Abstract
Introduction
Identification of factors associated with progression of cognitive symptoms in Parkinson’s disease (PD) is important for treatment planning, clinical care, and design of future clinical trials. The current study sought to identify whether prediction of cognitive progression is aided by examining baseline cognitive features, and whether this differs according to stage of cognitive disease.
Methods
Participants with PD in the Pacific Udall Center Clinical Consortium who had longitudinal data available and were nondemented at baseline were included in the study (n=418). Logistic and Cox regression models were utilized to examine the relationship between cognitive, demographic, and clinical variables with risk and time to progression from no cognitive impairment to mild cognitive impairment (PD-MCI) or dementia (PDD), and from PD-MCI to PDD.
Results
Processing speed (OR=1.05, p=0.009) and working memory (OR=1.01, p=0.03) were associated with conversion to PDD among those with PD-MCI at baseline, over and above demographic variables. Conversely, the primary predictive factor in the transition from no cognitive impairment to PD-MCI or PDD was male sex (OR = 4.47, p=0.004), and males progressed more rapidly than females (p=0.01). Further, among females with shorter disease duration, progression was slower than for their male counterparts, and poor baseline performance on semantic verbal fluency was associated with shorter time to cognitive impairment in females but not in males.
Conclusions
This study provides evidence for sex differences in the progression to cognitive impairment in PD, while specific cognitive features become more important indicators of progression with impending conversion to PDD.
Keywords: Parkinson’s disease, cognition, dementia, mild cognitive impairment, sex differences
Parkinson’s disease (PD) is strongly associated with the development of cognitive impairment, widely recognized as one of the most frequent nonmotor symptoms of the disease [1]. Among newly diagnosed individuals, the prevalence of mild cognitive impairment (PD-MCI) approaches 30%, and dementia (PDD) is increasingly recognized as an eventual and almost inevitable consequence of PD [2]. The development of cognitive symptoms during the course of PD is associated with decreased quality of life and loss of independence [3, 4]. Identification of specific features that predict progression of cognitive symptoms could have meaningful implications for treatment planning and clinical management for patients with PD, and may provide guidance for future clinical trial design.
Progression of cognitive symptoms in PD is recognized to vary widely, with some patients remaining relatively stable for many years, while others progress more rapidly to dementia [5]. In terms of specific cognitive features, PDD is often associated most strongly with cognitive functions that are largely mediated by dopamine-independent posterior-cortical brain regions [6]. Fronto-striatal deficits, on the other hand, which primarily impact executive abilities and attention, are considered nearly universal in PD but may be less closely associated with the dementia syndrome [6, 7]. This is not consistent, however, as progression to both PD-MCI and PDD is associated with decline in executive skills and attention in some studies [8, 9]. Given the small sample sizes of prior studies and the known heterogeneity of cognitive dysfunction in PD, however, it is difficult to generalize these results.
The current study compares baseline cognitive, demographic, and clinical characteristics of participants who remain cognitively stable and those who progress over the course of follow up in a large, well-characterized prevalent PD cohort. We seek to identify whether prediction of cognitive symptom progression is aided by examining baseline cognitive test performance, and whether this differs according to stage of cognitive disease.
Methods
Subjects
Participants were drawn from the Pacific Udall Center Clinical Consortium, a collaboration between multiple institutions that enroll prevalent idiopathic PD cohorts with a goal of harmonizing detailed clinical and neuropsychological evaluation, as previously described [10]. This study includes three sites with currently available longitudinal data: University of Washington/Veterans Affairs Puget Sound Health Care System and Oregon Health Sciences University/Veterans Affairs Portland Health Care System, together comprising the Pacific Udall Center, and the Udall Center at Johns Hopkins University.
All participants met the United Kingdom Parkinson’s Disease Society Brain Bank (UKBB) clinical diagnostic criteria for PD, had cognitive diagnostic information available, and had at least one follow up visit (n=567). Sixty-nine participants were diagnosed with dementia at baseline and one participant had “unknown” cognitive status due to potential confounding information that precluded a final cognitive diagnosis. Of the remaining 497 participants, 19 participants reverted from PD-MCI to no cognitive impairment (consistent with prior literature [11]) and 60 participants were missing data, for a total of 418 participants available for analyses. The institutional review board of each participating institution approved the study, and all participants provided written informed consent.
Cognitive diagnosis and variables
Participants were assigned motor and cognitive diagnoses at a clinical diagnostic consensus conference. Cognitive diagnoses were made using published diagnostic criteria for PDD [12] and PD-MCI [13]. Extensive neuropsychological and clinical assessments were available for determination of cognitive diagnosis and permitted assignment of diagnosis using PD-MCI Level II criteria [10].
A set of core cognitive variables that have been administered since the inception of the cohort and are given across all sites were included the current analyses. These include: 1) Montreal Cognitive Assessment; 2) Hopkins Verbal Learning Test-Revised, a list learning test that assesses immediate verbal learning, delayed recall, and recognition memory; 3) Letter-Number Sequencing and Trail Making Test (Part B minus Part A), which measure auditory and visuospatial working memory, respectively; 4) Digit Symbol, a measure of processing speed/working memory; 5) Judgment of Line Orientation, a measure of visuospatial ability, and 6) semantic and phonemic verbal fluency. All participants were rated in the ON state if they were taking PD medications.
Secondary variables
History of cardiovascular risk and hypertension (from the Hachinski Ischemic Index), head injury, and past alcohol and tobacco use were collected at baseline. Genomic DNA was prepared using standard procedures as described previously [14, 15]. Genes previously associated with cognitive function in the PD Cognitive Genetics Consortium were included in the current analyses and included 1) loss of function mutations in the glucocerebrosidase (GBA) gene as well as the E326K single nucleotide polymorphism in the GBA gene, and 2) presence of an apolipoprotein E ε4 (APOE ε4) allele.
