Abstract
Replacement of dead neurons following ischemia, either via enhanced endogenous neurogenesis or stem cell therapy, has long been sought. Unfortunately, while various therapies that enhance neurogenesis or stem cell therapies have proven beneficial in animal models, they have all uniformly failed to truly replace dead neurons in the ischemic core to facilitate long-term recovery. Remarkably, we observe robust repopulation of medium-spiny neurons within the ischemic core of juvenile mice following experimental stroke. Despite extensive neuronal cell death in the injured striatum of both juveniles and adults at acute time points after ischemia (24hr and 7d), mature newborn neurons replaced lost striatal neurons at 30d post-ischemia. This neuronal repopulation was found only in juveniles, not adults, and importantly, was accompanied by enhanced post-ischemic behavioral recovery at 30d. Ablation of neurogenesis using irradiation prevented neuronal replacement and functional recovery in MCAo-injured juvenile mice. In contrast, findings in adults were consistent with previous reports, that newborn neurons failed to mature and died, offering little therapeutic potential. These data provide support for neuronal replacement and consequent functional recovery following ischemic stroke and new targets in the development of novel therapies to treat stroke.
Keywords: cerebral ischemia, endogenous recovery, neural stem cells, neurogenesis, neuron replacement
Introduction
Approximately 800,000 Americans experience a new or recurrent stroke every year, and many survivors continue to live with permanent stroke-related disabilities, often leading to poor quality of life (Korda and Douglas, 1997, Mercier et al., 2001, Sun et al., 2014). Although stroke is one of the leading causes of death and disability worldwide, no successful long-term neuroprotective therapies have been found in clinical trials to date (Kidwell et al., 2001, Ginsberg, 2008, Minnerup et al., 2012), highlighting the need for novel therapeutic approaches. Neuronal replacement could result in direct recovery of function, since many post-ischemic impairments are due to neuronal damage or death. Neurogenesis (the birth of new neurons) is one emerging approach involving the generation of functionally integrated neurons from progenitor cells, and occurs throughout life in the brain of mammals, making it an appealing target for potential interventions to enhance post-stroke recovery.
Long-standing evidence indicates that cerebral ischemia initiates adult neurogenesis (Liu et al., 1998, Jin et al., 2001, Arvidsson et al., 2002, Nakatomi et al., 2002). Stroke-induced neurogenesis in adult mice involves vigorous proliferation and migration of neural progenitor cells, but most cells die within 4 weeks (Lichtenwalner and Parent, 2006, Liu et al., 2013, Tobin et al., 2014), unable to repair tissue and repopulate damaged areas (Zhao et al., 2008). The timeline for rapid proliferation is short-lived, peaking at 7 days post-injury (Lichtenwalner and Parent, 2006, Liu et al., 2013). Much research has focused on neonatal, perinatal, and adult rodents, yet few studies have assessed post-stroke neurogenesis in juveniles. Research centered on adult neurogenesis in rodent models of cerebral ischemia demonstrate little replacement of neurons lost following stroke-induced damage. Adult neurogenesis differs from developmental neurogenesis, where the brain is undergoing processes such as axon pathfinding, programmed cell death, and dendritic extension, which are limited in the mature neurons of the adult brain (Danzer, 2008). The juvenile brain is ideal for studying neurogenesis because 3–4 week old mice have a fully developed brain that has reached neuronal maturity like adults, yet isn’t vulnerable to the developmental processes found in the neonatal and perinatal brain. Differences between the juvenile and adult brain may shed light on potential interventions that could be used to enhance neurogenesis in adults, or help identify why newborn neurons do not survive in adults.
To test the hypothesis that neurogenesis and functional recovery may be enhanced in the juvenile brain, we compared an experimental ischemic stroke (Herson et al., 2013) model in juvenile and adult mice. We examined neurogenesis and neuronal replacement in the striatum with neurobehavioral assays of functional recovery and immunohistochemistry, including bromodeoxyuridine (BrdU) labeling and cell-type specific markers at 24hr, 7d, and 30d following 45min transient middle cerebral artery occlusion (MCAo). The vast majority of neurons lost in the striatum following stroke are GABAergic medium-sized spiny neurons (MSN), which are the primary neuronal type (90–95%) in the region and are essential for motor function (Arlotta et al., 2008). To determine if striatal neurogenesis plays a role in post-stroke recovery of function, we tested an array of motor and locomotive functions at baseline, 7d, and 30d after cerebral ischemia.
Despite equivalent injury between age groups at acute time points (24hr and 7d), we discovered a robust regenerative response in the juvenile brain at 30d post-injury not found in adults. We found substantial neuronal replacement in areas of ischemic damage unique to the juvenile brain, along with improved functional outcomes on behavioral tests, revealing improved limb use and motor responses in MCAo-injured juvenile mice, but not adults.
Experimental Procedures
Seventy-two male C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were randomly assigned to one of two groups for molecular experiments (MCAo Adult or MCAo Juvenile), one of four groups for behavior tests (MCAo Adult, Sham-operated Adult, MCAo Juvenile, or Sham-operated Juvenile), and the irradiation experiment included MCAo Juvenile+irradiation mice. Mice were single housed, in temperature (23 ± 3 °C) and light (12:12hr, light:dark) controlled rooms with ad libitum access to food and water. All procedures were performed in accordance with University of Colorado Institutional Animal Care and Use Committee guidelines for the humane use of laboratory animals in biological research.
Middle Cerebral Artery Occlusion (MCAo)
MCAo methods are as previously reported (Jia et al., 2011, Herson et al., 2013). Briefly, cerebral ischemia was induced under isoflurane anesthesia in juvenile (postnatal day 20–25, 10–15g) and adult (8 weeks, 25–30g) mice for 45min with reversible MCAo via the intraluminal suture method. Minor variations were incorporated to accommodate the small size of P20–25 mice (a 6-0 nylon suture was heat-blunted and coated with silicone gel to obtain a smaller filament diameter of ~0.18 mm). The adequacy of MCAo was confirmed by laser Doppler flowmetry (>70% drop required for inclusion) measured over the ipsilateral parietal cortex in all mice.
Bromodeoxyuridine (BrdU) Administration
Two injections of BrdU (50 mg/kg in 0.9% saline, i.p.; Sigma, St. Louis, MO) were given at 24hr and 48hr after stroke, at peak expression times reported in the literature following stroke. A synthetic analog of thymidine, BrdU is commonly used in the detection of proliferating cells in living tissues.
