Abstract
Most dermatologists are aware of the benefits of dermoscopy, and a few are familiar with laser-scanning confocal microscopy. Beyond confocal, there are fully 11 different categories of optical techniques that have been applied to clinical dermatology. This article first provides a comprehensive tabular overview of all these optical diagnostic technologies and then details 4 of the lesser known innovations that are already available or still in development (laser Doppler and speckle imaging, Raman spectroscopy, multiphoton microscopy, photoacoustic tomography), with some potential applications in clinical dermatology (blood flow monitoring, skin cancer diagnosis, composition measurements in atopic dermatitis, skin rejuvenation measurement, and noninvasive sentinel lymph node assessment in melanoma). These methods present many advantages, being non-invasive, portable, and rapid. The development of optics in biological and biomedical sciences (i.e. biophotonics) requires not only deep insight into the applications but also synergistic collaboration between engineers and clinicians.
Keywords: non-invasive imaging, laser Doppler and speckle imaging, Raman spectroscopy, multiphoton microscopy, photoacoustic tomography, melanoma
Optical instrumentation for imaging is an important component of biological and medical science progress. The range of applications of biomedical optics devices is very broad, from medical diagnostics, monitoring treatment to clinical research (1). Medical imaging has already dramatically transformed the practice of medicine in a variety of clinical settings. Optical methods and devices are indeed advantageous, because they are non-invasive, portable, relatively inexpensive, and they can provide rapid information to the physician (2).
In the field of dermatology, non-invasive imaging technologies are particularly useful for skin disease diagnosis and monitoring, including skin cancer detection. This is especially the case when cutaneous conditions present ambiguous features, leading to delays in treatment. Many optical imaging methodologies are currently developed to improve the diagnosis of a variety of skin conditions without the need for biopsy, which remains the gold standard for skin neoplasms (3–5). Patient burden would be greatly reduced if biopsy could be avoided for even a subset of benign lesions through reliable non-invasive optical tools. Recent breakthroughs in morphological and physiological diagnostic methods enable imaging with higher resolution and functional data. Advances in imaging devices (6) such as dermoscopy, ultrasound, optical coherence tomography, and most notably reflectance confocal microscopy (7) have allowed these techniques to be applied to skin neoplasms and inflammatory disease management. Other dermatologic applications include assessment of moisture, burn depth, wound healing, UV damage and atrophy. In fact, no fewer than 11 fundamentally different optical technologies have been applied to dermatology patients. For the reader, a convenient and annotated overview table is provided in Table I for reference of all of these different techniques.
Table I.
2016 overview of available and novel dermatologic non-invasive imaging devices
| Fundamental technique and synonyms or variations | Most likely user, Examples of CE devices (bold for FDA-approved), and price range | Clinical uses and highest quality in vivo clinical trial result | Features typically visualized and imaging time | Advantages & unique technologic capabilities | Limitations of currently available devices | Technological developments and anticipations |
|---|---|---|---|---|---|---|
| Polarization techniques (dermoscopy, polarimetry) | All dermatologists, DermLite (3Gen), EpiScope (Welch Allyn), NevoScope (TransLite), Dermascope (American Diagnostic Corp), MoleMax (Derma Medical Systems), DermoGenius (Dermoscan), handyscope (Fotofinder), Canfield; $0.1k to $2k | Assistance of dermatologic physical exam, especially for cancer screening; melanoma vs. benign naevi sens 89%, spec 84%a | Modestly magnified subsurface morphology including vessels; melanin distribution and other skin cancer features; instantaneous images | Rapid skin cancer screening; wide base of experienced dermoscopy users; significant improvement in sensitivity and specificity relative to unaided clinical exam; devices do not require FDA approval (Class I) | Added value highly user- and training-dependent; low resolution images; top view image (no cross- sectional images at depth) | Mobile phone mounts and apps; advanced polarimetry techniques will extend possibilities, e.g. automatic evaluation of average nuclear morphology or tissue heterogeneity; www.dermoscopyids.org |
| Total body digital photography (TBDP), regional imaging | Pigmented lesion experts, Dermspectra, Canfield, FotoFinder, Molemax, Molesafe, MoleMap, MelanoScan, Dermoscan, Visiomed; $10k to $250k | Monitoring melanocytic neoplasms in high risk pigmented lesion clinics, NMSC, and inflammatory diseases | Generally same features as clinical exam; 10 min for total body | Rapidly acquire and monitor large portion of skin surface; computer algorithms help track changes and suspicious features | Challenging to rapidly present and interpret resulting large data set in clinical setting | Increasing number of commercial devices with automated image acquisition; comprehensive resource at http://isdis.net/imaging-modalities/total-body-photography/ |
| Confocal microscopy (LSCM, CSLM, RCM) | All dermatologists willing to invest in necessary training, six category 1 CPT reimbursement codes; Vivascope (Caliber ID and Mavig, formerly Lucid), Stratum (Optiscan); $100k | Identify diverse lesions for which biopsy can be avoided; preoperative mapping of malignancies including lentigo maligna for reduced surgical defects; melanoma vs benign nevi sens 97%, spec 83%a diagnosis of equivocal lesions vs BCC sens 100%, spec 89%b | Microscopic structures as in H&E but only in horizontal (en face) sections; 25 min for 6 × 6 mm image stack (including prep time described in CPT 96932) | Highest accuracy; only imaging technology with Medicare reimbursement; video-rate single-lesion, histology-grade (<1 μm) resolution of cellular components based on scattered light; able to view dendrites on melanocytes (unachievable with standard H&E) | En face views best interpreted by experienced confocalist; difficult to detect invasion through dermal-epidermal junction and other depth-resolved features such as melanoma stage or HAK vs SCC; unable to image beneath papillary dermis (limited to 0.25 mm depth) | Intraoperative use, e.g. coupled to laser ablation; combination with fluorescent techniques; working group at http://www.confocal-icwg.com/ |
