Abstract
Myeloid-derived suppressor cells (MDSC) are present in most individuals with cancer where they inhibit adaptive and innate antitumor immunity and are an obstacle to cancer immunotherapies. Chronic inflammation is characteristic of adipose tissue and is a risk factor for the onset and progression of cancer in obese individuals. Because MDSC accumulate in response to inflammation, it has been hypothesized that one of the mechanisms by which obesity promotes malignancy is through the induction of MDSC. This article reviews the data supporting this hypothesis, the role of leptin and fatty acid metabolism in the induction of MDSC, and the surprising finding that although MDSC promote tumor progression, they are protective against some of the metabolic dysfunction associated with obesity.
Introduction
During the past approximately ten years myeloid-derived suppressor cells (MDSC) have been under intensive study because they are present in most cancer patients and are recognized as a significant obstacle to cancer immunotherapies. Chronic inflammation associated with solid tumors is the dominant driving force for the accumulation of tumor-induced MDSC, and led to the hypothesis that one of the mechanisms by which inflammation facilitates cancer progression is by the induction of MDSC which inhibit antitumor immunity [1]. Chronic inflammation is also associated with obesity, suggesting that MDSC may also be elevated in obese individuals. This article describes how obesity-driven MDSC protect against some of the metabolic dysfunction associated with obesity while simultaneously increasing the rate of tumor progression that is characteristic of obese cancer patients. Several recent review articles detail the induction, function, characterization, and nomenclature for MDSC [2–5], so only a brief overview of MDSC characteristics and function will be presented here.
MDSC are a heterogeneous population of immature myeloid cells that arise in the bone marrow (and in the spleen of mice) from the common myeloid progenitor (CMP) and migrate through the circulatory system to solid tumors. The two subsets of MDSC, monocytic and polymorphonuclear/granulocytic (M-MDSC and PMN-MDSC), suppress adaptive and innate immunity using a variety of mechanisms including inhibition of T cell activation, anergizing activated T cells, inhibition of NK cell cytotoxicity, polarization of macrophages towards a tumor-promoting phenotype, and perturbation of immune cell trafficking. Multiple redundant pro-inflammatory mediators drive MDSC accumulation, suppressive potency, and survival by regulating various transcription factors, and a diversity of immune and non-immune host cells produce factors that contribute to the accumulation of MDSC and/or are modified as a result of MDSC development (see figure 1). Most of the knowledge of MDSC biology is derived from studies of tumor-induced MDSC from mice and humans. As described in the following sections, since chronic low grade inflammation is also present in adipose tissue, many of the conditions that drive tumor-induced MDSC also drive MDSC accumulation and function in obese individuals.
Figure 1.
MDSC development, accumulation, suppressive activity, and survival are regulated by a complex network of transcription factors, cytokines, and non-cytokine immune regulatory factors produced by tumor cells and host cells. MDSC originate from the common myeloid progenitor (CMP) cell in the bone marrow (also in the spleen of mice) during myelopoiesis. There are two subtypes of MDSC: mononuclear (M-MDSC) and polymorphonuclear or granulocytice MDSC (PMN-MDSC). Tumor cells and/or host cells in the periphery produce cytokines and other factors that drive MDSC differentiation. From the bone marrow (and spleen of mice), MDSC circulate in the blood and home to sites of inflammation and to solid tumors. Within an inflammatory milieu such as the tumor microenvironment, a variety of factors promote MDSC suppressive activity. The survival of MDSC is facilitated by some of the same conditions and mediators that regulate MDSC accumulation, as well as genes that limit apoptosis. A variety of cells including tumor cells, adipocytes, macrophages, mast cells, dendritic cells, and cancer-associated fibroblasts produce molecules that regulate MDSC.
