Abstract
Stress and anxiety are states which sportsmen are continuously exposed to. Our study aimed to evaluate neuroelectrical peripheral and central nervous system responses of sportsmen (SPR) and sedentary individuals (SED) during concentration grid test (CGT) employed under time pressure. Forty three SPR and 33 SED participated in the study. Neuroelectrical responses were simultaneously obtained during baseline and CGT. All responses were observed to increase under stress in both SED and SPR. The SPR’s stress related peripheral responses were lower than SED’s. When central values were evaluated a stress related increase according to baseline was observed in all frequency powers in all of the participants. Statistical comparison of increase rates revealed a significantly greater increase in beta in SED compared to SPR. Beta has been associated to alertness and cortical arousal. As SED exhibit greater beta increase under stress compared to SPR their state of cortical arousal and alertness may be interpreted to be higher than SPR. However the SPR’s weak increase in beta and their lower peripheral responses taken together may imply that they are better in stress management. In fact according to their performance scores the SPR’s higher level of performance under stress compared to SED shows that they are better at maintaining and focusing their attention under stress than SED.
Keywords: Sportsmen, EEG spectral power, EDA, Concentration grid test, Cognitive stress
Introduction
Stress and anxiety are states which sportsmen are continuously exposed to. While being conditioned to win during a sports event is a source of stress in itself, there are many factors which increase stress such as competition, preservation of previous success and the effect of audience. The same stressor elicits different stress responses in different individuals (Jena 2015). In this study, as sportsmen are more exposed to the above mentioned stress generating factors than sedentary individuals, their psychophysiological responses to stress were predicted to differ from those of sedentary individuals.
Kaufman et al. (1996) list the most prominent stress factors as tests and exams, time pressure, completing a task by deadline and heavy work load. Besides psychophysiological effects such as increasing focused attention, wakefulness and vigilance, stress also elicits physiological effects such as alterations in feeding behavior, inhibition of appetite and reproductive functions. Due to increasing heart rate, blood pressure and respiration rate, metabolic energy is channeled to the necessary body regions. The brain’s electrical activity, blood pressure, heart rate (HR), Galvanic skin response, etc. can be quantitatively measured during stress. These physiological and psychophysiological variables can be observed non-invasively (Kurniawan et al. 2013). Activated by stress, the sympathetic nervous system affects the eccrine sweat glands in the periphery. This activity is called the Galvanic skin response (GSR) or electrodermal activity (EDA). It is obtained by measuring the resistance or change in conductivity between two electrodes placed on the surface of the skin. Villarejo et al. (2012) have shown that states of strain and relaxation are 91% distinguishable via the EDA method.
Electroencephalogram (EEG) is electrical brain activity which can be recorded via electrodes placed on the scalp. Due to its high time resolution, ease of use and low installation costs, EEG is the most commonly used non-invasive brain imaging technique. EEG enables the investigation of changes in cognitive activity on a scale of milliseconds. While processing information neurons generate electrical discharge in order to integrate communication with each other. These electrical discharges are exhibited in the form of repetitive oscillations over time. These oscillations are called EEG frequencies. Classically, these frequencies from low to high are named delta (δ; 0,5–3,5 Hz), theta (θ; 3,5–7 Hz), alpha (α; 8–14 Hz), beta (β; 15–30 Hz) and gamma (γ; 30–48 Hz) respectively. According to the conventional approach, delta is generated during sleep, theta, during superficial sleep, alpha during wakefulness with eyes closed and beta is generated while eyes are open, during the performance of cognitive tasks or muscular activity. According to the oscillative approach, EEG frequencies are associated to various cognitive functions. For example, delta as a frequency, plays a role in short term memory, theta in response to stress, alpha, in states of anxiety; beta in cortical stimulation and arousal, and gamma plays a role the communication between different brain regions during sensory information processing (Klimesch 1999; Kocaaslan Atlı et al. 2017).
Our study aimed to evaluate peripheral physiological (EDA and HR) and central nervous system responses (EEG frequencies) obtained from sportsmen and sedentary individuals during the “Concentration Grid Test” (CGT), which was employed in order to generate cognitive stress under time pressure. We hypothesized that sportsmen will be more successful in maintaining and focusing attention under stress compared to sedentary individuals and therefore peripheral and central neuroelectrical activity will differ between the two groups.