| Spectral (multispectral, hyperspectral, RGB, infrared thermography) imaging | Few dermatologists, category 3 CPT codes for research use; MelaFind (Melasciences) $30k; SIAscope (MedX) with SIMSYS or MoleMate software $6k - $8k; Dermlite II MS (3gen) $1k; TiVi (WheelsBridge) $20k | Help triage pigmented lesions for biopsy; for melanoma vs nevus, Melafind sens 98.3%, spec 9.9%c whereas SIAscope sens 80%, spec 76%d; clinical research with TiVi | Macroscopic views of erythema and blanching, oxy- & deoxyhaemoglobin and melanin; Siascope 5s for single 11 × 11 mm image; Melafind 45s for single image up to 22 × 22 mm; TiVi 30fps wide field or single lesion | Mapping of some chemical components through entire thickness of skin (to 2.5 mm deep) based on light collection at numerous frequencies; often combined with polarization technique | Large data set interpretation highly dependent on training set that computer algorithms use; top view image (no cross-sectional images at depth) | Research needed correlating spectral properties of skin to disease; handheld spectral polarization camera probes operating on tablets |
| Optical coherence tomography (low coherence interferometry, FF-OCT, GD-OCT) | Few academic dermatologists, Vivosight (Michelson Diagnostics), Light-CT (LLTech), Skintell (Agfa), Nitid (DermaLumics), SkinDex300 (ISIS Optronics); $130-$180k | Depth demarcation and reduction of presurgical biopsy rate for BCCs; dynamic blood flow imaging; as adjunct to expert dermoscopy exam, sens not significantly improved, but spec for BCC improved from 54% to 75%e | Macroscopic structures (e.g. blood vessels, DEJ, BCC border); Vivosight 20s for 6 × 6 mm image stack; Skintell <2s for 1.8 × 1.5 × 1 mm 3D volume; Light-CT 1 min for 10 × 10 mm image | Optical analogue of ultrasound; images relatively deep in dermis (~1 mm), able to image flow with speckle variance or Doppler; images in same plane of view (vertical) as traditional H&E | Diagnostic accuracy limited by lateral resolution (Vivosight 8 μm, Skintell 3 μm with adaptive optics). FF-OCT overcomes this (Light-CT resolution 1 μm) but in excised tissue and limited to 0.2 mm depth | Intraoperative Mohs margins with FF-OCT; OCT elastography; molecular imaging; polarization-sensitive OCT; potential resolution improvement with Gabor domain liquid lens or Mirau interferometer |
| Interferometry (dynamic light scattering, laser Doppler flowmetry, LDPI, laser speckle imaging, LSPI, LSFG, LASCA, MESI) | Research centres, FluxExplorer (Microvascular Imaging), Moor, Perimed, Lisca | Skin grafts, vascular lesion treatment monitoring, patch test quantification, Raynaud’s scoring, scar evaluation; in detection of active morphea sens 80% spec 77% in single-centre trialf | Colour-coded perfusion image reflecting blood flow level or velocity; imaged area adjustable; 1s for 50 × 50 mm | Low cost, non-contact; rapidly evaluates blood flow over a large area (up to 500 × 500 mm) | Lower resolution (>100 μm) | Combination with OCT |
| Vibrational spectroscopy (Raman, FTIR) | Research centres, gen2-SCA (RiverD) $100k to $250k; Aura (Verisante) $65k | Determining skin hydration, antioxidant levels, and distribution of cosmetics and other topical treatments; diagnostically, benign (including SK) vs. malignant (including AK) lesions had sens 90–99%, spec 75–20% in single-centre trialg | Molecular composition and biochemical information; single point or depth-resolved spectra acquired in seconds but without yielding actual images | Quantitative measurements of many known compounds already available, e.g. carotenoid antioxidants, NMF, urea, lactate; theoretically any molecule will have unique Raman signature | Rapid high resolution volumetric imaging impractical as Raman effect (inelastic scattering) several orders of magnitude weaker than reflectance (elastic scattering) or fluorescence; spectra are difficult to interpret for unknown compounds | Research needed correlating Raman signatures to disease; more complex non-linear implementations (e.g. CARS, stimulated Raman) enable rapid imaging for some specific chemical signature lines |
| Fluorescence (autofluorescence lifetime imaging, photodynamic diagnosis, fluorescence videomicroscopy) | Research centres, SkinSpect (Spectral Molecular Imaging) | Presently early research phase; not used as single modality; primarily used to enhance confocal images, especially in perioperative imaging | Images of added or intrinsic fluorescent compounds | Fluorescent agents can improve contrast of other modalities; fluorescence lifetime measurements, when used, are sensitive to microenvironment of detected compounds | Little dermatologic clinical data; limited by width of fluorescence absorption and emission lines and few FDA-approved exogenous fluorescent compounds (fluorescein, indocyanine green, methylene blue) | Much R&D needed |
| Diffuse optics (spatial frequency domain imaging) | Research centres and wound care physicians, Ox-Imager (Modulated Imaging) $100k | Presently early research phase; preliminary data in wound monitoring, burn thickness assessment, and surgical flap viability prediction based on blood supply | Haemoglobin total concentration and oxygenation; optical properties of skin (scattering, absorption); 1s for two frequency scan, 25s for full scan | Non-contact imaging of large area (size adjustable up to 200 × 150 mm) | Little dermatologic clinical data; low resolution (>100 μm) | Much R&D needed |
| Nonlinear optical imaging (multiphoton, SHG, two photon fluorescent, coherent Raman, CARS, stimulated emission imaging) | Few research centres, DermaIinspect, MPTflex (JenLab) $400k | Presently early research phase; preliminary data for diagnosis of melanoma vs benign nevi sens 75%, spec 80%h | Similar features as corresponding linear modalities above; molecular composition and microscopic structures; few seconds for 0.35 × 0.35 mm image at single depth | High resolution (<1 μm) sensitive and label-free quantitative measurements of many intrinsic chemicals (such as collagen, NADH, pheo- and eumelanin) | High laser cost; current CE device has higher laser intensities, much slower imaging, and slightly worse depth (about 0.2 mm) than confocal | Much R&D needed; impressive basic science results in questions of immune cell interactions, stem cell trafficking, and metabolism (including redox ratios); more advanced setups including pump-probe dynamics under development |
| Photoacoustic imaging (optoacoustic tomography, photoacoustic microscopy) | Few research centres, inSight, MSOT Acuity (iThera) $150k–$350k | Presently early research phase; preliminary data for detection of melanoma mets in sentinel lymph nodes sens 100%, spec 49%i | High-contrast, absorptionbased images of melanin, oxy-, deoxyhemoglobin, lipids, and external dyes (such as indocyanine green); 5 min for 5 × 5 × 1.5 mm 3D volume at 8 μm resolution | Combines optical and ultrasound imaging; excellent melanin contrast (50x better than light microscopy); only method that can tune between extremely high resolution images (to 0.1 μm) and depth (> 10 mm), e.g. 4 μm resolution at 5 mm depth | Little dermatologic clinical data | Much R&D needed; early data suggests sensitive detection of melanoma metastases in circulation and lymph nodes; high resolution microvasculature assessment; pilosebaceous unit imaging |
Langley RG. Dermatology 2007; 215: 365–372 – (125 patients single centre).
