Abstract
Background
Candida albicans is a dimorphic fungus to which humans are exposed early in life and by adulthood it is part of the mycobiome of skin and other tissues. Neonatal skin lacks resident memory T (TRM) cells, but in adults the C. albicans skin test is a surrogate for immunocompetence. Young adult mice raised under SPF conditions are naive to C. albicans, and have recently been shown to have an immune system resembling that of neonatal humans.
Objective
We studied the evolution of the adaptive cutaneous immune response to Candida.
Methods
We examined both human skin T cells and the de novo and memory immune responses in a mouse model of C. albicans skin infection.
Results
In mice, the initial IL-17 producing cells after C. albicans infection were dermal γδ T cells, but by day 7 αβ Th17 T effector cells were predominant. By day 30, the majority of C. albicans reactive IL-17 producing T cells were CD4 TRM cells. Intravital microscopy showed that CD4 effector T cells were recruited to the site of primary infection and were highly motile 10 days post infection. Between 30–90 days post infection, these CD4 T cells became increasingly sessile, acquired expression of CD69 and CD103, and localized to the papillary dermis. These established TRM produced IL-17 upon challenge, while motile migratory memory T (TMM) cells did not. TRM rapidly clear an infectious challenge with C. albicans more effectively than re-circulating T cells, though both populations participate. We found that in normal human skin, IL-17 producing CD4+ TRM that responded to C. albicans in an MHC Class II restricted fashion could be readily identified.
Conclusions
These studies demonstrate that C. albicans infection of skin preferentially generates CD4+ IL-17 producing TRM, which mediate durable protective immunity.
Keywords: resident memory T cells, TRM, Candida albicans, IL-17, Th17, CD4+ TRM
INTRODUCTION
Barrier tissues, including skin, have complex and distinct microbiomes and are the sites of entry for most pathogens that infect living organisms.1 After early observations that memory T cells circulated through barrier tissues,2 it was demonstrated that the site (and therefore draining lymph node (LN)) where a memory T cell was generated influences its ability to traffic to that tissue.3 While T cells had been identified in peripheral tissues in murine models of viral infection,4 recent studies in humans indicated that large numbers of T cells appear to reside indefinitely in peripheral tissues.1 For example, twice as many T cells reside in human skin as in blood, and that 50 fold more memory T cells with skin homing markers were present in skin.5 These skin resident T cells were enriched for CD4+ T cells, and they resided primarily in the dermis.5,6 Other human barrier epithelial tissues also contain abundant resident memory T (TRM) cells.7,8 In parallel, a series of experiments in mouse models of viral infection demonstrated the appearance of antigen specific CD8 T cells that entered tissue and persisted long term, mediating protective memory.9,10 However, young mice raised under SPF conditions have few non-recirculating T cells in skin and other tissues in the absence of intentional infection.11 A recent study showed that mice raised in the wild or obtained from pet stores, without the benefit of specific-pathogen free (SPF) housing, had abundant T cells in nearly all peripheral tissues examined.11 This study went on to show that with regard to T cell memory, the adaptive immune systems of SPF raised mice resembled that of neonatal humans, while “dirty” mice showed profiles of T cell memory in blood and tissues that resembled adult humans.12 This makes SPF mice an excellent system for studying the de novo acquisition of T cell memory in peripheral tissues.
In the present study, our goal was to study the generation, maintenance, and behavior of CD4 TRM cells generated by infection of skin by C. albicans, an organism to which most humans have skin resident immunity. C. ablicans is considered part of the skin mycobiome,13 and while it can cause pathogenic infections in patients with germ line mutations of genes involved in IL-17/IL-23 pathways,14–16 it is typically a commensal organism in man. Murine models of Candida infection have demonstrated that protective immunity against infection is mediated in part by Th17 CD4 T cells, but the contribution of TRM has not been studied.17 CD4+ TRM to non-viral pathogens have rarely been studied, and only recently have IFNγ producing CD4 skin TRM to parasitic infection been demonstrated.18 No mouse model of infection has revealed a population of skin Th17 CD4 TRM, although such cells are abundant in human skin.19,20 We set out to determine whether C. albicans infection generated a skin specific population of Th17 CD4 T cells.21 Moreover, we asked if protective immunity to skin infection with C. albicans was mediated by Th17 CD4 T cells, and whether these protective cells were TRM, re-circulating TMM, or a combination of these two cell types. Finally, we compared the phenotype of these murine Th17 TRM generated by C. alibicans infection to those found in normal human skin.6
METHODS
Cutaneous candida infection
We modified the techniques as described previously22 to generate a consistent skin infection with C. albicans. Mice were first anesthetized with a mixture of ketamine and xylazine (100/10 µg/kg body weight), and then the shaved back skin or ear skin was tape-stripped six times with Tegaderm (3M). C. albicans (SC5314) was grown in YPAD medium23 at 30°C until the OD600 reached 1.5–2.0. After washing with sterile PBS, 2×108 C. albicans in 50 µl of sterile PBS on to the shaved back skin or 1×108 C. albicans in 25 µl of sterile PBS was applied on to the ear skin.
In vivo imaging and analysis of T cell migration
Mice were anesthetized with isofluorane (3% for induction, 1–1.5% for maintenance), and placed in an electrically heated tube to maintain body temperature at 37°C during the continuous imaging. The ear was flattened and then stabilized for imaging by adhering the ventral ear skin to a steel platform using double-sided tape (3M). The steel platform was maintained at 37°C with an electronic heating pad. A cover slip was gently placed on the surface of the epidermis of the ear dorsal skin.
In vivo imaging of skin was performed by a custom-built microscope, and images were acquired under the control of software developed in-house.24 Images were acquired with a 60/1.0 NA water immersion objective. GFP and KAEDE-green were excited at 491 nm (Cobolt Dual Calypso), while DsRed and KAEDE-red were excited at 561 nm (Cobolt Jive). The dermis was imaged by second harmonic generation excited by a Ti:sapphire laser (MaiTai, SpectraPhysics) at 840 nm. Each xy-plane was scanned at 500 × 500 µm at a resolution of 1 pixel per µm. Stacks of images were acquired with a z-axis resolution of 2 µm per section. Three-dimensional stacks were repeated at a 1-min interval for 40–60 min to create time-series four-dimensional images. Cell migration spatiotemporally was analyzed through automatic cell tacking combined with manual corrections in Imaris (Biplane). In some cases, to minimize photo-bleaching of fluorescent proteins, only two-dimensional time-series images were taken at an interval of one-minute for 30–60 min at the area of interest. Each mouse was focused on the same depth (50 µm under the surf ace of skin if not indicated) for the two-dimensional time-series images. Cell velocity, displacement, and arrest coefficients (proportion of time cells were moving at less than 2.5 µm/min) were further calculated using the data produced by Imaris or ImageJ (NIH Image). Data were used to plot graphs Prism 4 (Graphpad).
See Supplementary Methods in this article’s Online Repository.
RESULTS
CD4 T cells are abundant in skin after C. albicans infection
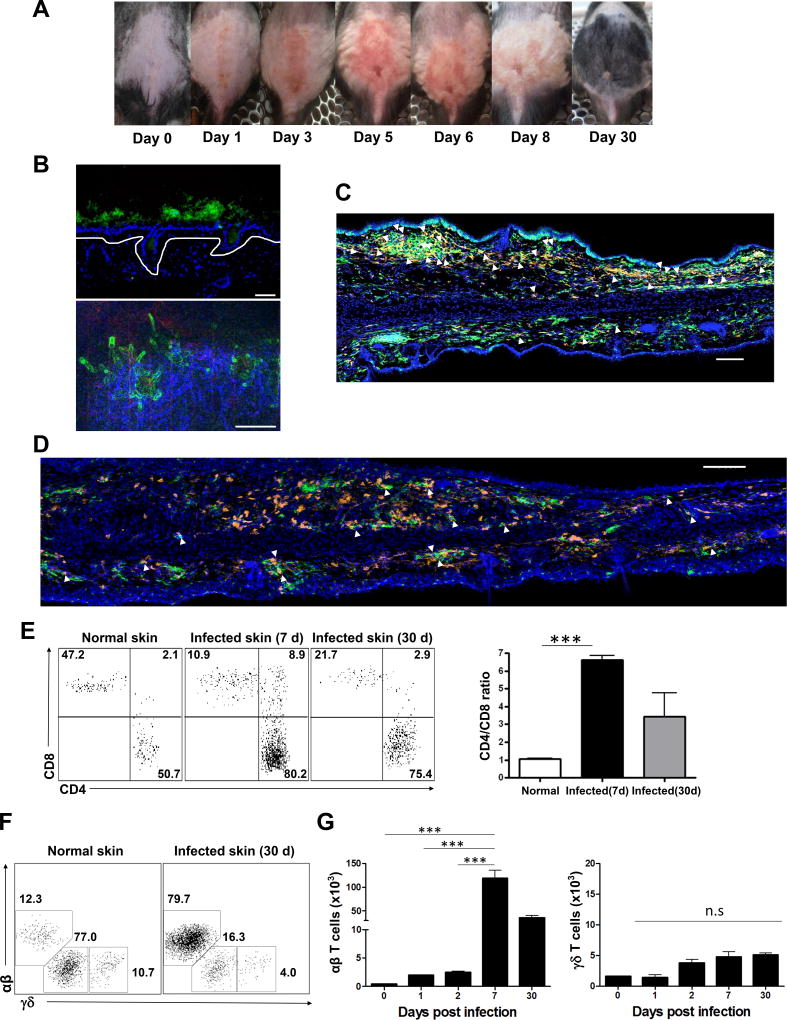
We infected the dorsal skin of wild-type B6 mice with C. albicans (Figure 1A), and created a fungal skin infection which was most florid at day 5 and had resolved completely by day 30. Similar kinetics were observed after infection of ear skin. While microscopic analysis of skin showed yeast forms of C. albicans hours after inoculation, by day 3 invasive hyphal forms of C. albicans were observed, consistent with pathogenic infection (Figure 1B). After C. albicans skin infection, all mice remained objectively healthy and no significant weight loss was observed. Confocal microscopy at day 10 (Figure 1C) and day 30 (Figure 1D) show many CD4 T cells, and many appeared contiguous to MHC Class II+ cells. In this setting, no obvious clustering of CD4 T cells around hair follicles was observed. By day 7, CD4 T cells had accumulated in infected skin (CD4/CD8 ratio 8:1), and remained abundant at day 30 (Figure 1E). Before C. albicans infection, normal skin had abundant IL-17 producing γδ T cells; however, αβ Th17 cells greatly outnumbered γδ T cells by 7 days after C. albicans infection (Figure 1F and 1G).
