zheng Y, Li P, Shang T, Ling Y, Wang F, Sun Y. A Laser-Activated Biocompatible Theranostic Nanoagent for Targeted Multimodal Imaging and Photothermal Therapy. Theranostics. 2017;7(18):4410–23. doi: 10.7150/thno.21283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chi YH, Hsiao JK, Lin MH, Chang C, Lan CH, Wu HC. Lung Cancer-Targeting Peptides with Multi-subtype Indication for Combinational Drug Delivery and Molecular Imaging. Theranostics. 2017;7(6):1612–32. doi: 10.7150/thno.17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Zhang L, Cai J, Li X, Cheng D, Su H, Zhang J, Liu S, Shi H, Zhang Y, Zhang C. Tumor Angiogenesis Targeted Radiosensitization Therapy Using Gold Nanoprobes Guided by MRI/SPECT Imaging. ACS Appl Mater Interfaces. 2016;8(3):1718–32. doi: 10.1021/acsami.5b09274. [DOI] [PubMed] [Google Scholar]
- 33.Daldrup-Link HE, Golovko D, Ruffell B, Denardo DG, Castaneda R, Ansari C, Rao J, Tikhomirov GA, Wendland MF, Corot C, Coussens LM. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin Cancer Res. 2011;17(17):5695–704. doi: 10.1158/1078-0432.CCR-10-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daldrup-Link HE, Meier R, Rudelius M, Piontek G, Piert M, Metz S, Settles M, Uherek C, Wels W, Schlegel J, Rummeny EJ. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur Radiol. 2005;15(1):4–13. doi: 10.1007/s00330-004-2526-7. [DOI] [PubMed] [Google Scholar]
- 35.Valable S, Barbier EL, Bernaudin M, Roussel S, Segebarth C, Petit E, Remy C. In vivo MRI tracking of exogenous monocytes/macrophages targeting brain tumors in a rat model of glioma. Neuroimage. 2008;40(2):973–83. doi: 10.1016/j.neuroimage.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM, Daldrup-Link HE. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11(11):986–94. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaluzova M, Bouras A, Machaidze R, Hadjipanayis CG. Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget. 2015;6(11):8788–806. doi: 10.18632/oncotarget.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouras A, Kaluzova M, Hadjipanayis CG. Radiosensitivity enhancement of radioresistant glioblastoma by epidermal growth factor receptor antibody-conjugated iron-oxide nanoparticles. J Neurooncol. 2015;124(1):13–22. doi: 10.1007/s11060-015-1807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theocharis AD, Karamanos NK. Proteoglycans remodeling in cancer: Underlying molecular mechanisms. Matrix Biol. 2017 doi: 10.1016/j.matbio.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Kakkad SM, Penet MF, Akhbardeh A, Pathak AP, Solaiyappan M, Raman V, Leibfritz D, Glunde K, Bhujwalla ZM. Hypoxic tumor environments exhibit disrupted collagen I fibers and low macromolecular transport. PLoS One. 2013;8(12):e81869. doi: 10.1371/journal.pone.0081869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakkad S, Zhang J, Akhbardeh A, Jacob D, Krishnamachary B, Solaiyappan M, Jacobs MA, Raman V, Leibfritz D, Glunde K, Bhujwalla ZM. Collagen fibers mediate MRI-detected water diffusion and anisotropy in breast cancers. Neoplasia. 2016;18(10):585–93. doi: 10.1016/j.neo.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raphael AP, Sisney JP, Liu DC, Prow TW. Enhanced delivery of nano- and submicron particles using elongated microparticles. Curr Drug Deliv. 2015;12(1):78–85. doi: 10.2174/1567201811666140904143542. [DOI] [PubMed] [Google Scholar]
