Summary
Polyamines are an essential class of metabolites found throughout all kingdoms in life. Borrelia burgdorferi harbors no enzymes to synthesize or degrade polyamines yet does contain a polyamine uptake system, potABCD. In this report, we describe the initial characterization of this putative transport system. After several unsuccessful attempts to inactivate potABCD, we placed the operon under the control of an inducible LacI promoter expression system. Analyses of this construct confirmed that potABCD was required for in vitro survival. Additionally, we demonstrated that the potABCD operon were upregulated in vitro by low osmolarity. Previously, we had shown that low osmolarity triggers the activation of the Rrp2/RpoN/RpoS regulatory cascade which regulates genes essential for the transmission of spirochetes from ticks to mammalian hosts. Interestingly, induction of the pot operon was only affected in an rpoS mutant but not in a rpoN mutant, suggesting that the genes were RpoS-dependent, RpoN-independent. Furthermore, potABCD was upregulated during tick feeding concomitant with the initiation of spirochete replication. Finally, uptake experiments determined the specificity of B. burgdorferi’s PotABCD for spermidine.
Keywords: Lyme disease, polyamine, potABCD, tick, regulation
Graphical Abstract
Borrelia burgdorferi, Lyme disease agent, harbors no enzymes to synthesize or degrade polyamines yet does contain the genes encoding a putative polyamine uptake system (potABCD). Here, we demonstrated that the PotABCD is a spermidine-specific transporter system that is essential for survival. The genes are upregulated during tick feeding concomitantly with the decrease of osmoliarity which has been shown to trigger virulence factor expression. The potABCD genes are regulated in a RpoN-dependent, RpoS-independent, BosR-independent fashion.
Introduction
Borrelia burgdorferi, one of the causative agents of Lyme disease (Pritt et al., 2016), is maintained in a life cycle that involves a vertebrate host (e.g. rodent) and a hard tick: Ixodes scapularis (Stewart & Rosa, 2017). During its infectious cycle, B. burgdorferi adapts its physiology and metabolism in response to changing environmental conditions in the mammal or the arthropod vector (Caimano et al., 2016, Gherardini et al., 2010). The mammalian host offers this spirochete a nutrient-rich, stable environment (temperature, pH, osmolarity, etc.) (Waymouth, 1970, The staff of the Jackson Laboratory, 1966). In addition, B. burgdorferi uses several mechanisms for evading the aggressive host immune system, allowing it to successfully colonize various mammalian species, including humans (Norris, 2014, Petzke & Schwartz, 2015, Radolf et al., 2012). In contrast, environmental parameters inside the arthropod vector are quite dynamic over the course of B. burgdorferi’s life cycle. Studies have shown that the I. scapularis midgut has shifting physio-chemical parameters (temperature: 28°C–> 37°C; osmolarity: 600–> 300–> 600 mOsM) (Bontemps-Gallo et al., 2016, Schwan et al., 1995, Stevenson et al., 1995) and, nitrosative and oxidative bursts that peak during the feeding process (Bourret et al., 2016, Sonenshine & Anderson, 2014). In response to fluctuating environmental conditions, B. burgdorferi modulates its gene expression program to allow it to successfully colonize both host and vector.
A small set of regulators allow B. burgdorferi to adapt to changing conditions throughout the infectious process. Upon colonization of I. scapularis, the spirochetes upregulate the expression of genes involved in carbohydrate utilization, cell envelope biosynthesis and long-term survival through the two-component system, Hk1-Rrp1 (Caimano et al., 2011, He et al., 2011, Kostick et al., 2011). This two-component system modulates the levels of c-di-GMP due to the diguanylate cyclase activity on Rrp1 (Caimano et al., 2011, He et al., 2011). In vitro, Hk1-Rrp1 is stimulated by high osmolarity (600 mOsM) (Bontemps-Gallo et al., 2016). Long-term survival of the spirochetes, after digestion of the blood meal and subsequent molt, is coordinated via RelBbu (RelA/SpoT homolog) (Drecktrah et al., 2015), BosR and RpoS (Samuels, 2011, Skare et al., 2010). After the molt, I. scapularis begins its next feeding which provides an influx of nutrients and affects multiple environmental parameters inside the midgut, including temperature and osmolarity (Blevins et al., 2009, Bontemps-Gallo et al., 2016, Caimano et al., 2004, Caimano et al., 2007, Elias et al., 2000, Fisher et al., 2005, Hubner et al., 2001). Growth and changes in environmental conditions activate the Rrp2/RpoN/RpoS regulatory cascade which is required for successful transmission from a tick to a mammal (Caimano et al., 2016). Over the last two decades, several signals responsible for activating this pathway have been identified including a temperature shift (23–> 34°C) (Schwan et al., 1995, Stevenson et al., 1995), cell density (3 x 108 cells ml−1) (Indest et al., 1997, Ramamoorthy & Philipp, 1998, Yang et al., 2000), low osmolarity (250 mOsM) (Bontemps-Gallo et al., 2016) and acid stress (Dulebohn et al., 2017).
In other bacteria, polyamines (e.g. putrescine, spermidine, spermine) are involved in both the oxidative and osmotic stress responses (Altendorf et al., 2009, Chattopadhyay et al., 2003, Cohen, 1998, Munro et al., 1972, Shah & Swiatlo, 2008), both of which are encountered by B. burgdorferi during tick feeding. In general, the polyamine content in bacterial cells is regulated by both biosynthesis and transport (Igarashi & Kashiwagi, 1999). In E. coli, the most commonly used bacterial model for polyamine research, the intracellular concentration of putrescine (22.1 nmol/109 cells) is higher than spermidine (3.8 nmol/109 cells) (Morris & Jorstad, 1970). Uptake experiments in E. coli demonstrate that spermidine/putrescine transport (via PotABCD) is a spermidine-preferential uptake system (Kashiwagi et al., 1993) that also transports putrescine but with lower affinity. Both molecules can bind nucleic acids and proteins to stabilize their interactions (Childs et al., 2003), modulate the rate of transcription (Thomas et al., 1995) and stimulate growth (Igarashi & Kashiwagi, 2010). Despite extensive research efforts, the role of putrescine and spermidine in bacterial cells remains unclear (Miller-Fleming et al., 2015).
Since B. burgdorferi’s genome does not appear to contain the genes encoding enzymes with the capacity to synthesize, modify or degrade either putrescine, spermidine or spermine, the bacteria is thought to be a spermidine auxotroph (Fraser et al., 1997, Wyss & Ermert, 1996). Only one putative spermidine/putrescine ABC-transporter, called PotABCD (encoded by bb0639-642) is annotated in B. burgdorferi’s genome (Fraser et al., 1997, Lin et al., 2017). bb0642 (potA) encodes a potential ATP-binding protein while bb0641 (potB) and bb0640 (potC) encode proteins with 6 transmembrane domains suggesting a role in membrane translocation. Finally, bb0639 (potD) encodes a putative spermidine/putrescine periplasmic binding protein which could bind polyamines facilitating transport from the periplasm space into the cytosol. It has been shown that these four genes are expressed as an operon in a polycistronic mRNA (Lin et al., 2017).
In this study, we further characterize PotABCD, the spermidine/putrescine transport system from B. burgdorferi, using an inducible expression system (lacUV5 p/o) to regulate potABCD expression. Using this genetic construct, we were able to demonstrate that this system was essential for survival in vitro. Interestingly, analyses of specific RpoS, BosR and RpoN mutants indicated that potABCD was regulated in an RpoS-dependent, RpoN-independent manner, and that regulation was concurrent with the drop in osmolarity measured in the midgut of feeding ticks (600–250 mOsM). Finally, uptake experiments strongly suggested that PotABCD was specific for spermidine in B. burgdorferi.