Obesity is a risk factor for cancer onset and a driver of tumor progression
Abundant epidemiological data demonstrates that obesity not only increases the risk of cancer but also increases the progression of established cancers [6]. A 2003 study concluded that overall, obese men and women have a 1.5–1.6 fold increase in the risk of dying from cancer [7]. Obesity is not an “equal opportunity” risk for all organ sites, however, since obese men have a 4.5 fold risk and 2.6 fold risk of dying from liver and pancreatic cancer, respectively, while obese women have a 4.8 and 5.3 fold risk of dying from kidney and gastrointestinal (GI) cancers, respectively [8,9]. Therefore, although there is an overall propensity of obesity to facilitate cancer onset and progression, there are also organ and tissue-specific, as well as sex-related factors involved. Despite the differences in obesity-driven conditions that lead to increased cancer risk, obesity-driven systemic and/or local inflammation appear to be a unifying condition that facilitates cancer onset and progression [10–13].
The chronic inflammatory environment of adipose tissue is similar to the inflammatory conditions that drive cancer-associated MDSC
Obese individuals typically have elevated levels of IL-6, TNFα, and prostaglandin E2 (PGE2) in their blood. These molecules are produced by adipose cells as well as by macrophages that invade adipose tissue. Elevation of these pro-inflammatory mediators is at least partially due to the adipokine leptin, which is over-expressed in obese individuals and an inducer of IL-1β, TNFα, and IL-6. IL-1β, the product of cellular inflammasomes [14] and TNFα, in turn, induce leptin, thereby creating a feed-back loop that sustains the inflammatory environment (reviewed in [13]). Since leptin lacks inflammatory activity in IL-1β-deficient mice, inflammasomes are probably key regulators of the pro-inflammatory adipose tissue environment [15]. Prevalence of this inflammatory milieu is particularly evident in breast tissue of obese women, but also occurs in intestinal epithelial cells of obese mice [16], in liver cells in non-alcoholic fatty liver disease [17], as well as in other organs of both humans and mice.
The pro-inflammatory mediators IL-6 [18], IL-1β [19–21], TNFα [22], and PGE2 [23,24] are major inducers of the differentiation, accumulation, and potency of tumor-induced MDSC. Since the same constellation of molecules are present in adipose tissue [25], it has been hypothesized that the pro-inflammatory environment of adipose tissue may support the induction and accumulation of MDSC [26,27].
Immune suppressive MDSC are elevated in obese individuals
The first indication that obesity increased the levels of MDSC came from studies with genetically obese mice and with mice maintained on a high fat diet (HFD). ob/ob mice on a C57BL/6 background are genetically obese because they are deficient for the leptin gene. Leptin is a 16KDa protein encoded by the ob gene and is predominantly produced by adipose cells. It regulates body mass by serving as an appetite inhibitor. The Leptin receptor (Ob-R) has multiple forms; however, leptin regulates appetite by binding to the long form of the Ob-R on cells in the hypothalamus [28]. By 28 weeks of age, ob/ob mice have approximately twice as many Gr1+CD11b+ cells in their spleens and adipose tissue compared to lean ob/+ mice. Mice on a HFD (60% fat) for 12 weeks accumulate excess body fat and have approximately 1.5 and 3 times as many Gr1+CD11b+ cells in their spleens and adipose tissue, respectively, as mice on a low fat diet (LFD; 11% fat). Gr1+CD11b+ cell levels in the liver of ob/ob and HFD mice are as much as 3 times higher than their lean counterparts. The Gr1+CD11b+ cells are MDSC as shown by their ability to suppress the activation/proliferation of CD8+ T cells. Both M-MDSC and PMN-MDSC were elevated in the ob/ob and HFD mice [26]. Gr1+CD11b+ cells in obese mice are also immune suppressive in vivo as shown by the reduced antigen-specific cellular and antibody responses in HFD mice following immunization with a hepatitis B vaccine [25]. Not surprisingly, obese tumor-bearing mice have higher levels of f MDSC than lean/control tumor-bearing mice [27,29].
