Abstract
Cervical gastric-type adenocarcinomas are aggressive non HPV-related carcinomas with a propensity for extracervical spread, including unusual sites such as the omentum, peritoneum and ovary. We report 7 cases of cervical gastric-type adenocarcinoma with fallopian tube involvement predominantly in the form of mucosal colonisation without underlying invasion. As far as we are aware, this has not been previously described and this report adds to the literature regarding metastatic neoplasms which may exhibit tubal mucosal involvement and mimic an in-situ lesion at this site. In all cases, there was associated ovarian involvement and in 6 of 7 cases, there was endometrial colonisation. We speculate that the fallopian tube (and ovarian) involvement is secondary to transuterine spread. Given the occasional occurrence of multifocal gastric-type glandular lesions (benign or malignant) involving different sites in the female genital tract, we discuss the distinction between synchronous independent and metastatic lesions.
Keywords: cervix, gastric-type adenocarcinoma, fallopian tube involvement, transuterine spread
INTRODUCTION
Gastric-type adenocarcinoma is an uncommon variant of cervical carcinoma which is included in the 2014 World Health Organization (WHO) Classification as a subtype of mucinous adenocarcinoma (1). Gastric-type adenocarcinomas are not associated with infection by high-risk human papillomavirus (HPV) and these neoplasms exhibit more aggressive behaviour than so-called usual HPV-related cervical adenocarcinomas with a particular propensity for ovarian, omental and peritoneal involvement (2–9). We report a series of cervical gastric-type adenocarcinomas exhibiting fallopian tube spread, predominantly in the form of mucosal involvement without underlying invasion, the morphological features potentially mimicking a tubal in-situ lesion. All cases were associated with ovarian and all but one with endometrial involvement and we speculate that the tubal and ovarian disease is secondary to transuterine spread of tumour. This phenomenon of tubal mucosal involvement has been previously documented in HPV-related cervical adenocarcinomas but not in gastric-type adenocarcinomas (10,11). Given that gastric-type glandular lesions in the female genital tract may occasionally involve multiple sites (so-called synchronous mucinous metaplasia and neoplasia of the female genital tract) (3,9,12), we discuss the distinction between independent synchronous fallopian tube and cervical lesions and spread of cervical tumour to the tube.
MATERIALS AND METHODS
The 7 cases derived from the pathology archives of the institutions to which the authors are affiliated together with the consultation practice of two of the authors (KJP, WGM). Haematoxylin and eosin and immunohistochemical stained slides were reviewed by the authors during the preparation of this manuscript.
RESULTS
The clinicopathological features of the 7 cases are summarised in Table 1. The patients ranged in age from 44 to 68 years (mean 56). None of the patients had a known history of Peutz-Jeghers syndrome (PJS). Most of the patients underwent hysterectomy and bilateral salpingo-oophorectomy with or without lymph node dissection but in 1 patient (case 3), cervical biopsy and bilateral salpingo-oophorectomy was performed.
Table 1.
Clinicopathological Features of Study Cases.
| Case | Age (years) |
Operative Procedure | Parametrial / Paracervical Involvement |
Endometrial Involvement |
Vaginal involvement |
Lymphovascular invasion | Laterality of Fallopian Tube Involvement |
Laterality of Ovarian Involvement |
Lymph Node Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | Radical hysterectomy, bilateral salpingo-oophorectomy, omental and peritoneal biopsy | Absent | Present | Absent | Present | Left | Bilateral | Not applicabl |
| 2 | 61 | Radical hysterectomy, bilateral salpingo-oophorectomy, omentectomy, appendicectomy and pelvic lymph node dissection | Present | Present | Absent | Present | Left | Right | Involved |
| 3 | 68 | Cervical and endometrial biopsy and bilateral salpingo-oophorectomy | Not known (no hysterectomy) | Present on endometrial biopsy | Not known (no hysterectomy) | Presen | Bilatera | Bi;ateral | Not applicable |
| 4 | 58 | Total pelvic exenteration (uterus, cervix, bilateral salpingo-oophorectomy, bladder, rectum, sigmoid colon and vagina) | Present | Present | Present | Present | Bilateral | Left(right ovary not identified) | Not applicable |
| 5 | 64 | Radical hysterectomy, bilateral salpingo-oophorectomy and pelvic lymph node dissection and mesenteric biopsy | Present | Present | Absent | Present | Right | Left | Involved |
| 6 | 48 | Simple hysterectomy and bilateral salpingo-oophorectomy, para-aortic, aorto-caval, and left infra-renal lymph node dissection (post-chemoradiotherapy) | Absent on imaging | Present on endometrial biopsy but not on hysterectomy post-chemoradiotherapy | Present on imaging | Present | Bilateral | Bilateral | Involved |
| 7 | 51 | Radical hysterectomy, bilateral salpingo-oophorectomy and pelvic lymph node dissection | Present | Absent | Absent | Present | Bilateral | Bilateral | Not involved |
In all 7 cases, the cervix contained a gastric-type adenocarcinoma. The pathological features of these are not described in detail but in all cases the morphology was typical of gastric-type adenocarcinoma with glands with abundant mucinous, eosinophilic or clear cytoplasm. Some tumours were predominantly composed of cytologically bland glands but in all cases, there were foci where the glands were lined by cells with atypical nuclei. Lymphovascular space invasion was identified in all cases. There was vaginal involvement in 2 of 6 cases and parametrial/ paracervical involvement in 4 of 6 where this information was available; in 1 case this information was only available from pretreatment imaging.