Materials and methods
Participants
The study participants were comprised of national sportsmen and sedentary individuals. There were 43 (22 female; mean age 21.49 ± 3.71) participants in the sportsmen group and 33 (23 female; mean age 21.97 ± 3.24) in the sedentary group. Informed consent to participate was obtained from all participants according to Declaration of Helsinki. The study was carried out with the approval of the local ethical committee (2011/22-03). None of the participants had a history of psychiatric or neurological disease or psychotropic drug use.
Procedure
The participants were allowed to rest sitting down for 2 min with their eyes closed, and 2 min with their eyes open (baseline); then the stressor test CGT was carried out. In this test, individuals were presented with a grid containing digits from 00 to 99 in mixed order and obliged to mark the digits in numerical order starting from 00 to the highest digit in 60 s. Peripheral and neuroelectrical responses were simultaneously recorded during the baseline and CGT conditions.
Electrophysiological recordings
All electrophysiological assessments were carried out in the sports psychophysiology laboratory using a bio potential amplifier (MindMedia Nexus II, Holland) connected to a computer. EEG recordings were taken according to the International 10–20 system from the frontal region (F3–F4) bilaterally. The ground electrode was designated as FCz. Electrode impedances were kept at less than 5 kΩ. EEG was recorded at 512 Hz/sec sampling rate with 0.05–48 Hz bandpass filter. Peripheral sympathetic nervous system activity was monitored by measuring electrodermal activity (EDA) with electrodes placed on the second and third fingers of the participant’s non-dominant hand. Pre-gelled electrodes were placed on the surface of the skin one each under the left and right shoulder (sub midclavicular point) and the points on the back corresponding to the left upper abdomen (between the last costal cartilage and the iliac spine). HR was calculated, in beats/min, from the ECG obtained with these electrodes using BioTrace + software. In data analysis, artifact rejection was performed visually in EEG data. The amplitudes which were higher than ± 50 V in continuous EEG were rejected. EEG segments that were less than 30 s were not further analyzed. Then from this data the power of delta (δ; 0.5–3.5 Hz), theta (θ; 3.5–7 Hz), alpha (α; 8–14 Hz), beta (β; 15–30 Hz) and gamma (γ; 30–48 Hz) frequency components were calculated using EEG power spectrum analysis tool of BioTrace + (Mindmedia, Netherlands). The power values were calculated from 1 s epochs of baseline and CGT EEG segments. An equal number of baseline and CGT power values of each subject were averaged and used for statistical analysis.
Statistical analysis
SPSS 15.00 (Leadtools, USA) program was used in the statistical analysis of data. For the peripheral neuroelectrical data analysis, EDA and HR values were subjected to a repeated measures of ANOVA, with TYPE (2 level: sedentary; sportsmen) × CONDITION (2 level: baseline; CGT) × PERIPHERAL ACTIVITY (2 level: EDA; HR) design. For the central neuroelectrical data analysis, the power of frequencies was analyzed by means of repeated measures ANOVAs, with TYPE (2 level: sedentary; sportsmen) × CONDITION (2 level: baseline; CGT) × CENTRAL ACTIVITY (5 level: δ, θ, α, β, γ) design. Greenhouse–Geisser correction was applied. Following a significant effect on the repeated measures, a paired samples t test was conducted in within-subject design for compare baseline and CGT condition of variables (EDA, HR, δ, θ, α, β, γ). An independent-samples t-test was conducted in between subject design to compare variables of sedentaries and sportsmen during baseline or CGT. The CGT performance score between sedentaries and sportsmen was compared with a paired samples t-test. A Pearson correlation coefficient was computed to assess the relationship between CGT performance score and other variables (EDA, HR, δ, θ, α, β, γ). Results with a p value less than 0.05 were accepted to be statistically significant.
Results
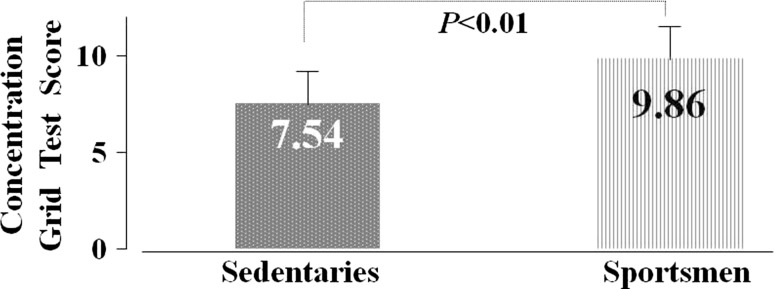
The sportsmen’s mean CGT performance scores were higher than the sedentary individual’s scores (T = − 2.68, p < 0.01), as shown in Fig. 1.
