Abstract
Immunotherapy strategies targeting immune checkpoints such as the CTLA4 and CD274 (programmed cell death 1 ligand 1, PD-L1)/PDCD1 (programmed cell death 1, PD-1) T-cell coreceptor pathways are revolutionising oncology. The approval of pembrolizumab use for solid tumours with high-level microsatellite instability or mismatch repair deficiency by the US Food and Drug Administration highlights promise of precision immuno-oncology. However, despite evidence indicating influences of exogenous and endogenous factors such as diet, nutrients, alcohol, smoking, obesity, lifestyle, environmental exposures and microbiome on tumour-immune interactions, integrative analyses of those factors and immunity lag behind. Immune cell analyses in the tumour microenvironment have not adequately been integrated into large-scale studies. Addressing this gap, the transdisciplinary field of molecular pathological epidemiology (MPE) offers research frameworks to integrate tumour immunology into population health sciences, and link the exposures and germline genetics (eg, HLA genotypes) to tumour and immune characteristics. Multilevel research using bioinformatics, in vivo pathology and omics (genomics, epigenomics, transcriptomics, proteomics and metabolomics) technologies is possible with use of tissue, peripheral blood circulating cells, cell-free plasma, stool, sputum, urine and other body fluids. This immunology-MPE model can synergise with experimental immunology, microbiology and systems biology. GI neoplasms represent exemplary diseases for the immunology-MPE model, given rich microbiota and immune tissues of intestines, and the well-established carcinogenic role of intestinal inflammation. Proof-of-principle studies on colorectal cancer provided insights into immunomodulating effects of aspirin, vitamin D, inflammatory diets and omega-3 polyunsaturated fatty acids. The integrated immunology-MPE model can contribute to better understanding of environment-tumour-immune interactions, and effective immunoprevention and immunotherapy strategies for precision medicine.
INTRODUCTION
Accumulating evidence indicates that innate and adaptive immunity profoundly influences the evolution of neoplasms.1–3 While cancer comprises transformed neoplastic cells that have accumulated somatic molecular alterations, there is a dynamic interplay of neoplastic and non-neoplastic cells including inflammatory and immune cells. A fraction of somatic mutations may result in the generation of new antigens (neoantigens) that can be recognised as non-self by the immune system. During an individual’s life-course, cells may acquire somatic molecular alterations, and some of these cells undergo clonal expansion, displaying hallmarks of early neoplasia. Many of these cells are likely kept in check or killed by the host immune system before they can develop into clinically detectable tumours. By the time a tumour is detected, it has often acquired mechanisms to suppress immune responses and evade host immune surveillance. These processes are referred to as cancer immunoediting.
Cancer immunology is a blossoming field that has garnered well-deserved attention because of the success of immunotherapy approaches that target immune checkpoint mechanisms such as the CTLA4 and CD274 (programmed cell death 1 ligand 1, PD-L1)/PDCD1 (programmed cell death 1, PD-1) pathways.4 Many types of cancers misappropriate physiologic immune checkpoint mechanisms to evade immune-mediated recognition and destruction. Notably, blockade of immune checkpoints has proven successful in treating multiple tumour types, underscoring the power of the immune system to keep neoplasia in check. Additional active areas within cancer immunology include the development of cancer vaccines, adoptive cell therapy and immunisation for prevention and therapy.5–8
The immune system and inflammation undoubtedly play an important role in cancer aetiology as indicated by IBD-associated cancers and post-transplant malignancies due to long-term immunosuppression. Hence, primary cancer prevention is possible through the use of the immune system.5,9 Evidence indicates that modifiable factors such as non-steroidal anti-inflammatory drug (NSAID) use, obesity, physical activity, cigarette smoking and systemic vitamin D levels influence cancer risk and outcome as well as immune system function.10–17 Hence, dietary, lifestyle and pharmacological immunomodulators may be used to enhance the immune system for cancer prevention and treatment.
Due to these multifaceted interactions, a comprehensive understanding of neoplasia requires a robust interrogation of the environment (inclusive of the exposome, ie, the totality of the exposures to various exogenous and endogenous factors) given environmental effects on both host immunity and neoplasms (illustrated in figure 1). Expert panels have recommended that integrative transdisciplinary studies of modifiable exposures, tumour characteristics (including tumour omics and immunity) and clinical outcomes are urgently needed to improve strategies for precision prevention and treatment.9,18–21 However, this area of research is still in its infancy. We propose an integrated multilevel analysis of environment, tumour and immunity for improving cancer prevention and treatment. In this review, we discuss values and potentials of new approaches of integrating cancer immunology into pathobiology-based population health science.
Figure 1.
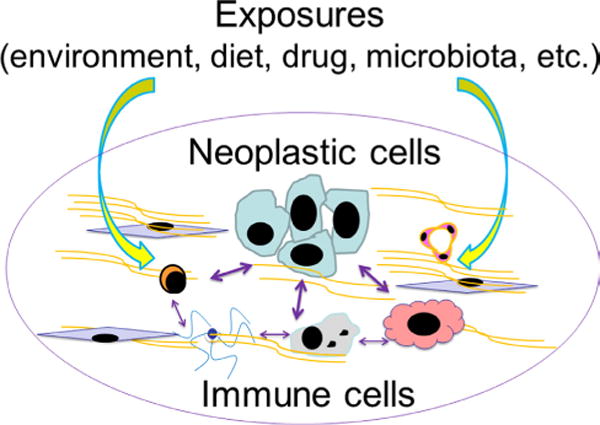
Various exogenous and endogenous factors (collectively called ‘exposures’) influence a tumour that has intrinsic and interacting components of neoplastic cells and the immune system. For simplicity, this does not depict complex interactions between the exposures and between neoplastic cells. There are ample research opportunities to decipher the interactions between these factors and components in human subjects and populations for better understanding of neoplasms. This figure illustrates potentials of integration of immunology and molecular pathological epidemiology.
CANCER IMMUNOLOGY AND PRECISION MEDICINE
The immune system is complex, consisting of many different and interacting cell types, which include (but are not limited to) T cells, B cells, NK cells, dendritic cells, macrophages, mast cells, polymorphonuclear neutrophils, eosinophils, basophils and other lymphoid and myeloid cells. While the importance of immunity for cancer is well recognised, the complexities of the immune system make its measurement and evaluation challenging in the clinical setting. Hence, clinical testing on tumour immunity have lagged behind tumour molecular testing for which the guidelines have been well developed.22 The clinical implications of the abundance and activities of various immune cell types in tumour tissue may vary in different tumour types. For example, intratumoural FOXP3+ regulatory T cells (Tregs) may have a different prognostic significance in different tumour types.23,24 In addition, heterogeneity in immune cell distribution and action can be influenced by local tumour cells, stroma and microenvironmental factors, including the microbiota.25 Spatial heterogeneity of the immune response poses further challenge when analysis is performed on small biopsy specimens rather than large resection specimens.
Despite these challenges, there are ample opportunities to develop clinically useful cancer immunology assays. For instance, international efforts have recently started to standardise immune cell analysis in cancer tissue; one of such efforts is the ‘immunoscore’ project,26,27 with an independent proof-of-principle study.28 In addition, a comprehensive evaluation of tumour immunity necessitates analyses of both tumour and immune cells.29 A number of tumour molecular analyses have been used in clinical practice, and tumour immunity evaluation can add prognostic information beyond tumour molecular features.24,30 For example, high-level microsatellite instability (MSI) and a high neoantigen load in colorectal cancers have been associated with a robust immune response and favourable clinical outcomes.31–34 MSI-high phenotype or mismatch repair deficiency predicts response to PDCD1 (PD-1) immune checkpoint blockade.35,36 Since May 2017, the US Food and Drug Administration (FDA) has approved use of the anti-PDCD1 (PD-1) antibody pembrolizumab for MSI-high or mismatch repair-deficient solid tumours regardless of primary organ site of tumour (https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm560040.htm; last visited on 5 December 2017). This represents the first FDA approval of a drug indication based solely on tumour molecular testing without consideration of primary body or organ site. Thus, MSI status (or mismatch repair protein expression) is now an established predictive biomarker of GI cancers for response to immune checkpoint inhibition.
Regarding other immune-related tumour markers, analyses of immune checkpoint pathways have become common for targeted immunotherapies. Some tumours express immune checkpoint ligands, including CD274 (PD-L1) and PDCD1LG2 (PD-L2), to suppress antitumour immunity.2 These ligands can bind to the T cell surface receptor PDCD1 (PD-1) and downregulate the immune response. In multiple cancers including melanoma, renal cell carcinoma, lung carcinoma, gastric carcinoma, lymphomas and other malignancies, blockade of the immune checkpoint pathway provides an effective treatment strategy. Accumulating evidence indicates that activation of the PI3K signalling pathway (by EGFR mutation, PTEN loss, PIK3CA mutation, etc) can upregulate CD274 expression in various tumour types.37–40 These tumour molecular features can be combined with immune cell status in tumour tissues, to subclassify tumours for precision intervention strategies.41,42 In addition to the immune checkpoint ligand-receptor interactions, some cancers have been shown to upregulate certain metabolic enzymes including IDO1 (indoleamine 2,3-dioxygenase 1), TDO2 (tryptophan 2,3-dioxygenase) and ARG1 (arginase 1) that can skew the immune response to suppress antitumour immunity.43–47 IDO1 expression in cancers has been associated with the levels of T cell infiltrates.48,49 Evidence also suggests that enhanced tumour cell metabolism depletes specific nutrients in the tumour microenvironment, which in turn, can suppress immune response and lead to tumour progression.50
To accelerate translation of our growing knowledge about cancer immunology into precision medicine, further efforts are needed to develop integrative tumour molecular pathology and immunity tests that can be useful for clinical and public health practices. To ensure rigour and reproducibility of these efforts, we cannot overemphasise the importance of data sciences, including epidemiology. The field of epidemiology primarily concerns methods of designing studies and analysing biomedical health data. Essentially, all clinical studies explicitly or implicitly rely on epidemiological principles to ensure the internal and external validity of results. Misuse or failure to properly use epidemiological and statistical principles is one of major reasons for non-reproducible study findings.51 Hence, the epidemiological principles must be properly used in clinical and translational research studies to promote robust evidence-based precision medicine.
MOLECULAR PATHOLOGICAL EPIDEMIOLOGY—INTEGRATING MOLECULAR AND POPULATION-LEVEL SCIENCE
Although molecular pathology analyses had been used in epidemiological research on non-communicable diseases since the 1990s, a more complete integration of pathology and epidemiology occurred only relatively recently.52 Molecular pathology has become a major subfield of pathology with molecular pathology diagnostics now playing a routine part in clinical practice. By virtue of advances in molecular pathology, disease classification systems have transformed patient treatment and management. Molecular pathological information can also transform epidemiology. Advances in molecular pathology have enabled testing of targeted epidemiological hypotheses based on biological mechanisms. This trend necessitates the development of new research frameworks and analytic methodologies to decipher disease at both the molecular and population levels.52
In parallel with this trend, the integration of molecular pathology and epidemiology has led to the emergence of the transdisciplinary field of ‘molecular pathological epidemiology (MPE)’.53–56 The MPE approach aims to connect potential risk factors to the molecular pathology of disease. As outcome variables, MPE research deals with disease incidence and mortality as well as health conditions and biomarkers that can predict future disease development or manifestations.57–59 Figure 2 illustrates the general approach that is typically taken for MPE research, using immune analysis as an example. The conceptual and practical rationale for hypothesis testing in MPE research has been described in detail elsewhere.55,60,61 Statistical analysis methods have been developed to test hypotheses on aetiological heterogeneity between disease subtypes in various study design settings,60–69 and to address missing data in MPE research.70 The paradigm of MPE has been widely recognised in the literature.71–113 Its relevance has been discussed in well-established scientific society meetings,114–117 and in the International MPE Meeting Series.118,119
Figure 2.
