Abstract
In vivo persistence of chimeric antigen receptor (CAR)-modified T cells correlates with therapeutic efficacy, yet CAR-specific factors that support persistence are not well resolved. Using a CD33-specific CAR in an acute myeloid leukemia (AML) model, we show how CAR expression alters T cell differentiation in a ligand independent manner. Ex vivo expanded CAR-T cells demonstrated decreased naïve and stem memory populations and increased effector subsets relative to vector-transduced control cells. This was associated with reduced in vivo persistence. Decreased persistence was not due to specificity or tumor presence, but to pre-transfer tonic signaling through the CAR CD3ζ ITAMs. We identified activation of the PI3K pathway in CD33 CAR-T cells as responsible. Treatment with a PI3K inhibitor modulated the differentiation program of CAR-T cells, preserved a less differentiated state without affecting T cell expansion, and improved in vivo persistence and reduced tumor burden. These results resolve mechanisms by which tonic signaling of CAR-T cells modulates their fate, and identifies a novel pharmacologic approach to enhance the durability of CAR-T cells for immunotherapy.
INTRODUCTION
Human T cells expressing tumor-specific chimeric antigen receptors (CARs) have demonstrated potency in the immunotherapy of acute lymphoblastic leukemia (ALL) and are being assessed for other malignancies.1, 2 CARs co-express tumor-specific recognition domains and signaling components triggering T cell activation. CAR therapy for B-cell ALL has improved prognosis for patients with refractory or recurrent disease, and therapeutic T cells expressing anti-CD19 receptors incorporating 4-1BB or CD28 and CD3ζ signaling domains induce high rates of remission.3
Preclinical data supports the application of CAR therapy for myeloid neoplasms,4–7 yet this is less developed. Our group generated an anti-CD33 CAR through the substitution of the CD19 scFv, present in an anti-CD19-41BB-CD3ζ CAR that is FDA approved for the immunotherapy of pediatric ALL, with a CD33-specific scFv.8–10 Whereas the resulting AML-specific CAR-T cells potently targeted tumor lines and primary AML samples ex vivo, inadequate persistence in preclinical models led to incomplete tumor clearance and disease recurrence.7
CAR-T cell persistence is associated with efficacy and sustained remission.10–14 CAR-T cell differentiation, exhaustion, and metabolic status impact the survival and efficacy of adoptively administered cells.15–19 These parameters are related to CAR structure. For instance, spontaneous clustering and tonic signaling in GD2-CD28-CD3ζ CAR-T cells induced early exhaustion, limiting therapeutic efficacy.19 Replacement of the CD28 domain with 4-1BB reduced but did not eliminate this, indicating that ligand-independent CAR signaling influences T cell state.19
In this study, we assessed additional determinants of CAR-T cell durability. We show that tonic CAR signaling during ex vivo T cell expansion rather than specificity or tumor presence leads to inadequate persistence. Signaling through CAR CD3ζ ITAMs led to activation of PI3K signaling and was associated with a more differentiated phenotype. This effect was diminished by PI3K inhibitor treatment during ex vivo expansion, which maintained a less differentiated state and heightened in vivo persistence and anti-tumor efficacy. These results demonstrate how tonic CAR signaling promotes ligand-independent terminal differentiation thereby limiting CAR-T cell survival, and support interventions to improve CAR-T cell survival and function.
RESULTS
CD19 and CD33 CAR-T cells control AML tumor growth
CD33-specific CAR-T cells fail to fully eradicate AML 7 despite the potency of its parental CD19-specific receptor against ALL. We first asked whether ligand specificity played a role. We assessed the ex vivo cytolytic potential of CD33 and CD19 CAR-T cells against an AML cell line that was stably transduced with CD19 to co-express CD33 and CD19 (MOLM-13-CD19). The CARs equivalently redirected CTLs against MOLM-13-CD19 cells, with near complete killing at low E:T ratios (Supplementary Figure 1).
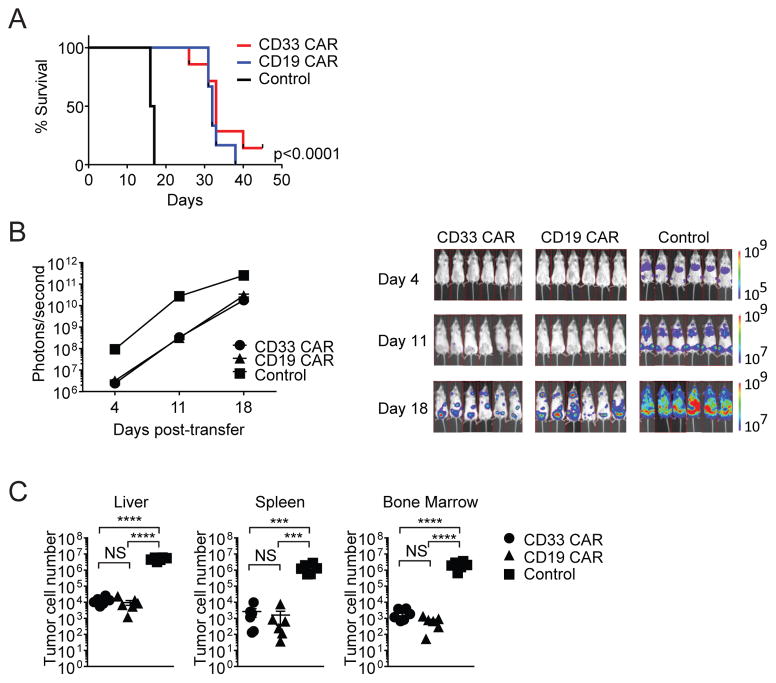
We next tested the efficacy of CD33- and CD19-specific CARs against AML in vivo. Treatment with either CAR equivalently delayed AML progression relative to vector-transduced control T cells (Figure 1A). At day 18, a large tumor burden was detected in the control cohort, and this was substantially reduced with both CD33 and CD19 CAR-T cell therapy (Figure 1B and C). Thus, there was no significant difference in anti-AML efficacy of T cells modified with the CD33 or clinically-established CD19 CAR.