Statistical analyses
Group differences in baseline clinical, demographic, and cognitive characteristics were assessed using t-tests, Kruskall-Wallis tests, or chi-square tests. To determine the association between baseline cognitive test scores and subsequent conversion from one diagnostic group to the next, separate logistic regression models were run for conversion from no cognitive impairment to cognitive impairment (PD-MCI or PDD) and from PD-MCI to PDD. Model 1 included age, sex, education, disease duration, site, and length of follow-up; Model 2 added the cognitive variables. Receiver operating characteristic (ROC) curves were calculated for all models; comparisons between Model 1 and Model 2 areas under the curve (AUC) were made using the DeLong, DeLong, and Clarke-Pearson algorithm. Hosmer-Lemeshow goodness of fit tests were calculated for each model. Separate Cox regression analyses were performed to assess time to conversion from no cognitive impairment to PD-MCI or PDD and from PD-MCI to PDD, including all cognitive variables and controlling for age, sex, education, disease duration, and site. Kaplan-Meier estimates were calculated for time to cognitive impairment for males and females, with log-rank tests performed to determine whether there was a significant difference between the curves. Kaplan–Meier curves were also generated separately for males and females for variables that interacted significantly with sex in the Cox regression analyses, using a median split for the variables of interest. Secondary analyses included genetic status and additional clinical variables, as well as more detailed examination of baseline differences between male and female participants. All analyses were performed using Stata 14.2.
Results
At baseline, cognitively impaired groups (PD-MCI and PDD) were older, more likely to be male, more likely to be an armed forces Veteran, and had more severe motor symptoms. Those with dementia had longer disease duration and were significantly more likely to carry a GBA variant. Group differences between cognitive diagnostic category were present for all neuropsychological measures (Supplemental data). Over an average of 3.2 years of follow up, 38.6% of those with no cognitive impairment at baseline converted to PD-MCI, and 4.8% progressed to PDD. Among those with PD-MCI at baseline, 24.4% progressed to PDD.
Mild cognitive impairment, stable vs. mild cognitive impairment, progressed
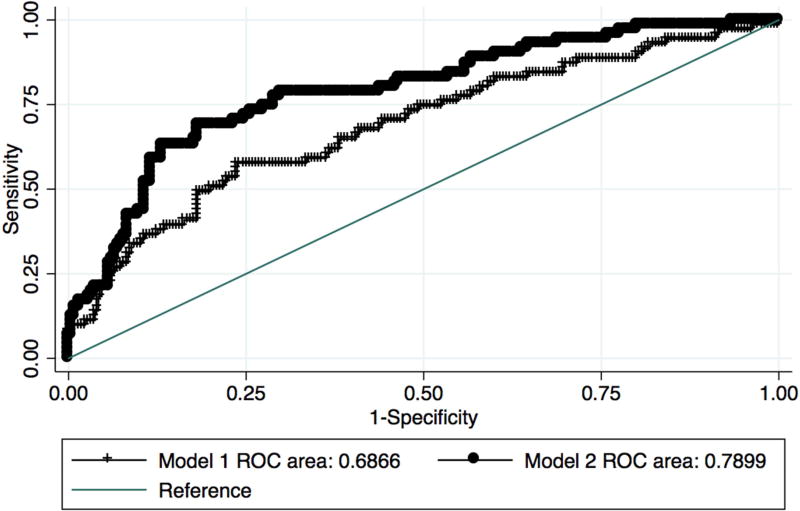
Baseline characteristics of those with PD-MCI at baseline who subsequently converted to PDD and those who remained stable are presented in Table 1. In logistic regression Model 1, conversion from PD-MCI to PDD was associated with older age (OR = 1.07 95% confidence interval 1.04 – 1.11, p<0.001). When the 10 cognitive variables were included, age was no longer statistically significant, while baseline processing speed (Digit Symbol: OR = 1.05 95 % confidence interval 1.01 – 1.09, p = 0.009) and visuospatial working memory (Trail Making Test B – A: OR = 1.01 95% confidence interval 1.00 – 1.01, p = 0.03) were associated with conversion to PDD. Prediction of conversion was significantly improved when cognitive scores were included as compared to the demographic-only model (Figure 1, AUC 0.69, 95% confidence interval 0.61 – 0.76 vs. 0.79, 95% confidence interval 0.73 – 0.85, χ2 = 11.71, p=0.0006).
Table 1.