Figure 1. CD4 T cells are predominant in skin after cutaneous candida infection.
The images of dorsal skin (A) of wild-type B6 mice infected with C. albicans. (B) Yeast forms of GFP-expressing C. albicans outside of epidermis at day 0 (top) and hyphae forms of GFP-expressing C. albicans in the dermis at day 3 after candida infection (bottom, blue, dermis). Scale bars, 30 µm. Confocal microscopy of infected ear skin at (C) day 10 (effector) and (D) day 30 (memory) after cutaneous candida infection. MHCII+ APCs were green, and CD4+ T cells were orange. Scale bars, 100 µm. Arrows indicate CD4+ T cells in contact with MHCII+ APCs. Data are representative of three experiments. (E) Expression of CD4 and CD8 T cells (gated on CD3+CD4+ and CD3+CD8+ cells) and ratios of CD4/CD8 T cells from normal skin, infected dorsal skin (day 7 and day 30). (F) Expression of γδ and αβ T cells (gated on CD3+γ δ+ and CD3+TCRβ+ cells) from normal skin and infected dorsal skin (day 30). (G) The number of γδ and αβ T cells (gated on CD3+γ δ+ and CD3+TCRβ+ cells) from normal skin and infected dorsal skin (day 1, 2, 7, 30). Data are representative of more than five experiments. *** p < 0.001; ns, not significant.
Skin CD4 T cells present after cutaneous C. albicans infection are pathogen-specific
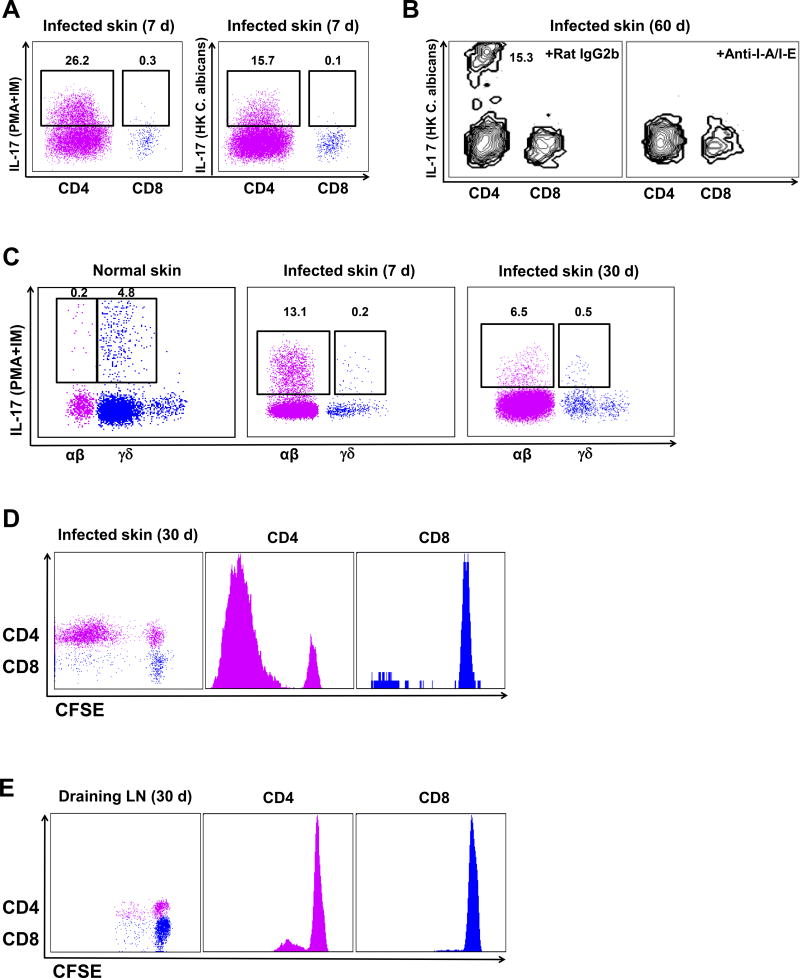
To further validate C. albicans specificity, we performed several ex vivo experiments measuring IL-17 production and proliferation. When incubated with heat-killed (HK) C. albicans, skin CD4 T cells produced significant IL-17 at day 7 (Figure 2A) and at day 60 (Figure 2B), while skin CD8 T cells produced little IL-17 (Figures 2A and 2B). When treated with anti-MHC Class II blocking Abs during incubation with HK C. albicans, IL-17 production was markedly reduced at day 60 (Figure 2B), indicating that antigen presentation was required for IL-17 production of skin CD4 memory T cells after cutaneous C. albicans infection and Th17 response was C. albicans antigen-specific. T cells extracted from skin of uninfected normal mice included both TCR αβ and γδ T cells (Figure 2C), but only γδ cells produced IL-17 when treated with PMA-ionomycin, suggesting that normal recirculating αβ T cells were not Th17 cells (Figure 2C). When incubated with HK C. albicans in vitro, normal skin CD4 and CD8 T cells did not produce IL-17 (Figure S1A and 1B). However, after C. albicans infection, skin αβ T cells were the predominant producers of IL-17 (Figure 2C). The majority of these IL-17 producing cells were CD4+ (Figure S1C). We then examined production of IL-9, 17 and IFNγ. Of these CD4 T cells, more than half produced IL-17, while CD8 T cells did not (Figure S2A). IL-9 was produced by a minority of CD4 T cells, and IFNγ was not detectable. Neither cytokine was produced by skin CD8 T cells (Figure S2C). We found that skin CD4 T cells at day 30 (memory phase) had significant proliferative activity when co-cultured 72 hours with CD11c+ DC’s purified from skin draining lymph nodes of day 3 infected mice, while skin CD8 T cells did not (Figure 2D). This C. albicans-specific IL-17 production and proliferative activity are consistent the generation of C. albicans-specific skin CD4 T cells. CD4 T cells at draining lymph nodes (day 30) showed less proliferation than skin CD4 T cells (Figure 2E), indicating that C. albicans-specific skin CD4 TRM cells are enriched in skin. A population of CD8 T cells that produced IFNγ in response to this stimulus could be identified in lymph node but not skin at day 30 after infection (Figures S2B and S2D).
Figure 2. Skin TRM cells present after cutaneous candida infection are C. albicans antigen-specific.