Figure 1. CD33 and CD19 CAR-T cells control AML growth.
Mice were injected with MOLM-13-CD19 tumor cells and treated with CD33 CAR, CD19 CAR, or control T cells. Mice were monitored for survival and tumor burden, or organs were harvested 18 days after transfer. (A) Kaplan-Meier survival analysis. (B) Xenogen images and bioluminescent signal intensities (C) MOLM-13-CD19 tumor cell numbers *** p<0.001, **** p<0.0001.
Poor CAR-T cell survival is tumor independent
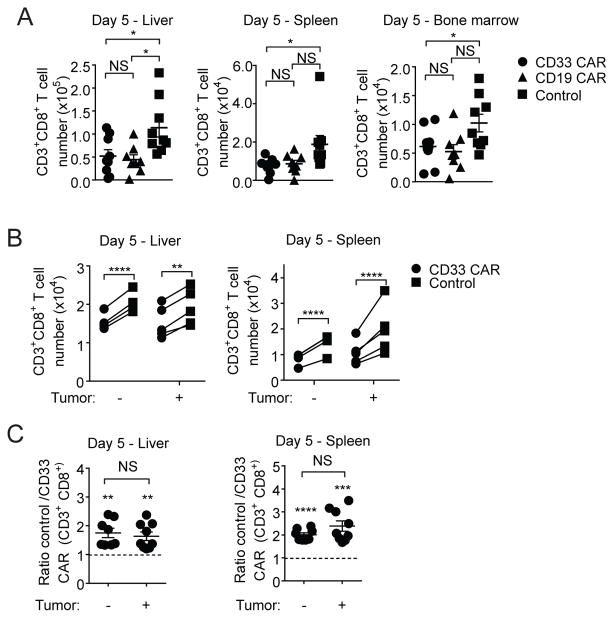
To assess CAR-T cell survival in this AML treatment model, absolute cell numbers were determined. At day 5, control CD8+ T cell numbers were significantly greater than CD33 CAR-T cell numbers (Figure 2A). At day 18, while control T cells persisted in all organs, few CAR-T cells were seen (Supplementary Figure 2A). CD33 and CD19 CAR-T cells had comparably reduced persistence relative to control T cells in vivo, indicating that CAR specificity did not impact T cell longevity.
Figure 2. Poor CAR-T cell survival is tumor independent.
(A) Mice were injected with MOLM-13-CD19 tumor cells and treated with CD33 CAR, CD19 CAR or control T cells. Organs were harvested 5 days after transfer. Total numbers of CD3+CD8+ CAR or control T cells are shown. (B–C) CD33 CAR-T cells (GFP) and control T cells (RFP) were co-transferred at ratios of 0.9:1–1.1:1 into mice with or without tumor. Organs were harvested 5 days after transfer. (B) Numbers of CD8+ CAR and control T cells with and without tumor. (C) Ratio of CD8+ control T cells to CD33 CAR-T cells with and without tumor, normalized to input ratio. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
To assess whether differential persistence resulted from tumor recognition or cell intrinsic effects of CAR expression, equal numbers of CD33-specific CAR and control T cells were co-transferred with or without tumor. Regardless of tumor presence, there were significantly fewer CD8+ and CD4+ CD33 CAR-T cells than control T cells in the liver and spleen of recipient mice (Figure 2B and Supplementary Figure 2B). The ratio of control to CD33 CAR-T cells in individual mice was unaltered by the presence of tumor (Figure 2C). Thus, the accelerated attrition of CD8+ CAR-T cells relative to control T cells in vivo is tumor-independent.
Ex vivo tonic CAR signaling alters T cell differentiation
We next assessed CD45RA+CCR7+ naïve (TN), CD45RA−CCR7+ central memory (TCM), CD45RA−CCR7− effector memory (TEM), and CD45RA+CCR7− effector (TEFF) subsets, as well as a subset of TN cells, CD62L+CCR7+CD45RA+CD45RO−CD95+ stem memory (TSCM) T cells, in the ex vivo activated populations.20 CAR and control T cells were stimulated pre-transfer with mitogen in the absence of cognate ligand, and should be identical unless CAR expression modulated T cell maturation.
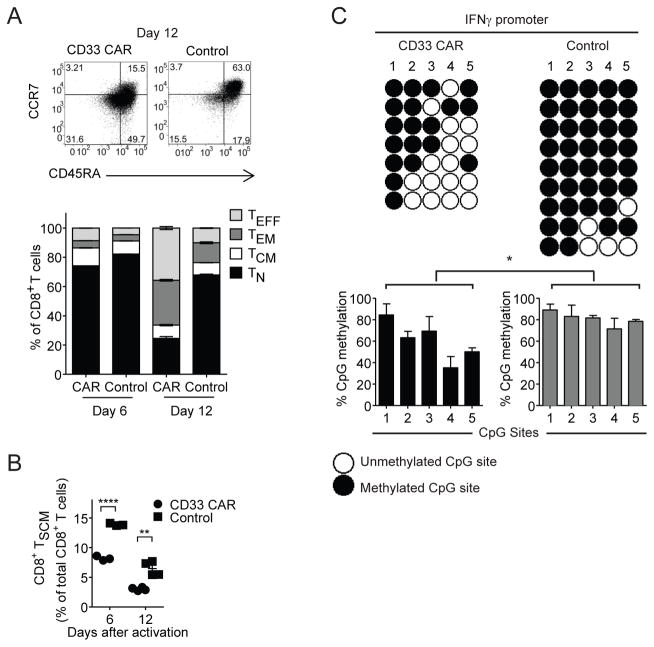
CD8+ CD33 CAR-T cells showed decreased CD45RA, CCR7 and CD62L expression, and increased CD45RO expression, relative to control T cells (Supplementary Figure 3A and B). Nearly 3-fold more control CD8+ T cells bore a TN phenotype at day 12, compared to CD33 CAR-T cells (Figure 3A). Correspondingly, increased proportions of CD33 CAR-T cells differentiated into TEFF and TEM cells. Moreover, control T cells contained more TSCM cells than CD33 CAR-T cells (Figure 3B, Supplementary Figure 3C). Transducing sorted naïve CD8+CD45RA+CD45RO−CCR7+CD95− T cells with CAR likewise resulted in reduction of the TN subset and increased TEM proportions relative to control cells (Supplementary Figure 3D). This was also correlated with enhanced expression of exhaustion and activation markers in pre-transfer CAR-T cells relative to controls on day 12 (Supplementary Figure 3E). Similar skewing of differentiation was observed during ex vivo expansion of CD19 CAR-T cells (Supplementary Figure 3F and G).
