Abstract
Purpose
Recent work suggests that key aspects of sensitive parenting (e.g., warmth, emotional attunement) may be shaped in part by biology, specifically the neuropeptide oxytocin. However, some studies have found that oxytocin may not act in expected ways in higher-risk populations (e.g., those with postnatal depression or borderline personality disorder). This study examined the relation between oxytocin and parenting among mothers with varying levels of early life stress.
Methods
Forty low-income mothers and their 34 to 48-month-old child participated in this study. Mother-child dyads were observed in an interaction task in their home, and videos of these interactions were later coded for parenting behaviors. Mothers' oxytocin production before and after the interaction task was assessed through saliva. Mothers' early stress was assessed via the Adverse Childhood Experiences Scale (ACES; Felitti et al., 1998).
Results
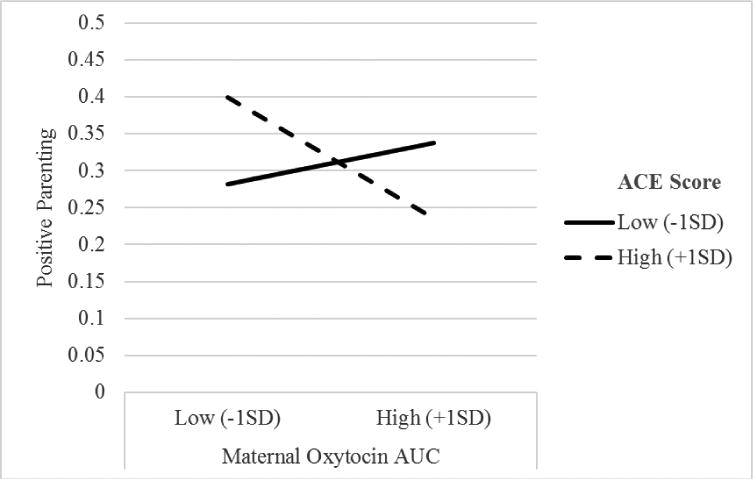
For mothers with low ACEs, higher oxytocin secretion was associated with more positive parenting. For mothers with high ACEs, higher oxytocin secretion was associated with lower levels of positive parenting.
Conclusions
Oxytocin may be operating differently for mothers who experienced harsh early social environments, supporting more defensive behaviors and harsh parenting than anxiolytic and prosocial behaviors.
Keywords: Parenting, Stress, Oxytocin, Parent-child interaction, Adverse Childhood Experiences
Young children depend on their caregivers to scaffold their development and help them cope with negative arousal by reading child cues, providing comfort, anticipating challenges, and promptly responding to the child's needs. Parenting can play a protective role for young children who are at higher familial (Raby, Roisman, Fraley, & Simpson, 2015) or genetic risk (Kochanska, Boldt, Kim, Yoon, & Philibert, 2015), and parenting behaviors are associated with children's self-regulation (Brophy-Herb, Stansbury, Bocknek, & Horodynski, 2012; Eiden, Edwards, & Leonard, 2007), behavior problems (Lunkenheimer, Olson, Hollenstein, Sameroff, & Winter, 2011), internalizing problems (Rose, Roman, Mwaba, & Ismail, 2017), and executive function (Lucassen et al., 2015). Recent work suggests that key aspects of sensitive parenting (e.g., warmth, emotional attunement) may be shaped in part by biology, specifically the neuropeptide oxytocin. In the current study we consider associations of oxytocin and observed positive and negative parenting in a low-income sample of mother-child dyads.
Biological basis of parenting
Studies examining oxytocin and parenting have increased rapidly in recent years. Animal studies suggest that oxytocin production is associated with maternal behavior and bonding (Dwyer, 2008; Febo, Numan, & Ferris, 2005), and rats who have been administered oxytocin provide more maternal care to their offspring (Pedersen & Boccia, 2002). In humans, oxytocin levels increase during pregnancy, and oxytocin facilitates mother-child bonding and reduces maternal stress reactivity (Galbally, Lewis, van IJzendoorn, & Permezel, 2011). The increase in oxytocin just prior to birth is thought to prime maternal care (Flinn, Ward, & Noone, 2015).
Oxytocin and maternal behavior are related during infancy and early childhood, yet associations are complex. During mother-child interaction, maternal salivary oxytocin increases, but only when mothers provide affectionate physical contact (Feldman, Gordon, Schneiderman, Weisman, & Zagoory-Sharon, 2010). Likewise, plasma and salivary oxytocin levels are both higher among parents who are more socially engaged, and have more synchronous and positive interactions with their infants (Feldman, Gordon, & Zagoory-Sharon, 2011). Relative to insecure-dismissing mothers, mothers with secure attachment styles show elevations in serum oxytocin after playing with their infants, and show more activation of dopaminergic reward circuits in the brain in response to a picture of their infant smiling or sad (Strathearn, Fonagy, Amico, & Montague, 2009). When shown a picture of their infant with sad affect, insecure/dismissing mothers show less activation of reward circuits, and more activation of neural circuits associated with negative feelings. Thus, for mothers with secure attachments, but not those with insecure attachments, oxytocin may contribute to the experience of reward when mothers interact positively with their infants, which may facilitate mothers' ability to continue to provide nurturing care (Galbally et al., 2011).