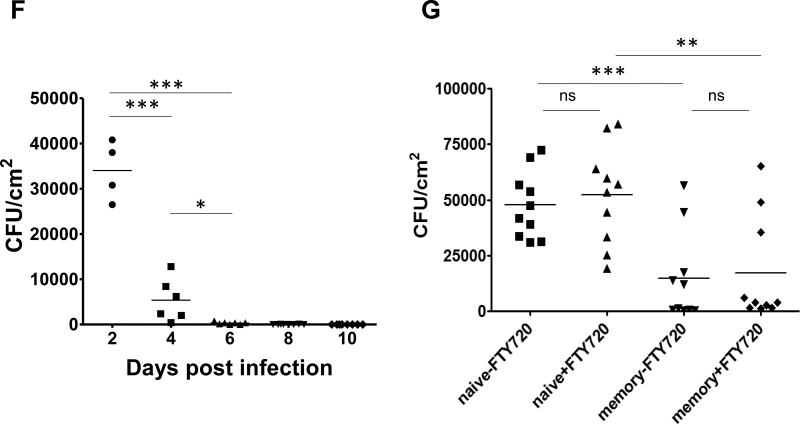
(A) Expression of IL-17 in CD4 and CD8 T cells (gated on CD3+CD4+ and CD3+CD8+ cells) of heat-killed (HK) C. albicans-treated group (right) and PMA-ionomycin-treated group (left) from infected ear skin of wild type B6 mice at day 7 after candida infection. Data are representative of five independent experiments. (B) Expression of IL-17 in CD4 and CD8 T cells (gated on CD3+CD4+ and CD3+CD8+ cells) from infected ear skin at day 60 after candida infection when incubated with HK C. albicans for 6 hours in the presence of 50 µg anti-I-A/I-E or 50 µg isotype Abs (rat IgG2b). (C) IL-17 production of γδ and αβ T cells from normal skin prior to candida infection, infected ear skin at day 7 and 30 after candida infection. Data are representative of three independent experiments. (D) Proliferation of CFSE-labeled skin CD4 (middle) and CD8 (right) T cells (gated on CD45.2+CD3+CD4+ and CD45.2+CD3+CD8+ cells) from infected ear skin at day 30 after candida infection. (E) Proliferation of CFSE-labeled LN CD4 (middle) and CD8 (right) T cells (gated on CD45.2+CD3+CD4+ and CD45.2+CD3+CD8+ cells) from draining LN at day 30 after candida infection. CFSE-labeled skin or LN cells were co-cultured 72 hours with CD45.1+CD11c+ DCs purified from skin draining lymph nodes of day 3 infected mice. Data are representative of three independent experiments. (F) Infected skin samples (n = 4–5) were cleaned with povidone-iodine prior to being harvested at the indicated time after candida infection. C. albicans CFU is expressed as colonies per cm2 of infected skin tissues. (G) Normal wild type B6 mice prior to candida infection (naive) and previously infected mice 45 days after candida infection (memory) were re-infected with C. albicans on the ear skin. Half mice were injected daily with FTY720 (1µg/g) during re-infection at day −1, 0, 1, 2. Two days later, skin samples (n = 10 at each group) were harvested and CFU obtained. * p < 0.05, ** p < 0.01, *** p < 0.001; ns, not significant.
An important functional test of pathogen specificity is protective immunity. Colony counts from biopsies of naive infected skin showed abundant live C. albicans at day 2, with rapid elimination of viable organisms by day 10 (Figure 2F). To test for protective immune memory, mice infected 45 days previously were re-infected with C. albicans and compared to naive mice at day 2 of a new infection measuring colony counts from skin biopsies, in the presence or absence of pretreatment with FTY720 (Figure 2G). More than half of previously infected mice had completely cleared the C. albicans by day 2, while none of the naive mice had cleared the infection at this time point. This protective immunity was unaffected by FTY720 administration, indicating that non-recirculating skin T cells were responsible for this protective effect. To further confirm the protective function of skin TRM cells, we performed parabiosis surgery using infected mice 2 months previously (memory) and naive mice. At 2 months after parabiosis using memory and naive mice, memory parabionts (memory-para) had significant protective immunity comparable to previously infected mice that did not receive parabiosis surgery (memory-only), but naive parabionts (memory-opp) had slightly less protection against re-infection (Figure S3A). To further confirm C. albicans specificity of skin TRM cells, we first used high throughput sequencing (HTS) of the tcrb gene. Of the top 7 unique tcrb clones (identified by unique CDR3 sequences) in activated draining lymph nodes after cutaneous C. albicans infection, five could be found in skin at day 30 (Figure S3B). This was not due to blood contamination, as only two of these clones could be detected at low levels in blood. Thus, skin infection with C. albicans generates a protective population of CD4+ T cells that persist in skin (i.e., TRM), produce IL-17, and protect against subsequent infection.
Characterization of skin TRM cells after cutaneous C. albicans infection
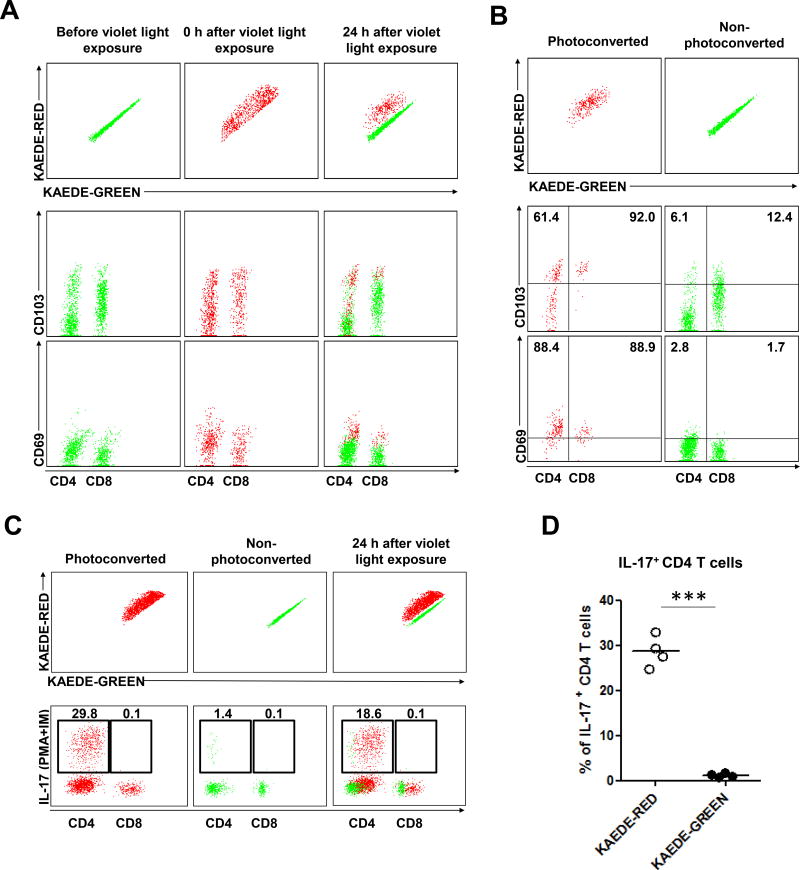
We next studied C. albicans infection induced skin T cells using KAEDE transgenic mice. Baseline KAEDE-green cells are irreversibly photoconverted to KAEDE-red cells after violet light exposure of skin in vivo.25 At day 60 after infection with C. albicans, we found that most of CD8 and some CD4 TRM cells expressed variable to high levels of CD103 (Figure 3A), both before photoconversion (KAEDE-green) and immediately after photoconversion (KAEDE-red, 0h) consistent with a previous study.26 At 24 hours after photoconversion, we observed a new population of KAEDE-green cells in skin (Figure 3A). These were not KAEDE-red cells that had lost their photoconverted pigment, as we and others has shown that these photoconverted cells remain red for >7 days.27 Neither did this reflect incomplete photoconversion of T cells in skin, as immediately after violet light exposure all skin T cells were KAEDE-red. We speculated that this population of KAEDE green-cells represented a population that was migrating from blood to skin, a hypothesis that would be tested by intravital microscopy in subsequent experiments. The stable non-migratory KAEDE-red CD4 and CD8 TRM cells expressed higher levels of CD103 than the newly appearing KAEDE-green cells, but some KAEDE-green cells expressed moderate levels of CD103 (Figures 3A and 3B). KAEDE-red CD4 TRM cells contained populations of CD103+ and CD103−cells (Figure 3B), similar to what is reported in human skin.6 However, KAEDE-red CD4 and CD8 TRM cells expressed CD69, while KAEDE-green cells were largely CD69 negative (Figures 3A and 3B).28 These results indicate at least two populations of CD4 memory T cells co-exist in previously infected skin: non-migratory (KAEDE-red) stable CD69+ CD103+/− TRM cells, and a KAEDE-green population that lacks CD69 and may represent recently emigrated memory T cells. These latter cells resemble migratory memory T (TMM) cells recently described in human skin6 and in mouse skin.21 We examined production of IL-17 at day 30 in KAEDE mice, focusing on KAEDE-red and KAEDE-green T cells 24 hours after photoconversion (Figure 3C). At this time point KAEDE-red cells were more abundant (Figure 3C), and virtually all IL-17 production was confined to the non-migratory KAEDE-red population (Figures 3C and 3D). Neither KAEDE-red nor green CD8 T cells produced significant IL-17. To further assess the abundance of non-migratory KAEDE-red CD4 TRM cells, we performed parabiosis surgery using KAEDE-memory (infected 2 months previously): KAEDE-naive vs KAEDE-naive: KAEDE-naive mice pairs. After 45 days of parabiosis, KAEDE-memory parabionts memory-para) have still significant non-migratory KAEDE-red CD4 TRM cells compared to KAEDE-naive parabionts (naive-para), even at 7 days after violet light exposure (Figure S3C and S3D). Thus, the most abundant IL-17 producing T cells in skin previously infected with C. albicans were non-migratory (KAEDE-red) CD4 TRM that expressed both CD69 and CD103.