Immunohistochemistry
Tissue collection, staining, and analyses were performed by a blinded investigator. Cellular proliferation and neurogenesis was assessed by BrdU co-localization with cell type-specific markers, since developing neurons express distinct markers during the maturation process. Immunofluorescence assays also included markers for GABAergic MSNs, the primary neuronal type in the striatum (90–95%), and for the remaining neuronal types (5–10%), cholinergic interneurons and GABAergic parvalbumin-immunoreactive interneurons (Chang and Kita, 1992, Arlotta et al., 2008). Staining of 50 μm sections consisted of phosphate-buffered saline washes (1X PBS, 3×5 min), 1hr incubation in blocking serum (5% normal donkey serum with 0.3% Triton X-100), overnight incubation at 4°C in primary antibody, PBS washes (3×5 min), 1hr incubation in secondary antibody, PBS washes (3×5 min), Hoechst counterstain 5 min (1:10,000 in PBS), PBS washes (3×5 min), mount and coverslip with anti-fade mounting medium (Vectashield). For BrdU staining, sections were washed with 1X PBS (3×5 min), denatured (2N HCl) for 20 min at 37°C, neutralized with 0.1M borate buffer (pH 8.5, 3×15 min), PBS washes (3×5 min), and finished using the protocol listed above. The following primary antibodies for cell-specific markers were used: rat anti-BrdU (1:300, Abcam), rabbit anti-doublecortin (DCX, 1:500, Abcam), mouse anti-NeuN (1:500, Millipore), rabbit anti-COUP-TF-interacting protein 2 (Ctip2, 1:300, Abcam), rat anti-Ctip2 (1:300, Abcam), rabbit anti-choline acetyltransferase (ChAT, 1:300, Millipore), mouse anti-parvalbumin (PV, 1:300, Sigma), goat anti-glial fibrillary acidic protein (GFAP, 1:500, Santa Cruz Biotech), and rabbit anti-oligodendrocyte transcription factor 2 (Olig2, 1:300, Millipore). The following secondary antibodies were used: Alexa Fluor 488, 594, or 647-conjugated IgG (1:500 or 1:600; Jackson Immuno) and Alexa Fluor 555 (1:500, Abcam). Cell death was assessed by a terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL, Cell Death Detection Kit, Roche) assay. Confocal microscopy was used to confirm co-localization of BrdU and cell type-specific markers using an Olympus FV1000 laser scanning confocal microscope and Olympus Fluoview imaging software (Center Valley, PA, USA). The Cell Counter plug-in on Fiji software (Schindelin et al., 2012) was used for cell count analyses of the lateral striatum, averaged across two sections per animal with 100 μm spacing between.
Neurobehavioral Testing
Stroke is commonly associated with sensory and motor deficits, including loss of coordination and partial paralysis (Mercier et al., 2001), and a leading goal of stroke treatment is the restoration of behavioral function to patients. Investigation of neurobehavioral recovery in juvenile compared to adult mice following cerebral ischemia will help to determine the functional significance of neurogenesis in enhanced post-stroke outcomes. Damage to the forelimb region of sensorimotor cortex causes deficits in limb and motor function that can be assessed with simple tasks that are not affected by repeated testing, do not require training or aversive motivation, and have been validated in unilateral models of stroke in rodents measuring post-ischemic recovery of function (Bland et al., 2000, Schallert et al., 2000, Hua et al., 2002, Zhang et al., 2002, Woodlee et al., 2005, Schaar et al., 2010). Multiple measures were chosen to evaluate different aspects of motor and sensory responses, which may have been undetected by a single test. Behavioral assessment was performed by a blinded investigator, and included a battery of tests (Table 1) measured at baseline, 7d and 30d following MCAo in juvenile, adult, and sham-operated controls.
Table 1.
Neurobehavioral Tests
| Behavioral Test | Scoring | References |
|---|---|---|
| Vibrissae-elicited forelimb placing test: detects impairments in visual placing and limb use asymmetry | The observed ability of ipsilateral (right) and contralateral (left) placing of the forepaw on top of the table was scored as: 0 = no attempt to place forepaw, 1 = weak attempt to place forepaw, or 2 = normal placing of forepaw. Scores were averaged across four consecutive trials of each limb and converted to percent placing. | Markgraf et al., 1992; Schallert et al., 2000; Hua et al., 2002; Woodlee et al., 2005 |
| Spontaneous forelimb use: used to assess walking score, general limb use, and limb neglect | The observed use of ipsilateral and contralateral forelimbs and hindlimbs, were scored and averaged as: 0 = no movement of limb; 1 = barely perceptible movement of limb; 2 = movement, but limb does not support weight; 3 = limb supports weight and animal takes a few steps; 4 = animal walks with mild paresis; 5 = normal limb use, no detectable deficits. Scores were averaged across four consecutive trials of each limb and converted to percentage limb use. | Burkey et al., 1996; Bland et al., 2000; Bland et al., 2001 |
| Toe Spread: used to assess gross motor function | Mice were elevated by the tail and the amount of toe spread observed in ipsilateral and contralateral forelimbs and hindlimbs were scored as: 0 = no spreading; 1 = intermediate spreading; 2 = sustained spreading of all toes. Scores were averaged across four consecutive trials of each limb and converted to percent toe spread. | Nitz et al., 1986; Brenneis et al., 2013 |
| Catalepsy Grid Test: used to assess rigid/cataleptic body postures | A wire grid 28 × 14 cm was tilted at a 45°angle inside a testing chamber and mice were placed on the grid facing down. The time before mice begin to move downward or turn and face upward (negative geotaxis) on the grid was recorded in seconds across a 2 min observation period. | Fuenmayor and Vogt, 1979; Saposnik et al., 1999 |
| Open Field Test: used to assess gross motor and exploratory locomotive behavior | Open field locomotor activity was assessed for 15 minutes in a circular open field (60 cm arena diameter, 30 cm wall height). Horizontal locomotion data was obtained with ANY-maze automated tracking software (Stoelting, Wood Dale, IL), total distance traveled and speed were recorded during spontaneous exploration. | Brooks and Dunnett, 2009; Seibenhener and Wooten, 2015 |
| Corner Test: used to assess sensory- motor deficits and postural asymmetry | Mice were placed in a Plexiglas chamber, with two walls (30 × 20 × 1 cm3), attached at a 30°angle with an opening along the joint to encourage corner entry. When vibrissae are stimulated upon entry into the corner, mice would rear along the wall and exit the corner, turning either right or left. Non-ischemic mice turn either left or right, but ischemic mice preferentially turn toward the non-impaired, ipsilateral (right) side. The number of left and right turns was recorded for 10 trials and score was calculated as percentage of right turns; turns in the absence of vertical rearing were not scored. | Zhang et al., 2002; Balkaya et al., 2013 |
General Motor and Limb Use Asymmetry Measures
Forelimb Placing
The vibrissae-elicited forelimb placing test is commonly used to assess deficits in visual placing and limb use asymmetry following stroke, damage to the motor system will elicit forelimb placing impairments (Markgraf et al., 1992, Schallert et al., 2000, Hua et al., 2002, Woodlee et al., 2005).