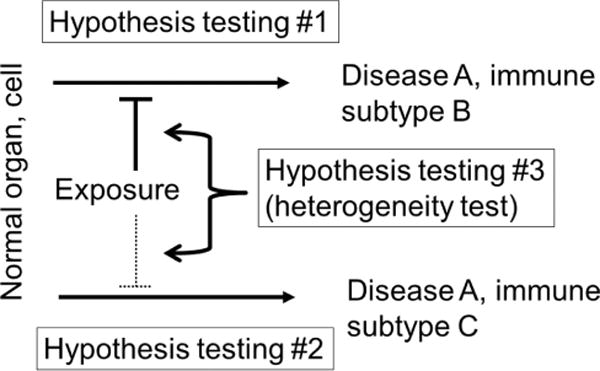
Hypothesis testing in molecular pathological epidemiology (MPE) in a study of immune subtypes of disease A, with the simplest binary subtyping (subtypes B and C). Note that disease subtyping systems are often more complicated than simple dichotomy. Hypothesis testing #1 or #2 is on the relationship between an exposure of interest and each subtype. In this illustration, the exposure is hypothesised to prevent subtype B. Hypothesis testing #3 is unique to MPE, and concerns on a difference (heterogeneity) between the relationships of the exposure with the immune subtypes B and C.
The MPE approach can contribute to precision medicine. Aspirin has been associated with reduction of colorectal cancer incidence and mortality.120–125 MPE analyses of patient survival have suggested potential of aspirin as a therapeutic agent specifically for PTGS2 (cyclooxygenase-2)-overexpressing colorectal cancers,126,127 PIK3CA-mutated colorectal cancers128–130 and CD274 (PD-L1)-low colorectal cancers.131 Some of these markers may be used to select patients for aspirin therapy. Because these MPE analyses have been based on observational cohorts, prospective clinical trials have been ongoing.76
MPE analyses to assess cancer incidence can also contribute to precision prevention.11,13,21,132–134 Although colonoscopy screening has been associated with lower incidence of colorectal cancer, it may be less effective for MSI-high, CpG island methylator phenotype (CIMP)-high and BRAF-mutated colorectal cancers as high levels of MSI and CIMP and BRAF mutations are common features of postcolonoscopy (or interval) colorectal cancers.135–138 MPE studies have shown that smoking is associated with increased risk of MSI-high, CIMP-high and BRAF-mutated colorectal cancers.139–143 These lines of evidence may raise a question whether tobacco smokers may need improved colonoscopy or other screening procedures. It is expected that future MPE analyses can reveal additional links of certain risk factors and specific disease subtypes, which will contribute to the development of precision prevention strategies.
Moreover, MPE is a versatile method-based discipline that can enhance other scientific fields by means of developing unique analytical frameworks and methodologies. For instance, MPE can be integrated into social science144 and pharmacology.145 In particular, integration of pharmacology and MPE can decipher the influence of common medications on incidence and progression of disease subtypes.128,146–149 Similarly, integration of immunology and MPE can also be achieved as detailed below.
MPE HELPS ESTABLISH CAUSALITY
Epidemiological associations between lifestyle factors and cancer risks are relatively weak because traditional epidemiological studies generally use overall organ-specific cancers as a single outcome, and controversies have existed on causality in many of the associations.54 By means of connecting an exposure to specific molecular pathology, MPE research can provide pathogenic insights, and determine the strength of the association for the specific subtype, thereby helping establish causality.54,150 MPE research can reveal hidden structures of causal relationships, which may not be observed in conventional epidemiological research.150 For example, in the so-called obesity paradox, obesity is associated with better clinical outcome among patients with a disease that is caused (at least in part) by obesity. Such paradoxical findings on obesity lead to confusion and questions on causality of the relationship, hindering the development of public health measures for obesity prevention. On the other hand, MPE research can provide unique insights into paradoxical observations. For example, studies on renal cell carcinoma have shown such paradoxical findings.151 MPE research has shown that obesity is associated with FASN-non-expressing subtype of renal cell carcinoma, which in turn is associated with better clinical outcome compared with FASN-overexpressing subtype.152 Hence, MPE research can potentially decipher paradoxical findings in conventional epidemiological research.150
It is also important to acknowledge limitations of observational studies in establishing causality. Hence, in addition to rigorous study design and analyses, the importance in acquiring multiple lines of evidence from research using different experimental biological models and different study settings, including experimental clinical trials, should be recognised.
INTEGRATING IMMUNOLOGY INTO MPE
In this section, we discuss the integration of cancer immunology and MPE.
The scientific link between MPE and immunology
MPE research addresses the interpersonal heterogeneity of disease processes and pathogenesis.53,118 As the unique disease principle153,154 indicates, a pathological process in each person is uniquely influenced by a combination of exogenous and endogenous factors, and their interactions with both disease cells and normal cells including immune cells. It is assumed that individuals with diseases that share similar molecular pathological features also share similar aetiologies.153 Accordingly, subclassification of patients based on similarities (and dissimilarities) of pathological and immunological markers may enable researchers to link putative risk factors to specific pathologies, including altered immune status. Ample evidence indicates an influence of tumour molecular pathological alterations on immune response to tumour, and an influence of immune status on biological aggressiveness and progression of tumour.24,26–29,155,156 Exogenous and endogenous factors can modify the biological nature of a given tumour that has intrinsic and interacting components of neoplastic cells and the immune system (figure 1). In this sense, immunology can be considered an integral part of MPE at the conceptual level, with the potential for providing valuable new insights when integrated into MPE at the practical level.
In addition, the importance of immunity analysis in human tissue cannot be overemphasised, because even the best animal models cannot fully recapitulate the human immune system or complex diseases.157 Hence, experimental immunology research on model systems need to be corroborated and augmented by human tissue and population research and vice versa.
How can immunology be integrated into MPE?
MPE research has been most commonly applied to neoplastic diseases.55 Neoplastic diseases provide us with clonally expanded cells for molecular analyses in both clinical practice and MPE research settings. However, analyses of immunity status in tumour tissue have been uncommon in epidemiology despite the important role of immunity in cancer. This is in part because immune cell assessment in tumour tissue has not been a common clinical test, and it remains a considerable challenge to accurately and reproducibly evaluate the interactions between the tumour and immune cells. Furthermore, in a typical population-scale research setting, it is not always practical or feasible to incorporate detailed characterisation of tumours beyond information present in medical records. Assessment of immune cells in tumour tissue currently requires considerable effort from a pathologist. Hence, the integration of immunological assessment of tissue into epidemiological studies remains limited.
Likely, the most commonly used immune-related analysis method in epidemiology settings is germline genetic testing. For example, specific HLA genotypes have been associated with risks for certain autoimmune disorders, including ankylosing spondylitis, rheumatoid arthritis and multiple sclerosis. Numerous genes and gene variants (including those in HLA loci) play roles in immune response to various antigens including microbial proteins and tumour neoantigens. Analysis of immune cells and inflammatory markers in peripheral blood specimens is another method that can provide information on systemic inflammation and immune status and is commonly used in epidemiological research. Nonetheless, germline genetic and peripheral blood analyses yield only limited information on the status of interactions between tumour and immune cells in the local tumour microenvironment. Immune cells in the normal colon mucosa and colorectal cancer tissue show substantial phenotypic differences compared with the same immune cell type isolated from the peripheral blood from the same individual.158,159 Hence, analysis of immune cells in the relevant tissue is essential to understand immune cells in the tumour microenvironment.160 Importantly, integrative multilevel analyses (including omics technologies) of available biospecimens, including germline DNA, peripheral blood cells, circulating molecules, microbes, neoplastic cells and immune cells in the local microenvironment can provide a better, holistic view of immune status within an individual and in relation to a tumour, compared with analysis of any single source of information. Ultimately, the full integration of immunology into MPE will help us gain considerable insights into cancer as a disease of immune dysfunction.
Integrative ‘immunology-MPE’ research model
As discussed above, the integration of immunology and MPE is a natural extension of MPE. It can generate insights into how lifestyle, dietary, genetic, microbial and environmental exposures influence disease processes through impacting the immune system and disease-immune interactions. Although data are currently scarce in this emerging area, this field is expected to expand in light of the increasing importance of cancer immunology. Figure 3 illustrates the multilevel framework of immunology-MPE research. Assessment of immunity can be performed using multilevel and multidimensional approaches, using germline genotypes, immune biomarkers in blood or other body fluids and normal and disease tissues of interest to evaluate the immune status in the tissue microenvironment. Immunology-MPE can and should synergise with basic experimental immunology, as these two research models offer complementary strengths (table 1).
Figure 3.
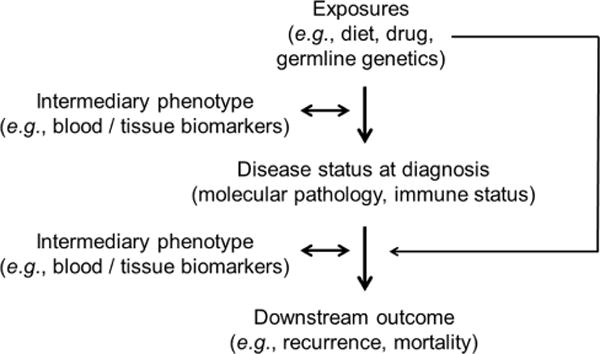
Overview of research designs to integrate immunity analyses into the framework of molecular pathological epidemiology. The word ‘exposures’ is broadly used as an epidemiological term for variables that can causally influence disease incidence, status or outcome. Immunity assessments include germline genotyping of immune-related genes, analyses of immunity status in disease tissue and analyses of biomarkers on tissue, blood and/or other body fluids as intermediary phenotypes. Intermediary phenotypes refer to phenotypes that are thought to precede full-blown disease phenotypes or subsequent clinical outcome (eg, mortality), and can be typically assessed in radiological images, biopsy tissue, peripheral blood or other body fluids. Intermediary phenotype variables can be used as exposures or outcomes in epidemiological terms.
Table 1.
Comparisons of basic experimental immunology and immunology-molecular pathological epidemiology (MPE)
| Basic experimental immunology | Immunology-MPE | |
|---|---|---|
| Research subjects | Immune system in in vivo or in vitro models | Human populations |
| Exposure data (diet, lifestyle, medications, etc.) | Easily control and record exposure data | Possible to collect exposure data |
| Control of environmental or other conditions | Yes | Difficult; a targeted control can be done by randomised trials |
| Evaluation of the human immune system | Can be done using human immune cells. It is still a challenge to recapitulate the human immune system in experimental models. Encompassing the complexity of systemic and local tumour-related immunity is difficult. | Can be done. It is still a challenge to evaluate immunity status in the local microenvironment if access to tissue is difficult. Encompassing the complexity of systemic and local tumour-related immunity may be possible. |
| In vivo evaluation | Possible | Difficult; can be done by in vivo pathology or molecular imaging |
| Large sample size | Difficult | Possible |
| Insight into mechanisms | Possible | Immunology-MPE can provide mechanistic insights, which need to be verified by basic experimental research |
| Generalisability and validity in humans | Generalisability and validity of findings from model systems need to be examined in human populations | As research on human populations, immunology-MPE can be a validation method for findings from experimental research |
Research on GI neoplasms, especially colorectal carcinoma, has considerable relevance in this emerging immunology-MPE area. GI neoplasms represent exemplary diseases to use this integrative research model. The digestive tract, especially the colon and rectum, has rich microbiota and immune tissues and is accessible by endoscopies. It is also sensitive to tumour-promoting effects of inflammation, as illustrated by the link between IBD and colorectal cancer. Furthermore, the external environment, including immune-related lifestyle factors, appears to have a stronger role in the development of GI cancers compared with many other types of malignancies. Colorectal cancer is a molecularly heterogenous group of neoplasms,161–168 which are influenced by exogenous and endogenous factors and immune response to tumour.54,169–172 Epigenetics can mediate effects of exposures on tumour plasticity and phenotypes.153,173–178 The continuum of differences in clinical and molecular characteristics of colorectal neoplasms according to bowel subsites is compatible with the interactive roles of the microbiota and immunity in colorectal carcinogenesis.179–191 As immunoediting appears to be a common event during colorectal carcinogenesis, a subset of colorectal carcinomas have been shown to overexpress CD274 (PD-L1)31,192–201 and PDCD1LG2 (PD-L2).202 However, immune checkpoint blockade has not been shown to be effective against most colorectal carcinomas, except for MSI-high (mismatch repair deficient) tumours.156,203,204 There are thus ample opportunities for the development of effective interventions, including immunoprevention and immunotherapy strategies against colorectal cancer.204,205
Colorectal cancer has been commonly studied in MPE research.54,206,207 There is an increasing trend of assessments of immune response to colorectal carcinoma, in addition to tumour molecular analyses, in the context of large-scale population-based studies.194,208–219 Table 2 lists proof-of-principle immunology-MPE studies on potential aetiological factors that may influence incidence of immune subtypes of cancer; below, we highlight several notable findings.