Figure 3. Characterization of skin TRM cells after cutaneous C. albicans infection.
(A) Expression of CD103 and CD69 in KAEDE-red and KAEDE-green CD4 and CD8 T cells (gated on CD3+CD4+ and CD3+CD8+ cells) from infected ear skin of KAEDE mice at day 60 after candida infection before (left), immediately (0 h, middle), and 24 hours (24 h, right) after violet light exposure. (B) Expression of CD103 and CD69 in KAEDE-red (left) and KAEDE-green (right) CD4 and CD8 T cells (gated on CD3+CD4+ and CD3+CD8+ cells) from infected ear skin of KAEDE mice at day 60 after candida infection 24 hours after violet light exposure. Data are representative of five independent experiments (A and B). (C) Expression of IL-17 in KAEDE-red (left) and KAEDE-green (middle) CD4 and CD8 T cells (gated on CD3+CD4+ and CD3+CD8+ cells) from infected ear skin (right) of KAEDE mice at day 30 after candida infection 24 hours after violet light exposure. Data are representative of four independent experiments. (D) The percentage of IL-17-producing KAEDE-red and KAEDE-green CD4 T cells from infected ear skin (n = 4) at day 30 after candida infection. The lines indicate the mean. *** p < 0.001.
Heterogeneous migratory properties of cutaneous CD4 TRM cells after C. albicans infection
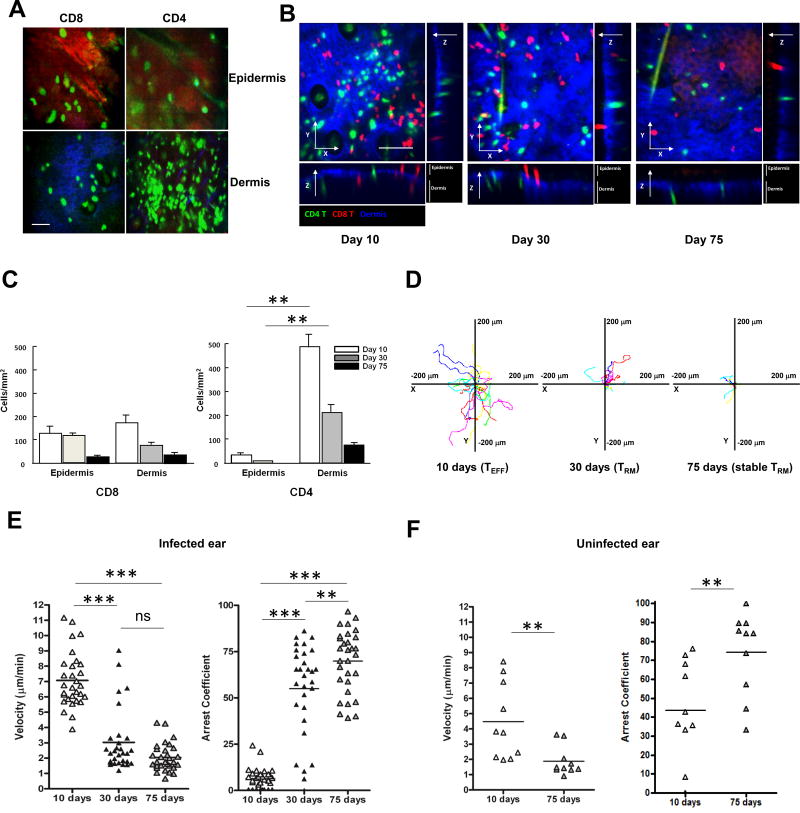
To further study skin infiltrating T cells after C. albicans skin infection, we performed in-vivo imaging studies in the ear skin. GFP+ CD4 T cells (and either DsRed+ or GFP+ CD8 T cells) were adoptively transferred i.v. into RAG1−/− mice (having no mature B and T cells, and in particular no γδ T cells), which were then infected with C. albicans on ear skin. RAG1−/− mice reconstituted by CD4 and/or CD8 T cells showed similar kinetics of candida clearance as wild-type mice while candida clearance was delayed in only CD8-reconstituted RAG1−/− mice (data not shown). This suggests that γδ T cells are not required for clearance of the primary infection. Intravital microscopy was performed initially at days 10, 30, and 75. Though less abundant, infiltrating CD8 T cells favored localization in the epidermis, while CD4 T cells populated the dermis at day 10 (Figures 4A and 4B). This remained the case at day 30 (Figure 4B and Movie S1). The relative locations of CD4 and CD8 cells at different time points are shown in Figure 4C. CD4 T cells always outnumbered CD8 T cells and were always more abundant in dermis and virtually absent in epidermis.
Figure 4. Heterogeneous migratory properties of cutaneous CD4 TRM cells after C. albicans infection.
(A) Intravital microscopy of GFP+ CD8 T cells in the epidermis (top, left) and dermis (bottom, left; blue, dermis), and GFP+ CD4 T cells in the epidermis (top, right) and dermis (bottom, right; blue, dermis) at day 10 (effector phase) after candida infection on the ear skin. Scale bar, 50 µm. Data are representative of more than five independent experiments. (B) Intravital microscopy of skin CD8 (DsRed, red) and CD4 (GFP, green) T cells after candida infection. Maximum intensity projections across x-y dimension, and section of x-z, and y-z dimensions of skin CD8 and CD4 T cells at day 10 (effector), day 30 and 75 (memory) after candida infection. Dermis was defined by the presence of collagen (second harmonic generation, blue). Scale bar, 100 µm. Data are representative of five independent experiments. (C) Quantification of skin CD8 (DsRed, red) and CD4 (GFP, green) T cells in the epidermis and dermis at day 10, 30, and 75 after candida infection. (D) Skin GFP+ CD4 T cell-migration tracks over 20 min in candida-infected dermis, presented as x-y projections (distance, in µm) at day 10, 30, and 75 after candida infection. Data are representative of five independent experiments. Skin GFP+ CD4 T cell mean velocity (left) and arrest coefficients (right) in (E) infected ear and (F) uninfected (contralateral) ear at day 10, 30, and 75 after candida infection. Points represent individual cells and the lines indicate the mean. ** p < 0.01, *** p < 0.001; ns, not significant.
At day 10 after C. albicans infection, we observed that CD4 T cells moved rapidly in the dermis of infected ear (Figures 4D, 4E and Movie S2). By day 30, most CD4 TRM cells moved more slowly, although fast-moving CD4 T cells were still detectable in the dermis of infected skin (Figures 4D, 4E and Movie S3). By day 75 after infection, 80% of dermal CD4 T cells were nearly sedentary (MV 1.69 µm/min; AC > 50) and the remaining 20% of CD4 T cells displayed an intermediate motility phenotype (Figures 4D, 4E and Movie S4). Sedentary CD4 TRM cells (MV 1.66 µm/min; AC > 50) were also observed in uninfected skin at day 75 (Figure 4F), though fewer cells were present.
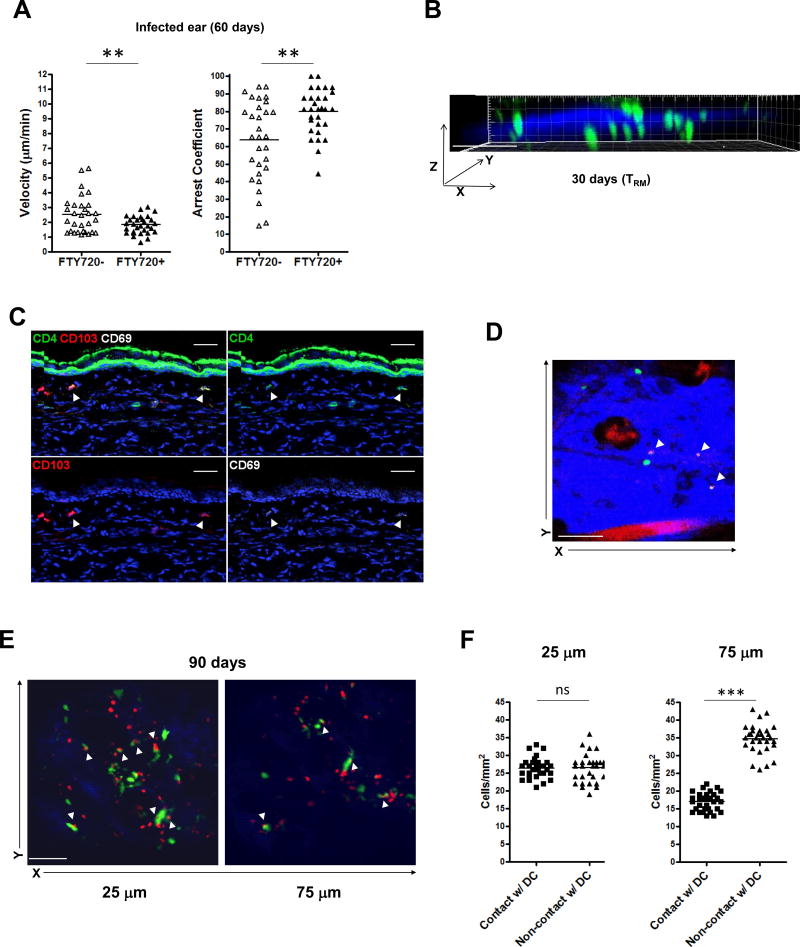
When we pre-treated the mice with FTY720, we found that an even greater proportion of skin-dwelling T cells acquired a sessile phenotype in infected ear at day 60 after C. albicans skin infection Figure 5A), implying that inhibiting the function of skin T cells to sense S1P gradients may affect the velocity of T cells within skin.29,30 Consistently, slow-moving CD4 T cells resided in the superficial papillary dermis and faster moving CD4 T cells were located in the deeper dermis at day 30 (Figure 5B and Movie S5). Even at day 180 after C. albicans infection, non-migratory sedentary CD4 T cells still populated in the superficial papillary dermis, compared to faster moving CD4 memory T cells appearing in the deeper dermis (Movie S6). We also observed that the sessile CD4 T cells located in the papillary dermis at day 30 were CD103+ CD69+ (Figure 5C). Finally, we confirmed through in vivo imaging that CD69-expressing CD4 T cells were sessile compared to migratory non-CD69-expressing CD4 T cells in skin at day 90 after C. albicans infection (Figure 5D and Movie S7).
