Abstract
Objective
This study evaluates potential compensation strategies under conditions of glottal insufficiency.
Method
Using a numerical respiratory-laryngeal model of voice production, Voice production under conditions of glottal insufficiency is investigated across a large range of voice conditions, and compared to normal voice production.
Results
This study shows that glottal insufficiency leads to increased noise production, reduced F0 range, and inability to produce very low-intensity voice. Glottal insufficiency also leads to significantly increased respiratory effort of phonation and difficulty in maintaining a normal breath group duration, which restricts high-intensity voice production and falsetto-like voice production. Although compensation strategies exist to alleviate these undesirable voice changes, they often require hyperfunctional laryngeal and respiratory muscle activities and thus are more likely to result in vocal fatigue.
Conclusion
The laryngeal and respiratory sub-systems need to be considered as a whole in order to fully understand the effect of glottal insufficiency on voice production. Strategies that compensate for laryngeal weakness at the cost of compromising the normal function of the respiratory sub-system are undesirable and may impose additional constraints on voice production and the effectiveness of available compensation strategies.
Keywords: glottal insufficiency, compensation strategies, respiratory-laryngeal coordination, voice production, Aging
I. INTRODUCTION
Changes to the vocal folds, due to either pathologies or aging [1–2], often lead to undesired voice changes (e.g., reduced vocal range or degraded voice quality) and increased vocal effort. Under such conditions, compensation strategies are often adopted in an attempt to alleviate such undesired voice changes and reduce vocal effort. While some compensation strategies may be effective in partially restoring desirable acoustic and perceptual goals or reducing vocal effort, other compensation strategies may not be as effective or even lead to hyperfunctional muscular activities and produce vocal fold vibration patterns that are prone to vocal fold injury. Because compensation is an integral component of voice production, a better understanding of available compensation strategies in humans and their effects on voice production is thus essential to fully understanding pathological voice production. Clinically, understanding the acoustic consequences of compensations may eventually allow clinicians to better identify counterproductive compensatory behaviors and recommend useful compensation strategies.
The present study aims to identify possible compensation strategies under conditions of glottal insufficiency, or the inability to sufficiently approximate the two folds during phonation, due to bilateral recurrent laryngeal nerve (RLN) paresis or aging. Although clinical symptoms of glottal insufficiency have been well documented [1–3], there have been few systematic studies of the underlying physical mechanisms and potential compensation strategies and their effects on phonation. On the other hand, although human voice production involves coordination among the respiratory, laryngeal, and articulatory sub-systems, modeling the physics of human voice production, under either normal or pathological conditions, often focuses on the laryngeal subsystem alone and respiratory-laryngeal interaction is often neglected. Such respiratory-laryngeal coordination is particularly important for pathological conditions resulting in glottal insufficiency, which may use too much airflow and require extremely high respiratory effort (particularly toward the end of the breath group). Thus, evaluation of the effectiveness of compensation strategies needs to consider their effect on all sub-systems as a whole. Strategies that compensate for laryngeal weakness at the cost of compromising the normal function of the respiratory sub-system are undesirable. In this study, a three-dimensional model of phonation incorporating respiratory-laryngeal coupling is used to systematically investigate the cause-effect relation between physiological changes and voice production across a large range of voice conditions. The capability to model respiratory-laryngeal interaction also allows us to evaluate the effect of glottal insufficiency and potential compensations on the respiratory effort of phonation, which we will show imposes additional constraints on voice production and available compensation strategies.