Table 2.
Immunology–molecular pathological epidemiology (MPE) studies on possible aetiological factors and incidence of neoplasia subgroups classified by immune response to tumour
| First author, Year, ref | Study design | Cases with tissue specimens (sample size*) | Study cohort | Exposure variables | Outcome variables | Main findings on exposures and risk of disease subtypes classified by local tissue immune status |
|---|---|---|---|---|---|---|
| Cao 2016289 | Prospective cohort study | Colorectal cancer (1458 cases in 1 34 981 participants) | Health Professionals Follow-up Study and Nurses’ Health Study | Aspirin use | Incidence of colorectal cancer subtype classified by lymphocytic infiltrates | Regular aspirin use is associated with lower incidence of colorectal cancer subtype with lower level tumour-infiltrating lymphocytes (TIL) but not that of subtype with higher level TIL. |
| Hanyuda 2016290 | Prospective cohort study | Colorectal cancer (1436 cases during 3 346 000 person-years of follow-up) | Health Professionals Follow-up Study and Nurses’ Health Study | Body mass index (BMI) | Incidence of colorectal cancer subtype classified by lymphocytic infiltrates | BMI is not associated differentially with risk of colorectal cancer subtypes classified by lymphocytic infiltrates. |
| Khalili 2015221 | Nested case-control study within prospective cohort study (subgroup analysis in consortium) | Colorectal cancer (288 cases and 1172 controls) | Health Professionals Follow-up Study and Nurses’ Health Study (in Genetics and Epidemiology of Colorectal Cancer Consortium) | Genetic polymorphism rs11676348 (risk allele for inflammatory bowel disease) | Incidence of colorectal cancer subtype classified by lymphocytic infiltrates | The rs11676348 C allele is associated with risk of colorectal cancer subtype showing Crohn’s-like lymphoid reaction but not risk of subtype showing no Crohn’s-like reaction. |
| Liu 2017291 | Prospective cohort study | Colorectal cancer (1311 cases in 1 24 433 participants) | Health Professionals Follow-up Study and Nurses’ Health Study | Dietary inflammatory index | Incidence of colorectal cancer subtype classified by lymphocytic infiltrates | Inflammatory diet is associated with higher incidence of colorectal cancer subtype with lower level intratumour periglandular reaction but not that of subtype with higher level reaction. |
| Song 2016223 | Nested case-control study (within prospective cohort study) | Colorectal cancer (318 cases and 624 controls) | Health Professionals Follow-up Study and Nurses’ Health Study | 25-Hydroxyvitamin D in plasma | Incidence of colorectal cancer subtype classified by lymphocytic infiltrates | Level of plasma 25-hydroxyvitamin D is associated with lower risk of colorectal cancer subtype with high-level tumour-infiltrating lymphocytes (TILs), but not with risk of subtype with low-level TILs. |
| Song 2016220 | Prospective cohort study | Colorectal cancer (614 cases in 1 25 172 participants) | Health Professionals Follow-up Study and Nurses’ Health Study | Dietary intake of omega-3 polyunsaturated fatty acids (ω-3 PUFA) | Incidence of colorectal cancer subtype classified by lymphocytic infiltrates | Intake of ω-3 PUFA is associated with lower risk of colorectal cancer subtype with high tissue FOXP3+ cell density, but not with risk of subtype with low tissue FOXP3+ cell density. |
Official symbols for genes and gene products including proteins are described in the HUGO Gene Nomenclature Committee (HGNC) website (www.genenames.org). Studies with <200 cases with tissue data are not listed.
Sample size is based on cases with available tissue data.
One study, using resources of the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), showed that a higher intake of marine omega-3 polyunsaturated fatty acids (ω-3 PUFA, rich in fish) was associated with a lower risk of colorectal cancer containing high-density FOXP3+ cells in tissue, but not with risk of colorectal cancer containing low-density FOXP3+ cells.220 Possibly, ω-3 PUFA may downregulate the function of FOXP3+ Treg cells, thereby enhancing the antitumour function of effector T cells even in the presence of abundant FOXP3+ cells.220 Consistent with this hypothesis, an in vitro experiment using coculture of naïve CD4+ T cells and colonic FOXP3+ Treg cells revealed that exposure to high ω-3 PUFA concentrations led to downregulation of FOXP3+ cell function, which resulted in increased naïve CD4+ T cell proliferation.220 As exemplified by this study,220 immunology-MPE research can synergise with basic experimental immunology research.
A second study221 based on the Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO) examined whether risk alleles for IBD identified by genome-wide association studies (GWAS) might be associated with colorectal cancer risk (figure 4). Although risk alleles for IBD, a well-established colorectal cancer risk factor, are conceivably also risk alleles for colorectal cancer, none of GWAS-identified IBD risk alleles was detected as a risk allele for colorectal cancer by agnostic GWAS.222 Using over 25 000 cases and controls, one IBD risk allele (rs11676348) was associated weakly with colorectal cancer with an OR (per allele) of 1.08 (95% CI 1.04 to 1.12)221; notably with this effect size, this polymorphism was not detected by an agnostic GWAS approach. Interestingly, when immune response to colorectal cancer was examined, the rs11676348 allele was associated with colorectal cancer exhibiting Crohn’s-like lymphoid reaction (with the OR 1.47; 95% CI 1.01 to 2.13) but not with cancer exhibiting no Crohn’s-like reaction.221 Crohn’s-like lymphoid reaction refers to transmural lymphoid aggregates that mimic Crohn’s disease (one manifestation of IBD).208 As Crohn’s-like reaction is associated with MSI-high colorectal carcinoma,208 the differential association of the rs11676348 allele according to MSI status was examined, revealing a consistent differential association of the rs11676348 allele with MSI-high tumours, but not with non-MSI-high tumours, in two independent datasets within GECCO.221 Possible heterogeneity of aetiological associations according to cancer immune subtypes may explain why the rs11676348 polymorphism is only weakly associated with colorectal cancer overall. This approach enables us to find many uncovered risk alleles for various diseases. This study221 represents a proof-of-principle analysis of the GWAS-MPE approach54 and immunology-MPE research.
Figure 4.
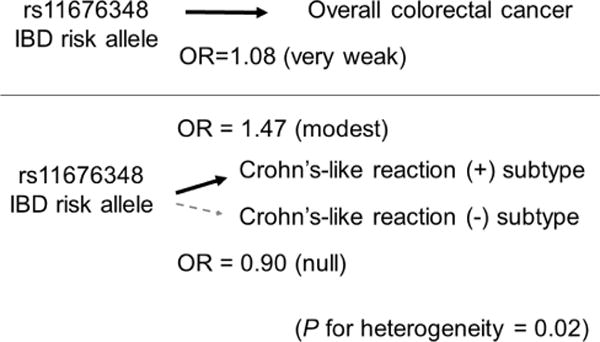
Illustration of the molecular pathological epidemiology approach using tumour immunity status. The germline DNA polymorphism rs11676348 is a risk allele for IBD. Because IBD is a risk factor for colorectal cancer, the rs11676348 polymorphism is considered to be a risk factor for colorectal cancer. The Genetics and Epidemiology of Colorectal Cancer Consortium study showed the OR estimate of 1.08 per rs11676348 risk allele, indicating a very weak association with overall colorectal cancer.221 When colorectal cancer was classified by immune response features, the association of the risk allele was stronger (OR estimate of 1.47 per risk allele) and specific for colorectal cancer subtype with Crohn’s-like lymphoid reaction, but not for subtype without Crohn’s-like lymphoid reaction.
A third study based on the NHS and the HPFS showed that high-level plasma vitamin D was associated with a lower risk of colorectal cancer with high-level lymphocytic infiltrates, but not with risk of colorectal cancer with low-level lymphocytic infiltrates.223 Hence, the cancer preventive effect of vitamin D appears to be stronger for cancer with high-level lymphocytic infiltrates.223 Some immune cells are capable of converting 25-hydroxyvitamin D [25(OH)D] to bioactive 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3], which may prevent neoplastic progression in individuals who can elicit high-level lymphocytic response to emerging tumours.223
TRANSLATION INTO IMMUNOPREVENTION AND IMMUNOTHERAPY
The integration of immunology and MPE can drive future research and clinical practice, to generate population-based evidence and novel insight for the development of effective immunotherapy and immunoprevention strategies. Immunoprevention (or immunoprophylaxis) embraces the use of immunomodulators and prophylactic vaccines. For example, the aforementioned research on ω-3 PUFA and vitamin D suggests their roles in preventing cancer through immune mechanisms. If replicated, the findings of this immunology-MPE study may provide the rationale for the use of nutritional supplementation for cancer immunoprevention. Identification of a dietary, lifestyle or pharmacological factor that can effectively improve outcomes in a specific disease subtype (classified by immune status) may lead to an effective immunotherapeutic strategy for that subtype. Hence, in addition to targeted immunotherapeutic agents, immunomodulators may have a role in clinical immuno-oncology practice. A recent study has shown that use of aspirin is associated with better clinical outcome in colorectal cancers that do not overexpress the CD274 (PD-L1) immune checkpoint ligand, but not with outcome in CD274 (PD-L1)-overexpressing cancers, suggesting a possible synergistic effect of immune checkpoint blockade and aspirin.131 Hence, immunology-MPE research has substantial roles in the development and refinement of strategies of immunoprevention and immunotherapy.
CHALLENGES
Challenges exist in the emerging immunology-MPE field. Some of those are relevant to the field of MPE as previously described.54 First, sample size is generally limited based on biospecimen availability, which can also lead to selection bias. Hence, investigators should make efforts to increase sample size and reduce sample selection bias in MPE research. In addition, a causal inference method such as inverse probability weighting can be used to reduce selection bias due to specimen availability.131,224,225 Second, MPE research uses disease subtyping (ie, multiple disease subtypes), hence inherently facing the issue of multiple hypothesis testing. To address the multiple hypothesis testing issue, it has been recommended that investigators set a heterogeneity test comparing subtype-specific associations as primary hypothesis testing.55 Proper utilisation and practice of statistical analysis can also mitigate this weakness. Third, there are measurement errors in bioassays. Hence, it is critical to ensure assay validity and performance. These challenges affect generalisability of findings. Currently, the studies described in table 2 represent rare examples of immunology-MPE research, for which a replication analysis may not easily be conducted. To increase overall sample size, statistical power and robustness of findings, a consortium should be formed to pool data from multiple studies. It may also be feasible to synthesise population health registries into global-scale MPE databases in the future.226
There are also challenges specific to the emerging field of immunology-MPE. While integration of tumour molecular pathology into epidemiology has been progressing since the 1990s,145 integration of cancer immunology into epidemiology has lagged behind due to several reasons. Importantly, the immune system and its interactions with tumour are inherently complex. Hence, it is very challenging to develop a standardised laboratory test for detailed phenotyping and precise measurement of immune status in the tumour microenvironment. For this reason, it is difficult to implement detailed analyses of tumour-immune interactions in clinical settings and large-scale studies. Nonetheless, the emerging field of immunology-MPE can address this unmet need. Tackling many unanswered questions on effects of exposures on tumour-immune interactions are important in our efforts to improve our understanding of cancer and develop strategies for cancer prevention and treatment. Indeed, efforts to perform cancer immunity analyses using tissue resources in population-based studies have been ongoing. A number of analytic methods that use high-throughput tumour omics platforms to assess tumour-immune cell interactions are available.227 Infiltrates of various immune cell types can be quantified using transcriptome profiling of tumour tissue that contains immune cells.228,229 In addition, DNA or RNA sequencing-based methods targeting T cell receptor genes (such as TRB; T cell receptor β)230–233 may have considerable potential in large-scale population-based studies. Data analysis methods such as network analysis can be applied to tumour immune response data in a large-scale study.189 There is also an opportunity to incorporate digital pathology and image analysis technologies on tissue specimens. In vivo microscopic technologies, which can be used together with endoscopies,234,235 will revolutionise biomedical and population studies of neoplasms, especially premalignant lesions.21,85,236–239 As various omics analysis platforms have recently been applied to single cell analyses,240 there are open opportunities to use single cell analyses on a large number of cells (including immune cells) within a tumour in a large number of people; however, there exist considerable challenges in cost and feasibility of such a study.