Guitera P. J Invest Dermatol 2012; 132: 2386–2394 – (663 patients multicentre).
Monheit G. Arch Dermatol 2011; 147: 188-194 – (1,251 patients multicentre).
Tomatis S. Phys Med Biol 2005; 21; 50: 1675–1687– (1,278 patients single centre).
Ulrich M. Br J Dermatol 2015; 173: 428–435 – (250 patients multicentre).
Weibel L. et al. Arthritis Rheum 2007; 56: 3489–3495 – (111 lesions).
Zhao J. et al. Analyst 2016; 141: 1034–1043– (127 lesions tested).
Dimitrow E. et al. J Invest Dermatol 2009; 129: 1752–1758 – (53 lesions).
Stoffels I. et al. Sci Transl Med 2015; 7: 317ra199 – (41 sentinel lymph nodes from 20 patients).
CE: European certification; AK: actinic keratosis; BCC: basal cell carcinoma; CARS: coherent anti-stokes Raman scattering; CLSM: confocal laser scanning microscopy; CPT: current procedure terminology; DEJ: dermoepidermal junction; FF-OCT: full-field optical coherence tomography; FTIR: Fourier transform infrared spectroscopy; GD-OCT: Gabor domain optical coherence tomography; H&E: hematoxilin and eosin; HAK: hyperkeratotic actinic keratosis; LASCA: laser speckle contrast analysis; LSCM: laser scanning confocal microscopy; MESI: multi-exposure speckle imaging; MSOT: multispectral opto-acoustic tomography; NMF: natural moisturising factor; OCT: optical coherence tomography; R&D: research and development; RCM: reflectance confocal microscopy; SCC: squamous cell carcinoma; Sens: sensitivity; SHG: second harmonic generation; SK: seborrhoeic keratosis; Spe: specificity.
In addition to the overview provided in the Table I, this paper will review the potential applications of 4 optical devices and imaging techniques that are not widely known in clinical dermatology. Applications that will be discussed include blood flow monitoring with laser Doppler and speckle imaging, mapping chemicals and water in skin with Raman spectroscopy, visualization of the composition and structure of the skin in high-resolution by multiphoton microscopy, and deep imaging of melanin by photo-acoustic tomography. Considerations for selecting the most appropriate existing or emerging imaging technique for evaluating specific cutaneous conditions will also be presented.
BLOOD FLOW MONITORING WITH LASER DOPPLER AND SPECKLE IMAGING
Two techniques, laser Doppler and laser speckle imaging, can be used for blood flow mapping and imaging in the skin (8). Laser Doppler velocimetry measures velocity of blood flow by using the frequency shift induced by the Doppler effect. It has already been used in many medical and surgical situations to monitor blood flow or other tissue movements in the body. Laser speckle is a random interference effect that gives a granular aspect to objects illuminated by laser light. For individual moving scatterers (such as blood cells), the speckle pattern waves lead to so-called time-varying laser speckle. These fluctuations reflect the velocity distribution of the scattering particles. Both techniques perform measurements at a single point and in this regime can be shown to be mathematically equivalent results of interference. The single point imaging drawback can be overcome to measure blood flow in real time by coupling the technique with scanning of the area (8). With the speckle technique it is also possible to apply a full-field technique that gives an instantaneous map of velocities in real time. In dermatology, blood flow imaging can be used to evaluate inflammation and erythema, by measuring quantitatively velocity and haemoglobin content in capillaries of the skin. Numerous autoimmune, drug-induced, genetic, and inflammatory skin diseases and even scars can benefit from such assessment. Herein we will discuss two examples: patch test quantification used in the diagnosis of contact dermatitis, and monitoring the efficacy of vascular lesion treatments.
Quantification of irritation after patch testing
In medical practice, blood flow measurement can be applied to patch testing, which is used to diagnose contact allergy. Indeed this in vivo test aims to mimic allergic contact dermatitis by reproducing the triggering step of the reaction to a contact allergen (9). The methodology is simple and consists in applying allergens under occlusion on the skin under standardized conditions. However it requires adequate training for the results to be correctly interpreted and used, especially when a too weakly positive reaction appears. In this case, an objective and user-independent assessment of the test reaction can be obtained using blood flow measurement by either laser Doppler or speckle imaging. Both technologies allow quantifying the amount of inflammation at each test site (10), thereby driving dermatologists’ final decision (Fig. 1).
Fig. 1. Laser doppler imaging of blood flow in a patch test.
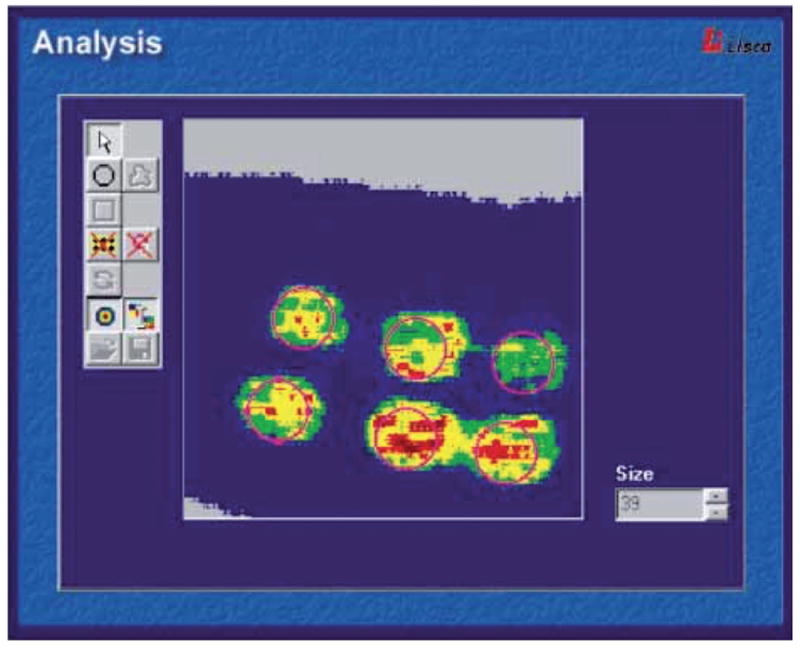
Printed with permission from Fullerton et al. (2002) (10). The perfusion image can be analysed by an integrated system software. The relative colour scale extends from the smallest and the largest perfusion value (from green to red). (https://www.perimed-instruments.com/skin-patch-testing).