II. METHOD
A sketch of the three-dimensional computational model used in this study is shown in Fig. 1. The model includes a respiratory system driven by a respiratory muscular pressure to provide the target subglottal pressure, a three-dimensional vocal fold model coupled with a one-dimensional glottal flow model, and a vocal tract model in which sound propagates. The fluid-structure-acoustic interaction within the glottis and sound propagation in the vocal tract are described in [4], and the respiratory model is described in detail in [5]. These two studies [4, 5] focused on the laryngeal and respiratory systems, respectively, with [4] investigating the cause-effect relation between changes in vocal fold physiology and the resulting changes in vocal fold vibration and output acoustics, and [5] investigating the mechanics of the respiratory system and the respiratory effort of phonation. In this study, the vocal fold model and the respiratory model are coupled through the instantaneous glottal volume flow rate using Equation (6) in [5], thus allowing systematic investigation of respiratory-laryngeal interaction during phonation. The input of this coupled model includes the vocal fold conditions (geometry, stiffness, and position), lung function parameters (total lung capacity TLC, vital capacity VC, functional residue capacity FRC, and lung compliance), and the respiratory muscular pressure or the target subglottal pressure.
Fig. 1.
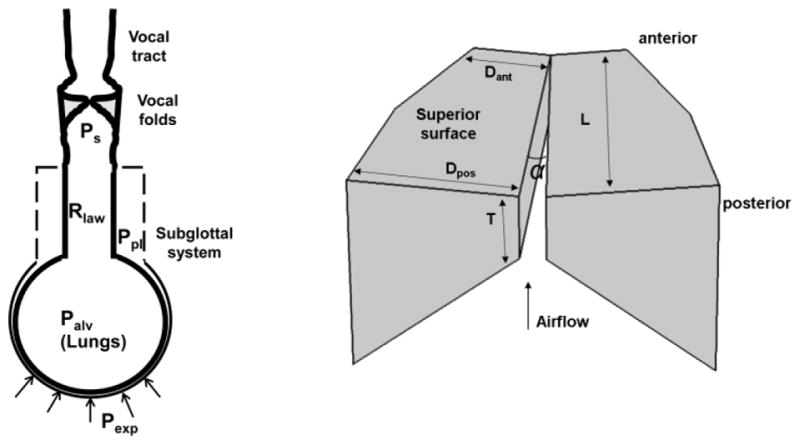
a) A sketch of the computational model used in this study; b) the three-dimensional vocal fold model and its primary geometric controls. L = 17 mm. More details of the geometry can be found in [4].
While glottal insufficiency may also refer to conditions in which the vocal folds are able to close the glottis without airflow but still vibrate with incomplete glottal closure, in this study we only consider pathological conditions with weakened control of the degree of vocal fold approximation, due to bilateral RLN paresis or vocal fold atrophy with aging, but normal control of vocal fold longitudinal stiffness and tension (through activation of the cricothyroid muscle). Specifically, glottal insufficiency is modeled by a large initial glottal angle of 4°, simulating inability to completely approximate the vocal folds. This initial glottal angle leads to a mid-membranous glottal width of about 1 mm and an initial glottal opening area of 10 mm2, which is slightly larger than the range of normal phonation reported by Isshiki [6] and those investigated in previous numerical simulations [4]. Voice production under such weakened adduction conditions is compared to voice production with normal control of vocal fold approximation, i.e., with the initial glottal angle varying in a range between 0° to 4°. Such comparison allows us to identify voice changes due to glottal insufficiency as well as potential compensations that, under the constraint of a large constant initial glottal angle of 4°, are able to at least partially restore the vocal range (fundamental frequency, sound pressure level, and spectral characteristics) of normal phonation produced with a large range of initial glottal angles.