Results
The putative PotABCD transport system is essential for B. burgdorferi survival
Spermidine and spermine have been shown to trigger the expression and synthesis of proteins required for mammalian invasion through RpoS and BosR (Lin et al., 2017). To better understand the significance of this transport system, we attempted to inactivate the potABCD operon. Unfortunately, multiple attempts were unsuccessful. In addition, the comprehensive STM mutant library did not detect any transposon insertions in any of the genes in the operon (Lin et al., 2012) supporting the assumption that the potABCD system could be essential to B. burgdorferi. Therefore, we generated an inducible pot operon using the inducible lac promoter expression system developed by Gilbert et al. (Gilbert et al., 2007). In this system, lacI under control of the flgB promoter leads to constitutive synthesis of the LacI repressor which binds the flacp promoter and represses the expression of the potABCD operon. When added, IPTG will bind LacI in a dose-dependent manner, allowing controlled expression of the potABCD operon (Fig. S1). Using B. burgdorferi strain B31-68-LS (Chu et al. 2016), we obtained three clones harboring the flacp promoter fused to potABCD and confirmed these putative mutants by sequencing. One clone, designated BBi-potABCD, was selected for further characterization.
To confirm that polyamine utilization and the pot operon were essential, the wild-type (B31-68-LS) parent and BBi-potABCD strains were grown in BSK-II, pH 7.5 with 0.5 mM IPTG to 5 x 107 cells/ml. Cells from the cultures were harvested, washed, sub-cultured (to ~ 5 x 105) and grown in BSK-II with or without IPTG. Growth of each culture was monitored every 24 h by dark-field microscopy and aliquots were removed and the cells were grown and quantified on BSK-II plates with IPTG (Fig. 1). In the presence of IPTG, the cells exhibited a normal growth rate and reached 108 cells/ml, while without IPTG, growth completely stopped. More importantly, the number of cells that could be recovered from cultures without IPTG decreased dramatically with time suggesting that the cells lost viability without the expression of potABCD. These data indicated that potABCD is essential in B. burgdorferi.
Fig. 1. The PotABCD uptake system is essential for B. burgdorferi.
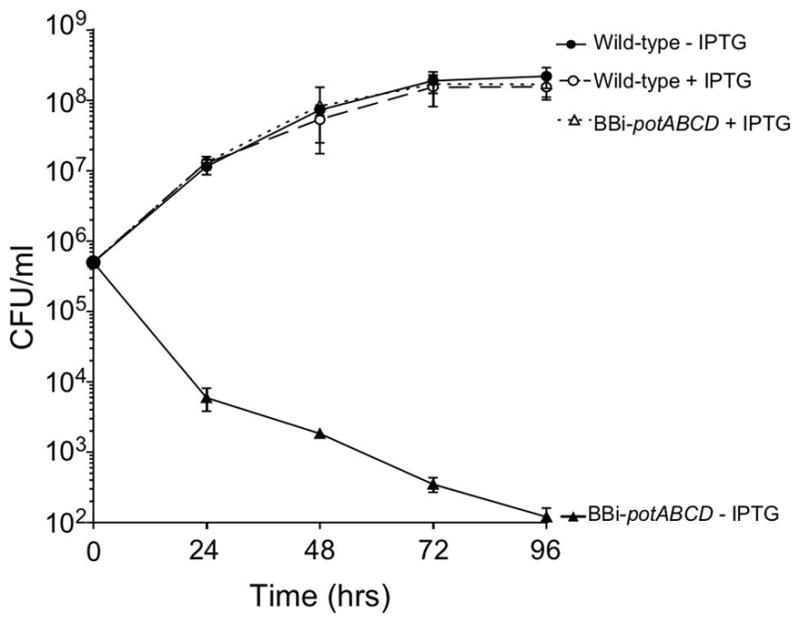
Growth and survival curve of wild-type B31-68-LS and BBi-potABCD strains with or without 500 μM IPTG. Cells were enumerated by plating on BSK-II plating. Data represent means ± standard deviation of three independent experiments.
Spermidine is specifically transported by B. burgdorferi
Previous studies of potABCD systems in other bacteria have demonstrated that these transporters are capable of a broad range of uptake selectivity for polyamines (Kashiwagi et al., 1993, Yao & Lu, 2014). Some transport spermine, spermidine and/or putrescine with equal efficiency while others demonstrate a selective preference. To determine if potABCD in B. burgdorferi demonstrated preferential uptake of polyamines, we performed kinetic uptake assays with tritium (3H) labelled polyamines. B. burgdorferi cells were grown to a density of ~5 x 107 cells/ml, harvested, washed thoroughly and resuspended to a cell density of 6 x 108 cells/ml. Cells were incubated with either 3H-spermidine or 3H-putrescine and cell-associated radioactivity was analyzed at 1, 2, 6 and 10 min (Fig. 2). Uptake of 3H-spermidine increased linearly 2.4-fold between 1 and 10 min, while no 3H-putrescine uptake was observed during the same time interval. Because the inducible strain lysed when IPTG was removed, we were unable to assay the uptake selected polyamines in BBi-potABCD. These data indicated that, unlike the characterized polyamine transport systems in other bacteria (Kashiwagi et al., 1993, Yao & Lu, 2014), potABCD in B. burgdorferi seems to be specific for the uptake of spermidine.
Fig. 2. Spermidine is the preferred polyamine transported by B. burgdorferi.
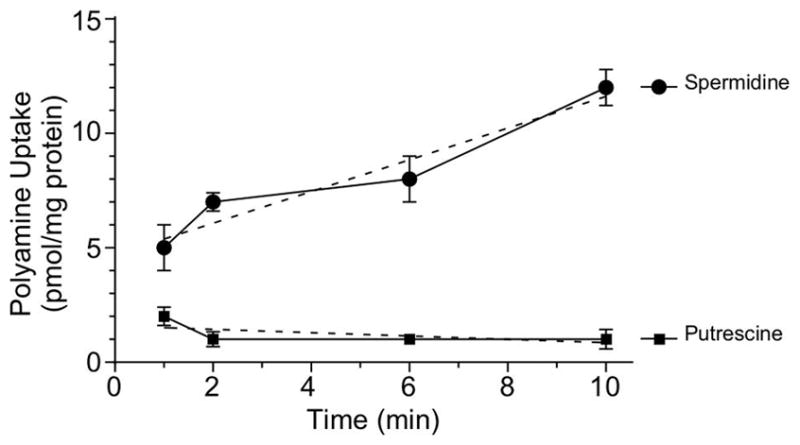
Curve of uptake of radioactive spermidine and putrescine by B. burgdorferi B31-A3 strain was measured over a 10-min time course. Cells were grown to mid-log phase, washed and resuspended to a cell density of 6 x 108 cells/ml in HN buffer supplemented with 6 mM glucose. Tritium labelled polyamine (5μCi) was added to the cell suspensions and incubated at 34°C for the duration of the experiment. Polyamine amounts (pmol) in samples were determined against a calibration performed with authentic labeled substrate. The protein concentration was determined using a BCA Protein Assay with bovine serum albumin as a standard. Dotted lines represent the linear regression. Data represent means ± standard deviation of three independent experiments.
