Abstract
DJ-1 is a redox-sensitive protein with several putative functions important in mitochondrial physiology, protein transcription, proteasome regulation, and chaperone activity. High levels of DJ-1 immunoreactivity are reported in astrocytes surrounding pathology associated with idiopathic Parkinson’s disease, possibly reflecting the glial response to oxidative damage. Previous studies showed that astrocytic over-expression of DJ-1 in vitro prevented oxidative stress and mitochondrial dysfunction in primary neurons. Based on these observations, we developed a pseudotyped lentiviral gene transfer vector with specific tropism for CNS astrocytes in vivo to overexpress human DJ-1 protein in astroglial cells. Following vector delivery to the substantia nigra and striatum of adult Lewis rats, the DJ-1 transgene was expressed robustly and specifically within astrocytes. There was no observable transgene expression in neurons or other glial cell types. Three weeks after vector infusion, animals were exposed to rotenone to induce Parkinson’s disease-like pathology, including loss of dopaminergic neurons, accumulation of endogenous α-synuclein, and neuroinflammation. Animals over-expressing hDJ-1 in astrocytes were protected from rotenone-induced neurodegeneration, and displayed a marked reduction in neuronal oxidative stress and microglial activation. In addition, α-synuclein accumulation and phosphorylation were decreased within substantia nigra dopaminergic neurons in DJ-1–transduced animals, and expression of LAMP-2A, a marker of chaperone mediated autophagy, was increased. Together, these data indicate that astrocyte-specific overexpression of hDJ-1 protects neighboring neurons against multiple pathologic features of Parkinson’s disease and provides the first direct evidence in vivo of a cell non-autonomous neuroprotective function of astroglial DJ-1.
Keywords: DJ-1, astrocyte, rotenone, Parkinson’s disease, chaperone-mediated autophagy, neuroinflammation, oxidative stress, gene therapy
Introduction
DJ-1 is a pleiotropic protein containing a ThiJ/PfpI domain characteristic of a protein chaperone, and is expressed ubiquitously in all human cell types (Nagakubo et al., 1997; S.-J. Lee et al., 2003; Tao and Tong, 2003; Wilson et al., 2005). Autosomal recessive mutations in the PARK7 gene encoding DJ-1 are linked to a rare, familial type of early-onset parkinsonism (Bonifati et al., 2002; 2003; Tao and Tong, 2003). However, even in cases of idiopathic Parkinson’s disease (PD) with no apparent PARK7 mutations, postmortem analysis reveals that high levels of oxidized DJ-1 accumulate in neurons and astrocytes of the ventral midbrain (Bandopadhyay et al., 2004; Choi et al., 2006).
Multiple lines of evidence suggest that, within neurons, DJ-1 is involved in maintaining mitochondrial function (Miller et al., 2003; Canet-Avilés et al., 2004; Blackinton et al., 2005; Hayashi et al., 2009; Junn et al., 2009; N. J. Larsen et al., 2011; Thomas et al., 2011), protein transcription and processing (Takahashi et al., 2001; Shinbo et al., 2005; J. Xu et al., 2005; Zhong et al., 2006; Lu et al., 2016;), neurotransmitter reuptake (Luk et al., 2015; Piston et al., 2017), proteasome activity (Saito et al., 2016; Moscovitz et al., 2017), and signal transduction (Aleyasin et al., 2010; Zhang et al., 2017; reviewed in Oh and Mouradian, 2017), abnormalities of which are implicated in the pathogenesis of PD. A highly conserved (S. Bandyopadhyay and Cookson, 2004; Bai et al., 2006;), and readily oxidized cysteine (Cys)106 residue suggests that DJ-1 functions in part as a redox sensor (Canet-Avilés et al., 2004; Taira et al., 2004; Kinumi et al., 2004; Ooe et al., 2006; Blackinton et al., 2009b), and is induced in substantia nigra (SN) dopamine neurons that experience a high degree of oxidative burden (Lin and Beal, 2006; X. Wang and Michaelis, 2010; Winklhofer and Haass, 2010; Surmeier et al., 2017). Additionally, DJ-1 expression has been inversely correlated with α-synuclein toxicity and accumulation, both through mechanisms of direct protein interaction (Shendelman et al., 2004; Meulener et al., 2005; W. Zhou et al., 2006; Zondler et al., 2017), as well as the transcriptional upregulation of chaperone proteins (i.e. HSP70; Batelli et al., 2008; C.-Y. Xu et al., 2017).
DJ-1 has also been shown to be important in maintaining astrocytic mitochondrial function (Ashley et al., 2009; N. J. Larsen et al., 2011), and impairment of DJ-1 in astrocytes deleteriously affects primary neurons following an oxidative insult (Mullett and Hinkle, 2011; 2009; Mullett et al., 2012). Conversely, in vitro overexpression of DJ-1 in astrocytes is protective against rotenone-induced mitochondrial dysfunction and reactive oxygen species (ROS) production in co-cultured neurons (Mullett et al., 2012). Collectively, these data suggest that astrocytic DJ-1 plays a cell non-autonomous role in the protection of neurons from the consequences of mitochondrial dysfunction.
In a previous study, we discovered that replication-defective HIV1-based vectors pseudotyped with the envelope glycoprotein from Moloney Murine Leukemia Virus (MuLV) showed specific tropism for astrocytes within the adult rat brain (Cannon et al., 2011). Here, we engineered a MuLV-pseudotyped HIV1-based vector to overexpress human DJ-1 (hDJ-1) protein selectively within astrocytes, but not in neurons or other glial cells (microglia or oligodendrocytes). This new vector allowed us to test whether modulation of astrocytic DJ-1 expression could attenuate neurodegeneration in vivo. Our data show unequivocal evidence that astroglial DJ-1 over-expression is neuroprotective in the rat rotenone model of PD, thereby providing the first direct evidence of a cell non-autonomous protective function of astrocyte DJ-1 in vivo.
Materials and Methods
Chemical reagents and supplies
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Antibodies are listed in Table 1.
Table 1.
Antibodies for immunohistoche mistry.
| Antibody | Catalog | Company |
|---|---|---|
| Tyrosine hydroxylase Anti-Nitrotyrosine |
AB1542 06-284 |
EMD Millipore (Burlington, MA) |
| Glial fibrillary acidic protein (GFAP) | mAb #3670 | Cell Signaling Technology (Danvers, MA) |
| Human DJ-1/PARK7 Ser129-α-Synuclein |
Ab18257 Ab51253 |
Abcam (Cambridge, MA) |
| Iba1 | 019-19741 | Wako Chemicals USA (Irvine, CA) |
| NDUFS3/OxPhos LAMP-2A |
459130 51-2200 |
Thermo Fisher (Waltham, MA) |
| TOM20 | SC-11415 | Santa Cruz Biotechnology (Santa Cruz, CA) |
| CD-68 (ED1) | MCA341 | BioRad (Hercules, CA) |
| α-Synuclein | 610787 | BD Biosciences (San Jose, CA) |
Lentiviral vectors
Total RNA (RNAqueous, Invitrogen, Carlsbad, CA) was isolated from human NT2 cells and reverse transcribed using oligo-dT primers (SuperScript III, Invitrogen). cDNA encoding human DJ-1 was amplified by PCR with Platinum Pfx polymerase (Invitrogen) using primers hDJ-1F-EcoR1 (5′-CGAATTCGGTGCAGGCTTGTAAACATA-3′) and hDJ-1R-KpnI (5′-CGGTACCTGAGAATGGATTCCTAACGG-3′). The PCR product was digested with EcoRI and KpnI and inserted into the EcoRI/KpnI sites of pBlueScript to make pBS-DJ-1, which was sequenced to verify that no mutations were introduced during amplification. The human DJ-1 ORF was released from pBS-DJ-1 and inserted into the EcoRI/SmaI sites of pIRES2-EGFP (Clontech. Mountain View, CA) to yield pDJ-1-IRES2-EGFP. This was digested with XbaI, the 5′ overhang filled in using Klenow fragment and then digested with EcoRI to liberate a fragment containing hDJ-1-IRES-EGFP. The HIV-1-based lentiviral gene transfer plasmid pHR′ (Naldini et al., 1996) was digested with BamHI/XhoI to release the LacZ insert and re-ligated, generating pHR′-ΔLacZ. pHR′-ΔLacZ was digested with KpnI, the 3′ overhang removed using Klenow fragment, and then digested with EcoRI, so that the hDJ-1-IRES-EGFP could be ligated into the resulting blunt and EcoRI ends to yield pHR′-hDJ-1-IRES2-EGFP, with the DJ-1 ORF immediately 3′ of the CMV promoter. The control vector pHR′-IRES2-EGFP was generated similarly except for the absence of the DJ-1 gene. Production of transduction-competent lentiviral particles from these plasmids was confirmed in vitro by co-transfection of 293T cells with packaging and VSV-G envelope plasmids. Expression of DJ-1 was verified by Western blot analysis of cells transduced by the resulting viral particles, expression of GFP was confirmed by epifluorescence microscopy of transduced cells, and biological activity of over-expressed DJ-1 was confirmed by protection of transduced PC6–3 cells from H2O2 exposure (supplemental figure 1). High titer stocks of MuLV-pseudotyped lentiviral vector particles of sufficient purity for in vivo transduction were generated by 3-plasmid transfection at the at the University of Pennsylvania Gene Therapy Vector Core. The presence of replication-competent lentivirus in the resulting viral stocks was excluded by monitoring p24 antigen levels in the culture medium for 30 days after transduction of 293T cells. DJ-1 and control vector stocks were diluted in PBS to the same titer (1.78×109 GC/ml) prior to experiments.
Animals and stereotaxic surgery
Adult, middle-age (7–9 month), male Lewis rats (Charles River Laboratories, Wilmington, MA) were used for all experiments. Conventional diet and water were given ad libitum. Animals were maintained under standard temperature-controlled conditions with 12:12 hour light-dark cycle.
Rats were randomly assigned to control and treatment groups and blinded throughout all study analyses. All experiments involving animal treatment and euthanasia were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Rodent stereotaxic surgery was performed under deep isoflurane anesthesia. Each animal was infused unilaterally with 2 μl of LV-hDJ-1/MuLV or LV-GFP/MuLV following standard Bregma coordinates for the substantia nigra (Bregma −5.8 mm A/P, −2.2 mm M/L, −8.5 mm V). Striatal infusion of 4 μl of either vector was performed at two injection depths (2 μl per site) to maximize vector expression throughout the dorsolateral striatum (Bregma +1.2 mm A/P, −3.4 mm M/L, −5; −7 mm V).
