Abstract
Fatalities from organophosphate (OP) insecticide result from both occupational and deliberate exposure; significantly impacting human health. Like nerve agents, insecticides are neurotoxins which target and inhibit acetylcholinesterases (AChE) in central and peripheral synapses in the cholinergic nervous system. Post-exposure therapeutic countermeasures generally include administration of atropine with a pyridinium aldoxime e.g. pralidoxime, to reactivate the OP-inhibited AChE. However, commonly used oximes inefficiently cross the bloodbrain barrier and are rapidly cleared and their benefit is debated. Recent findings have demonstrated the ability of a novel zwitterionic, centrally acting, brain penetrating oxime (RS194B) to reverse severe symptoms and rapidly reactivate sarin-inhibited AChE in macaques, but it has not been tested following OP pesticide poisoning. In the present study, the symptoms following a lethal dose of inhaled paraoxon (100 ug/kg), were shown to mimic those in insecticide poisoned individuals and were also rapidly reversed in macaques by post-exposure IM administration of 80 mg/kg of RS194B. This occurred with a concomitant reactivation of AChE to 40–100% in < 1 hr and BChE (40% in 8 h). These findings will be used to develop a macaque model with RS194 B as a post-exposure treatment for insecticide poisoning and generate efficacy data for approval under the FDA Animal rule.
Keywords: Oxime antidote, Nebulized paraoxon, Macaques, AChE, BChE, Reactivation
1. Introduction
Worldwide, the routine use of organophosphorus (OP) pesticides to control agricultural, household and structural pests has reached > 5 billion tons annually; potentially exposing > 1.8 billion civilians (Alavanja., 2009). This practice has resulted in > 200,000 occupational poisonings and > 100,000 deaths by intentional ingestion of insecticides each year (Hulse et al., 2014; Mew et al., 2017). Cardiovascular and nervous system symptoms associated with exposure result from irreversible inhibition of acetylcholinesterase (AChE) in neuromuscular junctions, peripheral autonomic and central nervous systems which may lead to hypoxia, bradycardia, seizures, cognitive and behavioural defects, and often death by respiratory failure (Hulse et al., 2014; Taylor, 2010; Gupta., 2006). Measurement of plasma butrylcholinesterase and erythrocyte AChE has been used to reflect exposure levels.
Standard post-exposure therapy typically involves administration of a muscarinic antagonist (atropine) to block receptors, pyridinium aldoximes e.g. pralidoxime (2-PAM), HI-6, obidoxime, MMB4, to reactivate the OP-inhibited AChE, with/without a benzodiazepine anticonvulsant to control seizures (Hulse et al., 2014; Eddleston et al., 2008; Eddleston and Chowdhury., 2016; Shih et al., 2012). While oximes are widely used globally, several animal model studies and recent clinical trials using pesticide-poisoned individuals have shown uneven clinical benefits with those currently used (Buckley et al., 2011; Eddleston et al., 2009; Steinritz et al., 2016) and the value of administering an oxime has therefore been seriously debated. Limitations of antidotal function of most synthetic oxime reactivators relate to their quaternary structure and their inability to rapidly cross the blood-brain barrier (BBB), in addition to rapid clearance from the circulation. Only recently has the search for broad spectrum, centrally acting oximes with enhanced efficacy met with some success (DeMar et al., 2010; Chambers et al., 2016; Mercey et al., 2012; Cadieux et al., 2016)
One leading candidate to emerge is a zwitterion of simplified structure, RS194B, whose neutral species efficiently crosses the BBB permitting favorable tissue disposition, CNS penetration and oral bioavailability in mice exposed to sarin and VX (Sit et al., 2011; Radić et al., 2012). More recently, a single treatment (62.5 mg/kg) of macaques with RS194B following head-only exposure to sarin vapor has also been shown to reactivate circulating red blood cell (RBC)-AChE and plasma BChE and dramatically reverse both early and advanced symptoms typical of nerve agent toxicity (Rosenberg et al., 2017).
New countermeasures to OP insecticides, require extensive testing for regulatory approval under the Animal Rule (21 CFR 601.90 for biological products). However, very few animal models exist, and published reports indicate that the in vivo toxicokinetics and oxime responsiveness to different OP insecticides and nerve agents may vary significantly with the offending OP and animal species (Worek et al., 2010a; Luo et al., 2010, 2008), often preventing direct extrapolation to humans. In most cases the inhibition and reactivation kinetics with swine, rat and GP AChE exposed to a variety of OP nerve agents as well as insecticides are slower than for humans and non-human primates.