Fig. 1.
Sedentary individual’s and sportsmen’s CGT performance scores
The repeated measures ANOVAs on EDA and HR responses revealed a significant effect for TYPE (F(1,14) = 21.12, p < 0.001), CONDITION (F(1,14) = 71.87, p < 0.001), PERIPHERAL ACTIVITY (F(1,14) = 19.90, p < 0.001). The repeated measures of ANOVA on the frequency power revealed a significant effect for TYPE (F(1,4) = 52.89, p < 0.05), CONDITION (F(1,4) = 56.46, p < 0.05), CENTRAL ACTIVITY (F(1.79,7.19) = 62.21, p < 0.001).
Comparison of resting and test conditions in sedentary individuals and sportsmen
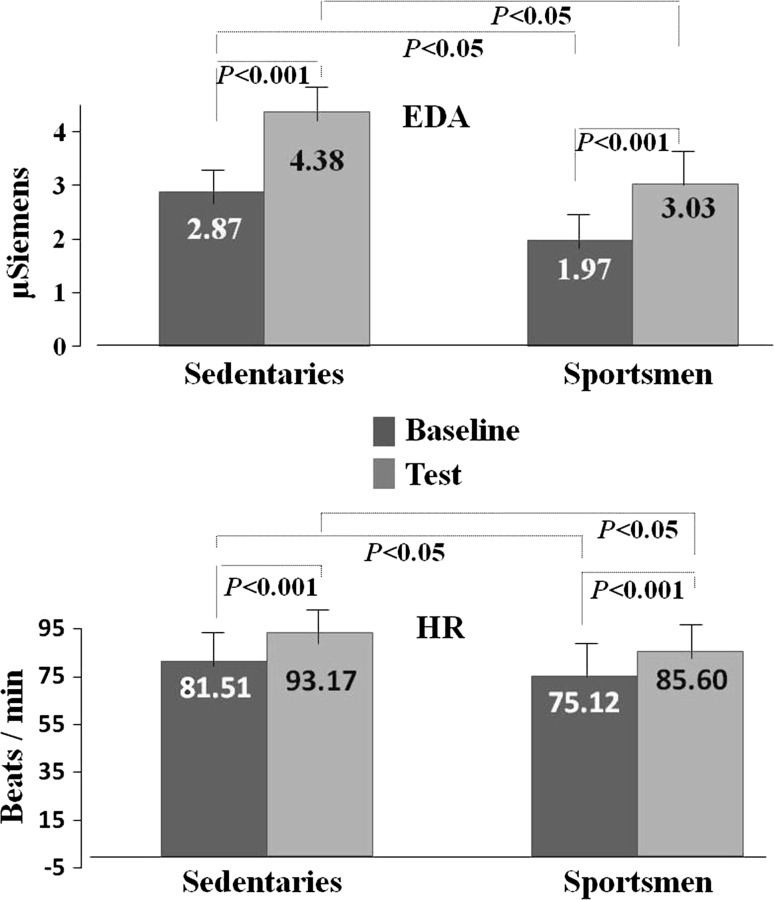
According to baseline state (2.87 ± 1.68), mean EDA values were statistically higher during the test (4.38 ± 2.14) in sedentary individuals (T = − 5.92, p < 0.001). Compared to baseline values (81.51 ± 8.64) HR mean values also increased during the test (93.17 ± 9.46) (T = − 7.48, p < 0.001), as shown in Fig. 2.
Fig. 2.