potABCD is up-regulated during nymph tick feeding
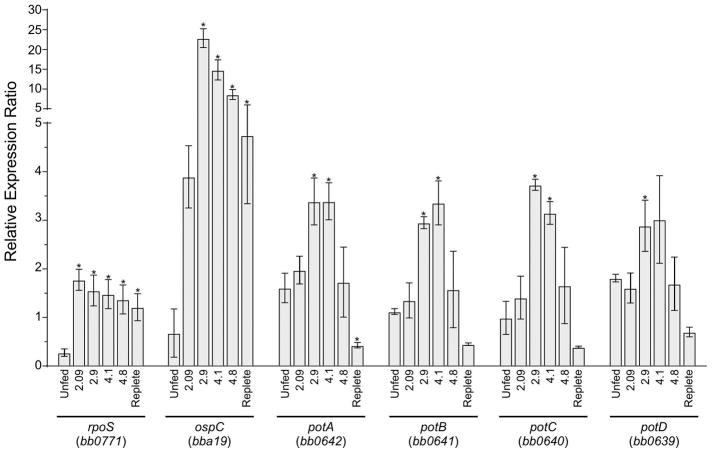
To shed light on the role of potABCD during tick feeding, we analyzed the expression of the four genes in the operon by RT-qPCR during nymph feeding. Additionally, rpoS and ospC were assayed as markers for the activation of the Rrp2/RpoN/RpoS pathway (Fig. 3). Ticks were removed daily from mice infected with wild-type B. burgdorferi (B31-A3) and the scutal index was measured to standardize feeding progress (Bontemps-Gallo et al., 2016, Falco et al., 1996) before pooling for RNA extraction. For each time-point examined, RNA was extracted from 3 groups of 5 I. scapularis ticks. The expression level of enoS was used as a housekeeping gene. As expected, rpoS transcript increased (6.1-fold) at the first day post attachment (scutal index 2.09) and stayed at a similar level during the feeding process (Fig. 3). Activation of the Rrp2-RpoN-RpoS pathway increases expression of RpoS-dependant genes (e.g. ospC) (Caimano et al., 2004). The expression of ospC increased 5.6-fold on day one post-attachment and 5.9-fold more on the second day post-attachment after which the expression slowly decreased. The four genes of the potABCD operon followed the same pattern of transcription (Fig. 3). On the second day of tick feeding (scutal index of 2.9), the expression of potABCD increased 1.9-fold, 2.3-fold, 3.1-fold and 1.8-fold, respectively. In contrast, the expression of the potABCD operon decreased about 7-fold in the replete ticks (scutal index of 5.1) compared to the value measured on the second day of feeding (scutal index of 2.9). These data showed that during tick feeding B. burgdorferi upregulated the expression of genes encoding the polyamine transport system (potABCD) and this expression corresponded with the increase of RpoS-dependent genes.
Fig. 3. Relative expression of potABCD operon genes during nymph feeding.
Graph of the expression of rpoS and ospC (as markers for the induction of the Rrp2-RpoN-RpoS pathway), and the four genes of the potABCD operon were analyzed by RT-qPCR. RNA was extracted from 3 pools of 5 ticks and cDNA was generated as described in the methods section. Scutal index was used to standardize feeding progress (Bontemps-Gallo et al., 2016, Falco et al., 1996). The scutal index is indicated under each bar. Relative gene expression was calculated using levels expression of enoS as reference. Data represent means ± standard deviation of three independent pools for each condition.
potABCD is regulated by low osmolarity
During the feeding process of the tick vector, B. burgdorferi encounters and must survive two significant stresses in the midgut: oxidative and osmotic (Bontemps-Gallo et al., 2016, Sonenshine & Anderson, 2014). Since putrescine and spermidine have been shown to be involved in protecting bacteria from both stresses (Chattopadhyay et al., 2003, Cohen, 1998, Munro et al., 1972, Shah & Swiatlo, 2008), we hypothesized that one or both stresses could be responsible for the activation of the potABCD operon observed during tick feeding. To test this hypothesis, we followed the expression of each gene by RT-qPCR at 250, 450, and 650 mOsM and after exposure to tert-butyl hydroperoxide (Fig. 4). Expression of the potA, potB and potC genes encoding the putative ATP-binding protein and the two membrane transport proteins increased 10-fold at low osmolarity compared to medium or high osmolality (Fig. 4A). Surprisingly, expression of the potD gene, encoding the periplasmic binding protein, increased 19-fold (Fig. 4A). The higher expression of potD compared to the three other genes suggested an internal promoter for potD. These data may also indicate that increased expression of the putative periplasmic binding protein (PotD) is required to increase the uptake of polyamines during a time of rapid growth in the tick midgut.
Fig. 4. Expression of potABCD operon under osmotic or oxidative stress.
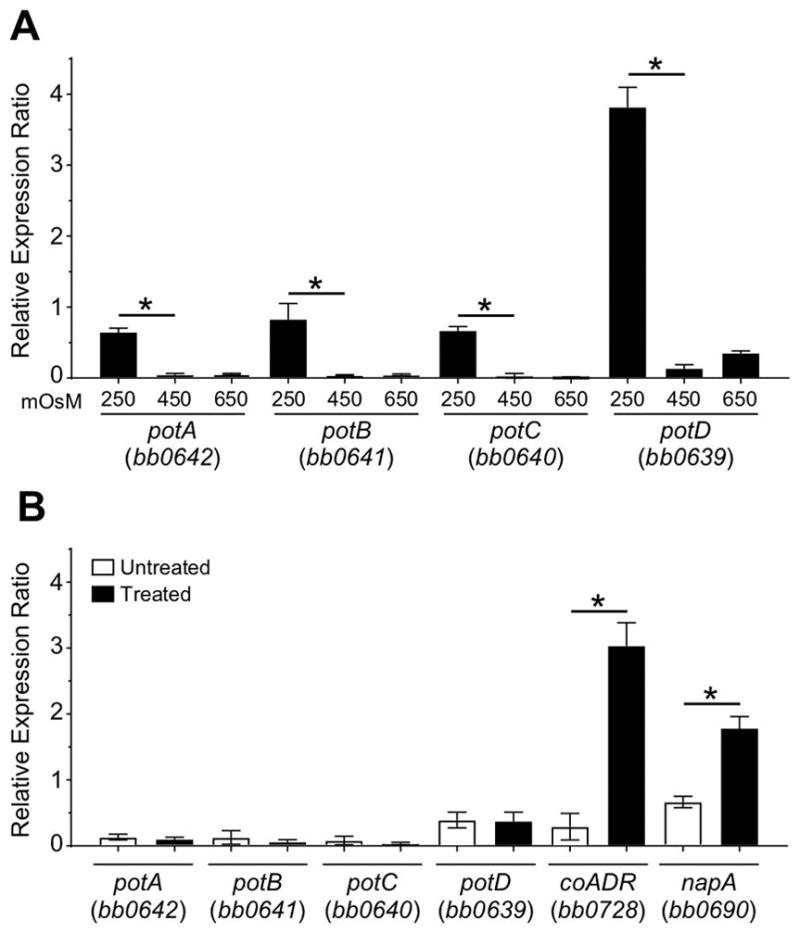
(A) Graph of the expression analyses of the potABCD operon of B. burgdorferi grown in BSK-II at 250, 450 and 650 mOsM. (B) Graph of the expression analyses of the potABCD operon of B. burgdorferi grown in BSK-II after a treatment with 5 mM tert-butyl hydroperoxide compared to an untreated culture. The expression of coADR, napA (as marker of the induction of the oxidative stress response), and the four genes of potABCD operon were analyzed by RT-qPCR. Relative gene expression was calculated using levels expression of enoS as reference. Data represent means ± standard deviation of three independent experiments.