Figure 5. Distinct migratory properties of cutaneous CD4 TRM cells after C. albicans infection.
(A) Skin GFP+ CD4 T cell mean velocity (left) and arrest coefficients (right) in infected ear from FTY720-treated (one week of daily injection) and non-FTY720-treated mice at day 60 after candida infection. Points represent individual cells and the lines indicate the mean. Data are representative of three independent experiments. (B) GFP+ CD4 T cells (green) in candida-infected dermis, presented as y projection at day 30 after candida infection (dermis, blue). Scale bar, 100 µm. Data are representative of five independent experiments. (C) CD4+ T cells (green; arrows) expressed CD103 (red; arrows) and CD69 (white; arrows) in the upper dermal area of infected ear from RAG1−/− mice adoptively transferred with CD45.1+ CD4 T cells 30 days after candida infection. Scale bar, 30 µm. (D) GFP+ CD4 T cells (green) and CD69+GFP+ CD4 T cells (arrows, in vivo stained with Alexa647 anti-CD69 Ab) in candida-infected dermis at day 90 after candida infection. Imaging depth was 50 µm below the skin surface (dermis, blue). Scale bar, 100 µm. (E) CD11c-YFP+ DCs (green) and DsRed+ CD4 T cells (red) in candida-infected dermis at day 90 after candida infection. Imaging depths were 25 µm (left), 75 µm (right) below the skin surface (dermis, blue). Scale bar, 100 µm. Data are representative of three independent experiments (C–E). (F) The number of DsRed+ CD4 T cells in contact or not in contact with CD11c-YFP+ DCs in infected ear at day 90 after C. albicans infection. Points represent individual cells and the lines indicate the mean. ** p < 0.01, *** p < 0.001; ns, not significant.
To further evaluate how skin CD4 TRM cells interact with DCs which are also enriched in the skin, we performed in-vivo imaging studies using CD11c-YFP+ (on RAG1−/−) mice adoptively transferred with DsRed+ CD4 T cells. At 90 days after C. albicans infection, we observed that CD11c+ DCs were present a higher frequency in the superficial papillary dermis than in the deeper dermis (Movie S8), but did not appear to form clusters. We found that skin CD4 T cells making most frequent contact with CD11c+ DCs in the papillary dermis were very slow moving (Figures 5E, 5F and Movie S9). Deep reticular dermal CD4 T cells showed significantly fewer contacts with CD11c+ DCs, and these T cells were fast movers (Figures 5E, 5F and Movie S10).
Two distinct populations of cutaneous CD4 memory T cells with different migratory properties after skin C. albicans infection
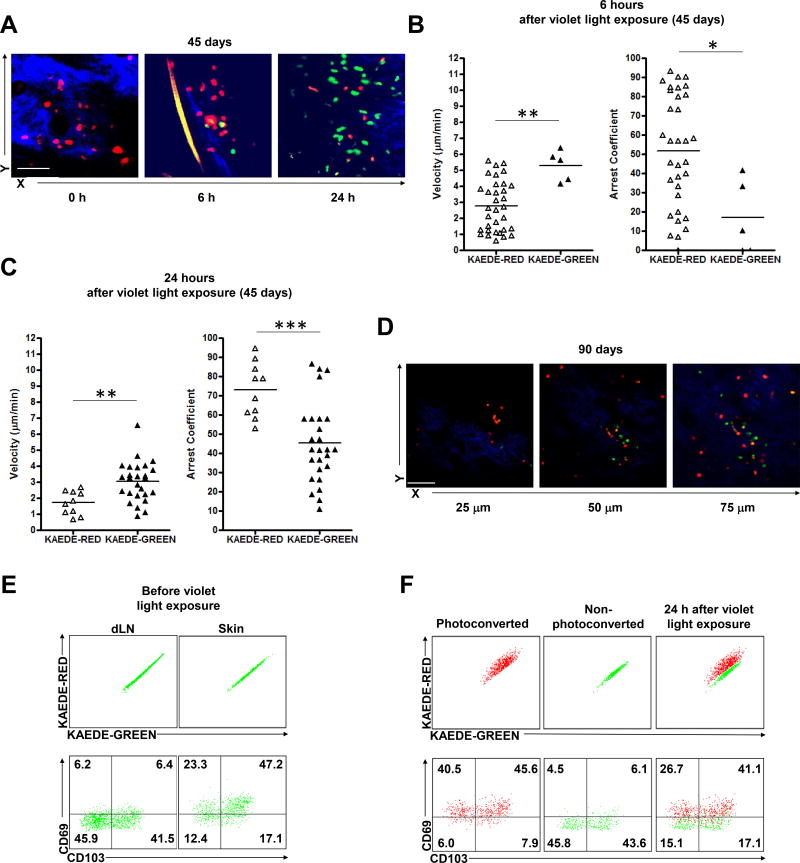
To further characterize the migratory patterns of CD4 TRM cells, we adoptively transferred with KAEDE CD4 T cells in the RAG1−/− mice prior to C. albicans infection. To test the hypothesis that KAEDE-green CD4 T cells migrated into skin from blood, we first examined skin at day 45 after infection, immediately after violet light exposure (0 h). At this time, 100% of the CD4 T cells were KAEDE-red (Figure 6A). These included both sessile and faster moving CD4 T cells in the infected ear skin (Movie S11). They had similar migratory patterns compared to non-photoconverted KAEDE-green CD4 T cells before violet light exposure (data not shown), validating that violet light does not affect cell function or mobility consistent with a previous study.25 At 6 hours after violet light exposure, we could identify a small population of rapidly moving KAEDE-green CD4 T cells appearing in the photoconverted area, consistent with recent entry into skin. These cells were rapid movers (MV 5.30 µm/min; AC < 50) and appeared in the deep dermis, while the persisting upper dermal KAEDE-red CD4 T cells continued to move slowly (Figures 6A, 6B and Movie S12). At 24 hours after violet light exposure, both KAEDE-red and KAEDE-green CD4 T cells could identified: KAEDE-red CD4 T cells populated in the superficial papillary dermis and moved slowly (MV 1.72 µm/min; AC > 50) while KAEDE-green CD4 T cells populated in the deep reticular dermis and were rapid movers (MV 3.05 µm/min) (Figures 6A, 6C and Movie S13). These data are consistent with CD4 T cells (KAEDE-green) emigrating into previously infected skin in the 24 hour period after photoconversion, moving rapidly in the deep dermis, while slow moving KAEDE-red CD4 T cells remained sessile in the papillary dermis (putative TRM). This emigration was not driven by inflammation, which was absent at day 45 after infection.
Figure 6. Cutaneous CD4 memory T cells with distinct migratory and heterogeneous functional properties after C. albicans.