There was fallopian tube involvement in all cases (2 left, 1 right, 4 bilateral). This was predominantly in the form of mucosal colonisation without stromal invasion but in 3 cases there was involvement of the submucosa and in 1 of these there was invasion of the muscle wall. The tubal colonisation varied from focal to involvement of almost the entire mucosa and was generally sharply demarcated from the normal tubal epithelium. It was mostly continuous but in 2 cases was discontinuous with normal tubal epithelium between the foci of mucosal colonisation. The non-fimbrial mucosa was predominantly involved but in some cases the fimbria was also colonised. The tubal involvement was in the form of mucinous epithelium and the degree of atypia was variable from case to case and within individual cases; in all cases, there were morphologically bland areas but there was at least focal mild to moderate nuclear atypia. In those cases where much of the tubal mucinous epithelium was bland, the adenocarcinomas within the cervix often contained areas composed of bland mucinous glands. In some areas, there was a single cell lining of mucinous epithelium but elsewhere there was multilayering with a papillary architecture and/ or tufting. A noticeable feature in some cases was focal surface denudation or areas where the mucinous epithelium had “lifted-off” and separated from the underlying stroma resulting in a subepithelial cleft. In one case, there was mucin extravasation within the submucosa.
In 6 of the 7 cases, there was endometrial involvement (usually including lower uterine segment) and in 3 cases there was myometrial invasion. In 2 of the cases, there was endometrial involvement on biopsy; in one of these hysterectomy was not undertaken (case 3) and in the other (case 6), there was no residual endometrial tumour in the post-chemoradiotherapy hysterectomy specimen. In all 7 cases, there was ovarian involvement (2 left, 1 right, 4 bilateral). In 2 cases (cases 2 and 5), where only a single ovary and fallopian tube was involved, these represented contralateral adnexa. Sometimes the ovarian involvement was in the form of multiple cysts lined by bland and variably atypical mucinous epithelium, the morphology mimicking a primary ovarian mucinous neoplasm with an admixture of benign and borderline foci. In some cases, there was surface tumour involvement and in most (but not all) cases, there were areas of obvious destructive stromal infiltration by atypical mucinous glands. As with the tubal involvement, a noticeable feature in the ovaries in some cases was the presence of areas where the mucinous epithelium had “lifted-off” and separated from the underlying stroma. In 3 of 4 cases where lymph node dissection was undertaken, there was tumour involvement.
Figures 1–3 show representative images of the cases.
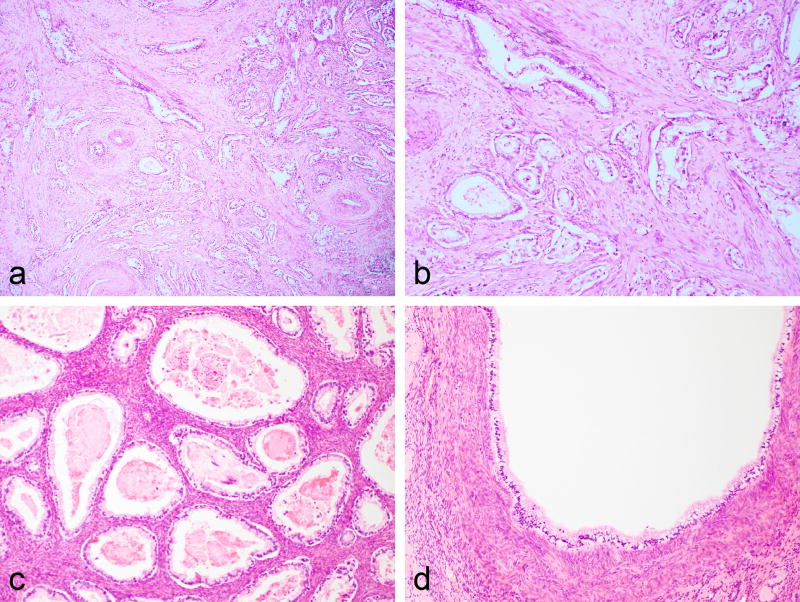
Figure 1.
Case showing cervical involvement by gastric-type adenocarcinoma with deeply invasive mucinous glands surrounded by a desmoplastic stroma (a and b). Ovarian metastasis composed of bland mucinous glands (c) and cystic spaces lined by bland mucinous epithelium (d).
Figure 3.