Spontaneous Forelimb Use
The use of the forelimbs was assessed in freely moving mice during exploratory activity in a clear, Plexiglas cube (20 × 20 × 20 cm) for 3 min to assess walking score, general limb use, and limb neglect after stroke (Burkey et al., 1996, Bland et al., 2000, Bland et al., 2001).
Toe Spread
This test has been used to detect impairments in gross motor function (Nitz et al., 1986, Brenneis et al., 2013).
Composite Score – a general measure of overall stroke-induced motor function impairment, highly correlated tests (r = 0.812–0.964) were combined (equally weighted, scores ranged from 0–100%) to form a motor construct across different aspects of limb use, motor response, and visual/vestibular function.
Corner Test
The corner test is commonly used to assess sensory-motor deficits and postural asymmetry in rodent models of stroke, and has sensitivity in detecting sensory-motor symmetry impairments at early and late time points post-ischemia (Zhang et al., 2002, Balkaya et al., 2013).
Open Field
This task was used to assess gross motor and exploratory locomotive behavior (Brooks and Dunnett, 2009, Seibenhener and Wooten, 2015).
Catalepsy Grid Test
Characterized by a tendency to maintain postures and rigidity of the body, catalepsy can occur after stroke (Saposnik et al., 1999), which could confound other behavioral measures.
Irradiation
In order to investigate the possible role of juvenile neurogenesis on enhanced post-stroke behavioral outcomes, we arrested neurogenesis in MCAo-injured juveniles with ionizing irradiation and assessed neuromotor outcomes at the following time points after irradiation: post-irradiation baseline, MCAo+irradiation 7d, and MCAo+irradiation 30d. A γ-emitting irradiator was used to arrest neurogenesis in juvenile mice at PN15. Following a 3d recovery, baseline behavioral testing was conducted at PN18, then MCAo was delivered at PN20–25. We performed whole body irradiation, delivered in a single dose (5Gy), based on minimum radiation exposure dosages reported for peak reductions in proliferating SVZ cells and immature neurons in a comprehensive dose-response investigation of adult neurogenesis (Mizumatsu et al., 2003). Irradiation was performed at the University of Colorado, using a γ-emitting irradiator, which allows for deep tissue irradiations with remarkable accuracy.
Statistics
All molecular and behavioral analyses were performed by a blinded investigator. For percent expression and mean cell counts, independent samples t-tests were utilized to assess differences in the injured striatum of the juvenile versus adult brain at 24hr, 7d, and 30d post- ischemia. One-way ANOVAs were used to compare the ipsilateral versus contralateral striatum of the juvenile versus adult brain at 24hr, 7d, and 30d post-ischemia. A mixed-design ANOVA (between-subjects factor: surgical group and within-subjects factor: time) and simple main effects analysis with Bonferroni correction was used to assess differences in behavioral scores of MCAo versus sham mice at baseline, 7d, and 30d post-injury. A repeated-measures ANOVA with Bonferroni correction for multiple comparisons was used to compare differences in behavioral scores of juvenile MCAo+Irradiation mice across baseline, 7d, and 30d post-injury. Data was analyzed with IBM SPSS Statistics (Armonk, NY) and differences with a p-value of <0.05 were considered significant; data represent mean ± SEM.
Results
Equivalent Injury at Acute Time Points
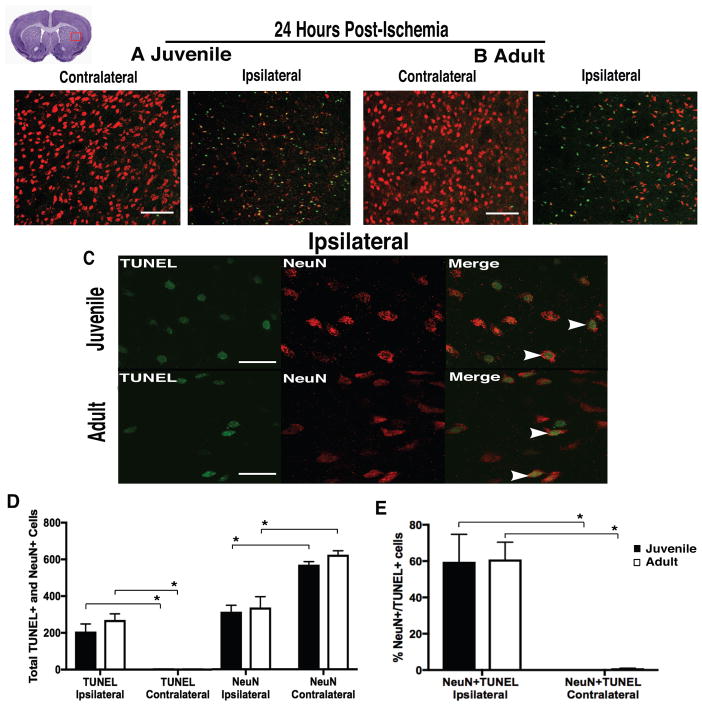
At 24hr following stroke, equivalent injury was found in the injured striatum of MCAo-injured juvenile and adult mice. No differences (p=0.254) were found in cell death (total TUNEL+ cells, marker of apoptotic signaling cascades) between juvenile 206±41 and adult 270±33 (Fig 1, A–D), neuronal loss (NeuN+ cells, p=0.743) between juvenile 314±35 and adult 337±59 (Fig 1, A–D), or neuronal cell death (%NeuN+/TUNEL+, p=0.943) between juvenile 59.6±15.1% and adult 60.9±9.5% (Fig 1, C and E) mice. The contralateral hemisphere of both MCAo-injured juvenile and adult mice had fewer TUNEL+ (p<0.001) and NeuN+/TUNEL+ cells (p<0.001), and more NeuN+ cells (p<0.001) compared to the injured, ipsilateral hemisphere.