No induction of the potABCD operon was observed under oxidative stress while coADR and napA, both known to be involved in the oxidative stress response (Boylan et al., 2003, Hyde et al., 2009), were induced (Fig. 4B). Additionally, we tested the potential of spermidine and putrescine to function as a protective molecule against osmotic or oxidative stress (Fig. S1). No effects on the growth or on the susceptibility to both osmotic and oxidative stresses were observed. We were unable to use our BBi-potABCD mutant to assay if the PotABCD system was required for protection against oxidative or osmotic stresses, because the strain did not grow without the IPTG (Fig. 1). It seems very likely that the in vitro conditions tested do not accurately reflect the combination of stresses and changing conditions that challenge B. burgdorferi in vivo.
potABCD is regulated by RpoS independent of RpoN
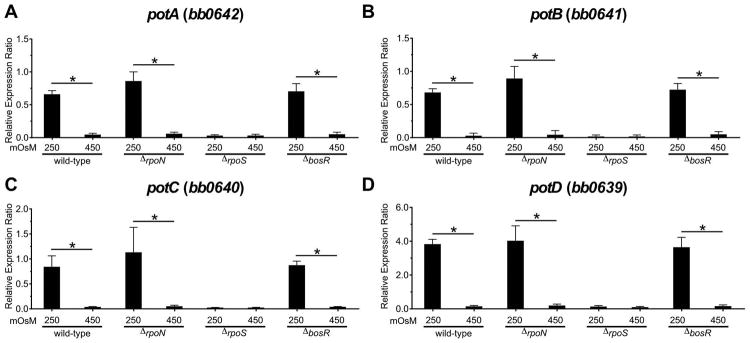
Because potABCD expression followed a similar pattern to genes regulated by the Rrp2/RpoN/RpoS regulon in a feeding tick under low osmolarity, we hypothesized that this regulatory pathway controlled potABCD expression. To test this hypothesis, we assayed the expression levels of potABCD in mutant strains deficient in the key regulatory factors (ΔrpoN, ΔrpoS, and ΔbosR) known to directly or indirectly modulate the Rrp2/RpoN/RpoS cascade (Caimano et al., 2016, Samuels, 2011). Wild-type B. burgdorferi, ΔrpoN, ΔrpoS, and ΔbosR mutant strains were grown in BSK-II at 250 or 450 mOsM to approximately ~5 x 107 cells/ml and RNA was harvested for RT-qPCR analyses. As observed previously, the potABCD operon was induced in wild-type cells at low osmolarity (Fig. 4 and 5). potABCD induction in the ΔrpoN mutant was similar to levels observed in WT cells, while the ΔrpoS mutant exhibited significantly reduced transcript levels under low osmolarity (Fig 5). These data were surprising since the current understanding is that the inactivation of either rpoN or rpoS results in a very similar phenotype (Caimano et al., 2016, Samuels, 2011). Our understanding of the regulation of this small set of genes by RpoS, independent of RpoN, is incomplete. More investigation will be required to evaluate the importance of this regulation beyond Rrp2/RpoN/RpoS regulation (Samuels, 2011).
Fig. 5. Expression of potABCD operon in wild-type, ΔrpoN, ΔrpoS and ΔbosR mutants.
Graph of the expression analyses of the potABCD operon of B. burgdorferi grown in BSK-II at 250 and 450 mOsM. Relative gene expression was calculated using levels expression of enoS as reference. Data represent means ± standard deviation of three independent experiments.
It has been shown that rpoS expression can be modulated by BosR, a transcription factor that is activated by oxidative stress (Boylan et al., 2003, Ouyang et al., 2011). We found that inactivation of bosR had no effect on the induction of potABCD at low osmolarity. This result was expected since oxidative stress had no effect on potABCD gene expression (Fig. 4). Taken together, these data suggest that potABCD is RpoS-dependent and BosR- and RpoN-independent. These results also demonstrate that various in vivo stresses can be regulated independently [e.g. oxidative stress via BosR (Boylan et al., 2003, Hyde et al., 2009) and acid stress via RpoS (Dulebohn et al., 2017)] from the Rrp2/RpoN/RpoS cascade.
Discussion
Polyamines such as putrescine, spermidine, spermine and cadaverine are found in both prokaryotes and eukaryotes and are essential for normal cell growth, modulating the rate of transcription (Childs et al., 2003), promoting efficient translation, altering and stabilizing the structure of RNA (Thomas et al., 1995) and protecting the cell from deleterious effects of acid, oxidative and nitrosative stresses (Miller-Fleming et al., 2015). Typically, bacterial cells transport, synthesize and/or modify polyamines to maintain an intracellular concentration that is optimal to promote maximum function and these processes are coordinately regulated (Miller-Fleming et al., 2015). Most bacteria maintain higher intercellular levels of spermidine than putrescine. E. coli is a notable exception in that higher levels of putrescine (22.1 nmol/109 cells) are detected compared to spermidine (3.8 nmol/109 cells) (Morris & Jorstad, 1970). The synthesis of putrescine and spermidine is accomplished by the decarboxylation of precursor amino acids such as ornithine (via ornithine decarboxylase, encoded by speC) or arginine (arginine decarboxylase, encoded by speA), while the synthesis of spermine or cadaverine are rare (Miller-Fleming et al., 2015).
The genome of B. burgdorferi lacks the genes encoding proteins for the de novo synthesis of putrescine or spermidine and it has been proposed that B. burgdorferi is a spermidine auxotroph (Wyss & Ermert, 1996). However, it does harbor genes encoding a single putative putrescine/spermidine transport system, potABCD, suggesting that this putrescine/spermidine system may be responsible for polyamine utilization in B. burgdorferi. Recently, Lin et al. showed that high concentration of spermidine (4 mM) in vitro triggers the activation of the Rrp2/RpoN/RpoS cascade (Lin et al., 2017), suggesting that this polyamine may affect the regulation of key virulence factors during the infectious cycle. During the infectious process, B. burgdorferi switches between an arthropod vector and a mammalian host. To be successful during this transition, the bacteria needs to adapt to take advantage of the resources available, optimize growth, correctly alter gene expression and protect itself from deleterious environmental conditions. The purpose of this study is to determine the role of potABCD and polyamines in these progressions.
To determine the role of potABCD in B. burgdorferi, we initially attempted to generate a mutant by deleting the entire operon. However, multiple attempts to inactivate the putative polyamine transport system were unsuccessful. Therefore, an inducible potABCD (strain BBi-potABCD) was generated. Growth studies indicated that without inducer, cell division ceased, and the cells lost viability after 24 h, suggesting that potABCD was required for bacterial replication and survival (Fig. 1) in vitro. Compared to other bacteria which can synthesize and/or transport putrescine, spermidine or spermine, B. burgdorferi is quite metabolically limited. Uptake experiments indicate that the bacteria use the PotABCD transport system to specifically transport spermidine Previous studies have shown that spermidine is more abundant in human blood (9 uM) than putrescine (0.13 uM) (Wishart et al., 2007, Wishart et al., 2009, Wishart et al., 2013) and is the only polyamine available in the midgut of nymphal ticks. The availability of spermidine in the various hosts and vectors harboring B. burgdorferi and its selective transport strongly suggests that spermidine is the essential polyamine required by B. burgdorferi for survival (Narasimhan et al., 2017).
Since we could not introduce strain BBi-potABCD directly into ticks, we evaluated the expression patterns of potABCD in ticks colonized with strain B31-A3. In the tick, spirochetes up-regulated the potABCD operon during the early stage of feeding concomitant with the activation of RpoS-dependent genes (Fig. 3). Recently, Arnold et al. demonstrated by RNA-seq that the potABCD operon used only one promoter regardless of the phase of growth (Arnold et al., 2016). However, Adams et al. showed by using an in vivo expression technology-based approach that the potABCD operon had one primary promoter and multiple secondary promoters (Adams et al., 2017). These data together with our data from feeding ticks (Fig. 3), strongly suggested that the potABCD operon could be expressed as one polycistronic mRNA from a single promoter or as multiple transcripts from secondary promoters to control the ratio of the mRNA of each gene and the stoichiometry of the different proteins. Additionally, we demonstrated that the potABCD operon is part of a small subset of genes that are RpoS-dependent, RpoN-independent (Fig. 5). Fisher et al. previously demonstrated using microarray analyses that a small set of genes in the Rrp2/RpoN/RpoS regulon were RpoS-dependent, RpoN-independent (Fisher et al., 2005). In their study, they found that potD expression decreased in an rpoS mutant but not in an rpoN mutant. Those previous observations and the data presented here demonstrate that potABCD is regulated in an RpoS-dependent, RpoN-independent manner.