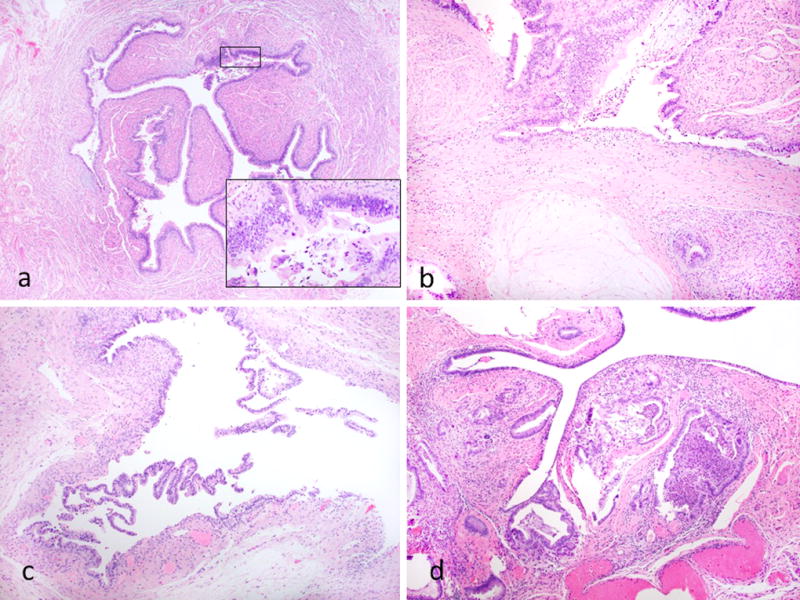
Other features of fallopian tube involvement. Case showing focal involvement of the non-fimbrial portion of the tube; there is epithelial proliferation with nuclear atypia and tufting (inset) (a). Case showing mucin extravasation within the tubal stroma (b). Case where the mucinous epithelium has lifted-off” and separated from the underlying stroma resulting in a subepithelial cleft (c). Case exhibiting involvement of submucosa (d).
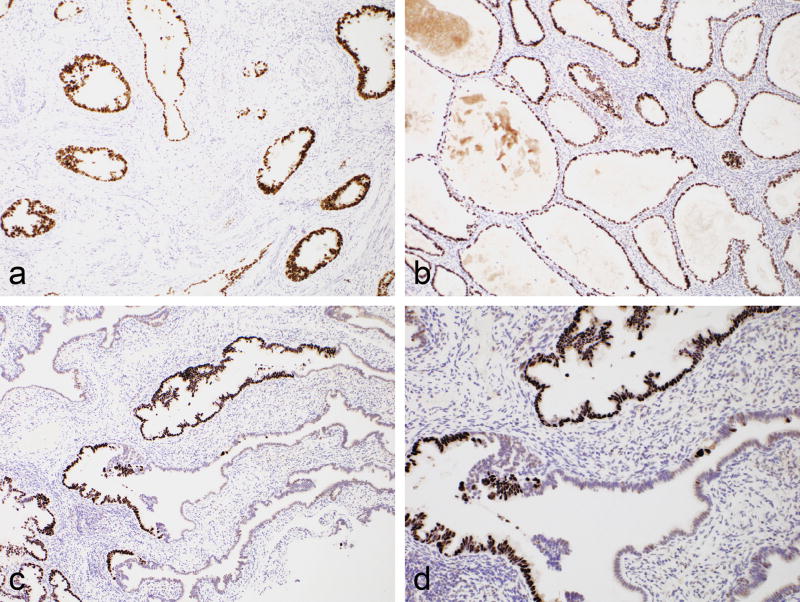
Various immunohistochemical studies were performed in all cases. The results are not presented since the markers used were variable and immunohistochemistry is not needed for the diagnosis of gastric-type adenocarcinoma. However, in all cases p16 was negative or exhibited patchy “non-block” type immunoreactivity. p53 immunohistochemistry was performed in all cases (either at the time of reporting or during the preparation of this manuscript) on both the cervical and tubal lesions. p53 exhibited mutation-type staining in both the cervical and tubal lesions in 5 cases (4 diffuse-type: 1 null-type) and wild-type expression in both in 2 cases. There was no case with different patterns of p53 immunoreactivity in the cervical and tubal lesions. Table 2 shows the p53 staining patterns in the cervical and tubal lesions in each case. Figure 4 shows diffuse mutation-type p53 staining in the cervical, ovarian and tubal involvement in 1 of the cases.
Table 2.
Results of p53 Immunohistochemistry.
| Case | Cervix | Fallopian tube |
|---|---|---|
| 1 | Wild-type | Wild-type |
| 2 | Mutation-type (diffuse) | Mutation-type (diffuse) |
| 3 | Mutation-type (diffuse) | Mutation-type (diffuse) |
| 4 | Wild-type | Wild-type |
| 5 | Mutation-type (diffuse) | Mutation-type (diffuse) |
| 6 | Mutation-type (null) | Mutation-type (null) |
| 7 | Mutation-type (diffuse) | Mutation-type (diffuse) |
Figure 4.
Case showing diffuse mutation-type p53 staining of tumour in cervix (a), ovary (b) and fallopian tube (c and d); the normal fallopian tube epithelium exhibits wild-type p53 immunoreactivity (d).