Measurement of port wine stain treatment efficacy
For cosmetic procedures, laser Doppler and speckle imaging can also be used to evaluate the performance of skin treatment by laser therapy, with the aim to improve its efficacy (2). In particular, in the treatment of port wine stain birthmarks, the efficacy of laser therapy is limited, with only 10% of cases resulting in complete disappearance of redness after ten laser sessions (11). One factor is that the procedure relies on the subjective impression and overall experience of the clinician. Therefore Doppler/speckle imaging may serve as a metric of the degree of photocoagulation after laser surgery of port wine stain, and will identify areas with persistent perfusion that are not visible to the naked eye. The Speckle Flow Index (SFI), obtained with laser speckle imaging instrumentation by converting raw speckle reflectance images to speckle contrast images, is a numerical value proportional to the degree of scatterer motion and is higher in feeding vessel regions (Fig. 2). This indicator of microvasculature provides real-time, quantitative feedback during laser surgery, enabling the clinician to go back to the persistent perfusion areas until complete blanching is reached. Both laser Doppler and speckle imaging are innovative and individualized methods that could reduce the number of laser treatment sessions, hence optimizing results and diminishing the cost and potential risks associated with general anaesthesia for this intervention.
Fig. 2. Laser Speckle Imaging (LSI) of a Caucasian female patient with a port wine stain involving the V2 dermatomal distribution.
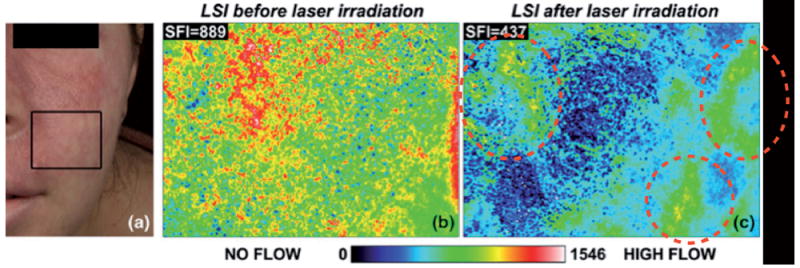
Printed with permission from Huang Y.C. et al. (2008) (11). (a) Photograph. (b) Speckle Flow Index images taken from the marked region of interest immediately before and (c) 15 min after laser therapy. Colour range indicates the level of blood flow in this area.
Overall, these approaches are low cost technologies and offer a non-contact method of mapping flow fields such as capillary blood flow, avoiding skin blanching by pressure which is a common drawback of measurement devices requiring skin contact (2). Meanwhile they present two main limitations: a low spatial resolution and a relative measurement of the blood flow only at a top surface view. Another potential limitation of laser speckle imaging is that the SFI values also are dependent on tissue optical properties. Some alternatives to improve resolution and sensitivity are to combine these techniques of visualization of tissue function, with optical coherence tomography or diffuse optic techniques, such as spatial frequency domain imaging (SFDI), which also enable visualization of changes in skin structure (see references in Table I, Overview of optical imaging techniques).
Laser Doppler imaging is on the market and has been for some time, whereas laser speckle contrast imaging is still used mostly in the research setting. It may be worth further developing laser speckle technique for commercialization, because of 3 main advantages over laser Doppler imaging: lower cost, truly real-time operation enabling movies of perfusion changes (8) and potential ease of integration into current laser systems (11).
MAPPING CHEMICALS AND WATER IN SKIN WITH RAMAN SPECTROSCOPY
In clinical dermatology research, it is important to evaluate the efficiency of topical therapeutic interventions and decipher the mechanism underlying their effect, how they distribute and at which concentration in the skin. For this purpose, it is necessary to quantify and follow the mapping of chemicals or biological compounds in the outermost layers of skin. However, most approaches that are able to provide direct information about skin components or the depth profiles of water content, such as tape-stripping and micro-dialysis, are invasive and/or time-consuming. Raman spectroscopy by contrast can identify skin constituents and provide spatially resolved molecular information under in vivo conditions in a non-invasive and rapid manner. It is based on depth-resolved vibrational spectra of molecules at optical wavelengths and can monitor conformational changes in lipids and proteins of the skin. Thus, Raman is accepted as a reliable method to quickly and noninvasively measure skin hydration, retinol, lactate and many other molecules (12, 13).
Natural moisturising factor content measurement in atopic dermatitis
Natural moisturising factor (NMF) is a breakdown product of filaggrin (encoded by the gene FLG) consisting mainly of a range of hydroscopic amino acids (14). It has been shown that FLG mutations are a major predisposing factor for atopic dermatitis (AD), with approximately 50% of moderate-to-severe AD cases harbouring filaggrin null-alleles (15). These mutations have a clear permissive effect in the early inflammatory phenomena that characterize eczema and affect both priming of disease and chronicity. Treating newborns with filaggrin mutations and low NMF levels with daily full-body emollient therapy was found to reduce the incidence of AD at 6 months of age by 50% (16). The early follow-up of NMF levels might therefore allow timely prophylactic treatment and appropriate surveillance.
Raman spectra can provide specific signatures of NMF levels that predict FLG mutation status, overcoming the need for the more technically demanding genotyping or invasive investigation. This technique accurately predicted newborn infants’ filaggrin genotype with 98–96% sensitivity and 87–67% specificity (Fig.3). Raman spectroscopy could also be applied to give fast and quantitative information before and after emollient application.
Fig. 3.
