Abstract
Introduction
Increased levels of circulating tissue factor (TF)-positive microvesicles (MVs) may increase the risk of thrombosis. Indeed, TF-positive MVs are detected in plasma of patients with various types of diseases. In this study, we measured levels of MV TF activity in non-cancer severely ill patients and cancer patients.
Methods
We used an in-house MV TF activity assay to measure MV TF activity.
Results
MV TF activity was significantly increased in a population of cancer patients but not in a population of non-cancer severely ill patients compared with healthy controls. However, in the population of severely ill patients, those with infection had significantly elevated levels of MV TF activity compared with controls. Interestingly, patients with adenocarcinoma had higher levels of MV TF activity compared with patients with non-adenocarcinoma tumors. Levels of MV TF activity were not associated with venous thromboembolism in cancer patients. MV TF activity was associated with reduced survival in cancer patients.
Conclusion
Cancer patients as well as severely ill patients with infection have higher levels of MV TF activity compared with healthy controls. Patients with adenocarcinoma have higher levels of MV TF activity compared with patients with other types of cancer. Elevated levels of MV TF activity were associated with reduced survival in cancer patients.
Keywords: cancer, microvesicles, severely ill, survival, tissue factor
Introduction
The tissue factor (TF)-factor VIIa (FVIIa) complex triggers the extrinsic pathway of coagulation [1]. TF containing microvesicles (MVs) are submicron vesicles released from activated, apoptotic or cancer cells [2, 3]. Increased levels of circulating MV TF activity have been detected in patients with various diseases, including acute liver injury [4], cirrhosis [5], urinary tract infection [6], influenza [7], endotoxemia [8] and cancer [9-11]. An early study analyzed the procoagulant activity of MVs in meningococcal sepsis [12]. Interestingly, MVs from non-surviving patients had significantly higher levels of thrombin generation compared with surviving patients. Another study found that MVs from patients with meningococcal septic shock had higher levels of TF-dependent thrombin generation than MVs from patients with meningococcal meningitis [13]. Similarly, we found higher levels of MV TF activity in non-surviving patients with severe influenza A/H1N1 infection compared with surviving patients [7]. It is likely that TF-positive (TF+) MVs may increase the risk of disseminated intravascular coagulation and venous thromboembolism (VTE) [14]. For instance, levels of MV TF activity are associated with VTE in patients with pancreatic cancer, but not in patients with other types of cancer, such as brain, stomach, colorectal cancer or multiple myeloma [9-11, 14, 15]. In addition, high levels of MV TF activity in cancer patients are associated with reduced survival [9, 15].
In this study, we compared the levels of MV TF activity in non-cancer severely ill patients with those in cancer patients. Our hypothesis was that all non-cancer severely ill patients would have elevated levels of MV TF activity regardless of the underlying illness.
Methods
Study design and study population
The study complied with the declaration of Helsinki, and the study protocol was approved by the Stockholm Ethical Review Board (Dnr 2015/1533-31/1). All study participants provided written informed consent. 60 cancer patients, 51 severely ill and hospitalized patients without known cancer, and 50 healthy individuals were prospectively recruited between October 2015 and March 2017. Cancer patients were recruited during hospitalization at the palliative in-patient unit at Stockholms Sjukhem, Stockholm. Inclusion criterion was active cancer, defined as either diagnosis <1 year prior to study inclusion and/or disseminated disease, comprising a variety of advanced malignancies. There were no exclusion criteria, and patients were recruited consecutively at all times when research personnel were available. Severely ill and hospitalized patients without known cancer were recruited at the Department of Medicine, Danderyd Hospital, Stockholm. Inclusion criterion was severe and in-hospital requisite illness, and exclusion criteria were active or prior cancer, and the group comprised patients with heterogeneous indications for hospitalization. For the patients with infection 5 patients had bacterial infections and 2 had viral infections. Exclusion criteria for healthy individuals were active or prior cancer or the presence of comorbidities with the exception of hypertension. The three groups were matched according to age and sex. Demographic data, comorbidity, routine laboratory data, and date of death were obtained from patient history documented on admission and medical records. Comorbidity burden other than cancer was assessed using the Charlson Comorbidity Index (CCI) score [16] excluding score points for cancer. Cancer patients and severely ill patients were followed for 90 days or until time of death, regardless of whether they were hospitalized during the whole follow-up period or dismissed to out-patient care.