DISCUSSION
Like cervical squamous carcinomas, most cervical adenocarcinomas are associated with infection by high-risk (oncogenic) HPV. However, there is a significant proportion of cervical adenocarcinomas (approximately 10–15% of cervical adenocarcinomas and 2–4% of cervical carcinomas overall) which are not associated with HPV (13–18). The most common of these is so-called gastric-type adenocarcinoma but non HPV-related adenocarcinomas also include mesonephric and clear cell carcinoma (13–18). Gastric-type adenocarcinoma is included in the 2014 WHO Classification as a subtype of mucinous adenocarcinoma (1); the category of gastric-type adenocarcinoma includes adenoma malignum (mucinous variant of minimal deviation adenocarcinoma), a neoplasm which is now recognised to constitute the well-differentiated end of the spectrum of gastric-type adenocarcinomas. Since admixtures of well-differentiated and poorly differentiated areas are common and there is no difference in the outcome between well differentiated and poorly differentiated neoplasms (8,19), the umbrella term gastric-type adenocarcinoma is preferred to encompass this group of neoplasms. The concept of gastric-type cervical glandular lesions has emerged over recent decades and it is now recognised that there is a spectrum of benign, premalignant and malignant lesions (3,9). Some of these occur in patients with PJS. Established benign lesions (which are rare) include simple gastric metaplasia and lobular endocervical glandular hyperplasia (LEGH) (complex gastric metaplasia) (3,9,20,21). Postulated premalignant lesions comprise so-called atypical LEGH and gastric-type adenocarcinoma in situ (3,6,9,22,23). The term “gastric-type” derives from the morphological resemblance of the glandular epithelium to that seen in the stomach and pancreaticobiliary tree; intestinal metaplasia with goblet cells and neuroendocrine cells can occur in the various benign, premalignant and malignant lesions. A gastric immunophenotype has been demonstrated using immunohistochemical markers of pyloric gland mucin, MUC6 and HIK1083. The immunophenotype of cervical gastric-type adenocarcinomas has been investigated in detail in a single study and these neoplasms are generally positive with cytokeratin (CK) 7 and CEA while CDX2, CA19.9, PAX8, CA125 and CK20 are positive in a proportion of cases (6). Oestrogen receptor (ER) and progesterone receptor (PR) are usually negative. p53 exhibits mutation-type staining in a significant proportion of cases (41% in the immunohistochemical study referred to) and p16 is generally negative or exhibits “non-block” type immunoreactivity (6). However, a diagnosis of gastric-type adenocarcinoma can be made on the characteristic morphology without immunohistochemical staining.
Cervical gastric-type adenocarcinomas are clinically aggressive neoplasms which are much more likely to present at advanced stage compared to HPV-related adenocarcinomas (2,8). For example, in one study, the 5-year survival was 30% versus 77% for HPV-related adenocarcinomas (2). In a more recent study, the 5-year survival was 42% versus 91% for HPV-related adenocarcinomas; in this study, 59% of gastric-type adenocarcinomas were stage II-IV at presentation, 50% had lymph node metastasis, 35% ovarian involvement and 20% abdominal involvement (8). Unlike HPV-related adenocarcinomas, which typically remain localised within the pelvis, gastric-type adenocarcinomas spread to unusual sites such as the peritoneum, omentum and ovary (2,3,8,9).
Ovarian metastasis is uncommon in cervical cancers and is more common in adenocarcinomas than squamous carcinomas (24,25). The particular propensity for gastric-type adenocarcinomas to spread to the ovary has already been discussed. Fallopian tube involvement in cervical carcinoma has received little attention in the literature but has been reported in HPV-related squamous and adenocarcinoma (11). Reyes et al reported 8 examples (7 unilateral, 1 bilateral) of fallopian tube mucosal involvement in HPV-related cervical adenocarcinomas (11); it was commented that the tubal involvement was often mucosal and mimicked a primary tubal lesion such as serous tubal intraepithelial carcinoma (STIC) (11).
To our knowledge, fallopian tube involvement by cervical gastric-type adenocarcinoma has not been specifically reported until now. In this series, the fallopian tubes showed mainly mucosal colonisation with submucosal involvement in 3 cases, 1 of which also exhibited invasion of the muscle wall. All 7 cases with fallopian tube involvement also had ovarian involvement, being bilateral at both sites in 4 cases each. The endometrium was also involved in 6 of 7 cases. Given the common presence of endometrial involvement and the fact that the tubal disease always involved and was sometimes confined to the mucosa, we feel it is highly likely that the tubal and ovarian involvement occurred by retrograde transuterine spread. An interesting observation in some of the cases was the tendency for the tumour involving the fallopian tube mucosa (and also that lining ovarian cystic metastatic foci) to “lift-off” or separate from the underlying stroma, resulting in a subepithelial cleft or denudation. This is somewhat similar to the subepithelial stromal clefts described in ovarian involvement by low-grade appendiceal mucinous neoplasms (26).