Rotenone administration and motor behavior
One week after virus infusion, rats started a 2-week behavioral training period, after which they were given single daily intraperitoneal (IP) injections of 2.8 mg/kg of rotenone suspended in 2% DMSO, 98% Miglyol 812 N as described (Cannon et al., 2009a). Motor deficits were assessed daily using the postural instability test (PIT; Woodlee et al., 2008) until each rat reached its motor behavioral endpoint, defined as the rat’s inability to successfully complete the PIT or loss of 25% body mass (Supplemental Figure 2). Behavioral tests were carried out by investigators blinded to treatment group throughout the study. Animals were euthanized using 0.3 mg/kg pentobarbital, followed by transcardial perfusion and 4% paraformaldehyde perfusion fixation.
Striatal terminal intensity
Brain sections (35 μm) through striatum were stained for tyrosine hydroxylase (TH) using immunofluorescence. Striatal tissue sections were analyzed using near-infrared imaging for density of dopamine neuron terminals (LiCor), and analyzed using Odyssey software (V3.0).
Stereology
Stereological analysis of dopamine neuron number in the SN was achieved using an adapted protocol from Tapias et al. (2013), employing an unbiased, automated system. Briefly, nigral tissue sections were stained for TH and counterstained with DAPI and NeuroTrace Dye (640; Life Technologies) and imaged using a Nikon 90i upright fluorescence microscope equipped with high N.A. plan fluor/apochromat objectives, Renishaw linear encoded microscope stage (Prior Electronics) and Q-imaging Retiga cooled CCD camera (Center for Biological Imaging, University of Pittsburgh). Images were processed using Nikon NIS-Elements software, and quantitative analysis was performed on fluorescent images colocalizing DAPI, TH, and Nissl-positive stains.
Immunohistochemistry and pathology
Brain sections (35 μm) were maintained at −20°C in cryoprotectant, stained while free-floating, and mounted to glass slides for imaging, using a “primary antibody delete” (secondary antibody only) stained section to subtract background fluorescence. Fluorescent immunohistochemical images were collected using an Olympus BX61 confocal microscope and Fluoview 1000 software (Melville, NY). Quantitative fluorescence measurements were thoroughly monitored using standard operating imaging parameters to ensure that images contained no saturated pixels.
For quantitative comparisons, all imaging parameters (e.g., laser power, exposure, pinhole) were held constant across specimens. For presentation, screenshots of rat brain images (Figure 1) were modified from brainmaps.org, and released under the Creative Commons Attribution 3.0 License.
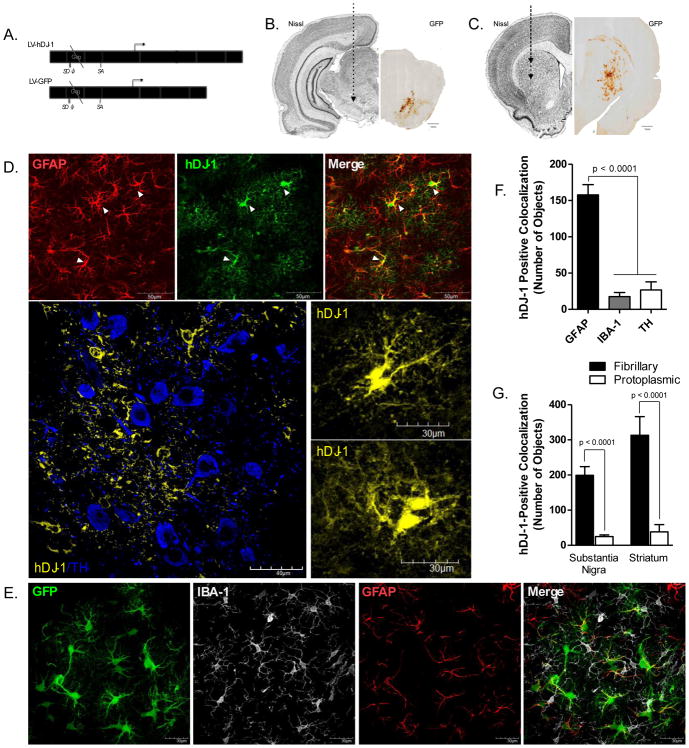
Figure 1. LV-hDJ-1/MuLV is expressed in astrocytes within the substantia nigra and striatum.
A. The Moloney Murine Leukemia Virus (MuLV) protein coat encapsulates a lentiviral expression vector driving bicistronic expression of human DJ-1 (CMV promoter) and green fluorescent protein (GFP; iRES promoter) enabling GFP localization to distinguish cells co-expressing human DJ-1 protein. B–C. Stereotaxic targets within the striatum and substantia nigra (SN) of adult male Lewis rats were selected as the areas most affected by dopamine neuron cell body and terminal loss following rotenone treatment. D. Glial fibrillary acidic protein (GFAP) marker for astrocytes (red) is colocalized with human DJ-1 (hDJ-1) overexpression (green) following LV-hDJ-1/MuLV expression vector transfection in the SN (100×). hDJ-1 immunoreactivity within the SN (60×) reveals hDJ-1 expression within astrocytic processes and cell bodies (hDJ-1, yellow) adjacent to dopamine neurons (TH, blue) but does not colocalize with TH; inset images of astrocytes overexpressing hDJ-1 (100×). E. Striatal expression of MuLV vector GFP (green) indicates colocalization with GFAP (red), but is excluded from microglia (IBA-1, white). F. Quantification of total hDJ-1 protein within cell types of the substantia nigra N=5 (F2,18 = 49.64, p < 0.0001). G. hDJ-1 colocalizes with fibrillary (GFAP-positive) astrocytes more than protoplasmic (ALDH1L1-positive) astrocytes within both the substantia nigra and striatum; N=5 (F3,30 = 16.26, p < 0.0001). Statistical analysis; one-way ANOVA, followed by Tukey post-hoc test.
Statistical analysis
All data were expressed as mean values ± standard error of the mean (SEM). Statistical significance was evaluated between normally distributed means by parametric one-way analysis of variance (ANOVA) with the Tukey post-hoc test to compare multiple data sets, unless otherwise stated. Statistical significance for group comparisons is represented in each Figure as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, or as specified on graph. Animal survival was analyzed using the Mantel-Cox comparison of survival curve (p < 0.05), and postural instability testing (PIT) was evaluated using the Kruskal-Wallis test, followed by Dunn’s multiple comparison test. To compare daily PIT performance following the first endpoint animal, a one-way ANOVA (p < 0.0001) with a Bonferroni multiple comparison test was performed between pairs of each vector treatment per day. Statistical analyses were carried out using GraphPad Prism software (V. 5.01).
Results
Overexpression of hDJ-1 in astrocytes
We generated an HIV1-based lentiviral vector, LV-hDJ-1, that over-expresses human DJ-1 and GFP in transduced cells (Figure 1a). The control vector LV-GFP is isogenic, with the exception of the deleted hDJ-1 gene (Figure 1a). Vectors were pseudotyped with MuLV envelope glycoprotein in order to direct tropism to astrocytes in the adult rat brain (Cannon et al., 2011). Stereotaxic injections of LV-hDJ-1/MuLV increased expression of DJ-1 through the ipsilateral SN and dorsolateral striatum (Figure 1b–c), whereas no change in DJ-1 expression was seen after transduction with LV-GFP/MuLV. hDJ-1 protein expression was found in all cellular compartments of the astrocyte (cell body and processes), but was not detectable in adjacent neurons or microglia, (Figure 1d–e). Quantification of total DJ-1 (human + endogenous rat) protein within the SN of animals injected with LV-hDJ-1 revealed a significant (9-fold, p < 0.0001) increase in DJ-1 expression within astrocytes relative to endogenous expression within microglia (IBA-1) or dopaminergic neurons (tyrosine hydroxylase, TH; Figure 1f).
The population of astrocytes expressing the hDJ-1 transgene were predominately immunoreactive for a marker of fibrillary-type astrocytes, glial fibrillary acidic protein (GFAP), and shared relatively low colocalization with a marker for protoplasmic astrocytes, aldehyde dehydrogenase (ALDH)-1L1 (p < 0.0001; Figure 1g). LV-DJ-1/MuLV or LV-GFP/MuLV infusion into the rat brain provoked no neurological abnormalities, induced minimal pathology immediately surrounding the injection site, and no apparent gliosis or cellular activation was observed beyond the immediate needle tract.
Astrocytic overexpression of hDJ-1 protects dopaminergic neurons
Rotenone, an organic pesticide, is a classic mitochondrial complex I inhibitor that reproduces key pathological features of PD in rat models, including the selective killing of dopaminergic neurons (Cannon et al., 2009a). As such, rotenone treatment induced progressive motor disability until all rats reached a predefined endpoint. During this endpoint study, animals that received LV-DJ-1/MuLV infusion did not significantly differ in total survival time post rotenone treatment from those receiving the LV-GFP/MuLV control vector, (p = 0.147; Figure 2b). Similarly, hDJ-1 expressing animals did not differ significantly in motor function compared to LV-GFP/MuLV control animals (p = 0.094; Figure 2c).
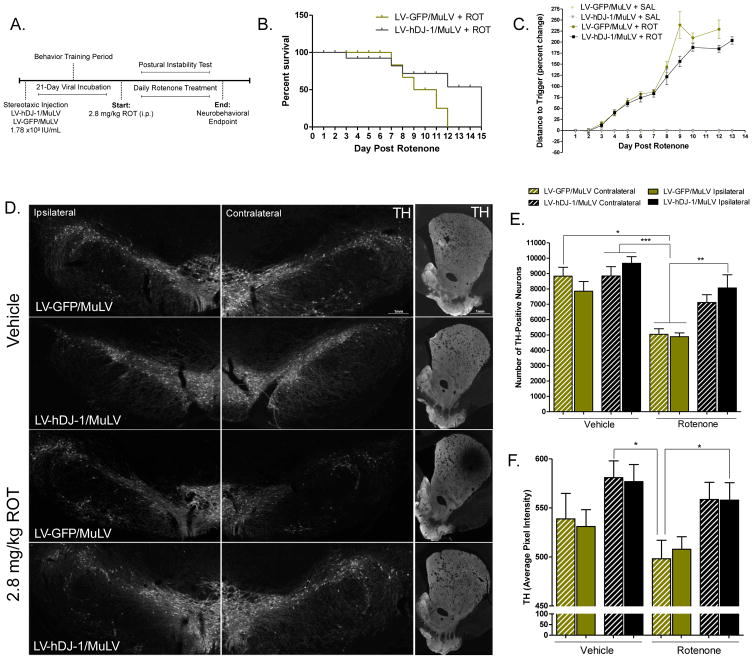
Figure 2. Overexpression of astrocytic hDJ-1 attenuates rotenone-induced neurotoxicity.