Baseline characteristics, by subsequent cognitive progression vs. cognitive stability
| NCI at baseline | PD-MCI at baseline | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| NCI, Progressed (n=63) |
NCI, Stable (n=81) |
p | PD-MCI, Progressed (n = 86) |
PD-MCI, Stable (n = 248) |
p | |
| Demographic/Clinical Variables | ||||||
|
| ||||||
| Age yrs | ||||||
| mean (sd) | 65.5 (7.3) | 60.7 (8.9) | 0.0008 | 71.2 (9.1) | 66.9 (8.0) | <0.0001 |
| range | 48.8 – 81.8 | 43.0 – 83.9 | 36.2 – 90.1 | 46.1 – 89.2 | ||
|
| ||||||
| Education yrs | ||||||
| mean (sd) | 16.0 (2.5) | 16.5 (2.5) | - | 15.6 (2.6) | 15.9 (2.5) | - |
| range | 12 – 20 | 12 – 20 | 9 – 20 | 8 – 20 | ||
|
| ||||||
| Sex | ||||||
| N, % male | 38, 60.3 | 32, 39.5 | 0.01 | 66, 76.7 | 177, 71.4 | - |
|
| ||||||
| MDS UPDRS, Part 3 | ||||||
| mean (sd) | 22.2 (9.4) | 20.5 (9.9) | - | 28.5 (10.3) | 27.0 (12.2) | - |
| range | 5 – 47 | 3 – 56 | 10 – 57 | 3 – 66 | ||
|
| ||||||
| Hoehn and Yahr | ||||||
| median (range) | 2 (1 – 4) | 2 (1 – 3) | - | 2.5 (1.5 – 5) | 2 (1 – 5) | 0.006 |
|
| ||||||
| Disease duration yrs | ||||||
| mean (sd) | 8.3 (4.8) | 7.2 (4.8) | - | 9.5 (6.0) | 8.7 (6.2) | - |
| range | 1 – 21 | 0 – 21 | 1 – 25 | 0 – 32 | ||
|
| ||||||
| Follow up yrs | ||||||
| mean (sd) | 3.6 (1.6) | 3.4 (1.6) | - | 3.2 (1.4) | 3.2 (1.5) | - |
| range | 1.3 – 6.9 | 1.1 – 6.9 | 1.0 – 6.8 | 0.9 – 7.2 | ||
|
| ||||||
| Veteran | ||||||
| N, % | 10, 23.8 | 8, 14.0 | - | 26, 44.1 | 60, 38.7 | - |
|
| ||||||
| APOE ε4 carrier | 11, 18.3 | 18, 21.0 | - | 17, 20.5 | 58, 23.7 | - |
| N, % | ||||||
|
| ||||||
| GBA mutation carrier | ||||||
| N, % | 7, 11.3 | 6, 7.4 | - | 6, 7.0 | 19, 7.7 | - |
|
| ||||||
| Head injury ever | ||||||
| N, % | 11, 29.7 | 12, 23.5 | - | 15, 30.0 | 41, 26.3 | - |
|
| ||||||
| Alcohol use, current | ||||||
| N, % | 28, 57.1 | 35, 53.9 | - | 22, 36.1 | 90, 46.9 | - |
|
| ||||||
| Alcohol use, ever | ||||||
| N, % | 37, 75.5 | 47, 72.3 | - | 44, 73.3 | 148, 75.9 | - |
|
| ||||||
| Tobacco use, ever | ||||||
| N, % | 20, 41.7 | 14, 24.1 | 0.05 | 28, 49.1 | 79, 41.2 | - |
|
| ||||||
| Tobacco use, current | ||||||
| N, % | 1, 2.0 | 1, 1.6 | - | 1, 1.7 | 5, 2.6 | - |
|
| ||||||
| Hachinski Ischemic Scale | ||||||
| median (range) | 0 (0 – 3) | 0 (0 – 6) | - | 0 (0 – 4) | 0 (0 – 4) | - |
|
| ||||||
| Hypertension (Y/N) | ||||||
| N,% | 21, 42.0 | 14, 21.2 | 0.02 | 24, 39.3 | 70, 36.8 | - |
|
| ||||||
| Cognitive Variables | ||||||
|
| ||||||
| MOCA | ||||||
| mean (sd) | 27.1 (2.0) | 27.9 (1.9) | 0.02 | 24.1 (2.4) | 25.1 (2.3) | 0.001* |
| range | 22 – 30 | 22 – 30 | 17 – 29 | 18 – 30 | ||
|
| ||||||
| HVLT-R Immediate Recall | ||||||
| mean (sd) | 26.4 (3.5) | 28.3 (3.0) | 0.001* | 19.1 (4.8) | 21.7 (4.4) | <0.0001* |
| range | 21 – 34 | 19 – 36 | 9 – 30 | 12 – 33 | ||
|
| ||||||
| HVLT-R Delayed Recall | ||||||
| mean (sd) | 9.3 (2.0) | 10.4 (2.1) | 0.004* | 5.5 (3.0) | 6.8 (3.2) | 0.001* |
| range | 1 – 12 | 0 – 12 | 0 – 11 | 0 – 12 | ||
|
| ||||||
| HVLT- R RDI | ||||||
| mean (sd) | 10.6 (2.0) | 11.1 (1.1) | - | 8.7 (2.5) | 9.3 (2.3) | 0.05 |
| range | 0 – 12 | 8 – 12 | 0 – 12 | 0 – 12 | ||
|
| ||||||
| Digit Symbol | ||||||
| mean (sd) | 48.4 (9.7) | 52.9 (10.4) | 0.01 | 34.1 (9.6) | 41.4 (10.6) | <0.0001* |
| range | 30 – 82 | 30 – 77 | 2 – 55 | 18 – 79 | ||
|
| ||||||
| Letter Number Sequencing | ||||||
| mean (sd) | 10. 6 (2.4) | 11.7 (2.4) | 0.01 | 7.9 (2.7) | 9.3 (2.4) | 0.0001* |
| range | 3 – 17 | 6 – 18 | 0 – 14 | 2 – 18 | ||
|
| ||||||
| Trailmaking Test, Part A | ||||||
| mean (sd) | 30.4 (10.6) | 27.8 (12.3) | - | 49.0 (23.9) | 36.9 (14.0) | <0.0001* |
| range | 13 – 65 | 15 – 116 | 17 – 150 | 16 – 112 | ||
|
| ||||||
| Trailmaking Test, Part B | ||||||
| mean (sd) | 71.3 (25.6) | 64.5 (36.7) | - | 153.1 (67.3) | 103.7 (52.7) | <0.0001* |
| range | 34 – 128 | 30 – 300 | 45 – 300 | 29 – 300 | ||
|
| ||||||
| Trailmaking Test, B - A | ||||||
| mean (sd) | 41.1 (21.7) | 36.7 (34.6) | - | 104.3 (60.6) | 66.3 (47.2) | <0.0001* |
| range | 13 – 110 | 7 – 272 | 20 – 258 | 7 – 264 | ||
|
| ||||||
| Semantic verbal fluency | ||||||
| mean (sd) | 21.4 (4.5) | 24.0 (4.3) | <0.001* | 16.2 (5.0) | 19.0 (4.7) | <0.0001* |
| range | 13 – 34 | 14 – 34 | 5 – 33 | 8 – 32 | ||
|
| ||||||
| Phonemic verbal fluency | ||||||
| mean (sd) | 46.6 (12.4) | 49.9 (12.1) | - | 35.9 (11.8) | 38.8 (11.1) | 0.04 |
| range | 23 – 93 | 22 – 90 | 15 – 66 | 8 – 81 | ||
|
| ||||||
| Judgment of Line Orientation | ||||||
| mean (sd) | 12.8 (2.0) | 13.2 (1.8) | - | 11.6 (2.0) | 12.2 (2.4) | 0.05 |
| range | 6 – 15 | 9 – 15 | 8 – 15 | 0 – 15 | ||
Abbrev: APOE, apolipoprotein; GBA, glucocerebrosidase; HVLT-R, Hopkins Verbal Learning Test-Revised; MCI, mild cognitive impairment; MDS UPDRS, Movement Disorders Society Unified Parkinson’s Disease Rating Scale; MoCA, Montreal Cognitive Assessment; NCI, no cognitive impairment; NSAIDs, non-steroidal anti-inflammatory drugs; PD, Parkinson’s disease; RDI, recognition discriminability index; sd, standard deviation
p-values remain significant after applying a Bonferroni correctiion for multiple comparisons
Figure 1. Predictors of progression from PD-MCI to PDD.