Blood sampling and plasma preparation
A total of 15 mL of venous blood was collected once, at the time of inclusion in the study. Blood was drawn from an antecubital vein with the study participant in the supine position 30 min prior to blood sampling. Platelet-poor plasma (PPP) was prepared from citrated whole blood by immediate centrifugation at 2000 × g for 20 minutes at room temperature, and stored immediately at −80°C until further analyses.
Microvesicle TF activity assay
The isolation of MVs and the MV TF activity assay was performed as described [17].
Statistics
Data are shown as median [interquartile range (IQR)]. For two-group comparisons, the Mann-Whitney U-test was used. For multiple-group comparisons, Kruskal-Wallis test with Dunn's multiple comparisons test was used. Survival analysis over a 90 days follow-up period was obtained using the log rank test comparing survival curves of patients with MV TF activity above and below median, and depicted as Kaplan Meier curves. These statistical analyses were performed with GRAPHPAD PRISM version 7.0.3 (GraphPad Software, La Jolla, CA, USA).
Results
Levels of MV TF activity in non-cancer severely ill patients and cancer patients
Fifty-one severely ill patients without cancer and 60 cancer patients were recruited for the study. Demographic data, tumor entities, comorbidities and indication for hospitalization are shown in Table 1 and 2. Fifty healthy individuals were used as controls. We found that MV TF activity was not increased in the population of non-cancer severely ill patients compared with healthy controls (Figure 1A). In contrast, the population of cancer patients had significantly higher levels of MV TF activity compared with healthy controls (Figure 1A). The median [IQR] of MV TF activity in the three groups were 0.08 [0.03-0.14] (healthy controls), 0.12 [0.06-0.28] (non-cancer severely ill) and 0.30 [0.05-0.58] (cancer).
Table 1. Clinical and demographic characteristics of study subjects.
| Non-cancer severely ill patients (n=51) | Cancer patients (n=60) | Healthy Individuals (n=50) | |
|---|---|---|---|
| Age, mean, (SD) | 76.7 (11.5) | 70.4 (12.4) | 68.1 (7.7) |
| Female (%) | 57 | 58 | 58 |
|
| |||
| Comorbidity Index Score*, mean (SD) | 5.9 (2.1) | 3.3 (1.8) | 0 |
|
| |||
| Comorbidity | |||
| Hypertension - no. (%) | 34 (67) | 18 (30) | 15 (30) |
| Cerebrovascular disease - no. (%) | 32 (63) | 10 (17) | 0 |
| Congestive heart disease- no. (%) | 10 (20) | 6 (10) | 0 |
| Renal insufficiency- no. (%) | 9 (18) | 7 (12) | 0 |
| Liver failure- no. (%) | 11 (22) | 3 (5) | 0 |
| Diabetes mellitus type 1- no. (%) | 11 (22) | 0 (0) | 0 |
| Diabetes mellitus type 2- no. (%) | 13 (25) | 9 (15) | 0 |
| Chronic pulmonary disease- no. (%) | 9 (18) | 4 (7) | 0 |
| Dementia- no. (%) | 8 (16) | 0 (0) | 0 |
| Acute infection- no. (%) | 7 (14) | 6 (10) | 0 |
Charlson Comorbidity Index [14] excluding score points for cancer
Table 2. MV TF activity in non-cancer severely ill patients.
