Abstract
In recent years, myeloid-derived suppressor cells (MDSC) have emerged as one of the major inhibitors of immune effector cell function in cancer. MDSC represent a heterogeneous population of largely immature myeloid cells that are characterized by a pathological state of activation and display potent immune suppressive activity. Two major subsets of MDSC have been identified: monocytic (M-MDSC) and polymorphonuclear (PMN-MDSC). PMN-MSDC share phenotypic and morphologic features with neutrophils, whereas M-MDSC are similar to monocytes and are characterized by high plasticity. Differentiation of M-MDSC to macrophages and dendritic cells is shaped by tumor microenvironment. In recent years, the mechanisms of this process start to emerge.
Keywords: Myeloid-derived suppressor cells, monocytes, macrophages, inflammation, cancer, immune suppression
Introduction
Cancer immunotherapies rely on the ability of activated T or NK cells to recognize and eliminate tumor cells. However, in the tumor microenvironment, effector cells encounter a wide variety of factors that substantially limit their activity. Myeloid-derived suppressor cells (MDSC) have recently emerged as one of the major mechanisms of blocking the function of immune effector cells in cancer. MDSC are largely immature myeloid cells that are characterized by a pathological state of activation and display potent immune suppressive activity [1]. Two major subsets of MDSC have been identified so far: monocytic (M-MDSC) and polymorphonuclear (PMN-MDSC). PMN-MSDC share phenotypic and morphologic features with neutrophils and M-MDSC are similar to monocytes [2]. MDSC have been implicated not only in the control of tumor immune responses but also in tumor progression by promoting tumor angiogenesis, tumor cell invasion, and formation of pre-metastatic niches [3]. In cancer patients, MDSC levels are closely associated with clinical outcomes and therapeutic effects [4]. Although much progress has been made in recent years towards understanding the functional, genomic, and biochemical characteristics of these cells, the nature of these cells is still debated. In this review, we will discuss the origin and plasticity of MDSC.
The origin of MDSC
Recent studies have suggested that PMN-MDSC and M-MDSC are generated from the normal progenitors of neutrophils and monocytes, respectively, with subsequent conversion to MDSC (reviewed in [5]). MDSC accumulation is governed by two groups of signals: the first is responsible for the expansion of immature myeloid cells, while the second converts these expanded cells into an immunosuppressive population[6]. However, it is still unknown why only a proportion of all immature neutrophils and monocytes are converted to MDSC. Recent studies in human patients suggest that normal neutrophils and monocytes coexist with PMN-MDSC and M-MDSC [7]. In a recent study, normal neutrophils were distinguished from PMN-MDSC by the expression of the lectin-type oxidized LDL receptor 1 (LOX-1). LOX-1+ neutrophils were potent suppressors of T cell proliferation, while LOX-1− neutrophils were not. LOX-1+ neutrophils had genomic and biochemical characteristics of MDSC and could be considered bona fide PMN-MDSC, whereas LOX-1− cells were probably classical neutrophils [8]. Using LOX-1 as a marker, it was possible to quantitatively determine the frequency of PMN-MDSC among the entire population of neutrophils in cancer patients. In most patients, the percentage ranged from 4 to 8%, although in some patients, it was as high as 20%. In tumor sites, LOX-1+ PMN-MDSC represented 30–45% of all neutrophils [8]. In mice, specific markers for MDSC have not yet been defined; therefore, the exact proportions of MDSC in neutrophil and monocyte populations are not currently clear.