Finally, we investigated the potential biological roles of polyamines in B. burgdorferi as well as the cues that trigger the activation of the potABCD in B. burgdorferi. Polyamines, such as putrescine or spermidine, are required for cell growth and, in some bacteria, for protecting cells from oxidative or osmotic stress (Altendorf et al., 2009, Igarashi & Kashiwagi, 2010, Miller-Fleming et al., 2015). First, we tested the ability of polyamines to protect B. burgdorferi in protecting the bacterium from the adverse effects of osmolarity or oxidative stress (Fig. S2). Under the conditions tested, spermidine was not effective at expanding the survivability of cells from osmotic conditions outside the range of those which have been shown to exist in the tick midgut before, during or after feeding (250–600 mOsM) (Bontemps-Gallo et al., 2016). Additionally, it did not increase the resistance of cells to ROS.
However, potABCD was up-regulated when the cells were exposed to low osmolarity but not when cells were exposed to ROS (Fig. 4). Also, potABCD was upregulated in the tick midgut during feeding, paralleling the drop in osmolarity that has been measured in ticks at the initial stages of feeding (Bontemps-Gallo et al., 2016). While these data suggest a role for potABCD in the tick, we cannot assign a specific function for spermidine in B. burgdorferi. It is interesting to note that the increase in expression of potABCD occurs at a time during tick feeding which corresponds with maximum cell growth. Because of the complexity of the environment in the tick midgut, it seems likely that the conditions tested in vitro do not accurately reflect the multiple parameters (osmolarity, ROS, RNS) to which B. burgdorferi is exposed in the tick midgut. While spermidine is essential for B. burgdorferi survival and growth in vitro, its exact biochemical role remains to be determined.
Experimental Procedures
Bacterial strains, media and growth conditions
The bacterial strains used in this study are described in the Table 1. B. burgdorferi strains were grown in BSK-II medium (Barbour, 1984) at 34°C under microaerobic conditions (3–5% O2, 5% CO2, 90% N2). Cell densities were determined by dark-field microscopy. The osmolality of BSK-II medium is 450 mOsM. High and low-osmolality media, were generated as previously described (Bontemps-Gallo et al., 2016). Every 24 h, an aliquot from each culture was examined by dark-field microscopy and plated on BSK-II. Plates were incubated at 34°C under a microaerobic atmosphere for 7–14 d to determine CFU. Spermidine and putrescine (Sigma-Aldrich, St. Louis, MO, USA) were added to BSK-II as indicated. Osmolarity of the growth media (mOsM) was measured with a vapor pressure osmometer (Model 3320, Advanced Instruments, Inc., Norwood, MA, USA).
Table 1.
Bacterial Strains and Plasmids used in this study
| Strains | Source |
|---|---|
| B. burgdorferi | |
| B31-A3 | (Elias et al., 2002) |
| B31-A3ΔrpoN | (Fisher et al., 2005) |
| B31-A3ΔrpoS | (Burtnick et al., 2007) |
| B31-A3ΔbosR (clone K18) | (Katona, 2015) |
| B31-68-LS | (Chu et al., 2016) |
| B31-68-LS–BBi-potABCD | This study |
| Escherichia coli | |
| Top10F′: mcrA, Δ(mrr-hsdRMS-mcrBC), Φ80lacZΔM15, ΔlacX74, recA1, araD139, Δ(ara- leu)7697, galU, galK, rpsL, endA1, nupG | Invitrogen |
| Plasmids | |
| pUC57 | Genscript |
| pBBi-potABCD | This Study |
Construction of the BBi-potABCD (B. burgdorferi inducible–pot operon) strain
Plasmids and primers designed for PCR are listed in Tables 1 and 2, respectively. A fragment containing bb0643-flaBp-Kan-flacp-potA was synthesized by Genscript USA and cloned into pUC57 and was named pBBi-potABCD. The plasmid was transformed into B. burgdorferi strain B31-68-LS (Chu et al., 2016) as described previously (Samuels, 1995) and kanamycin-resistant colonies were analyzed by PCR and sequenced to confirm that potABCD had been replaced by the inducible potABCD (lacUV5-potABCD) (Table 2) (Fig. S1). The mutant strain was designated as BBi-potABCD. The plasmid profiles of the wild-type and the BBi-potABCD were determined using the multiplex PCR system from Bergström laboratory (Bunikis et al., 2011). Wild-type strain B31-68-LS was missing cp9 and lp5 while strain BBi-potABCD was missing cp9, lp28-1 and lp5.
Table 2.
PCR primers used in this study.
| PCR Primersa | Sequence | Efficiency |
|---|---|---|
| potAF | GCCATATGGATAATTGTATTATCCTAGAGATTAA | |
| potAR | CGGATCCTTATTCCTTATGCATAACATGAAT | |
|
| ||
| GTPF-EcoRI | ACTGCTGAATTCTCCACCTATTTTGGCTGCCA | |
| GTPR-aatII | ACTGCTGACGTCACATCCGGATGGGCCTAGTA | |
|
| ||
| QrpoSF | CTGGACAAAGAAATAGAGGGATCTG | 1.838 |
| QrpoSR | CAAGGGTAATTTCAGGGTTAAAAGAA | |
|
| ||
| QospCF | TGGTACTAAAACTAAAGGTGCTGAAGAA | 1.951 |
| QospCR | GCATCTCTTTAGCTGCTTTTGACA | |
|
| ||
| QpotAF | GCTGGTTGTAAGTTTGCTTGG | 1.958 |
| QpotAR | ATCTTCTGGGCGTATTACAAGG | |
|
| ||
| QpotBF | AGATCTTGGAGCAAGAATGTG | 1.704 |
| QpotBR | GTTTAGAGCCTCCTAGCAAATC | |
|
| ||
| QpotCF | CGGAAATCGCAGGAAGCATAG | 1.891 |
| QpotCR | ATCCCTGTCCAGTGGTGAAA | |
|
| ||
| QpotDF | GCACAAAGCGCTATGCTAAA | 1.796 |
| QpotDR | TGGAGCATCAATAGGAATTACA | |
|
| ||
| QnapAF | CCCTCAATGGAAAGCATTGTTTG | 1.831 |
| QnapAR | GCATCCATAAATGTTTCTCAAGATCAC | |
|
| ||
| QcoADRF | AGCTGGGAATCATACAGCATTTA | 1.748 |
| QcoADRR | GTCCTGTTCTTGCAGCTTCT | |
|
| ||
| QenoSF | GTGCACACTCTGACAACTCT | 1.927 |
| QenoSR | ACCTCTGCTGCCATTCTTATT | |
All primers were generated for this study.
Uptake of 3H-Spermidine and 3H-Putrescine
Polyamine transport was determined using a previously described assay by Tilly et al. (Tilly et al., 2004). Briefly, cells were grown in BSK-II to mid log phase (~5 x 107 cells/ml), harvested by centrifugation, washed three times with an equal volume of HN buffer (50 mM Hepes, 20 mM NaCl, pH 7.6) supplemented with 6 mM glucose and resuspended to a cell density of 6x108 cells/ml in HN buffer supplemented with 6 mM glucose. A 50 μl aliquot was reserved for protein quantification. Tritium labeled polyamine (5 μCi) was added to 1.0 ml cell suspensions and incubated at 34°C for the duration of the experiment. 100 μl aliquots were removed at each time point and applied to a 0.22 μm AcetatePlus filter (Osmonics, Inc., Minnetonka, MN) using a vacuum manifold and washed three times with 5 ml HN buffer before measuring scintillation counts. Spermidine [terminal methylene-3H] trihydrochloride (ART1749) and Putrescine [2,3-3H] dihydrochloride (ART0279) were purchased from American Radiolabeled Chemicals, Inc, (St. Louis, MO). The polyamine concentrations (pmol) in samples were determined against a calibration performed with labeled authentic substrates. The protein concentrations were determined using the PierceTM BCA Protein Assay with bovine serum albumin as a standard (ThermoFisher Scientific, Rockford, IL, USA).