| Indication for hospitalization | MV TF activity (pg/mL), median [IQR] | vs healthy controls |
|---|---|---|
| Infection (n=7) | 0.28 [0.13-0.96] | P=0.0082 |
| Liver failure (n=5) | 0.28 [0.12-0.35] | NS |
| VTE (n=4) | 0.20 [0.03-0.46] | NS |
| Ischemic stroke (n=14) | 0.08 [0-0.15] | NS |
| Major fracture (n=3) | 0.31 [0-0.09] | NS |
| Confusion (n=6) | 0.07 [0.05-0.07] | NS |
| Hyperglycemia (n=2) | 0.32 [0.08-0.56] | - |
| COPD (n=2) | 0.19 [0-0.38] | - |
| Acute anemia (n=2) | 0.09 [0.07-0.11] | - |
| Intoxication (n=1) | 0.36 | - |
| Inflammatory bowel disease (n=1) | 0.25 | - |
| MI (n=1) | 0.12 | - |
| Renal failure (n=1) | 0.12 | - |
| Hyponatremia (n=1) | 0 | - |
| Epileptic seizure (n=1) | 0 | - |
Figure 1. MV TF activity in non-cancer severely ill patients, cancer patients, and healthy controls.
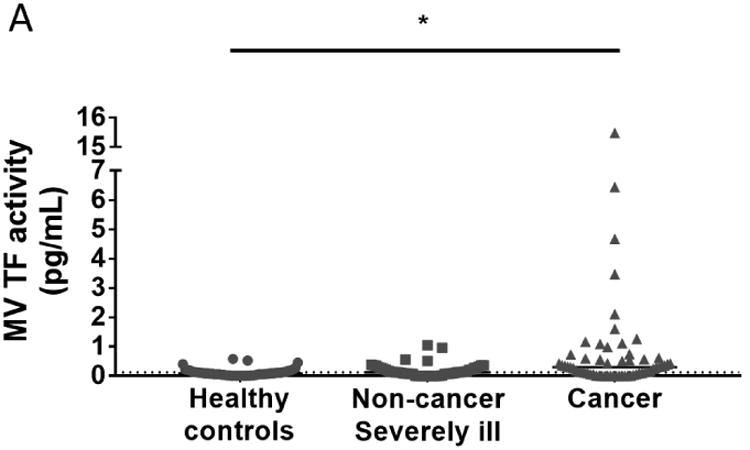

(A) We measured plasma levels of MV TF activity in healthy controls, non-cancer severely ill patients and cancer patients. Levels of MV TF activity in different non-cancer indications for hospitalization (B) and in each type of cancer (C) are shown (Asterisks represent significant difference compared with healthy controls). Gray dots represent patients with VTE. The black bar shows the median of each group. The dotted line represents the mean of healthy controls. The data were analyzed by Kruskal-Wallis test with Dunn's multiple comparisons test. *P<0.05. GI: gastrointestinal; Gyn: gynecological; VTE, venous thromboembolism.
The population of non-cancer severely ill patients contained a wide range of illnesses some of which had very low numbers (Table 2). Therefore, we focused on illnesses that had larger numbers of patients and would be predicted to have increased levels of MV TF activity. We found that patients with infection but not patients with liver failure, VTE or ischemic stroke had elevated levels of MV TF activity compared with healthy controls (Figure 1B) (Table 2).
Consistent with previous studies [14, 15, 18, 19], we observed variable levels of MV TF activity in different types of cancer (Figure 1C). Patients with gastrointestinal or prostate cancer had significantly elevated levels of MV TF activity compared with healthy controls (Figure 1C) (Table 3).
Table 3. Summary of MV TF activity and number of VTE in patients with different types of tumors.