One major question is whether accumulation of MDSC is the result of conversion from differentiated monocytes and neutrophils to immunosuppressive MDSC, or whether it is the result of changes that happen at the precursor stages of cell differentiation. In sepsis, conversion of monocytes to M-MDSC has been directly demonstrated. Bergenfelz et al. [9] compared total CD14+ cells from breast cancer patients with those from sepsis patients and found strong similarities. Since sepsis monocytes are believed to be reprogrammed monocytes, the authors concluded that the same may happen in breast cancer patients. However, caution should be taken in this interpretation of the data. By isolating a total population of CD14+ cells, the authors collected a mixed population of both monocytes (CD14+ HLA-DRhigh) and M-MDSC (CD14+ HLA-DR−/lo). The population of CD14+ cells in cancer patients can contain up to 60% M-MDSC, thus making any direct conclusions about the conversion of monocytes to M-MDSC under these conditions would be rather difficult [10,11]. Several studies have shown that healthy donor monocytes can acquire both MDSC phenotypes (mainly based on HLA-DR downregulation) and immunosuppressive properties when exposed to different tumor cells [12,13]. It appears that this process is mediated through the activity of certain cytokines, such as IL-10 or PGE2 [12,14,15]. However, the in vivo relevance of these studies remains hard to assess. Similarly, activation of normal neutrophils has been proposed as a possible mechanism of conversion into PMN-MDSC (discussed in [5,16,17]). Recent work has shown that conversion of neutrophils to immunosuppressive PMN-MDSC can be achieved by induction of ER stress [8]. However, synthetic inducers of ER stress were used, and it remains unclear what physiological signals can cause the same effect in mature cells. Thus, although conversion of neutrophils and monocytes to MDSC is certainly a possibility, at this time, the biological factors responsible for such conversion has not been determined.
Distinctive features of MDSC
Although MDSC are phenotypically and morphologically distinct from mature dendritic cells and macrophages, they share some phenotypic and morphologic features with neutrophils and monocytes [2]. Therefore, one of the major unresolved issues in the field is defining what characteristics separate MDSC from other myeloid cells. In recent years, knowledge of MDSC biology has increased considerably. As a result, an algorithm to define MDSC was suggested [2]. This algorithm includes phenotypic, functional, and biochemical criteria. However, not all parameters currently described have equal values in defining MDSC. Many studies use in vitro generated MDSC, where the true nature of the cells is difficult to ascertain. In this review, we focus on studies that compared cells directly isolated from tumor-free or tumor-bearing mice or cancer patients.
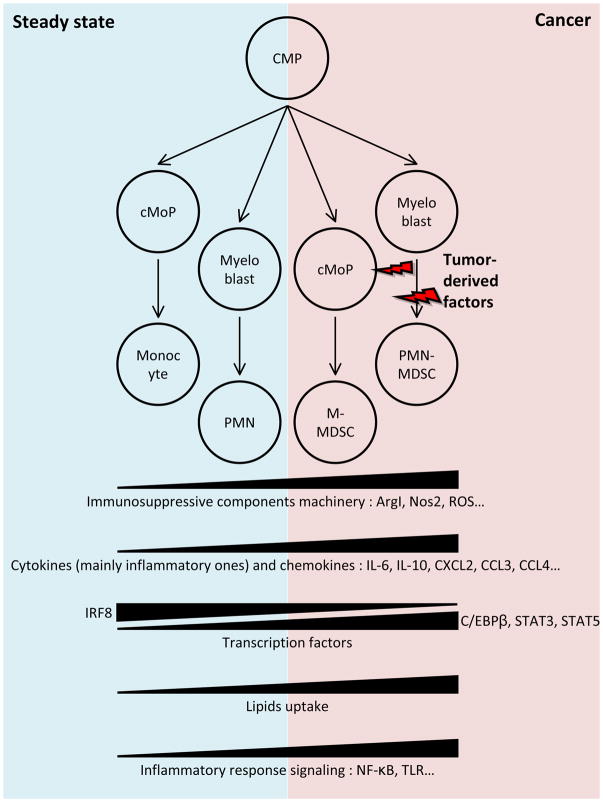
The most prominent factors implicated in MDSC suppressive activity include nitric oxide (NO) produced by Nos2, Arg1, ROS as a result of increased Nox2 activity and the ER stress response, and production of prostaglandin E2 (PGE2) as a result of increased expression of the pgs2 and ptges genes [12,18,19] (Fig. 1).
Figure 1. Origin of MDSC.
MDSC differentiate from common myeloid progenitors (CMP), and follow the pathway that involves granulocyte-macrophage progenitor (not shown) and various intermediate progenitors and precursors including common monocyte progenitors (cMOP). Figure shows most common changes observed in MDSC as compared to monocytes and neutrophils.