Figure 1. Equivalent injury following ischemic stroke.
(A–B) Representative images of FOV for analysis of TUNEL+ (green) and NeuN+ (red) cells, showing equivalent cell death and neuronal loss in the injured striatum of juvenile and adult mice at 24hr post-ischemia, compared to uninjured (contralateral) striatum. (C) Representative images at 100x magnification. (D) Comparable cell death (TUNEL+), neuron loss (NeuN+), and (E) neuronal cell death (NeuN+/TUNEL+) were found in the injured striatum of both groups. Data represent mean ± SEM, scale (A–B) = 100 μm, and scale (C) = 10 μm.
Newborn Neuron Survival and Replacement in Juveniles at Chronic Time Points
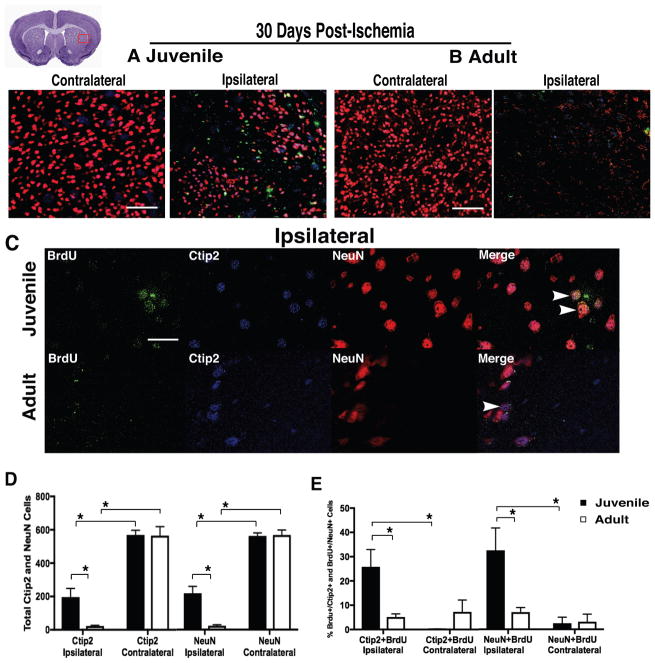
In contrast to equivalent neuronal loss and comparable production of newborn neurons in juvenile and adult mice at acute time points, at 30d post-ischemia we discovered striking differences between groups (Fig 2, A–E). Incredibly, neurons lost after ischemia appear to be replaced in the juvenile brain (Fig 2, A and C), but not in adults (Fig 2, B and D). The total number of mature neurons (NeuN+, Fig 2D) was greater (p=0.003) in the injured striatum of juvenile 239±45 mice compared to adults 42±9, and juveniles had more 36.9±4.8% mature newborn neurons (NeuN+/BrdU+, Fig 2E) than adults 9.4±5.5% (p=0.003); revealing a marked increase at P30 in newborn neurons that migrated, matured, and replaced those lost in the injured juvenile striatum compared to adult. The ipsilateral hemisphere of both MCAo-injured juvenile and adult mice had more DCX+ cells (<0.001) and fewer NeuN+ cells (p<0.001) compared to the non-injured contralateral hemisphere. There was a higher percentage of co-localized NeuN+/BrdU+ cells (p<0.001) found in the ipsilateral hemisphere compared to contralateral, but post-hoc analysis revealed that only juveniles had a higher percentage of NeuN+/BrdU+ cells (p<0.001) in the injured hemisphere over non-injured, while co-localization in adults did not differ between hemispheres (p=0.343).
Figure 2. Neuronal replacement after ischemic stroke.
(A–B) Representative images of FOV for analysis of BrdU+ (green), NeuN+ (red), and DCX (blue), showing neuronal replacement in juvenile mice, but not adult at 30d following stroke. (C) Representative images of lateral striatum at 100x magnification. (D) Juveniles had more mature neurons in the injured striatum compared to adult. (E) A marked increase in neuronal replacement in the juvenile brain, compared to adult. Data represent mean ± SEM, scale (A–B) = 100 μm, and scale (C) = 10 μm.
MSN Differentiation and Replacement
To determine if newborn neurons were region-specific medium spiny neurons (MSN), co-labeling of COUP-TF-interacting protein 2 (Ctip2, marker of MSNs) and BrdU was assessed (Fig 3, A–E). At 30d post-ischemia there were more mature neurons 219±38 (p=0.002) and MSNs 196±54 (p=0.014) in the injured hemisphere of the juvenile brain (Fig 3D) compared to adult (24±44 and 23±62, respectively). There were also more (=0.030) newly generated MSNs (Ctip2+/BrdU+, Fig 3E) found in the injured hemisphere of juveniles 25.8±6.4% compared to adult 5.1±6.9% at 30d post-stroke. Mature newborn neurons (NeuN+/BrdU+) were also increased (p=0.038) in the ipsilateral hemisphere of juveniles 32.6±7.5% (Fig 3E) compared to adult 7.1±8.0%, verifying earlier findings (Fig 2). The contralateral hemisphere of both MCAo-injured juvenile and adult mice had more Ctip2+ (p<0.001) and NeuN+ cells (p<0.001) compared to the injured hemisphere. A higher percentage of co-localized Ctip2+/BrdU+ cells (p=0.003) and NeuN+/BrdU+ cells (p=0.001) was found in the ipsilateral hemisphere compared to contralateral, but post-hoc analysis revealed that only juveniles had a significantly higher percentage of Ctip2+/BrdU+ cells (p=0.002) and NeuN+/BrdU+ cells (p=0.002) in the injured hemisphere compared to non-injured, while co-localization in adults did not differ between hemispheres (p=0.989 and p=0.959, respectively).
Figure 3. MSNs after ischemic stroke.
(A–B) Representative images of FOV for analysis of BrdU+ (green), Ctip2+ (blue), and NeuN+ (red), showing that newborn neurons differentiate into region-specific MSNs in juveniles, but not adults 30d post-ischemia. (C) Representative images of lateral striatum at 100x magnification. (D–E) Juvenile mice had more MSNs overall, and more newborn MSNs than adults. Data represent mean ± SEM, scale (A–B) = 100 μm, and scale (C) = 10 μm.