Based on the protective efficacy of RS194B in sarin vapor exposed macaques, we examined the capacity of a single dose of RS194 B to reactivate AChE and BChE and prevent death in macaques following lethal exposure to the insecticide oxon, paraoxon (Px), in order to establish a new non-human primate treatment model for insecticide poisoning. In previous studies, (Marchand et al., 2014; Rosenberg et al., 2015) it has been surprisingly demonstrated that aerosolized (aer) substances administered to macaques (and small children) using a nebulizer are deposited predominantly in the stomach (~40%) rather than the lungs (5–13%); presumably due to the swallowing of nasal and oral secretions. This model offers the advantage of mimicking both inhalation and oral OP insecticide exposures. The early results indicate that: (i) the clinical symptoms observed following a lethal dose of aer-Px (100 ug/kg) are very similar to those seen in insecticide-poisoned humans, and (ii) post-exposure administration of RS194B prevents death even in animals exhibiting signs of lethal toxicity. These results bode well for developing an inhalation macaque/RS194B model to aid in the treatment of insecticide-poisoned people.
2. Materials and methods
2.1. Chemicals
Paraoxon (99.1% pure) was obtained from ChemService Inc. (West Chester, PA), diluted in sterile water to obtain a stock solution of 1 mg/ml and maintained at RT protected from light. Inoculation doses were prepared 12–24 h prior to use in 1 ml in PBS, pH 6.8 at 0.7 mg/ml.
2.2. RS194B oxime
RS194B was prepared as previously described (Sit et al., 2011; Radic et al., 2012) and was formulated for IM injection at concentrations of 50–80 mg/ml. RS194 B was dispersed in sterile water and then titrated with ~ 18% HCl to a pH of 5.5–6.5, resulting in clarity of the solution. It was allowed to stand for at least 24 h at 4C before injection at room temperature. The concentration of ionized species is slightly above isotonicity.
2.3. Assays for reactivation of AChE and BChE
AChE and BChE activities were assayed using 1 mM acetylthiocholine or butyrylthiocholine (Sigma-Aldrich) and 0.5 mM 5,5"- dithiobis 2-nitrobenzoic acid (DTNB) in 50 mM sodium phosphate buffer, pH 8.0, at 22 °C. The formation of product was followed by monitoring the increase in absorbance of 5-thio-2- nitrobenzoic acid at 412 nm using a molar extinction coefficient of 13,600 cm−1 M−1. Activity was reported as U/ml where 1 U represents 1 µmole of acetyl- or butyrylthiocholine per min. In the AChE activity assay, 20 µM ethopropazine was used as a BChE-specific inhibitor. These assays were performed in triplicate by three different people and repeated several times. Background levels in macaque blood generally range from 2.5 to 6.5 U/ml AChE and 3.5–8.5 U/ml BChE.
2.4. Pharmacokinetics of RS194 B in macaques
Circulating RS194 B oxime was measured by liquid chromatography-tandem mass spectrometry (LC–MS/MS). Twenty ul of lysed blood from macaques, diluted 1/10, was initially used in this assay, which has a dynamic range is 1–20,000 ng/ml. Samples were de-proteinated by 1:3 dilution with acetronitrile, using a close congener, RS138 B as internal standard, before injection of 10 ul for analysis by MS/MS. Separation of oximes from other plasma constituents was performed with an HPLC gradient of methanol partitioning in water + 0.1% formic acid (by volume), in an ACE C18-Ar column (2.1 × 100 mm; 3 µM. Mac-Mod analytical), prior to MS/MS analysis. MS/MS transitions for RS194 B and RS138 B used for quantification were m/z 214 > 115 and m/z 266 > 115, respectively. The limit of detection for RS–194 B was 20 pg on the column.
2.5. Administration of aerosolized paraoxon
Animal studies were conducted at the IIT Research Institute, Chicago, in compliance with the Animal Welfare Act and other federal statutes and regulations stated in Guide for the Care and Use of Laboratory Animals (NRC Publication, 1996).
Three female rhesus macaques (#T767, T768 and T769), weighing 3.4, 3.66 and 4.14 kg, were anesthetized with Telazol (tiletamine/zolazepam) (5–8 mg/kg IM (Zoetis, Parsippany, NJ), and moved to a preparation room where they were bled for baseline cholinesterase activity. After bleeding, they were placed into a primate chair (Model 515-SASR, PlasLabs, Inc. Lansing, MI 48906) and moved into an enclosed inhalation chamber with glove access. EKG leads and a pulseoximeter sensor were attached to allow monitoring of heart rate and rhythm; respiratory rate and blood oxygen saturation. A pediatric facemask was used to administer an accumulating lethal dose of aer-Px (100 ug/kg) using an Aerogen Solo® nebulizer (Aerogen Ltd. Galway, Ireland) modified by the addition of a small reservoir which facilitated evaporation of droplets and thereby increased lung deposition (Marchand et al., 2014; Rosenberg et al., 2015; MacLoughlin et al., 2016). Using carrier airflow to the nebulizer, aerosol was supplied to the exposure mask at a flow rate of approximately 2 L/min. The Px solution was dispensed into the nebulizer reservoir as needed. In order to minimize loss of aerosol in transport, the aerosolization device and the mask combination was held manually at the nostril of the animal allowing it to breathe the test aerosol. Any excess aerosol was exhausted through a one way valve. Handling and holding of the aerosol generation/administration device was accomplished through seal tight gloves. The inhalation chamber was held at slightly negative pressure compared to the laboratory to prevent any leakage of test aerosols into the laboratory. This combination of face mask and modified nebulizer device has been shown to increase aerosolization rate and generate respirable aerosols (greater than 85%) with a Mass Median Aerodynamic Diameter (MMAD) of 2.1 ± 0.4 µm. Use of this modified nebulizer has been previously shown to increase lung deposition of an anti-ricin antibody to 13% in macaques (Marchand et al., 2014).