Figure 3. CAR-T cells exhibit increased effector differentiation.
(A) Composition of TN, TCM, TEM and TEFF CD8+ T cell subsets in CD33 CAR and control T cells after ex vivo activation. (B) Percent of TSCM cells after ex vivo activation. (C) Methylation analysis of genomic DNA CpG sites within the IFNγ promotor. Naïve CD8+CD45RA+CD45RO−CCR7+CD95− T cells were sorted from donor samples, transduced with CD33 CAR or control vector, and assessed 9 days after ex vivo activation. Each line represents an individual clone. Bar graphs show % CpG methylation at each site of the locus in CD33 CAR or control T cells. * p<0.05, ** p<0.01, **** p<0.0001.
To further examine the differentiation status of CD33 CAR-T cells, methylation of the IFNγ promoter was assessed. Five CpG sites within this locus are highly methylated in TN cells, and demethylated for rapid expression in memory and effector subsets.21 Control T cells were more heavily methylated at the IFNγ promoter locus compared to CD33 CAR-T cells (Figure 3C), further indicating that CD33 CAR-T cells more readily differentiate into memory or short-lived effector cells.
CAR CD3ζ signaling is responsible for altered T cell differentiation
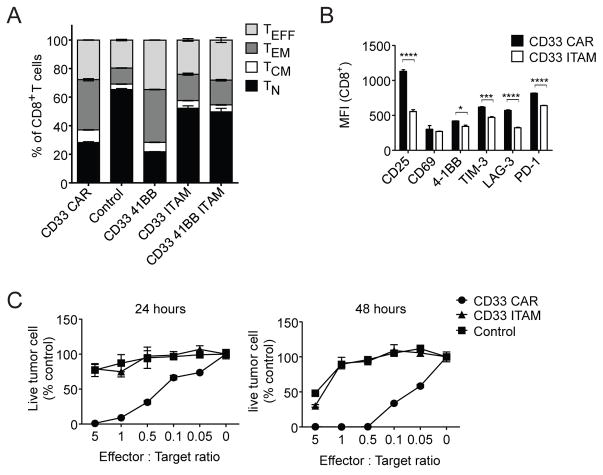
To determine which CAR signaling domains altered T cell differentiation, we generated constructs in which the signaling motifs of 4-1BB (CD33-41BB), CD3ζ (CD33-ITAM), or both (CD33-41BB-ITAM) were mutated (Supplementary methods). The CD33-ITAM and CD33-41BB-ITAM mutants had differentiation profiles similar to control T cells, with increased proportions of TN cells and reduced TEFF cells relative to CD33 CAR-T cells (Figure 4A). Mutation of 4-1BB alone did not impact differentiation status. This indicated that CAR CD3ζ ITAM signaling during ex vivo T cell expansion leads to a more effector-differentiated phenotype. The CD33-ITAM mutant also exhibited reduced expression of activation and exhaustion markers relative to CD33 CAR-T cells (Figure 4B). Although mutation of the CD3ζ domain produced CAR-T cells that were phenotypically similar to control T cells, this resulted in the abrogation of CAR-T cell activity in vitro (Figure 4C).
Figure 4. Altered CAR-T cell differentiation due to CAR CD3ζ ITAM signaling.
(A) Composition of CD8+ TN, TCM, TEM, and TEFF subsets in CD33 41BB, CD33 ITAM, and CD33 41BB ITAM T cells relative to CD33 CAR and control T cells 12 days after activation. (B) Activation and exhaustion marker expression on CD8+ CD33 CAR and CD33-ITAM T cells 12 days after activation. (C) MOLM-13-CD19 cells were incubated with CD33 CAR, CD33 CAR ITAM mutant, or control T cells as indicated for 24–48h. Tumor cells were quantified by flow cytometry and normalized to cultures without added T cells. * p<0.05, *** p<0.001, **** p<0.0001.