Comparison of area under the curve (AUC) for Model 1 (age, education, sex, disease duration, site, follow up time) and Model 2 (Model 1 variables plus cognitive variables).
Cox regression analyses were performed to determine which variables were associated with time to progression from PD-MCI to PDD. Again, poorer performance on processing speed (HR=1.01 95% confidence interval 1.00 – 1.01, p = 0.001) and visuospatial working memory (HR=1.03 95% confidence interval 1.00 – 1.06, p = 0.04) was significantly associated with faster time to conversion. Demographic variables were not associated with time to conversion over and above these cognitive measures.
No cognitive impairment, stable vs. no cognitive impairment, progressed
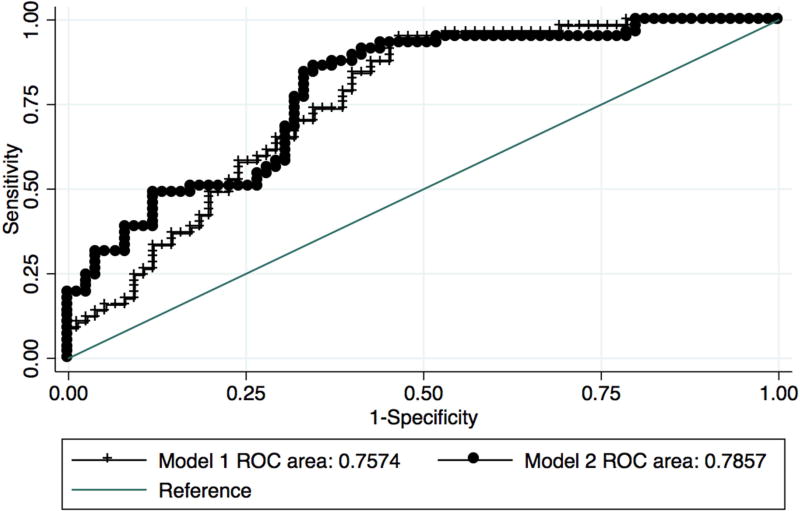
Baseline characteristics of those with no cognitive impairment at baseline who progressed and those who remained stable are presented in Table 1. Conversion from no cognitive impairment at baseline to PD-MCI or PDD was associated with older age (OR = 1.10 95% confidence interval 1.05 – 1.15, p<0.001), male sex (OR = 3.87 95% confidence interval 1.74 – 8.62, p = 0.001), and lower education (OR = 1.18 95% confidence interval 1.01 – 1.39, p=0.041) in Model 1. When neuropsychological test variables were added in Model 2, sex was the only demographic variable that remained significant (OR = 4.47 95% CI 1.61 – 12.39, p=0.004). Of the cognitive variables, only semantic verbal fluency was a significant predictor (OR = 1.13 95% confidence interval 1.01 – 1.27, p = 0.03). However, the addition of the cognitive variables did not significantly improve the predictive value of the model (Figure 2, AUC 0.76, 95% confidence interval 0.68 – 0.84 vs. 0.79, 95% confidence interval 0.71 – 0.86, χ2 = 2.09, ns). Goodness of fit tests indicated that Model 1 was a better fit for prediction of conversion.
Figure 2. Predictors of progression from no cognitive impairment to PD-MCI or PDD.
Comparison of area under the curve (AUC) for Model 1 (age, education, sex, disease duration, site, follow up time) and Model 2 (Model 1 variables plus cognitive variables).
Cox regression analyses indicated that older age (HR per year = 1.05 95% confidence interval 1.01 – 1.10, p=0.02), fewer years of education (HR=1.19 95% confidence interval 1.03 – 1.37, p = 0.02), male sex (HR= 2.96 95% confidence interval 1.51 – 5.80, p = 0.002), study site (Portland, HR= 2.59 95% confidence interval 1.09 – 6.17, p = 0.03), and poorer performance on semantic verbal fluency (HR=1.10 95% confidence interval 1.00 – 1.20, p = 0.04) were associated with faster time to conversion.