The target Aer-Px dose was (100ug/kg); however, the Px aerosol exposure had to be terminated early for macaques T768 (78% of dose) and T769 (92% of dose) when their heart rate dropped significantly to ~ 100 beats/min. Macaque (T767) received the full dose. Aerogen nebulizers are powered by electricity and aerosol generation can be stopped by switching off the power. Post exposure, as the symptoms became severe, each macaque was first administered 0.28 mg/kg atropine IM and then 80 mg/kg RS194B at 22 min (T767), at 7 min (T768) and at 6 mins (T769) following termination of the Px exposure.
Animals were monitored continuously throughout the exposure and clinical observations were recorded prior to administration, during administration and 1 h post administration. When exposure was complete, anesthesia monitoring equipment was removed, animals were removed from the primate restraint chair.
Animals were closely examined for cessation of symptoms typical of excessive cholinergic stimulation over the periods lasting up to 2 h, checked at 4,8 and 12 h and then for survival at 48 h after challenge. After macaques were removed from the inhalation chamber, blood was drawn from the femoral vein prior to and at 0.5, 1, 6, 24, 48 and 192 h following aer-Px and assessed for AChE and BChE levels. Whole blood samples (20 µl) from macaques were first diluted 10-fold in water, frozen and tested for plasma BChE and RBC-AChE activity. Animals were sedated for each blood collection with 10 mg/kg ketamine IM and allowed to recover in their cage.
3. Results
While monkeys have been employed to assess oxime potency of nerve agent-inhibited enzymes both in vitro and in vivo (Sit et al., 2011; Radić et al., 2012; Rosenberg et al., 2017; Worek et al., 2010a; Luo et al., 2010, 2008), they have been rarely used to evaluate oxime reactivation following phosphorothioate insecticide exposure in vivo. In this context, unlike nerve agents, OP insecticides such as chlorpyrifos, malathion and parathion, must be first metabolically converted by oxidative desulphuration into the active oxon (to replace P = S bond with a P= O) by liver cytochrome P450 for their insecticidal action (Murray and Butler., 1994; Atterberry et al., 1997; Mukhopadhyay and Saha., 2013). This results in a slow onset in clinical symptoms following insecticide-poisoning, providing more time to administer post-exposure atropine/oxime and achieve ChE reactivation and reversal of cholinergic symptoms. Using a macaque model and pulmonary system for delivery of 100ug/kg of active paraoxon, the present study has examined the effectiveness of a single IM injection of the centrally acting RS194 B oxime to reactivate AChE and BChE and reverse severe clinical symptoms resulting from a lethal exposure. It should be noted that a large study on parathion-poisoned patients (~70 kg) indicated that a lethal dose of parathion was in the range of 20–100 mg (Eyer et al., 2003).
3.1. Delivery parameters and onset of symptoms following paraoxon exposure
Table 1 describes the pulmonary exposure details and the impact on heart rate of a lethal dose of aer-Px (100ug/kg) delivered using a combination nebulizer/adaptor. It should be noted that while the duration of aer-Px exposure was ~21 min, only macaque T767 received 100% of the 100 ug/kg aer-Px dose before bradycardia occurred and the administration was stopped. Thus, doses of Px delivered to macaques T767, T768 and T769 were 320 ug, 250 ug and 313 ug, respectively
Table 1.