Pre-transfer differentiation of CD33 CAR-T cells diminishes in vivo persistence
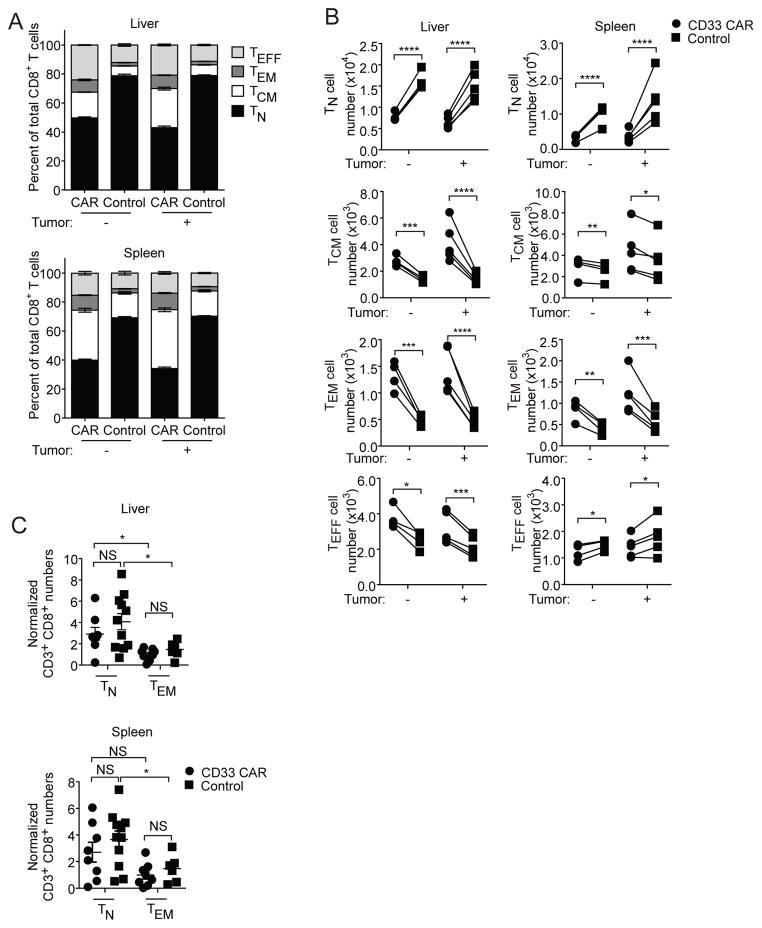
We next analyzed the differentiation status of CD33 CAR-T cells after co-transfer with control T cells. Regardless of tumor presence, both CD8+ and CD4+ CD33 CAR-T cells showed elevated frequencies of effector subsets while control T cells maintained increased percentages of TN cells (Figure 5A and B, Supplementary Figure 4A–C). Therefore, the perturbed subset proportions observed during ex vivo culture are maintained after transfer in vivo.
Figure 5. Differentiation status of CAR-T cells influences in vivo persistence.
(A–B) CD33 CAR and control T cells were mixed 1:1 and transferred with or without MOLM-13-CD19 tumor cells into mice. Cell numbers were determined 5 days after transfer. (A) Proportions of and (B) Numbers of CD8+ TN, TCM, TEM and TEFF subsets. (C) CD8+CCR7+CD45RA+ TN and CD8+CCR7−CD45RA− TEM cells were sorted from activated CD33 CAR or control T cells and transferred individually into mice. Cell numbers were normalized to the CD33 CAR TEM group. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
Several studies have associated high frequencies of effector subsets with diminished therapeutic T cell survival.22–24 We hypothesized that increased effector differentiation of CD33 CAR-T cells reduces their sustainability in vivo. We evaluated the persistence of sorted CD8+ TN and TEM subsets of CD33 CAR or control T cells. There were no differences between CAR and control groups for TN and TEM cells, and transferred TN cells were better sustained regardless of CAR expression (Figure 5C). Therefore poor in vivo persistence of CAR-T cells can be attributed to decreased TN and increased TEM proportions in pre-transfer populations, rather than an effect of the CAR itself on T cell survival after transfer.
CD33 CAR-T cells exhibit a distinct transcriptional profile
To further explore the impact of CAR signaling on T cell differentiation, we analyzed transcriptomes of CD33 CAR and control T cells during ex vivo expansion. Approximately 500 genes were differentially expressed and Ingenuity upstream regulator analysis identified TCR as the top upstream regulator of gene expression changes (P value: 2.71E-38, Activation z-score: 2.695), indicating increased TCR signaling in CD33 CAR relative to control T cells, despite the absence of cognate ligand during culture.25 Consistently, pathways associated with TCR signaling were also significantly up-regulated in CD33 CAR-T cells (Supplementary Figure 5A and B).
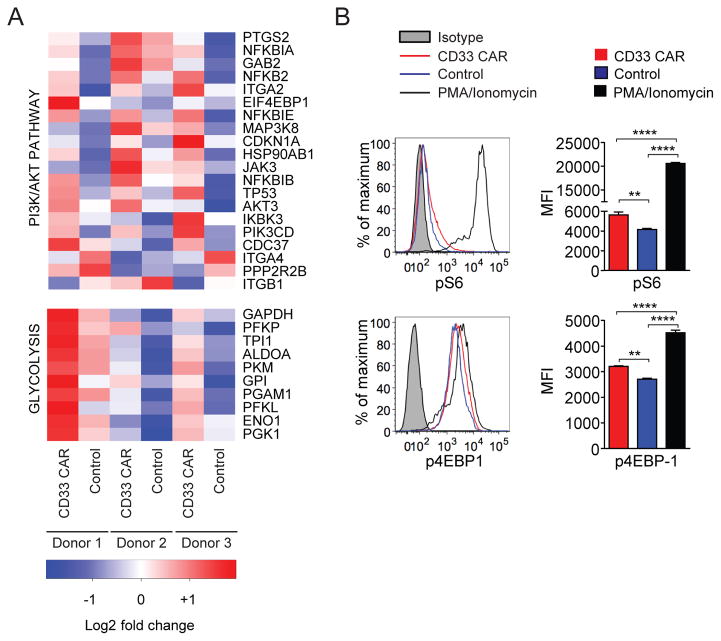
The PI3K/AKT and glycolysis pathways were highly upregulated in CD33 CAR-T cells (Figure 6A). These were of interest as PI3K/AKT signaling controls CD8+ T cell differentiation through mTOR-mediated effects on glycolysis.26 To further assess these pathways, we analyzed the phosphorylation state of downstream targets of PI3K and TCR signaling in T cells 12 days after activation. Levels of phospho-S6 and 4EBP-1 were higher in CD33 CAR than control T cells (Figure 6B). No detectable changes were observed in phospho-AKT or ERK levels (Supplementary Figure 5C). In addition to the constitutive activation seen, CAR-T cells also demonstrated elevations of pS6, 4EBP-1, and pERK relative to controls 10 minutes after PMA/ionomycin activation, indicating an impact of the CAR after acute stimulation (not shown). These data imply that tonic CAR signaling promotes constitutive activation of some components of the PI3K/AKT pathway and are consistent with persistent ligand-independent activation of TCR CD3ζ signaling through the CAR.