Peripheral neuroelectrical responses of sedentary individuals and sportsmen. In the above graph EDA responses are presented as µSiemens units and in the graph below HR values are presented as beats/min
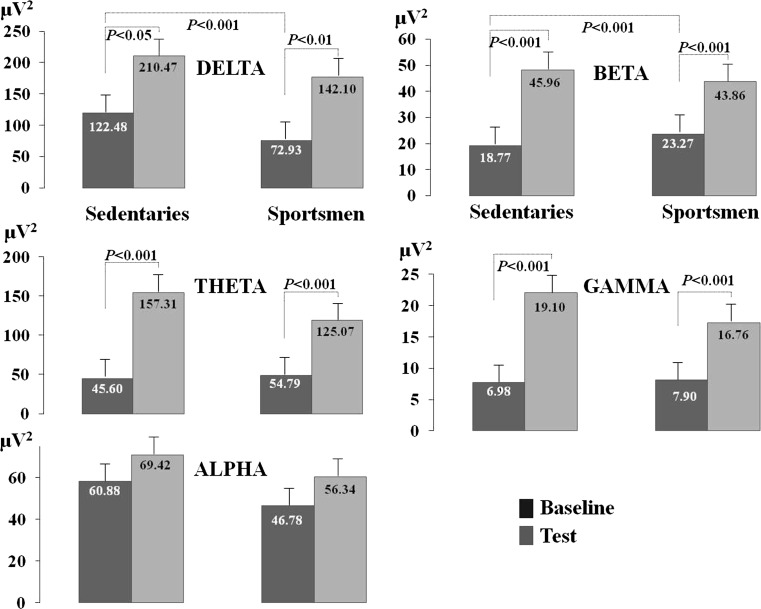
The sedentary individuals’ EEG delta power was found to increase during the test compared to the baseline condition (T = − 2.71, p < 0.05). EEG theta power was higher during testing in comparison to baseline (T = − 5.48, p < 0.001). EEG beta power was also found to increase during the test compared to baseline condition (T = − 7.47, p < 0.001). EEG gamma power statistically increased during testing when compared to baseline (T = − 6.95, p < 0.001). EEG alpha power was also observed to increase compared to baseline in the test condition although this increase was not statistically significant, as shown in Fig. 3.
Fig. 3.
The sportsmen’s and sedentary individual’s central neuroelectrical responses, Delta, Theta, Alpha, Beta and Gamma frequency powers are presented in µV2 units. The dark coloured blocks in the graph represent the baseline condition, while light coloured blocks depict the test condition
EDA value was found to increase during the test (3.03 ± 1.28), compared to baseline (1.97 ± 1.09) in sportsmen (T = − 9.35, p < 0.001). Mean HR value was higher compared to baseline (75.12 ± 12.49) during the test (85.60 ± 11.33) (T = − 9.03, p < 0.001), as shown in Fig. 2. The sportsmen’s EEG delta power increased during testing compared to the baseline condition (T = − 3.39, p < 0.01). EEG theta power was higher during testing compared to baseline (T = − 5.94, p < 0.001). EEG beta power increased during testing compared to baseline (T = − 6.21, p < 0.001). EEG gamma power was higher during testing compared to baseline (T = − 6.03, p < 0.001). Although an increase was observed in EEG alpha power during testing compared to baseline this increase did not reach statistical significance, as shown in Fig. 3.
Comparison of Electrophysiological Data from Sportsmen and Sedentary Individuals
In the baseline condition, EDA value was higher in sedentary individuals than in athletes (t(39.55) = 2.19, p < 0.05). Mean HR value was also higher in sedentary individuals compared to athletes (t(64.09) = 2.36, p < 0.05), as shown in Fig. 2.
During the testing, EDA value was higher in sedentary individuals than in athletes (t(37.14) = 2.80, p < 0.01). Mean HR value was also higher in sedentary individuals compared to athletes (t(65.68) = 3.09, p < 0.01), as shown in Fig. 2.
Baseline delta frequency power was found to be higher in sedentary individuals compared to athletes (t(40.36) = 2.50, p < 0.05). Beta frequency power was higher in athletes compared to sedentary individuals at baseline (t(64) = − 2.10, p < 0.05).
The test/baseline power increase rate in the beta frequency was higher in sedentary individuals when compared to sportsmen (t(60) = 2.08, p < 0.05).
Furthermore, a negative correlation was found between CGT performance and beta frequency increase rates in all of the participants (r = − 0.295, n = 65, p < 0.05).
Discussion
The major finding of our study is that there is a difference in peripheral and central neuroelectrical responses among sportsmen and sedentary individuals. Sportsmen are more successful in maintaining and focusing attention during cognitive stress compared to sedentary individuals, and peripheral and neuroelectrical activity differs between these two groups.