Sex differences
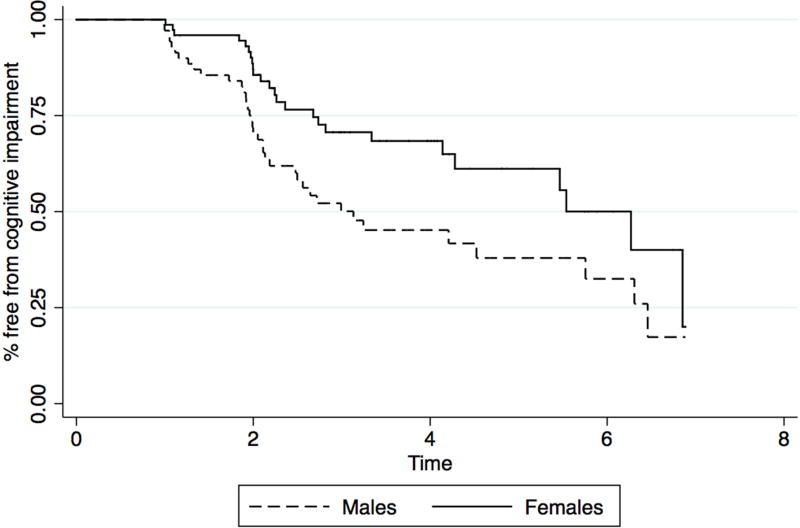
Given the consistent association between sex and cognitive diagnosis and cognitive progression, sex differences were further evaluated. Among those who were not cognitively impaired at baseline, females had longer disease duration (8.5 years vs. 6.8 years, p = 0.03), were less likely to endorse alcohol use (ever) (63% vs. 85%, p = 0.006), and were less likely to endorse veteran status (5% vs. 33%, p= 0.001). On cognitive measures, baseline sex differences were found for verbal learning and memory (women > men) and on visuospatial function (men > women), in line with previously reported sex differences in healthy populations [17]. Kaplan-Meier survival estimates showed that initially cognitively normal males progressed to cognitive impairment more rapidly than females (Figure 3), and the log rank test indicated this difference was statistically significant (χ2 = 6.47, p=0.01). Cox regression analyses yielded an interaction between sex and disease duration (p = 0.04), such that females with shorter disease duration progressed more slowly to cognitive impairment than males, while those with longer disease duration progressed at rates more similar to their male counterparts. A comparison of Kaplan-Meier curves indicates that there is a statistical trend for time to conversion based on a median split of disease duration for females but not for males (χ2 = 3.51, p=0.06 vs. χ2 = 0.61, ns). Analyses also indicated a trend for an interaction between sex and performance on semantic verbal fluency (p = 0.08), such that females with poorer performance on semantic verbal fluency was associated with shorter time to cognitive impairment, while a similar pattern was not found for males. A comparison of Kaplan-Meier curves indicated a statistically significant difference when a median split is applied to the test score for females, but not for males (χ2 = 10.89, p=0.001 vs. χ2 = 1.41, ns). There was no correlation or interaction between disease duration and semantic verbal fluency for males or females, suggesting that these variables are independent risk factors for cognitive progression in women.
Figure 3. Kaplan-Meier survival estimates for initially cognitively unimpaired males and females.
Males progressed more rapidly to cognitive impairment than females.
Secondary analyses
Genetic status, motor severity scores, past or current use of alcohol or tobacco, head injury history, veteran status, and hypertension were not associated with conversion or time to conversion from PD-MCI to PDD nor from no cognitive impairment to PD-MCI or PDD. However, higher total Hachinski score was associated with faster time to conversion from no cognitive impairment to PD-MCI or PDD (HR 1.58 95% confidence interval 1.18 – 2.12, p = 0.002) after controlling for demographic factors.
DISCUSSION
In this prevalent PD cohort, we found that cognitive tests measuring processing speed and working memory were predictive of the transition from PD-MCI to PDD over and above demographic and clinical factors. Conversely, the primary predictive factor in the transition from no cognitive impairment to cognitive impairment was male sex, while for this group the addition of cognitive variables did not add significantly to the predictive model.
The hallmark pathologic feature in PD involves dopamine depletion that impacts fronto-striatal pathways, and thus executive and attention features of cognition are most commonly adversely affected even early on in the disease.[18, 19] Dopamine-independent processes are also prominent, including loss of cholinergic neurons that impact projections to temporal-parietal regions and related cognitive functions, and are reported to be important for progression to PDD [6, 7]. However, recent longitudinal studies suggest a role for reduced executive function and attention in the prediction of decline to PDD as well [4, 11]. Here, we demonstrate that primarily frontal/executive functions (processing speed, working memory) are associated with progression from PD-MCI to PDD in our large, well-characterized sample.
Conversely, semantic verbal fluency, often considered to be mediated by more posterior cortical regions, was associated with progression from no cognitive impairment to cognitive impairment. Despite this association, the inclusion of cognitive variables could not overcome the powerful impact of the demographic model, driven primarily by male sex. Sex differences in PD are widely reported [20, 21], and are largely, although not uniformly, attributed to potential neuroprotective effects of estrogen on dopaminergic pathways and potential differences in the manner in which sex chromosomes contribute to the development of the dopaminergic system [22, 23]. Recent studies have shown that males are more likely to have prevalent cognitive impairment and may be at higher risk for developing dementia [24–26]. Consistent with our results, Cereda et al. [1] reported a higher prevalence of dementia among males with PD within a large PD database, and Pigott et al. [5] found that among initially cognitively normal PD participants, male sex was a significant predictor of subsequent cognitive decline. However, in a study of participants with PD that examined conversion from no cognitive impairment to MCI, and from MCI to PDD over the course of 5 years, no statistically significant sex differences were reported [27], although the total sample size for this study was smaller than ours, and there was a statistical trend for a higher proportion of initially cognitively intact males converting to MCI.
Further exploration into sex differences in the current study consistently support that female participants with PD progress more slowly to cognitive impairment than male participants. This is corroborated both by examination of cross-sectional baseline data, in which there was a significantly lower prevalence of cognitive impairment despite longer disease duration in females, and by the overall slower time to reach cognitive impairment in females compared to males in longitudinal follow up. Intriguingly, in secondary analyses, we found that worse performance on semantic fluency and longer disease duration were independently associated with a shorter time to cognitive impairment in females, but not in males. These results support the possibility that the underlying pathophysiology for cognitive impairment at least partially differ for men and women with PD.