Figure 6. CD33 CAR-T cells exhibit a distinct transcriptional profile.
(A) Differentially expressed genes from PI3K/AKT and glycolysis pathways identified by Ingenuity Pathway Analysis (IPA) of CD8+ CD33 CAR and control T cells 12 days after ex vivo activation. (B) Phospho-flow staining of pS6 and p4EBP-1 in CD33 CAR and control T cells. CD33 CAR-T cells were stimulated with PMA/Ionomycin as a positive control. ** p<0.01, **** p<0.0001.
PI3K/AKT signaling drives CD8+ CAR-T cell effector differentiation ex vivo
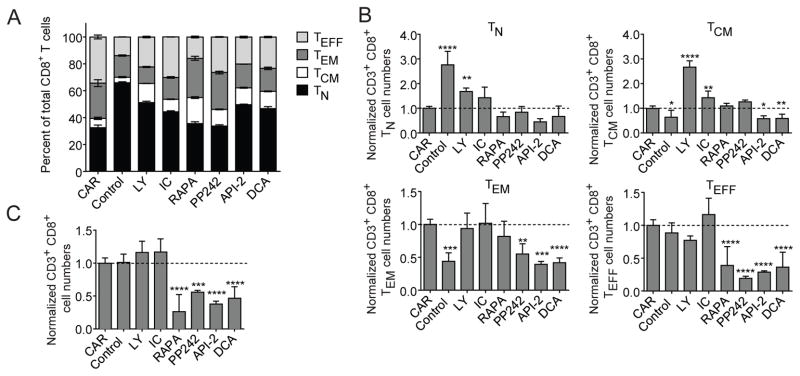
Constitutive activation of PI3K/AKT/mTOR promotes terminal differentiation of CD8+ T cells, while antagonism promotes memory development.27, 28 To evaluate the role of PI3K/AKT/mTOR activation in CD33 CAR-T cell differentiation, we added PI3K, AKT, mTOR, or glycolysis inhibitors to pre-transfer CAR-T cell cultures. Treatment with PI3K inhibitors increased TN and TCM percentages relative to untreated CD33 CAR-T cells (Figure 7A). Consistent with the role of mTOR in promoting effector differentiation,29 mTOR inhibitors reduced TEFF percentages. Treatment with AKT or glycolysis inhibitors also increased TN and TCM percentages. While absolute cell numbers were maintained with PI3K inhibitors, they were significantly diminished with all other treatments such that the absolute numbers of TN and TCM cells were decreased relative to untreated CD33 CAR-T cells (Figure 7B and C). PI3K inhibition also maintained CCR7 expression on cells undergoing division, such that there was no difference between control and LY294002 treated CAR-T cells (Supplementary Figure 6). Therefore, PI3K inhibitors appear unique in their ability to suppress effector differentiation and increase TN and TCM proportions without diminishing therapeutic T cell expansion.
Figure 7. PI3K inhibition restrains aberrant CAR-T cell differentiation ex vivo.
CD33 CAR or control T cells were stimulated for 5 days, treated with inhibitor for 4 days, and analyzed by flow cytometry. PI3K inhibitors LY294002 (LY) and IC87114 (IC), mTOR inhibitors rapamycin (RAPA) and PP242, AKT inhibitor API-2, and glycolysis inhibitor DCA were used as indicated. (A) Percentages of TN, TCM, TEM and TEFF subsets of CD8+ T cells. (B) Numbers of TN, TCM, TEM and TEFF subsets of CD8+ T cells normalized to the number of untreated CD33 CAR-T cells. (C) Relative numbers of total CD8+ T cells after 4 days of inhibitor treatment, normalized to untreated CAR-T cells. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
PI3K blockade enhances CD33 CAR-T cell persistence and reduces tumor burden
The PI3K inhibitor LY294002 was selected for further testing. A concentration of 10 μM optimally impacted T cell differentiation without reducing cell numbers (Supplementary Figure 7A and B). PI3K inhibitor-treated and untreated CD33 CAR-T cells were indistinguishable in in vitro killing assays (Supplementary Figure 8A). However, inhibitor-treated CD33 CAR-T cells expressed increased effector molecules relative to untreated CD33 CAR-T cells after co-culture with MOLM-13 cells, indicating an increased cytokine response after stimulation (Supplementary Figure 8B).