Tick rearing and feeding
I. scapularis egg masses (Oklahoma State University) were allowed to hatch and mature in a controlled temperature, humidity and photoperiod environment. RML mice, an outbred strain of Swiss-Webster mice reared at the Rocky Mountain Laboratories breeding facility, were needle inoculated by intradermal injection with 100 μl of BSK-II containing 1 x 105 B. burgdorferi B31-A3 and after three weeks, infection was confirmed by re-isolation of spirochetes from ear punch biopsies. Larval ticks were fed to repletion on infected mice, collected and allowed to molt into nymphs in a controlled environment. Nymphs were then fed on naïve RML mice, mechanically removed during the feeding and the scutal index was determined to standardize the feeding progression (Bontemps-Gallo et al., 2016, Falco et al., 1996).
RNA purification and RT-qPCR
RNA samples were extracted from B. burgdorferi cultures using the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Three independent culture samples were used for each condition. RNA samples were also extracted from B. burgdorferi infected ticks during feeding. Ticks were frozen at −80°C, crushed and resuspended in TRIzol (Life technologies, Carlsbad, CA) with chloroform. After centrifugation, the upper phase was mixed with 70% ethanol (1:1) and loaded onto RNeasy column (Qiagen) according to the manufacturer’s protocol. The removal of contaminating DNA was accomplished using TURBO DNA-free DNase I (Life technologies, Carlsbad, CA). The cDNA was synthesized from the purified RNA using Superscript IV reverse transcriptase with random primers (Invitrogen, Carlsbad, CA). To compare gene expression, a relative quantification method was employed using enoS as a reference gene (Bontemps-Gallo et al., 2016, Pfaffl, 2001). All samples were analyzed in triplicate on a Roche LightCycler 480 System (Indianapolis, IN) using Power SYBR® Green PCR Master Mix according to the manufacturer’s instructions (Life technologies, Carlsbad, CA). The primers used for the qPCR are listed in Table 2.
ROS susceptibility and protection assays
Strains were grown in pyruvate-free BSK-II medium under microaerobic conditions at 34°C to a cell density of 2 x107 cells/ml and cells were exposed to the different concentrations of tert-Butyl hydroperoxide (Luperox® TBH70X, Sigma-Aldrich, St. Louis, MO, USA) for 3 h in pyruvate-free BSK-II. Samples were plated in BSK-II and incubated at 34°C under microaerobic conditions for 7–14 d to allow enumeration of CFU. When indicated, Spermidine (Sigma-Aldrich, St. Louis, MO, USA) was added to the BSK-II.
Statistical Analysis
Prism 7 software (GraphPad Software, Inc.,La Jolla, CA, USA) was used. Data were analyzed by using a One-Way ANOVA with a Geisser-Greenhouse correction; a value of p < 0.05 was considered significant.
Supplementary Material
Genomic organization of the BBi-potABCD strain.
The flgB-lacI repressor was inserted into bbe02 (lp25) by double crossover generating the strainB31-68-LS (Chu et al., 2016). The promoter of the potABCD operon was replaced by the flacp promoter, the IPTG-inducible promoter generating the strain BBi-potABCD. Expression of lacI was maintained constitutive by the flgB promoter. The position of each construct on chromosome and plasmid is marked.
Spermidine is not an osmoprotective or oxidative protective molecule.
(A) Growth of wild-type B31-A3 strain at 150, 250, 450, 650, 850 mOsM with or without 5 mM spermidine (SPD). An aliquot of each culture was examined daily by dark-field microscopy and plated on solid BSK-II. (B) Early stationary phase (5 x 107 cells/ml) cultures were incubated for 3 h with 0, 1, 5 or 10 mM of tert-butyl hydroperoxide (t-butyl) and plated on BSK-II. Plates were incubated at 34°C under microaerobic conditions for 7–14 d to allow enumeration of CFU. Untreated samples were used to determine a 100% benchmark to which all other numbers were compared. Data represent means ± standard deviation of three independent experiments.
Acknowledgments
Funding
This study was supported by the Intramural Research Program of the National Institute for Allergy and Infectious Diseases, National Institutes of Health. The funders were not involved in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Drs. Jorge Benach and Laura Katona (Stony Brook University) for the B31-A3ΔbosR strain. We would like to acknowledge Drs. Patricia Rosa and Philip Stewart (RML/NIH) for providing the B31-68-LS strain and their critical review of the manuscript, Dr. Mollie Jewett (University of Central Florida - College of Medicine) for discussions and sharing data and Taylor Robinson (RML/NIH) for plagiarism checking. We are also grateful to Anita Mora and The Visual Medical Arts section (NIH) for help with figure preparation.
Footnotes
Author Contributions
S.B-G. designed and performed experiments, interpreted data and wrote manuscript.
K. L. and C. L. R. performed experiments and edited manuscript.
F. C. G. contributed to experimental design, data analysis, and wrote manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest with the contents of this article.
Ethics statement
Mouse infection studies were carried out in accordance with the Animal Welfare Act (AWA1990) and followed the guidelines of the National Institutes of Health, Public Health Service Policy on Humane Care (PHS 2002) and Use of Laboratory Animals and the United States Institute of Laboratory Animal Resources, National Research Council, Guide for the Care and Use of Laboratory Animals. All animal work was done according to protocols approved by the Rocky Mountain Laboratories, NIAID, NIH Animal Care and Use Committee. The Rocky Mountain Laboratories are accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All efforts were made to minimize animal suffering.