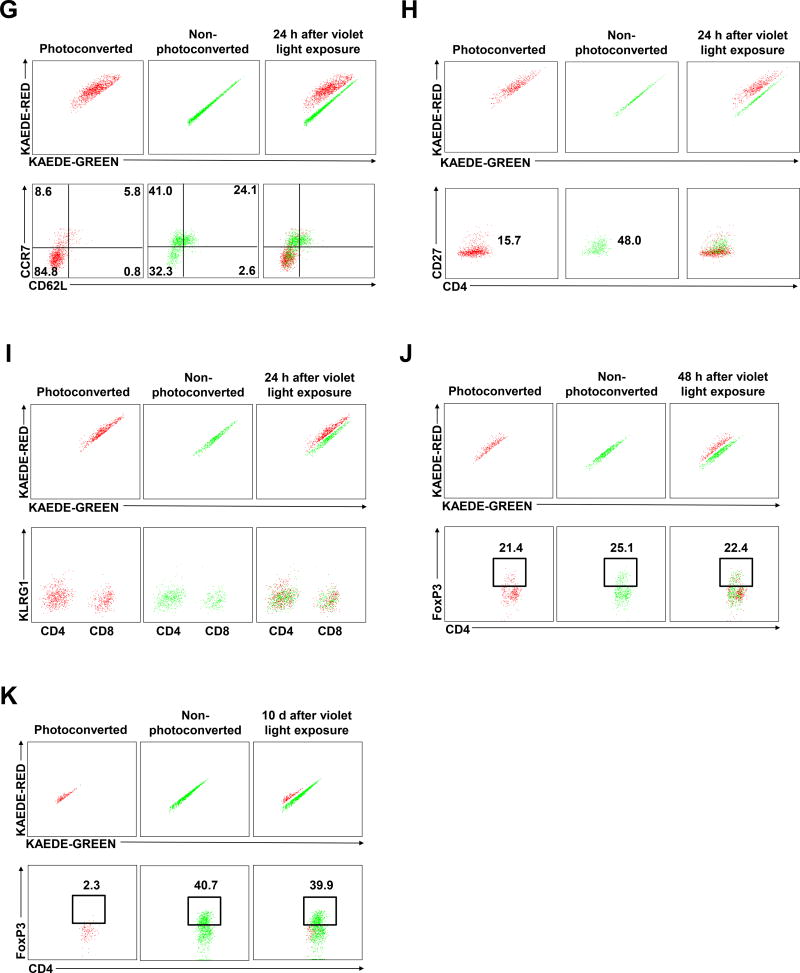
infection (A) KAEDE-red (red) and KAEDE-green (green) CD4 T cells in candida-infected dermis at day 45 after candida infection immediately (0 h, left), 6 hours (6 h, middle), and 24 hours (24 h, right) after violet light exposure. Imaging depth was 50 µm below the skin surface (dermis, blue). Scale bar, 100 µm. Skin KAEDE-red and KAEDE-green CD4 T cell mean velocity (left) and arrest coefficients (right) at (B) 6 hours and (C) 24 hours after violet light exposure at day 45 after candida infection on the ear skin. Points represent individual cells and the lines indicate the mean. Data are representative of five independent experiments (A–C). (D) KAEDE-red (red) and KAEDE-green (green) CD4 T cells in candida-infected dermis at day 90 after candida infection 24 hours after violet light exposure. Imaging depths were 25 µm (left), 50 µm (middle), 75 µm (right) below the skin surface (dermis, blue). Scale bar, 100 µm. Data are representative of three independent experiments. (E) Expression of CD103 and CD69 in KAEDE-green CD4 T cells in RAG1−/− mice (gated on CD3+CD4+ cells) from draining LN (left) and infected ear skin (right) at day 60 after candida infection before violet light exposure. (F) Expression of CD103 and CD69 in KAEDE-red (left) and KAEDE-green (middle) CD4 T cells in RAG1−/− mice (gated on CD3+CD4+ cells) from infected ear skin (right) at day 60 after candida infection 24 hours after violet light exposure. Data are representative of five experiments (E and F). Expression of CCR7, CD62L (G), and CD27 (H) in KAEDE-red (left) and KAEDE-green (middle) CD4 T cells (gated on CD3+CD4+ cells) from infected ear skin (right) at day 60 after candida infection 24 hours after violet light exposure. (I) Expression of KLRG1 in KAEDE-red (left) and KAEDE-green (middle) CD4 and CD8 T cells (gated on CD3+CD4+ and CD3+CD8+ cells) from infected ear skin (right) at day 60 after candida infection 24 hours after violet light exposure. Data are representative of five independent experiments (G–I). Expression of FoxP3 in KAEDE-red (left) and KAEDE-green (middle) CD4 T cells (gated on CD3+CD4+ cells) from infected ear skin (right) at day 60 after candida infection 48 hours (J) and 10 days (K) after violet light exposure. Data are representative of three independent experiments (J and K). * p < 0.05, ** p < 0.01, *** p < 0.001.
To determine if similar phenomenon could be observed at different time points after infection, we examined skin at day 10 and day 90. At day 10 after infection (during the effector phase), KAEDE-red and KAEDE-green CD4 T cells both mixed quickly and moved very rapidly in the photoconverted area 24 hours after violet light exposure (Figure S3E and Movie S14). However, at day 90 after C. albicans infection, stable KAEDE-red CD4 TRM cells were present in the superficial dermis (MV 1.58 µm/min; AC > 50), while KAEDE-green CD4 T cells (MV 2.69 µm/min) appeared in the deep dermal dermis at 24 hours after violet light exposure (Figure 6D and Figures S4A–C), similar to the day 45 post infection time point. This suggests that the emigration of CD4+ T cells into previously infected skin is continuous.
Heterogeneous CD4 migratory T cells rapidly and constitutively infiltrate skin and co-exist with populations of TRM cells
We have previously shown that continuous treatment with FTY 720 blocks emigration of T cells from lymph node to blood, and subsequently to skin. When we treated the mice with FTY720, the appearance of rapidly moving KAEDE-green CD4 T cells in skin 6 hours after violet light exposure was completely eliminated (Movies S15 and S16, 6 h), and the appearance of KAEDE-green CD4 T cells in skin at 24 hours after photoconversion was greatly reduced (Movies S17 and S18, 24 h). These data are consistent with the idea that the KAEDE-green T cells appearing at 6 hours and 24 hours post photoconversion had actively emigrated into skin from blood, presumably from a re-circulating pool of memory CD4 T cells. Consistent with our previous data, we also found that interference with S1P1 via FTY720 treatment reduced the velocity of KAEDE-red CD4 T cells within skin immediately after violet light exposure (Movies S19 and S20, 0 h).
We also evaluated CD103 and CD69, putative TRM markers, in this in vivo imaging model. Before violet light exposure, skin KAEDE-green CD4 T cells expressed higher levels of CD69 than KAEDE-green CD4 T cells in the draining lymph nodes, and more KAEDE-green CD4 T cells in the skin expressed CD103 than in the draining lymph nodes (Figure 6E). At 24 hours after violet light exposure, sessile photoconverted KAEDE-red CD4 TRM cells uniformly expressed CD69, while recently migrated KAEDE-green CD4 TRM cells did not express CD69, consistent with our previous results (Figure 6F). These studies showed that two populations of CD4 memory T cells co-existed in previously infected skin: slow-moving papillary dermal CD69+ CD103+ TRM cells that are sessile, and faster moving CD4 T cells that enter dermis from blood and at least initially lack CD69 and CD103. The appearance of a few of these CD4 T cells at 6 hours, and many more at 24 hours, speaks to the rapid kinetics of the process.
These migratory CD4 T cells expressed CCR7, CD27, were heterogeneous for L-selectin, and were negative for KLRG-1 (Figure 6G–I). This phenotypic profile suggests that they are a combination of skin homing central memory T (TCM) cells (CCR7+, CD62L+) and the recently described “migratory memory T” (TMM) cells6 (CCR7+, CD62L-), rather than effector memory T (TEM) cells which lack CCR7/CD62 co-expression and express KLRG-1. In contrast, Th17 CD4 TRM cells in the same field are negative for all of these markers (Figure 6G–I and S5A, S5B). Migratory CD4 T cells also expressed E-selectin ligand, but not α4β7 (Figure S5C and S5D). They produced IL-2 and TNF-α (Figure S5E and S5F). Later in the time course (48 hours and 10 days), CD4 FoxP3+ regulatory T (Treg) cells also infiltrated the skin and accumulated as a major population of TMM cells coexisting with Th17 CD4 TRM cells (Figure 6J, 6K and S5G). These results suggest that heterogeneous populations of CD4 T cells constitutively migrate into skin and may modify the immunosurveillance provided by TRM.