Peripheral nervous system responses
The increase in EDA and HR values during the test compared to baseline in sportsmen and sedentary individuals is a peripheral indicator of the stress response generated to the application of CGT. The lower baseline HR value in sportsmen compared to the sedentary individuals is due to the sportsmen having a non-pathological hypertrophic heart muscle (Erickson 2017). The sedentary individual’s EDA responses were higher compared to the sportsmen. This finding was interpreted to represent higher level of baseline stress in sedentary individuals. The increase in HR and EDA values during testing shows that the applied test generated stress. During the test, sedentary individuals had higher responses compared to sportsmen. This shows that the sympathetic nervous system was more active in sedentary individuals; in other words their arousal level was higher.
Central nervous system responses
All EEG frequency powers are noted to increase during the test. This increase indicates an increase in central neuroelectrical activity and greater interchange of information between neuron populations associated with various oscillatory systems (Knyazev et al. 2006a, b).
Peripheral responses show that stress condition was generated. In our study the delta and theta activities were found to be higher during the test compared to baseline. This has been noted in a study in which frontal delta and theta activity was found to increase during stress generating cold pressure test (Chang et al. 2002).
The CGT implemented in our study requires participants to visually store the positions of numerical digits presented in mixed order in memory and recall this information to mark the digits in numerical order. Therefore, short term memory and working memory needs to be employed during the test. Delta/theta and alpha rhythm have been reported to be associated to short term memory (Bastiaansen et al. 2002; Babiloni et al. 2004). Delta has been reported to be associated to signal detection, decision making (Başar-Eroğlu et al. 1992) and memory updating (Karakaş and Kafadar 1999). Theta has been reported to be associated to selective attention, and theta power has been noted to increase with increasing attentional demand and task difficulty. Furthermore, theta is associated to working memory (Balconi and Lucchiari 2006; Klimesch et al. 2008; Sammer et al. 2007). There are studies reporting theta power increase during mental arithmetic and working memory tasks (Boha et al. 2009; Molnár et al. 2008). Among our findings the increase in these three frequency powers (delta, theta and alpha) during the test is in line with findings in literature. In addition, as seen in Fig. 3, the higher rate of increase in delta frequency power in sportsmen may reflect that, compared to sedentary individuals, sportsmen are more successful in cognitive tasks such as those which require information processing and selective attention. Likewise, the sportsmen’s CGT performance is observed to be higher.
The theta frequency band is known to arise in response to stress (Chang et al. 2002). In our study the greatest increase in EEG frequencies during the test compared to baseline was observed in the theta frequency. The rate of increase in theta frequency is higher compared to the rest of the frequencies in both the sportsmen and sedentary individuals, as shown in Fig. 3. This finding suggests that theta can be evaluated as a stress marker. Furthermore, theta power increase was higher in sedentary individuals. Balconi and Lucchiari, report that theta power to increases with increasing attentional demand and task difficulty (Balconi and Lucchiari 2006). Accordingly, sedentary individuals can be considered to be more pressured during the test compared to the sportsmen.
Anxious individuals are more alert and vigilant in unfamiliar environments and their baseline alpha powers are higher and increases even more in anxiogenic environment (Bell et al. 1998; Knyazev et al. 2004a; Herrmann and Winterer 1996). The alpha frequency power was observed to be higher in sedentary individuals compared to sportsmen; although the difference was not statistically significant, as shown in Fig. 3. The higher baseline alpha power among sedentary individuals compared to sportsmen may imply that they were more anxious than the sportsmen.
Beta is a frequency that has been associated to alertness and cortical arousal (Knyazev et al. 2006b). In our study the test/baseline increase rate in beta frequency was higher in sedentary individuals than in sportsmen. Therefore sedentary individuals may be interpreted to have a higher state of cortical arousal and alertness compared to sportsmen. However considering the weak increase in beta power in sportsmen paired with lower peripheral neuroelectrical responses, sportsmen can be said to be more successful under stress on tasks requiring attention. In fact when CGT performance scores are examined, the sportsmen’s higher performance indicates that they are more successful in maintaining continuous attention and focused attention compared to sedentary individuals. Beta is known to play a role in cognitive processes involving mathematical tasks (Neuper and Pfurtscheller 2001; Kantor et al. 2002). The increase in beta power during the CGT in all participants in our study is in line with this information. Jung et al. report a high correlation between beta power increase and error rate (Jung et al. 1997). Wang et al. (2015), reported low beta power in high attentional performance. In our study, while the beta power increase rate was lower in sportsmen with high CGT scores, the beta power increase rate was higher in sedentary individuals who had low CGT scores. Thus, a negative association is statistically confirmed between CGT performance and beta power increase rate.