We also examined specific risk factors in secondary analyses, and found that higher total Hachinski score was associated with faster time to conversion from no cognitive impairment to PD-MCI or PDD, irrespective of sex. This is supported by other recent studies emphasizing an independent role of vascular risk factors on cognitive performance, particularly on measures of executive function and notably early on in the disease process [28, 29]. Interestingly, we did not find a relationship between vascular risk factors and conversion from PD-MCI to PDD, suggesting that focusing on control of vascular risk factors may be important earlier in the disease process. However, studies that explore vascular risk factors in more detail are important to determine at which stages these variables pose greatest risk for cognitive progression.
Despite the large sample size, this study has limitations. First, we were unable to follow the natural history of cognitive impairment from the time of first PD diagnosis in all participants. However, separating the cohort by baseline cognitive status allowed us to examine different stages of cognitive impairment over a shorter duration, while including disease duration in the analyses permitted additional control. Further, although our sample size was sufficient to assess the predictive value of demographic and cognitive factors, it limited our ability to make conclusions about the impact of genetic status on cognitive progression. In a related study in which we examined motor and cognitive progression in a larger genetic cohort, both APOE ε4 status and GBA variants were associated with progression to dementia [30]. Given that the additional cohorts included in the earlier study did not undergo identical consensus diagnosis procedures, they were not included in the current study. We were also unable to examine the potential mediating effects of specific medications, such as antidepressants and sedatives, on cognition in this study. Finally, information pertaining to vascular risk factors was limited in scope and to self-report data. Additional work related to the influence of vascular risk factors on cognitive impairment in PD is needed.
PD is associated with multiple parallel degenerative processes in the aging brain that result in variable cognitive profiles, but that ultimately lead to significant cognitive impairment or dementia in most patients. Identification of those patients at risk for imminent progression could impact treatment decisions and clinical management, as well as increase vigilance of caregivers and medical providers. Attention to decline in certain areas of cognitive function may be particularly useful, and this may vary according to sex. Control of vascular risk factors may be especially important, although additional investigation is needed to determine whether treatment and monitoring of specific vascular risk may ultimately temper progression of cognitive symptoms. Finally, further exploration into the pathologic processes that underlie the increasingly consistent finding that males with PD may progress more rapidly to cognitive impairment is a vital future endeavor.
Supplementary Material
Highlights.
Determining predictors of progression to cognitive impairment in Parkinson’s disease (PD) will aid individualized treatment planning for patients and future clinical trial design with a basis in personalized medicine.
Performance on specific cognitive tests predicts imminent conversion from mild cognitive impairments to dementia in PD patients, over and above other demographic factors.
Male sex is the most important predictor of cognitive decline in those who are initially not cognitively impaired, and females with PD may have slower progression to cognitive impairment. Cognitive and clinical features that predict cognitive decline differ between males and females.
Acknowledgments
This study was supported by the Department of Veterans Affairs and National Institutes of Neurological Disorders and Stroke (P50 NS0662684). L.S.R. and T.M.D. were supported by P50 NS38377 and U01 NS082133. The funding sources did not provide scientific input for the study. We sincerely thank our research subjects and family members for their participation in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORSHIP DISCLOSURE
All authors have materially participated in the research and/or article preparation (see roles, below). All authors have approved the final submitted article. This article represents original work by the authors, has not been published elsewhere, and is not under consideration for publication elsewhere.
1. A. Conception and design, B. Acquisition of data C. Analysis/interpretation of data; 2.
Drafting/revising the article; 3. Final approval of the submitted version
Dr. Cholerton: 1A, 1B, 1C, 2, 3
Dr. Johnson: 1A, 1C, 2, 3
Mr. Fish: 1C, 2, 3
Dr. Quinn: 1B, 2, 3
Dr. Chung: 1B, 2, 3
Dr. Peterson-Hiller: 1B, 2, 3
Dr. Rosenthal: 1B, 2, 3
Dr. Dawson: 1B, 2, 3
Dr. Albert: 1B, 2, 3
Dr. Hu: 1B, 2, 3
Dr. Mata: 1A, 1B, 2, 3
Dr. Leverenz: 1B, 2, 3
Dr. Poston: 1A, 2, 3
Dr. Montine: 1A, 2, 3
Dr. Zabetian: 1A, 1B, 1C, 2, 3
Dr. Edwards: 1A, 1C, 2, 3
DECLARATION OF INTEREST
The authors report no direct conflict of interest related to the work on this manuscript. Full financial disclosure is provided below:
Dr. Cholerton is funded by grants from the NIH.
Dr. Johnson is funded by grants from the NIH.
Mr. Fish is funded by grants from the NIH.
Dr. Quinn is reimbursed by Prothena and Roche for the conduct of clinical trials and by vTv Pharmaceuticals for DSMB service. Dr. Quinn is also funded by grants from the NIH and Department of Veterans Affairs.
Dr. Chung is funded by a VA Merit Pilot grant.
Dr. Peterson-Hiller is funded by a VA Career Development Award and by the Michael J. Fox Foundation and NIH.
Dr. Rosenthal receives a lecture honorarium from the Edmund Safra Foundation and is funded by grants from the NIH. Dr. Rosenthal also received one time consulting fees for Biohaven pharmaceuticals and Functional Neuromodulation.