Paraoxon exposure summary and effect on heart rate.
| Animal ID | Dose (mg/ kg) |
Aerosol Flowrate (l/ min) |
Exposure Start time |
Exposure End time |
Vol(ml) | Note |
|---|---|---|---|---|---|---|
| T767 | 0.1 | 2 | 9:02 AM | 9:23 AM | 3.65 ml loaded 3.65 ml used (100%) | Heart rate dropped from 212/min to 142/min |
| T768 | 0.1 | 2 | 10:28 AM | 10:45 AM | 3.75 ml loaded 2.93 ml used (78%) | Paused @10:43am, heart rate dropped from 194/min to 110/min |
| T769 | 0.1 | 2 | 11:25 AM | 11:47 AM | 3.8 ml loaded 3.49 ml used (92%) | Paused @11:36am due to low SpO2 (63%), resumed @ 11:40am, heart beat dropped from 185/min to 101/min |
The clinical symptoms which began within 30 mins following termination of aer-Px are shown in Table 2. and involved diarrhoea, miosis, irregular breathing, tail stiffness and salivation before atropine (0.28 mg/kg) and RS194B oxime (80 mg/kg) was administered IM at 43, 32, and 38 mins following initiation of the exposure and at 22, 7 and 6 mins following termination of the exposure to T767, T768 and T769 respectively. Tremors and labored breathing observed in 3/3 monkeys at 40–49 mins following oxime injection, had disappeared within 4 h post-exposure.
Table 2.
Clinical symptoms in macaques exposed to lethal inhaled paraoxon and treated post-exposure with RS194B oxime.
| Animal ID | BW (kg) | During Exposure | Immediately After Exposure | Hours Post Exposure | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 1–2 h | 4 | 8 | 24 | ||||
| T767 | 3.4 | N | 9:36am T = 96.3F | 10:45 am tremors | N | N | N |
| 10:00am T = 94.7F | |||||||
| 10:13am T = 95.1F | |||||||
| 9:30am Continuous defecation | |||||||
| 9:32am Irregular breathing, gasping | |||||||
| 9:45am Atropine and RS194B | |||||||
| 9:47am Respiratory stridor, pale | |||||||
| 10:18am Left pupil normal right pupil miotic | |||||||
| 10:58am Still present | |||||||
| T768 | 3.66 | SpO2 decreased, facial tremors | 10:45am SpO2 = 60% | 11:39am | N | N | N |
| 10:52am Pale, slight defecation, T = 97.2F, miotic bilateral, irregular breathing | tremors 11:45am | ||||||
| Labored breathing | |||||||
| 10:50am Atropine | |||||||
| 10:52am RS194B | |||||||
| T769 | 4.14 | rapid respiration, SpO2 decreased | 11:51am Muscle tremors, defecation, irregular breathing, gasping | 12:53pm | N | N | N |
| tremors, Labored breathing | |||||||
| 11:53am Atropine and RS194B | |||||||
| 11:55am SpO2 = 53%, T = 97.8F, slight eye twitching, miotic bilateral, tail stiffness, pale, salivation | |||||||
| 12:04pm Respiratory stridor, eyes more miotic | |||||||
Macaques were exposed to 100ug/kg aer-Px, SpO2–blood oxygen saturation levels. N = normal.
3.2. Rapid RS194 B oxime reactivation of blood AChE and BChE activities in macaques following paraoxon inhalation
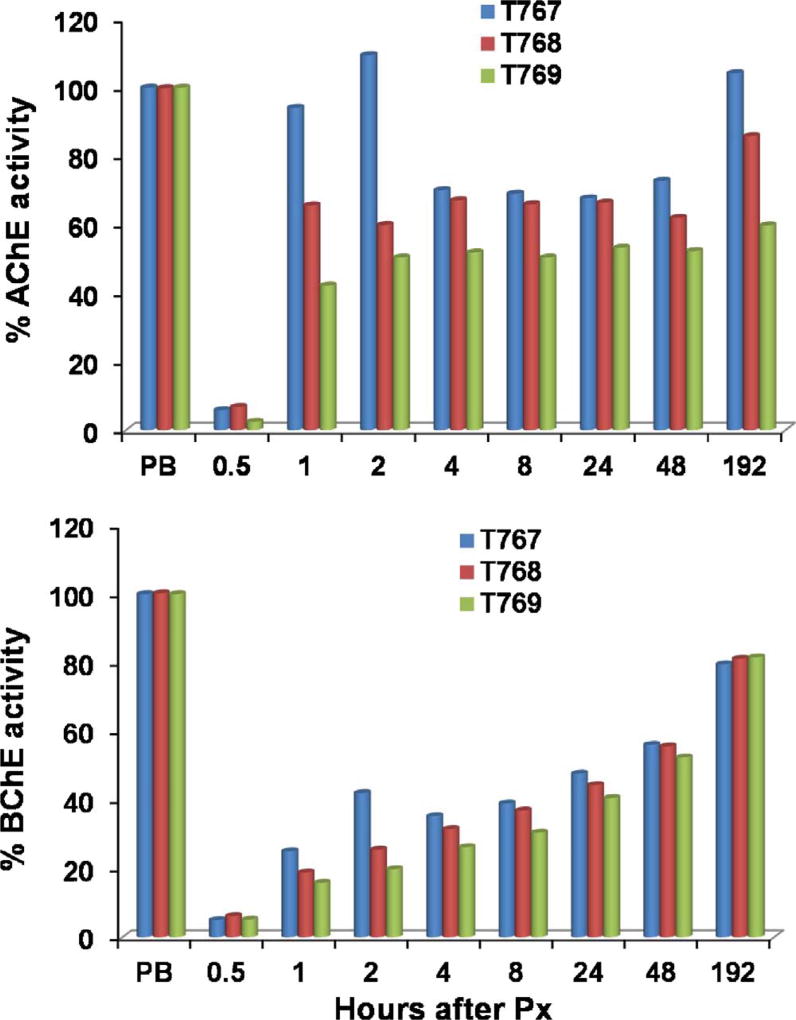
The macaques described in Tables 1 and 2 were also monitored for reactivation of RBC-AChE and plasma BChE. While all macaques exhibited rapid, sustained recovery of RBC-AChE and plasma BChE activities, the oxime responsiveness varied; macaque T767 reaching 100% of AChE baseline levels within an hour compared to 65% for T769 and 42% for T768 (Fig. 1). By day 8 all levels ranged from 60 to 100%. The reactivation of BChE was slower than that observed with AChE reaching 40% reactivation by ~8 h and > 80% by day 8 in all macaques. Once again, T767 exhibited the fastest recovery. Restoration of cholinesterase levels by RS194 B in the blood, along with the rapid cessation of gastrointestinal and respiratory symptoms, suggest a roughly parallel time course for RS194B-mediated reactivation of AChE in the brain.