M-MDSC are similarly elevated in obese humans. Chinese men with BMI >25kg/m2 but without diabetes or other complicating metabolic issues had the same number of monocytes in their blood as men with a BMI <25kg/m2, but had approximately 3.5 times as many M-MDSC (CD11b+CD33+CD14+HLADRlo/− cells) [30]. Down-regulation of the TCRζ chain is a hallmark of MDSC function in mice [31], and a similar decrease in TCRζ chain expression was found on naïve T cells in the obese group of men [30].
Obesity-induced MDSC enhance tumor progression
There is ample data demonstrating that malignancies grow more rapidly and that cancer-induced mortality is higher in obese than in non-obese individuals (reviewed in [32]). Given the immune suppressive activity of MDSC and the known ability of antitumor immunity to decrease tumor progression, the obvious question is whether the elevated levels of MDSC contribute to enhanced tumor progression in obese individuals. Three recent studies demonstrate that obesity-driven MDSC levels substantially increase tumor growth. In one study, BALB/c mice were rendered obese by feeding a HFD (diet-induced obesity) and then inoculated with the syngeneic RENCA renal cell tumor. Tumors progressed more rapidly in the obese mice and the tumors contained more MDSC compared to tumors of lean controls. There was no difference in the immune suppressive potency of MDSC between obese and lean mice. However, the tumors of the HFD mice had elevated levels of CCL2, a potent chemoattractant for CCR2+ MDSC, suggesting that obesity alters the tumor microenvironment to favor the accumulation of MDSC [29]. In a pancreatic cancer mouse study using diet-induced obese mice, Gr1+CD11b+ cells were recruited to the pancreas by adipocytes producing the pro-inflammatory mediator IL-1β. Pancreatic stellate cells were subsequently activated and produced additional IL-1β and recruited more Gr1+CD11b+ cells. Depletion of the Gr1+CD11b+ cells, IL-1β, or the pancreatic stellate cells prevented more rapid growth of cancer in the obese mice [33]. The authors attributed the increased tumor progression to cross-talk between adipocytes, Gr1+CD11b+ cells, and pancreatic stellate cells. The authors called the Gr1+CD11b+ cells tumor-associated neutrophils [33]. However, neutrophils and MDSC have similar phenotypes, and because IL-1β is an established inducer of MDSC and the Gr1+CD11b+ cells were not further characterized as neutrophils, it is likely that the Gr1+CD11b+ cells were MDSC.
The key study for establishing that obesity-induced MDSC promote more rapid tumor progression used diet-induced obese BALB/c mice carrying the 4T1 mammary carcinoma. MDSC in the blood and within tumors accumulated to higher levels in mice fed a HFD vs mice fed a low fat diet (LFD), and MDSC from HFD mice were more immune suppressive than MDSC from LFD mice. Primary tumors progressed more rapidly and survival time was significantly decreased in HFD mice. Depletion of MDSC reverted the rate of tumor progression in HFD mice to that of LFD controls, and restored T cell activation. Obese mice had higher levels of spontaneous liver metastases relative to LFD mice, and MDSC depletion of HFD mice reduced the levels of liver metastases to that of LFD mice [27].
Obesity-induced MDSC protect against some of the metabolic dysfunction that accompanies obesity
Obesity in both mice and humans is frequently associated with metabolic dysfunction that has significant health consequences. Blood glucose levels can be abnormally elevated when insulin does not eliminate glucose in the blood, a condition known as insulin tolerance. Two studies have examined whether obesity-driven MDSC enhance the metabolic dysfunction associated with obesity [26,27]. Surprisingly, the opposite effect was found. Using MDSC depletion approaches, MDSC in obese mice were shown to decrease serum glucose levels and to protect against insulin tolerance. MDSC were also protective in that they reduced both systemic inflammation and inflammation within adipose tissue. However, they also facilitated the accumulation of adipose tissue. Thus, obesity-driven MDSC simultaneously protect against some obesity-associated metabolic dysfunction, while enhancing obese conditions (Figure 2).
Figure 2.