MDSC development is also known to be driven by several transcription factors. Among the most prominent are STAT3, IRF8, and C/EBPβ [20,21]. Several components of the inflammatory response, such as NF-κB and TLR signaling, are also upregulated in MDSC [20,22,23], reinforcing the notion that MDSC are pathologically activated myeloid cells (Fig. 1). Another potential feature of MDSC is lipid accumulation, which can affect MDSC activity via a number of different mechanisms [24]. Lipid accumulation increases oxidative metabolism and immunosuppressive activity of MDSC. Inhibition of STAT3 or STAT5 signaling or genetic depletion of scavenger receptor CD36 also inhibited the activation of oxidative metabolism and the induction of immunosuppressive function in tumor-infiltrating MDSC[25]. However, these studies were performed with mixed population of myeloid cells, which raised some concerns regarding the interpretation of the data and should be addressed in future studies.
Recent studies demonstrated that the PMN-MDSC population has a rather distinct gene expression profile from neutrophils. Some prominent pathways include regulating eukaryotic translation initiation factors 2 and 4 (eIF2 and eIF4), mTOR signaling, MAPK pathway, CSF1, IFN-γ-regulated pathways, autophagy, G-protein signaling, and CREB pathway [26,27]. Quantitative proteomics of murine MDSC determined that these cells constitute a distinct myeloid population characterized by a “kinase signature” and well-defined interactomes [28,29].
Plasticity of M-MDSC in the tumor microenvironment
MDSC are attracted to tumor sites in response to various different cytokines (CCL2, CCL5, CSF1 for M-MDSCs; CXCL1, CXCL5, CXCL6, CXCL8, CXCL12 for PMN-MDSCs, respectively [30]). In tumors, the incoming cells dive into a fairly aggressive microenvironment, which is characterized by hypoxic conditions, high concentrations of oxidative agents (ROS, NO, peroxynitrite), pro-inflammatory cytokines, and limited supply of nutrients [30,31]. These conditions affect MDSC function and differentiation. Because PMN-MDSC are generally short-living cells [32], the differentiation of M-MDSC has been studied in more detail. Of note, most studies did not directly check the functionality of Ly-6Chi monocytic cells and sometimes refer to these cells as Ly-6Chi inflammatory monocytes. However, in the studies that evaluated functional activity of these cells at the tumor site, their potent suppressive activity was universally demonstrated [31,33,34]. Therefore, monocytic cells in tumor microenvironment most likely represent bona fide M-MDSC.
M-MDSC have been shown to differentiate into tumor-associated macrophages (TAMs) after migration to the tumor site [35]. Movahedi et al. described the differentiation of Ly-6ChiCX3CR1low monocytes into 3 distinct TAM subsets in transplantable mammary adenocarcinoma, mammary, and lung carcinoma models [36]. Franklin et al. described the generation of TAMs from Ly6C+CCR2+ inflammatory monocytes in the model of spontaneous mammary tumors [37]. Cortez-Retamozo et al. demonstrated TAM formation from Ly-6Chi monocytes in a spontaneous lung adenocarcinoma model. In the last study, these inflammatory monocytes were mostly produced by extramedullary hematopoiesis in the spleen. Analogous GMP-like splenic precursors of TAMs was found in cancer patients. In NOD/SCID mice bearing non-small cell lung carcinoma, a xenograft of these precursor cells gave rise to CD11b+CD68−CD11c− monocytic-like cells in the spleen and CD68+ macrophages in the tumor [38]. Similar results demonstrating differentiation of monocytes/M-MDSC into TAMs in tumors were obtained by a number of other groups in models of glioblastoma [39], pancreatic adenocarcinoma [40], and mammary PyMT tumors [41]. Additionally, a number of studies have documented the ability of MDSC to differentiate into DCs and fibrocytes during cancer progression [42]. The CCL2-CCR2 signaling pathway was implicated in the attraction of M-MDSC to the tumor site. The disruption of CCL2-CCR2 signaling dramatically decreased the monocyte influx into the tumor, reduced TAM numbers, and generally delayed tumor growth [36,39,41,43–45]. In addition, several groups demonstrated that M-MDSC-derived TAMs are constantly “regenerated” by the recruitment of new M-MDSC from peripheral organs [38,46,47].