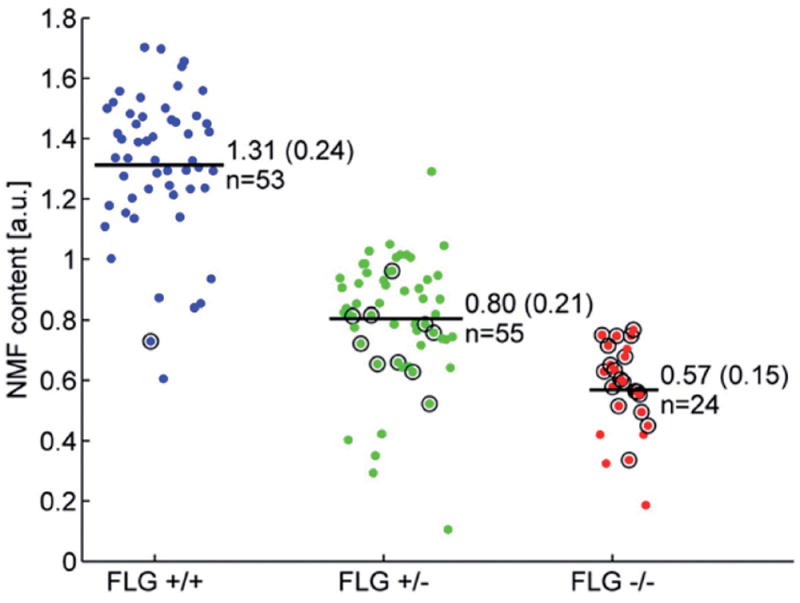
Cloud plot of natural moisturising factor (NMF) values in newborns, obtained from Raman spectra and categorized by filaggrin (FLG) genotype (final genotype after full screening: FLG+/+, FLG+/–, FLG−/−). From O’Regan GM et al. (2010) (15). For each group, the number of patients and NMF level (mean ± SD) are indicated in the figure. a.u.: arbitrary units.
Nonlinear Raman imaging of topical drug delivery
A wide range of research applications for in vivo Raman spectroscopy is now available (13). However, unlike other techniques that produce images, Raman is at present mainly used for single point measurement. One significant limitation is that the weak Raman effect gives insufficient signal strength to quickly acquire an image. This drawback can be overcome in the future through nonlinear implementations. In particular, multiplex stimulated Raman scattering microscopy may provide rapid dynamic imaging at video rate to monitor delivery and diffusion of topical agents, such as sunscreens, moisturizers, tretinoin and metronidazole, through skin tissue in vivo (17). As most chemicals of interest have known Raman spectra, nonlinear Raman technology should enable real-time ascertainment of their distribution without labelling and at the cellular scale in the skin.
HIGH-RESOLUTION COMPOSITION AND STRUCTURE OF SKIN WITH MULTIPHOTON MICROSCOPY
Multiphoton microscopy (MPM) is a femtosecond laser scanning microscopy technique based on nonlinear light-matter interactions to produce 3-dimensional (3D) images with submicron resolution (18). The most important signals are two-photon excited autofluorescence (AF) and second harmonic generation (SHG). By this way, images of endogenous biomolecules within tissue can be obtained without using specific fluorescent labels. Indeed, autofluorescence resulting from the distribution of endogenous fluorophores in tissue yields structural and biochemical information without fixation or staining procedures. The main compounds present in skin with two-photon autofluorescence are reduced nicotinamide adenine dinucleotide (NADH), flavine adenine dinucleotide (FAD), keratin, melanin, collagen, and elastin fibres (Fig. 4). Furthermore, SHG is able to visualize collagen fibres in the dermis. Therefore, MPM provides non-invasive in vivo imaging of the epidermis and superficial dermis, providing useful morphological and label-free molecular information (18).
Fig. 4. In vivo MPM imaging of normal human skin.
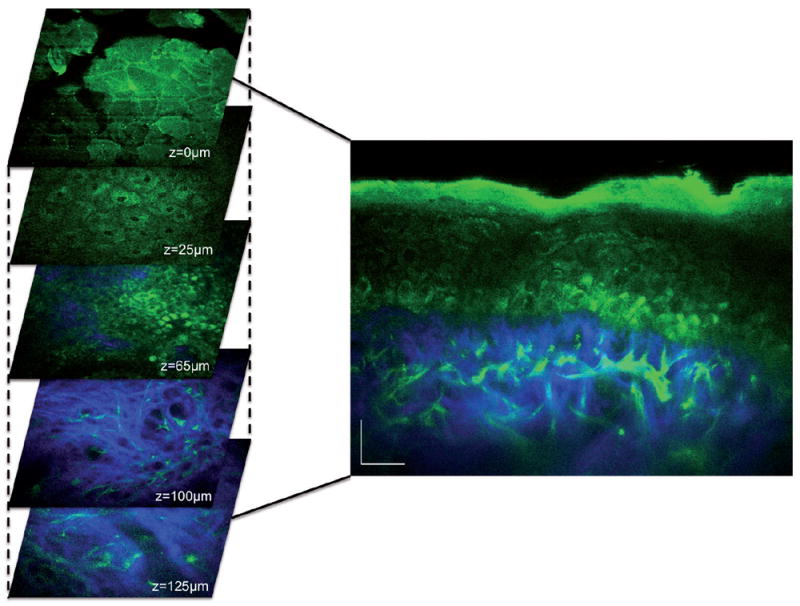
Left, horizontal sections of MPM images (x–y scans) at different depths showing images of: the stratum corneum (z = 0 μm), keratinocytes normally distributed in the stratum spinosum (z=25 μm), basal cells (green) surrounding dermal papilla (blue; z=65 μm), collagen (blue) and elastin fibers (green) in the dermis (z=100 μm;). Right, cross-sectional view (x–z scan) corresponding to a vertical plane through the horizontal sections on the left. Scale bar is 20 μm. Image kindly provided by Dr Mihaela Balu from University of California, Irvine/Beckman Laser Institute.
Diagnosis of skin lesions by multiphoton microscopy
One potential MPM application is melanoma, for which new and better treatments are now available but are also very expensive (as much as 36,000 USD/patient/month or more depending on the treatment (19)). In addition, melanoma incidence has been increasing for 30 years and is responsible for around 72% of skin cancer deaths in the USA (20). Early detection and accurate diagnosis of melanoma is therefore critical for a good prognosis and appropriate treatment. Current standard diagnosis is based on invasive biopsy and histopathologic examination. In addition, significant among pathologists has been often documented for the classification of melanocytic neoplasms (21), resulting in meaningful change in clinical management in about 18% of cases (22). This observation has highlighted the urgent need to find more consistent skin tumour staging parameters to improve the accuracy of diagnosis decisions by dermatologists.