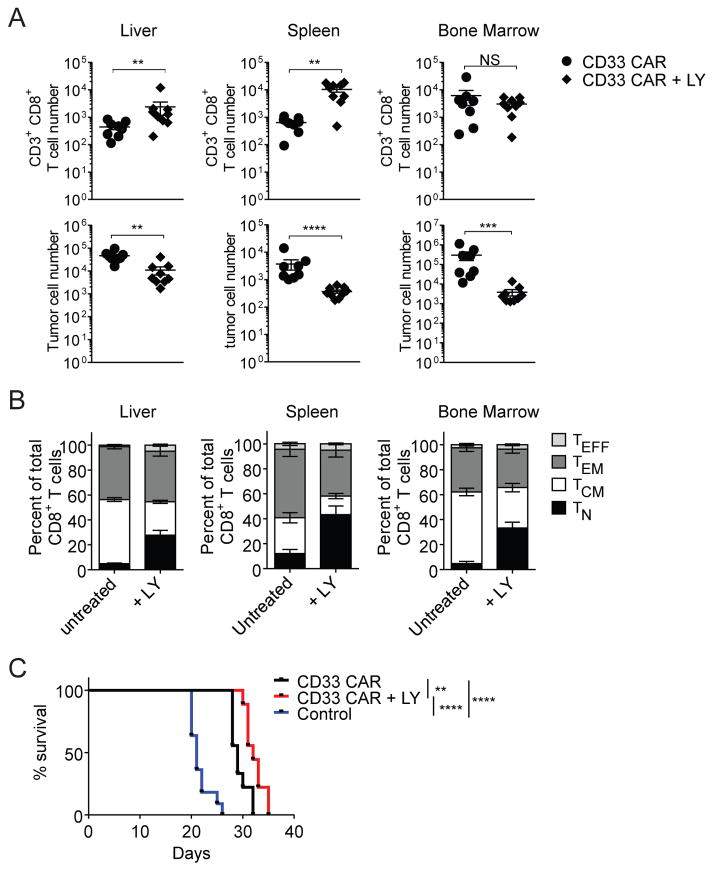
To determine if PI3K inhibition during ex vivo culture improved in vivo efficacy of CAR-T cells, inhibitor-treated or untreated CD33 CAR-T cells were each transferred into MOLM-13-CD19 tumor-bearing mice. At day 14 after transfer, PI3K inhibition improved the persistence of CD8+ CD33 CAR-T cells in liver and spleen, and resulted in reduced tumor burden in all organs (Figure 8A). LY-treated CD33 CAR-T cells also maintained their less differentiated phenotype in vivo, with an increased TN percentage and total cell number (Figure 8B and Supplementary Figure 8C). Median survival was increased by 3 days in the LY-treated CAR group, relative to the untreated CAR group under these conditions (Figure 8C). This modest increase emphasizes the need for further optimization of this approach. These results indicate that PI3K signaling can be targeted to restrain aberrant CAR-T cell activation without impacting expansion, and this results in significantly enhanced post-transfer persistence and anti-tumor efficacy at intermediate time points, and more modestly enhanced survival in this model system.
Figure 8. PI3K inhibition during ex vivo expansion improves CAR-T cell persistence and reduces tumor burden in vivo.
Mice were injected with MOLM-13-CD19 tumor cells and either untreated or LY-treated CD33 CAR-T cells. Organs were harvested 14 days after transfer. (A) Total CD8+ T cell numbers (top) and total MOLM-13-CD19 tumor cell numbers (bottom). (B) Proportions of CD8+ TN, TCM, TEM and TEFF subsets. (C) Kaplan-Meier survival analysis of mice with MOLM-13-CD19 tumors treated with CD33 CAR, CD33 CAR + LY treatment, or vector-transduced control T cells. ** p<0.01, *** p<0.001, **** p<0.0001.
DISCUSSION
CAR-T cells are effective treatments for CD19+ leukemias and lymphomas,3 but their application to other malignancies remains a challenge. CAR-T cell longevity and functional preservation are critical. We used a CD33-specific CAR incorporating an established framework for the 4-1BB-CD3ζ signaling domains to show that in vivo CAR-T cell persistence is limited by tonic signaling through the CAR CD3ζ ITAMs. This trait was acquired during ex vivo expansion and was independent of antigen specificity. CAR-T cells increasingly differentiated into shorter-lived effector forms, and PI3K signaling was responsible for altered differentiation and survival. PI3K inhibition improved CAR-T survival and efficacy in vivo.
TN and TCM subsets, including a sub-population of TSCM cells, have been associated with enhanced CAR persistence in vivo.16, 17, 30–32 We found that tonic CAR signaling during expansion reduced TN/TSCM and increased effector subsets that correlate with poor persistence in vivo. Transferred TN/TSCM cells showed similarly improved persistence relative to TEM cells regardless of the presence of the CAR. Our data implies that reduced CAR-T cell persistence is mediated through alteration of T cell maturation profiles ex vivo and prior to transfer. This was further correlated with enhanced expression of exhaustion and activation markers in pre-transfer CAR-T cells relative to controls.
Disruption of CD3ζ signaling restored the TN:TEM ratio of CD33 CAR-T cells relative to control cells. Disruption of 4-1BB signaling did not alter CAR-T cell differentiation. Therefore, tonic 4-1BB signaling does not produce a noticeable effect on differentiation, or effects of 4-1BB are superseded by CD3ζ signals. Genome-wide transcriptional profiling identified pathways downstream of TCR signaling that were upregulated in CD33 CAR-T cells, most notably the PI3K/AKT/mTOR and glycolytic pathways, which control T cell differentiation, metabolism, and fate.33,34 These findings illustrate the relationship between CAR structure and in vivo persistence, and how the therapeutic capacity of CAR-T cells is influenced by ligand-independent alterations in programming.
PI3K signaling may play different roles in CAR-T cell function at different times during manufacture and application. While antigen-dependent PI3K signaling has been correlated with increased in vivo efficacy,35 we show a link between antigen-independent constitutive PI3K activation during ex vivo culture and reduced in vivo persistence. Treatment with a PI3K inhibitor during ex vivo CAR-T cell expansion increased TN/TSCM and TCM populations, and decreased the TEFF population, resulting in improved in vivo persistence and cytokine production. PI3K inhibitor treatment was associated with unperturbed ex vivo CAR-T cell expansion, and can be incorporated into production regimens without adversely impacting yield.
Inhibition of other pathways may similarly alter CAR-T cell differentiation. Treatment of CAR-T cells with BET bromodomain inhibitors, which downregulate c-Myc-dependent target genes, resulted in expansion of TN and TCM phenotypes and extended survival in an ALL model.36,37 This is consistent with our result in that aberrant PI3K and mTOR signaling may also activate c-Myc resulting in altered metabolism and differentiation.38, 39 Downstream of PI3K, excessive activation of mTOR leads to increased glycolysis and terminal differentiation.28 While mTOR and glycolysis inhibition reduced the proportion of TEFF cells, they severely reduced proliferative capacity, making them undesirable targets for use during ex vivo CAR-T cell expansion.