Voice production simulations are performed for a large range of vocal fold and respiratory conditions, with parametric variations in the vertical thickness T (1, 2, 3, 4.5 mm), the shear modulus along the anterior-posterior direction Gap (from 4 kPa to 50 kPa in step of 2 kPa), the initial glottal angle α (from 0° to 4° in step of 0.4°, for simulation of normal phonation conditions), the subglottal pressure Ps (from 50 Pa to 2.4 kPa in 18 steps, see [4]), and the inspiratory muscular pressure Pins (−1.5 kPa and −2.4 kPa). The detailed simulation conditions are summarized in Table 1. A total of 19008 vocal fold conditions, including conditions both of glottal insufficiency and normal phonation, is considered for each of the two inspiratory conditions. Note that the glottal insufficiency conditions are a subset of the normal phonation conditions. The large number of conditions investigated allows us to understand the effect of glottal insufficiency on the entire vocal range and identify compensation strategies that are effective for a large range of voice conditions instead of one specific voice condition. Each simulation consists of a half-second period of inspiration, in which the lung volume starts at the FRC and an inspiratory muscular pressure is applied to the lungs [5]. The lung volume at the end of this inspiration period establishes the initial lung volume (LVI) for phonation. This inspiration period is followed by a 4-second expiration (typical breath group duration of normal speech, [7]), during which an expiratory muscle pressure is activated to maintain the target subglottal pressure and the vocal folds are set into desired geometry and stiffness condition. The lung volume at the end of this 4-second period of phonation determines the termination lung volume (LVT).
Table 1.
Simulations conditions for the control parameters: the vocal fold vertical thickness (T), shear modulus of the vocal folds along the anterior-posterior direction (Gap), initial glottal angle (α), subglottal pressure (Ps), and inspiratory muscular pressure (Pins). The vocal fold transverse Young’s modulus is set constant at 4 kPa. For the respiratory model, TLC=7L, RV=2L, FRC=3.5L, and lung compliance is 0.001 L/Pa.
| T (mm) | Gap (kPa) | α (°) | Ps (kPa) | Pins (kPa) |
|---|---|---|---|---|
| 1, 2, 3, 4.5 | 4 – 50 in step of 2 | 0 – 4 in step of 0.4 | 0.05–2.4 (18 steps) | −1.5, −2.4 |
For each vocal fold condition, the mean glottal flow (Qmean), closed quotient (CQ), vocal intensity (SPL), fundamental frequency (F0), the amplitude differences between the first harmonic and the second harmonic (H1–H2) and the harmonic nearest 2 kHz (H1-H2k), and harmonic-to-noise ratio (HNR) are calculated as described in [4]. Although the maximum respiratory muscular force required to maintain the target subglottal pressure in each simulation condition provides a direct evaluation of the respiratory effort of phonation, to normalize for individual differences in lung functions, the respiratory effort is quantified in this study by the LVT as a percentage of the VC. In normal human speech or singing, each breath group often finishes at a LVT around 30% of the VC, close to the FRC [8–10]). Zhang [5] demonstrated that for a given breath group duration, the maximum respiratory force required to maintain a target subglottal pressure increases significantly as the LVT falls below 30% of the VC and rapidly approaches the physiological limits of respiratory muscle activation. Based on these observations, in the discussion below, vocal fold conditions finishing at a LVT below 30% of the VC are considered to require a respiratory effort approaching physiological limits and thus should be avoided during phonation.
III. RESULTS
A. Voice changes
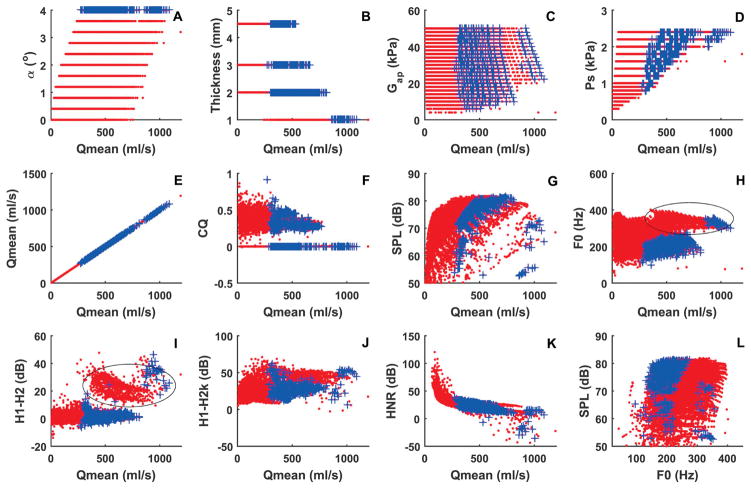
Fig. 2 compares voice production under glottal insufficiency (symbols + in blue; an initial glottal angle of 4°) to that for normal conditions in which the initial glottal angle varies between 0° to 4° (dot symbols in red). The first row of Fig. 2 plots the four control parameters (initial glottal angle, vertical vocal fold thickness, vocal fold stiffness, and subglottal pressure) against the corresponding mean glottal flow, for all vocal fold conditions investigated. The other two rows show selected measures of the resulting voice production as a function of the mean glottal flow. The right most panel in the bottom row shows the vocal ranges with the SPL plotted as a function of F0. Note that two clusters of voice conditions can be observed in the H1–H2 and F0 data shown in Figs. 1h and 1i, with one corresponding to a falsetto-like voice (high F0, high H1–H2, high H1-H2k, low HNR, and high glottal flow, produced with a thin vocal fold) and the other a chest-like voice (relatively low F0, low H1–H2, low H1-H2k, high HNR, low glottal flow, produced with a thick vocal fold).