In addition, the type of pathologic process and differing features of the tissue microenvironment can guide the differentiation of incoming M-MDSC down different paths. For example, it has been shown in infection models that Listeria monocytogenes and Trypanasoma brucei induced the differentiation of Ly6Chi monocytes into TNF-α and iNOS-producing DCs (TipDCs) with strong pro-inflammatory activity [46,48,49]. On the contrary, in models of skeletal muscle or spinal cord damage, the infiltrating Ly6Chi monocytes gave rise to macrophages with anti-inflammatory activity [46,50,51]. In cancer models, the Van Ginderachter group has described the formation of two distinct subsets of TAMs from Ly6Chi monocytes: M1-like MHC IIhi and M2-like MHC IIlow. Histological analyses revealed the enrichment of tumor hypoxic areas with M2-like TAMs, while M1-like TAMs were mainly localized in tumor normoxic regions [36]. In addition, we previously demonstrated that congenic MDSC murine donor cells after migrating to the spleen behave quite differently than those migrating to tumor tissue of the recipient mouse. 48 h after MDSC transfer, donor cells in the spleen gave rise to an equal amount of dendritic cells and macrophages. However, in the tumor site, the vast majority of donor MDSCs differentiated into TAMs, which emphasizes the role of the tumor microenvironment in driving M-MDSC differentiation [35]. The possible differentiation of M-MDSCs into inflammatory dendritic cells in tumor site was recently reviewed [52].
Although it is now widely accepted that TAMs are generated mostly by infiltrating monocytes/M-MDSCs, tissue-resident macrophages are also involved in TAM generation. In a few tumor models, it has been shown that TAMs can originate from tissue-resident macrophages proliferating in situ [40,53]. The overall pro-tumor actions of TAMs (with their immunosuppressive features, proangiogenic effect, etc.) were previously discussed in a number of reviews[46,54,55].
Mechanisms of MDSC differentiation
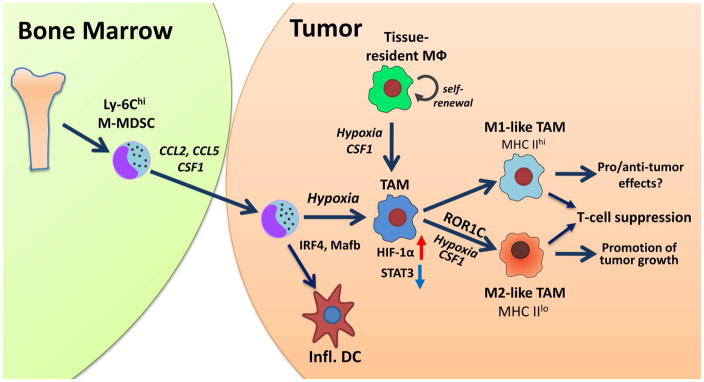
A number of factors have been shown to drive M-MDSC differentiation into TAMs. Among these factors, hypoxia, specifically HIF-1α, appears to be one of the most important [35,56]. Hypoxia was shown to be critical in M2-type TAM generation from Ly-6Chi monocytes inside the tumor [36], although later work from this group questions the role of hypoxia in TAM polarization [57]. More recently, it was shown that the fate of M-MDSC in tissue depended on the interplay between hypoxia and STAT3 transcription factor. Splenic M-MDSC had increased expression of STAT3, which subsequently prevented them from differentiation into macrophages or DCs. However, after migration to the hypoxic tumor microenvironment, these cells upregulated CD45 tyrosine phosphatase activity. This resulted in the selective decrease of STAT3 activity in myeloid cells, and M-MDSC quickly differentiated into TAMs [8]. These findings emphasize the fact that the plasticity of MDSC depends on the microenvironment. Evidently, hypoxia not only mediates the formation of TAMs from M-MDSCs but also is able to guide later events in tumor progression. For example, confining TAMs to normoxic regions by interrupting the Sema3A/Neuropilin-1 pathway reduced their pro-angiogenic and immunosuppressive functions and inhibited tumor growth and metastasis [58]. The effects of hypoxia on the host immune response inside the tumor were discussed in details elsewhere [59]. (Fig. 2)
Figure 2. Differentiation of M-MDSC in tissues.