Distribution of Striatal Neuron Types
Striatal cell types were examined using markers for GABAergic (Parvalbumin, PV) and Cholinergic (choline acetyltransferase, ChAT) interneurons, and the MSN marker Ctip2. At 30d post-ischemia there were more (p=0.014) MSNs in the injured hemisphere of the juvenile brain 184.8±12.2 (Fig 4D) compared to adult 44.7±14.5. Findings showed a normal distribution of MSNs 92.2±2.7% in ipsilateral striatum of juvenile mice at 30d post-ischemia (Fig 4, A–E). This is consistent with reports of healthy striatum, which has approximately 90–95% MSNs and 5–10% GABAergic and Cholinergic interneurons (Arlotta et al., 2008). Given the extensive tissue damage sustained from ischemia, the distribution data in adult mice was not very meaningful. In spite of the distribution data and neuronal repopulation of damaged brain regions, it could be argued that BrdU is labeling DNA repair in post-mitotic neurons or aberrant cell cycle reentry rather than neurogenesis (Cooper-Kuhn and Kuhn, 2002, Kuan et al., 2004), so we performed additional staining with BrdU and TUNEL. We did not find significant co-localization of BrdU+/TUNEL+ cells at 24hr and 7d post-injury (data not shown), indicating that DNA repair does not account for BrdU+ cells found following stroke. In order to further examine the cellular environment, an additional assay was performed including BrdU, GFAP (marker of astrocytes), and Olig2 (lineage marker of oligodendrocytes) to assess glial cell differentiation. Results did not reveal significant co-labeling of either BrdU+/GFAP+ cells or BrdU+/Olig2+ cells (data not shown), indicating that most newly generated cells in the injured striatum were neurons.
Figure 4. MSN distribution after ischemic stroke.
(A–B) Representative images of FOV for analysis of Ctip2+ (blue), ChAT+ (green), and Parvalbumin+ (red), showing striatal cells in juveniles and adults. (C) Representative images at 100x magnification. (D–E) Juvenile mice had more MSNs in the injured striatum than adults, and the distribution of striatal neuron types in recovering juveniles was consistent with reports in healthy tissue. Data represent mean ± SEM, scale (A–B) = 100 μm, and scale (C) = 10 μm.
Neurobehavioral Outcomes
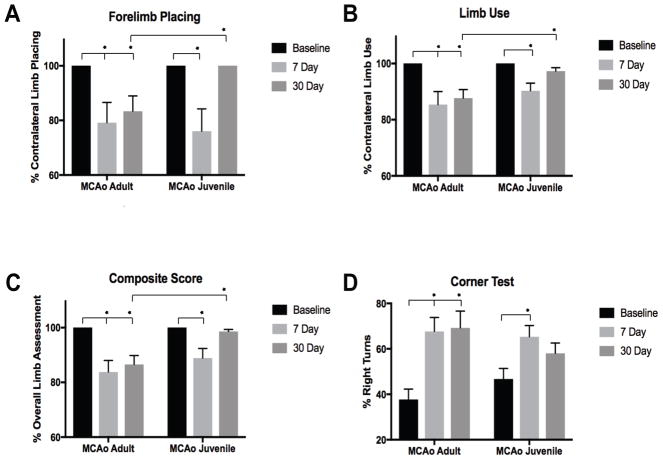
The analysis revealed significant reductions in use of the affected limb, motor coordination, and general motor functioning for MCAo-injured adults and juveniles at 7d post-injury, with only the juveniles returning to near baseline levels on most tasks by 30d. Sham-operated controls showed no neurological deficits or changes in behavior from baseline and are not included in the respective figures. There were no significant differences between stroke and sham-operated groups on the catalepsy grid task (p=0.512), or measures of horizontal locomotion: distance traveled (p=0.099) and speed (p=0.103) in the open field across time points, indicating that MCAo-injured mice did not have impaired ability to initiate movement or in locomotion, which may confound other behavioral measures.
On the forelimb placing task (Fig 5A), we found reductions (p=0.002) in the ability to place the affected limb for both adult 19.2±5.9% (p=0.006) and juvenile 25.8±5.5% (p<0.001) stroke-injured mice at 7d post-injury compared to baseline. However, by the 30d, impairment in adults 15.4±3.0% (p<0.001) continued, while juveniles demonstrated no deficits and had returned to baseline levels of functioning (p=1.000). Comparison of 7d and 30d in MCAo-injured adults and juvenile mice revealed no differences in forelimb placing deficits between groups at 7d post-injury (p=1.000), but at 30d, juveniles had greater recovery of limb placing compared to adults (p=0.003). Forelimb placing in MCAo-injured adult mice did not differ between 7d and 30d time points post-ischemia, while juveniles had recovery of placing in the affected forelimb from 7d to 30d (p=0.000). Further, at 30d following stroke, only adult MCAo-injured mice significantly differed from sham-operated controls (p=0.008), while juveniles had returned to similar levels of placing as sham-operated controls (p=1.000). On the forelimb limb use task (Fig 5B) we found reductions in limb use (p=0.006), adult 14.6±2.9% (p<0.001) and juvenile 9.7±2.7% (p=0.003) MCAo-injured mice used the affected, contralateral forelimb less at 7d compared to baseline. However, at 30d post-injury only the MCAo-injured adult mice 12.3±1.8% (p=0.000) had continued deficits in limb use, while juveniles no longer differed from baseline limb function 2.7±1.7% (p=0.345). At 30d only adult MCAo-injured mice significantly differed from sham-operated controls (p=0.000), while MCA0-injured juveniles displayed recovery of forelimb function (p=1.000). The composite score showed the same stroke-induced deficits at 7 days in both juvenile and adult mice that improved only in the juveniles, with no improvement in stroke-induced deficits observed in the adults (Fig 5C). Finally, figure 5D shows unilateral postural bias using the corner task in both juvenile and adults that recovers specifically in the juvenile mice 30 d after recovery from MCAO.
Figure 5. Neurobehavioral outcomes after ischemic stroke.
(A–B) Forelimb placing and spontaneous limb use, showing deficits in contralateral placing and limb use responses in both stroke groups at 7d post-ischemia; however, by 30d only the juvenile mice had returned to baseline levels, while adult mice showed continued impairment. (C) Composite score of overall impairment of the affected limb and general motor response measures, showing reduced limb function at 7d post-injury and recovery of functioning at 30d only in juvenile mice. (D) The corner test detected postural bias in both MCAo-injured adult and juvenile mice between baseline and 7d, but only adult mice had extended functional impairments at 30d.