Some human studies utilize intranasal oxytocin administration to test the effect of oxytocin on behavior. Intranasal oxytocin administration is associated with better recognition of facial expressions of emotions, and increased trust to one's in-group (Graustella & MacLeod, 2012; Van IJzendoorn & Bakermans-Kranenburg, 2012). Oxytocin may prime parents for positive parenting beyond infancy, yet results are somewhat mixed. When oxytocin is produced, low-risk mothers are typically in a more affiliative state and more attuned to facial expressions of emotions, which is hypothesized to make warm, sensitive caregiving more likely. Yet, associations between oxytocin and positive behavior may be attenuated or even reverse direction among high risk groups, as early aberrant social experiences have been associated with altered oxytocin activity throughout the lifespan (Bartz et al., 2010, 2011; Wismer Fries et al., 2005). Further, a study of oxytocin administration in postnatally depressed mothers found that oxytocin administration was associated with increased reports of positive relationships with their infant, but also increased negative affect and views of the infant as difficult (Mah, Van IJzendoorn, Smith, & Bakermans-Kranenburg, 2013). Thus, researchers should consider under what circumstances oxytocin promotes prosocial behavior and positive parenting; mothers' childhood experiences have been identified as a potentially important moderating factor (BakermansKranenburg, van IJzendoorn, Riem, Tops, & Alink, 2012; Huffmeijer et al., 2013).
Trauma and Parenting
Mothers who have experienced abuse, neglect, or other forms of childhood stress are at higher risk for poor parenting (Knutson, 1995; Maestripieri, 2005; Patterson, 1998), tend to be more intrusive, engage in more negative parenting strategies, and appear disengaged when interacting with their infant (Banyard, 1997; Lyons-Ruth & Block, 1996; Moehler, Biringen, & Poustka, 2007). Trauma may enact effects on parenting through modeling of harsh parenting practices (Serbin & Karp, 2003; Van Ijzendoorn, 1992) and increased psychopathology risks (Enns, Cox, & Clara, 2002), which are associated with negative parenting (Zahn-Waxler, Duggal, & Gruber, 2002). Animal studies have demonstrated that quality of early mothering affects the development of endocrine systems (Champagne & Meaney, 2001) and brain structures that are associated with later expressed maternal behavior (Barrett & Fleming, 2011). In human studies as well, early adversity has been shown to alter both biology (e.g., cortisol, adrenocorticotropic hormone) and parenting behavior (Barrett & Fleming, 2011). Yet, it is not known how biology, particularly oxytocin biology, may relate to parenting under conditions of high and low levels of early-life stress.
The Current Study
We examined the relation between oxytocin and parenting in a naturalistic setting among low-income mothers with varying levels of early stress. Prior research has shown that while oxytocin is typically associated with positive affect and behavior among low-risk samples, oxytocin may be associated with more negative affect and behavior among higher risk samples such as anxiously attached individuals (Bartz et al., 2010), individuals with borderline personality disorder (Bartz, Simeon, et al., 2011), and mothers with postnatal depression (Mah et al., 2013). Thus, it was hypothesized that higher oxytocin production would be associated with more positive parenting and less negative parenting in mothers with lower levels of early life stress, but that the reverse would be true among mothers with higher levels of early life stress.
Methods
Participants
Participants were a subset of families who had participated in a longitudinal study of child self-regulation and eating behavior (Miller, Rosenblum, Retzloff, & Lumeng, 2016). Families were low-income at original study enrollment, and were recruited from Women, Infants and Children (WIC) clinics and Early Head Start programs. Inclusion criteria for the original study were that the child was born at ≥35 weeks of gestation without significant complications at birth; had no history of food allergies, serious medical problems, or significant developmental delays; both mother and child were fluent in English; child was in custody of the biological mother; mother was at least 18 years old at recruitment and had less than a 4-year college degree; and family income was <185% of the federal poverty line. Families who had completed the final wave of the original study were recruited to participate in the current study. Additional eligibility criteria for this study were that the mother was not pregnant or nursing, as this can affect oxytocin production (Levine, Zagoory-Sharon, Feldman, & Weller, 2007; Matthiesen, Ransjo-Arvidson, Nissen, & Uvnas-Moberg, 2001).