Fig. 1.
Post-exposure reactivation of RBC-AChE and plasma BChE by RS194B in three macaques weighing between 3.4–4.14 kg (T767, T768, T769) exposed to inhaled Px (100 g/kg). Each monkey received 320, 250 and 313 µg, respectively. Values are shown as percent of pre-exposure levels in the individual macaque prior to paraoxon exposure. The time intervals between cessation of aer-Px administration and injection of RS194 B in the animals were 22, 7 and 6 min respectively. All animals were administered atropine (0.28 mg/kg) followed by oxime (80 mg/kg) IM in separate syringes. Doses were divided and ½ dose injected into each side. All macaques survived. Peak inhibition ranged from 2 to 7% of pre-bleed level.
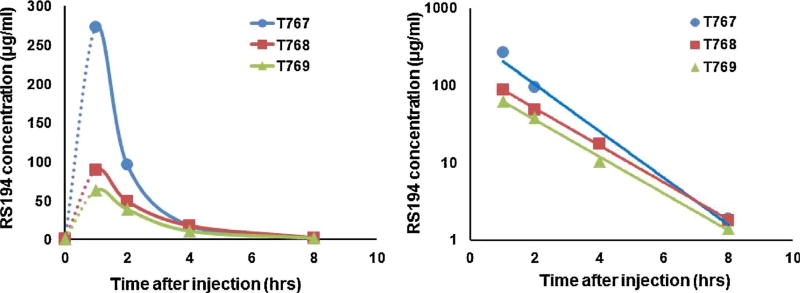
3.3. Kinetics of oxime clearance
Fig. 2 shows the clearance of RS194B in the macaques previously exposed to lethal aer-Px with a Cmax occurring at ~1 h and total elimination by 8 h. The high Cmax (270 ug/ml) in the circulation of macaque T767 correlated with the very high levels of reactivation above background.
Fig. 2.
Concentrations of IM administered RS 194B (80 mg/kg) extracted from blood samples of the three macaques following a lethal inhalation exposure of aer-Px (100ug/kg) (left). Measurements were made by High Pressure Liquid Chromatography and Mass spectrometry (17). (right) Logarithmic plots of the same data showing a similar decline of plasma concentrations. Oxime was delivered 22mins (blue), 7mins (orange) and 6 mins (green) after termination of the aer-Px. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
4. Discussion
Reactivation of plasma AChE, presumed reactivation in tissue, and the ultimate survival of the exposed macaques involve restoration of the inhibited enzyme activity in the brain and blood, through a nucleophilic attack by the oxime on the conjugated OP (Wilson and Ginsburg, 1955). Most oxime therapies for insecticide poisoned patients and animals achieve this outcome only if oxime treatment is initiated early after exposure and administered repetitively. In this context, in minipigs exposed to oral dimethoate, high doses of pralidoxime and obidoxime resulted in only a < 25% reactivation by 12 h (Worek et al., 2010b), whilst a steady state plasma concentration of 14.5uM of obidoxime, required infusion of a total of 2269 +/−1726 mg into a patient over 65 h+/−55 h to provide the necessary supportive care (Thiermann et al., 2010). In parathion poisoning of rats (Bunya et al., 2016), animals received IV 20 mg/kg (LD50), 40 mg/kg and 60 mg/kg (LD75) doses of the OP and were treated with the standard critical care used for humans made up of continuous atropine, midazolam (every 4 h) and either 15 mg/kg or 90 mg/kg doses of 2-PAM IV post exposure every 6 h. However, despite these high doses of 2-PAM, no reactivation of RBC-AChE was observed during the first for 24 h and 50% activity was not reached until days 4 and 5.