M-MDSCs are generated in bone marrow and migrate to tumor site attracted by chemokines (CCL2, CCL5) and growth factor CSF1. They differentiate to tumor associated macrophages (TAM) and inflammatory dendritic cells (DC). This process is controlled by a number of transcription factors (depicted in the figure). Among them HIF-1α is one of the most well studied. Hypoxia triggers down-regulation of STAT3 and promotes M-MDSC differentiation to TAM. Although TAM polarization towards M1 and M2 has been described in many conditions, clear dissociation between these states is difficult. Importantly, it appears that both populations can inhibit T-cell function using different mechanisms (NO for M1 and arginase1 for M2 TAM). The functional outcomes depend on the conditions of tumor microenvironment.
Growth factors have also been implicated in regulation of M-MDSC fate inside the tumor microenvironment. CSF1, CSF2, and VEGF are known to be secreted by many types of tumors and are also known to be involved in the differentiation of myeloid cells inside the tumor microenvironment [31]. It has also been demonstrated that CSF1 up-regulates PU.1 transcription factor, which is necessary for myeloid cell development and differentiation [60]. Recently, it was shown that blockade of the CSF1/CSFR1 pathway significantly decreases the numbers of tumor-infiltrating M-MDSC as well as TAMs [61,62]. Furthermore, the recent study by Van Overmeire et al. directly demonstrated the effect of CSF1 on M-MDSC migration and differentiation. The authors showed that the inhibition of CSF1 signaling with anti-CSF1R antibody blocked the migration of Ly-6Chi cells to tumor site. This inhibition impeded the differentiation of M2-like MHC IIlo TAMs, which are associated with tumor progression [63].
In a recent study, Strauss et al. demonstrated that RAR-related orphan receptor C (RORC1) is necessary for MDSC and TAM accumulation and survival. RORC1 guides myelopoiesis by suppressing negative regulators (Socs3 and Bcl3) and promoting positive regulators (C/EBPβ) of “emergency” granulopoiesis, as well as promoting the key transcriptional mediators of myeloid progenitor differentiation to the monocytic/macrophage lineage (IRF8 and PU.1). RORC1 supported TAM differentiation and M2 polarization, promoting tumor growth [64]. In metastatic lung cancer, CCR2+ M-MDSC were shown to differentiate into pro-tumor fibrocytes in a KLF4-dependent manner [65]. In an experimental atherosclerosis model, it was demonstrated that STAT6 drives Ly-6Chi polarization to M2-type macrophages [66]. Inflammatory monocytes were unable to terminally differentiate into TAMs in the absence of RBPJ, the key transcriptional regulator mediating the Notch signaling pathway [37], demonstrating another potential regulatory mechanism of MDSC differentiation. In addition, the recent study by Goudot et al. demonstrated that the “decision” of monocyte differentiation towards DC or macrophage depends on the balance of IRF4 and Mafb transcription factors, and that this “switch” is controlled by another transcription factor, AhR [67]. Another recent study has implicated monocyte heterogeneity and differential PU.1 expression in this process [68]. The relevance of these findings in cancer should be further investigated (Fig. 2).
Conclusions
It is now apparent that M-MDSC and TAM represent a closely connected continuum of myeloid cell differentiation in tumors, and the recent data has provided new insights into the underlying mechanisms governing this observation. M-MDSC represents a potential therapeutic target for cancer therapy, not only because of the ability to suppress immune responses, but also because of high plasticity and differentiation potential. Therapeutic targeting may not only include blockade of M-MDSC migration to the tumors, but it may also provide inhibition of M-MDSC differentiation into TAMs as well as TAM polarization.