Forty participants completed the study protocol (December 2012-May 2013). Five participants were excluded from analyses due to missing data. Two sets of twins were in the dataset; the first twin to complete the protocol was included in analyses. The resulting sample consisted of 33 mother-child dyads; this sample did not differ from the sample excluded with regard to maternal age, race/ethnicity, educational attainment, income-to-needs ratio, or child age, race or sex (ps>.05).
Participants were children aged 34 to 48 months (M = 39.21, SD = 3.37; 48.5% male; 48.5% non-Hispanic white) and their mothers (aged 21 to 38 years; M = 27.18, SD = 4.60). At enrollment, the average income-to-needs ratio was below the poverty line (M = 0.87, SD = 0.65), and the mean annual income was $20,148 (SD = $13,012). About half (48.5%) of mothers had more than a high school education.
Procedure & Measures
This study was approved by the [redacted for blind review] Institutional Review Board (protocol number [redacted for blind review]). Mothers provided written informed consent. Families were compensated $50 for participating. During a home visit, mother-child dyads participated in an interactive task during which saliva was sampled, then engaged in a 5-minute videotaped free play session, and then interviewers administered questionnaires to mothers. The interactive task consisted of storybook reading and games that were designed to promote positive mother-child interactions (20 minutes). Mother-child dyads were provided age-appropriate toys (e.g., plastic animals) for the free-play, and these videotapes were later coded for observed parenting.
Predictor: Oxytocin
Oxytocin was measured in saliva; salivary oxytocin correlates with serum values (Carter et al., 2007; Feldman et al., 2010). Saliva was collected by having mothers chew on a sorbette until it was saturated. No stimulants were used. Samples were gathered prior to the interactive task (after mothers and children had not interacted for at least 30 minutes) and 20 minutes after the end of the task, following others' similar behavioral paradigms to assess salivary oxytocin (e.g., Feldman et al., 2010).
Visits occurred in the afternoon to avoid interference with diurnal pattern of oxytocin that typically peaks in the morning and then declines across the day (Amico, Levin, & Cameron, 2008; Forsling, Montgomery, Halpin, Windle, & Treacher, 1998). Participants were asked to refrain from eating for at least 2 hours prior to the visit. We queried mothers at each saliva collection regarding factors that could influence oxytocin (e.g. medications, sleep, eating; Gunnar & Vazquez, 2001).
Once collected, samples were immediately placed on ice and refrigerated in a portable cooler. Upon arrival at the lab, they were centrifuged and stored at -20°C until assayed. Salivary oxytocin was determined utilizing a high sensitivity ELISA kit (Enzo Life Science™ RIA Kits, formally Assay Design, Ann Arbor, MI). The detection limit of this assay is 11.7 pg/mL, with little to no cross-reactivity with vasopressin. The intra (a) and inter assay (b) coefficients of variation vary with the concentration of oxytocin as follows: Low 9.1% a, 14.5% b; Medium 8.7%a, 8.7% b; High 12.4% a, 5.2% b. This study utilized a variable representing the total amount of oxytocin secreted during the assessment (in pg*minutes/mL), specifically area under the curve (AUC). The pre-assessment oxytocin level was used entered into the first step of regression analyses to control for baseline levels of oxytocin.
Predictor: Maternal Adverse Childhood Experiences
The Adverse Childhood Experiences Scale (ACES; Felitti et al., 1998) was used to assess mother's early life stress. Mothers responded yes or no to a series of questions about experiences prior to age 18 that covered physical and emotional abuse and neglect, domestic violence, household substance abuse, household mental illness, parental separation or divorce, and incarceration (9 items; the sexual abuse item was not administered). Responses were summed to create a total score (α=.82) with higher values indicating more ACEs. For some analyses, this variable was dichotomized into high (3+ ACEs endorsed; N=15) vs. low (0 to 2 ACEs endorsed; N=18) approximately equally sized groups.
Outcomes: Maternal Affect and Behavior
Maternal affect and behavior were coded from videotapes of the free play using a modified version of the Maternal Warmth and Control Scale (Booth, Rose-Krasnor, McKinnon, & Rubin, 1994). Videos were coded in 10-second intervals for: Positive Affect (e.g., pleasant, joyful; 0=none, 1=moderate positivity, 2=outright positivity), Negative Affect (e.g., sad, anxious; 0-2), Negative Control (e.g., intrusive, ill-timed behaviors; 0-2), Sensitivity & Guidance (e.g., supportive, well-timed behavior; -1=miss or inappropriate response, 0=none, 1=minimal, 2=extended) and Hostile Affect (e.g., anger, irritability; 0-2). These variables were averaged to create Positive Parenting (Positive Affect, Sensitivity & Guidance) and Negative Parenting (Negative Affect, Negative Control, Hostile Affect) composite variables.
Possible Covariates
Mothers provided demographic information and indicated time since waking, eating, or taking any medications/caffeine or hormonal birth control, date of last menses, and whether they were healthy or had a cold or fever, as these could influence oxytocin production (Gunnar & Vazquez, 2001).