Dr. Dawson acknowledges the Adrienne Helis Malvin and Diana Henry Helis Medical Research Foundations and their direct engagement in the continuous active conduct of medical research in conjunction with The Johns Hopkins Hospital and The Johns Hopkins University School of Medicine and the Foundation’s Parkinson’s Disease Programs. His work is also supported by Sanofi- Aventis Recherce and Development, NIH/NINDS P50NS038377, NIH/NINDS U01NS082133, NIH/NINDS R37NS067525, NIH/NIDA P50 DA00266, the JPB Foundation, the MDSCRF 2015-MSCRFE-1782, the Michael J. Fox Foundation and Abbvie Pharmaceuticals.
Dr. Dawson is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases. Dr. Dawson is a member of the Dystonia Prize committee of the Bachmann Strauss Dystonia and Parkinson’s Disease Foundation and the Michael J. Fox Foundation and a member of the Board of Directors of the Bachmann Strauss Dystonia and Parkinson’s Disease Foundation. Dr. Dawson is a member of Scientific Advisory Board of CurePSP. Dr. Dawson is a member of the Executive Scientific Advisory Board of Michael J. Fox Foundation for Parkinson’s Research. Dr. Dawson is a member of American Gene Technologies International Inc., advisory board. Dr. Dawson is a consultant to Inhibikase Therapeutics and owns stock options in the company. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. The terms of this arrangement are being managed by The Johns Hopkins University in accordance with its conflict of interest policies. Dr. Dawson is a founder of Valted, LLC and holds an ownership equity interest in the company. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
Dr. Albert has served on scientific advisory boards for Eli Lilly, Eisai, Genentech, Biogen and Agenebio, and has received research support from GE Healthcare.
Dr. Hu is funded by grants from the NIH and Michael J. Fox Foundation.
Dr. Mata is funded by grants from the Department of Veterans Affairs, NIH, and Parkinson's Disease Foundation.
Dr. Leverenz reports consulting fees from Boehringer-Ingelheim, Citibank, Piramal Healthcare, and Navidea Biopharmaceuticals and is funded by grants from the Department of Veterans Affairs, American Parkinson Disease Association, Michael J. Fox Foundation, NIH, and Parkinson’s Disease Foundation.
Dr. Poston is reimbursed by AstraZeneca and Sangamo BioSciences, Inc. for the conduct of clinical trials and is funded by grants from the NIH and the Michael J. Fox Foundation
Dr. Montine reports honoraria from invited scientific presentations to universities and professional societies not exceeding $5,000 per year and is funded by grants from the NIH.
Dr. Zabetian received support from the American Parkinson Disease Association, Department of Veterans Affairs, NIH, and the Dolsen Foundation
Dr. Edwards is funded by grants from the NIH.
References
- 1.Cereda E, Cilia R, Klersy C, Siri C, Pozzi B, Reali E, Colombo A, Zecchinelli AL, Mariani CB, Tesei S, Canesi M, Sacilotto G, Meucci N, Zini M, Isaias IU, Barichella M, Cassani E, Goldwurm S, Pezzoli G. Dementia in Parkinson's disease: Is male gender a risk factor? Parkinsonism Relat Disord. 2016;26:67–72. doi: 10.1016/j.parkreldis.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 2.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–44. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 3.Bjornestad A, Tysnes OB, Larsen JP, Alves G. Loss of independence in early Parkinson disease: A 5-year population-based incident cohort study. Neurology. 2016;87(15):1599–1606. doi: 10.1212/WNL.0000000000003213. [DOI] [PubMed] [Google Scholar]
- 4.Lawson RA, Yarnall AJ, Duncan GW, Breen DP, Khoo TK, Williams-Gray CH, Barker RA, Collerton D, Taylor JP, Burn DJ I.-P.s. group. Cognitive decline and quality of life in incident Parkinson's disease: The role of attention. Parkinsonism Relat Disord. 2016;27:47–53. doi: 10.1016/j.parkreldis.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pigott K, Rick J, Xie SX, Hurtig H, Chen-Plotkin A, Duda JE, Morley JF, Chahine LM, Dahodwala N, Akhtar RS, Siderowf A, Trojanowski JQ, Weintraub D. Longitudinal study of normal cognition in Parkinson disease. Neurology. 2015;85(15):1276–82. doi: 10.1212/WNL.0000000000002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130(Pt 7):1787–98. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 7.Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ, Barker RA. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(Pt 11):2958–69. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 8.Olde Dubbelink KT, Hillebrand A, Twisk JW, Deijen JB, Stoffers D, Schmand BA, Stam CJ, Berendse HW. Predicting dementia in Parkinson disease by combining neurophysiologic and cognitive markers. Neurology. 2014;82(3):263–70. doi: 10.1212/WNL.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 9.Taylor JP, Rowan EN, Lett D, O'Brien JT, McKeith IG, Burn DJ. Poor attentional function predicts cognitive decline in patients with non-demented Parkinson's disease independent of motor phenotype. J Neurol Neurosurg Psychiatry. 2008;79(12):1318–23. doi: 10.1136/jnnp.2008.147629. [DOI] [PubMed] [Google Scholar]