These results have broader implications for CAR-mediated immunotherapy. CD3ζ is a component of CAR constructs incorporating CD28, 4-1BB and other co-stimulatory domains, and all CAR constructs undergoing clinical trials,40 and its impact on T cell differentiation is likely intrinsically linked to signaling essential for CAR-T cell activation. Alternatively, inhibition of PI3K during ex vivo expansion is easily performed, and enhances CAR-T cell quality and in vivo persistence without affecting cell numbers. These findings form a basis for alternative approaches to improve cell-intrinsic features of therapeutic CAR-T cells and enhance the clinical efficacy of cell-based immunotherapeutics for cancer.
MATERIALS AND METHODS
Mice
NSG mice (NOD.Cg-Prkdcscid Il2rgtm1wj1/SzJ) were obtained from The Jackson Laboratory. Age matched, sex matched mice between 8–12 weeks of age were used in all studies.
Cell lines and culture
MOLM-13 transduced with firefly luciferase (Dr. Sharyn Baker, SJCRH) was transduced with human CD19 and single cell sorted to isolate stable cell line MOLM-13-CD19. MOLM-13-CD19 and Phoenix-AMPHO cells (ATCC-CRL-3213) were cultured in RPMI-1640 and DMEM (Life Technologies) with 10% FBS (Atlanta Biologicals), 100 U/ml penicillin, 100 μg/ml streptomycin and 292 μg/ml L-glutamine (Life Technologies).
CAR constructs and retrovirus production
Anti-CD3341 and anti-CD1942 scFv fragments were cloned in frame with the human CD8 leader sequence, CD8 transmembrane domain, and 41BB-CD3ζ signaling tail in MSCV-IRES-GFP or MSCV-IRES-RFP vectors. Empty vector was used as a negative control. Mutations to signaling domains were introduced by QuikChange mutagenesis (Agilent) (Supplementary methods). Phoenix-AMPHO cells were transfected and supernatant was collected 2–3 days later.
Transduction of human T cells
Leukocytes were isolated from apheresis rings by density gradient. T cells were isolated using the human Pan T Cell Isolation Kit (Miltenyi) and stimulated with anti-human CD3 and CD28 (eBioscience) in RPMI with IL-2 for 24h prior to transduction on RetroNectin-coated plates (TaKaRa). Viral supernatant from transfected Phoenix-AMPHO cells was used for transduction. GFP+ or RFP+ cells were sorted and expanded in culture with IL-2 for 5–7 days. All experiments were repeated with control and CAR-T cells generated from at least 2 donors.
Inhibitor treatment
CD33 CAR or control T cells were labeled with CellTrace Violet 5 days after activation and treated with 100U/ml IL-2 and small-molecule inhibitors of PI3K (LY294002, 10μM; IC87114, 10μM; Cell signaling)43, AKT (API-2, 1μM, Abcam), mTOR (rapamycin, 50nM; PP242, 1μM, Abcam), or glycolysis (DCA, 10mM, Sigma) every other day for 4 days, then analyzed in triplicate by flow cytometry or used in adoptive immunotherapy experiments.
Flow cytometry
Immunophenotypic analysis was performed with the indicated antibodies (supplementary methods). Non-viable cells were excluded with 7AAD. An LSR Fortessa (BD Biosciences) was used with FlowJo 9.6.6 software (Treestar) for analysis. Flow sorting was performed with a Reflection (iCyt) cytometer (Sony Biotechnology).
Co-culture killing assays
CAR or control T cells were incubated with MOLM-13-CD19 cells in duplicate as indicated and analyzed by flow cytometry at 24–48h. Cell numbers were normalized to TrucountTM beads (BD Biosciences).
Adoptive immunotherapy
All experiments were performed with least 5 mice per group without randomization or blinding. 1×106 MOLM-13-CD19 cells were administered i.v. and 6×106 CAR or control T cells were administered retro-orbitally the same day. For experiments with LY-treated CD33 CAR-T cells, 3×106 treated or untreated CAR T cells were administered reto-orbitally the same day as tumor. Mice were imaged using the Xenogen imaging system (Caliper Life Science). Organs were harvested as indicated after transfer and analyzed by flow cytometry. For co-transfers, 1.5×106 GFP+ CAR and 1.5×106 RFP+ control T cells were mixed and administered with or without tumor cells. To assess persistence of CAR-T cell subsets, 1.5×106 CD8+CCR7+CD45RA+ (TN) and CD8+CCR7−CD45RA− (TEM) CAR-T cells were sorted and transferred individually. Organs were harvested 5 days after transfer and analyzed by flow cytometry.
Bisulfite sequencing of human IFNγ promoter
Naïve CD8+CD45RA+CD45RO−CCR7+CD95− T cells were sorted from 3 separate donor samples and transduced with CD33 CAR or control vector. Genomic DNA was isolated 9 days after activation and subjected to bisulfite sequencing as previously described.21
RNA sequencing
RNA was extracted from CD33 CAR or control T cells generated from 3 separate donors 12 days after activation using TRIzol (Life Technologies). Paired-end sequencing was performed and analyzed by Ingenuity Pathway Analysis (QIAGEN) (Supplementary methods). All data are available at the Gene Expression Omnibus (GEO), accession code GSE93386.
Statistical analysis
All Figures are representative of at least two individual experiments performed on transduced cells from individual donors. Mean ± SEM is reported. Statistical analysis was performed with GraphPad Prism 6 (supplementary methods).