Irradiation
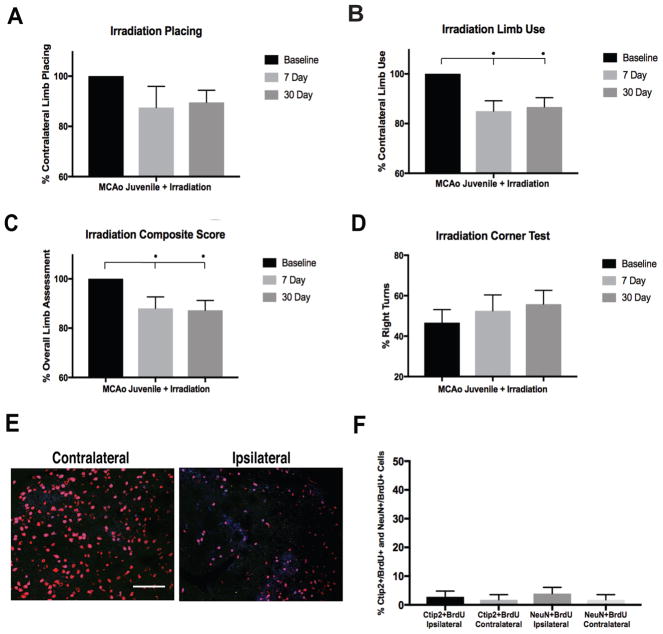
On the forelimb placing task (Fig 6A), we discovered that juvenile MCAo-injured+irradiation mice displayed reductions in the ability to place the affected forelimb at both 7d and 30d. We found decreased (p=0.001) use of the affected forelimb in juvenile MCAo-injured+irradiation mice (Fig 6B), at 7d post-injury+irradiation 15.0±4.2% (p=0.013) and 30d post-injury+irradiation 13.0±3.8% (p=0.014), demonstrating prolonged deficits in limb us compared to the recovery found at 30d in MCAo-nonirradiated, mice (p=1.000). Similarly, there was no recovery of behavioral function (composite score or corner task) found between the 7d and 30d time points (Fig 6D).
Figure 6. Loss of functional recovery on neurobehavioral outcomes following irradiation.
(A–B) Forelimb placing and spontaneous limb use, showing reduced contralateral placing responses in the MCAo+Irradiation group at 7d and 30d, with a reversal in recovery of function at 30d compared to non-irradiated juvenile mice. (C) On the composite measure MCAo+irradiation juvenile mice displayed decreased limb use and general motor functioning at both the 7d and 30d post-ischemic time points compared to baseline, with no recovery of motor function like non-irradiated mice. (D) Irradiated MCAo-injured mice had increased postural bias at 7d and 30d compared to baseline, showing continued deficits at chronic time points. (E–F) Representative images of FOV for analysis of BrdU+ (green), Ctip2+ (blue), and NeuN+ (red), showing that neurogenesis (Ctip2+/BrdU+ and NeuN+/BrdU+ positive cells) was abolished in the striatum following irradiation. Data represent mean ± SEM, scale = 100 μm.
Irradiation and newborn neuron maturation and survival
Following irradiation newborn neurons failed to mature and survive in the injured striatum of juveniles. At 30d post-ischemia, irradiated mice had no significant differences (p=0.839) between ipsilateral and contralateral hemispheres (Fig 6E–F) in the percentage newly generated MSNs (BrdU+/Ctip2+, 2.8±2.0%, ipsilateral and 1.7%±1.8, contralateral) or mature newborn neurons (BrdU+/NeuN+, 3.9±2.2% ipsilateral and 1.8%±1.8, contralateral). This is in sharp contrast to our earlier findings in MCAo-nonirradiated mice Fig 3E (25.8%, BrdU+/Ctip2+ and 32.6%, BrdU+/NeuN+), revealing that irradiation at 5Gy was sufficient to arrest neurogenesis in juveniles.
Discussion
Targeting neurogenesis to promote neuronal replacement and endogenous regenerative brain repair has long been intriguing, but the low survival rate of newborn neurons has left doubt about the therapeutic potential of neurogenesis. It has been widely accepted that our brains are not capable of significant self-repair and regeneration because most injury-induced newborn neurons die within 4 weeks of birth, lacking the ability to repair tissue and repopulate areas of damage (Zhao et al., 2008). Our findings show that juvenile brain appears to be capable of self-repair, as we observe a remarkable neuronal repopulation of the juvenile striatum that is coupled with improved behavioral outcomes after stroke that we do not observe in adults, providing strong evidence for enhanced neurogenesis and neuronal replacement following stroke in the injured juvenile brain.
From a clinical perspective, it is remarkable that newborn neurons migrate to the site of injury and repopulate areas where neurons have died. These observations not only increase our understanding of post-ischemic regeneration, but provide an opportunity to examine neuronal replacement following juvenile stroke as a novel therapeutic target for endogenous brain repair and recovery. These cells migrate, differentiate, and appear to replace the primary neurons lost following injury, region-specific medium spiny neurons, demonstrating the incredible potential for stroke-induced neurogenesis in the juvenile brain. This is in stark contrast with the multitude of studies in adult stroke that show a robust proliferation and migration of newborn neurons, which fail to survive within the ischemic core and die within 4 weeks (Jin et al., 2001, Zhang et al., 2001, Arvidsson et al., 2002, Parent et al., 2002, Lichtenwalner and Parent, 2006). In addition to the cellular findings, we also discovered differences in functional outcomes, revealing several deficits on neurobehavioral tasks that recovered only in the MCAo-injured juvenile mice and not adults, highlighting the role of neurogenesis in functional recovery after stroke. Our behavioral results provide powerful evidence for improved post-ischemic outcomes, with benefits seen across a variety of measures targeting region-specific behavioral changes. Investigating neurobehavioral deficits and potential mechanisms of repair in animal models of stroke is critical for developing translational applications that could promote improved recovery of function after injury. Following stroke that affects unilateral upper extremity function, humans tend to rely on their less-affected limb, which often undermines their ability to regain function in the affected limb (Taub et al., 2006). We found impairments on several measures of limb-use function in the affected limb of both juveniles and adults at 7d post-injury; however, we only found recovery of function in MCAo-injured juvenile mice at 30d post-injury, while MCAo-injured adults remained consistent or exhibited increased deficits at 30d. A sensorimotor function task (corner test) also revealed deficits in postural bias at 7d and 30d in MCAo-injured juvenile and adult mice, with MCAo-injured juveniles showing a reduction in right turns across time points compared to adults, and by 30d post-ischemia MCAo-injured juvenile mice performing similarly to sham-operated controls.