Adipose tissue drives the accumulation of MDSC which protect against some obesity-induced metabolic dysfunction, but promote tumor progression. The chronic inflammatory milieu of adipose tissue induces the accumulation of PMN-MDSC and M-MDSC. The induced MDSC reduce elevated blood glucose levels insulin tolerance associated with obesity, but simultaneously increase the accumulation of fat cells. Obesity-induced MDSC have enhanced potency and promote tumor growth by suppressing tumor-reactive T cells and preventing the entry of activated T cells into the tumor microenvironment.
Leptin, a key adipokine that regulates obesity, also impacts the immune system
In addition to its impact on appetite, leptin also effects the immune system since many immune cells, including CD4+ and CD8+ T cells, B cells, macrophages, and monocytes express the Ob-R [28]. Because there are multiple forms of the Ob-R, leptin signaling has been attributed to multiple signal transducers, including JAK2, STAT3, and the Akt/PI3K, mTOR, and p38 MAPK pathways. A detailed description of the signaling pathways can be found in [34].
Leptin mediates several immunologically beneficial effects. It confers mouse T and B cell resistance to FAS-mediated apoptosis and increases T cell proliferation via STAT3 activation [35]. The impaired cell-mediated immunity that accompanies starvation is due to the decrease in leptin since administration of leptin to starved mice restores T cell proliferation and cell-mediated immune responses. Leptin mediates these effects by stimulating the production of IFNγ and limiting production of IL-4 [36]. Leptin also impacts NK cell development. Immature and mature NK cells in the bone marrow of Ob-R-deficient (db/db) mice have an increased rate of apoptosis, resulting in abnormally low levels of NK cells. Treatment of wild type NK cells with leptin enhanced NK cell survival, indicating that leptin acts in the bone marrow to facilitate NK cell development and survival [37]. Macrophage phagocytic activity is also enhanced by leptin since mouse peritoneal macrophages displayed enhanced phagocytic activity for L. major promastigotes following treatment with leptin [38].
Human studies have confirmed mouse findings, and have provided additional functional information about leptin. For example, leptin activates STAT3 signaling and up-regulates production of IL-1β, IL-6, IL-12, TNFα, and MIP1α by human dendritic cells (DC), resulting in more potent antigen presenting cells with increased resistance to apoptosis [39]. Administration of leptin to morbidly obese, leptin-deficient children decreased body mass, and restored levels of circulating CD4+ T cells. Leptin therapy also increased serum levels of IFNγ which prior to treatment were undetectable, and decreased to normal levels the immune suppressive cytokine TGFβ [40].
Leptin also reduces T regulatory cells (Tregs). Both leptin-deficient and leptin receptor deficient mice have elevated levels of Foxp3+CD4+CD25+ Tregs, and human naturally occurring Tregs have high levels of Ob-R, produce leptin, and are resistant to anti-CD3 plus anti-CD28-induced activation. If neutralizing antibodies to leptin are included during anti-CD3 and anti-CD28 treatment, then Tregs proliferate, express higher levels of Foxp3, and maintain their suppressive phenotype [41].
MDSC and leptin regulate each other through cross-talk
To determine if leptin impacts MDSC, HFD mice were treated with a soluble form of the leptin receptor (Ob-Fc), thereby blocking leptin receptor signaling. Circulating MDSC levels were significantly reduced, indicating that leptin directly, or indirectly, drives the accumulation of MDSC. MDSC reciprocate and down-regulate serum levels of leptin, since depletion of MDSC in HFD mice increased blood levels of leptin (Figure 3A). Therefore, MDSC and leptin undergo cross-talk in which leptin increases MDSC levels while MDSC decrease leptin levels [27].