Fig. 2.
Comparison between normal voice production (dotted symbols in red) and voice production with glottal insufficiency (with a constant α = 4 °; symbols + in blue). Panels a-k show the four control parameters (initial glottal angle, vocal fold vertical thickness, shear modulus along the anterior-posterior direction, and subglottal pressure) and selected measures of voice production as a function of the mean glottal flow rate Qmean. Panel l shows the vocal intensity as a function of F0 or the vocal range profile. Inspiratory muscular pressure is −1.5 kPa, which provides an initial lung volume at 62% of the vital capacity. The ellipse in panels h and I indicates conditions with a falsetto-like phonation.
As expected, glottal insufficiency (symbols +) leads to significantly increased glottal flow, as shown in Fig. 2a. This leads to a reduced HNR (Fig. 2k), particularly for high vocal intensity productions. Another major acoustic effect of severe glottal insufficiency is a significantly restricted F0 range, particularly the upper limits, for both the chest-like and falsetto-like phonations (Fig. 2h). This is because that with glottal insufficiency, control of vocal fold approximation, one of the primary means of F0 control [4], is no longer effective. Glottal insufficiency also restricts the lower limits of the vocal intensity range (bottom left of Fig. 2g), because the production of very low vocal intensity requires a subglottal pressure that is too low to excite phonation for conditions of a large initial glottal angle [4] (note that voice with a very low vocal intensity is different from voice with limited harmonics but a strong noise component, as often observed in conditions of glottal insufficiency, which may be perceived to be weak or of low intensity but has a high vocal intensity due to the strong noise component). Surprisingly, Fig. 2g indicates that there is not much restriction on the upper limits of the vocal intensity range. Overall, glottal insufficiency restricts voice production of either low intensity or high F0. On the other hand, the spectral shape measures (Figs. 2i and 2j) for conditions of glottal insufficiency generally overlap in range with those for normal conditions, indicating a relatively small effect on voice quality other than a reduced HNR.