Conclusion
Considering the weak increase observed in beta power, low peripheral neuroelectrical responses and high CGT performance among sportsmen, sportsmen may be said to be more successful, than sedentary individuals, in cognitive processes such as maintaining continuous attention and focused attention under stress. In literature, expert sport performers are reported to increase attentional workload during performance and to be more advantaged in information processing while attaining cognitive skills and strategies (Eccles 2006; Furley and Dörr 2016; Furley and Wood 2016).
Some practical outputs that the study results can be support are listed as follow:
(1) The CGT performance can be use for measuring attention under stress before competition. (2) The peripheral neuroelectrical responses such as EDA and HR can be use for monitoring stress levels in the resting condition before competition. (3) Learning to strategies to control of EEG (delta, theta, beta and alpha) bands using a basic neurofeedback device may be useful to enhance continuous attention and focused attention under stress.
Further studies incorporating the use of multi channeled EEG systems are planned to investigate the beneficial effects of sports on focused and continuous attentional performance under stress, including the effects of various sport branches and exercise intensity. We will also try to develop simple neuroelectrical methods to enable mental strategies to be developed so that athletes can control their own EEG power (e.g., beta) under stress.
Acknowledgements
This study has been supported by Dokuz Eylül University Research Fund Accountancy, project number 2011.KB.SAG.059. Authors thank clinical psychologist Nur Evirgen for her contributions and language help.
References
- Babiloni C, Bares M, Vecchio F, Brazdil M, Jurak P, Moretti DV, Ubaldi A, Rossini PM, Rektor I. Synchronization of gamma oscillations increases functional connectivity of human hippocampus and inferior-middle temporal cortex during repetitive visuomotor events. Eur J Neurosci. 2004;19(11):3088–3098. doi: 10.1111/j.0953-816X.2004.03431.x. [DOI] [PubMed] [Google Scholar]
- Balconi M, Lucchiari C. EEG correlates (event-related desynchronization) of emotional face elaboration: a temporal analysis. Neurosci Lett. 2006;392:118–123. doi: 10.1016/j.neulet.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Başar-Eroğlu C, Başar E, Demiralp T, Schürmann M. P300 response: possible psychophysiological correlates in delta and theta frequency channels. Int J Psychophysiol. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-G. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, Posthuma D, Groot PF, de Geus EJ. Event-related alpha and theta responses in a visuo-spatial working memory task. Clin Neurophysiol. 2002;113:1882–1893. doi: 10.1016/S1388-2457(02)00303-6. [DOI] [PubMed] [Google Scholar]
- Bell IR, Schwartz GE, Hardin EE, Baldwin CM, Kline JP. Differential resting quantitative electroencephalographic alpha patterns in women with environmental chemical intolerance, depressives, and normals. Biol Psychiatry. 1998;43(5):376–388. doi: 10.1016/S0006-3223(97)00245-X. [DOI] [PubMed] [Google Scholar]
- Boha R, Molnár M, Gaál ZA, Czigler B, Róna K, Kass K, Klausz G. The acute effect of low-dose alcohol on working memory during mental arithmetic I. Behavioral measures and EEG theta band spectral characteristics. Int J Psychophysiol. 2009;73:133–137. doi: 10.1016/j.ijpsycho.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Chang PF, Arendt-Nielsen L, Chen ACN. Dynamic changes and spatial correlation of EEG activities during cold pressor test in man. Brain Res Bull. 2002;57:667–675. doi: 10.1016/S0361-9230(01)00763-8. [DOI] [PubMed] [Google Scholar]
- Eccles DW. Thinking outside of the box: the role of environmental adaptation in the acquisition of skilled and expert performance. J Sports Sci. 2006;24(10):1103–1114. doi: 10.1080/02640410500432854. [DOI] [PubMed] [Google Scholar]
- Erickson CC. Discrimination of the “Athlete’s Heart” from real disease by electrocardiogram and echocardiogram. Cardiol Young. 2017;27(Suppl. 1):S80–S88. doi: 10.1017/S1047951116002286. [DOI] [PubMed] [Google Scholar]