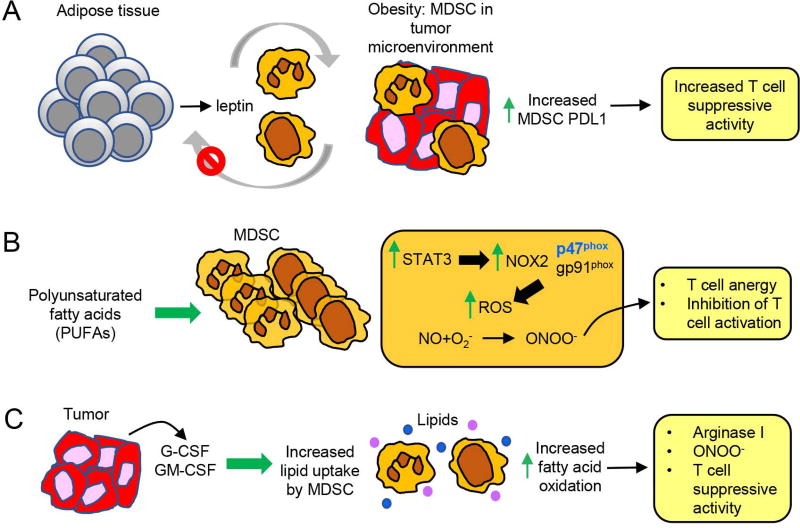
Figure 3.
Obesity-associated molecules increase the accumulation and potency of MDSC through at least three independent mechanisms. (a) Chronic inflammation in adipose tissue induces the over-production of leptin which drives the accumulation of MDSC in blood and in solid tumor. Elevated levels of MDSC decrease leptin production resulting in feedback, feed-forward regulation of both leptin and MDSC. Leptin-induced MDSC in the tumor microenvironment have elevated expression of PDL1, which may contribute to their increased suppressive activity. (b) PUFAs increase the differentiation of MDSC from bone marrow progenitor cells and enhance MDSC suppressive activity by activating STAT3 which increases the p47 subunit of NOX2. ROS are upregulated resulting in an increase in peroxynitrite which subsequently causes anergy of activated T cells and impairs activation of naïve T cells. (c) G-CSF and GM-CSF generated within the tumor microenvironment increase PMN-MDSC and M-MDSC uptake of lipids by upregulating MDSC synthesis of the lipid receptors CD36 and Msr1. Fatty acid oxidative enzymes are also upregulated in the MDSC, resulting in increased suppressive activity due to increased arginase I and peroxynitrite.
One of the pro-inflammatory mediators induced by leptin is IFNγ [36]. IFNγ is an established regulator of programmed death ligand 1 (PD-L1), a ligand for the checkpoint inhibitor PD-1 which causes T cell apoptosis and anergy [42]. The increased suppressive activity of HFD-induced MDSC has been partially attributed to increased PD-L1 expression [27], leading to the hypothesis that leptin may facilitate MDSC function by driving the production of IFNγ, which, in turn, increases MDSC expression of PD-L1.
MDSC accumulation and suppressive potency are facilitated by fatty acid metabolism
Obesity is frequently associated with an abundance of dietary fat, and recent studies have demonstrated that MDSC enhance their accumulation and/or suppressive activity through the metabolism of fatty acids and lipids. Polyunsaturated fatty acids (PUFAs), such as the omega-3 fatty acids, reduce the risk of cardiovascular disease (reviewed in [43]), and may also reduce cancer risk and limit tumor progression, although the cancer effects are controversial [44]. However, PUFAs also have immune suppressive effects which may be beneficial for individuals with autoimmune and inflammatory conditions, but potentially detrimental for cancer patients. In vitro and in vivo tumor studies have shown that PUFAs increase MDSC suppressive potency and the rate of MDSC differentiation from mouse bone marrow progenitor cells. PUFAs mediate their effect through JAK-STAT3 signaling which increases MDSC NADPH oxidase subunit p47(phox), a critical regulator of MDSC production of ROS [45] (Figure 3B).