A transuterine route has been speculated as a mode of spread of primary cervical HPV-related adenocarcinomas (27,28). In these neoplasms, ovarian involvement has been reported in the presence of small or even not recognisably invasive cervical adenocarcinomas (27,28). In some of these cases, especially with endometrial involvement, transuterine and transtubal spread has been implicated; the prognosis is generally favourable in these cases perhaps due to the mode of spread which is not via haematogenous routes. The tendency for metastatic HPV-related cervical adenocarcinomas involving the ovary to mimic primary ovarian mucinous or endometrioid neoplasms, borderline or malignant, has been stressed (11,28). "Borderline-like" patterns include cystic, confluent glandular, cribriform, and/or villoglandular. A “hybrid” of mucinous and endometrioid features with elongated nuclei and conspicuous apical mitoses and basal apoptotic bodies in an ovarian neoplasm are clues that one may be dealing with a metastatic HPV-related cervical adenocarcinoma. Other clues to a metastatic neoplasm include bilateral ovarian involvement (although this is present in a minority of metastatic cervical adenocarcinomas), surface tumour deposits and an infiltrative pattern of stromal invasion. Diffuse “block-type” immunoreactivity with p16 and/ or the demonstration of HPV by molecular techniques may also assist in confirming a metastatic HPV-related cervical adenocarcinoma. Although it has been speculated that such cases could represent synchronous HPV-related ovarian adenocarcinomas rather than metastatic cervical adenocarcinomas (29), this is not a widespread held view. The primary focus of this paper was not to describe the pattern of ovarian involvement in the cervical gastric-type adenocarcinomas but, similar to some other metastatic adenocarcinomas involving the ovary, there were often morphologically bland areas and cystic foci potentially mimicking a primary ovarian mucinous cystadenoma, borderline tumour or carcinoma.
In all the cases we report, the diagnosis of tubal metastasis was relatively easily established given the presence of an obvious infiltrative gastric-type adenocarcinoma within the cervix. However, in two of the cases received in consultation (cases 1 and 2), a synchronous independent fallopian tube lesion was considered by the referring pathologist; this distinction is discussed below. The phenomenon of tubal mucosal metastasis mimicking an in-situ lesion has already been discussed for HPV-related cervical adenocarcinomas and has recently been highlighted for other neoplasms (30). Rabban and colleagues reported a series of metastatic tumours from a variety of sites (gynaecological and non-gynaecological) involving the tube and sometimes exhibiting prominent and/ or exclusive mucosal involvement mimicking an in-situ lesion such as STIC (30). In such cases, immunohistochemistry may be of value, the panel of markers depending on the morphological features and the presence of a known tumour elsewhere. The propensity for uterine serous carcinoma to exhibit fallopian tube mucosal involvement and mimic STIC has also recently been highlighted (31). Endometrioid adenocarcinomas of the uterine corpus may also involve the tubal mucosa and mimic an independent synchronous tubal lesion.
Gastric-type mucinous glandular lesions may involve all sites in the female genital tract, occasionally with synchronous involvement of more than one site (3,9,12). This may be in the form of benign metaplastic or neoplastic lesions; this multifocal involvement has been referred to as synchronous mucinous metaplasia and neoplasia of the female genital tract and may occur in patients with PJS (3,9,12). For example, Mikami et al reported 6 cases of synchronous mucinous lesions with a gastric phenotype and immunophenotype (positive with HIK1083 and/ or MUC6) at different sites within the female genital tract (12). All 6 patients had mucinous metaplasia of the endometrium, which showed features of LEGH/pyloric gland metaplasia in 5 and was associated with mucinous adenocarcinoma in 3. Five patients had mucinous metaplasia of the fallopian tubes and 2 had mucinous borderline tumours of the ovary. In 5 patients, there were cervical lesions, including LEGH associated with either adenocarcinoma in situ or adenoma malignum. While in these cases, the various mucinous lesions were interpreted as representing synchronous independent proliferations, it may be extremely difficult to distinguish between this possibility and metastatic disease from one site to another. Older studies describe the coexistence of cervical adenoma malignum with independent ovarian benign or borderline mucinous neoplasms (32); however, it is possible that these represented bland ovarian metastasis of adenoma malignum. In the cases we report, given the obvious malignant nature of the cervical lesions and the presence of areas of significant nuclear atypia in the endometrial, tubal and ovarian lesions (and sometimes other features suggesting metastasis in the ovarian neoplasms), the extracervical disease was considered to represent metastatic spread rather than synchronous independent metaplasia or neoplasia. Since the mucinous lesions at various sites exhibit gastric differentiation, immunohistochemistry is of limited value in helping to determine the relationship between them (synchronous or metastatic). An exception is p53 since mutation-type staining is seen in a significant proportion of cervical gastric-type adenocarcinomas (6); thus an identical pattern of mutation-type p53 staining at the different sites would support a primary neoplasm at one site with metastasis to the others. Conversely, mutation-type staining at one site and wild-type immunoreactivity at another would lend support to synchronous independent lesions while wild-type staining at the various sites would be of no value. p53 staining was undertaken in all cases in our study. There was an identical pattern of p53 staining in the cervical and tubal lesions in all cases; in 5 cases this was mutation-type (4 diffuse, 1 null) and in 2 cases it was wild-type. One potential pitfall worthy of mention is that mutation-type p53 staining is characteristic of STIC and this could result in misdiagnosis of tubal involvement by cervical gastric-type adenocarcinoma as STIC; correlation with the findings in the cervix and the mucinous features should result in a correct diagnosis.