- 43.Koo H, Lee H, Lee S, Min KH, Kim MS, Lee DS, Choi Y, Kwon IC, Kim K, Jeong SY. In vivo tumor diagnosis and photodynamic therapy via tumoral pH-responsive polymeric micelles. Chem Commun (Camb) 2010;46(31):5668–70. doi: 10.1039/c0cc01413c. [DOI] [PubMed] [Google Scholar]
- 44.Wang D, Zhou J, Chen R, Shi R, Zhao G, Xia G, Li R, Liu Z, Tian J, Wang H, Guo Z, Wang H, Chen Q. Controllable synthesis of dual-MOFs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials. 2016;100:27–40. doi: 10.1016/j.biomaterials.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 45.Crespo-Ortiz MP, Wei MQ. Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. J Biomed Biotechnol. 2012;2012:247597. doi: 10.1155/2012/247597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan F, Yu Y, Zhong F, Gao M, Sun T, Liu J, Zhang H, Qian H, Tao W, Yang X. Design of Tumor Acidity-Responsive Sheddable Nanoparticles for Fluorescence/Magnetic Resonance Imaging-Guided Photodynamic Therapy. Theranostics. 2017;7(5):1290–302. doi: 10.7150/thno.18557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding Z, Liu P, Hu D, Sheng Z, Yi H, Gao G, Wu Y, Zhang P, Ling S, Cai L. Redox-responsive dextran based theranostic nanoparticles for near-infrared/magnetic resonance imaging and magnetically targeted photodynamic therapy. Biomater Sci. 2017;5(4):762–71. doi: 10.1039/c6bm00846a. [DOI] [PubMed] [Google Scholar]
- 48.Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2015;237(3):273–81. doi: 10.1002/path.4586. [DOI] [PubMed] [Google Scholar]
- 49.Mohanty S, Chen Z, Li K, Morais GR, Klockow J, Yerneni K, Pisani L, Chin FT, Mitra S, Cheshier S, Chang E, Gambhir SS, Rao J, Loadman PM, Falconer RA, Daldrup-Link HE. A Novel Theranostic Strategy for MMP-14-Expressing Glioblastomas Impacts Survival. Mol Cancer Ther. 2017;16(9):1909–21. doi: 10.1158/1535-7163.MCT-17-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kagoya Y, Tanaka S, Guo T, Anczurowski M, Wang CH, Saso K, Butler MO, Minden MD, Hirano N. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med. 2018 doi: 10.1038/nm.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eroglu Z, Zaretsky JM, Hu-Lieskovan S, Kim DW, Algazi A, Johnson DB, Liniker E, Ben K, Munhoz R, Rapisuwon S, Gherardini PF, Chmielowski B, Wang X, Shintaku IP, Wei C, Sosman JA, Joseph RW, Postow MA, Carlino MS, Hwu WJ, Scolyer RA, Messina J, Cochran AJ, Long GV, Ribas A. High response rate to PD-1 blockade in desmoplastic melanomas. Nature. 2018;553(7688):347–50. doi: 10.1038/nature25187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polasek M, Yang Y, Schuhle DT, Yaseen MA, Kim YR, Sung YS, Guimaraes AR, Caravan P. Molecular MR imaging of fibrosis in a mouse model of pancreatic cancer. Sci Rep. 2017;7(1):8114. doi: 10.1038/s41598-017-08838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vandsburger MH, Radoul M, Addadi Y, Mpofu S, Cohen B, Eilam R, Neeman M. Ovarian carcinoma: quantitative biexponential MR imaging relaxometry reveals the dynamic recruitment of ferritin-expressing fibroblasts to the angiogenic rim of tumors. Radiology. 2013;268(3):790–801. doi: 10.1148/radiol.13122053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Han Z, Roelle S, DeSanto A, Sabatelle R, Schur R, Lu ZR. Synthesis and Assessment of Peptide Gd-DOTA Conjugates Targeting Extradomain B Fibronectin for Magnetic Resonance Molecular Imaging of Prostate Cancer. Mol Pharm. 2017;14(11):3906–15. doi: 10.1021/acs.molpharmaceut.7b00619. [DOI] [PMC free article] [PubMed] [Google Scholar]