Other studies have shown that in contrast to effector T cells and M1 macrophages, which use glycolysis, MDSC use fatty acid oxidation as their energy supply [46]. Studies in mice demonstrated that tumor-infiltrating MDSC (TI-MDSC) are particularly fatty acid dependent. The tumor microenvironment increases MDSC oxygen consumption and the biogenesis of mitochondria, and upregulates MDSC expression of enzymes critical for fatty acid oxidation. Human peripheral blood and TI-MDSC display a similar dependence on fatty acid metabolism. In vivo mouse studies demonstrated that inhibition of fatty acid oxidation increases the antitumor efficacy of adoptive T cell therapy, especially when combined with low-dose chemotherapy. Subsequent studies revealed that tumor cells enhance lipid metabolism by TI-MDSC through their production of G-CSF and GM-CSF. These cytokines induce signaling through STAT3 and STAT5 which increases TI-MDSC synthesis of lipid transport receptors resulting in an enhanced uptake of tumor-localized lipids. The increased lipid metabolism not only raises the level of MDSC, but also enhances the ability of MDSC to suppress T cell activation [47] (Figure 3C).
Conclusions
MDSC were originally identified as immune suppressive cells that were induced by tumor-secreted factors and promoted tumor progression. It is now apparent that MDSC are also involved in other conditions including autoimmunity, infection, stress, and aging, as well as obesity. In contrast to tumor-induced MDSC that are exclusively detrimental to the host, obesity-induced MDSC are at least partially beneficial to the host because they protect against some of the metabolic dysfunction associated with obesity. Recent studies have demonstrated that MDSC are also beneficial to the host during pregnancy, since they facilitate implantation and protect the allogeneic early embryo from immune-mediated rejection [48]. They may also play a moderating role in autoimmunity by down-regulating both cellular and antibody-mediated anti-self responses [49]. Given these beneficial effects, MDSC may have evolved to protect the host against metabolic and immune dysfunction and to facilitate reproduction. However, as they have done for other cell populations such as macrophages, dendritic cells, and T regulatory cells, tumors have co-opted MDSC to promote their own growth.
Inflammation is the unifying condition that drives the accumulation of MDSC. Cancer, autoimmunity, infection, and obesity typically involve overlapping pro-inflammatory mediators, so it is not unexpected that MDSC should accumulate in obese individuals. What is unusual about MDSC in obesity is that they are simultaneously protective and detrimental. Since obese individuals have an increased risk of developing cancer and their tumors progress more rapidly, the pro-tumor effects of MDSC likely out-weigh the protective effects. MDSC are known to use a variety of disparate mechanisms to promote tumor growth. Studies of MDSC in obesity are at an early stage, and at present it is not understood how MDSC reduce glucose levels or protect against insulin tolerance. If MDSC use different mechanisms to deter metabolic dysfunction and promote tumor progression, then the beneficial effects of MDSC could be harnessed while eliminating MDSC tumor-promoting activity.
Highlights.
MDSC promote tumor growth by blocking antitumor immunity and cancer immunotherapies
Obesity promotes tumor growth by inducing the accumulation of MDSC
MDSC protect against some of the metabolic dysfunction associated with obesity
MDSC accumulation and function are driven by leptin and fatty acid metabolism
Acknowledgments
This work was supported by the National Institutes of Health (grant numbers RO1CA115880, RO1CA84232, and RO1GM021248).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Netherby CS, Abrams SI. Mechanisms overseeing myeloid-derived suppressor cell production in neoplastic disease. Cancer Immunol Immunother. 2017;66:989–996. doi: 10.1007/s00262-017-1963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **4.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. This group of leaders in the field provide a unifying nomenclature, functional characterization, and phenotypic characterization for the identification of mouse and hman MDSC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer Immunol Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donohoe CL, Lysaght J, O'Sullivan J, Reynolds JV. Emerging Concepts Linking Obesity with the Hallmarks of Cancer. Trends Endocrinol Metab. 2016 doi: 10.1016/j.tem.2016.08.004. [DOI] [PubMed] [Google Scholar]