Myeloid-derived suppressor cells (MDSC) are critically important for regulation of immune responses in cancer;
MDSC have distinct features separating them from neutrophils and monocytes;
Monocytic MDSC differentiate in tumor sites to macrophages and dendritic cells;
This process is shaped by tumor microenvironment;
In recent years, specific mechanisms regulating M-MDSC differentiation start to emerge
Acknowledgments
This work was supported by the National Institutes of Health CA084488 and CA100062 to DIG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer Immunol Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bronte V, Brandau S, Chen S-H, Colombo M, Frey A, Greten T, Mandruzzato S, Murray P, Ochoa A, Ostrand-Rosenberg S, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015;66:97–110. doi: 10.1146/annurev-med-051013-052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Ma X, Zhu C, Liu L, Wang G, Yuan X. The Role of Myeloid-Derived Suppressor Cells in Patients with Solid Tumors: A Meta-Analysis. PLoS One. 2016;11:e0164514. doi: 10.1371/journal.pone.0164514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millrud CR, Bergenfelz C, Leandersson K. On the origin of myeloid-derived suppressor cells. Oncotarget. 2017;8:3649–3665. doi: 10.18632/oncotarget.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol. 2015;98:913–922. doi: 10.1189/jlb.4RI0515-204R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann NY Acad Sci. 2014;1319:47–65. doi: 10.1111/nyas.12469. [DOI] [PubMed] [Google Scholar]
- 8*.Kumar V, Cheng P, Condamine T, Mony S, Languino LR, McCaffrey JC, Hockstein N, Guarino M, Masters G, Penman E, et al. CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Immunity. 2016;44:303–315. doi: 10.1016/j.immuni.2016.01.014. This study reports a mechanism of M-MDSC differentiation to TAM mediated by down-regulation of STAT3 activity dependent on hypoxia and CD45 phosphatase activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergenfelz C, Larsson AM, von Stedingk K, Gruvberger-Saal S, Aaltonen K, Jansson S, Jernstrom H, Janols H, Wullt M, Bredberg A, et al. Systemic Monocytic-MDSCs Are Generated from Monocytes and Correlate with Disease Progression in Breast Cancer Patients. PLoS One. 2015;10:e0127028. doi: 10.1371/journal.pone.0127028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Efron PA, Mohr AM. The Monocyte That Wasn’t. Crit Care Med. 2015;43:1532–1534. doi: 10.1097/CCM.0000000000001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kratofil RM, Kubes P, Deniset JF. Monocyte Conversion During Inflammation and Injury. Arterioscler Thromb Vasc Biol. 2017;37:35–42. doi: 10.1161/ATVBAHA.116.308198. [DOI] [PubMed] [Google Scholar]
- 12.Mao Y, Poschke I, Wennerberg E, Pico de Coana Y, Egyhazi Brage S, Schultz I, Hansson J, Masucci G, Lundqvist A, Kiessling R. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 2013;73:3877–3887. doi: 10.1158/0008-5472.CAN-12-4115. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, Lin Y, Dietz AB, Forsyth PA, Yong VW, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12:351–365. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118:5498–5505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiu B, Lin Y, Grote DM, Ziesmer SC, Gustafson MP, Maas ML, Zhang Z, Dietz AB, Porrata LF, Novak AJ, et al. IL-10 induces the development of immunosuppressive CD14(+)HLA-DR(low/−) monocytes in B-cell non-Hodgkin lymphoma. Blood Cancer J. 2015;5:e328. doi: 10.1038/bcj.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moses K, Brandau S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. 2016;28:187–196. doi: 10.1016/j.smim.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donkor MK, Lahue E, Hoke TA, Shafer LR, Coskun U, Solheim JC, Gulen D, Bishay J, Talmadge JE. Mammary tumor heterogeneity in the expansion of myeloid-derived suppressor cells. Int Immunopharmacol. 2009;9:937–948. doi: 10.1016/j.intimp.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, Bogner PN, Farren MR, Lee KP, Liu K, et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123:4464–4478. doi: 10.1172/JCI68189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonda N, Simonato F, Peranzoni E, Cali B, Bortoluzzi S, Bisognin A, Wang E, Marincola FM, Naldini L, Gentner B, et al. miR-142-3p prevents macrophage differentiation during cancer-induced myelopoiesis. Immunity. 2013;38:1236–1249. doi: 10.1016/j.immuni.