A few groups are exploring the ability of MPM to provide qualitative and quantitative information for diagnosis of skin lesions. In a small pilot clinical study, Balu and colleagues (18) derived a quantitative algorithm to distinguish multiphoton features of melanocytic naevi at different stages. The authors managed to derive a numerical multiphoton melanoma index (MMI), combining 3 main criteria, the melanocyte dendrite density, SHG signal strength (from collagen) and the autofluorescence, which was able to distinguish benign from atypical naevi or melanoma.
Studies in larger patient populations are still necessary to validate the diagnostic performance of MPM for both melanocytic and non-melanocytic tumours (23). Current MPM are unfortunately not suitable for widespread clinical use due to several limitations. Practical limitations include firstly an extraordinary high cost (> 400 000 EUR, quotation obtained by the author of this article in 2015 from Dermainspect), which is partially driven by the expense of the required femtosecond laser and so may fall with inevitable laser technology advances. Secondly, scan times are several orders of magnitude larger than for reflectance confocal microscopy, which provides similar resolution but no compositional information due to lack of discrimination of autofluorescence. Finally, technical challenges include limited field view (about 250 × 250 μm2) and penetration depth (about 200–300 μm) (23). The penetration depth is non-inferior to confocal microscopy and much less of a concern in practice than the tiny sampled area accommodated by the fraction of a mm2 field of view. Indeed, since skin lesions are often non-uniform, presenting focal dysplasia, skin areas in excess of a couple mm often have to be examined microscopically in order to avoid false-negative diagnoses. The scanning field can be increased by either acquiring mosaic images (i.e. adjacent field of views) or redesigning the optical parts of the microscope. However MPM is unlikely in the foreseeable future to be able in a matter of minutes to capture entire lesions on the skin that are several mm2, which by contrast is the case for confocal imaging. Therefore, MPM will need to undergo substantial advances before it can be used clinically for cancer diagnosis.
Monitoring skin aging
Multiphoton microscopy is increasingly used in applied dermatological research, in the fields of skin aging, nanoparticle imaging, tissue engineering, and in situ screening of pharmaceutical and cosmetic products. It is particularly useful in skin aging clinical studies to obtain quantitative information on extracellular matrix components under physiological conditions, by measuring both the two-photon autofluorescence of elastin and the SHG of collagen. The SAAID (SHG-to-AF Aging Index of Dermis) of skin is an indicator deduced from MPM data and calculated as the (Intensity of Collagen − Intensity of Elastin)/(Intensity of Collagen + Intensity of Elastin) (24). This index is well correlated with skin photo-aging in vivo, and is a valuable tool, e.g. to monitor anti-aging cosmetic treatments.
DEEP IMAGING OF MELANIN BY PHOTO-ACOUSTIC TOMOGRAPHY
Photo-acoustic tomography (PAT), also referred to as opto-acoustic tomography, is an emerging imaging technique with significant promise for biomedical applications. PAT is defined as 3D-imaging of a material based on the photo-acoustic effect and its principle can be summarized as “light in, sound out” (25). In this technology, light is absorbed by biological tissue and converted to transient heating, which subsequently creates an ultrasonic wave by thermo-elastic expansion. Ultrasound can then be detected by broadband ultrasonic transducers, and converted into tomographic images. Photo-acoustic imaging can be performed either by (i) relying on intrinsic tissue contrast alone (e.g., mapping endogenous chromophores such as melanin, haemoglobin, and lipids); or by (ii) using exogenous molecular imaging agents (25). This technology has the unique combined ability to make use of endogenous contrast alone to provide real-time images, at clinically relevant depths, with relatively high spatial resolution, all without the use of ionizing radiation. It can also be portable, is relatively cheap and safe, making it even more adoptable and integrable for clinicians. It can additionally monitor anatomical, functional, molecular and metabolic parameters that can potentially provide more comprehensive information for diagnosis, staging, and treatment of diseases (26).
Melanoma diagnosis with photo-acoustic tomography
In dermatology, PAT is most frequently explored for deep imaging of melanin to determine melanoma prognosis and staging. Most melanomas, even those that clinically appear pale and are classified as “amelanotic” actually contain melanin. This highly light-absorbing pigment naturally provides strong contrast for photo-acoustic imaging. PAT can distinguish and image both haemoglobin and melanin based on their respective absorption spectra, thereby allowing identification of early melanoma and angiogenic vessels. One implementation of PAT, sub-wavelength-resolution photo-acoustic microscopy (SW-PAM), has been developed to resolve subcellular organelles, and can image individual red blood cells and quantify individual melanosome melanin distribution in vivo (27). PAT can also accurately measure the metabolic rate of oxygen in melanoma, potentially improving early detection of this cancer (25). A handheld clinic-ready device has recently been pioneered as well, which is able to quantify the volume of pigmented lesions. This may herald photo-acoustic adaptation in high-risk melanoma clinics (28, 29).
PAT could some day in the future replace conventional sentinel lymph nodes excision. This important diagnostic procedure for metastatic melanoma, in the traditional method, is highly invasive and requires radioactive tracing. However, the sensitivity of PAT for melanin is roughly 50 times higher contrast than by light microscopy (27). This is so high that PAT can detect melanocytes in vivo in human lymph nodes, identifying microscopic metastatic disease. Combining the contrast agent indocyanine green, which tracks the lymphatic drainage, with PAT imaging of melanin has shown early promise in a clinical trial for sentinel lymph node detection (Fig. 5). In a trial of 20 melanoma patients, this approach identified all positive sentinel lymph nodes in vivo and ex vivo without a single false negative (189 total lymph nodes), with 100% sensitivity and 48 to 62% specificity (30). The low specificity that half of the time, the PAT-positive lymph nodes were false positives due to nonspecific haemorrhage or melanin in lymph nodes. However, the high sensitivity nevertheless indicates that a non-invasive, non-radioactive photo-acoustic approach can confidently rule out the presence of lymph node metastases in patients who test negative. This approach could potentially spare 50% of patients an unnecessary and inconvenient surgical procedure in the future (30). In the aforementioned study, lymph node metastasis of amelanotic melanomas contained enough melanin to be detected. However, this needs further validation as amelanotic melanomas have the risk to be the Achilles heel of this absorption-based technique.
Fig. 5. Preoperative assessment of sentinel lymph node melanin content using multispectral opto-acoustic tomography (MSOT).