In addition, there is a scarcity of interdisciplinary experts and education programmes integrating areas of epidemiology, pathology and immunology. This scarcity causes difficulty in planning and execution of transdisciplinary research including immunology-MPE projects. The scarcity of interdisciplinary experts is both a cause and consequence of the paucity of interdisciplinary training programmes. While population health scientists need education in immunology and pathobiology, wet laboratory scientists also need education in study design, epidemiology and statistics. Development of an interdisciplinary education system in public health and medical schools has been proposed to bridge this gap.52 Such a new system should encompass pathology and epidemiology, and naturally integrate immunology, considering its importance in many human diseases.
As another challenge, funding mechanisms also need to be adapted and optimised to fairly evaluate transdisciplinary science. The current peer-review system relies on evaluations of research proposals by researchers most of whom are experts in traditional disciplines. In addition, funding agencies (such as US National Institutes of Health and Cancer Research UK) have organisational structures mainly based on traditional disciplines, typically disease-based disciplines (but not method-based disciplines). Therefore, it is plausible that transdisciplinary research proposals may not be duly evaluated in light of their potential paradigm-shifting impact. Indeed, transdisciplinary research has been associated with lower funding success despite its stronger scientific impact compared with traditional discipline-bound research.241
OPPORTUNITIES AND FUTURE DIRECTIONS
There are ample opportunities, given the importance of immunity in cancer, evidence for immunomodulatory roles of many exposures and the early rising phase of the immunology-MPE field. Thus far, only a limited number of studies have been conducted in the area, and hence, novelty of the integrative immunology-MPE approach remains relatively high. In addition, new methodologies of immune cell analyses can be incorporated in research. For instance, in vivo pathology technologies are promising tools that can expand the immunology-MPE model as discussed above.
Combining tumour tissue assessment with analyses of normal tissue and other biospecimens including peripheral blood, sputum, urine and other body fluids can add new dimensions to immunology-MPE research. For instance, immune cell analyses such as multicolour flow cytometry can be performed on those body fluid specimens. In addition, omics approaches, such as epigenomic, transcriptomic, proteomic and metabolomic analysis of circulating peripheral blood cells (and/or plasma),242–245 may enable multilevel studies on the status of the immune system, and together with tissue analyses, improve our understanding of influences of the systemic and local immune system status on cancer development and progression. It should be noted that circulating peripheral blood cells consist of many different cell types, and those omic data on circulating cells substantially depend on cellular compositions and their activation status. Recent advances in liquid biopsy provide new approaches to repeatedly and non-invasively interrogate the dynamic evolution of the molecular profiles of human cancers, through analyses of circulating tumour cells (CTCs), circulating tumour DNA (ctDNA) and exosomes containing various biomolecules.3,246–251 Dysregulation of immune regulators has been detected in CTCs,252 and mutational profile in ctDNA has been suggested as a useful marker for monitoring immunotherapy response.253 Therefore, applications of liquid biopsies can lead to new approaches for studying the dynamics of the tumour-immune-environmental interaction.
As another opportunity, microbiology can be integrated into immunology-MPE research. Microorganisms have been the most important targets of the immune system during human evolution. Therefore, an improved understanding of microbes and their interactions with the immune system can advance broad areas of immunology and medicine. Accumulating evidence indicates that microorganisms play important roles in classical infectious diseases and many chronic diseases, including cancer.254–256 For example, many microorganisms have been implicated in tumourigenesis, including Epstein-Barr virus, HBV, HCV, HIV, human papillomavirus, human T-lymphotropic virus, polyoma viruses, Fusobacterium nucleatum, Helicobacter pylori, Schistosoma hematobium, among others. Notably, there is an intriguing link between the gut microbiota, including F. nucleatum and colorectal cancer.172,257–264 F. nucleatum may suppress the adaptive T cell response,265 and this immunosuppressive property may function in a way similar to the immune checkpoint in colorectal cancer. Supporting this, the amount of F. nucleatum in colorectal carcinoma has been inversely associated with CD3+ cell density in tumour.266 The abundance of F. nucleatum in colorectal cancer tissue has been associated with the serrated neoplasia pathway,236,267–270 a fibre-poor diet271 and unfavourable clinical outcomes.272,273 A recent study has shown persistence of F. nucleatum in metastatic colorectal tumour.274 The gut microbiota is a key factor in intestinal diseases and diseases in distant body sites, and even systemic diseases through their influences on metabolisms and immunity.254 Analyses of microbiota can be conducted using oral swab, stool and normal and tumour tissue,275–277 and integrated into immunology-MPE research.
Emerging evidence indicates inter-related links between drugs, nutrition, microbiota, immunity and tumour evolution. Data from integrative research can be used for drug repurposing.278 Studies have shown that the gut microbiota has a profound effect on the efficacy of cancer chemotherapy and immunotherapy.279–283 Frequent use of antibiotics has been associated with an increased risk of colorectal adenomas.284 Combined with pharmacological, nutritional, social and behavioural sciences,144,149,285–287 effects of medications, nutrients, socioeconomic status and other exposures on tumour-microbe-immune interactions can be examined.
CONCLUSIONS
Given the unquestionable importance of the immune system in health and disease, immunology needs to be fully integrated into pathobiological population health science such as MPE. This integration, which has the potential to facilitate the realisation of precision medicine, has been hindered by the complex nature of the immune system and difficulty in developing standardised laboratory methods to assess tumour-immune interactions. Nonetheless, these technical challenges can be surmounted, and research efforts have started to integrate tumour immunology into MPE. To foster this integration, we must develop transdisciplinary education systems and new funding mechanisms. In the next decade, recognised as a discrete scientific field, immunology-MPE will play an increasingly important role in medicine and health sciences, as this field represents one of the priority areas in cancer research.288 Immunology-MPE research will provide novel evidence for roles of the immune systems in health and diseases at a population scale. Figure 5 illustrates a roadmap of integrative immunology-MPE research in order for us to realise precision medicine and exert clinical impact. Ultimately, integrative immunology-MPE research will offer new insights for the development of intervention strategies harnessing the immune system towards precision prevention and treatment.
Figure 5.
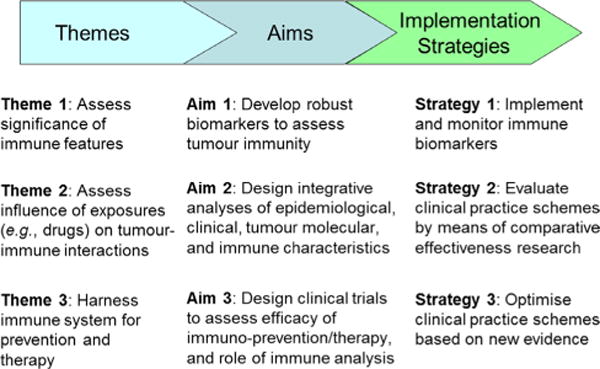
Roadmap of integrative immunology-molecular pathological epidemiology (MPE) to precision medicine. Three themes are set to start integrated immunology-MPE research and accomplish three specific aims. Based on data from research for the three specific aims, strategies 1 through 3 will help us implement, monitor and optimise tumour immunity testing for clinical use.
Acknowledgments
Funding
This work was supported in part by grants from the USA National Institutes of Health (R35 CA197735 (to SO), K07 CA172298 (to AIP), U01 CA137088 (to UP) and K07 CA190673 (to RN)) and Nodal Award (to SO) from the Dana-Farber Harvard Cancer Center.
Footnotes
Contributors
RN, MG, WSG and MS contributed equally. SO developed the main concept of the manuscript. SO, AIP, UP and RN wrote grant applications. All authors contributed to review and revision, and approved the final manuscript.
Competing interests
None declared.
Provenance and peer review
Commissioned; externally peer reviewed.
References
- 1.Galon J, Angell HK, Bedognetti D, et al. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Smyth MJ, Ngiow SF, Ribas A, et al. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13:143–58. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 3.Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. 2017;14:155–67. doi: 10.1038/nrclinonc.2016.144. [DOI] [PubMed] [Google Scholar]
- 4.Gubin MM. Cancer Immunology and Immunotherapy: taking a place in mainstream oncology keystone symposia meeting summary. Cancer Immunol Res. 2017;5:434–8. doi: 10.1158/2326-6066.CIR-17-0224. [DOI] [PubMed] [Google Scholar]
- 5.Marzbani E, Inatsuka C, Lu H, et al. The invisible arm of immunity in common cancer chemoprevention agents. Cancer Prev Res. 2013;6:764–73. doi: 10.1158/1940-6207.CAPR-13-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melief CJ, van Hall T, Arens R, et al. Therapeutic cancer vaccines. J Clin Invest. 2015;125:3401–12. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–21. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahin U, Derhovanessian E, Miller M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–6. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 9.Kensler TW, Spira A, Garber JE, et al. Transforming Cancer Prevention through Precision Medicine and Immune-oncology. Cancer Prev Res. 2016;9:2–10. doi: 10.1158/1940-6207.CAPR-15-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blomain ES, Waldman SA. Does obesity promote the development of colorectal cancer? Expert Rev Anticancer Ther. 2016;16:465–7. doi: 10.1586/14737140.2016.1162102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umar A, Steele VE, Menter DG, et al. Mechanisms of nonsteroidal anti-inflammatory drugs in cancer prevention. Semin Oncol. 2016;43:65–77. doi: 10.1053/j.seminoncol.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Friedenreich CM, Neilson HK, Farris MS, et al. Physical activity and cancer outcomes: a precision medicine approach. Clin Cancer Res. 2016;22:4766–75. doi: 10.1158/1078-0432.CCR-16-0067. [DOI] [PubMed] [Google Scholar]
- 13.Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16:173–86. doi: 10.1038/nrc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dou R, Ng K, Giovannucci EL, et al. Vitamin D and colorectal cancer: molecular, epidemiological and clinical evidence. Br J Nutr. 2016;115:1643–60. doi: 10.1017/S0007114516000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. 2017;18:843–50. doi: 10.1038/ni.3754. [DOI] [PubMed] [Google Scholar]
- 16.Koelwyn GJ, Quail DF, Zhang X, et al. Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer. 2017;17:620–32. doi: 10.1038/nrc.2017.78. [DOI] [PubMed] [Google Scholar]
- 17.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15:484–98. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 18.Palesh O, Demark-Wahnefried W, Mustian K, et al. Conducting cancer control and survivorship research via cooperative groups: a report from the American Society of Preventive Oncology. Cancer Epidemiol Biomarkers Prev. 2011;20:1050–5. doi: 10.1158/1055-9965.EPI-11-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 20.Elena JW, Travis LB, Simonds NI, et al. Leveraging epidemiology and clinical studies of cancer outcomes: recommendations and opportunities for translational research. J Natl Cancer Inst. 2013;105:85–94. doi: 10.1093/jnci/djs473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spira A, Yurgelun MB, Alexandrov L, et al. Precancer atlas to drive precision prevention trials. Cancer Res. 2017;77:1510–41. doi: 10.1158/0008-5472.CAN-16-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Febbo PG, Ladanyi M, Aldape KD, et al. NCCN Task Force report: evaluating the clinical utility of tumor markers in oncology. J Natl Compr Canc Netw. 2011;9(Suppl 5):S1–32. doi: 10.6004/jnccn.2011.0137. [DOI] [PubMed] [Google Scholar]