| Tumor entity | Histopathology (number) | Number of VTE | MV TF activity (pg/mL), median [IQR] | vs healthy controls |
|---|---|---|---|---|
| Gastrointestinal | Adenocarcinoma (n=7) | 3 | 0.35 [0.30-4.67] | P=0.033 |
| Prostate | Adenocarcinoma (n=5) | 0 | 0.74 [0.22-3.77] | P=0.024 |
| Gynecological | Adenocarcinoma (n=4) | 1 | 0.33 [0.03-2.74] | NS |
| Pancreas | Adenocarcinoma (n=2) | 0 | 0.86 [0.62-1.10] | - |
| Colon | Adenocarcinoma (n=11) | 2 | 0.19 [0.03-1.16] | NS |
| Lung | Adenocarcinoma (n=6) | 2 | 0.28 [0.10-0.58] | NS |
| Breast | Adenocarcinoma (n=10) | 1 | 0.20 [0.07-0.40] | NS |
| Unknown primary | Adenocarcinoma (n=1) | 0 | 0.34 | - |
| Melanoma | Non-adenocarcinoma (n=3) | 0 | 0 [0-0.01] | NS |
| Glioblastoma | Non-adenocarcinoma (n=3) | 0 | 0 [0-0.05] | NS |
| AML | Non-adenocarcinoma (n=1) | 0 | 0.59 | - |
| Lung | Non-adenocarcinoma (n=1) | 0 | 0.51 | - |
| Lymphoma | Non-adenocarcinoma (n=1) | 0 | 0.42 | - |
| Liposarcoma | Non-adenocarcinoma (n=1) | 0 | 0.42 | - |
| Sarcoma | Non-adenocarcinoma (n=1) | 0 | 0.13 | - |
| Neuroendocrine | Non-adenocarcinoma (n=1) | 0 | 0.05 | - |
| Gingival | Non-adenocarcinoma (n=1) | 1 | 0 | - |
| Gastrointestinal | Non-adenocarcinoma (n=1) | 1 | 0 | - |
Patients with adenocarcinoma had elevated levels of MV TF activity compared with patients with non-adenocarcinoma
Previous studies have reported that adenocarcinomas have a higher incidence of VTE compared with tumors of non-adenocarcinoma in patients with lung cancer [20, 21]. We found that 9 out of 11 cancer patients with VTE had adenocarcinoma (Table 3). Therefore, we compared MV TF activity in patients with adenocarcinoma and with non-adenocarcinoma tumors. Interestingly, we observed that patients with adenocarcinoma (n=46) had significantly higher MV TF activity compared with patients with non-adenocarcinoma tumors (n=14) (Figure 2). The median [IQR] of MV TF activity in patients with adenocarcinoma and non-adenocarcinoma tumors were 0.32 [0.11-0.80] and 0.03 [0.00-0.42], respectively. However, we did not see a significant difference in the levels of MV TF activity between adenocarcinoma patients without (n=37) and with VTE (n=9) (data not shown). The median [IQR] of MV TF activity in adenocarcinoma patients without and with VTE were 0.34 [0.12-0.86] and 0.24 [0.03-0.99], respectively.
Figure 2. MV TF activity in patients with adenocarcinoma and in patients with non-adenocarcinoma tumors.

Levels of MV TF activity in patients with adenocarcinoma (n=46, median [IQR], 0.32 [0.11-0.80]) and in patients with non-adenocarcinoma tumors (n=16, median [IQR], 0.03 [0.00-0.42]) were shown. Black bars represent the median of each group. The dotted line represents the mean of healthy controls. The data were analyzed by Mann-Whitney U-test. **P<0.01.
Cancer patients with elevated levels of MV TF activity have reduced survival
Finally, we analyzed the association between MV TF activity and survival. We compared the survival of cancer patients who had elevated levels of MV TF activity (≥ median) with cancer patients who had non-elevated levels of MV TF activity (< median). We included both adenocarcinoma and non-adenocarcinoma patients. The median [IQR] of ≥ median and < median groups were 0.58 [0.30-1.19] and 0.05 [0.00-0.13], respectively. Cancer patients with high levels of MV TF activity had a survival probability of 16.7% after 90 days whereas cancer patients with low levels of MV TF activity had a survival probability of 30.0% after 90 days (Figure 3, P=0.0433).
Figure 3. Survival of cancer patients with different levels of MV TF activity.