A. Dosing paradigm for MuLV injection followed by rotenone treatment. B. Animal survival following rotenone treatment in animals receiving hDJ-1 overexpression vector (MuLV-DJ-1) or GFP control vector (LV-GFP/MuLV); N=5 (χ2 = 2.104, p = .147), log-rank (Mantel-Cox) comparison of survival curves. C. Postural instability test (PIT) motor behavior scores during endpoint rotenone dosing paradigm; N=5 (p < 0.0001). Statistical analysis; Kruskal-Wallis test, followed by Dunn’s multiple comparison test. D. Representative montage images (20×) of SN and striatal tissue from LV-GFP/MuLV and MuLV-DJ-1 animals receiving saline or rotenone treatment. E. Stereological analysis of TH-positive neurons in the SN; N=5 (F7,26 = 9.61, p < 0.0001). F. Quantification of striatal TH intensity; N=5 (F3,382 = 21.44, p < 0.0001). Statistical analysis; one-way ANOVA, followed by Tukey post- hoc test (*p < 0.05, **p <0 .01, ***p < 0.001).
Stereological analysis of dopaminergic neuron survival within the SN following rotenone treatment revealed a ~38% loss of TH-expressing neurons in animals that received the control LV-GFP/MuLV vector (p < 0.05 versus vehicle-exposed LV-GFP/MuLV animals). In contrast, dopaminergic neuronal loss following rotenone exposure was significantly attenuated in animals overexpressing astrocytic hDJ-1 on the side of the brain ipsilateral to the vector injection (Figure 2d–e). Consequently, the number of nigral dopaminergic neurons in rats that received hDJ-1 and rotenone did not differ significantly from control rats that were treated with either LV-hDJ-1/MuLV or LV-GFP/MuLV but did not receive rotenone. Importantly, neither vector (LV-GFP/MuLV control or LV-DJ-1/MuLV) alone affected the number of dopaminergic neurons.
The density of dopaminergic neuronal terminals projecting to the dorsolateral striatum from the SN was estimated by quantitative near-infrared immunofluorescence to measure TH expression. Following rotenone exposure, animals receiving LV-DJ-1/MuLV showed approximately 10% more striatal TH expression that control animals (p < 0.05) indicating that dopaminergic terminals were protected by astrocytic DJ-1 over-expression (Figure 2d, f).
Neuronal mitochondrial dysfunction is attenuated by astrocytic hDJ-1
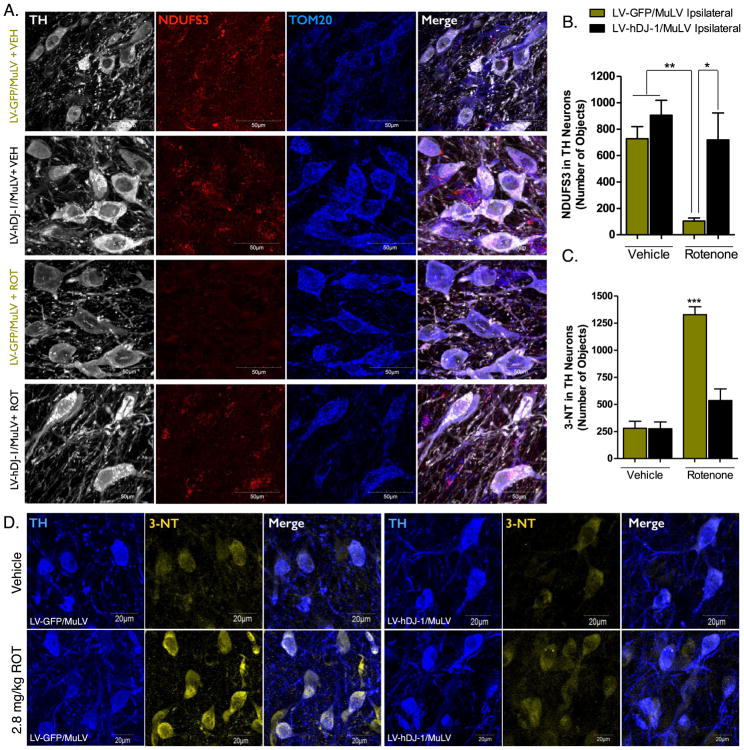
As a prototypical complex I inhibitor, rotenone induces significant mitochondrial dysfunction when administered in vivo. The reduction of complex I electron transfer and subsequent mitochondrial damage and oxidative stress is hypothesized to be the predominant cause of dopaminergic neuron death following rotenone exposure (Sherer et al., 2003b). The complex I subunit, NADPH:Ubiquinone Oxidoreductase Core Subunit S3 (NDUFS3), is imported into the mitochondria following nuclear transcription, a process that is downregulated in dysfunctional mitochondria (Devi et al., 2008; Taurino et al., 2012; Di Maio et al., 2016). Measurement of the NDUFS3 protein within the dopamine neurons of the SN of animals following endpoint rotenone administration revealed a reduction in protein expression and colocalization with the mitochondrial marker TOM20 (Figure 3a). This reduction was significantly attenuated in animals that received the hDJ-1 transgene, indicating that astrocytic DJ-1 expression protected neuronal mitochondrial function (p < 0.05; Figure 3b).
Figure 3. Dopaminergic neuron mitochondrial dysfunction and oxidative stress produced by rotenone is decreased by astrocytic hDJ-1 expression.
A. Representative images of the mitochondrial complex I protein NDUFS3 (NADH dehydrogenase [ubiquinone] iron-sulfur protein 3; red) in the SN, ipsilateral to LV/MuLV infusion. B. Quantification of NDUFS3 number of objects in TH-positive neurons ipsilateral to MuLV infusion; N=5 (F3,13 = 9.78, p < 0.0012). C. Quantification of 3-nitrotyrosine (3-NT) number of objects in TH-positive neurons of the SN ipsilateral to MuLV infusion; N=5 (F3,20 = 39.35, p < 0.0001). D. Representative images of 3-NT in TH-positive neurons (60×). Statistical analysis; one-way ANOVA, followed by Tukey post-hoc test (*p < 0.05, **p <0 .01, ***p < 0.001).
Astrocytic overexpression of hDJ-1 reduces dopaminergic neuron nitrosative stress
Oxidative stress produced by both the direct inhibition of complex I and downstream inflammatory signaling following rotenone treatment, produces significant damage to proteins and other cellular components. The production of peroxynitrite from NO and superoxide may lead to nitrosylation of proteins at tyrosine residues and is commonly assessed by 3-nitrotyrosine (3-NT) levels (Pennathur et al., 1999). Following rotenone treatment, 3-NT levels within dopamine neurons of the SN were significantly increased over vehicle treated animals; however, this was normalized in animals that received the hDJ-1 transgene (p < 0.05; Figure 3c–d).
Astrocytic hDJ-1 overexpression suppresses rotenone-induced neuroinflammation
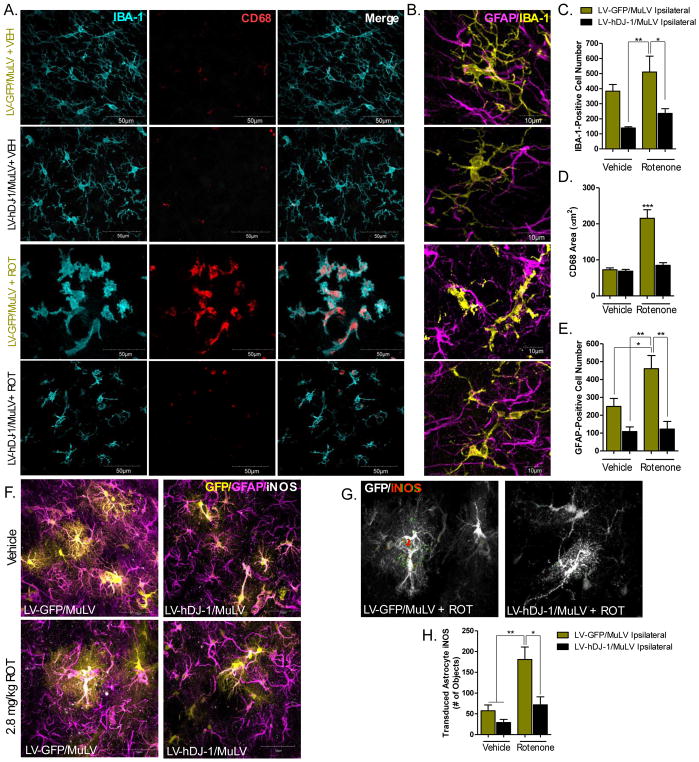
At baseline (no rotenone treatment), astrocytic hDJ-1 overexpression did not significantly alter IBA-1-positive microglial cell number within the SN (Figure 4c; p = 0.056). Following rotenone exposure, a robust activation of microglia was observed in the SN, which was indicated by a shift in morphology from a resting, ramified cell body expressing secondary and tertiary process branches to an amoeboid, phagocytic cell with little if any process branching (Figure 4a–c). The morphological shift to activated microglia was additionally measured using the lysosomal protein CD-68, indicating elevated phagocytic activity (p < 0.001; Figure 4a, d). Astrocytic overexpression of hDJ-1 reduced the abundance of activated microglia in SN; both the total number of IBA-1-postitive microglial cells, as well as the amount of CD-68 expressed per cell were reduced (p < 0.05; Figure 4a–c). Astrocyte activation was measured using total GFAP-positive cell number within the SN, and was significantly increased in animals treated with rotenone and the control vector LV-GFP/MuLV (p < 0.05), but suppressed in animals receiving rotenone and LV-hDJ-1/MuLV (p < 0.01; Figure 4b, d).
Figure 4. Astrocytic hDJ-1 overexpression suppresses neuroinflammation.
A. Representative images (60×) of microglia (IBA-1, cyan) and CD68 (red), ipsilateral to MuLV infusion in the SN. B. High resolution (100×) images of resting versus reactive microglia (IBA-1; yellow) and astrocytes (GFAP; magenta) within the SN. C. Total number of microglia (IBA-1-positive cells) within the SN; N=5 (F3,12 = 7.5, p < 0.0044). D. Quantification of microglial activation as measured using the area of CD68 expression within IBA-1-positive microglia; N=5 (F3,12 = 29.39, p < 0.0001). E. Total number of astrocytes (GFAP-positive cells) following rotenone treatment or overexpression of astrocytic hDJ-1; N=5 (F3,12 = 10.65, p < 0.0011). F. Representative striatal images of astrocytes (magenta), transduced astrocytes (yellow), and iNOS (white). G. Representative images of iNOS measurement in transduced striatal astrocytes. H. Quantification of LV-GFP/MuLV and LV- hDJ-1/MuLV striatal astrocyte iNOS expression; N=5 (F3,64 = 6.94, p < 0.0004). Statistical analysis; one-way ANOVA followed by Tukey post-hoc test *p < 0.05, **p < 0.01, *** p < 0.001).