- 23.deLeeuw RJ, Kost SE, Kakal JA, et al. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18:3022–9. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 24.Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–66. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito T, Nishikawa H, Wada H, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679–84. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 26.Galon J, Pagès F, Marincola FM, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wirta EV, Seppälä T, Friman M, et al. Immunoscore in mismatch repair-proficient and -deficient colon cancer. J Pathol Clin Res. 2017;3:203–13. doi: 10.1002/cjp2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogino S, Galon J, Fuchs CS, et al. Cancer immunology–analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8:711–9. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mlecnik B, Bindea G, Angell HK, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 31.Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giannakis M, Mu XJ, Shukla SA, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 2016;15:857–65. doi: 10.1016/j.celrep.2016.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li SK, Martin A. Mismatch repair and colon cancer: mechanisms and therapies explored. Trends Mol Med. 2016;22:274–89. doi: 10.1016/j.molmed.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Taieb J, Le Malicot K, Shi Q, et al. Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J Natl Cancer Inst. 2017;109:djw272. doi: 10.1093/jnci/djw272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–63. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–70. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 40.Xu C, Fillmore CM, Koyama S, et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell. 2014;25:590–604. doi: 10.1016/j.ccr.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teng MW, Ngiow SF, Ribas A, et al. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–45. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Chen L. Classification of advanced human cancers based on Tumor Immunity in the MicroEnvironment (TIME) for cancer immunotherapy. JAMA Oncol. 2016;2:1403–4. doi: 10.1001/jamaoncol.2016.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uyttenhove C, Pilotte L, Théate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 44.Antonioli L, Blandizzi C, Pacher P, et al. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer. 2013;13:842–57. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 45.Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72:5435–40. doi: 10.1158/0008-5472.CAN-12-0569. [DOI] [PubMed] [Google Scholar]
- 46.Sippel TR, White J, Nag K, et al. Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting arginase I. Clin Cancer Res. 2011;17:6992–7002. doi: 10.1158/1078-0432.CCR-11-1107. [DOI] [PubMed] [Google Scholar]
- 47.Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–51. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 49.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200r. doi: 10.1126/scitranslmed.3006504. a116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–41. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ioannidis JP. How to make more published research true. PLoS Med. 2014;11:e1001747. doi: 10.1371/journal.pmed.1001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogino S, King EE, Beck AH, et al. Interdisciplinary education to integrate pathology and epidemiology: towards molecular and population-level health science. Am J Epidemiol. 2012;176:659–67. doi: 10.1093/aje/kws226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogino S, Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst. 2010;102:365–7. doi: 10.1093/jnci/djq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogino S, Chan AT, Fuchs CS, et al. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogino S, Nishihara R, VanderWeele TJ, et al. Review article: the role of molecular pathological epidemiology in the study of neoplastic and non-neoplastic diseases in the era of precision medicine. Epidemiology. 2016;27:602–11. doi: 10.1097/EDE.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hughes LAE, Simons C, van den Brandt PA, et al. Lifestyle, diet, and colorectal cancer risk according to (epi)genetic instability: current evidence and future directions of molecular pathological epidemiology. Curr Colorectal Cancer Rep. 2017;13:455–69. doi: 10.1007/s11888-017-0395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lochhead P, Chan AT, Giovannucci E, et al. Progress and opportunities in molecular pathological epidemiology of colorectal premalignant lesions. Am J Gastroenterol. 2014;109:1205–14. doi: 10.1038/ajg.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roy HK, Turzhitsky V, Wali R, et al. Spectral biomarkers for chemoprevention of colonic neoplasia: a placebo-controlled double-blinded trial with aspirin. Gut. 2017;66:285–92. doi: 10.1136/gutjnl-2015-309996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lochhead P, Chan AT, Nishihara R, et al. Etiologic field effect: reappraisal of the field effect concept in cancer predisposition and progression. Mod Pathol. 2015;28:14–29. doi: 10.1038/modpathol.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang M, Spiegelman D, Kuchiba A, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35:782–800. doi: 10.1002/sim.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang M, Kuchiba A, Ogino S. A Meta-regression method for studying etiological heterogeneity across disease subtypes classified by multiple biomarkers. Am J Epidemiol. 2015;182:263–70. doi: 10.1093/aje/kwv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chatterjee N, Sinha S, Diver WR, et al. Analysis of cohort studies with multivariate and partially observed disease classification data. Biometrika. 2010;97:683–98. doi: 10.1093/biomet/asq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chatterjee N. A two-stage regression model for epidemiological studies with multivariate disease classification data. J Am Stat Assoc. 2004;99:127–38. [Google Scholar]
- 64.Begg CB, Orlow I, Zabor EC, et al. Identifying etiologically distinct sub-types of cancer: a demonstration project involving breast cancer. Cancer Med. 2015;4:1432–9. doi: 10.1002/cam4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zabor EC, Begg CB. A comparison of statistical methods for the study of etiologic heterogeneity. Stat Med. 2017;36:4050–60. doi: 10.1002/sim.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Begg CB, Seshan VE, Zabor EC, et al. Genomic investigation of etiologic heterogeneity: methodologic challenges. BMC Med Res Methodol. 2014;14:138. doi: 10.1186/1471-2288-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Begg CB, Zabor EC, Bernstein JL, et al. A conceptual and methodological framework for investigating etiologic heterogeneity. Stat Med. 2013;32:5039–52. doi: 10.1002/sim.5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosner B, Glynn RJ, Tamimi RM, et al. Breast cancer risk prediction with heterogeneous risk profiles according to breast cancer tumor markers. Am J Epidemiol. 2013;178:296–308. doi: 10.1093/aje/kws457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richiardi L, Barone-Adesi F, Pearce N. Cancer subtypes in aetiological research. Eur J Epidemiol. 2017;32:353–61. doi: 10.1007/s10654-017-0253-z. [DOI] [PubMed] [Google Scholar]
- 70.Nevo D, Nishihara R, Ogino S, et al. The competing risks Cox model with auxiliary case covariates under weaker missing-at-random cause of failure. Lifetime Data Anal. 2017 doi: 10.1007/s10985-017-9401-8. [Epub ahead of print 4 Aug 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rescigno T, Micolucci L, Tecce MF, et al. Bioactive nutrients and nutrigenomics in age-related diseases. Molecules. 2017;22:e105. doi: 10.3390/molecules22010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Curtin K, Slattery ML, Samowitz WS. CpG island methylation in colorectal cancer: past, present and future. Patholog Res Int. 2011;2011:1–8. doi: 10.4061/2011/902674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bishehsari F, Mahdavinia M, Vacca M, et al. Epidemiological transition of colorectal cancer in developing countries: environmental factors, molecular pathways, and opportunities for prevention. World J Gastroenterol. 2014;20:6055–72. doi: 10.3748/wjg.v20.i20.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang MJ, Dai JJ, Gu DN, et al. Aspirin in pancreatic cancer: chemopreventive effects and therapeutic potentials. Biochim Biophys Acta. 2016;1866:163–76. doi: 10.1016/j.bbcan.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Martinez-Useros J, Garcia-Foncillas J. Obesity and colorectal cancer: molecular features of adipose tissue. J Transl Med. 2016;14:21. doi: 10.1186/s12967-016-0772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chia WK, Ali R, Toh HC. Aspirin as adjuvant therapy for colorectal cancer–reinterpreting paradigms. Nat Rev Clin Oncol. 2012;9:561–70. doi: 10.1038/nrclinonc.2012.137. [DOI] [PubMed] [Google Scholar]
- 77.Campbell PT, Newton CC, Newcomb PA, et al. Association between body mass index and mortality for colorectal cancer survivors: overall and by tumor molecular phenotype. Cancer Epidemiol Biomarkers Prev. 2015;24:1229–38. doi: 10.1158/1055-9965.EPI-15-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Serafino A, Sferrazza G, Colini Baldeschi A, et al. Developing drugs that target the Wnt pathway: recent approaches in cancer and neurodegenerative diseases. Expert Opin Drug Discov. 2017;12:169–86. doi: 10.1080/17460441.2017.1271321. [DOI] [PubMed] [Google Scholar]
- 79.Patil H, Saxena SG, Barrow CJ, et al. Chasing the personalized medicine dream through biomarker validation in colorectal cancer. Drug Discov Today. 2017;22:111–9. doi: 10.1016/j.drudis.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 80.Kuroiwa-Trzmielina J, Wang F, Rapkins RW, et al. SNP rs16906252C>T is an expression and methylation quantitative trait locus associated with an increased risk of developing mgmt-methylated colorectal cancer. Clin Cancer Res. 2016;22:6266–77. doi: 10.1158/1078-0432.CCR-15-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Slattery ML, Lee FY, Pellatt AJ, et al. Infrequently expressed miRNAs in colorectal cancer tissue and tumor molecular phenotype. Mod Pathol. 2017;30:1152–69. doi: 10.1038/modpathol.2017.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alnabulsi A, Murray GI. Integrative analysis of the colorectal cancer proteome: potential clinical impact. Expert Rev Proteomics. 2016;13:917–27. doi: 10.1080/14789450.2016.1233062. [DOI] [PubMed] [Google Scholar]
- 83.Ku CS, Cooper DN, Wu M, et al. Gene discovery in familial cancer syndromes by exome sequencing: prospects for the elucidation of familial colorectal cancer type X. Mod Pathol. 2012;25:1055–68. doi: 10.1038/modpathol.2012.62. [DOI] [PubMed] [Google Scholar]
- 84.Hughes LA, Khalid-de Bakker CA, Smits KM, et al. The CpG island methylator phenotype in colorectal cancer: progress and problems. Biochim Biophys Acta. 2012;1825:77–85. doi: 10.1016/j.bbcan.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 85.Szylberg Ł, Janiczek M, Popiel A, et al. Serrated polyps and their alternative pathway to the colorectal cancer: a systematic review. Gastroenterol Res Pract. 2015;2015:1–7. doi: 10.1155/2015/573814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao C. Molecular pathological epidemiology in diabetes mellitus and risk of hepatocellular carcinoma. World J Hepatol. 2016;8:1119–27. doi: 10.4254/wjh.v8.i27.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mehta AM, Osse M, Kolkman-Uljee S, et al. Molecular backgrounds of ERAP1 downregulation in cervical carcinoma. Anal Cell Pathol. 2015;2015:1–5. doi: 10.1155/2015/367837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hagland HR, Berg M, Jolma IW, et al. Molecular pathways and cellular metabolism in colorectal cancer. Dig Surg. 2013;30:12–25. doi: 10.1159/000347166. [DOI] [PubMed] [Google Scholar]
- 90.Campbell PT, Deka A, Briggs P, et al. Establishment of the cancer prevention study II nutrition cohort colorectal tissue repository. Cancer Epidemiol Biomarkers Prev. 2014;23:2694–702. doi: 10.1158/1055-9965.EPI-14-0541. [DOI] [PubMed] [Google Scholar]
- 91.Buchanan DD, Win AK, Walsh MD, et al. Family history of colorectal cancer in BRAF p.V600E-mutated colorectal cancer cases. Cancer Epidemiol Biomarkers Prev. 2013;22:917–26. doi: 10.1158/1055-9965.EPI-12-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen IC, Lee KH, Hsu YH, et al. Expression Pattern and Clinicopathological Relevance of the Indoleamine 2,3-Dioxygenase 1/Tryptophan 2,3-Dioxygenase Protein in Colorectal Cancer. Dis Markers. 2016;2016:1–9. doi: 10.1155/2016/8169724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee DH, Keum N, Giovannucci EL. Colorectal Cancer Epidemiology in the Nurses’ Health Study. Am J Public Health. 2016;106:1599–607. doi: 10.2105/AJPH.2016.303320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li YY, Ge QX, Cao J, et al. Association of Fusobacterium nucleatum infection with colorectal cancer in Chinese patients. World J Gastroenterol. 2016;22:3227–33. doi: 10.3748/wjg.v22.i11.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Venniyoor A. The most important questions in cancer research and clinical oncology-question 2–5. Obesity-related cancers: more questions than answers. Chin J Cancer. 2017;36:18. doi: 10.1186/s40880-017-0185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li W, Qiu T, Ling Y, et al. Molecular pathological epidemiology of colorectal cancer in Chinese patients with KRAS and BRAF mutations. Oncotarget. 2015;6:39607–13. doi: 10.18632/oncotarget.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zaidi N, Lupien L, Kuemmerle NB, et al. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res. 2013;52:585–9. doi: 10.1016/j.plipres.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sampedro GR, Bubeck Wardenburg J. Staphylococcus aureus in the intensive care unit: are these golden grapes ripe for a new approach? J Infect Dis. 2017;215:S64–70. doi: 10.1093/infdis/jiw581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chu SK, Yang HC. Interethnic DNA methylation difference and its implications in pharmacoepigenetics. Epigenomics. 2017;9:1437–54. doi: 10.2217/epi-2017-0046. [DOI] [PubMed] [Google Scholar]