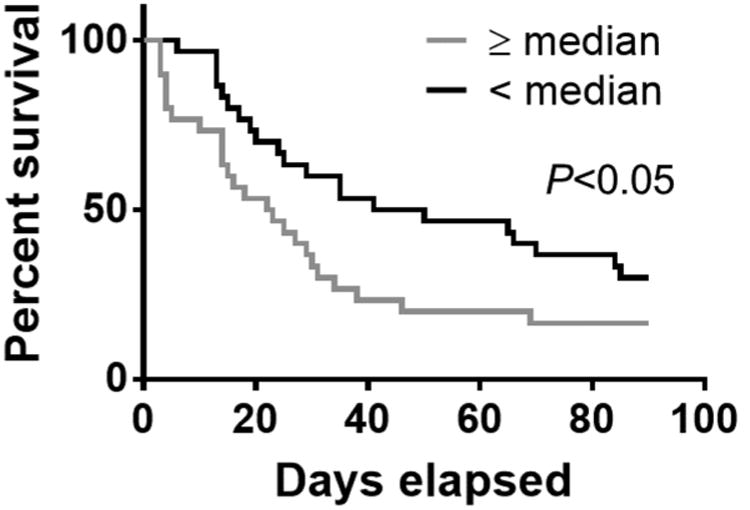
Kaplan-Meier survival curve of cancer patients with different levels of MV TF activity (≥ median [IQR], 0.58 [0.30-1.19] n=30, gray line; < median [IQR], 0.05 [0.00-0.13], n=30, black line). The data were analyzed by Log rank test. P<0.05.
Discussion
In the present study, we did not observe an increase in the level of MV TF activity in a population of non-cancer severely ill patients compared with healthy controls. As expected, MV TF activity was increased in a population of cancer patients. Gastrointestinal or prostate cancer patients had significantly higher levels of MV TF activity than healthy controls. Our hypothesis was that all non-cancer severely ill patients would have elevated levels of MV TF activity regardless of the underlying illness. However, the results did not support this hypothesis. Therefore, we re-analyzed the data by dividing the severely ill patients into the different diseases and found significantly elevated levels of MV TF activity in patients with infection. However, a limitation of the study is the small number of patients in many of the groups.
Previously, we and others showed that patients with acute liver injury or cirrhosis had increased levels of MV TF activity compared with healthy controls [4, 5]. In the current study, we did not see increased levels of MV TF in patients with liver failure. Although the number of patients was low, our results also suggest that a general comorbidity burden is not associated with increased levels of MV TF activity because the CCI score was higher in non-cancer severely ill patients compared with cancer patients.
Tumor-derived MVs and exosomes may increase the risk of VTE in cancer patients in a TF-independent manner. For instance, a recent study showed that tumor-derived exosomes induce neutrophil extracellular traps in mice [22]. Moreover, TF- MVs may also activate the contact pathway, which has been implicated in cancer-associated thrombosis [23, 24].
TF is highly expressed in barrier surfaces composed of epithelial cells [25] and therefore one might expect that adenocarcinomas would express high levels of TF. One study reported that both high TF+ and epithelial mucin+ MVs were detected in patients with breast and pancreatic cancer [18]. We found that patients with adenocarcinoma had significantly increased levels of MV TF activity compared with patients with non-adenocarcinoma tumors. These results indicate that epithelial cell-derived adenocarcinoma may be a major source of TF+ MVs in cancer.
Although 9 out of 11 cancer patients with VTE were categorized as adenocarcinomas, levels of MV TF activity were not associated with VTE in patients with adenocarcinoma. A previous in vitro study showed that cancer cells can release TF+ exosomes [26]. However, our assay does not measure TF activity on exosomes. We have observed an association between levels of MV TF activity and VTE in pancreatic cancer but not in other types of cancer [10, 15, 27, 28]. The current study included only 2 pancreatic cancer patients and neither had VTE. Therefore, the current data is consistent with our previous studies.
We found that cancer patients with higher levels of MV TF activity (>median) had reduced survival compared with those with lower levels of MV TF activity (<median). This observation is consistent with previous studies [9, 15, 27]. Notably, a previous study showed that aggressive and poorly differentiated pancreatic cancers have high MV TF activity [29]. These studies suggest that aggressive and poorly differentiated tumors release more TF+ MVs compared with non-aggressive or highly differentiated tumors.