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Fridlender ZG, Sun J, Mishalian I, Singhal S, Cheng G, Kapoor V, Horng W, Fridlender G, Bayuh R, Worthen GS, et al. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS One. 2012;7:e31524. doi: 10.1371/journal.pone.0031524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T, Zou W, et al. Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer Immunol Res. 2015;3:1236–1247. doi: 10.1158/2326-6066.CIR-15-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Khamia AA, Zheng L, Valle LD, Hossain F, Wyczechowska D, Zabaleta J, Sanchez MD, Deana MJ, Rodriguez PC, Ochoa AC. Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells. Oncoimmunology. 2017 doi: 10.1080/2162402X.2017.1344804. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016:1. doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91:167–181. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Gato M, Blanco-Luquin I, Zudaire M, de Morentin XM, Perez-Valderrama E, Zabaleta A, Kochan G, Escors D, Fernandez-Irigoyen J, Santamaria E. Drafting the proteome landscape of myeloid-derived suppressor cells. Proteomics. 2016;16:367–378. doi: 10.1002/pmic.201500229. This study, together with ref. 29, described proteomic characterstics of MDSC. [DOI] [PubMed] [Google Scholar]
- 29*.Gato-Canas M, Martinez de Morentin X, Blanco-Luquin I, Fernandez-Irigoyen J, Zudaire I, Liechtenstein T, Arasanz H, Lozano T, Casares N, Chaikuad A, et al. A core of kinase-regulated interactomes defines the neoplastic MDSC lineage. Oncotarget. 2015;6:27160–27175. doi: 10.18632/oncotarget.4746. This study, together with ref. 29, described proteomic characterstics of MDSC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016;37:208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Condamine T, Kumar V, Ramachandran IR, Youn JI, Celis E, Finnberg N, El-Deiry WS, Winograd R, Vonderheide RH, English NR, et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J Clin Invest. 2014;124:2626–2639. doi: 10.1172/JCI74056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 35.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 37.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, Ryan RJ, Iwamoto Y, Marinelli B, Gorbatov R, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109:2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17:170–182. doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, Cullinan DR, Luo J, Bearden AR, Lavine KJ, et al. Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity. 2017;47:323–338. e326. doi: 10.1016/j.immuni.2017.07.014. This work reveals the different origin of several TAM subsets in pancreatic cancer. The authors suggest different role of tissue-resident and monocyte-derived TAMs in progression of pancreatic cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart KM, Bak SP, Alonso A, Berwin B. Phenotypic and functional delineation of murine CX(3)CR1 monocyte-derived cells in ovarian cancer. Neoplasia. 2009;11:564–573. 561–573. doi: 10.1593/neo.09228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Movahedi K, Schoonooghe S, Laoui D, Houbracken I, Waelput W, Breckpot K, Bouwens L, Lahoutte T, De Baetselier P, Raes G, et al. Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res. 2012;72:4165–4177. doi: 10.1158/0008-5472.CAN-11-2994. [DOI] [PubMed] [Google Scholar]
- 45.Hartwig T, Montinaro A, von Karstedt S, Sevko A, Surinova S, Chakravarthy A, Taraborrelli L, Draber P, Lafont E, Arce Vargas F, et al. The TRAIL-Induced Cancer Secretome Promotes a Tumor-Supportive Immune Microenvironment via CCR2. Mol Cell. 2017;65:730–742. e735. doi: 10.1016/j.molcel.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Movahedi K, Van Ginderachter JA. The Ontogeny and Microenvironmental Regulation of Tumor-Associated Macrophages. Antioxid Redox Signal. 2016;25:775–791. doi: 10.1089/ars.2016.6704. [DOI] [PubMed] [Google Scholar]
- 47.Shand FH, Ueha S, Otsuji M, Koid SS, Shichino S, Tsukui T, Kosugi-Kanaya M, Abe J, Tomura M, Ziogas J, et al. Tracking of intertissue migration reveals the origins of tumor-infiltrating monocytes. Proc Natl Acad Sci U S A. 2014;111:7771–7776. doi: 10.1073/pnas.1402914111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guilliams M, Movahedi K, Bosschaerts T, VandenDriessche T, Chuah MK, Herin M, Acosta-Sanchez A, Ma L, Moser M, Van Ginderachter JA, et al. IL-10 dampens TNF/inducible nitric oxide synthase-producing dendritic cell-mediated pathogenicity during parasitic infection. J Immunol. 2009;182:1107–1118. doi: 10.4049/jimmunol.182.2.1107. [DOI] [PubMed] [Google Scholar]