To further investigate neurogenesis as a mechanism of neuronal replacement and recovery following stroke, we arrested neurogenesis using ionizing irradiation and assessed differences in post-ischemic behavioral recovery. Ionizing irradiation is a commonly used method of myeloablation in the mouse that causes breaks in the DNA double-strand of mitotically active cells, and this damage leads to cell death through apoptosis and necrosis (Duran-Struuck and Dysko, 2009). Neurogenesis is sensitive to irradiation in a dose-dependent fashion. Following a 5–10Gy irradiation dose, apoptosis peaks at 12hr post-irradiation and production of new neurons is abolished at 48hr, proliferating cells are reduced by over 95% and immature migrating neuroblasts decreased by 55%–70% (Mizumatsu et al., 2003, Achanta et al., 2012). Following irradiation exposure to arrest hippocampal neurogenesis, memory and cognitive impairments have been reported (Raber et al., 2004, Rola et al., 2004, Raber et al., 2011, Olsen et al., 2017), and following irradiation+brain injury (Rosi et al., 2012, Allen et al., 2014). While less is known about effects of irradiation on motor functioning, we found that irradiation alone was well tolerated in mice and associated with loss of recovery, similar to previous reports. When we arrested neurogenesis in MCAo-injured juveniles with a single dose (5Gy) of ionizing irradiation, newborn neurons failed to mature and survive in the injured striatum of juveniles at 30d post-ischemia, and the behavioral recovery previously found at 30d was lost. In addition to diminished newborn neuron survival, irradiated juvenile mice had deficits on most tasks at 7d after stroke. However, in stark contrast to non-irradiated mice, there was continued impairment at 30d with no recovery observed at chronic time points. The irradiation findings implicate juvenile neurogenesis and endogenous neuronal renewal in functional recovery after stroke, supported by recovery of behavioral function only in non-irradiated MCAo-injured juvenile mice.
While the mechanisms underlying juvenile neurogenesis remain to be elucidated, it is evident that differences exist between the juvenile and adult brain, with the juvenile environment supporting neuronal survival. These findings support our previous observations of glial and white matter responses using this same model of ischemic stroke, which revealed very different glial responses and pathological sequelae after MCAo in juvenile and adult mice (Ahrendsen et al., 2016). In this study, equivalent cell death, neuronal loss, and diffuse astrogliosis were seen in the striatum of both groups at early time points following stroke (24hr, 3d, and 7d); however, oligodendrocyte progenitor cells, mature oligodendrocytes, and myelinated axons were spared in juveniles while significant decreases were found in adults. At 30d post-ischemia, dramatic differences in tissue damage were observed between juveniles and adults, with long-term preservation of brain parenchyma only observed in juvenile mice. Adults had severe axon pathology, ultrastructural damage, demyelination, glial scar formation, and major loss of striatal and cortical tissue compared to the juvenile brain, which had remarkable resistance to ischemic injury with no glial scarring and preservation of all major brain regions. These findings further define the unique environment that results in enhanced repair and recovery in the juvenile brain compared to adult.
That newborn neurons survive and repopulate stroke-damaged brain regions, and contribute to improved behavioral outcomes, is an innovative finding that supports the role of juvenile neurogenesis in neuronal replacement and enhanced functional recovery following ischemic stroke. Such studies are essential for understanding the role of neurogenesis as an intrinsic mechanism of brain repair and recover. Newborn neuron production, maturation, survival, and replacement in the developing brain has exciting implications for promoting post-stroke neurogenesis and functional recovery in adults and children alike.
Highlights.
Juvenile neurogenesis and neuronal replacement following cerebral ischemia.
Newborn cells found in adult mice post-ischemia, but most failed to reach maturity.
Enhanced motor recovery accompanied molecular results only in juveniles.
Neurogenesis arrested with irradiation and motor recovery in juveniles reversed.
New insights into mechanisms of post-ischemic regeneration, repair, and recovery.
Acknowledgments
Funding: This work was supported by the American Heart Association (Grant Number 16SDG30320001), the National Institute of Neurological Disorders and Stroke (Grant Number RO1NS092645), and the Henrietta B. and Frederick H. Bugher Foundation (Grant Number 14BFSC17690001). Imaging experiments were performed in the University of Colorado Anschutz Medical Campus Advance Light Microscopy Core supported in part by the National Center for Advancing Translational Sciences at the National Institutes of Health, Colorado Translational Sciences Institute (Grant Number UL1 TR001082).
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achanta P, Capilla-Gonzalez V, Purger D, Reyes J, Sailor K, Song H, Garcia-Verdugo JM, Gonzalez-Perez O, Ford E, Quinones-Hinojosa A. Subventricular zone localized irradiation affects the generation of proliferating neural precursor cells and the migration of neuroblasts. Stem Cells. 2012;30:2548–2560. doi: 10.1002/stem.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrendsen JT, Grewal HS, Hickey SP, Culp CM, Gould EA, Shimizu T, Strnad FA, Traystman RJ, Herson PS, Macklin WB. Juvenile striatal white matter is resistant to ischemia-induced damage. Glia. 2016 doi: 10.1002/glia.23036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AR, Eilertson K, Chakraborti A, Sharma S, Baure J, Habdank-Kolaczkowski J, Allen B, Rosi S, Raber J, Fike JR. Radiation exposure prior to traumatic brain injury induces responses that differ as a function of animal age. Int J Radiat Biol. 2014;90:214–223. doi: 10.3109/09553002.2014.859761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, Macklis JD. Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:622–632. doi: 10.1523/JNEUROSCI.2986-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Balkaya M, Krober JM, Rex A, Endres M. Assessing post-stroke behavior in mouse models of focal ischemia. J Cereb Blood Flow Metab. 2013;33:330–338. doi: 10.1038/jcbfm.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Pillai RN, Aronowski J, Grotta JC, Schallert T. Early overuse and disuse of the affected forelimb after moderately severe intraluminal suture occlusion of the middle cerebral artery in rats. Behav Brain Res. 2001;126:33–41. doi: 10.1016/s0166-4328(01)00243-1. [DOI] [PubMed] [Google Scholar]