The mucosal involvement of the fallopian tube mucinous lesions we report could potentially result in consideration of a primary tubal mucinous adenocarcinoma. However, primary mucinous adenocarcinomas of the fallopian tube are extremely rare. Wheal et al reported a primary mucinous adenocarcinoma of the fimbria of the left fallopian tube exhibiting gastric differentiation (33); this was associated with adjacent mucinous metaplasia, a mucinous borderline tumour of the left ovary and a benign mucinous cystadenoma of the right ovary (32). As discussed previously, we believe the tubal lesions we report to be metastatic. Benign mucinous metaplasia also uncommonly occurs within the fallopian tubes, sometimes in patients with PJS (34,35). This may occur as a solitary lesion or in association with mucinous metaplasia or neoplasia elsewhere within the female genital tract. In such cases, it may be extremely difficult or even impossible to determine whether this represents mucinous metaplasia of the fallopian tube or bland metastasis from a mucinous adenocarcinoma elsewhere. Wong et al reported 23 cases of fallopian tubes with mucinous lesions, including 11 patients without evidence of malignancy, 4 with mucinous ovarian tumours, 5 with non-mucinous gynaecological tumours, 2 with mucinous appendiceal neoplasms and 1 with colonic carcinoma (34). The authors concluded that in most cases the tubal mucinous lesion represented a primary metaplastic process.
Staging of a cervical carcinoma with adnexal involvement (tubal and/ or ovarian) is sometimes controversial since adnexal involvement does not specifically appear in the FIGO or TNM staging systems (36). Adnexal involvement is FIGO stage IIA (pT2a); TNM states that distant metastasis (M1) excludes metastasis to vagina, pelvic serosa and adnexa.
In summary, we report 7 cases of cervical gastric-type adenocarcinoma with fallopian tube involvement in the form of mucosal colonisation, sometimes without underlying invasion. As far as we are aware, this phenomenon has not been previously reported. This report adds to the literature regarding neoplasms which may exhibit tubal mucosal involvement and mimic an in-situ lesion at this site. We speculate that the fallopian tube involvement is secondary to transuterine spread.
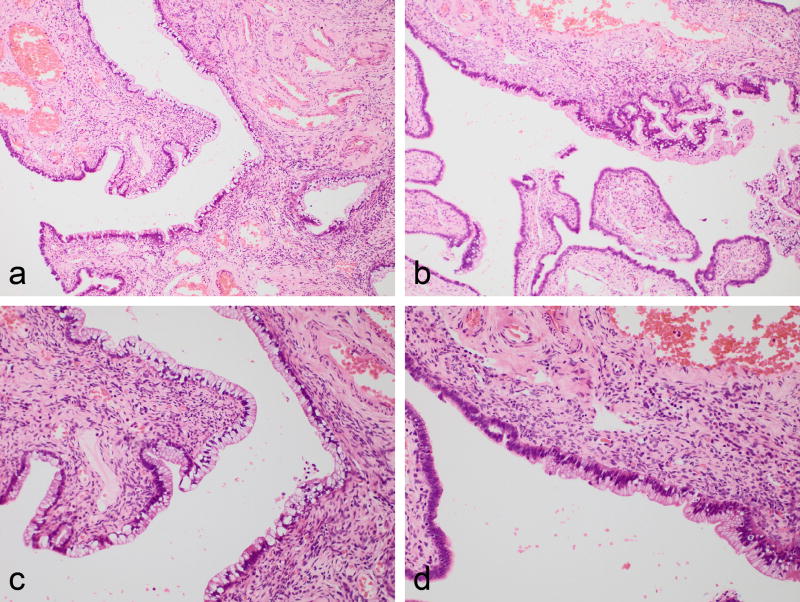
Figure 2.
Case showing fallopian tube involvement confined to the mucosa and predominantly comprising a single layer of mucinous epithelium (a and b). On higher power, the morphology is bland and the cells have abundant mucinous cytoplasm (c). There is a sharp demarcation between normal fallopian tube epithelium (left of photomicrograph) and gastric-type adenocarcinoma involving the mucosa (right of photomicrograph).
Acknowledgments
We would like to thank Dr Angela Ralte (Gateshead, United Kingdom) and Dr Shaun Roberts (Swansea, United Kingdom) for referring cases and providing clinical details.