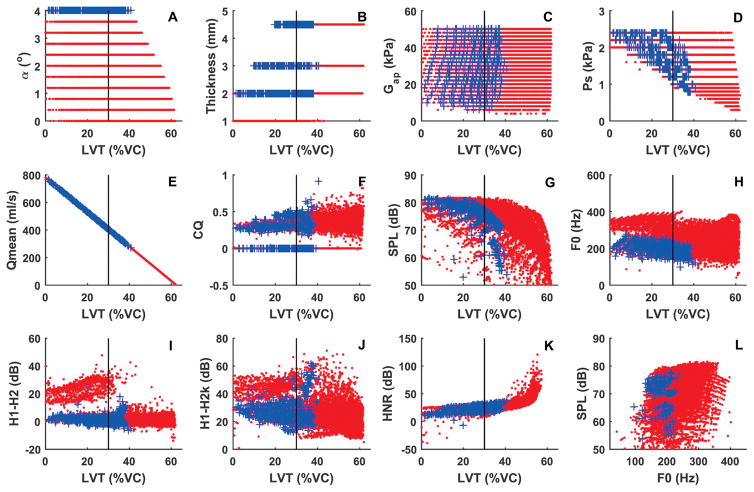
The increased glottal flow due to glottal insufficiency requires increased respiratory support. Fig. 3 shows the same set of data but as a function of the LVT as a percentage of the VC, which provides a relative measure of respiratory effort. The data are obtained with an inspiratory muscle pressure of −1.5 kPa (the minus sign indicates a net inspiratory pressure) in the inspiration phase, which leads to a LVI at about 62% of the VC. As discussed earlier, in this study, we consider a LVT below 30% as an indicator of significantly high respiratory effort that should be avoided. Note that some conditions cannot be sustained for a 4-second duration for the given initial lung volume, and thus do not show up in Fig. 3. One group of conditions that fall into this category is the falsetto-like voice production under conditions of glottal insufficiency, which appears in Fig. 2h but not in Fig. 3h. This is because the production of a falsetto-like voice requires a small vocal fold vertical thickness (e.g., conditions T = 1 mm in Fig. 2b), which results in too much airflow to be sustained for a 4-second breath group duration of phonation. With the falsetto-like phonation no longer possible, the F0 range is practically reduced to the lower-half of that under normal conditions. In addition, the glottal insufficiency conditions that produce the highest vocal intensity (and also the highest F0 in the chest-like phonations) finish the 4-second phonation at a lung volume below 30% of the vital capacity (Fig. 3g), indicating that these conditions require significantly high respiratory effort. Thus, while a falsetto-like phonation or phonation with a very high vocal intensity is theoretically still possible, such phonation would not be sustained for a typical breath group duration of speech, and if attempted, each breath group would finish at a very low lung volume so that one is likely to experience shortness of breath and significantly increased respiratory effort of phonation.
Fig. 3.
Respiratory requirement of normal voice production (dotted symbols in red) and voice production with glottal insufficiency (symbols + in blue). Panels a-k show the four control parameters (initial glottal angle, vocal fold vertical thickness, AP shear modulus, and subglottal pressure) and selected measures of voice production as a function of the termination lung volume (LVT) after 4-second phonation as a percentage of the lung vital capacity. Panel l shows the vocal intensity as a function of F0 for conditions with LVT>30%VC. The vertical lines in all panels indicate LVT=30%VC. Inspiratory muscular pressure is −1.5 kPa, which provides an initial lung volume at 62% of the vital capacity.
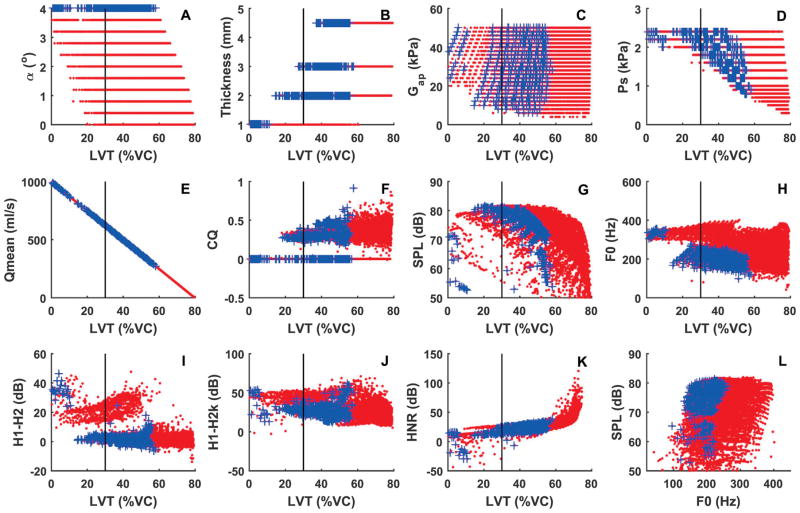
The high respiratory effort during phonation under conditions of glottal insufficiency can be compensated for by an increased respiratory effort during the inspiration phase. Fig. 4 shows similar data as in Fig. 3, but with an inspiratory muscle pressure of −2.4 kPa, which provides a LVI at 80% of the VC. Taking a deeper inspiration shifts the data to a higher LVT and reduces the respiratory effort during phonation, thus allowing more vocal fold conditions to be sustained for a normal breath group duration, particularly conditions producing a high vocal intensity. Note that, however, the falsetto-like phonation still finishes at a LVT close to the lung residual volume and thus still requires significantly high respiratory effort.