DJ-1 reportedly modulates the expression of astrocytic inducible nitric oxide synthase (iNOS) (Waak et al., 2009), which affects the levels of NO, peroxynitrite, and superoxide within the midbrain (Iravani et al., 2002). To identify whether astrocytic hDJ-1 expression is protective against rotenone-induced iNOS induction, we measured the amount of iNOS expression within transduced astrocytes (LV-GFP/MuLV or LV-hDJ-1/MulV) in the striatum (Figure 4f–h). iNOS is highly expressed within reactive astrocytes, but also neurons (Heneka and Feinstein, 2001) and other glial cells (Dehmer et al., 2000). Therefore, we measured iNOS only in transduced cells to ensure cell-type specific measurement of the enzyme (Figure 4g). Animals treated with the control vector (LV-GFP/MuLV) displayed approximately a 3-fold induction of iNOS within GFP-positive astrocytes following rotenone exposure (p < 0.01), however LV-hDJ-1/MuLV treated animals had significantly lower astrocytic iNOS in cells expressing the hDJ-1 transgene (p < 0.05; Figure 4h).
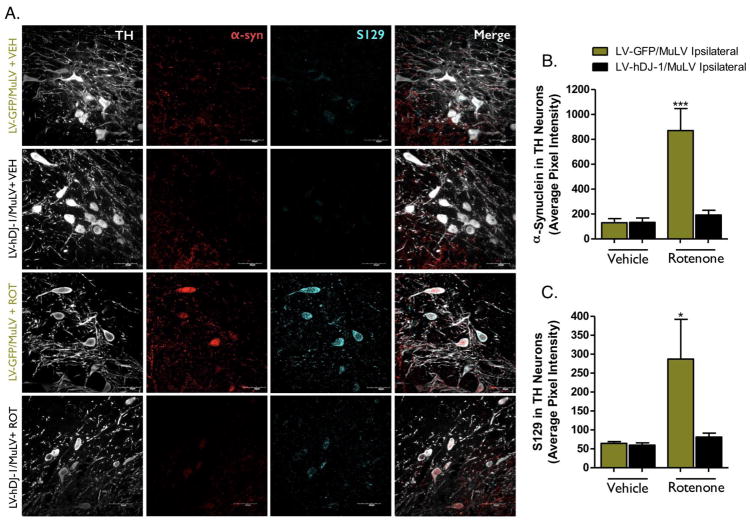
Astrocytic hDJ-1 overexpression prevents dopaminergic neuron α-synuclein pathology
Accumulation, aggregation, and phosphorylation at Ser-129 (S129) of endogenous wildtype α-synuclein within dopaminergic neurons are reproducible characteristics of the rotenone rat model (Cannon et al., 2009b; Di Maio et al., 2016). Accordingly, following rotenone treatment, animals receiving the LV-GFP/MuLV control vector displayed a significant increase in total α-synuclein protein in dopamine neurons of the SN (p < 0.0001; Figure 5a, b). Similarly, levels of S129-phosphorylated α-synuclein also increased in the dopaminergic cell bodies of the SN following rotenone treatment (Figure 5a, c). Astrocytic overexpression of hDJ-1 significantly attenuated the rotenone-induced increase in total and S129-phosphorylated α-synuclein (p < 0.05; Figure 5c).
Figure 5. Astrocytic hDJ-1 limits α-synuclein toxicity within dopaminergic neurons. A.
Representative images (60×) within the SN of total α-synuclein (α-Syn; red) and phosphorylated α-synuclein (S129; cyan) ipsilateral to MuLV infusion. B–C. Animals receiving MuLV-DJ-1 have a significantly less total α-synuclein; N=5 (F3,20 = 14.7, p < 0.0001), and a reduction in phosphorylated α-synuclein; N=5 (F3,7 = 8.03, p < 0.0115). Statistical analysis; one-way ANOVA followed by Tukey post-hoc test (*p < 0.05, *** p < 0.001).
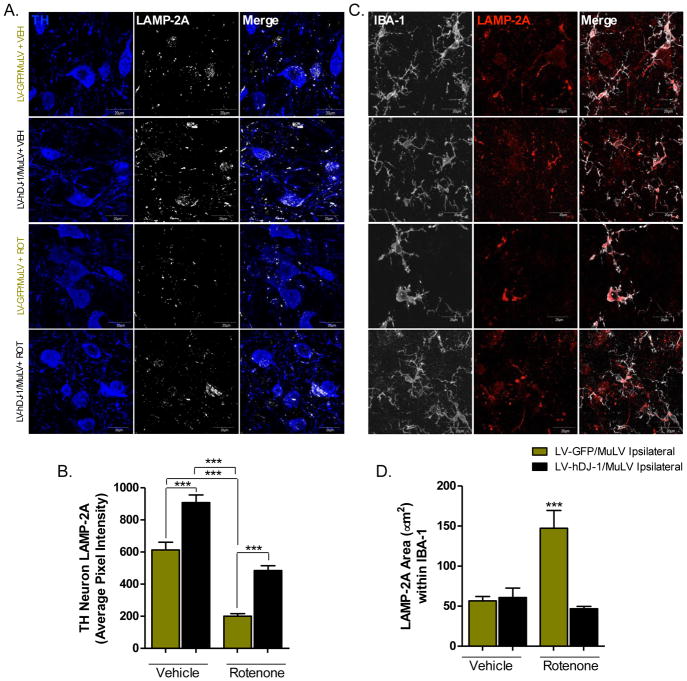
Astrocytic hDJ-1 overexpression prevents chaperone-mediated autophagy deficits
Recent evidence suggests that DJ-1 protects mitochondrial function, in part, through effects on chaperone-mediated autophagy (CMA; B. Wang et al., 2016). To investigate whether astrocytic DJ-1 may influence neuronal CMA following rotenone exposure, we examined the lysosomal surface protein LAMP-2A, a receptor for chaperones trafficking dysfunctional proteins destined for degradation (Massey et al., 2006; U. Bandyopadhyay and Cuervo, 2008; U. Bandyopadhyay et al., 2008). In dopaminergic neurons within the SN, LAMP-2A protein levels were significantly reduced in control LV-GFP/MuLV-injected animals receiving rotenone (p < 0.001; Figure 6a). In contrast, in animals overexpressing hDJ-1 in astrocytes, LAMP-2A-positive immunostaining in dopamine neurons was substantially increased at baseline and significantly preserved after rotenone (p < 0.0001; Figure 6a–b). LAMP-2A protein levels were significantly elevated in microglia following rotenone treatment (p < 0.0001; Figure 6c–d), similar to another lysosomal protein, CD68 (Figure 4). Astrocytic overexpression of hDJ-1 attenuated this microglial inflammatory phenotype.
Figure 6. Chaperone-mediated autophagy deficits in dopaminergic neurons induced by rotenone are ameliorated by astrocytic hDJ-1 overexpression.
A. Representative images of dopamine neurons in the SN (60×) immunopositive for the lysosomal surface protein LAMP-2A (white) which is induced in chaperone mediated autophagy. B. Rotenone treatment causes a significant decrease in neuronal LAMP-2A expression, which is protected in hDJ-1 treated animals; N=5 (F3,427 = 47.32, p < 0.0001). C. Representative images (100×) of microglia in the SN (white) expressing LAMP-2A (red). D. Rotenone treatment significantly increases LAMP-2A protein levels in activated microglia; N=5 (F3,20 = 17.99, p < 0.0001). Statistical analysis; one-way ANOVA followed by Tukey post-hoc test (***p < 0.0001).
Discussion
PD presents an inviting target for gene therapy approaches designed to modify disease progression. In this regard, it is especially appealing to investigate a gene therapy vector that may ameliorate several aspects of PD pathogenesis, including oxidative/nitrosative stress, mitochondrial impairment, neuroinflammation, and α-synuclein accumulation. DJ-1 is often described as a multifunctional protein, both because it harbors a master regulator function controlling central physiological processes (i.e. interaction with mRNA; (van der Brug et al., 2008; Blackinton et al., 2009a), and because it is directly involved in multiple protein functions key to cell survival under oxidative stress conditions (Shendelman et al., 2004; Girotto et al., 2012; Piston et al., 2017; Frøyset et al., 2018 ). For example, DJ-1 has been shown to interact with mitochondria to modulate mitophagy (Gao et al., 2012; McCoy and Cookson, 2014; Thomas et al., 2011), physically restrict α-synuclein monomers from oligomerizing (Zondler et al., 2017), and bind to p47(phox) of the NADPH oxidase complex (NOX), causing assembly disruption and degradation of NOX (Amatullah et al., 2017; Liu et al., 2015). It is therefore plausible that overexpression of DJ-1 can act in multiple oxidation-dependent pathways and exert protection against several aspects of PD pathology.
Elevated levels of DJ-1 immunoreactivity within activated astrocytes is a pathological feature of several neurodegenerative diseases (e.g. Alzheimer’s disease, multiple system atrophy, Pick’s disease, and progressive supranuclear palsy), and was originally thought to be a compensatory response to oxidative damage (Neumann et al., 2004; Rizzu et al., 2004 Kumaran et al., 2007; reviewed in (Antipova and Bandopadhyay, 2017). However, evidence from primary astrocyte-neuron cocultures suggests that astrocytic DJ-1 maintains neuronal mitochondrial function following complex I inhibition, and this protection extends beyond augmenting antioxidant capacity (Mullett and Hinkle, 2011; 2009). Here, we show that human DJ-1, expressed specifically in astrocytes within the SN and striatum, prevents several PD-like neuropathological abnormalities caused by the prototypical complex I inhibitor rotenone. In LV-hDJ-1/MuLV-injected animals, the soma and axons of dopamine neurons within the nigrostriatal tract were significantly protected from rotenone-induced degeneration compared to controls (Figure 2).
As previously observed (Cannon et al., 2009b), the endpoint rotenone dosing paradigm induces rapid motor impairment as measured using a postural instability test (PIT; Woodlee et al., 2008; Khaing et al., 2013). Thus, it is not surprising that all rotenone-treated animals (LV-GFP/MuLV and LV-hDJ-1/MuLV) exhibited motor behavior deficits, as well as rapid weight loss, associated with rotenone-induced systemic toxicity and gastrointestinal neuropathology (Drolet et al., 2009) (Supplemental Figure 2). Stereotactic infusion of the LV-hDJ-1/MuLV vector resulted in local expression of astrocytic hDJ-1 (or GFP control vector; Figure 1b–c) therefore, it is unlikely that this targeted area would provide fully effective protection against rotenone toxicity. Interestingly, unilateral injection of LV-hDJ-1/MuLV protected both the ipsilateral, and to a lesser extent, the contralateral SN and striatum from rotenone-induced neurodegeneration (Figure 2). These observations support previous hypotheses that soluble factors released from astrocytes are dynamically regulated by DJ-1 expression, and may be manipulated to provide neuroprotection (Mullett and Hinkle, 2009; Hauser and Cookson, 2011; Mullett and Hinkle, 2011). To date, it remains unclear which signaling molecules are involved in this process. One hypothesized mechanism for bilateral protection by LV-hDJ-1/MuLV is via neurotrophic factor regulation (e.g., glial-derived neurotrophic factor; GDNF), which can be globally neuroprotective if elevated within the intraventricular space (Kobayashi et al., 1998; Lapchak et al., 1998). There is evidence that supports DJ-1 as a modulator of neurotrophic signaling (Foti et al., 2010), which may represent one pathway for bilateral protection in this model. In this regard, we observed transduced astrocytes adjacent to the lateral ventricle in the striatum (Figure 2c), providing a potential avenue for neurotrophic factor release into the intraventricular space. At present, physiologic limitations of this in vivo model prevented the measurement of GDNF, or other neurotrophic factors, within the ventricles or cerebrospinal fluid, however investigations into the specific signaling mechanisms of hDJ-1 overexpression in astrocytes are currently underway.