This requirement for very high doses or continuous 2-PAM and obidoxime treatment is in marked contrast to the rapid 42–100% reactivation of AChE within 1hr and the rapid reversal of severe symptoms observed following a single post-exposure IM injection of RS194B (80 mg/kg) with low-dose atropine (0.28 mg/kg) in macaques exposed to inhaled paraoxon. These results are similar to those previously observed using 62.5 mg/kg RS194 B following inhaled sarin vapor (Rosenberg et al., 2017). This dramatic outcome is consistent with the ability of RS194 B to rapidly cross the BBB. In the current study, oxime was administered when all macaques experienced diarrhoea, hypothermia and severe bradycardia. Symptoms were expected to proceed to fatality. Immediately before and following oxime administration, exposed macaques also exhibited distinct tremors and/or muscle rigidity, and difficulty breathing from upper respiratory stridor and/or apneic episodes before normalization of symptoms within four hours.
In this context, these symptoms are very similar to those observed in insecticide poisoned people exposed dermally, by inhalation or by ingestion (Eddleston et al., 2008). The initial observed abdominal manifestations of toxicity e.g. diarrhoea, is consistent with deposition of the inhaled Px in the gastrointestinal tract as previously indicated bv gamma scintigraphy (Marchand et al., 2014). It should be noted that Px was diluted in water and PBS which is absorbed less readily than oil-based liquid formulations.
The levels of reactivation of Px-inhibited AChE were, in general, similar to that observed with sarin-inhibited AChE in exposed macaques; 70% for aer-Px vs 40–60% for sarin vapor. Although reactivation of BChE was slower than AChE, RS194B still achieved a significant 40% level by 8 h. In previous in vitro and in vivo studies with 2-PAM and other oximes, monitoring of pesticide inhibited BChE was determined not to be clinically useful as a therapeutic marker (Aurbek et al., 2009; Konickx et al., 2013). It is possible that RS194B reactivation of AChE and BChE may be more valuable blood markers in this respect. Recent studies using phenoxyalkyl pyridinium, detergent-like oximes have also shown AChE reactivation levels to 25% in the rat brain homogenates and 70–80% protection in rats against sarin and VX surrogates when given at the time of onset of seizure (25–30 mins)(Chambers et al., 2016).
The ability of oximes to reverse AChE inhibition in vivo is known to vary with the pesticide type ingested or inhaled (Eddleston and Chowdhury., 2016). In this context, diethyl phosphoryl conjugates (paraoxon-ethyl, parathion, chlorpyrifos, diazinon, quinalphos) are more efficiently reactivated than dimethyl conjugates (malathion, dimethoate, paraoxon-methyl, dichlorvos) which are generally resistant to reactivation by oximes such as 2-PAM and obidoxime (Worek et al., 1999). Further studies will determine how effective RS194B will be within a larger family of chemically diverse OPs.
5. Conclusion
Identifying efficacious antidotes capable of reactivating OP insecticide-inhibited brain AChE in the central and peripheral nervous systems has proven elusive despite many years of intense effort. In addition few animal models with biochemical/pharmacological similarities to humans exist. This initial study has demonstrated that a lethal dose of inhaled Px in macaques resulted in symptoms very similar to insecticide poisoned humans exposed by either inhalation or ingestion, and that these symptoms could be rapidly reversed and inhibited-RBC-AChE rapidly reactivated by IM administration of a centrally acting oxime RS194B. This model may serve as a valuable tool for developing a protective treatment in humans, as well as a NHP model for regulatory approval.
Acknowledgments
Funding
This work was supported by the National Institutes of HealthR44NS064608 to YJR and National Institutes of Health NS UO1-058046 to PT.