Fig. 4.
Comparison between normal voice production (dotted symbols in red) and voice production with glottal insufficiency (symbols + in blue) with deep inspiration (with an inspiratory muscular pressure of -2.4 kPa, which provides an initial lung volume at 80% of the vital capacity). Panels a-k show the four control parameters (initial glottal angle, vocal fold vertical thickness, AP shear modulus, and subglottal pressure) and selected measures of voice production as a function of the termination lung volume (LVT) after 4-second phonation as a percentage of the lung vital capacity. Panel l shows the vocal intensity as a function of F0 for conditions with LVT>30%VC. The vertical lines in all panels indicate LVT=30%VC.
B. Available compensation strategies and limitations
Compensation strategies that alleviate the undesirable voice changes can be identified from Figs. 2–4. Table 2 lists such compensation strategies and their limitations. The increased noise production can be compensated for by reducing the subglottal pressure [4], which would also reduce the respiratory effort. However, such compensation would also limit voice production to not-so-high intensity or F0.
Table 2.
Summary of undesirable voice changes, corresponding potential compensation strategies, and their limitations. * denotes voice changes due to respiratory system constraints.
| Voice changes | Compensation | Maintain normal breath group duration | Limitations |
|---|---|---|---|
| Increased noise (reduced HNR, Fig. 3k) | Reduced Ps | Yes | Not so high intensity or F0 |
| *Restricted falsetto-like voice production or phonation with extremely high F0 (Fig., 3h) | High Ps and deep inspiration | No | Excessively high expiratory effort |
| Reduced F0 and F0 range of chest-like phonation (Fig. 3h) | Rely solely on AP stiffness control by CT muscles for F0 control | Yes | Reduced F0 modulation; Strong CT activation may lead to vocal fold thinning and increased noise, and thus high respiratory effort |
| Restricted low-intensity voice production (Fig. 3g) | Not possible | ||
| *Restricted high- intensity voice production (Fig. 3g) | High Ps and deep inspiration | Yes but require deep inspiration | Increased noise and high expiratory effort |
Severe glottal insufficiency restricts the use of both the falsetto-like voice production and the upper-half F0 range of the chest-like voice production. There are no effective compensation strategies to completely restore the F0 range while still maintaining a normal breath group duration. Attempting a falsetto-like phonation or an extremely high F0 even for a much shorter breath group duration would still require excessively high expiratory effort. On the other hand, for chest-like phonation with not-so-high F0, to compensate for the loss of a primary means of F0 control (i.e., adjustment of vocal fold approximation), F0 control has to almost exclusively rely on adjusting vocal fold longitudinal stiffness through cricothyroid muscle activation (F0 control can be also assisted by controlling the vocal fold thickness and subglottal pressure, but to a much lesser degree). As a result, it is expected that more effort of the cricothyroid muscles is required to achieve the same amount of F0 modulation. On the other hand, because of this reduced F0 range and increased effort of F0 modulation, the amount of F0 modulation during speech is also expected to be reduced, likely resulting in a more monotonic speech. Note that increased activation of the cricothyroid muscles often leads to thinning of the vocal folds, which again leads to increased glottal flow, noise production, and respiratory effort.