- 100.Juárez M, Egoavil C, Rodríguez-Soler M, et al. KRAS and BRAF somatic mutations in colonic polyps and the risk of metachronous neoplasia. PLoS One. 2017;12:e0184937. doi: 10.1371/journal.pone.0184937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi YJ, Lee DH, Han KD, et al. The relationship between drinking alcohol and esophageal, gastric or colorectal cancer: a nationwide population-based cohort study of South Korea. PLoS One. 2017;12:e0185778. doi: 10.1371/journal.pone.0185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dong X, Hou Q, Chen Y, et al. Diagnostic value of the methylation of multiple gene promoters in serum in hepatitis B virus-related hepatocellular carcinoma. Dis Markers. 2017;2017:1–6. doi: 10.1155/2017/2929381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang J, Shen C, Fu Y, et al. The associations between five polymorphisms of vascular endothelial growth factor and renal cell carcinoma risk: an updated meta-analysis. Onco Targets Ther. 2017;10:1725–34. doi: 10.2147/OTT.S125965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suh SS, Kim TK, Kim JE, et al. Anticancer activity of ramalin, a secondary metabolite from the antarctic lichen ramalina terebrata, against colorectal cancer cells. Molecules. 2017;22:1361. doi: 10.3390/molecules22081361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yuan W, Chen J, Shu Y, et al. Correlation of DAPK1 methylation and the risk of gastrointestinal cancer: a systematic review and meta-analysis. PLoS One. 2017;12:e0184959. doi: 10.1371/journal.pone.0184959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Szpiech ZA, Strauli NB, White KA, et al. Prominent features of the amino acid mutation landscape in cancer. PLoS One. 2017;12:e0183273. doi: 10.1371/journal.pone.0183273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu CS, Huang WY, Pinsky PF, et al. The Prostate, Lung, Colorectal and Ovarian Cancer (PLCO) screening trial pathology tissue resource. Cancer Epidemiol Biomarkers Prev. 2016;25:1635–42. doi: 10.1158/1055-9965.EPI-16-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ciesielski TH, Aldrich MC, Marsit CJ, et al. Transdisciplinary approaches enhance the production of translational knowledge. Transl Res. 2017;182:123–34. doi: 10.1016/j.trsl.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuller LH. Commentary: epidemiology - then and now. Am J Epidemiol. 2016;183:372–80. doi: 10.1093/aje/kwv158. [DOI] [PubMed] [Google Scholar]
- 110.Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9–32. doi: 10.20892/j.issn.2095-3941.2016.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lewis C, McQuaid S, Hamilton PW, et al. Building a ‘repository of science’: the importance of integrating biobanks within molecular pathology programmes. Eur J Cancer. 2016;67:191–9. doi: 10.1016/j.ejca.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 112.Ryan E, Sheahan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: a rationale for routine testing. Crit Rev Oncol Hematol. 2017;116:38–57. doi: 10.1016/j.critrevonc.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 113.Micolucci L, Rippo MR, Olivieri F, et al. Progress of research on microRNAs with diagnostic value in asbestos exposure: a call for method standardization. Biosci Trends. 2017;11:105–9. doi: 10.5582/bst.2016.01249. [DOI] [PubMed] [Google Scholar]
- 114.Ogino S. Molecular pathological epidemiology (MPE): Overview of its paradigm and wide applicability even without tumor tissue [abstract]. Proceedings of the Twelfth Annual AACR International Conference on Frontiers in Cancer Prevention Research; National Harbor, MD:Cancer Prev Res (Phila). 27-30 Oct 2013; 2013. 6: CN06-1. [Google Scholar]
- 115.Kuller LH, Bracken MB, Ogino S, et al. The role of epidemiology in the era of molecular epidemiology and genomics: summary of the 2013 AJE-sponsored Society of Epidemiologic Research Symposium. Am J Epidemiol. 2013;178:1350–4. doi: 10.1093/aje/kwt239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Epplein M, Bostick RM, Mu L, et al. Challenges and opportunities in international molecular cancer prevention research: an ASPO molecular epidemiology and the environment and international cancer prevention interest groups report. Cancer Epidemiol Biomarkers Prev. 2014;23:2613–7. doi: 10.1158/1055-9965.EPI-14-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ogino S. Molecular pathological epidemiology of risk factors and CRC microbial and immune characteristics. [abstract]. Proceedings of the AACR Special Conference on Colorectal Cancer: From Initiation to Outcomes; Tampa, FL:Cancer Res. 17-20 Sep 2016; 2017. p. IA28. [Google Scholar]
- 118.Ogino S, Campbell PT, Nishihara R, et al. Proceedings of the second international molecular pathological epidemiology (MPE) meeting. Cancer Causes Control. 2015;26:959–72. doi: 10.1007/s10552-015-0596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Campbell PT, Rebbeck TR, Nishihara R, et al. Proceedings of the third international molecular pathological epidemiology (MPE) meeting. Cancer Causes Control. 2017;28:167–76. doi: 10.1007/s10552-016-0845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Drew DA, Chin SM, Gilpin KK, et al. ASPirin Intervention for the REDuction of colorectal cancer risk (ASPIRED): a study protocol for a randomized controlled trial. Trials. 2017;18:50. doi: 10.1186/s13063-016-1744-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Frouws MA, van Herk-Sukel MPP, Maas HA, et al. The mortality reducing effect of aspirin in colorectal cancer patients: Interpreting the evidence. Cancer Treat Rev. 2017;55:120–7. doi: 10.1016/j.ctrv.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 122.Herbert K, Kerr R, Kerr DJ, et al. Are NSAIDs coming back to colorectal cancer therapy or not? Curr Colorectal Cancer Rep. 2014;10:363–71. [Google Scholar]
- 123.Tougeron D, Sha D, Manthravadi S, et al. Aspirin and colorectal cancer: back to the future. Clin Cancer Res. 2014;20:1087–94. doi: 10.1158/1078-0432.CCR-13-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hua X, Phipps AI, Burnett-Hartman AN, et al. Timing of aspirin and other nonsteroidal anti-inflammatory drug use among patients with colorectal cancer in relation to tumor markers and survival. J Clin Oncol. 2017;35:2806–13. doi: 10.1200/JCO.2017.72.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li P, Wu H, Zhang H, et al. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut. 2015;64:1419–25. doi: 10.1136/gutjnl-2014-308260. [DOI] [PubMed] [Google Scholar]
- 126.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–58. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gray RT, Cantwell MM, Coleman HG, et al. Evaluation of PTGS2 expression, PIK3CA mutation, aspirin use and colon cancer survival in a population-based cohort study. Clin Transl Gastroenterol. 2017;8:e91. doi: 10.1038/ctg.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Domingo E, Church DN, Sieber O, et al. Evaluation of PIK3CA mutation as a predictor of benefit from NSAID therapy in colorectal cancer. J Clin Oncol. 2013;31:4297–305. doi: 10.1200/JCO.2013.50.0322. [DOI] [PubMed] [Google Scholar]
- 130.Mei ZB, Duan CY, Li CB, et al. Prognostic role of tumor PIK3CA mutation in colorectal cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27:1836–48. doi: 10.1093/annonc/mdw264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hamada T, Cao Y, Qian ZR, et al. Aspirin use and colorectal cancer survival according to tumor CD274 (programmed cell death 1 ligand 1) expression status. J Clin Oncol. 2017;35:1836–44. doi: 10.1200/JCO.2016.70.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stewart BW, Bray F, Forman D, et al. Cancer prevention as part of precision medicine: ‘plenty to be done’. Carcinogenesis. 2016;37:2–9. doi: 10.1093/carcin/bgv166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meyskens FL, Mukhtar H, Rock CL. Cancer prevention: obstacles, challenges and the road ahead. J Natl Cancer Inst. 2016;108:djv309. doi: 10.1093/jnci/djv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wild CP, Bucher JR, de Jong BW, et al. Translational cancer research: balancing prevention and treatment to combat cancer globally. J Natl Cancer Inst. 2015;107:1–5. doi: 10.1093/jnci/dju353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology. 2006;131:1700–5. doi: 10.1053/j.gastro.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 136.Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 2010;105:1189–95. doi: 10.1038/ajg.2009.699. [DOI] [PubMed] [Google Scholar]
- 137.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stoffel EM, Erichsen R, Frøslev T, et al. Clinical and molecular characteristics of post-colonoscopy colorectal cancer: a population-based study. Gastroenterology. 2016;151:870–8. doi: 10.1053/j.gastro.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Samowitz WS, Albertsen H, Sweeney C, et al. Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst. 2006;98:1731–8. doi: 10.1093/jnci/djj468. [DOI] [PubMed] [Google Scholar]
- 140.Limsui D, Vierkant RA, Tillmans LS, et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst. 2010;102:1012–22. doi: 10.1093/jnci/djq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Poynter JN, Haile RW, Siegmund KD, et al. Associations between smoking, alcohol consumption, and colorectal cancer, overall and by tumor microsatellite instability status. Cancer Epidemiol Biomarkers Prev. 2009;18:2745–50. doi: 10.1158/1055-9965.EPI-09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nishihara R, Morikawa T, Kuchiba A, et al. A prospective study of duration of smoking cessation and colorectal cancer risk by epigenetics-related tumor classification. Am J Epidemiol. 2013;178:84–100. doi: 10.1093/aje/kws431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rozek LS, Herron CM, Greenson JK, et al. Smoking, gender, and ethnicity predict somatic BRAF mutations in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:838–43. doi: 10.1158/1055-9965.EPI-09-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nishi A, Milner DA, Giovannucci EL, et al. Integration of molecular pathology, epidemiology and social science for global precision medicine. Expert Rev Mol Diagn. 2016;16:11–23. doi: 10.1586/14737159.2016.1115346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hamada T, Keum N, Nishihara R, et al. Molecular pathological epidemiology: new developing frontiers of big data science to study etiologies and pathogenesis. J Gastroenterol. 2017;52:265–75. doi: 10.1007/s00535-016-1272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–42. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 147.Nishihara R, Lochhead P, Kuchiba A, et al. Aspirin use and risk of colorectal cancer according to BRAF mutation status. JAMA. 2013;309:2563–71. doi: 10.1001/jama.2013.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fink SP, Yamauchi M, Nishihara R, et al. Aspirin and the risk of colorectal cancer in relation to the expression of 15-hydroxyprostaglandin dehydrogenase (HPGD) Sci Transl Med. 2014;6:233re2. doi: 10.1126/scitranslmed.3008481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ogino S, Jhun I, Mata DA, et al. Integration of pharmacology, molecular pathology, and population data science to support precision gastrointestinal oncology. NPJ Precis Oncol. 2017;1:40. doi: 10.1038/s41698-017-0042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nishihara R, VanderWeele TJ, Shibuya K, et al. Molecular pathological epidemiology gives clues to paradoxical findings. Eur J Epidemiol. 2015;30:1129–35. doi: 10.1007/s10654-015-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Choi Y, Park B, Jeong BC, et al. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer. 2013;132:625–34. doi: 10.1002/ijc.27639. [DOI] [PubMed] [Google Scholar]
- 152.Hakimi AA, Furberg H, Zabor EC, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst. 2013;105:1862–70. doi: 10.1093/jnci/djt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ogino S, Lochhead P, Chan AT, et al. Molecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and disease. Mod Pathol. 2013;26:465–84. doi: 10.1038/modpathol.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ogino S, Fuchs CS, Giovannucci E. How many molecular subtypes? Implications of the unique tumor principle in personalized medicine. Expert Rev Mol Diagn. 2012;12:621–8. doi: 10.1586/erm.12.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van Rooij N, van Buuren MM, Philips D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31:e439–42. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Basile D, Garattini SK, Bonotto M, et al. Immunotherapy for colorectal cancer: where are we heading? Expert Opin Biol Ther. 2017;17:709–21. doi: 10.1080/14712598.2017.1315405. [DOI] [PubMed] [Google Scholar]