Abbreviations
- AChE
acetylcholinesterase
- BChE
butyrylcholinesterase
- Ma
macaque
- OP
organophosphate
- Px
paraoxon
- IM
intramuscular
References
- Alavanja MC. Pesticides use and exposure extensive worldwide. Rev. Environ. Health. 2009;4:303–309. doi: 10.1515/reveh.2009.24.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atterberry TT, Burnett WT, Chambers JE. Age-related differences in parathion and chlorpyrifos toxicity in male rats: target and non-target esterase sensitivity and cytochrome P450-mediated metabolism. Toxicol. Appl. Pharmacol. 1997;147(2):411–418. doi: 10.1006/taap.1997.8303. [DOI] [PubMed] [Google Scholar]
- Aurbek N, Thiermann H, Eyer F, Eyer P, Worek F. Suitability of human butyrylcholinesterase as therapeutic marker and pseudo catalytic scavenger in organophosphate poisoning: a kinetic analysis. Toxicology. 2009;259(3):133–139. doi: 10.1016/j.tox.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Buckley NA, Eddleston M, Li Y, Bevan M, Robertson J. Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst. Rev. 2011;2:CD005085. doi: 10.1002/14651858.CD005085.pub2. [DOI] [PubMed] [Google Scholar]
- Bunya N, Sawamoto K, Benoit H, Bird SB. The effect of parathion on red blood cell acetylcholinesterase in the wistar rat. J. Toxicol. 2016;2016:1–5. doi: 10.1155/2016/4576952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadieux CL, Wang H, Zhang Y, Koenig JA, Shih T-M, McDonough J, Koh J, Cerasoli D. Probing the activity of a non-oxime reactivator for acetylcholinesterase inhibited by organophosphate nerve agents. Chem. Biol. Interact. 2016;259:133–141. doi: 10.1016/j.cbi.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JE, Chambers HW, Funck KE, Meek EC, Pringle RB, Ross MK. Efficacy of novel phenoxyalkyl pyridinium oximes as brain-penetrating reactivators of cholinesterase inhibited by surrogates of sarin and VX. Chem. Biol. Interact. 2016;259(Pt B):154–159. doi: 10.1016/j.cbi.2016.07.004. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Clarkson ED, Ratcliffe RH, Campbell AJ, Thangavelu SG, Herdman CA, Leader H, Schulz SM, Marek E, Medynets MA, Ku TC, Evans SA, Khan FA, Owens RA, Nambiar MP, Gordon RK. Pro-PAM therapy for central and peripheral cholinesterases. Chem. Biol. Interact. 2010;187:191–198. doi: 10.1016/j.cbi.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M, Chowdhury FR. Pharmacological treatment of organophosphorus insecticide poisoning: the old and the (possible) new. Br. J. Clin. Pharmacol. 2016;81(3):462–470. doi: 10.1111/bcp.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371(9612):597–607. doi: 10.1016/S0140-6736(07)61202-1. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M, Eyer P, Worek F, Juszczak E, Alder N, Mohamed F, Senarathna L, Hittarage A, Azher S, Jeganathan K, Jayamanne S, von Meyer L, Dawson AH, Sheriff MH, Buckley NA. Pralidoxime in acute organophosphorus insecticide poisoning – a randomised controlled trial. PLoS Med. 2009;6(6):1–12. doi: 10.1371/journal.pmed.1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyer F, Meischner V, Kiderlen D, Thiermann H, Worek F, Haberkorn M, Felgenhauer N, Zilker T, Eyer P. Human parathion poisoning. A toxicokinetic analysis. Toxicol. Rev. 2003;22(3):143–163. doi: 10.2165/00139709-200322030-00003. [DOI] [PubMed] [Google Scholar]
- Gupta RC. Toxicology of Organophosphate & Carbamate Compounds. Academic Press; New York: 2006. p. 763. [Google Scholar]
- Hulse EJ, James OJ, Davies A, Simpson J, Sciuto AM, Eddleston M. Respiratory complications of organophosphorus nerve agent and insecticide poisoning implications for respiratory and critical care. Am. J. Respir. Crit. Care Med. 2014;190(12):1342–1354. doi: 10.1164/rccm.201406-1150CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konickx LA, Worek F, Jayamanne S, Thiermann H, Buckely HA, Eddleston M. Reactivation of plasma butyrylcholinesterase by pralidoxime chloride in patients poisoned by WHO class II toxicity organophosphorus insecticides. Toxicol. Sci. 2013;136(2013) doi: 10.1093/toxsci/kft217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Tong M, Maxwell DM, Saxena A. Comparison of oxime reactivation and aging of nerve agent-inhibited monkey and human acetylcholinesterases. Chem. Biol. Interact. 2008;175(1–3):261–266. doi: 10.1016/j.cbi.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Luo C, Chambers C, Yang Y, Saxena A. Mechanism for potent reactivation ability of H oximes analyzed by reactivation kinetic studies with cholinesterases from different species. Chem. Biol. Interact. 2010;187(1–3):185–190. doi: 10.1016/j.cbi.2010.01.018. [DOI] [PubMed] [Google Scholar]