Results
Preliminary Analyses
Correlations revealed that maternal oxytocin AUC was not associated with mothers' medications, sleep, or eating (all ps >.05). There were no associations between oxytocin AUC and demographic variables (race/ethnicity, maternal education, maternal age, income-to-needs ratio; all ps >.05). Thus, these variables not were included as covariates in the regression models.
Main Analyses
Results are presented for effects that attain at least a “small” effect size. Effect sizes were considered small, medium, or large if they surpassed the cutoffs of .02, .13, and .26 for R2, or .10, .30, and .50 for r (Cohen, 1992).
Correlations
In the full sample, maternal oxytocin AUC was positively associated with pre-assessment maternal oxytocin, r = .92, and negatively associated with observed negative parenting, r = -.30. Pre-assessment maternal oxytocin was positively associated with Total ACEs, r = .10 and observed positive parenting, r = .10, and negatively associated with observed negative parenting, r = -.29. There was a negative association between observed positive parenting and observed negative parenting, r = -.19.
Correlations with Maternal Adversity Groups
When correlations were examined separately in the low (0-2) vs. high (3+) ACEs subsamples, observed positive parenting was positively associated with maternal oxytocin AUC, r = .41, and maternal pre-assessment oxytocin, r = .53, among mothers with low ACE scores. The association was of a similar magnitude but inversely associated among mothers with high ACE scores, rs = -.33, -.41. The association between observed negative parenting and maternal oxytocin AUC or maternal pre-assessment oxytocin was negative in both the low ACE, rs = -.34, -.34, and high ACE rs = -.29, -.26, subsamples. Among mothers with high ACE scores, but not those with low ACE scores, there was also a negative association between observed positive parenting and observed negative parenting, r = -.31.
Regressions
Linear regression analyses were conducted to test how oxytocin AUC and ACEs related to observed parenting. Pre-assessment maternal oxytocin was entered in Step 1, and maternal oxytocin AUC and Total ACEs were entered in Step 2. The oxytocin AUC and ACEs interaction was added in Step 3. The final model (Table 2), though nonsignificant, F(4,32) = 1.28, p = .30, explained a medium effect size of variance, R2 = .15, with most of the variance attributable to the maternal oxytocin AUC × ACEs interaction, ΔR2 = .14. The interaction effect was statistically significant, β = -.66, p = .04, sr = -.38, and suggests a positive association between positive parenting and oxytocin production among mothers with low ACEs, but a negative association for mothers with high ACEs (Figure 1). A parallel regression run with negative parenting as the outcome variable was nonsignificant for all variables (Table 2).
Table 2.
Hierarchical regressions of observed parenting behavior on pre-assessment maternal oxytocin, maternal oxytocin AUC, ACEs, and maternal oxytocin AUC × ACEs interaction.
| Positive Parenting | Negative Parenting | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| F | R2 | ΔR2 | β | sr | F | R2 | ΔR2 | β | sr | |
| Model 1 | 0.29 | .01 | .01 | 2.82 | .08 | .08 | ||||
| Pre-Assessment Maternal Oxytocin | .10 | .10 | -.29 | -.29 | ||||||
|
| ||||||||||
| Model 2 | 0.10 | .01 | .01 | 1.07 | .10 | .02 | ||||
| Pre-Assessment Maternal Oxytocin | .09 | .03 | -.10 | -.04 | ||||||
| Maternal Oxytocin AUC | .01 | .00 | -.22 | -.08 | ||||||
| Total ACEs | .03 | -.03 | .09 | .09 | ||||||
|
| ||||||||||
| Model 3 | 1.28 | .15 | .14 | 0.78 | .10 | .00 | ||||
| Pre-Assessment Maternal Oxytocin | .31 | .11 | -.12 | -.04 | ||||||
| Maternal Oxytocin AUC | .34 | .12 | -.25 | -.09 | ||||||
| Total ACEs | .07 | .07 | .08 | .08 | ||||||
| Maternal Oxytocin AUC × Total ACEs | -.66* | -.38 | .06 | .03 | ||||||
p < .05, sr = semipartial r
Small effect sizes (R2 ≥ .02, sr ≥ .10) are bolded. Medium effect sizes (R2 ≥ .13, sr ≥ .30) are bolded and underlined.
Figure 1. Positive Parenting as a function of maternal oxytocin production and mothers' adverse childhood experiences, controlling for pre-test maternal oxytocin level.
Discussion
This study examined the relation between oxytocin and observed parenting in a naturalistic setting in low-income mothers with varying levels of early adversity. While the sample size is small, and only some effects attained statistical significance, effect sizes were in the small to medium range. The primary finding of this study was that the effect of maternal oxytocin on positive parenting, differs based on maternal early adversity. For mothers with low ACEs, oxytocin related to positive parenting in the expected manner—higher oxytocin was associated with more positive parenting. For mothers with high ACEs, however, higher oxytocin was associated with lower levels of positive parenting. While there was a direct negative association between maternal oxytocin AUC and observed negative parenting, this association was no longer detected in the full regression model.