Supplementary Material
Acknowledgments
This research was supported by ALSAC/SJCRH. The authors thank the Howard Hughes Medical Institute for their support of J.H.B., The Assisi Foundation of Memphis, Richard Cross, Grieg Lennon, Parker Ingle, Tammar Williams, and the SJCRH Blood Donor Center.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Srivastava S, Riddell SR. Engineering CAR-T cells: Design concepts. Trends in immunology. 2015 Aug;36(8):494–502. doi: 10.1016/j.it.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013 Jul 25;39(1):49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016 Jun 30;127(26):3312–3320. doi: 10.1182/blood-2016-02-629063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casucci M, Nicolis di Robilant B, Falcone L, Camisa B, Norelli M, Genovese P, et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013 Nov 14;122(20):3461–3472. doi: 10.1182/blood-2013-04-493361. [DOI] [PubMed] [Google Scholar]
- 5.Pizzitola I, Anjos-Afonso F, Rouault-Pierre K, Lassailly F, Tettamanti S, Spinelli O, et al. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia. 2014 Aug;28(8):1596–1605. doi: 10.1038/leu.2014.62. [DOI] [PubMed] [Google Scholar]
- 6.Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJ, et al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia. 2015 Aug;29(8):1637–1647. doi: 10.1038/leu.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Hear C, Heiber JF, Schubert I, Fey G, Geiger TL. Anti-CD33 chimeric antigen receptor targeting of acute myeloid leukemia. Haematologica. 2015 Mar;100(3):336–344. doi: 10.3324/haematol.2014.112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004 Apr;18(4):676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 9.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science translational medicine. 2011 Aug 10;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England journal of medicine. 2013 Apr 18;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014 Oct 16;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011 Nov 3;118(18):4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science translational medicine. 2014 Feb 19;6(224):224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. The Journal of clinical investigation. 2011 May;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Z, Condomines M, van der Stegen SJ, Perna F, Kloss CC, Gunset G, et al. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer cell. 2015 Oct 12;28(4):415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016 Feb;30(2):492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Zhang M, Ramos CA, Durett A, Liu E, Dakhova O, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014 Jun 12;123(24):3750–3759. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawalekar OU, O’Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD, Jr, et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016 Feb 16;44(2):380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nature medicine. 2015 Jun;21(6):581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annual review of immunology. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 21.Abdelsamed HA, Moustaki A, Fan Y, Dogra P, Ghoneim HE, Zebley CC, et al. Human memory CD8 T cell effector potential is epigenetically preserved during in vivo homeostasis. The Journal of experimental medicine. 2017 Jun 05;214(6):1593–1606. doi: 10.1084/jem.20161760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proceedings of the National Academy of Sciences of the United States of America. 2009 Oct 13;106(41):17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011 Feb 10;117(6):1888–1898. doi: 10.1182/blood-2010-10-310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013 Jan 24;121(4):573–584. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 25.Kramer A, Green J, Pollard J, Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014 Feb 15;30(4):523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim EH, Suresh M. Role of PI3K/Akt signaling in memory CD8 T cell differentiation. Frontiers in immunology. 2013;4:20. doi: 10.3389/fimmu.2013.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009 Jul 2;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek A, Choi KY, et al. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. Journal of immunology. 2012 May 1;188(9):4305–4314. doi: 10.4049/jimmunol.1103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nature reviews Immunology. 2012 Nov;12(11):749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nature medicine. 2011 Oct;17(10):1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flynn JK, Gorry PR. Stem memory T cells (TSCM)-their role in cancer and HIV immunotherapies. Clinical & translational immunology. 2014 Jul;3(7):e20. doi: 10.1038/cti.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabatino M, Hu J, Sommariva M, Gautam S, Fellowes V, Hocker JD, et al. Generation of clinical-grade CD19-specific CAR-modified CD8+ memory stem cells for the treatment of human B-cell malignancies. Blood. 2016 Jul 28;128(4):519–528. doi: 10.1182/blood-2015-11-683847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frauwirth KA, Thompson CB. Regulation of T lymphocyte metabolism. Journal of immunology. 2004 Apr 15;172(8):4661–4665. doi: 10.4049/jimmunol.172.8.4661. [DOI] [PubMed] [Google Scholar]
- 34.Pollizzi KN, Powell JD. Regulation of T cells by mTOR: the known knowns and the known unknowns. Trends in immunology. 2015 Jan;36(1):13–20. doi: 10.1016/j.it.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong XS, Matsushita M, Plotkin J, Riviere I, Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2010 Feb;18(2):413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011 Sep 16;146(6):904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kagoya Y, Nakatsugawa M, Yamashita Y, Ochi T, Guo T, Anczurowski M, et al. BET bromodomain inhibition enhances T cell persistence and function in adoptive immunotherapy models. The Journal of clinical investigation. 2016 Sep 01;126(9):3479–3494. doi: 10.1172/JCI86437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapman NM, Chi H. mTOR Links Environmental Signals to T Cell Fate Decisions. Frontiers in immunology. 2014;5:686. doi: 10.3389/fimmu.2014.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011 Dec 23;35(6):871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunological reviews. 2014 Jan;257(1):107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Co MS, Avdalovic NM, Caron PC, Avdalovic MV, Scheinberg DA, Queen C. Chimeric and humanized antibodies with specificity for the CD33 antigen. Journal of immunology. 1992 Feb 15;148(4):1149–1154. [PubMed] [Google Scholar]
- 42.Nicholson IC, Lenton KA, Little DJ, Decorso T, Lee FT, Scott AM, et al. Construction and characterisation of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Molecular immunology. 1997 Nov-Dec;34(16–17):1157–1165. doi: 10.1016/s0161-5890(97)00144-2. [DOI] [PubMed] [Google Scholar]
- 43.Kong D, Yamori T. Advances in development of phosphatidylinositol 3-kinase inhibitors. Current medicinal chemistry. 2009;16(22):2839–2854. doi: 10.2174/092986709788803222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.