References
- Adams PP, Flores Avile C, Popitsch N, Bilusic I, Schroeder R, Lybecker M, Jewett MW. In vivo expression technology and 5′ end mapping of the Borrelia burgdorferi transcriptome identify novel RNAs expressed during mammalian infection. Nucleic Acids Res. 2017;45:775–792. doi: 10.1093/nar/gkw1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altendorf K, Booth IR, Gralla J, Greie JC, Rosenthal AZ, Wood JM. Osmotic Stress. EcoSal Plus. 2009:3. doi: 10.1128/ecosalplus.5.4.5. [DOI] [PubMed] [Google Scholar]
- Arnold WK, Savage CR, Brissette CA, Seshu J, Livny J, Stevenson B. RNA-Seq of Borrelia burgdorferi in Multiple Phases of Growth Reveals Insights into the Dynamics of Gene Expression, Transcriptome Architecture, and Noncoding RNAs. PLoS One. 2016;11:e0164165. doi: 10.1371/journal.pone.0164165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- Blevins JS, Xu H, He M, Norgard MV, Reitzer L, Yang XF. Rrp2, a sigma54-dependent transcriptional activator of Borrelia burgdorferi, activates rpoS in an enhancer-independent manner. J Bacteriol. 2009;191:2902–2905. doi: 10.1128/JB.01721-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps-Gallo S, Lawrence K, Gherardini FC. Two Different Virulence-Related Regulatory Pathways in Borrelia burgdorferi Are Directly Affected by Osmotic Fluxes in the Blood Meal of Feeding Ixodes Ticks. PLoS Pathog. 2016;12:e1005791. doi: 10.1371/journal.ppat.1005791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourret TJ, Lawrence KA, Shaw JA, Lin T, Norris SJ, Gherardini FC. The Nucleotide Excision Repair Pathway Protects Borrelia burgdorferi from Nitrosative Stress in Ixodes scapularis Ticks. Front Microbiol. 2016;7:1397. doi: 10.3389/fmicb.2016.01397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan JA, Posey JE, Gherardini FC. Borrelia oxidative stress response regulator, BosR: a distinctive Zn-dependent transcriptional activator. Proc Natl Acad Sci U S A. 2003;100:11684–11689. doi: 10.1073/pnas.2032956100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikis I, Kutschan-Bunikis S, Blonde M, Bergström S. Multiplex PCR as a tool for validating plasmid content of Borrelia burgdorferi. J of Microbiol Methods. 2011;86:243–247. doi: 10.1016/j.mimet.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Burtnick MN, Downey JS, Brett PJ, Boylan JA, Frye JG, Hoover TR, Gherardini FC. Insights into the complex regulation of rpoS in Borrelia burgdorferi. Mol Microbiol. 2007;65:277–293. doi: 10.1111/j.1365-2958.2007.05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Drecktrah D, Kung F, Samuels DS. Interaction of the Lyme disease spirochete with its tick vector. Cell Microbiol. 2016;18:919–927. doi: 10.1111/cmi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Eggers CH, Hazlett KR, Radolf JD. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect Immun. 2004;72:6433–6445. doi: 10.1128/IAI.72.11.6433-6445.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, Schwartz I, Radolf JD. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol. 2007;65:1193–1217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Kenedy MR, Kairu T, Desrosiers DC, Harman M, Dunham-Ems S, Akins DR, Pal U, Radolf JD. The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infect Immun. 2011;79:3117–3130. doi: 10.1128/IAI.05136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JA, Garon CF, Schwan TG. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect Immun. 1999;67:3181–3187. doi: 10.1128/iai.67.7.3181-3187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay MK, Tabor CW, Tabor H. Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc Natl Acad Sci U S A. 2003;100:2261–2265. doi: 10.1073/pnas.2627990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs AC, Mehta DJ, Gerner EW. Polyamine-dependent gene expression. Cell Mol Life Sci. 2003;60:1394–1406. doi: 10.1007/s00018-003-2332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CY, Stewart PE, Bestor A, Hansen B, Lin T, Gao L, Norris SJ, Rosa PA. Function of the Borrelia burgdorferi FtsH Homolog Is Essential for Viability both In Vitro and In Vivo and Independent of HflK/C. MBio. 2016;7:e00404–00416. doi: 10.1128/mBio.00404-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SS. Bacterial Metabolism and Polyamines. In: Cohen SS, editor. A guide to the polyamines. New York, USA: Oxford University Press; 1998. pp. 94–121. [Google Scholar]
- Drecktrah D, Lybecker M, Popitsch N, Rescheneder P, Hall LS, Samuels DS. The Borrelia burgdorferi RelA/SpoT Homolog and Stringent Response Regulate Survival in the Tick Vector and Global Gene Expression during Starvation. PLoS Pathog. 2015;11:e1005160. doi: 10.1371/journal.ppat.1005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulebohn DP, Richards CL, Su H, Lawrence KA, Gherardini FC. Weak Organic Acids Decrease Borrelia burgdorferi Cytoplasmic pH, Eliciting an Acid Stress Response and Impacting RpoN- and RpoS-Dependent Gene Expression. Front Microbiol. 2017;8:1734. doi: 10.3389/fmicb.2017.01734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias AF, Bono JL, Carroll JA, Stewart P, Tilly K, Rosa P. Altered stationary-phase response in a Borrelia burgdorferi rpoS mutant. J Bacteriol. 2000;182:2909–2918. doi: 10.1128/jb.182.10.2909-2918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias AF, Stewart PE, Grimm D, Caimano MJ, Eggers CH, Tilly K, Bono JL, Akins DR, Radolf JD, Schwan TG, Rosa P. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect Immun. 2002;70:2139–2150. doi: 10.1128/IAI.70.4.2139-2150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco RC, Fish D, Piesman J. Duration of tick bites in a Lyme disease-endemic area. Am J Epidemiol. 1996;143:187–192. doi: 10.1093/oxfordjournals.aje.a008728. [DOI] [PubMed] [Google Scholar]
- Fisher MA, Grimm D, Henion AK, Elias AF, Stewart PE, Rosa PA, Gherardini FC. Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization. Proc Natl Acad Sci U S A. 2005;102:5162–5167. doi: 10.1073/pnas.0408536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fuji C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- Gherardini FC, Boylan JA, Lawrence K, Skare J. Metabolism and Physiology of Borrelia. In: Samuels DS, Radolf J, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 103–138. [Google Scholar]
- Gilbert MA, Morton EA, Bundle SF, Samuels DS. Artificial regulation of ospC expression in Borrelia burgdorferi. Mol Microbiol. 2007;63:1259–1273. doi: 10.1111/j.1365-2958.2007.05593.x. [DOI] [PubMed] [Google Scholar]
- He M, Ouyang Z, Troxell B, Xu H, Moh A, Piesman J, Norgard MV, Gomelsky M, Yang XF. Cyclic di-GMP is essential for the survival of the lyme disease spirochete in ticks. PLoS Pathog. 2011;7:e1002133. doi: 10.1371/journal.ppat.1002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci U S A. 2001;98:12724–12729. doi: 10.1073/pnas.231442498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JA, Shaw DK, Smith R, III, Trzeciakowski JP, Skare JT. The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol Microbiol. 2009;74:1344–1355. doi: 10.1111/j.1365-2958.2009.06951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K, Kashiwagi K. Polyamine transport in bacteria and yeast. Biochem J. 1999;344(Pt 3):633–642. [PMC free article] [PubMed] [Google Scholar]
- Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42:39–51. doi: 10.1016/j.biocel.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Indest KJ, Ramamoorthy R, Sole M, Gilmore RD, Johnson BJ, Philipp MT. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect Immun. 1997;65:1165–1171. doi: 10.1128/iai.65.4.1165-1171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi K, Miyamoto S, Nukui E, Kobayashi H, Igarashi K. Functions of potA and potD proteins in spermidine-preferential uptake system in Escherichia coli. J Biol Chem. 1993;268:19358–19363. [PubMed] [Google Scholar]