Our results suggest that the expected connection between oxytocin and positive parenting behavior is disrupted among mothers who have had high levels of early adversity. These findings are in line with previous work that has found that oxytocin may not function as expected in individuals who had harsh early experiences (Bartz et al., 2010; Bartz, Simeon, et al., 2011). Oxytocin administration can increase social awareness and perception (Graustella & MacLeod, 2012), has also been shown to increase perceived social stress in men (Eckstein et al., 2014), and has been associated with negative affect in depressed mothers (Mah et al., 2013). Thus, it is possible that the inconsistencies in prior research on oxytocin (Bartz, Zaki, Bolger, & Ochsner, 2011; Graustella & MacLeod, 2012) are in part due to studies not taking into account an individual's social context, particularly for individuals who have experienced early-life adversity.
Mothers with low levels of early adversity may get a “boost” from their biology that helps them to exhibit positive parenting behaviors. Consistent with intranasal oxytocin studies of (low risk) individuals' behaviors (Graustella & MacLeod, 2012; Van IJzendoorn & BakermansKranenburg, 2012), close, affectionate interaction with their children is associated with higher oxytocin levels, which is then associated with more positive parenting behaviors. For mothers with high levels of early adversity, oxytocin levels are associated with lower levels of positive parenting. These mothers may have to work against their biology to provide positive parenting, thus, it may be more difficult for high-risk mothers to show positive parenting behaviors.
A possible explanation for oxytocin by ACEs interaction lies in the association between oxytocin and vasopressin, a related hormone that is linked to anxiety and defense behaviors. A large increase in oxytocin may lead to occupation of vasopressin receptors, producing defensive behaviors typically associated with vasopressin release (Carter, 2014), which could contribute to the current findings for mothers with high ACEs. Similar paradoxical effects of oxytocin have been reported in other studies of at-risk mothers; in mothers with postnatal depression, oxytocin administration is associated with increased negative affect, presumably because oxytocin can make an individual's emotional state (in this case depressed) more salient (Mah et al., 2013). The early social context has been associated with individual differences in oxytocin release and its role in mediating or buffering distress (Bartz, Zaki, et al., 2011; Neumann, 2007; UvnäsMoberg, 1998; Wismer Fries, Ziegler, Kurian, Jacoris, & Pollak, 2005; Zheng et al., 2010). As a result, oxytocin may be operating differently for mothers who experienced harsh early social environments, perhaps promoting more defensive behaviors and harsh parenting than anxiolytic and prosocial behaviors.
Recently, oxytocin has received attention as a potential target for intervention. Studies have used intranasal oxytocin to improve emotion recognition in individuals with autism spectrum disorders (Guastella et al., 2010), improve higher-level social cognition in individuals with schizophrenia (Davis et al., 2013), improve trust toward one's ingroup (Van IJzendoorn & Bakermans-Kranenburg, 2012), and improve positive communication during couple conflict (Ditzen et al., 2009). Yet, our findings suggest that oxytocin may not operate as expected in individuals with high levels of early adversity. Oxytocin interventions should therefore carefully consider individuals' social context, as effects of oxytocin may differ among different subgroups (Graustella & MacLeod, 2012). Future work might also consider exploring whether the negative relationship between oxytocin and positive parenting among high-risk parents might be ameliorated through trauma-informed interventions that target early parent-child relationships (e.g., Child-Parent Psychotherapy).
Strengths and Limitations
This study is limited by its small sample size, so findings will need to be replicated with larger samples. Although a strength of the study is the low-income sample, which is uncommon in existing studies of oxytocin and parenting, findings may not generalize to higher-income samples. Other strengths include use of observational and biological data in a naturalistic home setting, which builds upon extant literature that primarily uses lab-based protocols. Thus, despite the small sample size, this study expands our knowledge about how oxytocin relates to parenting in high-risk populations, and suggests that maternal early adversity may affect the association between oxytocin and parenting.
Table 1. Descriptive statistics and correlations of maternal oxytocin AUC (pg/mL), pre-assessment maternal oxytocin (pg/mL), ACES total score, CES-D total score, and observed parenting (N = 33).
| 1 | 2 | 3 | 4 | M (SD) | |
|---|---|---|---|---|---|
| 1. Maternal Oxytocin AUC | 325,306.68 (145,570.51) | ||||
| 2. Pre-Assessment Maternal Oxytocin | .92* | 100.89 (49.55) | |||
| 3. Total ACEs | .05 | .10 | 2.88 (2.41) | ||
| 4. Observed Positive Parenting | .09 | .10 | .04 | .31 (.12) | |
| 5. Observed Negative Parenting | -.30+ | -.29 | .07 | -.19 | .02 (.03) |
p < .05
p < .10
Small effect sizes (r ≥ .10) are bolded. Medium effect sizes (r ≥ .30) are bolded and underlined. Large effect sizes are bolded, underlined, and italicized.