The strong association between complex I inhibition and PD (Schapira et al., 1989; Greenamyre et al., 2001; Keeney, 2006; Schapira, 2010), and the discovery that autosomal recessive forms of parkinsonism are caused by mutations in PINK1 (Valente et al., 2004; 2002) and Parkin (Lücking et al., 1998), suggest that protection against mitochondrial dysfunction may be a key target to prevent dopamine neuron degeneration (S. B. Larsen et al., 2018). DJ-1 has been linked with the PTEN-induced putative kinase-1 (PINK1)/Parkin pathway to confer mitochondrial protection through both antioxidant function and mitochondrial fusion (Moore et al., 2005; Thomas et al., 2011; Joselin et al., 2012). In our model, we found a significant preservation of the complex I subunit NDUFS3 within dopamine neurons that were in proximity to astrocytes overexpressing hDJ-1 (Figure 3). We did not observe increased DJ-1 expression in these same neurons (Figure 1); therefore, it is unlikely that the DJ-1 protein itself has directly influenced neuronal mitochondrial dynamics. Instead, astrocytic DJ-1 may protect neurons from rotenone-induced mitochondrial toxicity by modulating antioxidants with known astrocyte-to-neuron transfer, such as glutathione (Dringen et al., 2000; 1999; X. F. Wang and Cynader, 2000). New evidence confirms that overexpression of astrocytic DJ-1 upregulates genes/proteins involved in response to oxidative stress, such as Nrf2-reglated peroiredoxin 2, thioredoxin, and superoxide dismutase (Frøyset et al., 2018).
In addition, DJ-1 has been shown to modulate astrocyte inflammatory signaling by reducing the amount of inducible nitric oxide synthase (iNOS) produced following LPS treatment (Waak et al., 2009). Glial upregulation of iNOS is an abundant source of NO production within the brain, a contributor to nitrosative damage, and a pathological hallmark found in both human PD tissue as well as in animal models (Liberatore et al., 1999; Calabrese et al., 2004; Chung et al., 2005). We found that rats receiving the hDJ-1 infusion had significantly less rotenone-induced nitrosative damage within TH-positive neurons than LV-GFP/MuLV control animals (Figure 3). Consistent with this, we observed that hDJ-1 expression attenuated iNOS protein levels within LV-hDJ-1/MuLV transduced striatal astrocytes (Figure 4f–h), supporting that downregulation of iNOS within astrocytes is protective against neuronal nitrosative stress (Ebadi and Sharma, 2003; Tieu et al., 2003; Carreras et al., 2004). Of note, these results are consistent with a recently published report which showed that astrocytic DJ-1 overexpression in zebrafish reduced iNOS protein expression following exposure to MPP+ (compared to wildtype fish; Frøyset et al., 2018).
Oxidative stress is also a strong inducer of microglial activation, driving neuroinflammation within the ventral midbrain (Lull and Block, 2010; Peterson and Flood, 2012; Dias et al., 2013). In addition to causing neurodegeneration, rotenone has also been shown to directly activate microglia via nuclear factor kappa (NF-κ)-B (Sherer et al., 2003a; Yuan et al., 2013; F. Zhou et al., 2017). A high degree of microglial activation was observed in the SN following rotenone treatment in animals receiving the control LV-GFP/MuLV infusion. This was marked by a morphological change to an amoeboid appearance and a substantial increase in the lysosomal protein CD68, which is associated with active phagocytosis in macrophages (Perego et al., 2011; Bodea et al., 2014; Figure 4). This inflammatory response was significantly attenuated in animals receiving rotenone and LV-hDJ-1/MuLV infusion. Gliosis following neurotoxic insult in models of PD is a well-characterized sequential event, with microglial activation typically preceding astrocytosis, and ultimately resulting in feed-forward signaling that drives neurotoxicity (Iravani et al., 2005; Farina et al., 2007; Hirsch and Hunot, 2009). Mitigation of microglial activation has been unequivocally neuroprotective in animal models of PD (Dehmer et al., 2003; Kim and Joh, 2006; Vijitruth et al., 2006). However, no small-molecule anti-inflammatory therapeutic has been successful in modulating the human disease, notably minocycline and pioglitazone, which showed no benefit over placebo in clinical trials (Glass et al., 2010; NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators, 2015; NINDS NET-PD Investigators, 2008). In this context, the use of cell type-specific gene therapy may provide a way to examine blockade of inflammatory signaling within a given cell population. Astrocytic hDJ-1 expression presents several novel advantages in this regard: (i) reduction of oxidative stress produced by mitochondrial dysfunction, (ii) transcriptional activation of astrocytic anti-inflammatory proteins (i.e. Nrf2; Gan et al., 2010; Bell et al., 2011; Im et al., 2012; Zhang et al., 2017), and (iii) decreased α-synuclein aggregation (Figures 5–6), all of which have been shown to modulate glial activation (Hayashi et al., 2009; Gan et al., 2010; Zondler et al., 2017).
Whether DJ-1 directly interacts with α-synuclein has been controversial (Jin et al., 2007; Zhu et al., 2017). Despite this, there is evidence that DJ-1 plays a role in autophagy as well as the detoxification of α-synuclein (Batelli et al., 2008; Jaramillo-Gómez et al., 2015; Burbulla et al., 2017; Zondler et al., 2017). Neuropathological analysis of the first human PARK7 (L172Q DJ-1) case to come to autopsy confirms that Lewy body accumulation occurs when DJ-1 is dysfunctional (Taipa et al., 2016). The rotenone model employed in this study induces the accumulation of endogenous α-synuclein within dopamine neurons of the SN, an affect that was significantly ameliorated in hDJ-1 treated animals (Figure 5). As there is evidence for both neuron-to-astrocyte (H.-J. Lee et al., 2010; Loria et al., 2017), as well as astrocyte-to-astrocyte α-synuclein transfer (Rostami et al., 2017), astrocytic DJ-1 overexpression may be protective against the propagation and aggregation of α-synuclein.
Rotenone treatment also caused a reduction in LAMP-2A expression within dopamine neurons, indicating dysfunction of chaperone mediated autophagy (CMA; U. Bandyopadhyay et al., 2008) – and this was prevented by overexpression of hDJ-1 (Figure 6). In addition to its chaperone activity, DJ-1 has been shown to modulate mitochondrial fusion via autophagy, and it protects against mitochondrial fragmentation caused by an oxidative insult (Krebiehl et al., 2010; McCoy and Cookson, 2014). There is recent evidence that CMA-mediated protection of mitochondria against MPP+ is lost in DJ-1 deficient neurons (B. Wang et al., 2016), and knockdown of DJ-1 in microglia impairs phagocytosis of α-synuclein (Nash et al., 2017). Conversely, overexpression of DJ-1 was protective against mitochondrial dysfunction when CMA was blocked (B. Wang et al., 2016). In parallel, DJ-1 has been shown to increase cellular levels of HSP70, a protein chaperone inversely correlated with α-synuclein levels (Li et al., 2005; Batelli et al., 2008). In our study, astrocytic overexpression of hDJ-1 was associated with elevated levels of the CMA receptor, LAMP-2A, at baseline (without rotenone). Rotenone caused a reduction of LAMP-2A-positive lysosomes in nigrostriatal neurons, which was prevented by overexpression of hDJ-1 in astrocytes. It is notable that rotenone-induced loss of LAMP-2A (Figure 6) was associated with elevated levels of α-synuclein in the same neurons (Figure 5), which indicates that disruption of CMA may be pivotal for α-synuclein accumulation in the rotenone model. Moreover, these results suggest that astrocytic DJ-1 levels can influence neuronal autophagic processes.
To some extent, the current study is consistent with prior evidence for modulating DJ-1 as a protective strategy. In a cell-autonomous system, DJ-1 is described to induce autophagy via activation of the ERK1/2 pathway following oxidative stress (Oh and Mouradian, 2017). In addition, DJ-1 has been explored as a therapeutic to restore autophagy and cardioprotection, using AAV9-mediated overexpression of hDJ-1 (B. Zhou et al., 2017). Alternately, the impaired ability of microglia deficient in DJ-1 to phagocytose α-synuclein (Nash et al., 2017) may have multiple consequences for both protein aggregation and inflammation in dynamically regulated phagocytic cells. Together, these data imply that DJ-1 plays a pleomorphic role in the cellular activities regulating protein handling and degradation, in both cell and cell non-autonomous pathways.
Conclusions
We have shown that overexpression of hDJ-1 in astrocytes potently protects dopamine neurons against neurodegeneration, apparently by multiple mechanisms, including preservation of mitochondrial function, inhibition of nitrosative stress, suppression of neuroinflammation, preservation of CMA, and prevention of α-synuclein accumulation. The specific targeting of astrocytes with the MuLV-pseudotyped lentiviral gene therapy vector may mitigate neurodegeneration without over-burdening or relying on expression within a vulnerable population of neurons, and may provide a novel avenue for therapeutic development.
Supplementary Material
Highlights.
DJ-1 is a redox-sensitive protein, upregulated in astrocytes in Parkinson’s disease
Using a pseudotyped lentiviral vector, DJ-1 was overexpressed in astrocytes in vivo
Rats expressing astrocytic DJ-1 were protected from rotenone model of PD
Astrocytic DJ-1 attenuated rotenone-induced oxidative stress and neuroinflammation
Astrocyte DJ-1 expression decreased α-synuclein accumulation in dopaminergic neurons
Acknowledgments
This work was supported by the National Institutes of Health [1R01 ES020327, 1R01 NS095387, T32 NS086749] and the US Department of Veterans’ Affairs [1I01 BX000548]. The contents of this article do not represent the views of the US Department of Veterans Affairs or the United States Government.