- Katona LI. The Fur homologue BosR requires Arg39 to activate rpoS transcription in Borrelia burgdorferi and thereby direct spirochaete infection in mice. Microbiology. 2015;161:2243–2255. doi: 10.1099/mic.0.000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostick JL, Szkotnicki LT, Rogers EA, Bocci P, Raffaelli N, Marconi RT. The diguanylate cyclase, Rrp1, regulates critical steps in the enzootic cycle of the Lyme disease spirochetes. Mol Microbiol. 2011;81:219–231. doi: 10.1111/j.1365-2958.2011.07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Gao L, Zhang C, Odeh E, Jacobs MB, Coutte L, Chaconas G, Philipp MT, Norris SJ. Analysis of an ordered, comprehensive STM mutant library in infectious Borrelia burgdorferi: insights into the genes required for mouse infectivity. PLoS One. 2012;7:e47532. doi: 10.1371/journal.pone.0047532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Romo JA, Smith TC, 2nd, Reyes AN, Karna SL, Miller CL, Van Laar TA, Yendapally R, Chambers JP, Seshu J. Spermine and spermidine alter gene expression and antigenic profile of Borrelia burgdorferi. Infect Immun. 2017;85:e00684–16. doi: 10.1128/IAI.00684-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Fleming L, Olin-Sandoval V, Campbell K, Ralser M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J Mol Biol. 2015;427:3389–3406. doi: 10.1016/j.jmb.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Morris DR, Jorstad CM. Isolation of conditionally putrescine-deficient mutants of Escherichia coli. J Bacteriol. 1970;101:731–737. doi: 10.1128/jb.101.3.731-737.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro GF, Hercules K, Morgan J, Sauerbier W. Dependence of the putrescine content of Escherichia coli on the osmotic strength of the medium. J Biol Chem. 1972;247:1272–1280. [PubMed] [Google Scholar]
- Narasimhan S, Schuijt TJ, Abraham NM, Rajeevan N, Coumou J, Graham M, Robson A, Wu MJ, Daffre S, Hovius JW, Fikrig E. Modulation of the tick gut milieu by a secreted tick protein favors Borrelia burgdorferi colonization. Nat Commun. 2017;8:184. doi: 10.1038/s41467-017-00208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris SJ. vls Antigenic Variation Systems of Lyme Disease Borrelia: Eluding Host Immunity through both Random, Segmental Gene Conversion and Framework Heterogeneity. Microbiol Spectr. 2014;2 doi: 10.1128/microbiolspec.MDNA3-0038-2014. MDNA3-0038-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z, Deka RK, Norgard MV. BosR (BB0647) controls the RpoN-RpoS regulatory pathway and virulence expression in Borrelia burgdorferi by a novel DNA-binding mechanism. PLoS Pathog. 2011;7:e1001272. doi: 10.1371/journal.ppat.1001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzke M, Schwartz I. Borrelia burgdorferi Pathogenesis and the Immune Response. Clin Lab Med. 2015;35:745–764. doi: 10.1016/j.cll.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Mead PS, Johnson DK, Neitzel DF, Respicio-Kingry LB, Davis JP, Schiffman E, Sloan LM, Schriefer ME, Replogle AJ, Paskewitz SM, Ray JA, Bjork J, Steward CR, Deedon A, Lee X, Kingry LC, Miller TK, Feist MA, Theel ES, Patel R, Irish CL, Petersen JM. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556–564. doi: 10.1016/S1473-3099(15)00464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy R, Philipp MT. Differential expression of Borrelia burgdorferi proteins during growth in vitro. Infect Immun. 1998;66:5119–5124. doi: 10.1128/iai.66.11.5119-5124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol Biol. 1995;47:253–259. doi: 10.1385/0-89603-310-4:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS. Gene regulation in Borrelia burgdorferi. Annu Rev Microbiol. 2011;65:479–499. doi: 10.1146/annurev.micro.112408.134040. [DOI] [PubMed] [Google Scholar]
- Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Swiatlo E. A multifaceted role for polyamines in bacterial pathogens. Mol Microbiol. 2008;68:4–16. doi: 10.1111/j.1365-2958.2008.06126.x. [DOI] [PubMed] [Google Scholar]
- Skare JT, Carroll JA, Yang X, Samuels DS, Akins DR. Gene regulation, Transcriptomics and Proteomics. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 67–101. [Google Scholar]
- Sonenshine DE, Anderson JM. Mouthparts and digestive system. In: Sonenshine DE, Roe RM, editors. Biology of Ticks. New-York (USA): Oxford University Press; 2014. pp. 122–162. [Google Scholar]
- Stevenson B, Schwan TG, Rosa PA. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PE, Rosa PA. Curr Top Microbiol Immunol. Springer; Berlin, Heidelberg: 2017. Physiologic and Genetic Factors Influencing the Zoonotic Cycle of Borrelia burgdorferi; pp. 1–20. [DOI] [PubMed] [Google Scholar]
- The staff of the Jackson Laboratory. Biology of the Laboratory Mouse. Dover Publications, Inc; New York: 1966. [Google Scholar]
- Thomas T, Gallo MA, Klinge CM, Thomas TJ. Polyamine-mediated conformational perturbations in DNA alter the binding of estrogen receptor to poly(dG-m5dC).poly(dG-m5dC) and a plasmid containing the estrogen response element. J Steroid Biochem Mol Biol. 1995;54:89–99. doi: 10.1016/0960-0760(95)00126-k. [DOI] [PubMed] [Google Scholar]
- Tilly K, Grimm D, Bueschel DM, Krum JG, Rosa P. Infectious cycle analysis of a Borrelia burgdorferi mutant defective in transport of chitobiose, a tick cuticle component. Vector Borne Zoonotic Dis. 2004;4:159–168. doi: 10.1089/1530366041210738. [DOI] [PubMed] [Google Scholar]
- Waymouth C. Osmolality of mammalian blood and of media for culture of mammalian cells. In Vitro. 1970;6:109–127. doi: 10.1007/BF02616113. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:D801–807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Knox C, Guo AC, Eisner R, Young N, Gautam B, Hau DD, Psychogios N, Dong E, Bouatra S, Mandal R, Sinelnikov I, Xia J, Jia L, Cruz JA, Lim E, Sobsey CA, Shrivastava S, Huang P, Liu P, Fang L, Peng J, Fradette R, Cheng D, Tzur D, Clements M, Lewis A, De Souza A, Zuniga A, Dawe M, Xiong Y, Clive D, Greiner R, Nazyrova A, Shaykhutdinov R, Li L, Vogel HJ, Forsythe I. HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res. 2009;37:D603–610. doi: 10.1093/nar/gkn810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, Cheng D, Jewell K, Arndt D, Sawhney S, Fung C, Nikolai L, Lewis M, Coutouly MA, Forsythe I, Tang P, Shrivastava S, Jeroncic K, Stothard P, Amegbey G, Block D, Hau DD, Wagner J, Miniaci J, Clements M, Gebremedhin M, Guo N, Zhang Y, Duggan GE, Macinnis GD, Weljie AM, Dowlatabadi R, Bamforth F, Clive D, Greiner R, Li L, Marrie T, Sykes BD, Vogel HJ, Querengesser L. HMDB: the Human Metabolome Database. Nucleic Acids Res. 2007;35:D521–526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss C, Ermert P. Borrelia burgdorferi is an Adenine and Spermidine Auxotroph. Microbial Ecology in Health and Disease. 1996;9:181–185. [Google Scholar]
- Yang X, Goldberg MS, Popova TG, Schoeler GB, Wikel SK, Hagman KE, Norgard MV. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol Microbiol. 2000;37:1470–1479. doi: 10.1046/j.1365-2958.2000.02104.x. [DOI] [PubMed] [Google Scholar]
- Yao X, Lu CD. Functional characterization of the potRABCD operon for spermine and spermidine uptake and regulation in Staphylococcus aureus. Curr Microbiol. 2014;69:75–81. doi: 10.1007/s00284-014-0556-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genomic organization of the BBi-potABCD strain.
The flgB-lacI repressor was inserted into bbe02 (lp25) by double crossover generating the strainB31-68-LS (Chu et al., 2016). The promoter of the potABCD operon was replaced by the flacp promoter, the IPTG-inducible promoter generating the strain BBi-potABCD. Expression of lacI was maintained constitutive by the flgB promoter. The position of each construct on chromosome and plasmid is marked.
Spermidine is not an osmoprotective or oxidative protective molecule.
(A) Growth of wild-type B31-A3 strain at 150, 250, 450, 650, 850 mOsM with or without 5 mM spermidine (SPD). An aliquot of each culture was examined daily by dark-field microscopy and plated on solid BSK-II. (B) Early stationary phase (5 x 107 cells/ml) cultures were incubated for 3 h with 0, 1, 5 or 10 mM of tert-butyl hydroperoxide (t-butyl) and plated on BSK-II. Plates were incubated at 34°C under microaerobic conditions for 7–14 d to allow enumeration of CFU. Untreated samples were used to determine a 100% benchmark to which all other numbers were compared. Data represent means ± standard deviation of three independent experiments.