Acknowledgments
This research was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development grants 1R01HD069179 (PIs JC Lumeng, AL Miller), T32 HD079350 (PI: JC Lumeng), F32HD088029 (PI: JR Doom), National Institute of Diabetes and Digestive Kidney Diseases grant T32 DK071212 (PI: DM Vazquez), and National Institutes of Health CTSA pilot grant UL1TR000433 (PI: AL Miller). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank the families for their participation in our research and to our research team at the University of Michigan for their assistance with data collection and coding.
Footnotes
Conflict of Interest: The authors declare that they have no conflicts of interest.
References
- Amico JA, Levin SC, Cameron JL. Circadian rhythm of oxytocin in the cerebrospinal fluid of rhesus and cynomolgus monkeys: Effects of castration and adrenalectomy and presence of a caudal-rostral gradient. Neuroendocrinology. 2008;50(6):624–632. doi: 10.1159/000125291. http://doi.org/10.1159/000125291. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH, Riem MME, Tops M, Alink LRA. Oxytocin decreases handgrip force in reaction to infant crying in females without harsh parenting experiences. Social Cognitive and Affective Neuroscience. 2012;7(8):951–957. doi: 10.1093/scan/nsr067. http://doi.org/10.1093/scan/nsr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banyard VL. The impact of childhood sexual abuse and family functioning on four dimensions of women's later parenting. Child Abuse and Neglect. 1997;21(11):1095–1107. doi: 10.1016/s0145-2134(97)00068-9. http://doi.org/10.1016/S0145-2134(97)00068-9. [DOI] [PubMed] [Google Scholar]
- Barrett J, Fleming AS. Annual research review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2011;52(4):368–397. doi: 10.1111/j.1469-7610.2010.02306.x. http://doi.org/10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, … Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Social Cognitive and Affective Neuroscience. 2011;6(5):556–563. doi: 10.1093/scan/nsq085. http://doi.org/10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: Context and person matter. Trends in Cognitive Sciences. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. http://doi.org/10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Ochsner KN, Bolger N, Kolevzon A, Ludwig N, Lydon JE. Effects of oxytocin on recollections of maternal care and closeness. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(50):21371–5. doi: 10.1073/pnas.1012669107. http://doi.org/10.1073/pnas.1012669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth CL, Rose-Krasnor L, McKinnon JA, Rubin KH. Predicting social adjustment in middle childhood: The role of preschool attachment security and maternal style. Social Development. 1994;3(3):189–204. http://doi.org/10.1111/j.1467-9507.1994.tb00040.x. [Google Scholar]
- Brophy-Herb HE, Stansbury K, Bocknek E, Horodynski MA. Modeling maternal emotion-related socialization behaviors in a low-income sample: Relations with toddlers' self-regulation. Early Childhood Research Quarterly. 2012;27(3):352–364. http://doi.org/10.1016/j.ecresq.2011.11.005. [Google Scholar]
- Carter CS. Oxytocin Pathways and the Evolution of Human Behavior. Annual Review of Psychology. 2014;65(1):17–39. doi: 10.1146/annurev-psych-010213-115110. http://doi.org/10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, Schwertz D. Oxytocin: Behavioral associations and potential as a salivary biomarker. Annals of the New York Academy of Sciences. 2007;1098:312–322. doi: 10.1196/annals.1384.006. http://doi.org/10.1196/annals.1384.006. [DOI] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: Evidence for non-genomic transmission of parental behavior and stress responsivity. Progress in Brain Research. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. http://doi.org/10.1016/S0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Davis MC, Lee J, Horan WP, Clarke AD, McGee MR, Green MF, Marder SR. Effects of single dose intranasal oxytocin on social cognition in schizophrenia. Schizophrenia Research. 2013;147(2–3):393–397. doi: 10.1016/j.schres.2013.04.023. http://doi.org/10.1016/j.schres.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal Oxytocin Increases Positive Communication and Reduces Cortisol Levels During Couple Conflict. Biological Psychiatry. 2009;65(9):728–731. doi: 10.1016/j.biopsych.2008.10.011. http://doi.org/10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Dwyer CM. Individual variation in the expression of maternal behaviour: A review of the neuroendocrine mechanisms in the sheep. Journal of Neuroendocrinology. 2008;20(4):526–534. doi: 10.1111/j.1365-2826.2008.01657.x. http://doi.org/10.1111/j.1365-2826.2008.01657.x. [DOI] [PubMed] [Google Scholar]