- *7.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. This early publication is frequently cited for its compelling clinical epidemiological data demonstrating the linkage between obesity and cancer. [DOI] [PubMed] [Google Scholar]
- 8.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 9.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 10.Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, Inflammation, and Cancer. Annu Rev Pathol. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- *11.Font-Burgada J, Sun B, Karin M. Obesity and Cancer: The Oil that Feeds the Flame. Cell Metab. 2016;23:48–62. doi: 10.1016/j.cmet.2015.12.015. Using examples from liver, pancreatic, and gastrointestinal tract cancer, this review article summarizes the current knowledge of how hypernutrition induces local and systemic inflammation that leads to incrased cancer risk and enhanced tumor progression. [DOI] [PubMed] [Google Scholar]
- 12.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annu Rev Med. 2015;66:297–309. doi: 10.1146/annurev-med-050913-022228. [DOI] [PubMed] [Google Scholar]
- 13.Kolb R, Sutterwala FS, Zhang W. Obesity and cancer: inflammation bridges the two. Curr Opin Pharmacol. 2016;29:77–89. doi: 10.1016/j.coph.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W. Inflammasomes in cancer: a double-edged sword. Protein & cell. 2014;5:12–20. doi: 10.1007/s13238-013-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1 beta mediates leptin induction during inflammation. Amer J Physiol. 1998;274:R204–208. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- 16.Fazolini NP, Cruz AL, Werneck MB, Viola JP, Maya-Monteiro CM, Bozza PT. Leptin activation of mTOR pathway in intestinal epithelial cell triggers lipid droplet formation, cytokine production and increased cell proliferation. Cell cycle. 2015;14:2667–2676. doi: 10.1080/15384101.2015.1041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 20.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song X, Krelin Y, Dvorkin T, Bjorkdahl O, Segal S, Dinarello CA, Voronov E, Apte RN. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J Immunol. 2005;175:8200–8208. doi: 10.4049/jimmunol.175.12.8200. [DOI] [PubMed] [Google Scholar]
- 22.Sade-Feldman M, Kanterman J, Ish-Shalom E, Elnekave M, Horwitz E, Baniyash M. Tumor necrosis factor-alpha blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity. 2013;38:541–554. doi: 10.1016/j.immuni.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S, Akbar SM, Miyake T, Abe M, Al-Mahtab M, Furukawa S, Bunzo M, Hiasa Y, Onji M. Diminished immune response to vaccinations in obesity: role of myeloid-derived suppressor and other myeloid cells. Obes Res Clin Pract. 2015;9:35–44. doi: 10.1016/j.orcp.2013.12.006. [DOI] [PubMed] [Google Scholar]
- *26.Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem. 2011;286:23591–23599. doi: 10.1074/jbc.M111.237123. Using genetically obese Ob/Ob mice and diet-induced obese mice, the authors demonstrate that obesity induces the accumulation of MDSC in the circulation and in adipose tissue. This is the seminal study demonstrating that obesity drives MDSC accumulation and function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **27.Clements VK, Long T, Long R, Figley C, Smith D, Ostrand-Rosenberg S. High fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells. J Leukoc Biol. 2018 doi: 10.1002/JLB.4HI0517-210R. in press. Using mice fed a high fat diet the authors demonstrate that leptin drives the accumulation of immune suppressive MDSC that promote tumor progression but also provide some protection against the metabolic dysfunction that accompanies obesity. This is the seminal paper demonstrating that obesity-induced MDSC are one of the mechanisms by which obesity promotes tumor progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conde J, Scotece M, Abella V, Lopez V, Pino J, Gomez-Reino JJ, Gualillo O. An update on leptin as immunomodulator. Exp Rev Clin Oncol. 2014;10:1165–1170. doi: 10.1586/1744666X.2014.942289. [DOI] [PubMed] [Google Scholar]