- MacLoughlin RJ, van Aergongen G, Fink JB, et al. Optimization and dose estimation of aerosol delivery to nonhuman primates. J. Aerosol Med. Pulm. Drug Deliv. 2016;29:1–7. doi: 10.1089/jamp.2015.1250. [DOI] [PubMed] [Google Scholar]
- Marchand D, Parent C, Respaud R, et al. Nebulization of antibodies against ricin poisoning by inhalation: drug and development. Respir. Drug Deliv. 2014;3:605–608. [Google Scholar]
- Mercey G, Verdelet T, Renou J, Kliachyna M, Baati R, Nachon F, Jean L, Renard PY. Reactivators of acetylcholinesterase inhibited by organophosphorus nerve agents. Acc. Chem. Res. 2012;45:756–766. doi: 10.1021/ar2002864. [DOI] [PubMed] [Google Scholar]
- Mew EJ, Padmanathan P, Konradsen F, Eddleston M, Chang SS, Phillips MR, Gunnell D. The global burden of fatal self-poisoning with pesticides 2006–15: Systematic review. J. Affect. Disord. 2017;219:93–104. doi: 10.1016/j.jad.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, Saha D. Insecticide resistance mechanisms in three sucking insect pests of tea with reference to North-East India: an appraisal. Inter. J. Trop. Sci. 2013;33(1):46–70. [Google Scholar]
- Murray M, Butler AM. Hepatic biotransformation of parathion: role of cytochrome P450 in NADPH-and NADH-mediated microsomal oxidation in vitro. Chem. Res. Toxicol. 1994;7:782–799. doi: 10.1021/tx00042a012. [DOI] [PubMed] [Google Scholar]
- Sit RK, Radić Z, Gerardi V, Zhang L, Garcia E, Katalinić M, Amitai G, Kovarik K, Fokin VV, Sharpless KB, Taylor P. New structural scaffolds for centrally acting oxime reactivators of phosphylated cholinesterases. J. Biol. Chem. 2011;22:19422–19430. doi: 10.1074/jbc.M111.230656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radić Z, Sit RK, Kovarik Z, Berend S, Garcia E, Zhang L, Amitai G, Green C, Radić B, Fokin VV, Sharpless KB, Taylor P. Refinement of structural leads for centrally acting oxime reactivators of phosphylated cholinesterases. J. Biol. Chem. 2012;15:11798–11809. doi: 10.1074/jbc.M111.333732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg YJ, Walker J, Jiang X, Donahue S, Robosky J, Sack M, Lees J, Urban L. A highly stable minimally processed plant-derived recombinant acetylcholin-esterase for nerve agent detection in adverse conditions. Sci. Rep. 2015;5:1–8. doi: 10.1038/srep13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg YJ, Mao L, Jiang X, Lees J, Zhang L, Radic Z, Taylor P. Postexposure treatment with the oxime RS194 B rapidly reverses early and advanced symptoms in macaques exposed to sarin vapor. Chem. Biol. Interact. 2017;274:50–57. doi: 10.1016/j.cbi.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T-M, Koplovitz I, Kan RK, McDonough JH. In search of an effective in vivo reactivator for organophosphorus nerve agent-inhibited acetylcholinesterase in the central nervous system. Adv. Stud. Biol. 2012;4:451–478. [Google Scholar]
- Steinritz D, Eyer F, Worek F, Thiermann H, John H. Repetitive obidoxime treatment induced increase of red blood cell acetylcholinesterase activity even in a late phase of a severe methamidophos poisoning: a case report. Toxicol. Lett. 2016;244:121–123. doi: 10.1016/j.toxlet.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Taylor P. Anticholinesterase agents. In: Brunton LL, editor. Goodman and Gilman's Pharmacological Basis of Therapeutics. twelfth. 2010. pp. 239–254. [Google Scholar]
- Thiermann H, Eyer F, Felgenhauer N, Pfab R, Zilker T, Eyer P, Worek F. Pharmaco-kinetics of obidoxime in patients poisoned with organophosphorus compounds. Toxicol. Lett. 2010;197(3):236–242. doi: 10.1016/j.toxlet.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Wilson B, Ginsburg S. Reactivation of acetylcholinesterase inhibited by alkylphosphates. Arch. Biochem. Biophys. 1955;54:569–571. doi: 10.1016/0003-9861(55)90075-8. [DOI] [PubMed] [Google Scholar]
- Worek F, Diepold C, Eyer P. Dimethylphosphoryl-inhibited human cholin-esterases: inhibition, reactivation, and aging kinetics. Arch. Toxicol. 1999;73(1):7–14. doi: 10.1007/s002040050580. [DOI] [PubMed] [Google Scholar]
- Worek F, Aurbek N, Wille T, Eyer P, Thiermann H. Kinetic analysis of interactions of paraoxon and oximes with human, Rhesus monkey, swine, rabbit, rat and guinea pig acetylcholinesterase. Toxicol. Lett. 2010a;200(1–2):19–23. doi: 10.1016/j.toxlet.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Worek F, Aurbek N, Herkert NM, John H, Eddleston M, Eyer P, Thiermann H. Evaluation of medical countermeasures against organophosphorus compounds: the value of experimental data and computer simulations. Chem. Biol. Interact. 2010b;187(1–3):259–264. doi: 10.1016/j.cbi.2009.11.009. [DOI] [PubMed] [Google Scholar]