- Furley P, Dörr J. Eddie would(n’t) go! Perceptual-cognitive expertise in surfing. Psychol Sport Exerc. 2016;22:66–71. doi: 10.1016/j.psychsport.2015.06.008. [DOI] [Google Scholar]
- Furley P, Wood G. Working memory, attentional control, and expertise in sports: a review of current literature and directions for future research. J Appl Res Mem Cogn. 2016;5(4):415–425. doi: 10.1016/j.jarmac.2016.05.001. [DOI] [Google Scholar]
- Herrmann WM, Winterer G. Electroencephalography in psychiatry-current status and outlook. Nervenarzt. 1996;67:348–359. [PubMed] [Google Scholar]
- Jena SK. Examination stress and its effect on EEG. Int J Med Sci Public Health. 2015;11(4):1493–1497. doi: 10.5455/ijmsph.2015.23042015308. [DOI] [Google Scholar]
- Jung TP, Makeig S, Stensmo M, Sejnowski TJ. Estimating alertness from the EEG power spectrum. IEEE Trans Biomed Eng. 1997;44(1):60–69. doi: 10.1109/10.553713. [DOI] [PubMed] [Google Scholar]
- Kantor S, Jakus R, Bodizs R, Halasz P, Bagdy G. Acute and long term effects of the 5-HT2 receptor antagonist ritanserin on EEG power spectra, motor activity, and sleep: changes at the light-dark phase shift. Brain Res. 2002;5:105–111. doi: 10.1016/S0006-8993(02)02698-7. [DOI] [PubMed] [Google Scholar]
- Karakaş S, Kafadar H. Şizofrenideki bilişsel süreçlerin değerlendirilmesinde nöropsikolojik testler: bellek ve dikkatin ölçülmesi. Şizofreni Dizisi. 1999;4:132–152. [Google Scholar]
- Kaufman DM, Day V, Mensink D. Stressors in 1st-year medical school: comparison of a conventional and problem-based curriculum. Teach Learn Med. 1996;8(4):188–194. doi: 10.1080/10401339609539796. [DOI] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999;29:169–195. doi: 10.1016/S0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Freunberger R, Sauseng P, Gruber W. A short review of slow phase synchronization and memory: evidence for control processes in different memory systems? Brain Res. 2008;1235:31–44. doi: 10.1016/j.brainres.2008.06.049. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Savostyanov AN, Levin EA. Alpha oscillations as a correlate of trait anxiety. Int J Psychophysiol. 2004;53(2):47–160. doi: 10.1016/j.ijpsycho.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Savostyanov AN, Levin EA. Alpha synchronization and anxiety: implications for inhibition vs. alertness hypotheses. Int J Psychophysiol. 2006;59:151–158. doi: 10.1016/j.ijpsycho.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Schutter DJLG, van Honk J. Anxious apprehension increases coupling of delta and beta oscillations. Int J Psychophysiol. 2006;61:283–287. doi: 10.1016/j.ijpsycho.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Kocaaslan Atlı S, Bayazıt O, Kahya MC. Biophysical bases of electroencephalography (article in Turkish with an abstract in English) Turk Klinikleri J Neurol Spec Top. 2017;10(2):110–114. [Google Scholar]
- Kurniawan H, Maslov AV, Pechenizkiy M. Stress detection from speech and galvanic skin response signals. Proc IEEE Int Symp Comput Based Med Syst. 2013 [Google Scholar]
- Molnár M, Csuhaj R, ZsA Gaál, Czigler B, Ulbert I, Boha R, Kondákor I. Spectral characteristics and linear–nonlinear synchronization changes of different EEG frequency bands during the CNV. Psychophysiology. 2008;45:412–419. doi: 10.1111/j.1469-8986.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol. 2001;43(1):41–58. doi: 10.1016/S0167-8760(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Sammer G, Blecker C, Gebhardt H, Bischoff M, Stark R, Morgen K, Vaitl D. Relationship between regional hemodynamic activity and simultaneously recorded EEG-theta associated with mental arithmetic-induced workload. Hum Brain Mapp. 2007;28:793–803. doi: 10.1002/hbm.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarejo MV, Zapirain BG, Zorrilla AM. A Stress sensor based on Galvanic skin response (GSR) controlled by ZigBee. Sensors. 2012;12:6075–6101. doi: 10.3390/s120506075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Tsai CL, Tu KC, Muggleton NG, Juan CH, Liang WK. Modulation of brain oscillations during fundamental visuo-spatial processing: a comparison between female collegiate badminton players and sedentary controls. Psychol Sport Exerc. 2015;16:121–129. doi: 10.1016/j.psychsport.2014.10.003. [DOI] [Google Scholar]