C. albicans-specific Th17 cells are abundant in normal human skin
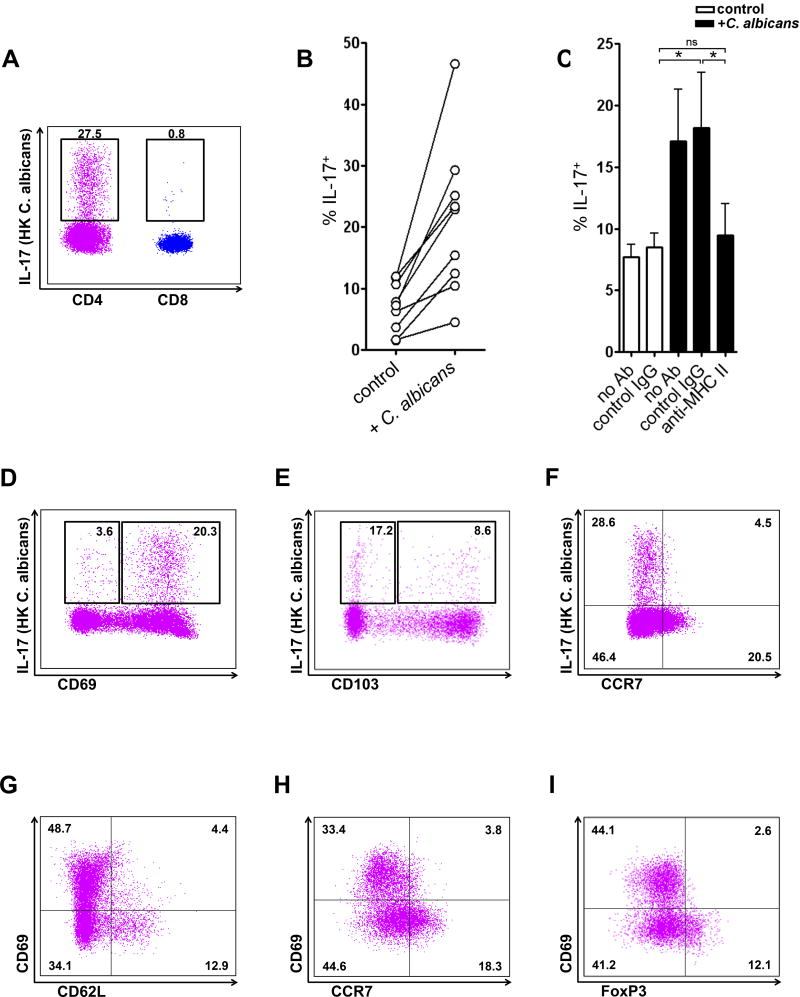
Next, we demonstrated that normal human skin CD4 T cells also produced significant IL-17, while skin CD8 T cells produced little IL-17 when incubated with HK C. albicans (Figure 7A). When cultured with HK C. albicans, skin CD4 T cells increased IL-17 production in 9/9 subjects (Figure 7B), and this increase was MHC Class II dependent (Figure 7C). CD69+ CD4 TRM cells produced significant IL-17, while CD69− CD4 T cells produced less IL-17, which was very consistent with our mouse data (Figure 7D). IL-17 production of CD103+ and CD103− CD4 T cells was, however, not significantly different (Figure 7E). Skin Th17 cells were CCR7 negative (Figure 7F). Consistent with our mouse data, we also found that CD69 negative CD4 T cells expressed CD62L and CCR7 (Figure 7G and 7H) and a significant population of FoxP3+ Treg cells was CD69 negative (Figure 7I), thus suggesting that these CD69− migratory CD4 T cells could enter and exit human skin at steady state.
Figure 7. C. albicans-specific Th17 cells are abundant in normal human skin.
(A) Expression of IL-17 in CD4 and CD8 T cells (gated on CD3+CD4+ and CD3+CD8+ cells) from normal human skin when treated with HK C. albicans. Data are representative of five independent experiments. (B) Expression of IL-17 in CD4 T cells from normal human skin when cultured in the presence (+ C. albicans) or absence (control) of HK C. albicans. (C) C. albicans mediated increased IL-17 production was dependent on MHC class II. Neutralizing antibodies to MHC II (anti-MHC II) blocked increases in IL-17 production in C. albicans-treated cultures (black bars). The mean and SEM of 5 donors are shown. *p < 0.05; ns, not significant. (D) Expression of IL-17 in CD69+ and CD69− CD4 T cells (gated on CD3+CD4+ cells) from normal human skin. (E) Expression of IL-17 in CD103+ and CD103− CD4 T cells (gated on CD3+CD4+ cells) from normal human skin. (F) Expression of IL-17 in CCR7+ and CCR7− CD4 T cells (gated on CD3+CD4+ cells) from normal human skin. (G) Expression of CD62L in CD69+ and CD69− CD4 T cells (gated on CD3+CD4+ cells) from normal human skin. (H) Expression of CCR7 in CD69+ and CD69− CD4 T cells (gated on CD3+CD4+ cells) from normal human skin. (I) Expression of FoxP3 in CD69+ and CD69− CD4 T cells (gated on CD3+CD4+ cells) from normal human skin. Data are representative of three independent experiments (D–I). * p < 0.05; ns, not significant.
DISCUSSION
The recent demonstration that adult human skin contains both CD4 TRM and as well as two phenotypically distinct CD4 T cells that migrate out of skin helps to clarify the decades-old observation of MacKay and colleagues that afferent lymph draining skin contains abundant CD4 memory T cells.2,6 These data suggest that in human skin at steady state, there is a heterogeneity of CD4 memory T cells with regard to migratory properties. The present study was designed to study the process by which CD4+ skin TRM and other CD4 memory T cells present in skin are generated, and how they behave both immediately and remotely after skin infection with a ubiquitous fungal pathogen. We chose C. albicans in part because most humans have skin resident immunity to this pathogen mediated by CD4+ memory T cells.19,20,31 In a recently developed murine model, skin infection was shown to generate Th17 CD4+ T cells in draining lymph nodes, a process dependent upon antigen presentation by Langerhans cells.22 We used a modification of this model to study the putative generation of skin dwelling T cells after C. albicans infection, whether TRM or migratory. In this model, we demonstrated pathogenic C. albicans hyphal invasion of dermis, and observed that the protective immune response evolved rapidly, such that by day 10 after infection, no viable C. albicans could be isolated from previously infected skin. Infected lesions were clinically normal in appearance by 14 days. The T cell infiltrate appearing in infected skin was predominantly CD4+, and while less abundant than at day 10, was still obvious at days 30 and later.
Murine skin is distinct from human skin in that two abundant populations of γδ T cells exist—a sessile epidermal population with a monomorphic T cell receptor, and a more heterogeneous dermal population that can produce abundant IL-17.32–34 We showed that prior to infection, IL-17 producing cells in murine skin are exclusively γδ T cells. Indeed, γδ T cells are critical to clearance of C. albicans after a primary infection. At 30 days after C. albicans infection, however, CD4 αβ T cells become the predominant producers of IL-17.17,35 These data are consistent with the idea that after infection of murine skin, resident γδ populations become replaced by resident αβ T cell populations. The replacement of epidermal γδ DETC by αβ CD8 T cells was demonstrated after HSV infection. An analogous replacement of dermal γδ T cells by αβ CD4 resident T cells after C. albicans infection may be a general phenomenon of murine peripheral tissue immunology. The skin dwelling population of CD4 T cells generated after C. albicans infection conferred protective immunity to re-infection even in the absence of T cell recruitment from blood, reminiscent of the protective immunity conferred by CD8 TRM after skin viral infection.9,37
We used intravital microscopy experiments of previously infected skin to study the in situ motility of the CD4 T cells recruited after C. albicans infection. At day 10, a time point at which no viable C. albicans could be isolated and inflammation was subsiding, CD4 T cells moved very rapidly within skin. By day 30, their movement was much slower, and by day 75 after infection, the majority of T cells were nearly sessile. The sessile population had the motility properties of TRM, and was largely confined to papillary dermis, while faster moving T cells were present in deep dermis. In these intravital microscopy experiments, we also compared CD4 and CD8 T cells after C. albicans infection. CD8 T cells were more abundant in the epidermis at days 10 and 30, but persisted in both epidermis and dermis even 75 days after infection. CD8 skin dwelling T cells produced little to no IL-17. CD4 T cells were essentially absent from epidermis, and were most abundant in dermis at day 10, though they were still numerous at day 75. Virtually of the IL-17 production was confined to CD4 T cells. In addition, populations of CD4 TRM in papillary dermis that co-expressed CD69 and CD103 could be identified in situ.
Our initial experiments in KAEDE mice infected with C. albicans are the first to suggest that CD4 T cells in murine skin are heterogeneous, with an apparent TRM population of CD4+CD69+ T cells that produced IL-17 and showed heterogeneous CD103 expression. Another population of CD4 T cells was identified at 24 hours after photoconversion; this population made little IL-17, and expressed less CD103 and virtually no CD69. That they were absent immediately after photoconversion, but present at 24 hours after photoconversion, suggests that these KAEDE-green cells had recently entered skin from blood. This hypothesis was validated by intravital microscopy experiments in previously C. albicans infected mice in which KAEDE cells had been adoptively transferred. While all skin dwelling T cells were photoconverted at time zero, within 6 hours rapidly moving KAEDE-green T cells could be seen in the deep dermis (but not the papillary dermis), and their number increased by 24 hours. The KAEDE-red and green populations at this point differed not only in terms of velocity and location within the dermis, but also by expression of CD69 (exclusively on KAEDE-red cells). Interestingly, both populations expressed CD103, indicating that this marker could not be used to distinguish long term residents from recent emigrants. The appearance of the KAEDE-green cells in previously photoconverted skin could be almost completely blocked by FTY720, consistent with the idea that they had just entered the skin from blood. These rapidly migrating T cells are reminiscent of those shown by Collins et al, which were in virtual equilibrium with blood.21 Thus, our data are consistent with a pathogen specific population of Th17 CD4 TRM that reside long term in the papillary dermis after C. albicans infection, and mediate protective memory responses. At least one other population of CD4 T cells can be isolated from previously infected skin at the same time—these are faster moving CD4 T cells that appear to rapidly enter the skin but are largely confined to the deep dermis and are not Th17 CD4 T cells. Our experiments do not allow us to demonstrate their exit from skin, but our data in combination with that of Collins et al suggest that these cells continuously re-circulate between skin and blood.