- 29.Hale M, Itani F, Buchta CM, Wald G, Bing M, Norian LA. Obesity triggers enhanced MDSC accumulation in murine renal tumors via elevated local production of CCL2. PLoS One. 2015;10:e0118784. doi: 10.1371/journal.pone.0118784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Bao Y, Mo J, Ruan L, Li G. Increased monocytic CD14(+)HLADRlow/− myeloid-derived suppressor cells in obesity. Mol Med Rep. 2015;11:2322–2328. doi: 10.3892/mmr.2014.2927. This is the first study that demonstrates that similar to mice, obesity drives the accumulation of MDSC in humans. [DOI] [PubMed] [Google Scholar]
- 31.Ezernitchi AV, Vaknin I, Cohen-Daniel L, Levy O, Manaster E, Halabi A, Pikarsky E, Shapira L, Baniyash M. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J Immunol. 2006;177:4763–4772. doi: 10.4049/jimmunol.177.7.4763. [DOI] [PubMed] [Google Scholar]
- 32.Berger NA. Obesity and cancer pathogenesis. Ann N Y Acad Sci. 2014;1311:57–76. doi: 10.1111/nyas.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, Ng MR, Nia HT, Grahovac J, Kao S, et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016;6:852–869. doi: 10.1158/2159-8290.CD-15-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naylor C, Petri WA., Jr Leptin Regulation of Immune Responses. Trends Molec Med. 2016;22:88–98. doi: 10.1016/j.molmed.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–7752. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]
- 36.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 37.Lo CK, Lam QL, Yang M, Ko KH, Sun L, Ma R, Wang S, Xu H, Tam S, Wu CY, et al. Leptin signaling protects NK cells from apoptosis during development in mouse bone marrow. Cell Mol Immunol. 2009;6:353–360. doi: 10.1038/cmi.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gainsford T, Willson TA, Metcalf D, Handman E, McFarlane C, Ng A, Nicola NA, Alexander WS, Hilton DJ. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci U S A. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–6828. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- 40.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Rosa V, Procaccini C, Cali G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe Y, Tatsuno I. Omega-3 polyunsaturated fatty acids for cardiovascular diseases: present, past and future. Exp Rev Clin Pharmacol. 2017;10:865–873. doi: 10.1080/17512433.2017.1333902. [DOI] [PubMed] [Google Scholar]
- 44.Lenihan-Geels G, Bishop KS, Ferguson LR. Cancer Risk and Eicosanoid Production: Interaction between the Protective Effect of Long Chain Omega-3 Polyunsaturated Fatty Acid Intake and Genotype. J Clin Med. 2016;5 doi: 10.3390/jcm5020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **45.Yan D, Yang Q, Shi M, Zhong L, Wu C, Meng T, Yin H, Zhou J. Polyunsaturated fatty acids promote the expansion of myeloid-derived suppressor cells by activating the JAK/STAT3 pathway. Eur J Immunol. 2013;43:2943–2955. doi: 10.1002/eji.201343472. A high fat diet consisting of polyunsaturated fatty acids (PUFAs) induces mice to accumulate high levels of MDSC with increased immune suppressive activity. This is the first publication demonstrating that fatty acid metabolism is an independent inducer of MDSC. [DOI] [PubMed] [Google Scholar]
- **46.Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T, Zou W, et al. Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer Immunol Res. 2015;3:1236–1247. doi: 10.1158/2326-6066.CIR-15-0036. Using multiple mouse tumor models, the authors demonstrate that tumor-infiltrating MDSC increase their suppressive potency by enhanced fatty acid uptake and activation of fatty acid oxidation. These findings are confirmed in human MDSC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Khami AA, Zheng L, Del Valle L, Hossain F, Wyczechowska D, Zabaleta J, Sanchez MD, Dean MJ, Rodriguez PC, Ochoa AC. Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells. Oncoimmunology. 2017;6:e1344804. doi: 10.1080/2162402X.2017.1344804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostrand-Rosenberg S, Sinha P, Figley C, Long R, Park D, Carter D, Clements VK. Frontline Science: Myeloid-derived suppressor cells (MDSCs) facilitate maternal-fetal tolerance in mice. J Leukoc Biol. 2017;101:1091–1101. doi: 10.1189/jlb.1HI1016-306RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boros P, Ochando J, Zeher M. Myeloid derived suppressor cells and autoimmunity. Human Immunol. 2016;77:631–636. doi: 10.1016/j.humimm.2016.05.024. [DOI] [PubMed] [Google Scholar]