- 50.Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Curr Opin Immunol. 2017;45:43–51. doi: 10.1016/j.coi.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tymoszuk P, Evens H, Marzola V, Wachowicz K, Wasmer MH, Datta S, Muller-Holzner E, Fiegl H, Bock G, van Rooijen N, et al. In situ proliferation contributes to accumulation of tumor-associated macrophages in spontaneous mammary tumors. Eur J Immunol. 2014;44:2247–2262. doi: 10.1002/eji.201344304. [DOI] [PubMed] [Google Scholar]
- 54.Bolli E, Movahedi K, Laoui D, Van Ginderachter JA. Novel insights in the regulation and function of macrophages in the tumor microenvironment. Curr Opin Oncol. 2017;29:55–61. doi: 10.1097/CCO.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 55.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu G, Bi Y, Shen B, Yang H, Zhang Y, Wang X, Liu H, Lu Y, Liao J, Chen X, et al. SIRT1 limits the function and fate of myeloid-derived suppressor cells in tumors by orchestrating HIF-1alpha-dependent glycolysis. Cancer Res. 2014;74:727–737. doi: 10.1158/0008-5472.CAN-13-2584. [DOI] [PubMed] [Google Scholar]
- 57.Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, Movahedi K, Houbracken I, Schouppe E, Elkrim Y, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014;74:24–30. doi: 10.1158/0008-5472.CAN-13-1196. [DOI] [PubMed] [Google Scholar]
- 58.Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA, Tamagnone L, Mazzone M. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 2014;143:512–519. doi: 10.1111/imm.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mossadegh-Keller N, Sarrazin S, Kandalla PK, Espinosa L, Stanley ER, Nutt SL, Moore J, Sieweke MH. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature. 2013;497:239–243. doi: 10.1038/nature12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, Wu L. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63*.Van Overmeire E, Stijlemans B, Heymann F, Keirsse J, Morias Y, Elkrim Y, Brys L, Abels C, Lahmar Q, Ergen C, et al. M-CSF and GM-CSF Receptor Signaling Differentially Regulate Monocyte Maturation and Macrophage Polarization in the Tumor Microenvironment. Cancer Res. 2016;76:35–42. doi: 10.1158/0008-5472.CAN-15-0869. This study demonstrates direct regulation of Ly-6Chi monocyte fate inside the tumor by CSF1. It documents different role of CSF1 and CSF2 in monocyte differentiation inside the tumor. [DOI] [PubMed] [Google Scholar]
- 64.Strauss L, Sangaletti S, Consonni FM, Szebeni G, Morlacchi S, Totaro MG, Porta C, Anselmo A, Tartari S, Doni A, et al. RORC1 Regulates Tumor-Promoting “Emergency” Granulo-Monocytopoiesis. Cancer Cell. 2015;28:253–269. doi: 10.1016/j.ccell.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 65.Shi Y, Ou L, Han S, Li M, Pena MM, Pena EA, Liu C, Nagarkatti M, Fan D, Ai W. Deficiency of Kruppel-like factor KLF4 in myeloid-derived suppressor cells inhibits tumor pulmonary metastasis in mice accompanied by decreased fibrocytes. Oncogenesis. 2014;3:e129. doi: 10.1038/oncsis.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rahman K, Vengrenyuk Y, Ramsey SA, Vila NR, Girgis NM, Liu J, Gusarova V, Gromada J, Weinstock A, Moore KJ, et al. Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J Clin Invest. 2017;127:2904–2915. doi: 10.1172/JCI75005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67*.Goudot C, Coillard A, Villani AC, Gueguen P, Cros A, Sarkizova S, Tang-Huau TL, Bohec M, Baulande S, Hacohen N, et al. Aryl Hydrocarbon Receptor Controls Monocyte Differentiation into Dendritic Cells versus Macrophages. Immunity. 2017;47:582–596. e586. doi: 10.1016/j.immuni.2017.08.016. This study describes molecular mechanism regulating switch of monocytic differentiation to DCs as oppose to macrophages. [DOI] [PubMed] [Google Scholar]
- 68*.Menezes S, Melandri D, Anselmi G, Perchet T, Loschko J, Dubrot J, Patel R, Gautier EL, Hugues S, Longhi MP, et al. The Heterogeneity of Ly6Chi Monocytes Controls Their Differentiation into iNOS+ Macrophages or Monocyte-Derived Dendritic Cells. Immunity. 2016;45:1205–1218. doi: 10.1016/j.immuni.2016.12.001. This report describes monocytic populations specialized on differentiation to macrophages and dendritic cells. [DOI] [PMC free article] [PubMed] [Google Scholar]