- Eckstein M, Scheele D, Weber K, Stoffel-Wagner B, Maier W, Hurlemann R. Oxytocin facilitates the sensation of social stress. Human Brain Mapping. 2014;35(9):4741–4750. doi: 10.1002/hbm.22508. http://doi.org/10.1002/hbm.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Edwards EP, Leonard KE. A conceptual model for the development of externalizing behavior problems among kindergarten children of alcoholic families: Role of parenting and children's self-regulation. Developmental Psychology. 2007;43(5):1187–1201. doi: 10.1037/0012-1649.43.5.1187. http://doi.org/10.1037/0012-1649.43.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns MW, Cox BJ, Clara I. Parental bonding and adult psychopathology: results from the US National Comorbidity Survey. Psychol Med. 2002;32(6):997–1008. doi: 10.1017/s0033291702005937. http://doi.org/10.1017/S0033291702005937. [DOI] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional Magnetic Resonance Imaging Shows Oxytocin Activates Brain Regions Associated with Mother – Pup Bonding during Suckling. The Journal of Neuroscience. 2005;25(50):11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. http://doi.org/10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology. 2010;35(8):1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. http://doi.org/10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: Considering stress and affiliation components of human bonding. Developmental Science. 2011;14(4):752–761. doi: 10.1111/j.1467-7687.2010.01021.x. http://doi.org/10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz aM, Edwards V, … Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. http://doi.org/http://dx.doi.org/10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Flinn MV, Ward CV, Noone RJ. Hormones and the Human Family. The Handbook of Evolutionary Psychology. 2015:552–580. http://doi.org/10.1002/9780470939376.ch19.
- Forsling ML, Montgomery H, Halpin D, Windle RJ, Treacher DF. Daily patterns of secretion of neurohypophysial hormones in man: Effect of age. Experimental Physiology. 1998;83:409–418. doi: 10.1113/expphysiol.1998.sp004124. http://doi.org/10.1113/expphysiol.1998.sp004124. [DOI] [PubMed] [Google Scholar]
- Galbally M, Lewis AJ, van IJzendoorn MH, Permezel M. The Role of Oxytocin in Mother-Infant Relations: A Systematic Review of Human Studies. Harvard Review of Psychiatry. 2011;19(1):1–14. doi: 10.3109/10673229.2011.549771. http://doi.org/10.3109/10673229.2011.549771. [DOI] [PubMed] [Google Scholar]
- Graustella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: Evidence and future directions. Hormones and Behavior. 2012;61(3):410–418. doi: 10.1016/j.yhbeh.2012.01.002. http://doi.org/10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal Oxytocin Improves Emotion Recognition for Youth with Autism Spectrum Disorders. Biological Psychiatry. 2010;67(7):692–694. doi: 10.1016/j.biopsych.2009.09.020. http://doi.org/10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and Psychopathology. 2001;13(3):515–38. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Huffmeijer R, Alink LRA, Tops M, Grewen KM, Light KC, Bakermans-Kranenburg MJ, van IJzendoorn MH. The impact of oxytocin administration and maternal love withdrawal on event-related potential (ERP) responses to emotional faces with performance feedback. Hormones and Behavior. 2013;63(3):399–410. doi: 10.1016/j.yhbeh.2012.11.008. http://doi.org/10.1016/j.yhbeh.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Knutson JF. Psychological characteristics of maltreated children: Putative risk factors and consequences. Annual Review of Psychology. 1995;46(1):401–431. doi: 10.1146/annurev.ps.46.020195.002153. http://doi.org/10.1146/annurev.ps.46.020195.002153. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Boldt LJ, Kim S, Yoon JE, Philibert RA. Developmental interplay between children's biobehavioral risk and the parenting environment from toddler to early school age: Prediction of socialization outcomes in preadolescence. Development and Psychopathology. 2015;27(3):775–790. doi: 10.1017/S0954579414000777. http://doi.org/10.1017/S0954579414000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: Individual patterns and maternal-fetal attachment. Peptides. 2007;28(6):1162–1169. doi: 10.1016/j.peptides.2007.04.016. http://doi.org/10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Lucassen N, Kok R, Bakermans-Kranenburg MJ, Van Ijzendoorn MH, Jaddoe VWV, Hofman A, et al. Tiemeier H. Executive functions in early childhood: The role of maternal and paternal parenting practices. British Journal of Developmental Psychology. 2015;33(4):489–505. doi: 10.1111/bjdp.12112. http://doi.org/10.1111/bjdp.12112. [DOI] [PubMed] [Google Scholar]