- Bland ST, Schallert T, Strong R, Aronowski J, Grotta JC, Feeney DM. Early exclusive use of the affected forelimb after moderate transient focal ischemia in rats: functional and anatomic outcome. Stroke. 2000;31:1144–1152. doi: 10.1161/01.str.31.5.1144. [DOI] [PubMed] [Google Scholar]
- Brenneis C, Kistner K, Puopolo M, Segal D, Roberson D, Sisignano M, Labocha S, Ferreiros N, Strominger A, Cobos EJ, Ghasemlou N, Geisslinger G, Reeh PW, Bean BP, Woolf CJ. Phenotyping the function of TRPV1-expressing sensory neurons by targeted axonal silencing. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:315–326. doi: 10.1523/JNEUROSCI.2804-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci. 2009;10:519–529. doi: 10.1038/nrn2652. [DOI] [PubMed] [Google Scholar]
- Burkey AR, Carstens E, Wenniger JJ, Tang J, Jasmin L. An opioidergic cortical antinociception triggering site in the agranular insular cortex of the rat that contributes to morphine antinociception. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:6612–6623. doi: 10.1523/JNEUROSCI.16-20-06612.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HT, Kita H. Interneurons in the rat striatum: relationships between parvalbumin neurons and cholinergic neurons. Brain Res. 1992;574:307–311. doi: 10.1016/0006-8993(92)90830-3. [DOI] [PubMed] [Google Scholar]
- Cooper-Kuhn CM, Kuhn HG. Is it all DNA repair? Methodological considerations for detecting neurogenesis in the adult brain. Brain research Developmental brain research. 2002;134:13–21. doi: 10.1016/s0165-3806(01)00243-7. [DOI] [PubMed] [Google Scholar]
- Danzer SC. Postnatal and adult neurogenesis in the development of human disease. Neuroscientist. 2008;14:446–458. doi: 10.1177/1073858408317008. [DOI] [PubMed] [Google Scholar]
- Duran-Struuck R, Dysko RC. Principles of bone marrow transplantation (BMT): providing optimal veterinary and husbandry care to irradiated mice in BMT studies. J Am Assoc Lab Anim Sci. 2009;48:11–22. [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson PS, Bombardier CG, Parker SM, Shimizu T, Klawitter J, Quillinan N, Exo JL, Goldenberg NA, Traystman RJ. Experimental pediatric arterial ischemic stroke model reveals sex-specific estrogen signaling. Stroke. 2013;44:759–763. doi: 10.1161/STROKEAHA.112.675124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- Jia J, Verma S, Nakayama S, Quillinan N, Grafe MR, Hurn PD, Herson PS. Sex differences in neuroprotection provided by inhibition of TRPM2 channels following experimental stroke. J Cereb Blood Flow Metab. 2011;31:2160–2168. doi: 10.1038/jcbfm.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell CS, Liebeskind DS, Starkman S, Saver JL. Trends in acute ischemic stroke trials through the 20th century. Stroke. 2001;32:1349–1359. doi: 10.1161/01.str.32.6.1349. [DOI] [PubMed] [Google Scholar]
- Korda RJ, Douglas JM. Attention deficits in stroke patients with aphasia. J Clin Exp Neuropsychol. 1997;19:525–542. doi: 10.1080/01688639708403742. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Schloemer AJ, Lu A, Burns KA, Weng WL, Williams MT, Strauss KI, Vorhees CV, Flavell RA, Davis RJ, Sharp FR, Rakic P. Hypoxia-ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:10763–10772. doi: 10.1523/JNEUROSCI.3883-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab. 2006;26:1–20. doi: 10.1038/sj.jcbfm.9600170. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Chopp M, Zhang RL, Zhang ZG. MicroRNAs in cerebral ischemia-induced neurogenesis. J Neuropathol Exp Neurol. 2013;72:718–722. doi: 10.1097/NEN.0b013e31829e4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markgraf CG, Green EJ, Hurwitz BE, Morikawa E, Dietrich WD, McCabe PM, Ginsberg MD, Schneiderman N. Sensorimotor and cognitive consequences of middle cerebral artery occlusion in rats. Brain Res. 1992;575:238–246. doi: 10.1016/0006-8993(92)90085-n. [DOI] [PubMed] [Google Scholar]
- Mercier L, Audet T, Hebert R, Rochette A, Dubois MF. Impact of motor, cognitive, and perceptual disorders on ability to perform activities of daily living after stroke. Stroke. 2001;32:2602–2608. doi: 10.1161/hs1101.098154. [DOI] [PubMed] [Google Scholar]
- Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C. Neuroprotection for stroke: current status and future perspectives. Int J Mol Sci. 2012;13:11753–11772. doi: 10.3390/ijms130911753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Nitz AJ, Dobner JJ, Matulionis DH. Pneumatic tourniquet application and nerve integrity: motor function and electrophysiology. Exp Neurol. 1986;94:264–279. doi: 10.1016/0014-4886(86)90101-9. [DOI] [PubMed] [Google Scholar]
- Olsen RH, Weber SJ, Akinyeke T, Raber J. Enhanced cued fear memory following post-training whole body irradiation of 3-month-old mice. Behav Brain Res. 2017;319:181–187. doi: 10.1016/j.bbr.2016.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Raber J, Villasana L, Rosenberg J, Zou Y, Huang TT, Fike JR. Irradiation enhances hippocampus-dependent cognition in mice deficient in extracellular superoxide dismutase. Hippocampus. 2011;21:72–80. doi: 10.1002/hipo.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Rosi S, Ferguson R, Fishman K, Allen A, Raber J, Fike JR. The polyamine inhibitor alpha-difluoromethylornithine modulates hippocampus-dependent function after single and combined injuries. PLoS One. 2012;7:e31094. doi: 10.1371/journal.pone.0031094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saposnik G, Bueri JA, Rey RC, Sica RE. Catalepsy after stroke. Neurology. 1999;53:1132–1135. doi: 10.1212/wnl.53.5.1132. [DOI] [PubMed] [Google Scholar]
- Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Exp Transl Stroke Med. 2010;2:13. doi: 10.1186/2040-7378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibenhener ML, Wooten MC. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015:e52434. doi: 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med. 2014;2:80. doi: 10.3978/j.issn.2305-5839.2014.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys. 2006;42:241–256. [PubMed] [Google Scholar]
- Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab. 2014;34:1573–1584. doi: 10.1038/jcbfm.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodlee MT, Asseo-Garcia AM, Zhao X, Liu SJ, Jones TA, Schallert T. Testing forelimb placing “across the midline” reveals distinct, lesion-dependent patterns of recovery in rats. Exp Neurol. 2005;191:310–317. doi: 10.1016/j.expneurol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]