Footnotes
Declaration of Interest
The authors have declared no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleyasin H, Rousseaux MWC, Marcogliese PC, Hewitt SJ, Irrcher I, Joselin AP, Parsanejad M, Kim RH, Rizzu P, Callaghan SM, Slack RS, Mak TW, Park DS. DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad Sci USA. 2010;107:3186–3191. doi: 10.1073/pnas.0914876107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatullah H, Shan Y, Beauchamp BL, Gali PL, Gupta S, Maron-Gutierrez T, Speck ER, Fox-Robichaud AE, Tsang JLY, Mei SHJ, Mak TW, Rocco PRM, Semple JW, Zhang H, Hu P, Marshall JC, Stewart DJ, Harper M-E, Liaw PC, Liles WC, Santos Dos CC Canadian Critical Care Translational Biology Group. DJ-1/PARK7 Impairs Bacterial Clearance in Sepsis. Am J Respir Crit Care Med. 2017;195:889–905. doi: 10.1164/rccm.201604-0730OC. [DOI] [PubMed] [Google Scholar]
- Antipova D, Bandopadhyay R. Expression of DJ-1 in Neurodegenerative Disorders. Adv Exp Med Biol. 2017;1037:25–43. doi: 10.1007/978-981-10-6583-5_3. [DOI] [PubMed] [Google Scholar]
- Ashley AK, Hanneman WH, Katoh T, Moreno JA, Pollack A, Tjalkens RB, Legare ME. Analysis of targeted mutation in DJ-1 on cellular function in primary astrocytes. Toxicology Letters. 2009;184:186–191. doi: 10.1016/j.toxlet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Q, Mullett SJ, Garver JA, Hinkle DA, Burton EA. Zebrafish DJ-1 is evolutionarily conserved and expressed in dopaminergic neurons. Brain Research. 2006;1113:33–44. doi: 10.1016/j.brainres.2006.07.057. [DOI] [PubMed] [Google Scholar]
- Bandopadhyay R, Kingsbury AE, Cookson MR, Reid AR, Evans IM, Hope AD, Pittman AM, Lashley T, Canet-Aviles R, Miller DW, McLendon C, Strand C, Leonard AJ, Abou-Sleiman PM, Healy DG, Ariga H, Wood NW, de Silva R, Revesz T, Hardy JA, Lees AJ. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Cookson MR. BMC Evolutionary Biology. BMC Evol Biol. 2004;4:6–9. doi: 10.1186/1471-2148-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay U, Cuervo AM. Entering the lysosome through a transient gate by chaperone-mediated autophagy. Autophagy. 2008;4:1101–1103. doi: 10.4161/auto.7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay U, Kaushik S, Varticovski L, Cuervo AM. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol Cell Biol. 2008;28:5747–5763. doi: 10.1128/MCB.02070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batelli S, Albani D, Rametta R, Polito L, Prato F, Pesaresi M, Negro A, Forloni G. DJ-1 Modulates α-Synuclein Aggregation State in a Cellular Model of Oxidative Stress: Relevance for Parkinson’s Disease and Involvement of HSP70. PLoS ONE. 2008;3:e1884–11. doi: 10.1371/journal.pone.0001884. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bell KF, Al-Mubarak B, Fowler JH, Baxter PS, Gupta K, Tsujita T, Chowdhry S, Patani R, Chandran S, Horsburgh K, Hayes JD, Hardingham GE. Mild oxidative stress activates Nrf2 in astrocytes, which contributes to neuroprotective ischemic preconditioning. Proc Natl Acad Sci USA. 2011;108:E1–E2. doi: 10.1073/pnas.1015229108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackinton J, Ahmad R, Miller DW, van der Brug MP, Canet-Avilés RM, Hague SM, Kaleem M, Cookson MR. Effects of DJ-1 mutations and polymorphisms on protein stability and subcellular localization. Molecular Brain Research. 2005;134:76–83. doi: 10.1016/j.molbrainres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Blackinton J, Kumaran R, van der Brug MP, Ahmad R, Olson L, Galter D, Lees A, Bandopadhyay R, Cookson MR. Post-transcriptional regulation of mRNA associated with DJ-1 in sporadic Parkinson disease. Neuroscience Letters. 2009a;452:8–11. doi: 10.1016/j.neulet.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackinton J, Lakshminarasimhan M, Thomas KJ, Ahmad R, Greggio E, Raza AS, Cookson MR, Wilson MA. Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the parkinsonism protein DJ-1. J Biol Chem. 2009b;284:6476–6485. doi: 10.1074/jbc.M806599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodea L-G, Wang Y, Linnartz-Gerlach B, Kopatz J, Sinkkonen L, Musgrove R, Kaoma T, Muller A, Vallar L, Di Monte DA, Balling R, Neumann H. Neurodegeneration by activation of the microglial complement-phagosome pathway. J Neurosci. 2014;34:8546–8556. doi: 10.1523/JNEUROSCI.5002-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V, Breedveld GJ, Squitieri F, Vanacore N, Brustenghi P, Harhangi BS, Montagna P, Cannella M, Fabbrini G, Rizzu P, van Duijn CM, Oostra BA, Meco G, Heutink P. Localization of autosomal recessive early-onset parkinsonism to chromosome 1p36 (PARK7) in an independent dataset. Ann Neurol. 2002;51:253–256. doi: 10.1002/ana.10106. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MCJ, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, Santos DP, Blanz J, Obermaier CD, Strojny C, Savas JN, Kiskinis E, Zhuang X, Krüger R, Surmeier DJ, Krainc D. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science. 2017;357:1255–1261. doi: 10.1126/science.aam9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Boyd-Kimball D, Scapagnini G, Butterfield DA. Nitric oxide and cellular stress response in brain aging and neurodegenerative disorders: the role of vitagenes. In Vivo. 2004;18:245–267. [PubMed] [Google Scholar]
- Canet-Avilés RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JR, Sew T, Montero L, Burton EA, Greenamyre JT. Pseudotype-dependent lentiviral transduction of astrocytes or neurons in the rat substantia nigra. Experimental Neurology. 2011;228:41–52. doi: 10.1016/j.expneurol.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson’s disease. Neurobiology of Disease. 2009a;34:279–290. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson’s disease. Neurobiology of Disease. 2009b;34:279–290. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras MC, Franco MC, Peralta JG, Poderoso JJ. Nitric oxide, complex I, and the modulation of mitochondrial reactive species in biology and disease. Mol Aspects Med. 2004;25:125–139. doi: 10.1016/j.mam.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Choi J, Sullards MC, Olzmann JA, Rees HD, Weintraub ST, Bostwick DE, Gearing M, Levey AI, Chin L-S, Li L. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem. 2006;281:10816–10824. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KKK, Dawson TM, Dawson VL. Nitric oxide, S-nitrosylation and neurodegeneration. Cell Mol Biol (Noisy-le-grand) 2005;51:247–254. [PubMed] [Google Scholar]
- Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with IκBα induction and block of NFκB and iNOS activation. Journal of Neurochemistry. 2003;88:494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB. Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. Journal of Neurochemistry. 2000;74:2213–2216. doi: 10.1046/j.1471-4159.2000.0742213.x. [DOI] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio R, Barrett PJ, Hoffman EK, Barrett CW, Zharikov A, Borah A, Hu X, McCoy J, Chu CT, Burton EA, Hastings TG, Greenamyre JT. α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Science Translational Medicine. 2016;8:342ra78–342ra78. doi: 10.1126/scitranslmed.aaf3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis. 2013;3:461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267:4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. Journal of Neuroscience. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet RE, Cannon JR, Montero L, Greenamyre JT. Chronic rotenone exposure reproduces Parkinson’s disease gastrointestinal neuropathology. Neurobiology of Disease. 2009;36:96–102. doi: 10.1016/j.nbd.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Ebadi M, Sharma SK. Peroxynitrite and mitochondrial dysfunction in the pathogenesis of Parkinson’s disease. Antioxidants & Redox Signaling. 2003;5:319–335. doi: 10.1089/152308603322110896. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Foti R, Zucchelli S, Biagioli M, Roncaglia P, Vilotti S, Calligaris R, Krmac H, Girardini JE, Del Sal G, Gustincich S. Parkinson disease-associated DJ-1 is required for the expression of the glial cell line-derived neurotrophic factor receptor RET in human neuroblastoma cells. J Biol Chem. 2010;285:18565–18574. doi: 10.1074/jbc.M109.088294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøyset AK, Edson AJ, Gharbi N, Khan EA, Dondorp D, Bai Q, Tiraboschi E, Suster ML, Connolly JB, Burton EA, Fladmark KE. Astroglial DJ-1 over-expression up-regulates proteins involved in redox regulation and is neuroprotective in vivo. Redox Biology. 2018;16:237–247. doi: 10.1016/j.redox.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Johnson DA, Johnson JA. Keap1-Nrf2 activation in the presence and absence of DJ-1. European Journal of Neuroscience. 2010;31:967–977. doi: 10.1111/j.1460-9568.2010.07138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Yang W, Qi Z, Lu L, Duan C, Zhao C, Yang H. DJ-1 protects dopaminergic neurons against rotenone-induced apoptosis by enhancing ERK-dependent mitophagy. Journal of Molecular Biology. 2012;423:232–248. doi: 10.1016/j.jmb.2012.06.034. [DOI] [PubMed] [Google Scholar]
- Girotto S, Sturlese M, Bellanda M, Tessari I, Cappellini R, Bisaglia M, Bubacco L, Mammi S. Dopamine-derived quinones affect the structure of the redox sensor DJ-1 through modifications at Cys-106 and Cys-53. J Biol Chem. 2012;287:18738–18749. doi: 10.1074/jbc.M111.311589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms Underlying Inflammation in Neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenamyre JT, Sherer TB, Betarbet R, Panov AV. Complex I and Parkinson’s disease. IUBMB Life (International Union of Biochemistry and Molecular Biology: Life) 2001;52:135–141. doi: 10.1080/15216540152845939. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Ishimori C, Takahashi-Niki K, Taira T, Kim YC, Maita H, Maita C, Ariga H, Iguchi-Ariga SMM. DJ-1 binds to mitochondrial complex I and maintains its activity. Biochemical and Biophysical Research Communications. 2009;390:667–672. doi: 10.1016/j.bbrc.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Feinstein DL. Expression and function of inducible nitric oxide synthase in neurons. Journal of Neuroimmunology. 2001;114:8–18. doi: 10.1016/s0165-5728(01)00246-6. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? The Lancet Neurology. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Im JY, Lee KW, Woo JM, Junn E, Mouradian MM. DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Human Molecular Genetics. 2012;21:3013–3024. doi: 10.1093/hmg/dds131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani MM, Kashefi K, Mander P, Rose S, Jenner P. Involvement of inducible nitric oxide synthase in inflammation-induced dopaminergic neurodegeneration. NSC. 2002;110:49–58. doi: 10.1016/s0306-4522(01)00562-0. [DOI] [PubMed] [Google Scholar]