- 157.Cramer DW, Finn OJ. Epidemiologic perspective on immune-surveillance in cancer. Curr Opin Immunol. 2011;23:265–71. doi: 10.1016/j.coi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rocca YS, Roberti MP, Arriaga JM, et al. Altered phenotype in peripheral blood and tumor-associated NK cells from colorectal cancer patients. Innate Immun. 2013;19:76–85. doi: 10.1177/1753425912453187. [DOI] [PubMed] [Google Scholar]
- 159.Raine T, Liu JZ, Anderson CA, et al. Generation of primary human intestinal T cell transcriptomes reveals differential expression at genetic risk loci for immune-mediated disease. Gut. 2015;64:250–9. doi: 10.1136/gutjnl-2013-306657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fridman WH, Pagès F, Sautès-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 161.Colussi D, Brandi G, Bazzoli F, et al. Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. Int J Mol Sci. 2013;14:16365–85. doi: 10.3390/ijms140816365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kocarnik JM, Shiovitz S, Phipps AI. Molecular phenotypes of colorectal cancer and potential clinical applications. Gastroenterol Rep. 2015;3:269–76. doi: 10.1093/gastro/gov046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kudryavtseva AV, Lipatova AV, Zaretsky AR, et al. Important molecular genetic markers of colorectal cancer. Oncotarget. 2016;7:53959–83. doi: 10.18632/oncotarget.9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Phipps AI, Limburg PJ, Baron JA, et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology. 2015;148:77–87. doi: 10.1053/j.gastro.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bijlsma MF, Sadanandam A, Tan P, et al. Molecular subtypes in cancers of the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2017;14:333–42. doi: 10.1038/nrgastro.2017.33. [DOI] [PubMed] [Google Scholar]
- 166.Dienstmann R, Vermeulen L, Guinney J, et al. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17:79–92. doi: 10.1038/nrc.2016.126. [DOI] [PubMed] [Google Scholar]
- 167.Sepulveda AR, Hamilton SR, Allegra CJ, et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology. J Mol Diagn. 2017;19:187–225. doi: 10.1016/j.jmoldx.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Giannakis M, Hodis E, Jasmine Mu X, Mu J, et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat Genet. 2014;46:1264–6. doi: 10.1038/ng.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 170.Di Caro G, Marchesi F, Laghi L, et al. Immune cells: plastic players along colorectal cancer progression. J Cell Mol Med. 2013;17:1088–95. doi: 10.1111/jcmm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Koshiol J, Lin SW. Can tissue-based immune markers be used for studying the natural history of cancer? Ann Epidemiol. 2012;22:520–30. doi: 10.1016/j.annepidem.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Drewes JL, Housseau F, Sears CL. Sporadic colorectal cancer: microbial contributors to disease prevention, development and therapy. Br J Cancer. 2016;115:273–80. doi: 10.1038/bjc.2016.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:686–700. doi: 10.1038/nrgastro.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bardhan K, Liu K. Epigenetics and colorectal cancer pathogenesis. Cancers. 2013;5:676–713. doi: 10.3390/cancers5020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zoratto F, Rossi L, Verrico M, et al. Focus on genetic and epigenetic events of colorectal cancer pathogenesis: implications for molecular diagnosis. Tumour Biol. 2014;35:6195–206. doi: 10.1007/s13277-014-1845-9. [DOI] [PubMed] [Google Scholar]
- 176.Okugawa Y, Grady WM, Goel A. Epigenetic Alterations in Colorectal Cancer: Emerging Biomarkers. Gastroenterology. 2015;149:1204–25. doi: 10.1053/j.gastro.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ng JM, Yu J. Promoter hypermethylation of tumour suppressor genes as potential biomarkers in colorectal cancer. Int J Mol Sci. 2015;16:2472–96. doi: 10.3390/ijms16022472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Suzuki H, Yamamoto E, Maruyama R, et al. Biological significance of the CpG island methylator phenotype. Biochem Biophys Res Commun. 2014;455:35–42. doi: 10.1016/j.bbrc.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 179.Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–54. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yamauchi M, Lochhead P, Morikawa T, et al. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61:794–7. doi: 10.1136/gutjnl-2012-302014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bae JM, Kim JH, Cho NY, et al. Prognostic implication of the CpG island methylator phenotype in colorectal cancers depends on tumour location. Br J Cancer. 2013;109:1004–12. doi: 10.1038/bjc.2013.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jess P, Hansen IO, Gamborg M, et al. A nationwide Danish cohort study challenging the categorisation into right-sided and left-sided colon cancer. BMJ Open. 2013;3:e002608. doi: 10.1136/bmjopen-2013-002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rosty C, Young JP, Walsh MD, et al. Colorectal carcinomas with KRAS mutation are associated with distinctive morphological and molecular features. Mod Pathol. 2013;26:825–34. doi: 10.1038/modpathol.2012.240. [DOI] [PubMed] [Google Scholar]
- 184.Rosty C, Young JP, Walsh MD, et al. PIK3CA activating mutation in colorectal carcinoma: associations with molecular features and survival. PLoS One. 2013;8:e65479. doi: 10.1371/journal.pone.0065479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Phipps AI, Buchanan DD, Makar KW, et al. BRAF mutation status and survival after colorectal cancer diagnosis according to patient and tumor characteristics. Cancer Epidemiol Biomarkers Prev. 2012;21:1792–8. doi: 10.1158/1055-9965.EPI-12-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ito M, Mitsuhashi K, Igarashi H, et al. MicroRNA-31 expression in relation to BRAF mutation, CpG island methylation and colorectal continuum in serrated lesions. Int J Cancer. 2014;135:2507–15. doi: 10.1002/ijc.28920. [DOI] [PubMed] [Google Scholar]
- 187.Mima K, Cao Y, Chan AT, et al. Fusobacterium nucleatum in colorectal carcinoma tissue according to tumor location. Clin Transl Gastroenterol. 2016;7:e200. doi: 10.1038/ctg.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lee MS, Menter DG, Kopetz S. Right versus left colon cancer biology: integrating the consensus molecular subtypes. J Natl Compr Canc Netw. 2017;15:411–9. doi: 10.6004/jnccn.2017.0038. [DOI] [PubMed] [Google Scholar]
- 189.Nishihara R, Glass K, Mima K, et al. Biomarker correlation network in colorectal carcinoma by tumor anatomic location. BMC Bioinformatics. 2017;18:304. doi: 10.1186/s12859-017-1718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Loree JM, Pereira AA, Lam M, et al. Classifying colorectal cancer by tumor location rather than sidedness highlights a continuum in mutation profiles and Consensus Molecular Subtypes. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-17-2484. [Epub ahead of print 27 Nov 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Phipps AI, Chan AT, Ogino S. Anatomic subsite of primary colorectal cancer and subsequent risk and distribution of second cancers. Cancer. 2013;119:3140–7. doi: 10.1002/cncr.28076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Droeser RA, Hirt C, Viehl CT, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49:2233–42. doi: 10.1016/j.ejca.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 193.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Masugi Y, Nishihara R, Yang J, et al. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. 2017;66:1463–73. doi: 10.1136/gutjnl-2016-311421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lee KS, Kwak Y, Ahn S, et al. Prognostic implication of CD274 (PD-L1) protein expression in tumor-infiltrating immune cells for microsatellite unstable and stable colorectal cancer. Cancer Immunol Immunother. 2017;66:927–39. doi: 10.1007/s00262-017-1999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Inaguma S, Lasota J, Wang Z, et al. Clinicopathologic profile, immunophenotype, and genotype of CD274 (PD-L1)-positive colorectal carcinomas. Mod Pathol. 2017;30:278–85. doi: 10.1038/modpathol.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, et al. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol. 2016;29:1104–12. doi: 10.1038/modpathol.2016.95. [DOI] [PubMed] [Google Scholar]
- 198.Lee LH, Cavalcanti MS, Segal NH, et al. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol. 2016;29:1433–42. doi: 10.1038/modpathol.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Koganemaru S, Inoshita N, Miura Y, et al. Prognostic value of programmed death-ligand 1 expression in patients with stage III colorectal cancer. Cancer Sci. 2017;108:853–8. doi: 10.1111/cas.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Marisa L, Svrcek M, Collura A, et al. The balance between cytotoxic T-cell lymphocytes and immune checkpoint expression in the prognosis of colon tumors. J Natl Cancer Inst. 2018;110:68–77. doi: 10.1093/jnci/djx136. [DOI] [PubMed] [Google Scholar]
- 201.Inaguma S, Lasota J, Felisiak-Golabek A, et al. Histopathological and genotypic characterization of metastatic colorectal carcinoma with PD-L1 (CD274)-expression: Possible roles of tumour micro environmental factors for CD274 expression. J Pathol Clin Res. 2017;3:268–78. doi: 10.1002/cjp2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Masugi Y, Nishihara R, Hamada T, et al. Tumor PDCD1LG2 (PD-L2) expression and the lymphocytic reaciton to colorecal cancer. Cancer Immunol Res. 2017;5:1046–55. doi: 10.1158/2326-6066.CIR-17-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim JH, Kang GH. Molecular and prognostic heterogeneity of microsatellite-unstable colorectal cancer. World J Gastroenterol. 2014;20:4230–43. doi: 10.3748/wjg.v20.i15.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Benson AB, Venook AP, Cederquist L, et al. Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:370–98. doi: 10.6004/jnccn.2017.0036. [DOI] [PubMed] [Google Scholar]
- 205.Le DT, Hubbard-Lucey VM, Morse MA, et al. A blueprint to advance colorectal cancer immunotherapies. Cancer Immunol Res. 2017;5:942–9. doi: 10.1158/2326-6066.CIR-17-0375. [DOI] [PubMed] [Google Scholar]
- 206.Lin JH, Giovannucci E. Environmental exposure and tumor heterogeneity in colorectal cancer risk and outcomes. Curr Colorectal Cancer Rep. 2014;10:94–104. [Google Scholar]
- 207.Marley AR, Nan H. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet. 2016;7:105–14. [PMC free article] [PubMed] [Google Scholar]
- 208.Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412–20. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Edin S, Wikberg ML, Dahlin AM, et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7:e47045. doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ling A, Lundberg IV, Eklöf V, et al. The infiltration, and prognostic importance, of Th1 lymphocytes vary in molecular subgroups of colorectal cancer. J Pathol Clin Res. 2016;2:21–31. doi: 10.1002/cjp2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ling A, Edin S, Wikberg ML, et al. The intratumoural subsite and relation of CD8(+) and FOXP3(+) T lymphocytes in colorectal cancer provide important prognostic clues. Br J Cancer. 2014;110:2551–9. doi: 10.1038/bjc.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wangefjord S, Sundström M, Zendehrokh N, et al. Sex differences in the prognostic significance of KRAS codons 12 and 13, and BRAF mutations in colorectal cancer: a cohort study. Biol Sex Differ. 2013;4:17. doi: 10.1186/2042-6410-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rozek LS, Schmit SL, Greenson JK, et al. Tumor-infiltrating lymphocytes, crohn’s-like lymphoid reaction, and survival from colorectal cancer. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mima K, Nishihara R, Nowak JA, et al. MicroRNA MIR21 and T cells in colorectal cancer. Cancer Immunol Res. 2016;4:33–40. doi: 10.1158/2326-6066.CIR-15-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dou R, Nishihara R, Cao Y, et al. MicroRNA let-7, T Cells, and patient survival in colorectal cancer. Cancer Immunol Res. 2016;4:927–35. doi: 10.1158/2326-6066.CIR-16-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kosumi K, Masugi Y, Yang J, et al. Tumor SQSTM1 (p62) expression and T cells in colorectal cancer. Oncoimmunology. 2017;6:e1284720. doi: 10.1080/2162402X.2017.1284720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Prizment AE, Vierkant RA, Smyrk TC, et al. Cytotoxic T-cells and granzyme B associated with improved colorectal cancer survival in a prospective cohort of older women. Cancer Epidemiol Biomarkers Prev. 2017;26:622–31. doi: 10.1158/1055-9965.EPI-16-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Berntsson J, Svensson MC, Leandersson K, et al. The clinical impact of tumour-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: A cohort study. Int J Cancer. 2017;141:1654–66. doi: 10.1002/ijc.30869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Prizment AE, Vierkant RA, Smyrk TC, et al. Tumor eosinophil infiltration and improved survival of colorectal cancer patients: Iowa Women’s Health Study. Mod Pathol. 2016;29:516–27. doi: 10.1038/modpathol.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Song M, Nishihara R, Cao Y, et al. Marine ω-3 polyunsaturated fatty acid intake and risk of colorectal cancer characterized by tumor-infiltrating T cells. JAMA Oncol. 2016;2:1197–206. doi: 10.1001/jamaoncol.2016.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Khalili H, Gong J, Brenner H, et al. Identification of a common variant with potential pleiotropic effect on risk of inflammatory bowel disease and colorectal cancer. Carcinogenesis. 2015;36:999–1007. doi: 10.1093/carcin/bgv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Peters U, Bien S, Zubair N. Genetic architecture of colorectal cancer. Gut. 2015;64:1623–36. doi: 10.1136/gutjnl-2013-306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Song M, Nishihara R, Wang M, et al. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut. 2016;65:296–304. doi: 10.1136/gutjnl-2014-308852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22:278–95. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 225.Liu L, Nevo D, Nishihara R, et al. Utility of inverse probability weighting in molecular pathological epidemiology. Eur J Epidemiol. 2017 doi: 10.1007/s10654-017-0346-8. [Epub ahead of print 20 Dec 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ogino S, Lochhead P, Giovannucci E, et al. Discovery of colorectal cancer PIK3CA mutation as potential predictive biomarker: power and promise of molecular pathological epidemiology. Oncogene. 2014;33:2949–55. doi: 10.1038/onc.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hackl H, Charoentong P, Finotello F, et al. Computational genomics tools for dissecting tumour-immune cell interactions. Nat Rev Genet. 2016;17:441–58. doi: 10.1038/nrg.2016.67. [DOI] [PubMed] [Google Scholar]