In conclusion, cancer patients have higher levels of MV TF activity compared with non-cancer severely ill patients. Patients with adenocarcinoma have higher levels of MV TF activity compared with patients with other tumor types. The elevated levels of MV TF activity were associated with reduced survival in cancer patients.
Highlights.
Levels of microvesicle tissue factor activity are elevated in patients with infection
Adenocarcinoma patients have increased levels of microvesicle tissue factor activity
High levels of microvesicle tissue factor activity are associated with poor survival
Acknowledgments
This work was supported by grants from the NIH (Y.H. T32 HL007149-41), the Helleday Foundation (C.T., H.W.), and from the Jochnick Foundation (C.T., H.W.) and the John C. Parker Professorship (N.M.).
Footnotes
Conflict of interest statement: The authors state that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006;36(2):104–107. doi: 10.1016/j.bcmd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, Buzas EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122(11):1873–1880. doi: 10.1182/blood-2013-04-460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stravitz RT, Bowling R, Bradford RL, Key NS, Glover S, Thacker LR, Gabriel DA. Role of procoagulant microparticles in mediating complications and outcome of acute liver injury/acute liver failure. Hepatology. 2013;58(1):304–313. doi: 10.1002/hep.26307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rautou PE, Vion AC, Luyendyk JP, Mackman N. Circulating microparticle tissue factor activity is increased in patients with cirrhosis. Hepatology. 2014;60(5):1793–1795. doi: 10.1002/hep.27033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woei AJFJ, van der Starre WE, Tesselaar ME, Garcia Rodriguez P, van Nieuwkoop C, Bertina RM, van Dissel JT, Osanto S. Procoagulant tissue factor activity on microparticles is associated with disease severity and bacteremia in febrile urinary tract infections. Thromb Res. 2014;133(5):799–803. doi: 10.1016/j.thromres.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Rondina MT, Tatsumi K, Bastarache JA, Mackman N. Microvesicle Tissue Factor Activity and Interleukin-8 Levels are Associated with Mortality in Patients with Influenza A/H1N1 Infection. Crit Care Med. 2016;44(7):e574–578. doi: 10.1097/CCM.0000000000001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mooberry MJ, Bradford R, Hobl EL, Lin FC, Jilma B, Key NS. Procoagulant microparticles promote coagulation in a factor XI-dependent manner in human endotoxemia. J Thromb Haemost. 2016;14(5):1031–1042. doi: 10.1111/jth.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5(3):520–527. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 10.Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, Mackman N, Taubman MB. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6(11):1983–1985. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auwerda JJ, Yuana Y, Osanto S, de Maat MP, Sonneveld P, Bertina RM, Leebeek FW. Microparticle-associated tissue factor activity and venous thrombosis in multiple myeloma. Thromb Haemost. 2011;105(1):14–20. doi: 10.1160/TH10-03-0187. [DOI] [PubMed] [Google Scholar]
- 12.Nieuwland R, Berckmans RJ, McGregor S, Boing AN, Romijn FP, Westendorp RG, Hack CE, Sturk A. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95(3):930–935. [PubMed] [Google Scholar]