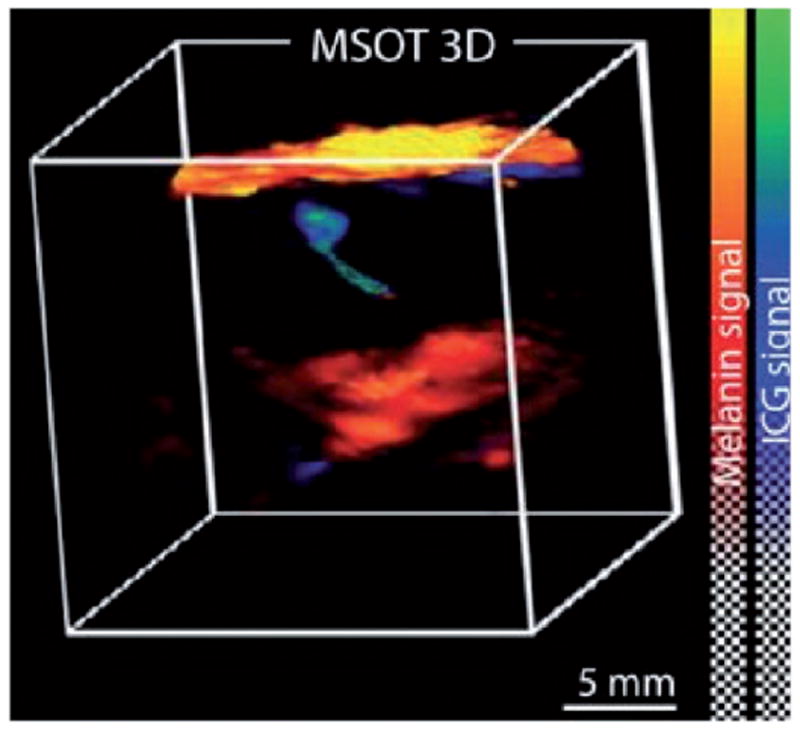
Printed with permission from Stoffels I. et al. (2015), (30). Sentinel lymph node of a patient with metastasis per a combined 3D rendering of an MSOT image taken by the 3D detector that shows both melanin (red) and indocyanine green (ICG) localization. The skin pigment appears in yellow. MSOT imaging was able to localize sentinel lymph node and melanin to provide information on the metastatic status of the lymph node.
Detection of circulating tumour cells with photo-acoustic flowmetry
Circulating tumour cells have proven to have important prognostic implication in most major cancers and have driven the birth of the field of non-invasive flow cytometry (31). Melanoma is no exception. PAT has been applied to basic science studies of in vivo flow cytometry. Photo-acoustic flowmetry can perform label-free imaging of single flowing red blood cells and circulating tumour cells in skin capillaries with millisecond-scale temporal resolution and micrometer-scale spatial resolution. Thus, it is able to provide a reliable enumeration of melanoma cells in blood samples (32). Up to now, photo-acoustic flow imaging has not been applied clinically, but it could be a significant step toward both an efficient disease monitoring technique and a diagnostic tool. It will also enable scientists to study melanoma metastatic cells at the molecular and genetic levels in hope of better understanding the processes by which they metastasize, which could result in the development of more efficient therapies for cancer patients, including the prevention of metastatic cancer by novel targeted destruction of circulating melanoma cells.
Technologies based on photo-acoustics are still in their early research phase and face some limitations. For instance, light attenuation limits the ultimate imaging depth. To address this limitation, novel light illumination schemes have to be explored, such as illuminating the object from both sides or internal delivery of light (26). Quantitative PA imaging also faces challenges because of the difficulty in measuring local fluence distribution. Advanced spectral separation algorithms have been proposed to address this issue.
With its unique combination of optical absorption contrast and ultrasonic imaging depth and resolution scalability, PAT shows great potential in the future for both biomedical research and clinical practice, and more specifically, for facilitating the diagnosis of both primary and metastatic melanoma.
CONCLUSION
Medical imaging has dramatically transformed how clinicians evaluate, diagnose, monitor, and treat disease. The highly visual nature of cutaneous diseases makes digital imaging valuable in everyday practice of dermatologists. Thus, biophotonics has become a useful tool to assess a variety of skin conditions. Table I summarizes the clinical uses, advantages and limitations of all optical imaging technologies that we are aware of in dermatology, including the 4 techniques reviewed here. In practice, no single imaging method will be ideal for all skin conditions. Many clinical problems will require multimodal in vivo imaging. With advancements and improved standardization of non-invasive imaging in dermatology, clinical practitioners may be able to better capture and monitor skin conditions over time and achieve better diagnostic accuracy, resulting in fewer biopsies, decreased morbidity, and ultimately less cost. Moreover, since great innovations for skin imaging have often been the result of the interplay between engineers and clinicians, they have to continue to work together and to define common roadmaps to maximize the benefit of innovative technologies for actual clinical needs.
Acknowledgments
Dr. Tkaczyk is grateful for support from NIH K12 CA090625. Cécile Desjobert and Marielle Romet (Santé Active Edition) provided medical writing assistance funded by Pierre Fabre Dermocosmetique. We are grateful to Dr Mihaela Balu from University of California, Irvine/Beckman Laser Institute for providing multiphoton microscopy images.
Footnotes
The author declares no conflicts of interest.