Studies in both human and murine skin suggest that TRM accumulate in skin as a function of number of infectious and inflammatory events over time.1,38 Neonatal foreskin contains virtually no T cells, while adult human skin contains abundant T cells.6 In young mice housed in SPF facilities and not iatrogenically infected on skin, the accumulation of non re-circulating TRM, whether epidermal CD8 or dermal CD4, is likely to occur slowly and indeed may not be evident in mice that are 8–12 weeks old. One human year is equivalent to roughly 14 mouse days,39 so that even allowing for discontinuous maturation, the skin of such young mice may be analogous to that of juvenile rather than adult humans, in addition to the differences linked to housing in SPF facilities. Jiang et al have shown that such mice contain significant dermal γδ T cells and in parabiosis experiments, these γδ T cells showed limited but measurable recirculation when compared to αβ T cells in the same mice, which showed more rapid recirculation.40 We speculate that these γδ T cells in murine dermis are gradually replaced by αβ TRM over time in mice raised in the wild, where skin trauma and infections are certainly much more frequent than in SPF facilities.
In summary, we have demonstrated the generation of long lived protective Th17 CD4 TRM appearing after a skin C. albicans infection. These cells localize to the papillary dermis in both interfollicular and follicular regions, do not appear to cluster with DC, are nearly sessile by intravital microscopy, and express CD69. Their expression of CD103 is variable. We have also shown that these TRM co-exist in skin with a population of CD4 memory T cells that rapidly re-circulate, reminiscent of the T cells described by Collins et al that are in equilibrium with the circulation.21 These latter T cells are not specific for the pathogen that generated the TRM, and in our case did not produce IL-17, a protective cytokine for skin C. albicans infection. Whether these Th17 CD4 TRM have the same dependence on exogenous free fatty acids, as was shown for CD8 TRM generated by viral infection, is presently unknown.41 This heterogeneity of memory T cells dwelling in previously infected skin is very consistent with observations in human skin by Watanabe et al, who described TRM coexisting with recirculating populations of memory T cells.6 Whether these findings apply to other barrier tissues such as lung and GI tract is unknown, but clearly pathogen specific CD4 TRM that mediate protective immunity to non-viral pathogens can be demonstrated in skin.
Supplementary Material
Key Messages.
Mucocutaneous candidiasis is associated with a number of rare genetic diseases that all share one feature—dysregulation or inhibition of the IL-23/IL-17 pathway. This data show that TCRαβ Th17 TRM cells mediate protective immunity to Candida albicans under normal conditions.
Acknowledgments
Funding Sources
This work was supported by NIH grants R01AI041707 (T.S.K.), R01AI127654 (T.S.K.), TR01AI097128 (T.S.K. and R.A.C.) and R01AR063962 (R.A.C.). C.O.P. was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C1324, HI14C1799).
Abbreviation list
- APC
antigen-presenting cell
- C. albicans
Candida albicans
- DC
dendritic cell
- HK
heat-killed
- HTS
high throughput sequencing
- IV
intravenous
- LN
lymph node
- SPF
specific-pathogen free
- TCR
T cell receptor
- Treg
regulatory T
- TCM
central memory T cell
- TEM
effector memory T cell
- TRM
resident memory T cell
- TMM
migratory memory T cell.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared no conflict of interest.
References
- 1.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med. 2015;21:688–97. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–17. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–22. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–7. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 5.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–9. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med. 2015;7:279ra39. doi: 10.1126/scitranslmed.3010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thome JJ, Bickham KL, Ohmura Y, Kubota M, Matsuoka N, Gordon C, et al. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med. 2016;22:72–7. doi: 10.1038/nm.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thome JJ, Farber DL. Emerging concepts in tissue-resident T cells: lessons from humans. Trends Immunol. 2015;36:428–35. doi: 10.1016/j.it.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–31. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–30. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 11.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–6. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reese TA, Bi K, Kambal A, Filali-Mouhim A, Beura LK, Burger MC, et al. Sequential Infection with Common Pathogens Promotes Human-like Immune Gene Expression and Altered Vaccine Response. Cell Host Microbe. 2016;19:713–9. doi: 10.1016/j.chom.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagunes L, Rello J. Invasive candidiasis: from mycobiome to infection, therapy, and prevention. Eur J Clin Microbiol Infect Dis. 2016;35:1221–6. doi: 10.1007/s10096-016-2658-0. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, van de Veerdonk FL, Netea MG. Basic Genetics and Immunology of Candida Infections. Infect Dis Clin North Am. 2016;30:85–102. doi: 10.1016/j.idc.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O, Casanova JL, et al. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr. 2013;25:736–47. doi: 10.1097/MOP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soltesz B, Toth B, Sarkadi AK, Erdos M, Marodi L. The Evolving View of IL-17-Mediated Immunity in Defense Against Mucocutaneous Candidiasis in Humans. Int Rev Immunol. 2015;34:348–63. doi: 10.3109/08830185.2015.1049345. [DOI] [PubMed] [Google Scholar]
- 17.Kashem SW, Kaplan DH. Skin Immunity to Candida albicans. Trends Immunol. 2016;37:440–50. doi: 10.1016/j.it.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med. 2015;212:1405–14. doi: 10.1084/jem.20142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlapbach C, Gehad A, Yang C, Watanabe R, Guenova E, Teague JE, et al. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci Transl Med. 2014;6:219ra8. doi: 10.1126/scitranslmed.3007828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–84. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins N, Jiang X, Zaid A, Macleod BL, Li J, Park CO, et al. Skin CD4(+) memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat Commun. 2016;7:11514. doi: 10.1038/ncomms11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–72. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 24.Kim P, Puoris'haag M, Cote D, Lin CP, Yun SH. In vivo confocal and multiphoton microendoscopy. J Biomed Opt. 2008;13:010501. doi: 10.1117/1.2839043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomura M, Honda T, Tanizaki H, Otsuka A, Egawa G, Tokura Y, et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J Clin Invest. 2010;120:883–93. doi: 10.1172/JCI40926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346:93–8. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomura M, Yoshida N, Tanaka J, Karasawa S, Miwa Y, Miyawaki A, et al. Monitoring cellular movement in vivo with photoconvertible fluorescence protein "Kaede" transgenic mice. Proc Natl Acad Sci U S A. 2008;105:10871–6. doi: 10.1073/pnas.0802278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol. 2013;190:970–6. doi: 10.4049/jimmunol.1202805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, et al. Cutting Edge: CD69 Interference with Sphingosine-1-Phosphate Receptor Function Regulates Peripheral T Cell Retention. J Immunol. 2015 doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 30.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14:1285–93. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moesgaard F, Lykkegaard Nielsen M, Norgaard Larsen P, Christophersen S, Mosbech H. Cell-mediated immunity assessed by skin testing (Multitest). I. Normal values in healthy Danish adults. Allergy. 1987;42:591–6. doi: 10.1111/j.1398-9995.1987.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 32.Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186:6091–5. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, et al. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med. 2011;208:505–18. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartwig T, Pantelyushin S, Croxford AL, Kulig P, Becher B. Dermal IL-17-producing gammadelta T cells establish long-lived memory in the skin. Eur J Immunol. 2015;45:3022–33. doi: 10.1002/eji.201545883. [DOI] [PubMed] [Google Scholar]
- 35.Kashem SW, Igyarto BZ, Gerami-Nejad M, Kumamoto Y, Mohammed J, Jarrett E, et al. Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation. Immunity. 2015;42:356–66. doi: 10.1016/j.immuni.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaid A, Mackay LK, Rahimpour A, Braun A, Veldhoen M, Carbone FR, et al. Persistence of skin-resident memory T cells within an epidermal niche. Proc Natl Acad Sci U S A. 2014;111:5307–12. doi: 10.1073/pnas.1322292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2010;16:224–7. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark RA. Resident memory T cells in human health and disease. Sci Transl Med. 2015;7:269rv1. doi: 10.1126/scitranslmed.3010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quinn R. Comparing rat's to human's age: how old is my rat in people years? Nutrition. 2005;21:775–7. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Jiang X, Park CO, Geddes Sweeney J, Yoo MJ, Gaide O, Kupper TS. Dermal gammadelta T Cells Do Not Freely Re-Circulate Out of Skin and Produce IL-17 to Promote Neutrophil Infiltration during Primary Contact Hypersensitivity. PLoS One. 2017;12:e0169397. doi: 10.1371/journal.pone.0169397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan Y, Tian T, Park CO, Lofftus SY, Mei S, Liu X, et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature. 2017;543:252–6. doi: 10.1038/nature21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.