- 228.Yoshihara K, Shahmoradgoli M, Martínez E, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4:2612. doi: 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–45. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Benichou J, Ben-Hamo R, Louzoun Y, et al. Rep-Seq: uncovering the immunological repertoire through next-generation sequencing. Immunology. 2012;135:183–91. doi: 10.1111/j.1365-2567.2011.03527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Safonova Y, Lapidus A, Lill J. IgSimulator: a versatile immunosequencing simulator. Bioinformatics. 2015;31:3213–5. doi: 10.1093/bioinformatics/btv326. [DOI] [PubMed] [Google Scholar]
- 232.Howie B, Sherwood AM, Berkebile AD, et al. High-throughput pairing of T cell receptor α and β sequences. Sci Transl Med. 2015;7:301ra131. doi: 10.1126/scitranslmed.aac5624. [DOI] [PubMed] [Google Scholar]
- 233.Li B, Li T, Pignon JC, et al. Landscape of tumor-infiltrating T cell repertoire of human cancers. Nat Genet. 2016;48:725–32. doi: 10.1038/ng.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sako T, Kudo SE, Miyachi H, et al. A novel ability of endocytoscopy to diagnose histological grade of differentiation in T1 colorectal carcinomas. Endoscopy. 2018;50:69–74. doi: 10.1055/s-0043-117403. [DOI] [PubMed] [Google Scholar]
- 235.Tearney GJ, Kang D. Introduction to biomedical optical imaging. Lasers Surg Med. 2017;49:214. doi: 10.1002/lsm.22658. [DOI] [PubMed] [Google Scholar]
- 236.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–29. doi: 10.1038/ajg.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Haumaier F, Sterlacci W, Vieth M. Histological and molecular classification of gastrointestinal polyps. Best Pract Res Clin Gastroenterol. 2017;31:369–79. doi: 10.1016/j.bpg.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 238.Ryan BM, Faupel-Badger JM. The hallmarks of premalignant conditions: a molecular basis for cancer prevention. Semin Oncol. 2016;43:22–35. doi: 10.1053/j.seminoncol.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bettington M, Walker N, Rosty C, et al. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut. 2017;66:97–106. doi: 10.1136/gutjnl-2015-310456. [DOI] [PubMed] [Google Scholar]
- 240.Prakadan SM, Shalek AK, Weitz DA. Scaling by shrinking: empowering single-cell ‘omics’ with microfluidic devices. Nat Rev Genet. 2017;18:345–61. doi: 10.1038/nrg.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bromham L, Dinnage R, Hua X. Interdisciplinary research has consistently lower funding success. Nature. 2016;534:684–7. doi: 10.1038/nature18315. [DOI] [PubMed] [Google Scholar]
- 242.Barrow TM, Michels KB. Epigenetic epidemiology of cancer. Biochem Biophys Res Commun. 2014;455:70–83. doi: 10.1016/j.bbrc.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 243.Zhou L, Wang K, Li Q, et al. Clinical proteomics-driven precision medicine for targeted cancer therapy: current overview and future perspectives. Expert Rev Proteomics. 2016;13:367–81. doi: 10.1586/14789450.2016.1159959. [DOI] [PubMed] [Google Scholar]
- 244.Thiele JA, Bethel K, Králíčková M, et al. Circulating tumor cells: fluid surrogates of solid tumors. Annu Rev Pathol. 2017;12:419–47. doi: 10.1146/annurev-pathol-052016-100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Townsend MK, Aschard H, De Vivo I, et al. Genomics, telomere length, epigenetics, and metabolomics in the nurses health studies. Am J Public Health. 2016;106:1663–8. doi: 10.2105/AJPH.2016.303344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Tan CR, Zhou L, El-Deiry WS. Circulating tumor cells versus circulating tumor DNA in colorectal cancer: pros and cons. Curr Colorectal Cancer Rep. 2016;12:151–61. doi: 10.1007/s11888-016-0320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Grillet F, Bayet E, Villeronce O, et al. Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut. 2017;66:1802–10. doi: 10.1136/gutjnl-2016-311447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Olmedillas López S, García-Olmo DC, García-Arranz M, et al. KRAS G12V mutation detection by droplet digital PCR in circulating cell-free DNA of colorectal cancer patients. Int J Mol Sci. 2016;17:484. doi: 10.3390/ijms17040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Nagai Y, Sunami E, Yamamoto Y, et al. LINE-1 hypomethylation status of circulating cell-free DNA in plasma as a biomarker for colorectal cancer. Oncotarget. 2017;8:11906–16. doi: 10.18632/oncotarget.14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531–48. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 251.Strickler JH, Loree JM, Ahronian LG, et al. Genomic landscape of cell-free DNA in patients with colorectal cancer. Cancer Discov. 2017 doi: 10.1158/2159-8290.CD-17-1009. [Epub ahead of print 1 Dec 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mazel M, Jacot W, Pantel K, et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol. 2015;9:1773–82. doi: 10.1016/j.molonc.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Khagi Y, Goodman AM, Daniels GA, et al. Hypermutated circulating tumor DNA: correlation with response to checkpoint inhibitor-based immunotherapy. Clin Cancer Res. 2017;23:5729–36. doi: 10.1158/1078-0432.CCR-17-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 255.Chen J, Domingue JC, Sears CL. Microbiota dysbiosis in select human cancers: Evidence of association and causality. Semin Immunol. 2017;32:25–34. doi: 10.1016/j.smim.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chen J, Pitmon E, Wang K. Microbiome, inflammation and colorectal cancer. Semin Immunol. 2017;32:43–53. doi: 10.1016/j.smim.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 257.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–8. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Dejea CM, Wick EC, Hechenbleikner EM, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A. 2014;111:18321–6. doi: 10.1073/pnas.1406199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Nakatsu G, Li X, Zhou H, et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6:8727. doi: 10.1038/ncomms9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Nosho K, Sukawa Y, Adachi Y, et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol. 2016;22:557–66. doi: 10.3748/wjg.v22.i2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yamaoka Y, Suehiro Y, Hashimoto S, et al. Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J Gastroenterol. 2017 doi: 10.1007/s00535-017-1382-6. [Epub ahead of print 19 Aug 2017] [DOI] [PubMed] [Google Scholar]
- 263.Purcell RV, Visnovska M, Biggs PJ, et al. Distinct gut microbiome patterns associate with consensus molecular subtypes of colorectal cancer. Sci Rep. 2017;7:11590. doi: 10.1038/s41598-017-11237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Abed J, Emgård JE, Zamir G, et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe. 2016;20:215–25. doi: 10.1016/j.chom.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–15. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Mima K, Sukawa Y, Nishihara R, et al. Fusobacterium nucleatum and T-cells in colorectal carcinoma. JAMA Oncol. 2015;1:653–61. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.East JE, Atkin WS, Bateman AC, et al. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut. 2017;66:1181–96. doi: 10.1136/gutjnl-2017-314005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ito M, Kanno S, Nosho K, et al. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int J Cancer. 2015;137:1258–68. doi: 10.1002/ijc.29488. [DOI] [PubMed] [Google Scholar]
- 269.Park CH, Han DS, Oh YH, Yh O, et al. Role of Fusobacteria in the serrated pathway of colorectal carcinogenesis. Sci Rep. 2016;6:25271. doi: 10.1038/srep25271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.IJspeert JE, Vermeulen L, Meijer GA, et al. Serrated neoplasia-role in colorectal carcinogenesis and clinical implications. Nat Rev Gastroenterol Hepatol. 2015;12:401–9. doi: 10.1038/nrgastro.2015.73. [DOI] [PubMed] [Google Scholar]
- 271.Mehta RS, Nishihara R, Cao Y, et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by Fusobacterium nucleatum in tumor tissue. JAMA Oncol. 2017;3:921–7. doi: 10.1001/jamaoncol.2016.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tahara T, Yamamoto E, Suzuki H, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014;74:1311–8. doi: 10.1158/0008-5472.CAN-13-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Mima K, Nishihara R, Qian ZR, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65:1973–80. doi: 10.1136/gutjnl-2015-310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bullman S, Pedamallu CS, Sicinska E, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358:1443–8. doi: 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Mima K, Ogino S, Nakagawa S, et al. The role of intestinal bacteria in the development and progression of gastrointestinal tract neoplasms. Surg Oncol. 2017;26:368–76. doi: 10.1016/j.suronc.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Yu J, Feng Q, Wong SH, et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. 2017;66:70–8. doi: 10.1136/gutjnl-2015-309800. [DOI] [PubMed] [Google Scholar]
- 277.Flemer B, Warren RD, Barrett MP, et al. The oral microbiota in colorectal cancer is distinctive and predictive. Gut. 2017 doi: 10.1136/gutjnl-2017-314814. [Epub ahead of print 7 Oct 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Bertolini F, Sukhatme VP, Bouche G. Drug repurposing in oncology–patient and health systems opportunities. Nat Rev Clin Oncol. 2015;12:732–42. doi: 10.1038/nrclinonc.2015.169. [DOI] [PubMed] [Google Scholar]
- 279.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–9. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–84. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170:548–63. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Geller LT, Barzily-Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–60. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2017 doi: 10.1136/gutjnl-2016-313413. [Epub ahead of print 4 Apr 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ashktorab H, Kupfer SS, Brim H, et al. Racial disparity in gastrointestinal cancer risk. Gastroenterology. 2017;153:910–23. doi: 10.1053/j.gastro.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Augustus GJ, Ellis NA. Race in cancer health disparities theme issue colorectal cancer disparity in African Americans: risk factors and carcinogenic mechanisms. Am J Pathol. 2017 doi: 10.1016/j.ajpath.2017.07.023. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Gardner K. The Science of Cancer Health Disparities: A young discipline with an old heritage. Am J Pathol. 2017 doi: 10.1016/j.ajpath.2017.08.037. [Epub ahead of print 11 Nov 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Jaffee EM, Dang CV, Agus DB, et al. Future cancer research priorities in the USA: a Lancet Oncology Commission. Lancet Oncol. 2017;18:e653–706. doi: 10.1016/S1470-2045(17)30698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Cao Y, Nishihara R, Qian ZR, et al. Regular aspirin use associates with lower risk of colorectal cancers with low numbers of tumor-infiltrating lymphocytes. Gastroenterology. 2016;151:879–92. doi: 10.1053/j.gastro.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Hanyuda A, Ogino S, Qian ZR, et al. Body mass index and risk of colorectal cancer according to tumor lymphocytic infiltrate. Int J Cancer. 2016;139:854–68. doi: 10.1002/ijc.30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Liu L, Nishihara R, Qian ZR, et al. Association between inflammatory diet pattern and risk of colorectal carcinoma subtypes classified by immune responses to tumor. Gastroenterology. 2017;153:1517–30. doi: 10.1053/j.gastro.2017.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]