References
- 1.Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO Classification of Tumours of Female Reproductive Organs. International Agency for Research on Cancer; Lyon: 2014. [Google Scholar]
- 2.Kojima A, Mikami Y, Sudo T, et al. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol. 2007;31:664–72. doi: 10.1097/01.pas.0000213434.91868.b0. [DOI] [PubMed] [Google Scholar]
- 3.Mikami Y, McCluggage WG. Endocervical glandular lesions exhibiting gastric differentiation: an emerging spectrum of benign, premalignant, and malignant lesions. Adv Anat Pathol. 2013;20:227–37. doi: 10.1097/PAP.0b013e31829c2d66. [DOI] [PubMed] [Google Scholar]
- 4.Stewart CJ, Frost F, Leake R, Mohan GR, Tan J. Foamy gland changes in gastric-type endocervical neoplasia. Pathology. 2015;47:653–8. doi: 10.1097/PAT.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 5.McCluggage WG. Recent developments in non-HPV-related adenocarcinomas of the lower female genital tract and their precursors. Adv Anat Pathol. 2016;23:58–69. doi: 10.1097/PAP.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 6.Carleton C, Hoang L, Sah S, et al. A detailed immunohistochemical analysis of a large series of cervical and vaginal gastric-type adenocarcinomas. Am J Surg Pathol. 2016;40:636–44. doi: 10.1097/PAS.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilks CB, Young RH, Aquirre P, DeLellis RA, Scully RE. Adenoma malignum (minimal deviation adenocarcinoma) of the uterine cervix. A clinicopathological and immunohistochemical analysis of 26 cases. Am J Surg Pathol. 1989;13:717–29. doi: 10.1097/00000478-198909000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Karamurzin YS, Kiyokawa T, Parkash V, et al. Gastric-type endocervical adenocarcinoma: an aggressive tumor with unusual metastatic patterns and poor prognosis. Am J Surg Pathol. 2015;39:1449–57. doi: 10.1097/PAS.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talia KL, McCluggage WG. The developing spectrum of gastric-type cervical glandular lesions. Pathology. doi: 10.1016/j.pathol.2017.09.009. In Press. [DOI] [PubMed] [Google Scholar]
- 10.Deel CD, Allen RA, Holman LL, Zuna RE. Adenocarcinoma of the cervix involving the fallopian tube mucosa: report of a case. Diagn Pathol. 2016;11:77. doi: 10.1186/s13000-016-0529-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reyes C, Murali R, Park KJ. Secondary involvement of the adnexa and uterine corpus by carcinomas of the uterine cervix: a detailed morphologic description. Int J Gynecol Pathol. 2015;34:551–63. doi: 10.1097/PGP.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikami Y, Kiyokawa T, Sasajima Y, et al. Reappraisal of synchronous and multifocal mucinous lesions of the female genital tract: a close association with gastric metaplasia. Histopathology. 2009;54:184–91. doi: 10.1111/j.1365-2559.2008.03202.x. [DOI] [PubMed] [Google Scholar]
- 13.Holl K, Nowakowski AM, Powell N, et al. Human papillomavirus prevalence and type-distribution in cervical glandular neoplasias: Results from a European multinational epidemiological study. Int J Cancer. 2015;137:2858–68. doi: 10.1002/ijc.29651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houghton O, Jamison J, Wilson R, Carson J, McCluggage WG. p16 immunoreactivity in unusual types of cervical adenocarcinoma does not reflect human papillomavirus infection. Histopathology. 2010;57:342–50. doi: 10.1111/j.1365-2559.2010.03632.x. [DOI] [PubMed] [Google Scholar]
- 15.Toki T, Zhai YL, Park JS, Fujii S. Infrequent occurrence of high-risk human papillomavirus and of p53 mutation in minimal deviation adenocarcinoma of the cervix. Int J Gynecol Pathol. 1999;18:215–9. doi: 10.1097/00004347-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson AW, Svoboda-Newman SM, Frank TS. Analysis of human papillomavirus infection and molecular alterations in adenocarcinoma of the cervix. Mod Pathol. 1998;11:11–8. [PubMed] [Google Scholar]
- 17.Kenny SL, McBride HA, Jamison J, McCluggage WG. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-β. Am J Surg Pathol. 2012;36:799–807. doi: 10.1097/PAS.0b013e31824a72c6. [DOI] [PubMed] [Google Scholar]
- 18.Park KJ, Kiyokawa T, Soslow RA, et al. Unusual endocervical adenocarcinomas: an immuno histochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol. 2011;35:633–46. doi: 10.1097/PAS.0b013e31821534b9. [DOI] [PubMed] [Google Scholar]
- 19.McCluggage WG, Harley I, Houghton JP, Geyer FC, MacKay A, Reis-Filho JS. Composite cervical adenocarcinoma composed of adenoma malignum and gastric type adenocarcinoma (dedifferentiated adenoma malignum) in a patient with Peutz Jeghers syndrome. J Clin Pathol. 2010;63:935–41. doi: 10.1136/jcp.2010.080150. [DOI] [PubMed] [Google Scholar]