References
- 1.Tkaczyk T, Xu C. Introduction to the bio-optics: design and application. Biomed Opt Express. 2015;6:4899–4900. doi: 10.1364/BOE.6.004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharif SA, Taydas E, Mazhar A, Rahimian R, Kelly KM, Choi B, et al. Noninvasive clinical assessment of port-wine stain birthmarks using current and future optical imaging technology: a review. Br J Dermatol. 2012;167:1215–1223. doi: 10.1111/j.1365-2133.2012.11139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, Bastholt L, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline – Update 2016. Eur J Cancer. 2016;63:201–217. doi: 10.1016/j.ejca.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Stratigos A, Garbe C, Lebbe C, Malvehy J, del Marmol V, Pehamberger H, et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989–2007. doi: 10.1016/j.ejca.2015.06.110. [DOI] [PubMed] [Google Scholar]
- 5.Trakatelli M, Morton C, Nagore E, Ulrich C, Del Marmol V, Peris K, et al. Update of the European guidelines for basal cell carcinoma management. Eur J Dermatol. 2014;24:312–329. doi: 10.1684/ejd.2014.2271. [DOI] [PubMed] [Google Scholar]
- 6.Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35:2–8. doi: 10.12788/j.sder.2016.001. [DOI] [PubMed] [Google Scholar]
- 7.Guitera P, Menzies SW, Longo C, Cesinaro AM, Scolyer RA, Pellacani G. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases. J Invest Dermatol. 2012;132:2386–2394. doi: 10.1038/jid.2012.172. [DOI] [PubMed] [Google Scholar]
- 8.Briers JD. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol Meas. 2001;22:R35–66. doi: 10.1088/0967-3334/22/4/201. [DOI] [PubMed] [Google Scholar]
- 9.Lindberg M, Matura M. Contact Dermatitis. 5. Springer; 2010. Patch Testing; pp. 439–464. [Google Scholar]
- 10.Fullerton A, Stucker M, Wilhelm KP, Wardell K, Anderson C, Fischer T, et al. Guidelines for visualization of cutaneous blood flow by laser Doppler perfusion imaging. A report from the Standardization Group of the European Society of Contact Dermatitis based upon the HIRELADO European community project. Contact Dermatitis. 2002;46:129–140. doi: 10.1034/j.1600-0536.2002.460301.x. [DOI] [PubMed] [Google Scholar]
- 11.Huang YC, Ringold TL, Nelson JS, Choi B. Noninvasive blood flow imaging for real-time feedback during laser therapy of port wine stain birthmarks. Lasers Surg Med. 2008;40:167–173. doi: 10.1002/lsm.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egawa M, Tagami H. Comparison of the depth profiles of water and water-binding substances in the stratum cor-neum determined in vivo by Raman spectroscopy between the cheek and volar forearm skin: effects of age, seasonal changes and artificial forced hydration. Br J Dermatol. 2008;158:251–260. doi: 10.1111/j.1365-2133.2007.08311.x. [DOI] [PubMed] [Google Scholar]
- 13.Pudney PDA. In vivo Raman spectroscopy of skin. Spectroscopy Europe. 2015;27:3. [Google Scholar]
- 14.Kezic S, O’Regan GM, Yau N, Sandilands A, Chen H, Campbell LE, et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy. 2011;66:934–940. doi: 10.1111/j.1398-9995.2010.02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Regan GM, Kemperman PM, Sandilands A, Chen H, Campbell LE, Kroboth K, et al. Raman profiles of the stratum corneum define 3 filaggrin genotype-determined atopic dermatitis endophenotypes. J Allergy Clin Immunol. 2010;126:574–580 e1. doi: 10.1016/j.jaci.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818–823. doi: 10.1016/j.jaci.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao CS, Slipchenko MN, Wang P, Li J, Lee SY, Oglesbee RA, et al. Microsecond scale vibrational spectroscopic imaging by multiplex stimulated raman scattering microscopy. Light Sci Appl. 2015;4 doi: 10.1038/lsa.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balu M, Kelly KM, Zachary CB, Harris RM, Krasieva TB, Konig K, et al. Distinguishing between benign and malignant mela-nocytic nevi by in vivo multiphoton microscopy. Cancer Res. 2014;74:2688–2697. doi: 10.1158/0008-5472.CAN-13-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toy EL, Vekeman F, Lewis MC, Oglesby AK, Duh MS. Costs, resource utilization, and treatment patterns for patients with metastatic melanoma in a commercially insured setting. Curr Med Res Opin. 2015;31:1561–1572. doi: 10.1185/03007995.2015.1062356. [DOI] [PubMed] [Google Scholar]
- 20.American cancer Society. [05/06/2017];Cancer facts & figures. 2017 Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures.
- 21.Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center. J Am Acad Dermatol. 2010;62:751–756. doi: 10.1016/j.jaad.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 22.Patrawala S, Maley A, Greskovich C, Stuart L, Parker D, Swerlick R, et al. Discordance of histopathologic parameters in cutaneous melanoma: Clinical implications. J Am Acad Dermatol. 2016;74:75–80. doi: 10.1016/j.jaad.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Balu M, Zachary CB, Harris RM, Krasieva TB, Konig K, Tromberg BJ, et al. In Vivo Multiphoton Microscopy of Basal Cell Carcinoma. JAMA Dermatol. 2015;151:1068–1074. doi: 10.1001/jamadermatol.2015.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koehler MJ, Konig K, Elsner P, Buckle R, Kaatz M. In vivo assessment of human skin aging by multiphoton laser scanning tomography. Opt Lett. 2006;31:2879–2881. doi: 10.1364/ol.31.002879. [DOI] [PubMed] [Google Scholar]
- 25.Zackrisson S, van de Ven SM, Gambhir SS. Light in and sound out: emerging translational strategies for photoacoustic imaging. Cancer Res. 2014;74:979–1004. doi: 10.1158/0008-5472.CAN-13-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia J, Yao J, Wang LV. Photoacoustic tomography: principles and advances. Electromagn Waves. 2014;147:1–22. doi: 10.2528/pier14032303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Maslov K, Wang LV. Subwavelength-resolution label-free photoacoustic microscopy of optical absorption in vivo. Opt Lett. 2010;35:3195–3197. doi: 10.1364/OL.35.003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y, Li G, Zhu L, Li C, Cornelius LA, Wang LV. Handheld photoacoustic probe to detect both melanoma depth and volume at high speed in vivo. J Biophotonics. 2015;8:961–967. doi: 10.1002/jbio.201400143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Xing W, Maslov KI, Cornelius LA, Wang LV. Handheld photoacoustic microscopy to detect melanoma depth in vivo. Opt Lett. 2014;39:4731–4734. doi: 10.1364/OL.39.004731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoffels I, Morscher S, Helfrich I, Hillen U, Leyh J, Burton NC, et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Sci Transl Med. 2015;7:317ra199. doi: 10.1126/scitranslmed.aad1278. [DOI] [PubMed] [Google Scholar]
- 31.Tkaczyk ER, Tkaczyk AH. Multiphoton flow cytometry strategies and applications. Cytometry A. 2011;79:775–788. doi: 10.1002/cyto.a.21110. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien CM, Rood KD, Bhattacharyya K, DeSouza T, Sengupta S, Gupta SK. Capture of circulating tumor cells using photoacoustic flowmetryand two phase flow. J Biomed Opt. 2012;17:061221. doi: 10.1117/1.JBO.17.6.061221. [DOI] [PMC free article] [PubMed] [Google Scholar]