- 10.Cholerton BA, Zabetian CP, Quinn JF, Chung KA, Peterson A, Espay AJ, Revilla FJ, Devoto J, Watson GS, Hu SC, Edwards KL, Montine TJ, Leverenz JB. Pacific Northwest Udall Center of excellence clinical consortium: study design and baseline cohort characteristics. J Parkinsons Dis. 2013;3(2):205–14. doi: 10.3233/JPD-130189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santangelo G, Vitale C, Picillo M, Moccia M, Cuoco S, Longo K, Pezzella D, di Grazia A, Erro R, Pellecchia MT, Amboni M, Trojano L, Barone P. Mild Cognitive Impairment in newly diagnosed Parkinson's disease: A longitudinal prospective study. Parkinsonism Relat Disord. 2015;21(10):1219–26. doi: 10.1016/j.parkreldis.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689–707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- 13.Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Rodriguez-Oroz MC, Troster AI, Weintraub D. MDS Task Force on mild cognitive impairment in Parkinson's disease: critical review of PD-MCI. Mov Disord. 2011;26(10):1814–24. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mata IF, Leverenz JB, Weintraub D, Trojanowski JQ, Chen-Plotkin A, Van Deerlin VM, Ritz B, Rausch R, Factor SA, Wood-Siverio C, Quinn JF, Chung KA, Peterson-Hiller AL, Goldman JG, Stebbins GT, Bernard B, Espay AJ, Revilla FJ, Devoto J, Rosenthal LS, Dawson TM, Albert MS, Tsuang D, Huston H, Yearout D, Hu SC, Cholerton BA, Montine TJ, Edwards KL, Zabetian CP. GBA Variants are associated with a distinct pattern of cognitive deficits in Parkinson's disease. Mov Disord. 2016;31(1):95–102. doi: 10.1002/mds.26359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mata IF, Leverenz JB, Weintraub D, Trojanowski JQ, Hurtig HI, Van Deerlin VM, Ritz B, Rausch R, Rhodes SL, Factor SA, Wood-Siverio C, Quinn JF, Chung KA, Peterson AL, Espay AJ, Revilla FJ, Devoto J, Hu SC, Cholerton BA, Wan JY, Montine TJ, Edwards KL, Zabetian CP. APOE, MAPT, and SNCA genes and cognitive performance in Parkinson disease. JAMA Neurol. 2014;71(11):1405–12. doi: 10.1001/jamaneurol.2014.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 17.Caselli RJ, Dueck AC, Locke DE, Baxter LC, Woodruff BK, Geda YE. Sex-based memory advantages and cognitive aging: a challenge to the cognitive reserve construct? J Int Neuropsychol Soc. 2015;21(2):95–104. doi: 10.1017/S1355617715000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudlicka A, Clare L, Hindle JV. Executive functions in Parkinson's disease: systematic review and meta-analysis. Mov Disord. 2011;26(13):2305–15. doi: 10.1002/mds.23868. [DOI] [PubMed] [Google Scholar]
- 19.Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson's disease: a review. J Neuropsychol. 2013;7(2):193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- 20.Picillo M, Nicoletti A, Fetoni V, Garavaglia B, Barone P, Pellecchia MT. The relevance of gender in Parkinson's disease: a review. J Neurol. 2017 doi: 10.1007/s00415-016-8384-9. [DOI] [PubMed] [Google Scholar]
- 21.Miller IN, Cronin-Golomb A. Gender differences in Parkinson's disease: clinical characteristics and cognition. Mov Disord. 2010;25(16):2695–703. doi: 10.1002/mds.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Sweidi S, Sanchez MG, Bourque M, Morissette M, Dluzen D, Di Paolo T. Oestrogen receptors and signalling pathways: implications for neuroprotective effects of sex steroids in Parkinson's disease. J Neuroendocrinol. 2012;24(1):48–61. doi: 10.1111/j.1365-2826.2011.02193.x. [DOI] [PubMed] [Google Scholar]
- 23.Bourque M, Dluzen DE, Di Paolo T. Neuroprotective actions of sex steroids in Parkinson's disease. Front Neuroendocrinol. 2009;30(2):142–57. doi: 10.1016/j.yfrne.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Liu R, Umbach DM, Peddada SD, Xu Z, Troster AI, Huang X, Chen H. Potential sex differences in nonmotor symptoms in early drug-naive Parkinson disease. Neurology. 2015;84(21):2107–15. doi: 10.1212/WNL.0000000000001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szewczyk-Krolikowski K, Tomlinson P, Nithi K, Wade-Martins R, Talbot K, Ben-Shlomo Y, Hu MT. The influence of age and gender on motor and non-motor features of early Parkinson's disease: initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism Relat Disord. 2014;20(1):99–105. doi: 10.1016/j.parkreldis.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Hu MT, Szewczyk-Krolikowski K, Tomlinson P, Nithi K, Rolinski M, Murray C, Talbot K, Ebmeier KP, Mackay CE, Ben-Shlomo Y. Predictors of cognitive impairment in an early stage Parkinson's disease cohort. Mov Disord. 2014;29(3):351–9. doi: 10.1002/mds.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Domellof ME, Ekman U, Forsgren L, Elgh E. Cognitive function in the early phase of Parkinson's disease, a five-year follow-up. Acta Neurol Scand. 2015;132(2):79–88. doi: 10.1111/ane.12375. [DOI] [PubMed] [Google Scholar]
- 28.Doiron M, Langlois M, Dupre N, Simard M. The influence of vascular risk factors on cognitive function in early Parkinson's disease. Int J Geriatr Psychiatry. 2017 doi: 10.1002/gps.4735. [DOI] [PubMed] [Google Scholar]
- 29.Malek N, Lawton MA, Swallow DM, Grosset KA, Marrinan SL, Bajaj N, Barker RA, Burn DJ, Hardy J, Morris HR, Williams NM, Wood N, Ben-Shlomo Y, Grosset DG P.R.C. Consortium. Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson's disease. Mov Disord. 2016;31(10):1518–1526. doi: 10.1002/mds.26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis MY, Johnson CO, Leverenz JB, Weintraub D, Trojanowski JQ, Chen-Plotkin A, Van Deerlin VM, Quinn JF, Chung KA, Peterson-Hiller AL, Rosenthal LS, Dawson TM, Albert MS, Goldman JG, Stebbins GT, Bernard B, Wszolek ZK, Ross OA, Dickson DW, Eidelberg D, Mattis PJ, Niethammer M, Yearout D, Hu SC, Cholerton BA, Smith M, Mata IF, Montine TJ, Edwards KL, Zabetian CP. Association of GBA Mutations and the E326K Polymorphism With Motor and Cognitive Progression in Parkinson Disease. JAMA Neurol. 2016;73(10):1217–1224. doi: 10.1001/jamaneurol.2016.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.