- 20.Nucci MR, Clement PB, Young RH. Lobular endocervical glandular hyperplasia, not otherwise specified: a clinicopathologic analysis of thirteen cases of a distinctive pseudoneoplastic lesion and comparison with fourteen cases of adenoma malignum. Am J Surg Pathol. 1999;23:886–91. doi: 10.1097/00000478-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Mikami Y, Hata S, Fujiwara K, Imajo Y, Kohno I, Manabe T. Florid endocervical glandular hyperplasia with intestinal and pyloric gland metaplasia: worrisome benign mimic of "adenoma malignum". Gynecol Oncol. 1999;74:504–11. doi: 10.1006/gyno.1999.5462. [DOI] [PubMed] [Google Scholar]
- 22.Talia KL, Stewart CJR, Howitt BE, Nucci MR, McCluggage WG. HPV-negative Gastric Type Adenocarcinoma In Situ of the Cervix: A Spectrum of Rare Lesions Exhibiting Gastric and Intestinal Differentiation. Am J Surg Pathol. 2017;41:1023–1033. doi: 10.1097/PAS.0000000000000855. [DOI] [PubMed] [Google Scholar]
- 23.Mikami Y, Kiyokawa T, Hata S, et al. Gastrointestinal immunophenotype in adenocarcinomas of the uterine cervix and related glandular lesions: a possible link between lobular endocervical glandular hyperplasia/pyloric gland metaplasia and 'adenoma malignum'. Mod Pathol. 2004;17:962–72. doi: 10.1038/modpathol.3800148. [DOI] [PubMed] [Google Scholar]
- 24.Shimada M, Kigawa J, Nishimura R, et al. Ovarian metastasis in carcinoma of the uterine cervix. Gynecol Oncol. 2006;101:234–7. doi: 10.1016/j.ygyno.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Toki N, Tsukamoto N, Kaku T, et al. Microscopic ovarian metastasis of the uterine cervical cancer. Gynecol Oncol. 1991;41:46–51. doi: 10.1016/0090-8258(91)90253-2. [DOI] [PubMed] [Google Scholar]
- 26.Stewart CJ, Ardakani NM, Doherty DA, Young RH. An evaluation of the morphologic features of low-grade mucinous neoplasms of the appendix metastatic in the ovary, and comparison with primary ovarian mucinous tumors. Int J Gynecol Pathol. 2014;33:1–10. doi: 10.1097/PGP.0b013e318284e070. [DOI] [PubMed] [Google Scholar]
- 27.Chang MC, Nevadunsky NS, Viswanathan AN, Crum CP, Feltmate CM. Endocervical adenocarcinoma in situ with ovarian metastases: a unique variant with potential for long-term survival. Int J Gynecol Pathol. 2010;29:88–92. doi: 10.1097/PGP.0b013e3181acefbf. [DOI] [PubMed] [Google Scholar]
- 28.Ronnett BM, Yemelyanova AV, Vang R, et al. Endocervical adenocarcinomas with ovarian metastases: analysis of 29 cases with emphasis on minimally invasive cervical tumors and the ability of the metastases to simulate primary ovarian neoplasms. Am J Surg Pathol. 2008;32:1835–53. doi: 10.1097/PAS.0b013e3181758831. [DOI] [PubMed] [Google Scholar]
- 29.Reichert RA. Synchronous and metachronous endocervical and ovarian neoplasms: a different interpretation of HPV data. Am J Surg Pathol. 2005;29:1686–7. doi: 10.1097/01.pas.0000183569.71269.01. [DOI] [PubMed] [Google Scholar]
- 30.Rabban JT, Vohra P, Zaloudek CJ. Nongynecologic metastases to fallopian tube mucosa: a potential mimic of tubal high-grade serous carcinoma and benign tubal mucinous metaplasia or nonmucinous hyperplasia. Am J Surg Pathol. 2015;39:35–51. doi: 10.1097/PAS.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 31.Kommoss F, Farugi A, Gilks CB, et al. Uterine serous carcinomas frequently metastasize to the fallopian tube and can mimic serous tubal intraepithelial carcinoma. Am J Surg Pathol. 2017;41:161–70. doi: 10.1097/PAS.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 32.Young RH, Scully RE. Mucinous ovarian tumors associated with mucinous adenocarcinomas of the cervix. A clinicopathological analysis of 16 cases. Int J Gynecol Pathol. 1988;7:99–111. doi: 10.1097/00004347-198805000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Wheal A, Jenkins R, Mikami Y, Das N, Hirschowitz L. Primary Mucinous Carcinoma of the Fallopian Tube: Case Report and Review of Literature. Int J Gynecol Pathol. 2017;36:393–399. doi: 10.1097/PGP.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 34.Wong AK, Seidman JD, Barbuto DA, McPhaul LW, Silva EG. Mucinous metaplasia of the fallopian tube: a diagnostic pitfall mimicking metastasis. Int J Gynecol Pathol. 2011;30:36–40. doi: 10.1097/PGP.0b013e3181f45f28. [DOI] [PubMed] [Google Scholar]
- 35.Seidman JD. Mucinous lesions of the fallopian tube. A report of seven cases. Am J Surg Pathol. 1994;18:1205–12. doi: 10.1097/00000478-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. 2009;105:107–8. doi: 10.1016/j.ijgo.2009.02.009. [DOI] [PubMed] [Google Scholar]