Due to the high phonation threshold pressure associated with glottal insufficiency, there are no effective compensation strategies to facilitate voice production with very low intensity. On the other hand, although high vocal intensity is possible, a deeper inspiration, at the cost of increased inspiratory effort, is required to compensate for the otherwise high expiratory effort during phonation, particularly if one intends to maintain a normal breath group duration.
IV. DISCUSSION AND CONCLUSION
This study shows that glottal insufficiency or inability to adduct sufficiently leads to reduced HNR, loss of the upper F0 range, reduced F0 modulation, and reduced intensity range, which are consistent with clinical observations [1–3]. This study also shows that the laryngeal and respiratory sub-systems need to be considered as a whole in order to fully understand the effect of glottal insufficiency on voice production. For example, glottal insufficiency does not impose any restrictions on high-intensity voice production or a falsetto-like phonation, as far as the laryngeal sub-system is concerned. However, due to the finite amount of the lung volume, such voice production is difficult to sustain for a normal breath group duration under conditions of glottal insufficiency because it uses too much airflow, which would lead to significantly high respiratory effort, especially toward the end of the breath group.
The results show that weakened control of vocal fold approximation may lead to compensation in the form of increased activation of the cricothyroid muscles (to achieve sufficient F0 modulation) and increased respiratory effort (during both inspiration and expiration to produce high intensity voice). Thus, in addition to increased noise production and reduced F0 range, the adoption of these compensation strategies would also cause the laryngeal and respiratory sub-systems to function at an increased level, which may make one more susceptible to vocal fatigue, discomfort, or even vocal fold injury when such compensation persists for an extended period [11]. The relation between compensation strategies and possibilities of vocal fatigue and injury will be the focus of future investigations.
Acknowledgments
This study was supported by research grants R01 DC009229 and R01 DC011299 from the National Institute on Deafness and Other Communication Disorders, the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubin A, Sataloff R. Vocal fold paresis and paralysis. Otolaryngol Clin N Am. 2007;40:1109–1131. doi: 10.1016/j.otc.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Kendall K. Presbyphonia: a review. Current opinion in otolaryngology & head and neck surgery. 2007;15(3):137–140. doi: 10.1097/MOO.0b013e328166794f. [DOI] [PubMed] [Google Scholar]
- 3.Awan SN. The aging female voice: Acoustic and respiratory data. Clinical Linguistics & Phonetics. 2006;20:171–180. doi: 10.1080/02699200400026918. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z. Cause-effect relationship between vocal fold physiology and voice production in a three-dimensional phonation model. J Acoust Soc Am. 2016;139(4):1493–1507. doi: 10.1121/1.4944754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z. Respiratory laryngeal coordination in airflow conservation and reduction of respiratory effort of phonation. J Voice. 2016;30(6):760.e7–760.e13. doi: 10.1016/j.jvoice.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isshiki N. Phonosurgery: Theory and Practice. Chap. 3 Springer-Verlag; Tokyo: 1989. [Google Scholar]
- 7.Wang Y, Green J, Nip I, Kent R, Kent J. Breath group analysis for reading and spontaneous speech in healthy adults. Folia Phoniatr Logop. 2010;62:297–302. doi: 10.1159/000316976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hixon TJ. Respiratory Function in Speech and Song. Chapter 1 Boston, MA: College-Hill Press; 1987. [Google Scholar]
- 9.Hoit JD, Hixon TJ. Age and speech breathing. J Speech Hear Res. 1987;30:351–366. doi: 10.1044/jshr.3003.351. [DOI] [PubMed] [Google Scholar]
- 10.Thomasson M, Sundberg J. Lung volume levels in professional classical singing. Logoped Phoniatr Vocol. 1997;22:61–70. [Google Scholar]
- 11.Hillman RE, Holmberg EB, Perkell JS, Walsh M, Vaughan C. Objective assessment of vocal hyperfunction: an experimental framework and initial results. J Speech Hear Res. 1989;32:373–392. doi: 10.1044/jshr.3202.373. [DOI] [PubMed] [Google Scholar]