- Iravani MM, Leung CCM, Sadeghian M, Haddon CO, Rose S, Jenner P. The acute and the long-term effects of nigral lipopolysaccharide administration on dopaminergic dysfunction and glial cell activation. Eur J Neurosci. 2005;22:317–330. doi: 10.1111/j.1460-9568.2005.04220.x. [DOI] [PubMed] [Google Scholar]
- Jaramillo-Gómez J, Niño A, Arboleda H, Arboleda G. Overexpression of DJ-1 protects against C2-ceramide-induced neuronal death through activation of the PI3K/AKT pathway and inhibition of autophagy. Neuroscience Letters. 2015;603:71–76. doi: 10.1016/j.neulet.2015.07.032. [DOI] [PubMed] [Google Scholar]
- Jin J, Li GJ, Davis J, Zhu D, Wang Y, Pan C, Zhang J. Identification of novel proteins associated with both alpha-synuclein and DJ-1. Mol Cell Proteomics. 2007;6:845–859. doi: 10.1074/mcp.M600182-MCP200. [DOI] [PubMed] [Google Scholar]
- Joselin AP, Hewitt SJ, Callaghan SM, Kim RH, Chung YH, Mak TW, Shen J, Slack RS, Park DS. ROS-dependent regulation of Parkin and DJ-1 localization during oxidative stress in neurons. Human Molecular Genetics. 2012;21:4888–4903. doi: 10.1093/hmg/dds325. [DOI] [PubMed] [Google Scholar]
- Junn E, Jang WH, Zhao X, Jeong BS, Mouradian MM. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res. 2009;87:123–129. doi: 10.1002/jnr.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney PM. Parkinson’s Disease Brain Mitochondrial Complex I Has Oxidatively Damaged Subunits and Is Functionally Impaired and Misassembled. Journal of Neuroscience. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaing ZZ, Geissler SA, Schallert T, Schmidt CE. Assessing forelimb function after unilateral cervical SCI using novel tasks: limb step-alternation, postural instability and pasta handling. J Vis Exp. 2013:e50955. doi: 10.3791/50955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- Kinumi T, Kimata J, Taira T, Ariga H, Niki E. Cysteine-106 of DJ-1 is the most sensitive cysteine residue to hydrogen peroxide-mediated oxidation in vivo in human umbilical vein endothelial cells. Biochemical and Biophysical Research Communications. 2004;317:722–728. doi: 10.1016/j.bbrc.2004.03.110. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Ogren SO, Hoffer BJ, Olson L. Dopamine D1 and D2 receptor-mediated acute and long-lasting behavioral effects of glial cell line-derived neurotrophic factor administered into the striatum. Experimental Neurology. 1998;154:302–314. doi: 10.1006/exnr.1998.6952. [DOI] [PubMed] [Google Scholar]
- Krebiehl G, Ruckerbauer S, Burbulla LF, Kieper N, Maurer B, Waak J, Wolburg H, Gizatullina Z, Gellerich FN, Woitalla D, Riess O, Kahle PJ, Proikas-Cezanne T, Krüger R. Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson’s disease-associated protein DJ-1. PLoS ONE. 2010;5:e9367. doi: 10.1371/journal.pone.0009367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran R, Kingsbury A, Coulter I, Lashley T, Williams D, de Silva R, Mann D, Revesz T, Lees A, Bandopadhyay R. DJ-1 (PARK7) is associated with 3R and 4R tau neuronal and glial inclusions in neurodegenerative disorders. Neurobiology of Disease. 2007;28:122–132. doi: 10.1016/j.nbd.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Hilt DC, Jiao S, Collin F, Miyoshi Y, Yi A, Zhang Z, Gash DM. Topographical distribution of [125I]-glial cell line-derived neurotrophic factor in unlesioned and MPTP-lesioned rhesus monkey brain following a bolus intraventricular injection. Brain Research. 1998;789:9–22. doi: 10.1016/s0006-8993(97)01495-9. [DOI] [PubMed] [Google Scholar]
- Larsen NJ, Ambrosi G, Mullett SJ, Berman SB, Hinkle DA. DJ-1 knock-down impairs astrocyte mitochondrial function. NSC. 2011;196:251–264. doi: 10.1016/j.neuroscience.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen SB, Hanss Z, Krüger R. The genetic architecture of mitochondrial dysfunction in Parkinson’s disease. Cell Tissue Res. 2018;78:417–17. doi: 10.1007/s00441-017-2768-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Suk J-E, Patrick C, Bae E-J, Cho J-H, Rho S, Hwang D, Masliah E, Lee S-J. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-J, Kim SJ, Kim I-K, Ko J, Jeong C-S, Kim G-H, Park C, Kang S-O, Suh P-G, Lee H-S, Cha S-S. Crystal structures of human DJ-1 and Escherichia coli Hsp31, which share an evolutionarily conserved domain. J Biol Chem. 2003;278:44552–44559. doi: 10.1074/jbc.M304517200. [DOI] [PubMed] [Google Scholar]
- Li HM, Niki T, Taira T, Iguchi-Ariga SMM, Ariga H. Association of DJ-1 with chaperones and enhanced association and colocalization with mitochondrial Hsp70 by oxidative stress. Free Radical Research. 2005;39:1091–1099. doi: 10.1080/10715760500260348. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu W, Wu H, Chen L, Wen Y, Kong X, Gao W-Q. Park7 interacts with p47(phox) to direct NADPH oxidase-dependent ROS production and protect against sepsis. Cell Res. 2015;25:691–706. doi: 10.1038/cr.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria F, Vargas JY, Bousset L, Syan S, Salles A, Melki R, Zurzolo C. α-Synuclein transfer between neurons and astrocytes indicates that astrocytes play a role in degradation rather than in spreading. Acta Neuropathol. 2017;134:789–808. doi: 10.1007/s00401-017-1746-2. [DOI] [PubMed] [Google Scholar]
- Lu L, Zhao S, Gao G, Sun X, Zhao H, Yang H. DJ-1/PARK7, But Not Its L166P Mutant Linked to Autosomal Recessive Parkinsonism, Modulates the Transcriptional Activity of the Orphan Nuclear Receptor Nurr1 In Vitro and In Vivo. Mol Neurobiol. 2016;53:7363–7374. doi: 10.1007/s12035-016-9772-y. [DOI] [PubMed] [Google Scholar]
- Luk B, Mohammed M, Liu F, Lee FJS. A Physical Interaction between the Dopamine Transporter and DJ-1 Facilitates Increased Dopamine Reuptake. PLoS ONE. 2015;10:e0136641. doi: 10.1371/journal.pone.0136641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7:354–365. doi: 10.1016/j.nurt.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lücking CB, Abbas N, Dürr A, Bonifati V, Bonnet AM, de Broucker T, De Michele G, Wood NW, Agid Y, Brice A. Homozygous deletions in parkin gene in European and North African families with autosomal recessive juvenile parkinsonism. The European Consortium on Genetic Susceptibility in Parkinson’s Disease and the French Parkinson’s Disease Genetics Study Group. The Lancet. 1998;352:1355–1356. doi: 10.1016/s0140-6736(05)60746-5. [DOI] [PubMed] [Google Scholar]
- Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci USA. 2006;103:5805–5810. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Cookson MR. DJ-1 regulation of mitochondrial function and autophagy through oxidative stress. Autophagy. 2014;7:531–532. doi: 10.4161/auto.7.5.14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulener MC, Graves CL, Sampathu DM, Armstrong-Gold CE, Bonini NM, Giasson BI. DJ-1 is present in a large molecular complex in human brain tissue and interacts with alpha-synuclein. Journal of Neurochemistry. 2005;93:1524–1532. doi: 10.1111/j.1471-4159.2005.03145.x. [DOI] [PubMed] [Google Scholar]
- Miller DW, Ahmad R, Hague S, Baptista MJ, Canet-Aviles R, McLendon C, Carter DM, Zhu P-P, Stadler J, Chandran J, Klinefelter GR, Blackstone C, Cookson MR. L166P mutant DJ-1, causative for recessive Parkinson’s disease, is degraded through the ubiquitin-proteasome system. J Biol Chem. 2003;278:36588–36595. doi: 10.1074/jbc.M304272200. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Zhang L, Troncoso J, Lee MK, Hattori N, Mizuno Y, Dawson TM, Dawson VL. Association of DJ-1 and parkin mediated by pathogenic DJ-1 mutations and oxidative stress. Human Molecular Genetics. 2005;14:71–84. doi: 10.1093/hmg/ddi007. [DOI] [PubMed] [Google Scholar]
- Moscovitz O, Ben-Nissan G, Fainer I, Pollack D, Mizrachi L, Sharon M. The Parkinson’s-associated protein DJ-1 regulates the 20S proteasome. Nature Communications. 2017:1–13. doi: 10.1038/ncomms7609. [DOI] [PubMed] [Google Scholar]
- Mullett SJ, Di Maio R, Greenamyre JT, Hinkle DA. DJ-1 Expression Modulates Astrocyte-Mediated Protection Against Neuronal Oxidative Stress. J Mol Neurosci. 2012;49:507–511. doi: 10.1007/s12031-012-9904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullett SJ, Hinkle DA. DJ-1 deficiency in astrocytes selectively enhances mitochondrial Complex I inhibitor-induced neurotoxicity. Journal of Neurochemistry. 2011;117:375–387. doi: 10.1111/j.1471-4159.2011.07175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullett SJ, Hinkle DA. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiology of Disease. 2009;33:28–36. doi: 10.1016/j.nbd.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochemical and Biophysical Research Communications. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- Nash Y, Schmukler E, Trudler D, Pinkas-Kramarski R, Frenkel D. DJ-1 Deficiency Impairs Autophagy and Reduces Alpha-synuclein Phagocytosis by Microglia. Journal of Neurochemistry. 2017 doi: 10.1111/jnc.14222. [DOI] [PubMed] [Google Scholar]
- Neumann M, Müller V, Görner K, Kretzschmar HA, Haass C, Kahle PJ. Pathological properties of the Parkinson“s disease-associated protein DJ-1 in alpha-synucleinopathies and tauopathies: relevance for multiple system atrophy and Pick”s disease. Acta Neuropathol. 2004;107:489–496. doi: 10.1007/s00401-004-0834-2. [DOI] [PubMed] [Google Scholar]
- NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators. Pioglitazone in early Parkinson’s disease: a phase 2, multicentre, double-blind, randomised trial. The Lancet Neurology. 2015;14:795–803. doi: 10.1016/S1474-4422(15)00144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NINDS NET-PD Investigators. A pilot clinical trial of creatine and minocycline in early Parkinson disease: 18-month results. Clin Neuropharmacol. 2008;31:141–150. doi: 10.1097/WNF.0b013e3181342f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SE, Mouradian MM. Cytoprotective mechanisms of DJ-1 against oxidative stress through modulating ERK1/2 and ASK1 signal transduction. Redox Biology. 2017;14:211–217. doi: 10.1016/j.redox.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooe H, Maita C, Maita H, Iguchi-Ariga SMM, Ariga H. Specific cleavage of DJ-1 under an oxidative condition. Neuroscience Letters. 2006;406:165–168. doi: 10.1016/j.neulet.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Pennathur S, Jackson-Lewis V, Przedborski S, Heinecke JW. Mass spectrometric quantification of 3-nitrotyrosine, ortho-tyrosine, and o,o“-dityrosine in brain tissue of 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine-treated mice, a model of oxidative stress in Parkinson”s disease. J Biol Chem. 1999;274:34621–34628. doi: 10.1074/jbc.274.49.34621. [DOI] [PubMed] [Google Scholar]
- Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. Journal of Neuroinflammation. 2011;8:174. doi: 10.1186/1742-2094-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LJ, Flood PM. Oxidative Stress and Microglial Cells in Parkinson’s Disease. Mediators of Inflammation. 2012;2012:1–12. doi: 10.1155/2012/401264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piston D, Alvarez-Erviti L, Bansal V, Gargano D, Yao Z, Szabadkai G, Odell M, Puno MR, Björkblom B, Maple-Grødem J, Breuer P, Kaut O, Larsen JP, Bonn S, Møller SG, Wüllner U, Schapira AHV, Gegg ME. DJ-1 is a redox sensitive adapter protein for high molecular weight complexes involved in regulation of catecholamine homeostasis. Human Molecular Genetics. 2017;26:4028–4041. doi: 10.1093/hmg/ddx294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzu P, Hinkle DA, Zhukareva V, Bonifati V, Severijnen L-A, Martinez D, Ravid R, Kamphorst W, Eberwine JH, Lee VM-Y, Trojanowski JQ, Heutink P. DJ-1 colocalizes with tau inclusions: a link between parkinsonism and dementia. Ann Neurol. 2004;55:113–118. doi: 10.1002/ana.10782. [DOI] [PubMed] [Google Scholar]
- Rostami J, Holmqvist S, Lindström V, Sigvardson J, Westermark GT, Ingelsson M, Bergström J, Roybon L, Erlandsson A. Human astrocytes transfer aggregated alpha-synuclein via tunneling nanotubes. J Neurosci. 2017;37:11835–11853. doi: 10.1523/JNEUROSCI.0983-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Akazawa-Ogawa Y, Matsumura A, Saigoh K, Itoh S, Sutou K, Kobayashi M, Mita Y, Shichiri M, Hisahara S, Hara Y, Fujimura H, Takamatsu H, Hagihara Y, Yoshida Y, Hamakubo T, Kusunoki S, Shimohama S, Noguchi N. Oxidation and interaction of DJ-1 with 20S proteasome in the erythrocytes of early stage Parkinson’s disease patients. Sci Rep. 2016;6:30793. doi: 10.1038/srep30793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. The Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Schapira AHV. Complex I: Inhibitors, inhibition and neurodegeneration. Experimental Neurology. 2010;224:331–335. doi: 10.1016/j.expneurol.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Kim JH, Greenamyre JT. Selective microglial activation in the rat rotenone model of Parkinson’s disease. Neuroscience Letters. 2003a;341:87–90. doi: 10.1016/s0304-3940(03)00172-1. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim J-H, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003b;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinbo Y, Taira T, Niki T, Iguchi-Ariga SMM, Ariga H. DJ-1 restores p53 transcription activity inhibited by Topors/p53BP3. Int J Oncol. 2005;26:641–648. [PubMed] [Google Scholar]
- Surmeier DJ, Obeso JA, Halliday GM. Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci. 2017;18:101–113. doi: 10.1038/nrn.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipa R, Pereira C, Reis I, Alonso I, Bastos-Lima A, Melo-Pires M, Magalhães M. DJ-1 linked parkinsonism (PARK7) is associated with Lewy body pathology. Brain. 2016;139:1680–1687. doi: 10.1093/brain/aww080. [DOI] [PubMed] [Google Scholar]
- Taira T, Saito Y, Niki T, Iguchi-Ariga SMM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Taira T, Niki T, Seino C, Iguchi-Ariga SM, Ariga H. DJ-1 positively regulates the androgen receptor by impairing the binding of PIASx alpha to the receptor. J Biol Chem. 2001;276:37556–37563. doi: 10.1074/jbc.M101730200. [DOI] [PubMed] [Google Scholar]
- Tao X, Tong L. Crystal structure of human DJ-1, a protein associated with early onset Parkinson’s disease. J Biol Chem. 2003;278:31372–31379. doi: 10.1074/jbc.M304221200. [DOI] [PubMed] [Google Scholar]
- Taurino F, Stanca E, Siculella L, Trentadue R, Papa S, Zanotti F, Gnoni A. Mitochondrial proteome analysis reveals depression of the Ndufs3 subunit and activity of complex I in diabetic rat brain. J Proteomics. 2012;75:2331–2341. doi: 10.1016/j.jprot.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Thomas KJ, McCoy MK, Blackinton J, Beilina A, van der Brug M, Sandebring A, Miller D, Maric D, Cedazo-Minguez A, Cookson MR. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Human Molecular Genetics. 2011;20:40–50. doi: 10.1093/hmg/ddq430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu K, Ischiropoulos H, Przedborski S. Nitric oxide and reactive oxygen species in Parkinson’s disease. IUBMB Life (International Union of Biochemistry and Molecular Biology: Life) 2003;55:329–335. doi: 10.1080/1521654032000114320. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MMK, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Valente EM, Brancati F, Ferraris A, Graham EA, Davis MB, Breteler MMB, Gasser T, Bonifati V, Bentivoglio AR, De Michele G, Durr A, Cortelli P, Wassilowsky D, Harhangi BS, Rawal N, Caputo V, Filla A, Meco G, Oostra BA, Brice A, Albanese A, Dallapiccola B, Wood NW European Consortium on Genetic Susceptibility in Parkinson’s Disease. PARK6-linked parkinsonism occurs in several European families. Ann Neurol. 2002;51:14–18. [PubMed] [Google Scholar]
- van der Brug MP, Blackinton J, Chandran J, Hao L-Y, Lal A, Mazan-Mamczarz K, Martindale J, Xie C, Ahmad R, Thomas KJ, Beilina A, Gibbs JR, Ding J, Myers AJ, Zhan M, Cai H, Bonini NM, Gorospe M, Cookson MR. RNA binding activity of the recessive parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proc Natl Acad Sci USA. 2008;105:10244–10249. doi: 10.1073/pnas.0708518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijitruth R, Liu M, Choi DY, Nguyen XV, Hunter RL, Bing G. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson’s disease. Journal of Neuroinflammation. 2006;3:6. doi: 10.1186/1742-2094-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waak J, Weber SS, Waldenmaier A, Görner K, Alunni-Fabbroni M, Schell H, Vogt-Weisenhorn D, Pham T-T, Reumers V, Baekelandt V, Wurst W, Kahle PJ. Regulation of astrocyte inflammatory responses by the Parkinson’s disease-associated gene DJ-1. FASEB J. 2009;23:2478–2489. doi: 10.1096/fj.08-125153. [DOI] [PubMed] [Google Scholar]
- Wang B, Cai Z, Tao K, Zeng W, Lu F, Yang R, Feng D, Gao G, Yang Q. Essential control of mitochondrial morphology and function by chaperone-mediated autophagy through degradation of PARK7. Autophagy. 2016;12:1215–1228. doi: 10.1080/15548627.2016.1179401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Cynader MS. Astrocytes provide cysteine to neurons by releasing glutathione. Journal of Neurochemistry. 2000;74:1434–1442. doi: 10.1046/j.1471-4159.2000.0741434.x. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Ringe D, Petsko GA. The Atomic Resolution Crystal Structure of the YajL (ThiJ) Protein from Escherichia coli: A Close Prokaryotic Homologue of the Parkinsonism-associated Protein DJ-1. Journal of Molecular Biology. 2005;353:678–691. doi: 10.1016/j.jmb.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochim Biophys Acta. 2010;1802:29–44. doi: 10.1016/j.bbadis.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Woodlee MT, Kane JR, Chang J, Cormack LK, Schallert T. Enhanced function in the good forelimb of hemi-parkinson rats: compensatory adaptation for contralateral postural instability? Experimental Neurology. 2008;211:511–517. doi: 10.1016/j.expneurol.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CY, Kang WY, Chen YM, Jiang TF, Zhang J, Zhang LN, Ding JQ, Liu J, Chen SD. DJ-1 Inhibits α-Synuclein Aggregation by Regulating Chaperone-Mediated Autophagy. Front Aging Neurosci. 2017;9:308. doi: 10.3389/fnagi.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhong N, Wang H, Elias JE, Kim CY, Woldman I, Pifl C, Gygi SP, Geula C, Yankner BA. The Parkinson’s disease-associated DJ-1 protein is a transcriptional co-activator that protects against neuronal apoptosis. Human Molecular Genetics. 2005;14:1231–1241. doi: 10.1093/hmg/ddi134. [DOI] [PubMed] [Google Scholar]
- Yuan YH, Sun JD, Wu MM, Hu JF, Peng SY, Chen NH. Rotenone Could Activate Microglia Through NFκB Associated Pathway. Neurochem Res. 2013;38:1553–1560. doi: 10.1007/s11064-013-1055-7. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Yuan YH, Shao QH, Wang ZZ, Zhu CG, Shi JG, Ma KL, Yan X, Chen NH. DJ-1 regulating PI3K-Nrf2 signaling plays a significant role in bibenzyl compound 20C-mediated neuroprotection against rotenone-induced oxidative insult. Toxicology Letters. 2017;271:74–83. doi: 10.1016/j.toxlet.2017.02.022. [DOI] [PubMed] [Google Scholar]
- Zhong N, Kim CY, Rizzu P, Geula C, Porter DR, Pothos EN, Squitieri F, Heutink P, Xu J. DJ-1 transcriptionally up-regulates the human tyrosine hydroxylase by inhibiting the sumoylation of pyrimidine tract-binding protein-associated splicing factor. J Biol Chem. 2006;281:20940–20948. doi: 10.1074/jbc.M601935200. [DOI] [PubMed] [Google Scholar]
- Zhou F, Wu JY, Sun XL, Yao HH, Ding JH, Hu G. Distinct Role for Microglia in Rotenone-Induced Degeneration of Dopaminergic Neurons. Neuropsychopharmacology. 2017;32:1–9. doi: 10.1038/sj.npp.1301381. [DOI] [PubMed] [Google Scholar]
- Zhou W, Zhu M, Wilson MA, Petsko GA, Fink AL. The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein. Journal of Molecular Biology. 2006;356:1036–1048. doi: 10.1016/j.jmb.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Zhu M, Patel HS, Han S. DJ-1, a Parkinson’s disease related protein, aggregates under denaturing conditions and co-aggregates with α-synuclein through hydrophobic interaction. Biochim Biophys Acta. 2017;1861:1759–1769. doi: 10.1016/j.bbagen.2017.03.013. [DOI] [PubMed] [Google Scholar]
- Zondler L, Miller-Fleming L, Repici M, alves SGC, Tenreiro S, Rosado-Ramos R, Betzer C, Straatman KR, Jensen PH, Giorgini F, Outeiro TF. DJ-1 interactions with alpha-synuclein attenuate aggregation and cellular toxicity in models of Parkinson’s disease. 2017;5:e1350–10. doi: 10.1038/cddis.2014.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.