- 13.Hellum M, Ovstebo R, Brusletto BS, Berg JP, Brandtzaeg P, Henriksson CE. Microparticle-associated tissue factor activity correlates with plasma levels of bacterial lipopolysaccharides in meningococcal septic shock. Thromb Res. 2014;133(3):507–514. doi: 10.1016/j.thromres.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Hisada Y, Alexander W, Kasthuri R, Voorhees P, Mobarrez F, Taylor A, McNamara C, Wallen H, Witkowski M, Key NS, Rauch U, Mackman N. Measurement of microparticle tissue factor activity in clinical samples: A summary of two tissue factor-dependent FXa generation assays. Thromb Res. 2016;139:90–97. doi: 10.1016/j.thromres.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, Key NS, Barcel DA, Scheithauer W, Kornek G, Zielinski C, Pabinger I. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10(7):1363–1370. doi: 10.1111/j.1538-7836.2012.04754.x. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Lee RD, Barcel DA, Williams JC, Wang JG, Boles JC, Manly DA, Key NS, Mackman N. Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thromb Res. 2012;129(1):80–85. doi: 10.1016/j.thromres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tesselaar ME, Romijn FP, van der Linden IK, Bertina RM, Osanto S. Microparticle-associated tissue factor activity in cancer patients with and without thrombosis. J Thromb Haemost. 2009;7(8):1421–1423. doi: 10.1111/j.1538-7836.2009.03504.x. [DOI] [PubMed] [Google Scholar]
- 19.Manly DA, Wang J, Glover SL, Kasthuri R, Liebman HA, Key NS, Mackman N. Increased microparticle tissue factor activity in cancer patients with Venous Thromboembolism. Thromb Res. 2010;125(6):511–512. doi: 10.1016/j.thromres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chew HK, Davies AM, Wun T, Harvey D, Zhou H, White RH. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost. 2008;6(4):601–608. doi: 10.1111/j.1538-7836.2008.02908.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee YG, Kim I, Lee E, Bang SM, Kang CH, Kim YT, Kim HJ, Wu HG, Kim YW, Kim TM, Lee KW, Lee SH, Kim DW, Heo DS. Risk factors and prognostic impact of venous thromboembolism in Asian patients with non-small cell lung cancer. Thromb Haemost. 2014;111(6):1112–1120. doi: 10.1160/TH13-11-0956. [DOI] [PubMed] [Google Scholar]
- 22.Leal AC, Mizurini DM, Gomes T, Rochael NC, Saraiva EM, Dias MS, Werneck CC, Sielski MS, Vicente CP, Monteiro RQ. Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications For The Establishment of Cancer-Associated Thrombosis. Sci Rep. 2017;7(1):6438. doi: 10.1038/s41598-017-06893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swystun LL, Mukherjee S, Liaw PC. Breast cancer chemotherapy induces the release of cell-free DNA, a novel procoagulant stimulus. J Thromb Haemost. 2011;9(11):2313–2321. doi: 10.1111/j.1538-7836.2011.04465.x. [DOI] [PubMed] [Google Scholar]
- 24.Nickel KF, Ronquist G, Langer F, Labberton L, Fuchs TA, Bokemeyer C, Sauter G, Graefen M, Mackman N, Stavrou EX, Ronquist G, Renne T. The polyphosphate-factor XII pathway drives coagulation in prostate cancer-associated thrombosis. Blood. 2015;126(11):1379–1389. doi: 10.1182/blood-2015-01-622811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134(5):1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 26.Garnier D, Magnus N, Lee TH, Bentley V, Meehan B, Milsom C, Montermini L, Kislinger T, Rak J. Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J Biol Chem. 2012;287(52):43565–43572. doi: 10.1074/jbc.M112.401760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bharthuar A, Khorana AA, Hutson A, Wang JG, Key NS, Mackman N, Iyer RV. Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers. Thromb Res. 2013;132(2):180–184. doi: 10.1016/j.thromres.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Cohen JG, Prendergast E, Geddings JE, Walts AE, Agadjanian H, Hisada Y, Karlan BY, Mackman N, Walsh CS. Evaluation of venous thrombosis and tissue factor in epithelial ovarian cancer. Gynecol Oncol. 2017;146(1):146–152. doi: 10.1016/j.ygyno.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 29.Thaler J, Ay C, Mackman N, Metz-Schimmerl S, Stift J, Kaider A, Mullauer L, Gnant M, Scheithauer W, Pabinger I. Microparticle-associated tissue factor activity in patients with pancreatic cancer: correlation with clinicopathological features. Eur J Clin Invest. 2013;43(3):277–285. doi: 10.1111/eci.12042. [DOI] [PubMed] [Google Scholar]