- Lunkenheimer ES, Olson SL, Hollenstein T, Sameroff AJ, Winter C. Dyadic flexibility and positive affect in parent-child coregulation and the development of child behavior problems. Development and Psychopathology. 2011;23(2011):577–591. doi: 10.1017/S095457941100006X. http://doi.org/10.1017/S095457941100006X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons-Ruth K, Block D. The disturbed caregiving system: Relations among childhood trauma, maternal caregiving, and infant affect and attachment. Infant Mental Health Journal. 1996;17(3):257–275. http://doi.org/10.1002/(SICI)1097-0355(199623)17:3<257∷AID-IMHJ5>3.0.CO;2-L. [Google Scholar]
- Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9726–9729. doi: 10.1073/pnas.0504122102. http://doi.org/10.1073/pnas.0504122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah BL, Van IJzendoorn MH, Smith R, Bakermans-Kranenburg MJ. Oxytocin in postnatally depressed mothers: Its influence on mood and expressed emotion. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;40(1):267–272. doi: 10.1016/j.pnpbp.2012.10.005. http://doi.org/10.1016/j.pnpbp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Matthiesen AS, Ransjo-Arvidson AB, Nissen E, Uvnas-Moberg K. Postpartum maternal oxytocin release by newborns: Effects of infant hand massage and sucking. Birth. 2001;28(1):13–19. doi: 10.1046/j.1523-536x.2001.00013.x. http://doi.org/10.1046/j.1523-536x.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- Miller AL, Rosenblum KL, Retzloff LB, Lumeng JC. Observed self-regulation is associated with weight in low-income toddlers. Appetite. 2016;105:705–712. doi: 10.1016/j.appet.2016.07.007. http://doi.org/10.1016/j.appet.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehler E, Biringen Z, Poustka L. Emotional availability in a sample of mothers with a history of abuse. The American Journal of Orthopsychiatry. 2007;77(4):624–628. doi: 10.1037/0002-9432.77.4.624. http://doi.org/10.1037/0002-9432.77.4.624. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochemical Society Transactions. 2007;35:1252–1257. doi: 10.1042/BST0351252. http://doi.org/10.1042/BST0351252. [DOI] [PubMed] [Google Scholar]
- Patterson GR. Continuities--a search for causal mechanisms: Comment on the special section. Developmental Psychology. 1998;34(6):1263–1268. doi: 10.1037//0012-1649.34.6.1263. http://doi.org/10.1037/0012-1649.34.6.1263. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin links mothering received, mothering bestowed and adult stress responses. Stress (Amsterdam, Netherlands) 2002;5(4):259–267. doi: 10.1080/1025389021000037586. http://doi.org/10.1080/1025389021000037586. [DOI] [PubMed] [Google Scholar]
- Raby KL, Roisman GI, Fraley RC, Simpson JA. The enduring predictive significance of early maternal sensitivity: Social and academic competence through age 32 years. Child Development. 2015;86(3):695–708. doi: 10.1111/cdev.12325. http://doi.org/10.1111/cdev.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J, Roman N, Mwaba K, Ismail K. The relationship between parenting and internalizing behaviours of children: a systematic review. Early Child Development and Care. 2017;4430(March):1–19. http://doi.org/10.1080/03004430.2016.1269762. [Google Scholar]
- Serbin L, Karp J. Intergenerational Studies of Parenting and the Transfer of Risk From Parent to Child. Current Directions in Psychological Science. 2003;12(4):138–142. http://doi.org/10.1111/1467-8721.01249. [Google Scholar]
- Strathearn L, Fonagy P, Amico JA, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2009;34(13):2655–2666. doi: 10.1038/npp.2009.103. http://doi.org/10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnäs-Moberg K. Antistress Pattern Induced by Oxytocin. News in Physiological Sciences : An International Journal of Physiology Produced Jointly by the International Union of Physiological Sciences and the American Physiological Society. 1998;13(1):22–25. doi: 10.1152/physiologyonline.1998.13.1.22. http://doi.org/10.1016/0304-3940(95)11335-t. [DOI] [PubMed] [Google Scholar]
- Van Ijzendoorn MH. Intergenerational transmission of parenting: A review of studies in nonclinical populations. Developmental Review. 1992;12(1):76–99. http://doi.org/10.1016/0273-2297(92)90004-L. [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ. A sniff of trust: Meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology. 2012;37(3):438–443. doi: 10.1016/j.psyneuen.2011.07.008. http://doi.org/10.1016/j.psyneuen.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceedings of the National Academy of Sciences. 2005;102(47):17237–17240. doi: 10.1073/pnas.0504767102. http://doi.org/10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn-Waxler C, Duggal S, Gruber R. Parental Psychopathology. In: Bornstein MH, editor. Handbook of Parenting 2. Vol. 3. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. pp. 295–327. [Google Scholar]
- Zheng J, Babygirija R, Bülbül M, Cerjak D, Ludwig K, Takahashi T. Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2010;299(4):G946–G953. doi: 10.1152/ajpgi.00483.2009. http://doi.org/10.1152/ajpgi.00483.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


